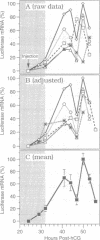
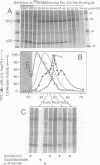
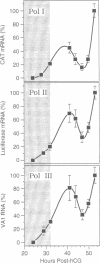
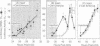Abstract
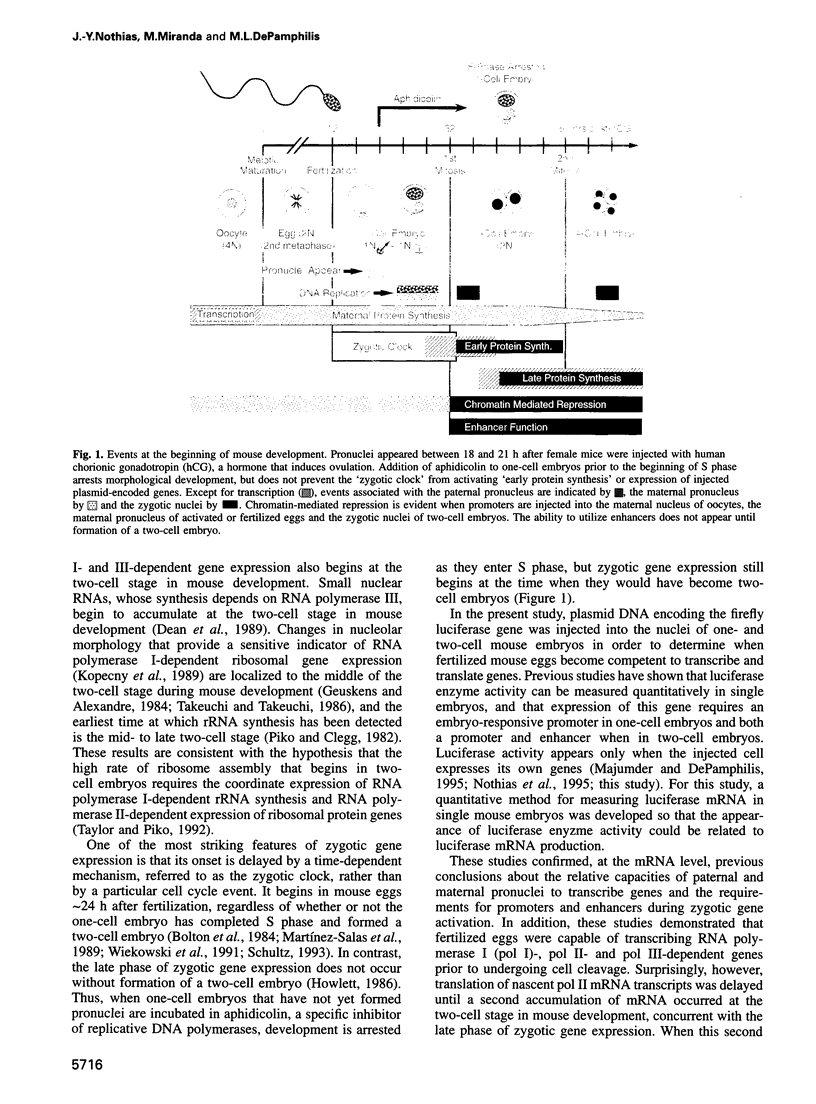
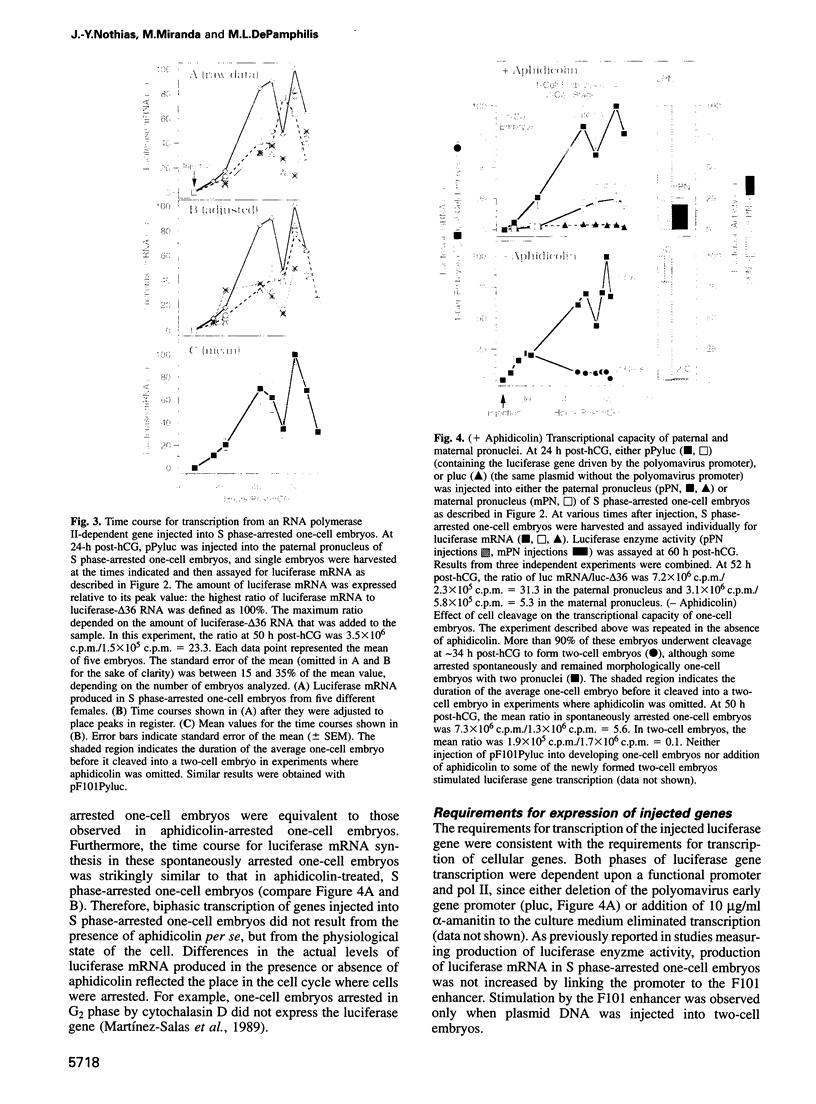
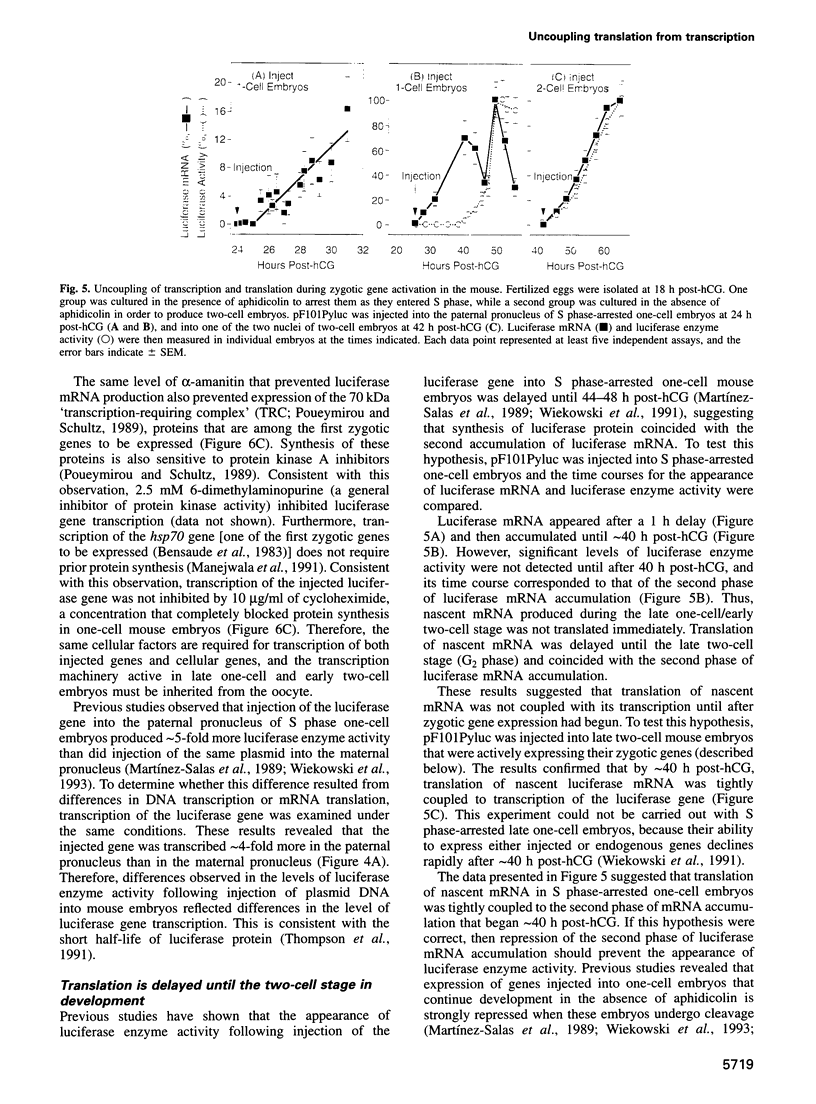
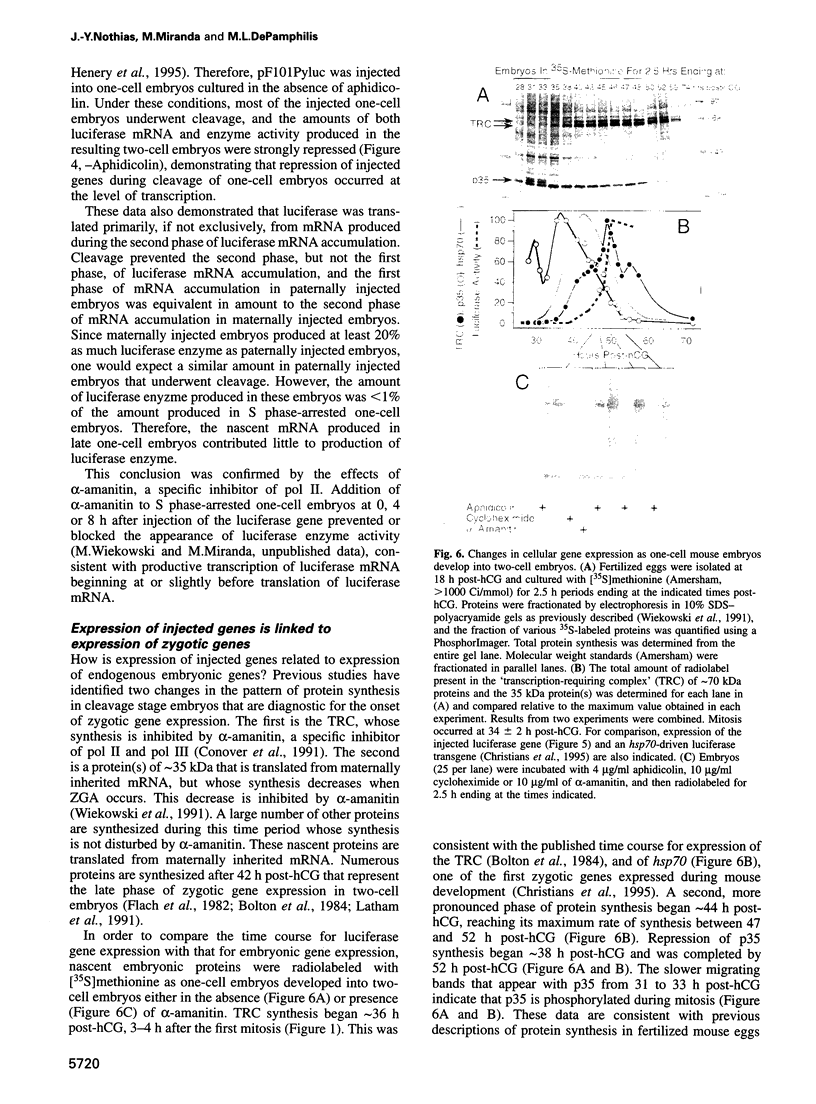
Zygotic gene expression in mice is delayed by a time-dependent mechanism until the two-cell stage in development. To investigate the basis of this 'zygotic clock', the firefly luciferase gene was injected into mouse embryos, and quantitative assays were used to monitor luciferase gene transcription and translation in individual embryos from single mothers. These studies confirmed, at the mRNA level, previous conclusions about the relative capacities of paternal and maternal pronuclei to transcribe genes, and the requirements for promoters and enhancers during zygotic gene activation. Furthermore, these studies revealed that fertilized mouse eggs can delay expression of zygotic genes by uncoupling translation from transcription. An RNA polymerase II-dependent gene could be translated until zygotic gene expression began (a delay of up to 15 h after injection). The time course for nascent mRNA accumulation was biphasic, with the second phase occurring during zygotic gene expression. If the luciferase gene was injected after zygotic gene expression had begun, then translation was tightly linked to transcription. If the second phase of mRNA accumulation was repressed, then luciferase was not produced. Therefore, translation was linked to the accumulation of mRNA during the onset of zygotic gene expression. Similar biphasic time courses also were observed for RNA polymerase I- and III-dependent transcription. These and other results reveal that the zygotic clock regulates the onset of both transcription and translation of zygotic genes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
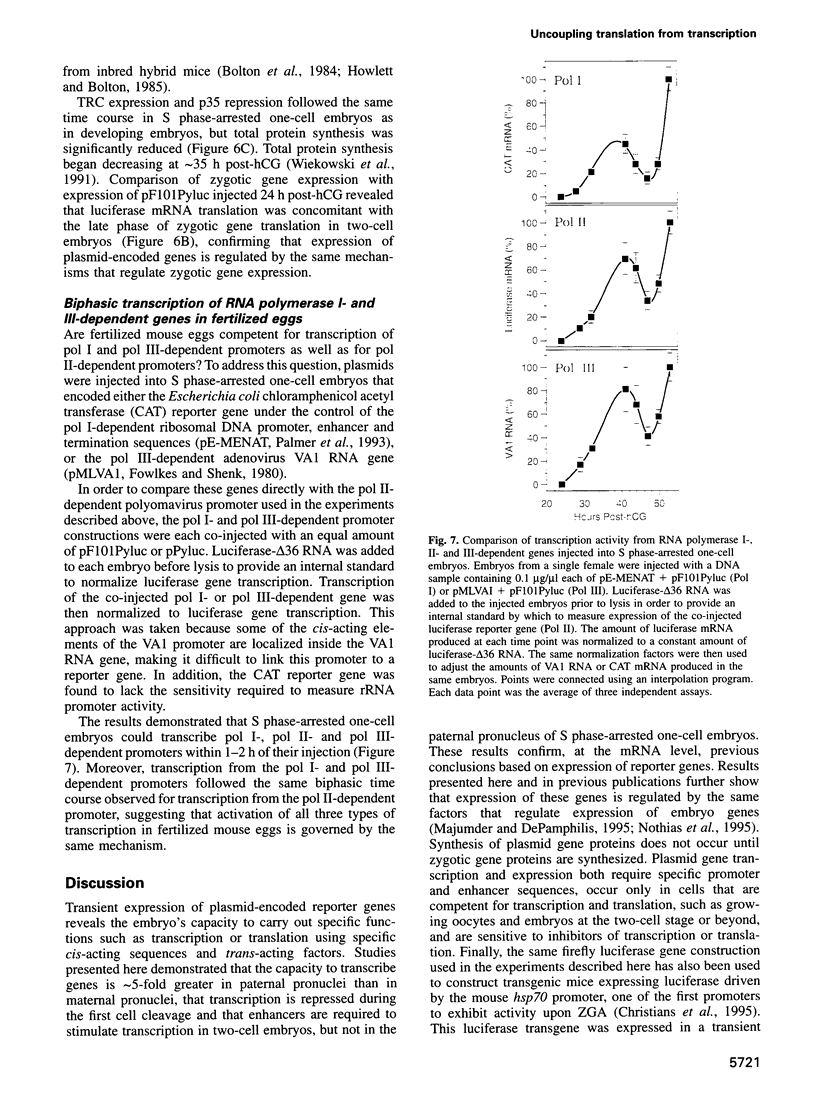
- Adenot P. G., Szöllösi M. S., Geze M., Renard J. P., Debey P. Dynamics of paternal chromatin changes in live one-cell mouse embryo after natural fertilization. Mol Reprod Dev. 1991 Jan;28(1):23–34. doi: 10.1002/mrd.1080280105. [DOI] [PubMed] [Google Scholar]
- Almouzni G., Wolffe A. P. Constraints on transcriptional activator function contribute to transcriptional quiescence during early Xenopus embryogenesis. EMBO J. 1995 Apr 18;14(8):1752–1765. doi: 10.1002/j.1460-2075.1995.tb07164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensaude O., Babinet C., Morange M., Jacob F. Heat shock proteins, first major products of zygotic gene activity in mouse embryo. Nature. 1983 Sep 22;305(5932):331–333. doi: 10.1038/305331a0. [DOI] [PubMed] [Google Scholar]
- Bolton V. N., Oades P. J., Johnson M. H. The relationship between cleavage, DNA replication, and gene expression in the mouse 2-cell embryo. J Embryol Exp Morphol. 1984 Feb;79:139–163. [PubMed] [Google Scholar]
- Bouniol C., Nguyen E., Debey P. Endogenous transcription occurs at the 1-cell stage in the mouse embryo. Exp Cell Res. 1995 May;218(1):57–62. doi: 10.1006/excr.1995.1130. [DOI] [PubMed] [Google Scholar]
- Bouvet P., Wolffe A. P. A role for transcription and FRGY2 in masking maternal mRNA within Xenopus oocytes. Cell. 1994 Jun 17;77(6):931–941. doi: 10.1016/0092-8674(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques. 1993 Sep;15(3):532-4, 536-7. [PubMed] [Google Scholar]
- Christians E., Campion E., Thompson E. M., Renard J. P. Expression of the HSP 70.1 gene, a landmark of early zygotic activity in the mouse embryo, is restricted to the first burst of transcription. Development. 1995 Jan;121(1):113–122. doi: 10.1242/dev.121.1.113. [DOI] [PubMed] [Google Scholar]
- Clegg K. B., Pikó L. Poly(A) length, cytoplasmic adenylation and synthesis of poly(A)+ RNA in early mouse embryos. Dev Biol. 1983 Feb;95(2):331–341. doi: 10.1016/0012-1606(83)90034-9. [DOI] [PubMed] [Google Scholar]
- Conover J. C., Temeles G. L., Zimmermann J. W., Burke B., Schultz R. M. Stage-specific expression of a family of proteins that are major products of zygotic gene activation in the mouse embryo. Dev Biol. 1991 Apr;144(2):392–404. doi: 10.1016/0012-1606(91)90431-2. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Herman S. A., Martínez-Salas E., Chalifour L. E., Wirak D. O., Cupo D. Y., Miranda M. Microinjecting DNA into mouse ova to study DNA replication and gene expression and to produce transgenic animals. Biotechniques. 1988 Jul-Aug;6(7):662–680. [PubMed] [Google Scholar]
- Dean W. L., Seufert A. C., Schultz G. A., Prather R. S., Simerly C., Schatten G., Pilch D. R., Marzluff W. F. The small nuclear RNAs for pre-mRNA splicing are coordinately regulated during oocyte maturation and early embryogenesis in the mouse. Development. 1989 Jun;106(2):325–334. doi: 10.1242/dev.106.2.325. [DOI] [PubMed] [Google Scholar]
- Ebert K. M., Brinster R. L. Rabbit alpha-globin messenger RNA translation by the mouse ovum. J Embryol Exp Morphol. 1983 Apr;74:159–168. [PubMed] [Google Scholar]
- Ebert K. M., Paynton B. V., McKnight G. S., Brinster R. L. Translation and stability of ovalbumin messenger RNA injected into growing oocytes and fertilized ova of mice. J Embryol Exp Morphol. 1984 Dec;84:91–103. [PubMed] [Google Scholar]
- Flach G., Johnson M. H., Braude P. R., Taylor R. A., Bolton V. N. The transition from maternal to embryonic control in the 2-cell mouse embryo. EMBO J. 1982;1(6):681–686. doi: 10.1002/j.1460-2075.1982.tb01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlkes D. M., Shenk T. Transcriptional control regions of the adenovirus VAI RNA gene. Cell. 1980 Nov;22(2 Pt 2):405–413. doi: 10.1016/0092-8674(80)90351-7. [DOI] [PubMed] [Google Scholar]
- Geuskens M., Alexandre H. Ultrastructural and autoradiographic studies of nucleolar development and rDNA transcription in preimplantation mouse embryos. Cell Differ. 1984 Jun;14(2):125–134. doi: 10.1016/0045-6039(84)90037-x. [DOI] [PubMed] [Google Scholar]
- Henery C. C., Miranda M., Wiekowski M., Wilmut I., DePamphilis M. L. Repression of gene expression at the beginning of mouse development. Dev Biol. 1995 Jun;169(2):448–460. doi: 10.1006/dbio.1995.1160. [DOI] [PubMed] [Google Scholar]
- Howlett S. K., Bolton V. N. Sequence and regulation of morphological and molecular events during the first cell cycle of mouse embryogenesis. J Embryol Exp Morphol. 1985 Jun;87:175–206. [PubMed] [Google Scholar]
- Kopecný V., Fléchon J. E., Camous S., Fulka J., Jr Nucleologenesis and the onset of transcription in the eight-cell bovine embryo: fine-structural autoradiographic study. Mol Reprod Dev. 1989;1(2):79–90. doi: 10.1002/mrd.1080010202. [DOI] [PubMed] [Google Scholar]
- Latham K. E., Garrels J. I., Chang C., Solter D. Quantitative analysis of protein synthesis in mouse embryos. I. Extensive reprogramming at the one- and two-cell stages. Development. 1991 Aug;112(4):921–932. doi: 10.1242/dev.112.4.921. [DOI] [PubMed] [Google Scholar]
- Latham K. E., Solter D., Schultz R. M. Acquisition of a transcriptionally permissive state during the 1-cell stage of mouse embryogenesis. Dev Biol. 1992 Feb;149(2):457–462. doi: 10.1016/0012-1606(92)90300-6. [DOI] [PubMed] [Google Scholar]
- Majumder S., DePamphilis M. L. A unique role for enhancers is revealed during early mouse development. Bioessays. 1995 Oct;17(10):879–889. doi: 10.1002/bies.950171010. [DOI] [PubMed] [Google Scholar]
- Manejwala F. M., Logan C. Y., Schultz R. M. Regulation of hsp70 mRNA levels during oocyte maturation and zygotic gene activation in the mouse. Dev Biol. 1991 Apr;144(2):301–308. doi: 10.1016/0012-1606(91)90423-z. [DOI] [PubMed] [Google Scholar]
- Martínez-Salas E., Linney E., Hassell J., DePamphilis M. L. The need for enhancers in gene expression first appears during mouse development with formation of the zygotic nucleus. Genes Dev. 1989 Oct;3(10):1493–1506. doi: 10.1101/gad.3.10.1493. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Anzai M., Nakagata N., Takahashi A., Takahashi Y., Miyata K. Onset of paternal gene activation in early mouse embryos fertilized with transgenic mouse sperm. Mol Reprod Dev. 1994 Oct;39(2):136–140. doi: 10.1002/mrd.1080390203. [DOI] [PubMed] [Google Scholar]
- Miranda M., DePamphilis M. L. Preparation of injection pipettes. Methods Enzymol. 1993;225:407–412. doi: 10.1016/0076-6879(93)25028-z. [DOI] [PubMed] [Google Scholar]
- Miranda M., Majumder S., Wiekowski M., DePamphilis M. L. Application of firefly luciferase to preimplantation development. Methods Enzymol. 1993;225:412–433. doi: 10.1016/0076-6879(93)25029-2. [DOI] [PubMed] [Google Scholar]
- Mélin F., Miranda M., Montreau N., DePamphilis M. L., Blangy D. Transcription enhancer factor-1 (TEF-1) DNA binding sites can specifically enhance gene expression at the beginning of mouse development. EMBO J. 1993 Dec;12(12):4657–4666. doi: 10.1002/j.1460-2075.1993.tb06154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport J., Kirschner M. A major developmental transition in early Xenopus embryos: II. Control of the onset of transcription. Cell. 1982 Oct;30(3):687–696. doi: 10.1016/0092-8674(82)90273-2. [DOI] [PubMed] [Google Scholar]
- Nothias J. Y., Majumder S., Kaneko K. J., DePamphilis M. L. Regulation of gene expression at the beginning of mammalian development. J Biol Chem. 1995 Sep 22;270(38):22077–22080. doi: 10.1074/jbc.270.38.22077. [DOI] [PubMed] [Google Scholar]
- Palmer T. D., Miller A. D., Reeder R. H., McStay B. Efficient expression of a protein coding gene under the control of an RNA polymerase I promoter. Nucleic Acids Res. 1993 Jul 25;21(15):3451–3457. doi: 10.1093/nar/21.15.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paynton B. V., Bachvarova R. Polyadenylation and deadenylation of maternal mRNAs during oocyte growth and maturation in the mouse. Mol Reprod Dev. 1994 Feb;37(2):172–180. doi: 10.1002/mrd.1080370208. [DOI] [PubMed] [Google Scholar]
- Paynton B. V., Rempel R., Bachvarova R. Changes in state of adenylation and time course of degradation of maternal mRNAs during oocyte maturation and early embryonic development in the mouse. Dev Biol. 1988 Oct;129(2):304–314. doi: 10.1016/0012-1606(88)90377-6. [DOI] [PubMed] [Google Scholar]
- Pikó L., Clegg K. B. Quantitative changes in total RNA, total poly(A), and ribosomes in early mouse embryos. Dev Biol. 1982 Feb;89(2):362–378. doi: 10.1016/0012-1606(82)90325-6. [DOI] [PubMed] [Google Scholar]
- Poueymirou W. T., Schultz R. M. Regulation of mouse preimplantation development: inhibition of synthesis of proteins in the two-cell embryo that require transcription by inhibitors of cAMP-dependent protein kinase. Dev Biol. 1989 Jun;133(2):588–599. doi: 10.1016/0012-1606(89)90061-4. [DOI] [PubMed] [Google Scholar]
- Prioleau M. N., Huet J., Sentenac A., Méchali M. Competition between chromatin and transcription complex assembly regulates gene expression during early development. Cell. 1994 May 6;77(3):439–449. doi: 10.1016/0092-8674(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Ram P. T., Schultz R. M. Reporter gene expression in G2 of the 1-cell mouse embryo. Dev Biol. 1993 Apr;156(2):552–556. doi: 10.1006/dbio.1993.1101. [DOI] [PubMed] [Google Scholar]
- Rodman T. C., Pruslin F. H., Hoffmann H. P., Allfrey V. G. Turnover of basic chromosomal proteins in fertilized eggs: a cytoimmunochemical study of events in vivo. J Cell Biol. 1981 Aug;90(2):351–361. doi: 10.1083/jcb.90.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz G. A., Heyner S. Gene expression in pre-implantation mammalian embryos. Mutat Res. 1992 Dec;296(1-2):17–31. doi: 10.1016/0165-1110(92)90029-9. [DOI] [PubMed] [Google Scholar]
- Schultz R. M. Regulation of zygotic gene activation in the mouse. Bioessays. 1993 Aug;15(8):531–538. doi: 10.1002/bies.950150806. [DOI] [PubMed] [Google Scholar]
- Takeuchi I. K., Takeuchi Y. K. Ultrastructural localization of Ag-NOR proteins in full-grown oocytes and preimplantation embryos of mice. J Electron Microsc (Tokyo) 1986;35(3):280–287. [PubMed] [Google Scholar]
- Taylor K. D., Pikó L. Expression of ribosomal protein genes in mouse oocytes and early embryos. Mol Reprod Dev. 1992 Mar;31(3):182–188. doi: 10.1002/mrd.1080310304. [DOI] [PubMed] [Google Scholar]
- Temeles G. L., Ram P. T., Rothstein J. L., Schultz R. M. Expression patterns of novel genes during mouse preimplantation embryogenesis. Mol Reprod Dev. 1994 Feb;37(2):121–129. doi: 10.1002/mrd.1080370202. [DOI] [PubMed] [Google Scholar]
- Thompson J. F., Hayes L. S., Lloyd D. B. Modulation of firefly luciferase stability and impact on studies of gene regulation. Gene. 1991 Jul 22;103(2):171–177. doi: 10.1016/0378-1119(91)90270-l. [DOI] [PubMed] [Google Scholar]
- Vasseur M., Condamine H., Duprey P. RNAs containing B2 repeated sequences are transcribed in the early stages of mouse embryogenesis. EMBO J. 1985 Jul;4(7):1749–1753. doi: 10.1002/j.1460-2075.1985.tb03846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vautier D., Besombes D., Chassoux D., Aubry F., Debey P. Redistribution of nuclear antigens linked to cell proliferation and RNA processing in mouse oocytes and early embryos. Mol Reprod Dev. 1994 Jun;38(2):119–130. doi: 10.1002/mrd.1080380202. [DOI] [PubMed] [Google Scholar]
- Vernet M., Bonnerot C., Briand P., Nicolas J. F. Changes in permissiveness for the expression of microinjected DNA during the first cleavages of mouse embryos. Mech Dev. 1992 Feb;36(3):129–139. doi: 10.1016/0925-4773(92)90064-q. [DOI] [PubMed] [Google Scholar]
- White R. J., Jackson S. P. The TATA-binding protein: a central role in transcription by RNA polymerases I, II and III. Trends Genet. 1992 Aug;8(8):284–288. doi: 10.1016/0168-9525(92)90255-3. [DOI] [PubMed] [Google Scholar]
- Wiekowski M., Miranda M., DePamphilis M. L. Regulation of gene expression in preimplantation mouse embryos: effects of the zygotic clock and the first mitosis on promoter and enhancer activities. Dev Biol. 1991 Oct;147(2):403–414. doi: 10.1016/0012-1606(91)90298-h. [DOI] [PubMed] [Google Scholar]
- Wiekowski M., Miranda M., DePamphilis M. L. Requirements for promoter activity in mouse oocytes and embryos distinguish paternal pronuclei from maternal and zygotic nuclei. Dev Biol. 1993 Sep;159(1):366–378. doi: 10.1006/dbio.1993.1248. [DOI] [PubMed] [Google Scholar]
- Winslow S. G., Henkart P. A. Polyinosinic acid as a carrier in the microscale purification of total RNA. Nucleic Acids Res. 1991 Jun 25;19(12):3251–3253. doi: 10.1093/nar/19.12.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormington M. Unmasking the role of the 3' UTR in the cytoplasmic polyadenylation and translational regulation of maternal mRNAs. Bioessays. 1994 Aug;16(8):533–535. doi: 10.1002/bies.950160804. [DOI] [PubMed] [Google Scholar]
- Worrad D. M., Ram P. T., Schultz R. M. Regulation of gene expression in the mouse oocyte and early preimplantation embryo: developmental changes in Sp1 and TATA box-binding protein, TBP. Development. 1994 Aug;120(8):2347–2357. doi: 10.1242/dev.120.8.2347. [DOI] [PubMed] [Google Scholar]
- Yasuda G. K., Schubiger G. Temporal regulation in the early embryo: is MBT too good to be true? Trends Genet. 1992 Apr;8(4):124–127. doi: 10.1016/0168-9525(92)90369-F. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]