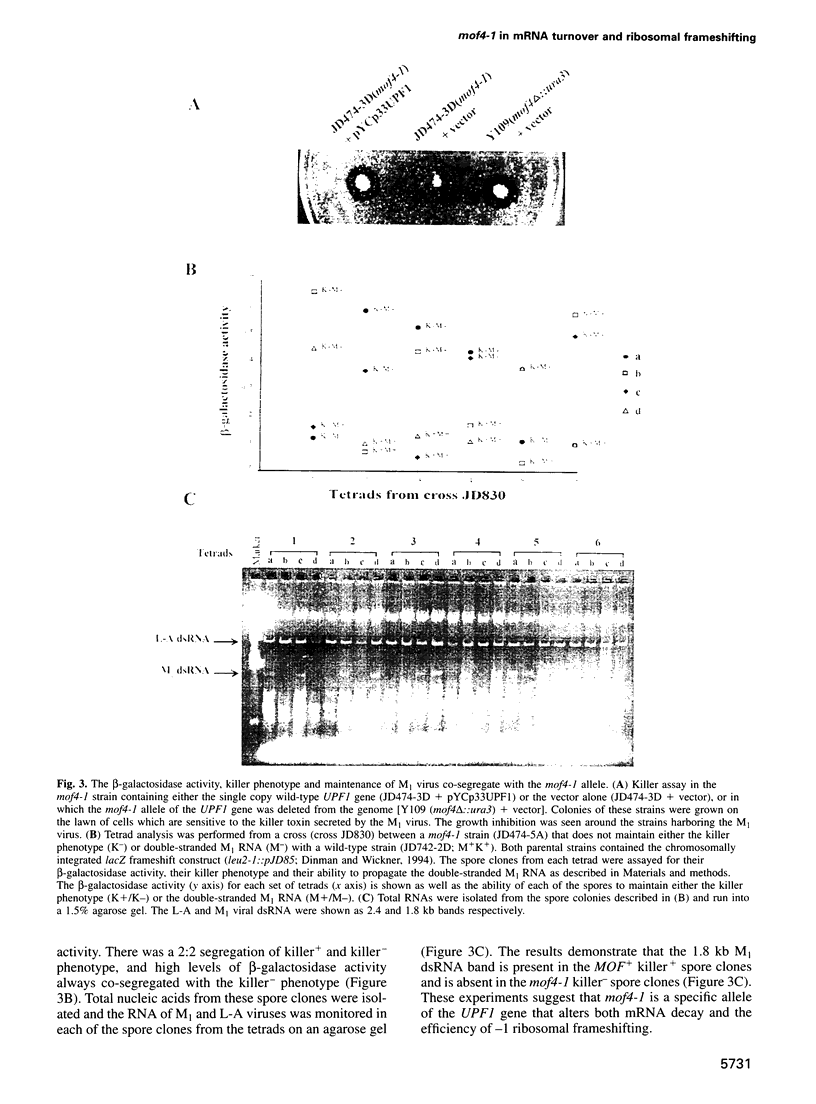
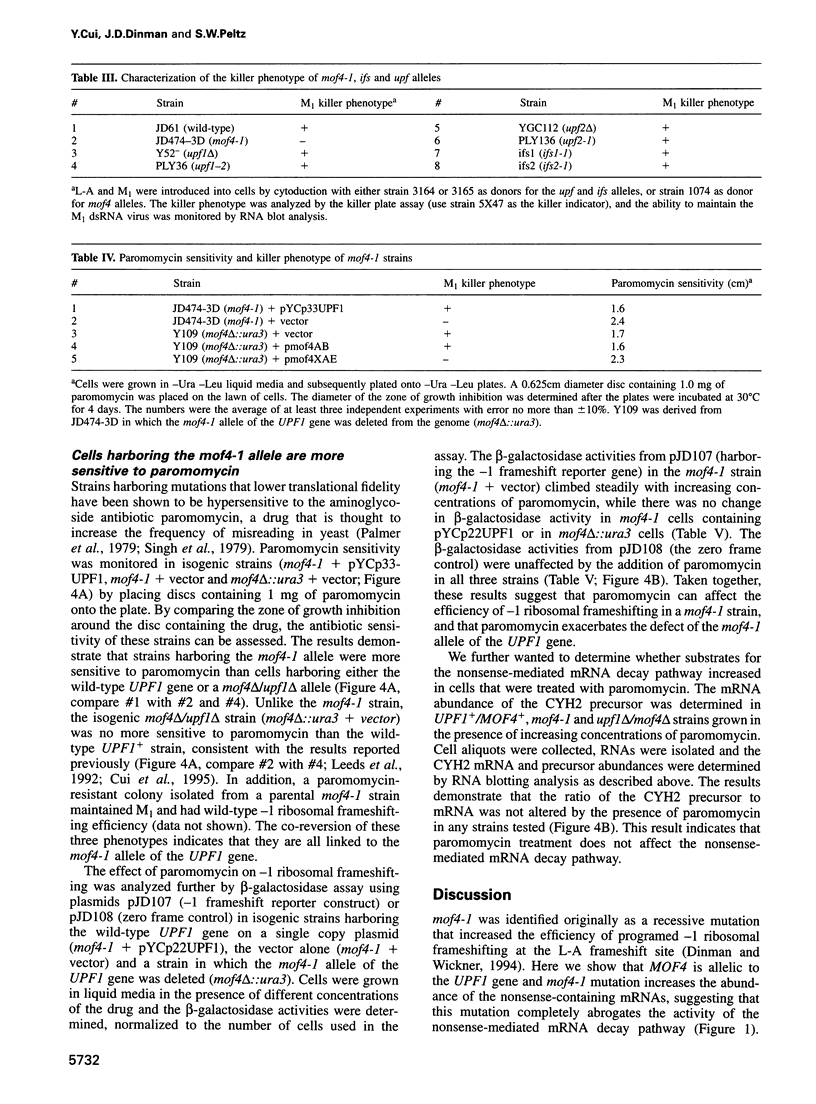
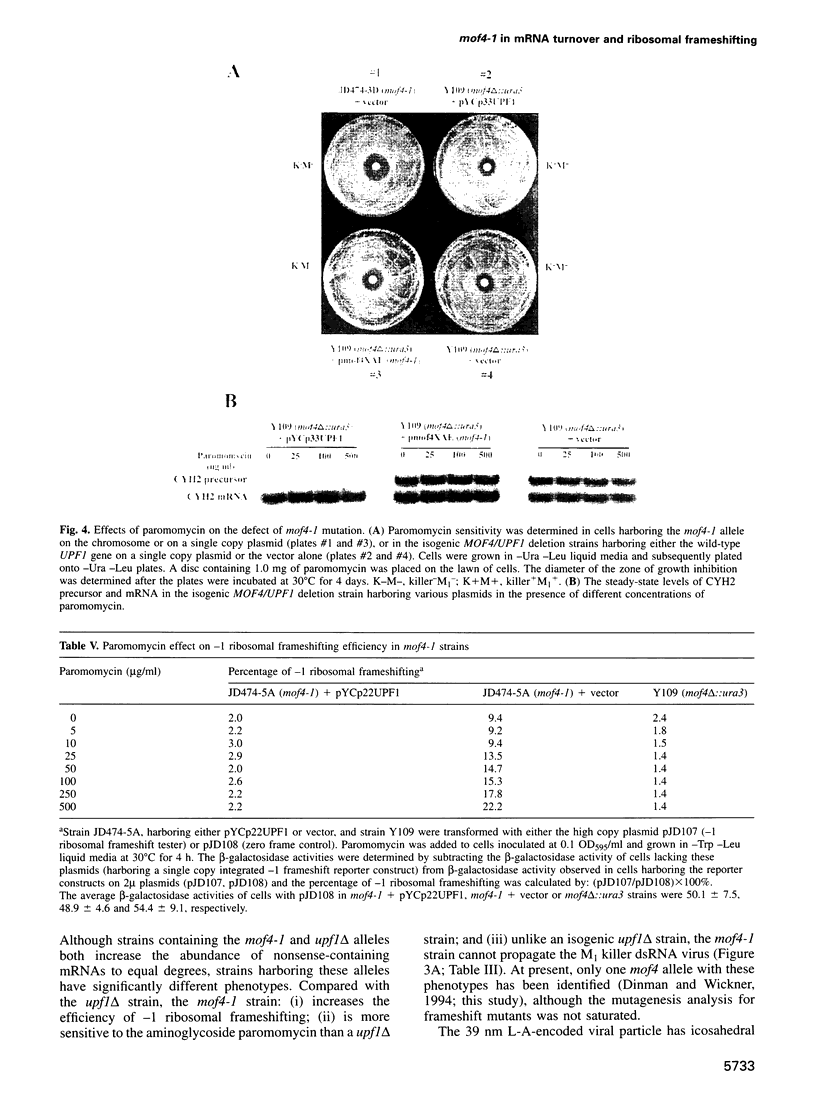
Abstract
The mof4-1 (maintenance of frame) allele in the yeast Saccharomyces cerevisiae was isolated as a chromosomal mutation that increased the efficiency of -1 ribosomal frameshifting at the L-A virus frameshift site and caused loss of M1, the satellite virus of L-A. Here, we demonstrate that strains harboring the mof4-1 allele inactivated the nonsense-mediated mRNA decay pathway. The MOF4 gene was shown to be allelic to UPF1, a gene whose product is involved in the nonsense-mediated mRNA decay pathway. Although cells harboring the mof4-1 allele of the UPF1 gene lose the M1 virus, mutations in other UPF genes involved in nonsense-mediated mRNA decay maintain the M1 virus. The mof4-1 strain is more sensitive to the aminoglycoside antibiotic paromomycin than a upf1 delta strain, and frameshifting efficiency increases in a mof4-1 strain grown in the presence of this drug. Further, the ifs1 and ifs2 alleles previously identified as mutations that enhance frameshifting were shown to be allelic to the UPF2 and UPF1 genes, respectively, and both ifs strains maintained M1. These results indicate that mof4-1 is a unique allele of the UPF1 gene and that the gene product of the mof4-1 allele affects both -1 ribosomal frameshifting and mRNA turnover.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altamura N., Groudinsky O., Dujardin G., Slonimski P. P. NAM7 nuclear gene encodes a novel member of a family of helicases with a Zn-ligand motif and is involved in mitochondrial functions in Saccharomyces cerevisiae. J Mol Biol. 1992 Apr 5;224(3):575–587. doi: 10.1016/0022-2836(92)90545-u. [DOI] [PubMed] [Google Scholar]
- Chandler M., Fayet O. Translational frameshifting in the control of transposition in bacteria. Mol Microbiol. 1993 Feb;7(4):497–503. doi: 10.1111/j.1365-2958.1993.tb01140.x. [DOI] [PubMed] [Google Scholar]
- Cheng R. H., Caston J. R., Wang G. J., Gu F., Smith T. J., Baker T. S., Bozarth R. F., Trus B. L., Cheng N., Wickner R. B. Fungal virus capsids, cytoplasmic compartments for the replication of double-stranded RNA, formed as icosahedral shells of asymmetric Gag dimers. J Mol Biol. 1994 Dec 2;244(3):255–258. doi: 10.1006/jmbi.1994.1726. [DOI] [PubMed] [Google Scholar]
- Christianson T. W., Sikorski R. S., Dante M., Shero J. H., Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992 Jan 2;110(1):119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- Cui Y., Hagan K. W., Zhang S., Peltz S. W. Identification and characterization of genes that are required for the accelerated degradation of mRNAs containing a premature translational termination codon. Genes Dev. 1995 Feb 15;9(4):423–436. doi: 10.1101/gad.9.4.423. [DOI] [PubMed] [Google Scholar]
- Czaplinski K., Weng Y., Hagan K. W., Peltz S. W. Purification and characterization of the Upf1 protein: a factor involved in translation and mRNA degradation. RNA. 1995 Aug;1(6):610–623. [PMC free article] [PubMed] [Google Scholar]
- Dinman J. D., Icho T., Wickner R. B. A -1 ribosomal frameshift in a double-stranded RNA virus of yeast forms a gag-pol fusion protein. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):174–178. doi: 10.1073/pnas.88.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinman J. D. Ribosomal frameshifting in yeast viruses. Yeast. 1995 Sep 30;11(12):1115–1127. doi: 10.1002/yea.320111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinman J. D., Wickner R. B. Ribosomal frameshifting efficiency and gag/gag-pol ratio are critical for yeast M1 double-stranded RNA virus propagation. J Virol. 1992 Jun;66(6):3669–3676. doi: 10.1128/jvi.66.6.3669-3676.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinman J. D., Wickner R. B. Translational maintenance of frame: mutants of Saccharomyces cerevisiae with altered -1 ribosomal frameshifting efficiencies. Genetics. 1994 Jan;136(1):75–86. doi: 10.1093/genetics/136.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabaugh P. J. Post-transcriptional regulation of transposition by Ty retrotransposons of Saccharomyces cerevisiae. J Biol Chem. 1995 May 5;270(18):10361–10364. doi: 10.1074/jbc.270.18.10361. [DOI] [PubMed] [Google Scholar]
- Fried H. M., Fink G. R. Electron microscopic heteroduplex analysis of "killer" double-stranded RNA species from yeast. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4224–4228. doi: 10.1073/pnas.75.9.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan K. W., Ruiz-Echevarria M. J., Quan Y., Peltz S. W. Characterization of cis-acting sequences and decay intermediates involved in nonsense-mediated mRNA turnover. Mol Cell Biol. 1995 Feb;15(2):809–823. doi: 10.1128/mcb.15.2.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Murakami Y. Rapid and regulated degradation of ornithine decarboxylase. Biochem J. 1995 Feb 15;306(Pt 1):1–10. doi: 10.1042/bj3060001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F., Jacobson A. Identification of a novel component of the nonsense-mediated mRNA decay pathway by use of an interacting protein screen. Genes Dev. 1995 Feb 15;9(4):437–454. doi: 10.1101/gad.9.4.437. [DOI] [PubMed] [Google Scholar]
- He F., Peltz S. W., Donahue J. L., Rosbash M., Jacobson A. Stabilization and ribosome association of unspliced pre-mRNAs in a yeast upf1- mutant. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7034–7038. doi: 10.1073/pnas.90.15.7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin E. V. A new group of putative RNA helicases. Trends Biochem Sci. 1992 Dec;17(12):495–497. doi: 10.1016/0968-0004(92)90338-a. [DOI] [PubMed] [Google Scholar]
- Lee S. I., Umen J. G., Varmus H. E. A genetic screen identifies cellular factors involved in retroviral -1 frameshifting. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6587–6591. doi: 10.1073/pnas.92.14.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeds P., Peltz S. W., Jacobson A., Culbertson M. R. The product of the yeast UPF1 gene is required for rapid turnover of mRNAs containing a premature translational termination codon. Genes Dev. 1991 Dec;5(12A):2303–2314. doi: 10.1101/gad.5.12a.2303. [DOI] [PubMed] [Google Scholar]
- Leeds P., Wood J. M., Lee B. S., Culbertson M. R. Gene products that promote mRNA turnover in Saccharomyces cerevisiae. Mol Cell Biol. 1992 May;12(5):2165–2177. doi: 10.1128/mcb.12.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquat L. E. When cells stop making sense: effects of nonsense codons on RNA metabolism in vertebrate cells. RNA. 1995 Jul;1(5):453–465. [PMC free article] [PubMed] [Google Scholar]
- Masison D. C., Blanc A., Ribas J. C., Carroll K., Sonenberg N., Wickner R. B. Decoying the cap- mRNA degradation system by a double-stranded RNA virus and poly(A)- mRNA surveillance by a yeast antiviral system. Mol Cell Biol. 1995 May;15(5):2763–2771. doi: 10.1128/mcb.15.5.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer E., Wilhelm J. M., Sherman F. Phenotypic suppression of nonsense mutants in yeast by aminoglycoside antibiotics. Nature. 1979 Jan 11;277(5692):148–150. doi: 10.1038/277148a0. [DOI] [PubMed] [Google Scholar]
- Peltz S. W., Brown A. H., Jacobson A. mRNA destabilization triggered by premature translational termination depends on at least three cis-acting sequence elements and one trans-acting factor. Genes Dev. 1993 Sep;7(9):1737–1754. doi: 10.1101/gad.7.9.1737. [DOI] [PubMed] [Google Scholar]
- Peltz S. W., He F., Welch E., Jacobson A. Nonsense-mediated mRNA decay in yeast. Prog Nucleic Acid Res Mol Biol. 1994;47:271–298. doi: 10.1016/s0079-6603(08)60254-8. [DOI] [PubMed] [Google Scholar]
- Singh A., Ursic D., Davies J. Phenotypic suppression and misreading Saccharomyces cerevisiae. Nature. 1979 Jan 11;277(5692):146–148. doi: 10.1038/277146a0. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. Double-stranded RNA virus replication and packaging. J Biol Chem. 1993 Feb 25;268(6):3797–3800. [PubMed] [Google Scholar]
- Wickner R. B., Icho T., Fujimura T., Widner W. R. Expression of yeast L-A double-stranded RNA virus proteins produces derepressed replication: a ski- phenocopy. J Virol. 1991 Jan;65(1):155–161. doi: 10.1128/jvi.65.1.155-161.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Ruiz-Echevarria M. J., Quan Y., Peltz S. W. Identification and characterization of a sequence motif involved in nonsense-mediated mRNA decay. Mol Cell Biol. 1995 Apr;15(4):2231–2244. doi: 10.1128/mcb.15.4.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]