Abstract
Idelalisib (Zydelig™, also known as CAL-101 and GS-1101) was approved in 2014 in the United States and European Union for the treatment of three indolent B-cell neoplasms: relapsed/refractory chronic lymphocytic leukemia (CLL, in combination with rituximab), relapsed follicular lymphoma, and relapsed small lymphocytic lymphoma (as monotherapy). Furthermore, it was approved in the European Union as first-line therapy for poor-prognosis CLL with 17p deletions or TP53 mutations and in patients unsuitable for chemo-immunotherapy. Idelalisib is an orally bioavailable ATP-competitive kinase inhibitor that targets the phosphoinositide 3-kinase p110 isoform δ (PI3Kδ) with high potency and selectivity. PI3Kδ is hyperactivated in B-cell malignancies and plays a pivotal role in the B-cell receptor (BCR) pathway, a key oncogenic driver in B-cell malignancies. The near exclusive expression of the PI3Kδ isoform in hematopoietic cells and the selectivity of idelalisib for the PI3Kδ isoform are essential for its efficacy and tolerability, even in elderly patients unfit for chemotherapy. Idelalisib is the first PI3K inhibitor approved by the regulatory agencies; this approval will change the treatment landscape of indolent B-cell malignancies.
Introduction
Biochemical, cellular and genetic evidences have accumulated for the past three decades defining the phosphoinositide 3-kinase (PI3K) and downstream signaling as an important oncogenic driver in human cancers and have fueled attempts at targeting this axis by pan-PI3K (targeting all four class I isoforms: PI3Kα, PI3Kβ, PI3Kδ, or PI3Kγ) or isoform-specific inhibitors (1). Pan PI3K isoforms, although first to be tested in the clinic, have yet to demonstrate robust clinical efficacy as single agents. Ubiquitous expression and essential function of PI3Kα and PI3Kβ isoforms may limit the tolerability of these agents. Such limitations were not observed for inhibitors specific for the PI3K catalytic subunit p110δ (PI3Kδ) isoform, an isoform almost exclusively expressed in the hematopoietic lineage, and an important regulator of normal and malignant B-cell survival, proliferation, and homing (2, 3). The clinical evaluation of PI3Kδ selective inhibitors recently culminated with the milestone approval of the first of such agent, idelalisib, by the FDA and European Medicines Agency (EMA) for the treatment of relapsed indolent B-cell malignancies.
In 2014, the FDA granted approval of idelalisib for three disease indications: full approval for the treatment of relapsed CLL in combination with rituximab, and accelerated approval as monotherapy for patients with relapsed follicular lymphoma (FL) or small lymphocytic leukemia (SLL) who have received at least two prior systemic therapies. In parallel, the EMA granted marketing authorization for the use of idelalisib in combination with rituximab for patients with CLL who have received at least one prior therapy or as first-line treatment in CLL patients with a 17p deletion or TP53 mutation unsuitable for chemo-immunotherapy. Idelalisib monotherapy was also approved for the treatment of FL that is refractory to two prior therapies.
PI3K isoforms and expression
The PI3K plays a major role in many aspects of cellular biology and is often hyper-activated in human cancers (1, 4). The PI3K family of enzymes have multifunctional roles regulating cellular growth, proliferation, differentiation, motility, intracellular trafficking, and metabolism (4). Three distinct classes of PI3K (class I, II and III) have been characterized and grouped according to their structure and function. The class IA PI3Ks, which have been implicated in many human cancers, are activated downstream of receptor tyrosine kinases and protein G-coupled receptors (GPCR) and via interaction with activated RAS or Rho family of GTPases. Class IA PI3Ks are heterodimers and each consists of a regulatory subunit p85 (p85α, p55α or p50α isoforms encoded by PIK3R1, PIK3R2 or PIK3R3, respectively) and a catalytic subunit p110 (p110α, p110β, or p110δ isoforms encoded by PIK3CA, PIK3CB, or PIK3CD, respectively) (1, 4). Class IB comprises a single catalytic subunit, p110δ that associates with the regulatory subunit p101 (encoded by PIK3R5) or p87 (encoded by PIK3R6) to form PI3Kγ. Similar to class IA PI3Ks, activation of PI3Kγ is mediated by chemokine signaling through GPCR and binding to RAS (5).
PI3K catalytic subunit mediates phosphorylation of phosphatidylinositol-4,5-bisphosphate (PIP2) to yield phosphatidylinositol-3,4,5-trisphosphate (PIP3), a second messenger that functions as an anchor at the cellular membrane to assemble and activate downstream signaling complexes, including the protein kinase B (AKT) kinase (6). The class IA PI3K signaling pathway is activated in human cancers due to mutational activation, or amplification of genes encoding the catalytic subunits, or by inactivation of phosphatases such as PTEN that catabolize PIP3 (4, 7).
The PI3K class I catalytic isoforms have non-overlapping functions. Genetic ablation of the ubiquitously expressed p110α or p110β results in embryonic lethality, indicating their essential and non-redundant roles during development (8). In contrast, p110δ is mostly expressed in the hematopoietic system, including myeloid cells, B- and T-cells and play key roles in leukocyte signaling, proliferation, differentiation, activation, and chemotaxis (9, 10). Mice lacking p110δ function have severely impaired B-cell development and response (11–13). PIK3CD knockout and p110δ kinase-dead mice show complete impairment in proliferative responses to B-cell receptor (BCR) stimulation, and partial inhibition to CD40, interleukin (IL) -4 receptor or toll-like receptor (TLR) 4 stimulation leading to defects in T-cell-dependent and -independent antigen-stimulated antibody generation (11–13). In addition, mice with a kinase-dead mutation in p110δ develop progressive inflammatory bowel disease (11). Gain-of-function mutations in p110δ have been reported in rare cases of diffuse large B-cell lymphomas (DLBCL) but also surprisingly in immune-deficient human patients (7, 14, 15). These activating mutations severely impair the development and function of memory B- and T-cells by increasing activation-induced cell death, senescence and by altering differentiation further supporting an essential role of PI3Kδ in the regulation of normal and pathologic immune response (14, 15). Recent evidence suggests a role of PI3Kδ in the generation and function of regulatory T-cells (Treg) and myeloid-derived suppressor cells (MDSCs), and PI3Kδ inhibitors may stimulate anti-tumor immune responses, providing a rationale for the evaluation of PI3Kδ inhibitors in solid tumors in addition to hematologic malignancies (16). Class 1B catalytic subunit p110δ, is also exclusively expressed in the hematopoietic lineage and has essential functions in chemo-attractant-mediated migration of macrophages and neutrophils, thymocyte survival and mature T-cell activation (5).
Numerous reports have identified the pivotal role of class IA PI3Ks in leukemia and lymphoma. In CLL, the constitutive activation of the PI3K pathway is dependent on p110δ isoform (17). In this context, p110δ is generally not mutated, but signals downstream of the BCR pathway, leading to increased expression of anti-apoptotic proteins (17, 18). PI3Kδ expression is found at higher levels in Hodgkin’s lymphoma (HL) (19) and in mantle cell lymphoma (MCL) (20, 21). It has also been reported that constitutive activation of the PI3K pathway through BCR leads to dysregulation of cell-cycle progression in MCL cell lines and in primary MCL and CLL cells (18, 20).
PI3Kδ regulation and function
In normal and malignant B-cells, PI3Kδ critically regulates a number of signaling pathways driven by receptors, including BCR, Fc-gamma receptor (FcγR), TLR, C-X-C chemokine receptor type 4/5 (CXCR4/5), and the tumor necrosis factor (TNF) receptor family (9, 22). PI3Kδ functions to integrate and transduce these signals from the microenvironment, thus promoting malignant B-cell proliferation, growth, survival, adhesion, and homing. BCR, a prominent activator of PI3Kδ in B-cells, is chronically activated in various B-cell leukemias and lymphomas, including CLL, DLBCL, and MCL (23–25).
BCR activation recruits and activates the tyrosine kinase Lyn and spleen tyrosine kinase (SYK) at the plasma membrane (25). Phosphorylation of a key tyrosine residue in CD79A recruits the adaptor proteins (Nck) and B-cell PI3K adaptor protein (BCAP) (25, 26). PI3Kδ recruitment to the cell membrane is mediated by the association of p85 regulatory subunit to the phosphorylated tyrosine motifs in the B-cell antigen CD19 and BCAP (25). Both CD19 and BCAP contain YXXM sequences, which, upon phosphorylation on the tyrosine residue, become docking sites for the p85 regulatory subunits and a necessary step to the recruitment and activation of the p110δ catalytic subunit (27). In addition, PI3Kδ regulates B-cell responses to CD40-ligand, B-cell activating factor (BAFF), IL-4, and to the homing chemokines CXCL12/13 (2, 3, 9).
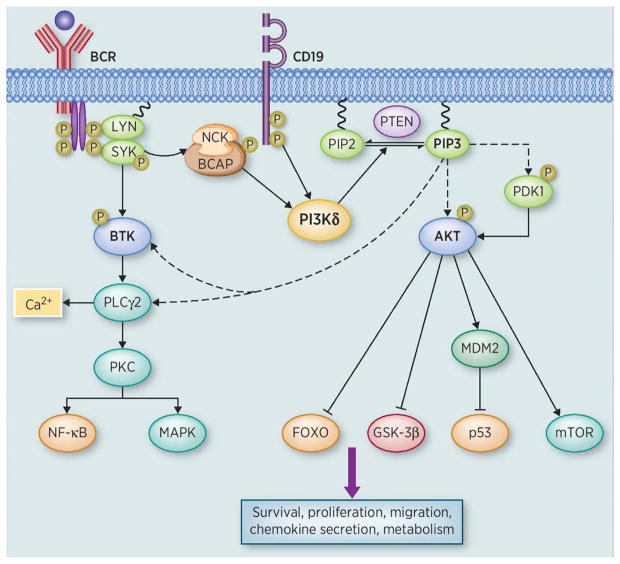
Key pathways orchestrated by PI3Kδ and turned on in B-cell malignancies upon BCR activation, include membrane trafficking, AKT/mammalian target of rapamycin (mTOR), mitogen-activated protein kinases (MAPK), and nuclear factor kappa light-chain enhancer of activated B cells (NF-κB) (12, 23). AKT is the best characterized downstream effector of PI3Kδ and is the central modulator of PI3K-regulated oncogenic signaling. Many oncogenic effectors downstream of AKT play critical roles in regulating cell cycle and cell survival (mouse double minute 2 homolog (MDM2), p53, forkhead box O (FOXO)), DNA repair (MDM2, p53), chemoresistance (NF-κB), and energy metabolism (GSK-3β, mTOR); many of these targets are inhibited by pan-PI3K or PI3Kδ-specific inhibitors (28) (Fig. 1).
Figure 1. BCR pathway and the role of PI3K.
Antigen binding activates the BCR pathway through recruitment and activation of Lyn/Syk, which phosphorylates tyrosine residues on CD19, Nck and BCAP. PI3K regulatory subunit is recruited to the cell membrane by docking to BCAP and CD19 leading to the activation of p110δ. Activated PI3K phosphorylates PIP2 into PIP3. PIP3 recruits and activates PH domain containing signaling molecules including AKT and PDK1. AKT activates various downstream effectors such as MDM2 and mTOR, whereas it inhibits the functions of FOXO and GSK-3β upon phosphorylation. Activation of the BCR pathway also leads to BTK and MAPK pathways activation. BTK initiates a transduction cascade leading to the activation of PLCγ2, intracellular calcium release, PKC activation and initiation of NF-κB transcriptional program. This process is facilitated through interaction with PIP3 at PH domain to recruit BTK and PLCγ2 to the cell membrane. P on various molecules indicates that they are phosphorylated. Solid arrows indicates recruitment and/or activation, dotted arrows indicate interaction through PH domain, and inhibitory signs indicates inhibition.
Discovery and preclinical development of idelalisib
Following the discovery of p110δ (10, 29), ICOS corporation identified the first PI3Kδ selective inhibitor, IC87114, which has been extensively used in vitro and in vivo to probe the function of PI3Kδ (30). Idelalisib (5-fluoro-3-phenyl-2-[(S)-1-(9H-purin-6-ylamino)-propyl]-3H-quinazolin-4-one) belongs to the same chemotype as IC87114 but has improved potency and metabolic stability. Idelalisib and IC87114 bind the ATP-binding pocket of PI3Kδ, which is responsible for the selectivity for PI3Kδ (31). The idelalisib IC50 for PI3Kδ determined at 2x Km for ATP is 19 nM. The IC50 for PI3Kα, β and γ were 8600 nM, 4000 nM, and 2100 nM, respectively (17, 32). In addition, idelalisib at 10 μM did not significantly bind or inhibit any other kinases besides the PI3Ks in a broad panel of kinase assays (17).
Idelalisib’s selectivity and potency translated to PI3K isoform-selective cellular assays. Human basophil activation by anti-FcεR1, as measured by the surface expression of CD69, and B-cell proliferation in response to BCR crosslinking provided two read-outs that are potently inhibited by idelalisib with an EC50 of 8.9 nM and 6 nM, respectively. Idelalisib was 281, 159 and >1124 fold less potent in cellular assays dependent on PI3Kα, β and γ, respectively (32). In patients treated with the 150mg BID dose, idelalisib’s free maximal plasma concentration is 13-fold higher than the p110δ IC50, but is only 0.12-fold the biochemical IC50 for p110γ and less than 0.10-fold the biochemical IC50 for p110α and p110β, indicating that idelalisib selectivity for PI3Kδ is maintained in the clinical setting.
Initial studies demonstrated sensitivity to idelalisib in cell lines and primary cells from patients with B-acute lymphocytic leukemia (ALL) and CLL (17). Phosphorylation of AKT on Ser473 and Thr308, two downstream markers of PI3K pathway activation, was inhibited by idelalisib in primary CLL and MCL and in HL cell lines, demonstrating a role for PI3Kδ in the regulation of intrinsic or stimulated AKT activity (17, 19). The understanding of idelalisib’s mode of action in CLL originated from seminal work from the laboratories of Byrd and Burger, who demonstrated the key role played by PI3Kδ in the transmission of survival, proliferation and homing signals produced by the tumor microenvironment (2, 3). Idelalisib potently negated the trophic influence of these factors as demonstrated by the inhibition of the survival benefits provided by ex vivo stimulation of CLL primary cells with BCR crosslinking, CD40L, BAFF, TNF-α, or fibronectin and co-culture with a stromal cell line or monocyte-derived nurse-like cells. In vitro idelalisib reduced CLL migration beneath a layer of bone marrow-derived stromal cells, inhibited CLL adhesion to stromal and endothelial cells, and decreased chemotaxis toward CXCL12 and CXCL13. These observations are consistent with the rapid decrease in lymph node size and increase in lymphocytosis in idelalisib-treated patients and are a possible indication of tumor cells being separated and migrating away from the tumor niche (3, 33, 34). Chemokines such as C-C motif ligand 3 and 4 (CCL3/4), as well as stroma-/T-cell-produced factors including CD40L, TNFa, IL-6 and -10, were also impacted (3, 35). Idelalisib may thus simultaneously target the malignant B-cells by inhibiting their response to stromal factors and the tumor niche by limiting its ability to support the tumor cell growth.
The interplay between transformed B-cells, tumor-associated macrophages, MDSCs, follicular dendritic cells, Tregs, and follicular helper T cells with the tumor stroma is also known to be important for the development and prognosis of FL (36). While most of these cell types predominantly express the PI3Kδ isoform (10), their contribution to idelalisib’s mode of action in FL remains to be fully characterized.
Pharmacokinetic and pharmacodynamic studies of idelalisib
Initial pharmacokinetic investigation of idelalisib was performed in healthy individuals at ascending doses. Similar pharmacokinetic parameters have been reported in patients with CLL or indolent non-Hodgkin lymphoma (iNHL) receiving doses of idelalisib ranging from 50 mg to 350 mg BID or 150 mg QD (33, 37). Over 28 days of dosing, accumulation was minimal and there was little variation in exposure by age or sex. Plasma exposure was less than dose-proportional above 150 mg BID. Trough exposure levels were markedly lower for QD dosing.
Investigations of pharmacodynamic activity have focused on the effect of idelalisib on AKT activity and plasma concentrations of an array of chemokines, stroma-derived factors, and cytokines often elevated in patients with CLL (33). As expected, baseline levels of phospho-AKT were elevated in B-cells from all patients with CLL who were evaluated (n= 27). Within 7 days of idelalisib dosing, AKT activation was reduced to levels approaching that observed in normal B-cells (33). Likewise, plasma concentrations of CCL3, CCL4, CCL17, CCL22, CD40 ligand, CCL2, CXCL13, and TNF-α also decreased significantly after 4 weeks of treatment with idelalisib.
Clinical studies
CLL
The pivotal trial supporting the approval of idelalisib in combination with rituximab included 220 patients with relapsed CLL who were not suitable for cytotoxic therapy due to myelotoxicity from previous therapy, reduced creatinine clearance, or the presence of significant co-morbidities not related to CLL(34). This was a multicenter, randomized, double-blind, placebo-controlled, phase 3 comparison of 150 mg BID idelalisib plus rituximab versus rituximab plus an oral placebo. The primary endpoint was progression-free survival (PFS).
Patients in this study had long-standing disease (approximately 9 years median time since initial diagnosis) and a median Cumulative Illness Rating Scale (CIRS) of 8, indicating a high degree of comorbidity. The median age was 71 years and patients had received a median of 3 prior therapies for CLL. A large majority (80%) of patients had unfavorable unmutated IGHV status and more than 40% had a 17p deletion or another mutation in TP53.
An interim analysis was pre-specified to occur after approximately 50% of 119 anticipated events (disease progression or death) had occurred. At this analysis, the study was stopped early by a safety monitoring board due to overwhelming efficacy in the idelalisib plus rituximab arm of the trial. When the study was stopped, the median duration of progression-free survival had not been reached in patients receiving idelalisib plus rituximab. Median duration of progression-free survival was 5.5 months in those receiving rituximab plus placebo. At 24 weeks, the rate of progression-free survival in the idelalisib plus rituximab arm was 93% versus 46% in the rituximab plus placebo arm (P<0.001). The favorable treatment effect of idelalisib plus rituximab was observed across the pre-specified patient subgroups, with similar benefits being observed regardless of the presence or absence of 17p deletion, other TP53 mutation or IGHV status. Idelalisib plus rituximab also had a superior survival benefit. The median duration of overall survival in the two arms was not reached but the overall survival rate was 92% in the idelalisib plus rituximab arm versus 80% in the rituximab plus placebo arm at 12 months (P = 0.02).
The most common adverse events in the idelalisib plus rituximab group were pyrexia, fatigue, nausea, chills, and diarrhea. In the rituximab plus placebo group, the common adverse events were infusion-related reactions, fatigue, cough, nausea, and dyspnea. Among more severe events, grade 3 or higher diarrhea was reported in 4 patients and grade 3 or higher rash was reported in 2 patients in the idelalisib plus placebo groups whereas no such events were reported in the rituximab plus placebo group. Among laboratory abnormalities, grade 3 or higher transaminase elevations occurred in 6 patients (5%) receiving idelalisib plus rituximab versus 1 patient (1%) in the rituximab plus placebo arm. Grade 3 or higher neutropenia was also somewhat more frequent in the idelalisib plus rituximab arm (34%) than in the comparator arm (22%).
NHL
The pivotal trial supporting the accelerated approval of idelalisib as monotherapy in patients with FL and SLL was a single-arm, open-label study in 125 patients with iNHL, all of whom received idelalisib 150 mg BID as oral monotherapy (38). As is common in single-arm oncology trials, the primary endpoint was overall response rate (ORR), with duration of response (DOR) being a key secondary endpoint.
Patients had 1 of 4 subtypes of iNHL: FL (n = 72), SLL (n = 28), marginal-zone lymphoma (n = 15), or lymphoplasmacytic lymphoma with or without Waldenström’s macroglobulinemia (n = 10). Per inclusion criteria, patients were those who were refractory to both rituximab and an alkylating agent, defined as having either no response or a response of limited duration (relapse within 6 months) after prior treatment with these agents.
Consistent with the inclusion criteria, subjects enrolled in the study were heavily pretreated with highly refractory disease. Subjects had received a median of 4 prior therapies; 100% had disease refractory to rituximab and 99% to an alkylating agent, with 91% refractory to both when administered as part of the same regimen. The majority of patients had failed regimens considered cornerstones of therapy, including bendamustine plus rituximab (78% of patients were refractory) and R-CHOP (71% of patients were refractory). The median age of enrolled patients was 64 years.
After a median treatment duration of 6.6 months, patients receiving idelalisib had an ORR of 57% (71 responders among 125 patients), with 6% having a complete response. Responses to idelalisib were rapid (median time to response was 1.9 months) and durable, with a median duration of response of 12.5 months. Median PFS was 11 months. Consistent benefit was observed across patient subgroups, including age, sex, iNHL subtype, number of prior therapies, prior bendamustine use or refractory status, and the presence or absence of bulky disease at baseline.
The most common adverse events of any severity were diarrhea (43%), fatigue (30%), nausea (30%), cough (in 29%), and pyrexia (28%). Among events that were grade 3 or higher, the most common were diarrhea (13%), pneumonia (7%), and dyspnea (3%). Grade 3 or higher rash was reported in 2% of patients. As in patients with CLL, grade 3 or higher elevations in transaminase were reported in some patients (13%). Grade 3 or higher neutropenia was reported in 27% of patients.
Other PI3K Delta or Delta/Gamma Inhibitors
Besides idelalisib, several other PI3Kδ-specific inhibitors are in clinical development. INCB40093 (Incyte Pharmaceuricals) entered phase I evaluation for refractory B-cell malignancies and may be further tested in combination with Incyte Pharmaceuticals’s Janus kinase 1 (JAK1) inhibitor (NCT01905813; clinicaltrials.gov). TGR-1202 (TG Therapeutics) is in phase I clinical trial with the most notable combinations being with brentuximab vedotin in HL and with chlorambucil and obinutuzumab in CLL (NCT02164006, NCT02100852; clinicaltrials.gov). AMG 319 (Amgen Inc.-Acerta Pharma) is currently in phase 1 trials for patients with relapsed or refractory CLL in combination with Acerta’s Bruton’s tyrosine kinase (BTK) inhibitor (39). Duvelisib (IPI-145, Infinity Pharmaceuticals), a dual PI3Kδ and γ inhibitor, is currently being evaluated as a single agent in phase 3 trials for patients with advanced CLL (40) and indolent NHL (41). It is also a more potent inhibitor of PI3Kδ. Duvelisib’s activity in primary CLL ex vivo is comparable to idelalisib when adjusted for potency (42, 43). However, a head to head comparison of idelalisib and duvelisib is needed to identify role of PI3K gamma inhibition in B-cell neoplasia. Consistent with the presence of PI3Kγ in T-cells and with the T-cell phenotype in PIK3CD PIK3CG double mutant mice, duvelisib decreases the viability of T- and NK-cells in vitro and demonstrates activity in T-cell malignancies (42, 44).
Future
The success of inhibiting the PI3Kδ isoform and the approval of idelalisib has opened a new chapter in targeting the PI3K pathway. Already there is a flurry of new PI3Kδ or δ/γ inhibitors being evaluated in both the preclinical and clinical settings. Several investigators are probing the mechanisms responsible for the activity of idelalisib (45) as well as the mechanisms that will define resistance to these agents. Idelalisib clinical program encompasses 2 major directions: the evaluation of this agent in first-line CLL and, FL, and novel combinations (32). Remarkably, idelalisib in the EU as well as the BTK inhibitor ibrutinib are already approved for the first-line treatment of CLL with the poor prognosis feature of 17p deletion or p53 mutation. Idelalisib already demonstrated clinical efficacy in combination with anti-CD20 monoclonal antibody rituximab and chemo-immunotherapy such as bendamustine-rituximab (34, 46). The landscape of the treatment of indolent B-cell malignancies is undergoing rapid transformation with the advent of exciting clinically active molecules that include Fc-enhanced anti-CD20 monoclonal antibodies such as obinutuzumab, inhibitors of BTK, SYK, BCL2 and programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors. The goal of future clinical research will be to identify the optimal combination regimen for idelalisib and sequence of these novel agents to achieve durable complete remission in chemotherapy fit and unfit patients.
Acknowledgments
Grant Support
This work was supported in part by grant P01CA81534 from the National Cancer Institute, a CLL Global Research Foundation Alliance grant award, and a sponsored research agreement from Gilead Sciences to Varsha Gandhi.
The authors are grateful to Daniel Tumas and Tim DiChiara for their critical review of the manuscript.
Footnotes
Disclosure of Potential Conflicts of Interest
T. Newcomb is an employee of Gilead Sciences. C. Quéva is an employee of, reports receiving a commercial research grant from, and has ownership interest in Gilead Sciences. V. Gandhi reports receiving a commercial research grant from Gilead Sciences. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140–56. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herman SE, Gordon AL, Wagner AJ, Heerema NA, Zhao W, Flynn JM, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116:2078–88. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118:3603–12. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075–83. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nature reviews Molecular cell biology. 2010;11:329–41. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 6.Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, et al. Synthesis and function of 3-phosphorylated inositol lipids. Annual review of biochemistry. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Grubor V, Love CL, Banerjee A, Richards KL, Mieczkowski PA, et al. Genetic heterogeneity of diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A. 2013;110:1398–403. doi: 10.1073/pnas.1205299110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanhaesebroeck B, Ali K, Bilancio A, Geering B, Foukas LC. Signalling by PI3K isoforms: insights from gene-targeted mice. Trends in biochemical sciences. 2005;30:194–204. doi: 10.1016/j.tibs.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Puri KD, Gold MR. Selective inhibitors of phosphoinositide 3-kinase delta: modulators of B-cell function with potential for treating autoimmune inflammatory diseases and B-cell malignancies. Front Immunol. 2012;3:256. doi: 10.3389/fimmu.2012.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanhaesebroeck B, Welham MJ, Kotani K, Stein R, Warne PH, Zvelebil MJ, et al. P110delta, a novel phosphoinositide 3-kinase in leukocytes. Proc Natl Acad Sci U S A. 1997;94:4330–5. doi: 10.1073/pnas.94.9.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–4. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 12.Clayton E, Bardi G, Bell SE, Chantry D, Downes CP, Gray A, et al. A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in B cell development and activation. J Exp Med. 2002;196:753–63. doi: 10.1084/jem.20020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jou ST, Carpino N, Takahashi Y, Piekorz R, Chao JR, Wang D, et al. Essential, nonredundant role for the phosphoinositide 3-kinase p110delta in signaling by the B-cell receptor complex. Mol Cell Biol. 2002;22:8580–91. doi: 10.1128/MCB.22.24.8580-8591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucas CL, Kuehn HS, Zhao F, Niemela JE, Deenick EK, Palendira U, et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110delta result in T cell senescence and human immunodeficiency. Nat immunology. 2014;15:88–97. doi: 10.1038/ni.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angulo I, Vadas O, Garcon F, Banham-Hall E, Plagnol V, Leahy TR, et al. Phosphoinositide 3-kinase delta gene mutation predisposes to respiratory infection and airway damage. Science. 2013;342:866–71. doi: 10.1126/science.1243292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali K, Soond DR, Pineiro R, Hagemann T, Pearce W, Lim EL, et al. Inactivation of PI(3)K p110delta breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014;510:407–11. doi: 10.1038/nature13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lannutti BJ, Meadows SA, Herman SE, Kashishian A, Steiner B, Johnson AJ, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117:591–4. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longo PG, Laurenti L, Gobessi S, Sica S, Leone G, Efremov DG. The Akt/Mcl-1 pathway plays a prominent role in mediating antiapoptotic signals downstream of the B-cell receptor in chronic lymphocytic leukemia B cells. Blood. 2008;111:846–55. doi: 10.1182/blood-2007-05-089037. [DOI] [PubMed] [Google Scholar]
- 19.Meadows SA, Vega F, Kashishian A, Johnson D, Diehl V, Miller LL, et al. PI3Kdelta inhibitor, GS-1101 (CAL-101), attenuates pathway signaling, induces apoptosis, and overcomes signals from the microenvironment in cellular models of Hodgkin lymphoma. Blood. 2012;119:1897–900. doi: 10.1182/blood-2011-10-386763. [DOI] [PubMed] [Google Scholar]
- 20.Dal Col J, Zancai P, Terrin L, Guidoboni M, Ponzoni M, Pavan A, et al. Distinct functional significance of Akt and mTOR constitutive activation in mantle cell lymphoma. Blood. 2008;111:5142–51. doi: 10.1182/blood-2007-07-103481. [DOI] [PubMed] [Google Scholar]
- 21.Iyengar S, Clear A, Bodor C, Maharaj L, Lee A, Calaminici M, et al. P110alpha-mediated constitutive PI3K signaling limits the efficacy of p110delta-selective inhibition in mantle cell lymphoma, particularly with multiple relapse. Blood. 2013;121:2274–84. doi: 10.1182/blood-2012-10-460832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3:317–30. doi: 10.1038/nri1056. [DOI] [PubMed] [Google Scholar]
- 23.Davis RE, Ngo VN, Lenz G, Tolar P, Young RM, Romesser PB, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez A, Villuendas R, Yanez L, Gomez ME, Diaz R, Pollan M, et al. Molecular heterogeneity in chronic lymphocytic leukemia is dependent on BCR signaling: clinical correlation. Leukemia. 2007;21:1984–91. doi: 10.1038/sj.leu.2404831. [DOI] [PubMed] [Google Scholar]
- 25.Rickert RC. New insights into pre-BCR and BCR signalling with relevance to B cell malignancies. Nat Rev Immunol. 2013;13:578–91. doi: 10.1038/nri3487. [DOI] [PubMed] [Google Scholar]
- 26.Castello A, Gaya M, Tucholski J, Oellerich T, Lu KH, Tafuri A, et al. Nck-mediated recruitment of BCAP to the BCR regulates the PI(3)K-Akt pathway in B cells. Nat immunology. 2013;14:966–75. doi: 10.1038/ni.2685. [DOI] [PubMed] [Google Scholar]
- 27.Aiba Y, Kameyama M, Yamazaki T, Tedder TF, Kurosaki T. Regulation of B-cell development by BCAP and CD19 through their binding to phosphoinositide 3-kinase. Blood. 2008;111:1497–503. doi: 10.1182/blood-2007-08-109769. [DOI] [PubMed] [Google Scholar]
- 28.Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–9. doi: 10.1038/nrc1753. [DOI] [PubMed] [Google Scholar]
- 29.Chantry D, Vojtek A, Kashishian A, Holtzman DA, Wood C, Gray PW, et al. p110delta, a novel phosphatidylinositol 3-kinase catalytic subunit that associates with p85 and is expressed predominantly in leukocytes. J Biol Chem. 1997;272:19236–41. doi: 10.1074/jbc.272.31.19236. [DOI] [PubMed] [Google Scholar]
- 30.Sadhu C, Masinovsky B, Dick K, Sowell CG, Staunton DE. Essential role of phosphoinositide 3-kinase delta in neutrophil directional movement. J Immunol. 2003;170:2647–54. doi: 10.4049/jimmunol.170.5.2647. [DOI] [PubMed] [Google Scholar]
- 31.Berndt A, Miller S, Williams O, Le DD, Houseman BT, Pacold JI, et al. The p110 delta structure: mechanisms for selectivity and potency of new PI(3)K inhibitors. Nat Chem Biol. 2010;6:117–24. doi: 10.1038/nchembio.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Q, Modi P, Ramanathan S, Queva C, VG Idelalisib for the treatment of B-cell malignancies. Expert Opin Orphan Drugs. 2015;3:109–23. [Google Scholar]
- 33.Brown JR, Byrd JC, Coutre SE, Benson DM, Flinn IW, Wagner-Johnston ND, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123:3390–7. doi: 10.1182/blood-2013-11-535047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herman SE, Lapalombella R, Gordon AL, Ramanunni A, Blum KA, Jones J, et al. The role of phosphatidylinositol 3-kinase-delta in the immunomodulatory effects of lenalidomide in chronic lymphocytic leukemia. Blood. 2011;117:4323–7. doi: 10.1182/blood-2010-11-315705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dave SS, Wright G, Tan B, Rosenwald A, Gascoyne RD, Chan WC, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351:2159–69. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- 37.Flinn IW, Kahl BS, Leonard JP, Furman RR, Brown JR, Byrd JC, et al. Idelalisib, a selective inhibitor of phosphatidylinositol 3-kinase-delta, as therapy for previously treated indolent non-Hodgkin lymphoma. Blood. 2014;123:3406–13. doi: 10.1182/blood-2013-11-538546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gopal AK, Kahl BS, de Vos S, Wagner-Johnston ND, Schuster SJ, Jurczak WJ, et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370:1008–18. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lanasa MC, Glenn M, Mato AR, Allgood SD, Wong S, Amore B, et al. First-In-Human Study Of AMG 319, a Highly Selective, Small Molecule Inhibitor Of PI3Kδ, In Adult Patients With Relapsed Or Refractory Lymphoid Malignancies. Blood (55th ASH Annual Meeting Abstracts) 2013;642:abstr 678. [Google Scholar]
- 40.O’Brien S, Patel M, Kahl B, Horwitz SM, Foss FM, Porcu P, et al. Duvelisib (IPI-145), a PI3K-δ,γ Inhibitor, Is Clinically Active in Patients with Relapsed/Refractory Chronic Lymphocytic Leukemia. Blood (ASH Annual Meeting Abstracts) 2014:abstr 3334. [Google Scholar]
- 41.Flinn I, Oki Y, Patel M, Horwitz SM, Foss FM, Sweeney J, et al. a Phase 1 Evaluation of Duvelisib (IPI-145), a PI3K-δ,γ Inhibitor, in Patients with Relapsed/Refractory iNHL. Blood (ASH Annual Meeting Abstracts) 2014:abstr 802. [Google Scholar]
- 42.Dong S, Guinn D, Dubovsky JA, Zhong Y, Lehman A, Kutok J, et al. IPI-145 antagonizes intrinsic and extrinsic survival signals in chronic lymphocytic leukemia cells. Blood. 2014;124:3583–6. doi: 10.1182/blood-2014-07-587279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balakrishnan K, Peluso M, Fu M, Rosin NY, Burger JA, Wierda W, et al. Inhibition Of PI3K-δ and -γ Isoforms By IPI-145 In Chronic Lymphocytic Leukemia Overcomes Signals From PI3K/AKT/S6 Pathway and Promotes Apoptosis. Blood (55th ASH Annual Meeting Abstracts) 2013:abstr 4167. [Google Scholar]
- 44.Horwitz SM, Porcu P, Flinn I, Kahl B, Sweeney J, Stern HM, et al. Duvelisib (IPI-145), a phosphoinositide-3-kinase-δ,γ inhibitor, shows activity in patients with relapsed/refractory T-cell lymphoma. Blood (ASH Annual Meeting Abstracts) 2014:abstr 803. [Google Scholar]
- 45.Yang Q, Chen LS, Neelapu SS, Gandhi V. The PI3Kδ inhibitor, idelalisib, inhibits transcription and translation through PI3K/Akt pathway in mantle cell lymphoma [abstract]. Proceedings of the 105th Annual Meeting of the American Association for Cancer Research; 2014; p. abst 4529. [Google Scholar]
- 46.de Vos S, Wagner-Johnston ND, Coutre SE, Flinn I, Schreeder MT, Fowler NH, et al. Durable Responses Following Treatment with the PI3K-Delta Inhibitor Idelalisib in Combination with Rituximab, Bendamustine, or Both, in Recurrent Indolent Non-Hodgkin Lymphoma: Phase I/II Results. Blood (ASH Annual Meeting Abstracts) 2014:abstr 3063. [Google Scholar]