Abstract
Background
A self-enhancing loop between impaired inhibitory control under alcohol and alcohol consumption has been proposed as a possible mechanism underlying dysfunctional drinking in susceptible people. However, the neural underpinnings of alcohol-induced impairment of inhibitory control are widely unknown.
Methods
We measured inhibitory control in fifty young adults with a stop-signal task (SST) during functional magnetic resonance imaging (fMRI). In a single-blind placebo-controlled cross-over design, all participants performed the SST once under alcohol with a breath alcohol concentration (BrAC) of 0.6 g/kg, and once under placebo. In addition, alcohol consumption was assessed using a free-access alcohol self-administration (ASA) paradigm in the same participants.
Results
Inhibitory control was robustly decreased under alcohol compared to placebo indicated by longer stop-signal reaction times (SSRTs). On the neural level, impaired inhibitory control under alcohol was associated with attenuated brain responses in the right fronto-temporal portion of the inhibition network that supports the attentional capture of infrequent stop-signals, and subsequent updating of action plans from response execution to inhibition. Furthermore, the extent of alcohol-induced impairment of inhibitory control predicted free-access alcohol consumption.
Conclusion
We suggest that during inhibitory control alcohol affects cognitive processes preceding actual motor inhibition. Under alcohol, decreased brain responses in right fronto-temporal areas might slow down the attentional capture of infrequent stop-signals and subsequent updating of action plans which leads to impaired inhibitory control. In turn, pronounced alcohol-induced impairment of inhibitory control may enhance alcohol consumption in young adults which might promote future alcohol problems.
Keywords: response inhibition, inhibitory control, stop-signal task, acute alcohol intoxication, fMRI, alcohol consumption
Introduction
Under the influence of alcohol, individuals are more likely to engage in risky behaviors such as risky driving (e.g., 1; 2), gambling (3), and aggression (4; 5). Harmful alcohol use is related to an increased risk of premature death and injuries, especially in young people (WHO, 2010).
Experimental studies demonstrate that alcohol impairs inhibitory motor control in stop-signal (SST) (6-9), and Go/Nogo tasks (10-12) that measure the ability to inhibit prepotent motor responses. Recently, alcohol consumption has been directly linked to alcohol-related impairment of inhibitory control in a Go/Nogo task: People with lower inhibitory control under alcohol consumed more alcohol in a free-access ASA experiment (13). Additionally, inhibitory control of binge drinkers was decreased in a Go/Nogo task under alcohol but not under placebo compared to moderate drinkers (14). A self-enhancing feedback loop between alcohol-induced impairment of inhibitory control and alcohol consumption has been suggested as a possible mechanism underlying loss of control during excessive drinking with negative long-term effects in susceptible people (cf, 12; 13).
Previous fMRI studies showed that alcohol decreased conflict-, and error-related activation of the anterior cingulate cortex (ACC) in a Go/Nogo (15) and Stroop task (16). During a SST, people at risk for alcoholism showed differential neural responses to moderate alcohol levels (BrAC ∼60 mg/dl). People with a low level of response to alcohol showed lower neural activation under alcohol in the left precentral gyrus, and higher activation in the left ACC (17), whereas people with a positive family history of alcoholism (FHA) showed no attenuation of brain responses under alcohol in anterior inferior frontal gyrus (IFG) compared to controls (18). However, both studies did not report overall alcohol effects on the neural response in inhibition-related brain areas. Thus, the neural mechanisms underlying the well-described alcohol-induced impairment of inhibitory control in healthy people (6-9) are still unknown.
Inhibitory control measured with a SST activates a right-dominant fronto-subcortical network including the right (R)IFG, bilateral anterior insulae, the pre-supplementary motor area (pre-SMA), the ACC, thalamic and striatal brain areas (19-23). This network was not only active during successful inhibitions as proposed earlier (24), but also during failed inhibitions indicating that response inhibition is triggered irrespective of the outcome of inhibition trials during a tracking SST (22; 25), in which the probability of inhibition converges to 50% across the experiment. Further, the inhibition-related network has been delineated into functionally distinct parts: (i) a right ventral fronto-parietal portion including the RIFG/insula assumed to support the attentional capture of infrequent stop-signals (26; 27) and subsequent updating of action plans from response execution to inhibition (23; 28), (ii) the pre-SMA associated with the outright motor inhibition process via connections to the subthalamic/caudate nuclei (26; 27), and (iii) a bilateral frontal error-monitoring network including the ACC (24; 26) and anterior insulae during failed inhibition (29). A number of studies highlighted that decreased activation of the RIFG was linked to impaired inhibitory control (comparison of bad vs. good inhibitors, adolescents vs. adults, ADHD patients vs. controls, 19; 21; 29-31). Correspondingly, improved inhibitory control was associated with increased activation of the RIFG induced by pharmacological interventions (32), and transcranial current stimulation of the RIFG (33). Precise functional localization within the RIFG/insula in inhibitory control is still debated (19; 22; 23; 26; 28).
The present fMRI study is part of the “Dresden Longitudinal study on Alcohol use in Young Adults” (D-LAYA), which investigates the relation between laboratory free-access ASA and the early phase of drinking trajectories in young adults. This is one of the few studies investigating acute alcohol effects in healthy emerging adults at the beginning of their drinking “careers”. At this age, alcohol use is very common (34; 35), and high alcohol consumption might be indicative of future alcohol problems (36). However, the exact mechanisms why explorative drinking proceeds into risky and abusive forms in some people, and not in others (37), remains an unsolved question. Here, we investigated the effects of alcohol on inhibition-related brain responses using a tracking SST (25; 38) during fMRI. Alcohol was administered in a placebo-controlled cross-over design with alcohol levels clamped at 0.6 g/kg. We tested the hypothesis that alcohol decreases brain responses in the right frontal portion of the inhibition-related fronto-subcortical network that has been shown to be sensitive to impaired inhibitory control (21; 29-31), and thereby leads to alcohol-induced impairment of inhibitory control. Additionally, we measured cerebral perfusion using arterial spin labeling (ASL) MRI (39) to test whether alcohol effects on task-related blood oxygenation level-dependent (BOLD) responses were confounded by vasoactive alcohol effects on perfusion (40-42). Furthermore, we tested in the same sample whether alcohol-induced impairment of inhibitory control predicted alcohol consumption levels in a separate free-access ASA experiment (cf, 43).
Methods
Participants
Fifty healthy social drinkers performed the SST twice during fMRI within the framework of the D-LAYA study. Of those, 47 also took part in the free-access ASA experiment of the D-LAYA study that preceded the fMRI experiment (see supplement, “Recruitment”/“Sample characteristics”). For safety reasons, participants were only considered for fMRI if they had no MR-contraindications and if their maximum BrAC during one of the free-access sessions exceeded 0.5 g/kg. Further inclusion criteria were physical and mental health, habitual social drinking (>=2 drinks/week, at least one lifetime occasion of getting drunk), drug/alcohol abstinence (at least one week/24h prior to each experimental day), positive or negative FHA (FHP: at least one first-degree biological relative affected by alcoholism; FHN: no first- or second-degree relative affected by alcoholism; see supplement, “Recruitment”). However, FHA was not the focus of the fMRI part. Exclusion criteria were a history of alcohol/“illicit drug” abuse/dependence, and pregnancy or breast-feeding in females.
For fMRI analysis, we excluded 8 data sets (reasons: head movement/sleepiness) resulting in a final sample of 42 right-handed participants (11 females, 15 FHP, mean age=19.1 years ±0.7, SD). Of those, 38 participants had valid free-access data for correlation with behavioral SST data from fMRI alcohol clamping (10 females, 15 FHP, mean age=18.9 years ±0.4, SD). All participants provided written consent and were paid 10€/hour. All study procedures were approved by the Ethics Committee of the Technische Universität Dresden.
Experimental procedures
On arrival, all participants had a BrAC of 0.0 g/kg (Dräger® Alcotest 6810 breathalyzer, Lübeck, Germany), and were tested negative for “illicit drug use” (see supplement “Sample characteristics”), and females for pregnancy.
Alcohol administration
In both experiments (free-access ASA/fMRI alcohol clamping), alcohol was administered intravenously using a 6% alcohol solution (v/v; mixture of normal saline with 95% ethanol [Braun, Melsungen, Germany]). Infusion rates were controlled using computer-assisted alcohol infusion systems (CAIS, 44).
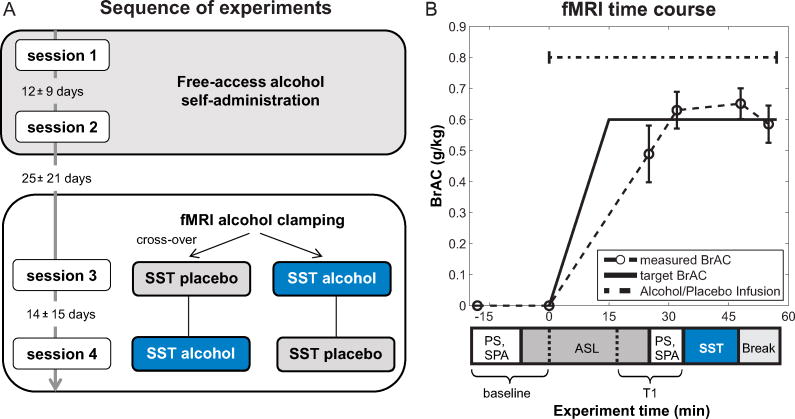
For fMRI alcohol clamping, alcohol was administered in a single-blind, placebo-controlled cross-over design (placebo first: n=25; alcohol first: n=17; see Figure 1A). CAIS was used to reach a BrAC of 0.6 g/kg within 15 minutes after starting the infusion, and to maintain that level for the rest of the experiment by adjusting infusion rates based on BrAC measurements (Figure 1B; cf, 44). The placebo infusion consisted of normal saline.
Figure 1.
Sequence of experiments and fMRI time course. A: The experiment consisted of four sessions. First, we conducted free-access alcohol self-administration on two separate days (session 1+2). Second, the SST was performed during fMRI alcohol clamping, once under a constant alcohol exposure of 0.6 g/kg, and once under placebo (session 3+4). The order of the alcohol and placebo condition in the fMRI alcohol clamping part was randomized across participants. B: Timing of the fMRI alcohol clamping experiment with target and measured BrACs (mean, Error bars represent standard deviations). We also measured subjective perceptions of alcohol (SPA) and saccadic eye-movements (PS) at baseline and before the SST at T1 to track the “Level of alcohol intoxication” (see supplement). After the break, the experiment continued with other tasks (see supplement Figure S1 for complete time course). Abbreviations: ASL = arterial spin labeling, BrAC = breath alcohol concentration, PS = prosaccades, SPA = subjective perception of alcohol, SST = stop-signal task, T1 = time 1.
Alcohol consumption was measured using an established free-access ASA paradigm (cf, 43; 45). Participants were instructed to produce pleasant alcohol effects like they would at a party with alcohol available for free, but to avoid unpleasant alcohol effects. Alcohol was requested by pressing a button which increased participants' arterial blood alcohol concentration by 7.5 mg%. A safety limit was set to 120 mg%.
BrAC was sampled regularly during the experiments. We developed a new method to obtain precise BrAC readings while participants lay in the MR-scanner (see supplement “Measurement of BrAC”).
Sequence of experiments (Figure 1A)
First, participants took part in two free-access ASA experiments that lasted approximately 145 minutes on separate days. Second, participants underwent fMRI alcohol clamping on two additional days. Imaging data were acquired with a 3T MR-scanner (Magnetom TrioTim; Siemens, Germany) equipped with a 12-channel head-coil (see supplement for “MRI data acquisition”). On both days, MR-scanning started with measurement of absolute perfusion using ASL MRI at baseline before the infusion was started, continuously for 15 minutes while BrAC-levels increased, and before the SST (Figure 1B for fMRI timing). After reaching the target BrAC, the SST was performed (see “Stop-signal task”).
On all days, alcohol administration started at the same time of day to control for circadian alcohol effects. Participants were sent home by taxi after BrAC dropped below 0.45 g/kg.
Stop-signal task
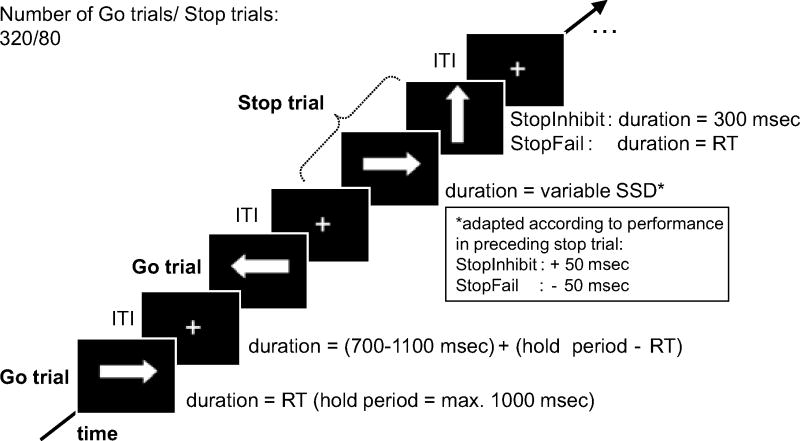
Figure 2 illustrates the SST. Participants responded to the direction of white arrows pointing left or right (go-signal; stimulus design adapted from Rubia et al. (24)) by pressing a button with their left or right index finger. Infrequently (20%), a white arrow pointing upwards (stop-signal) followed the presentation of the go stimulus with a time delay (stop-signal delay [SSD]). In this case, participants had to withhold the already triggered motor response. After every stop trial, the length of the SSD was adapted dynamically (+/- 50 ms) according to the participants' performance in the preceding stop trial (successful/failed inhibition; Figure 2) using a previously described tracking algorithm (25; 38). Using this tracking algorithm, the probability of inhibition (PI: #stop success/#all stop trials) converged to 50% after 10-15 stop trials, and fluctuated around 50% for the remaining trials. We estimated the SSRT, the latency of response inhibition, by rank ordering Go RTs and subtracting the mean SSD (reflecting the start of motor inhibition) from the nth Go RT corresponding to the percentile of the probability of response in stop trials (reflecting the finishing time of motor inhibition; cf, 18; 29; 46; for a review, 47). This SSRT estimation method accounts for deviations of “PI” from 50% that may occur among participants.
Figure 2.
Timing of the SST. In go trials, the go stimulus was displayed until a response was recorded, but for a maximum of 1000 ms. In stop trials, the go stimulus was presented for the duration of the variable stop-signal delay (SSD; mean ±SD: alcohol = 184 ms ± 91; placebo = 196 ms ±85) followed by the stop-signal for 300 ms in successful stop trials (StopInhibit) or until a response was recorded in failed stop trials (StopFail). Go and stop trials were followed by the presentation of a central fixation cross for the duration of a jittered inter-trial interval (ITI, mean=900ms). In stop trials, the SSD (initial SSD=200 ms) was adapted dynamically according to the performance in the preceding stop trial (cf, 36): If participants successfully inhibited the response, the SSD was increased by 50 ms, if they failed to inhibit the response, the SSD was decreased by 50 ms. The SST lasted for 13 minutes. ms = milliseconds, occ/temp = occipito-temporal cortex, RT = reaction time, SD = standard deviation.
Trials were separated by short jittered inter-trial intervals (mean=900 ms, range: 700-1100 ms; adopted from Whelan et al. (29)). Stop trials appeared every 2 to 7 go trials (on average every 8.6 seconds; range=3.7–13.4s) allowing for hemodynamic separation of stop trials. Direction of go stimuli (left/right) was equally distributed in go and stop trials. For timing/task specifications, see Figure 2. We used Presentation® (Neurobehavioral Systems, Albany, USA) for task presentation and recording of motor responses.
Data analysis
Behavioral data were analyzed with SPSS21 (IBM, NY, USA), and fMRI data with statistical parametric mapping (SPM8, Wellcome Trust Centre for Neuroimaging, London, UK). In the following, “alcohol effect” depicts the difference of “alcohol-placebo”.
fMRI alcohol clamping
Behavioral data
Our main emphasis was placed on alcohol effects on inhibitory control reflected by the SSRT. Additional SST-variables were mean Go RT, PI, and go trial accuracy. We compared alcohol to placebo responses with paired t-tests, and computed alcohol-induced impairment of inhibitory control (SSRTalcohol–SSRTplacebo) for additional analyses.
Imaging data (for “Preprocessing”, see supplement)
On the first level, we modeled successful (StopInhibit), and failed (StopFail) stop trials, as well as go error trials (4%/go trials) as separate events for placebo and alcohol using a general linear model (GLM) with realignment parameters included as nuisance variables (3 translation, 3 rotation parameters). Correct go trials were represented within the implicit baseline of the GLM (cf, 24; 29-31). They were not modeled explicitly because they appeared with high-frequency during the rapid event-related fMRI experiment (1.7-2.1 seconds). In 1% (SD=3%) of stop trials, a response was given before the stop-signal would have appeared. In these trials, the presentation of the stop-signal was omitted and the SSD for the next stop trial was decreased by 50 ms. Since participants perceived these trials as “normal” go trials, we modeled brain responses accordingly. For each regressor, the onsets of the go-signals were convolved with SPM8's canonical hemodynamic response function. We corrected for serial auto-correlations using an AR(1)-model.
On the second level, we subjected the first-level contrasts “StopInhibit” and “StopFail” above the implicit go baseline (i.e., contrasted against correct go trials) for placebo and alcohol to a 2×2 full-factorial model with the within-subject factors stopping (StopInhibit, StopFail) and drug (alcohol, placebo). According to Boehler et al. (22), we first computed a whole-brain conjunction analysis of “StopInhibit” and “StopFail” across both drug conditions to confirm that the fronto-subcortical motor inhibition network (i.e., bilateral IFG, insulae, thalamus, pre-SMA, basal ganglia) was active in stop trials irrespective of the outcome. We refer to findings from this conjunction analysis as “inhibition-related” brain areas/responses. Additionally, we compared activity between “StopInhibit” and “StopFail”. We expected activation differences in brain areas associated with error- and conflict-monitoring (bilateral insulae, ACC) for “StopFail>StopInhibit”, and less prominent differences for “StopInhibit>StopFail” as both stop conditions trigger motor inhibition (22).
Second, to identify inhibition-related brain areas affected by alcohol, we performed a whole-brain conjunction analysis of the contrasts “alcohol<placebo”, “StopInhibit”, and “StopFail”. Then, we extracted brain responses in the resulting areas (two regions: RIFG/Insula, volume=4048.3 mm3, occipito-temporal cortex, volume=2790.6 mm3) for first-level contrasts (“StopInhibit” and “StopFail” for alcohol and placebo) to test whether alcohol effects within these regions ([StopInhibit+StopFail]alcohol – [StopInhibit+StopFail]placebo) were correlated with alcohol-induced impaired inhibitory control.
For each participant, we computed global and local (i.e., inhibition-related areas affected by alcohol) perfusion measured before the SST (see supplement for “ASL data-analysis”), and assessed alcohol effects on perfusion using paired t-tests. To check whether alcohol effects on inhibition-related BOLD responses were confounded by alcohol effects on perfusion (global/local), we calculated path analyses (Figure S2, supplement) using AMOS 21 (IBM).
Alcohol-induced impairment of inhibitory control and free-access alcohol consumption
We correlated alcohol-induced impairment of inhibitory control with number of alcohol requests (NoAR) during free-access ASA. We focused on NoAR of the second free-access session because the first session may be biased by unspecific exploratory behavior (see, 43; 45). This was supported by the fact that drinks per drinking day assessed by a time-line follow-back interview (48) significantly correlated with NoAR from the second day (Spearman's r(36)=.44, p=.006), but not the first (Spearman's r(36)=.25, p=.13). We used NoAR as a measure of free-access alcohol consumption for association with alcohol-induced impaired inhibitory control because it also involved a motor response. NoAR was also highly correlated with free-access BrAC-levels (r>.90).
Before statistical analysis, we verified that potentially confounding variables such as FHA, gender, drug order, current smoking, and “illicit drug use” did not significantly influence alcohol effects on inhibitory control and inhibition-related brain responses, and free-access alcohol consumption (see supplement “Results, Between-subject variables”). Thus, we did not include those covariates into statistical analyses. Imaging data were thresholded at p<.001 (uncorrected) with at least 50 connected voxels.
Results
fMRI alcohol clamping
Alcohol effects on behavior
Alcohol significantly increased the SSRT by 18.4 ms, while the mean Go RT was not affected by alcohol (Table 1). Acute alcohol intoxication decreased the PI (-1.9%), and the proportion of correct go trials (-1.4%, Table 1). BrAC pre and post SST are shown in Figure 1B.
Table 1.
Effects of alcohol on behavioral SST outcome variables during fMRI alcohol clamping. Alcohol and placebo responses were compared with paired t-tests (t).
| Placebo | Alcohol | test-statistics | |||
|---|---|---|---|---|---|
|
| |||||
| Mean | (SEM) | Mean | (SEM) | placebo vs. Alcohol | |
| SSRT (ms)*** | 207.6 | (6.3) | 226.0 | (8.1) | t(41) = -4.07, p<.001 |
| Probability of inhibition (%)** | 48.2 | (0.5) | 46.2 | (0.8) | t(41) = 2.97, p=.005 |
| mean Go RT (ms)n.s. | 414.9 | (9.6) | 419.1 | (10.0) | t(41) = -.88, p=.383 |
| Correct Go trials (%)** | 96.9 | (0.4) | 95.5 | (0.5) | t(41) = 3.35, p=.002 |
Abbreviations:
= p<.001,
= p <.01,
= not significant,
SEM = standard error of the mean
Whole-brain analyses (2×2 full-factorial model)
Inhibition-related brain responses
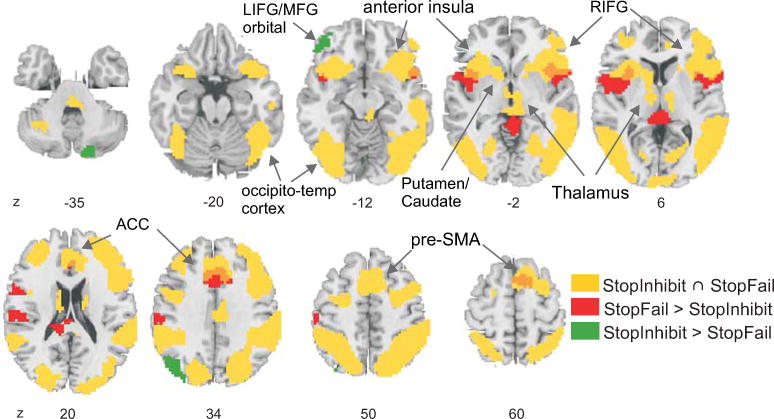
The whole-brain conjunction of “StopInhibit” and “StopFail” (above the implicit go baseline) revealed robust inhibiton-related activation of a right dominant fronto-subcortical network and bilateral occipito-temporo-parietal cortex (Figure 3; Table 2A). Comparison of stop conditions: “StopInhibit” elicited increased activation in left middle, and inferior frontal cortex, in occipito-parietal cortex, and the cerebellum compared to “StopFail” (Figure 3, Table 2B). In contrast, “StopFail” increased brain responses in bilateral precentral gyrus and anterior insulae, medial frontal, temporal and motor-related brain areas compared to “StopInhibit” (Figure 3, Table 2C).
Figure 3.
Activation of the fronto-subcortical motor inhibition network and bilateral occipito-temporo-parietal cortex during stop trials. Brain areas that were active across both stopping conditions (conjunction of StopInhibit and StopFail) are shown in yellow. Increased brain activation for StopInhibit is shown in green (StopInhibit > StopFail) and for StopFail in red (StopFail > StopInhibit). Some brain areas showed overlapping activation for both stopping conditions and increased activation for StopFail > StopInhibit, this is shown in orange. Voxel-wise significance threshold: p<.001 uncorrected with at least 50 connected voxels.
Table 2.
Results from the 2 (stopping: StopInhibit, StopFail)×2 (drug: alcohol, placebo) full-factorial model. Brain areas that showed (A) overlapping activation in both stop conditions, (B+C) differential activation between StopInhibit, and StopFail, and (D) decreased inhibition-related brain responses under alcohol compared to placebo. P-values corrected for multiple comparisons (FWE at cluster and peak level), T-values and MNI coordinates are shown. Whole-brain significance threshold: p <.001 (uncorrected) with at least 50 connected voxels.
| MNI coordinates | cluster level | peak level | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Brain area | BA | x | y | z | FWE-corr. | k | FWE-corr. | T | |
| (A) Inhibition-related brain responses (Conjunction of StopInhibit and StopFail above the implicit go baseline) | |||||||||
| L | MOG | 19 | -45 | -76 | 1 | <0.001 | 3253 | <0.001 | 11.46 |
| L | Fusiform G | 37 | -42 | -58 | -14 | 8.89 | |||
| L | MTG | 39 | -54 | -58 | 7 | 8.76 | |||
| R | ITG | 37 | 51 | -67 | -2 | <0.001 | 4804 | <0.001 | 11.39 |
| R | IPL | 40 | 33 | -49 | 46 | 9.42 | |||
| R | MOG | 19 | 30 | -73 | 28 | 9.38 | |||
| R | IFG/Insula | 47 | 33 | 23 | -5 | <0.001 | 4088 | <0.001 | 11.29 |
| R | IFG | 9 | 48 | 11 | 28 | 7.69 | |||
| R | Cingulate G | 32 | 9 | 29 | 31 | 7.48 | |||
| L | Insula | - | -30 | 20 | 4 | <0.001 | 1138 | <0.001 | 8.91 |
| L | IFG/Insula | 47 | -36 | 17 | -8 | 8.91 | |||
| R | Thalamus | - | 6 | -28 | -5 | 6.12 | |||
| L | MFG | 46 | -39 | 35 | 28 | <0.001 | 457 | <0.001 | 6.15 |
| L | MFG | 10 | -33 | 50 | 19 | 5.90 | |||
| L | MFG | 6 | -24 | -4 | 49 | <0.001 | 422 | <0.001 | 5.63 |
| L | IFG | 9 | -42 | 5 | 25 | 5.47 | |||
| L | IFG | 9 | -48 | 2 | 34 | 5.19 | |||
| R | Cingulate G | 23 | 3 | -22 | 28 | 0.001 | 168 | <0.001 | 5.59 |
| L | Post. Cingulate | 23 | -3 | -31 | 25 | 4.57 | |||
| R | Post. Cingulate | 23 | 6 | -40 | 22 | 3.74 | |||
| (B) StopInhibit > StopFail | |||||||||
| R | Cerebellum/Uvula | - | 15 | -85 | -35 | 0.141 | 51 | 0.035 | 4.86 |
| L | MFG | 11 | -45 | 44 | -11 | 0.062 | 69 | 0.166 | 4.41 |
| L | IFG | 47 | -51 | 38 | -14 | 0.362 | 4.14 | ||
| L | IFG | 47 | -36 | 32 | -17 | 0.949 | 3.51 | ||
| L | MOG | 19 | -36 | -82 | 40 | 0.008 | 118 | 0.341 | 4.17 |
| L | Angular G | 39 | -45 | -76 | 28 | 0.669 | 3.86 | ||
| L | SOG | 19 | -36 | -85 | 31 | 0.906 | 3.59 | ||
| (C) StopFail > StopInhibit | |||||||||
| L | Precentral G | 6 | -57 | 5 | 13 | <0.001 | 417 | 0.001 | 5.68 |
| L | Insula | - | -36 | 8 | 4 | 0.034 | 4.86 | ||
| L | STG | 22 | -57 | -7 | 7 | 0.976 | 3.42 | ||
| L | Postcentral G | 43 | -63 | -19 | 22 | 0.001 | 191 | 0.003 | 5.44 |
| L | Precentral G | 4 | -60 | -22 | 43 | 0.078 | 4.64 | ||
| R | Insula | 13 | 45 | 11 | -2 | <0.001 | 251 | 0.007 | 5.26 |
| R | Precentral G | 44 | 48 | 2 | 10 | 0.121 | 4.51 | ||
| R | STG | 22 | 60 | 8 | 4 | 0.213 | 4.33 | ||
| L | Cingulate G | 32 | -6 | 20 | 34 | <0.001 | 205 | 0.008 | 5.23 |
| L | ACC | 24 | 0 | 29 | 25 | 0.719 | 3.81 | ||
| Cerebellum | - | 0 | -31 | 10 | <0.001 | 205 | 0.165 | 4.42 | |
| L | Culmen | - | 0 | -43 | -2 | 0.353 | 4.15 | ||
| L | Pulvinar | - | -12 | -37 | 16 | 0.525 | 3.99 | ||
| R | SMA | 6 | 9 | 11 | 61 | 0.148 | 50 | 0.209 | 4.34 |
| (D) Alcohol<placebo in inhibition-related brain areas (Conjunction of alcohol<placebo, StopInhibit, and StopFail) | |||||||||
| R | IFG | 45 | 36 | 26 | 7 | 0.015 | 103 | 0.103 | 4.56 |
| R | Insula | - | 33 | 14 | 7 | 0.14 | 4.47 | ||
| R | IFG | 47 | 51 | 20 | 1 | 0.773 | 3.76 | ||
| R | MTG | 21 | 57 | -52 | 4 | 0.056 | 71 | 0.372 | 4.13 |
| R | MTG | 21 | 66 | -49 | 4 | 0.95 | 3.51 | ||
| R | MOG | 37 | 51 | -70 | 1 | 0.994 | 3.3 | ||
Abbreviations: ACC = anterior cingulate cortex, BA=Brodmann area, G=gyrus, IFG = inferior frontal gyrus, IPL = inferior parietal lobe, ITG = inferior temporal gyrus, MOG = middle occipital gyrus, MTG = middle temporal gyrus, SOG = superior occiptal gyrus; SMA = supplementary motor area, STG = superior temporal gyrus
Alcohol effects on inhibition-related brain responses
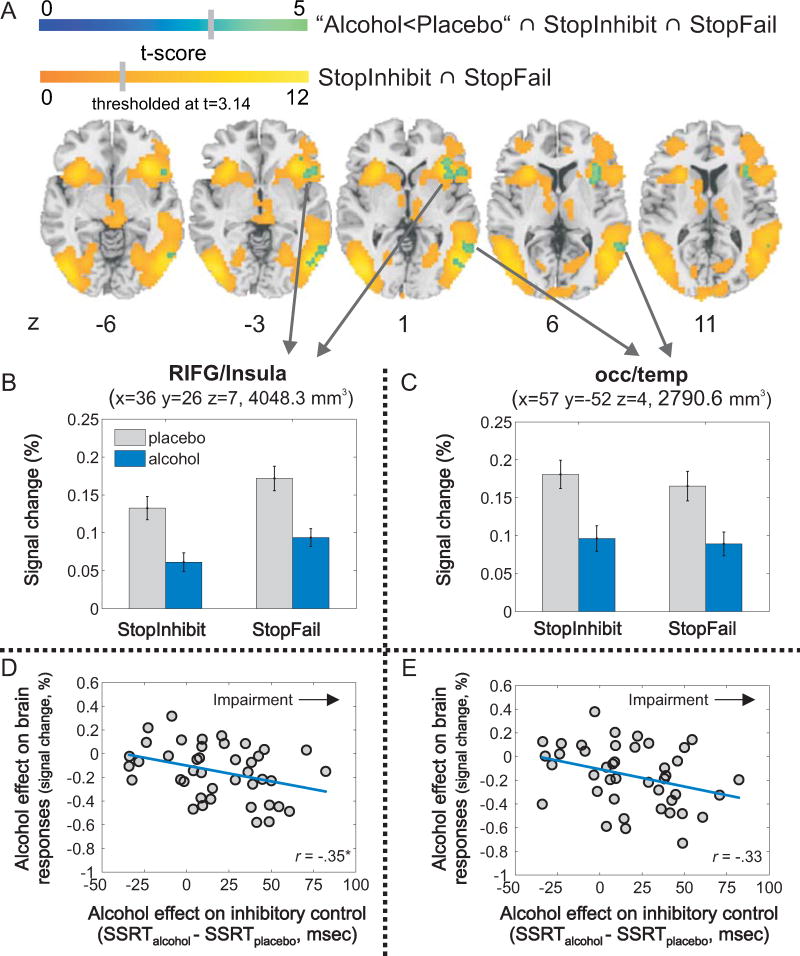
The whole-brain conjunction analysis of “alcohol<placebo”, “StopInhibit”, and “StopFail” revealed that alcohol decreased inhibition-related brain responses in two clusters: the RIFG/anterior insula, and the right middle occipito-temporal cortex (Figure 4ABC, Table 2D). Further, no brain area showed increased inhibitory activation under alcohol (versus placebo), and no interaction between stopping and drug emerged indicating that alcohol effects did not differ between stopping conditions (see supplement, Table S2).
Figure 4. Whole-brain conjunction analysis.
(A) Alcohol effects (alcohol<placebo) on inhibition-related brain responses (StopInhibit ∩ StopFail). Brain maps showing the alcohol effect (blue color scale) overlaid on inhibition-related brain responses (yellow-orange color scale; significance threshold: p<.001 uncorrected, k>50). Mean brain responses for StopInhibit and StopFail (above the implicit go baseline) for alcohol and placebo are displayed for the two inhibition-related brain areas that exhibited decreased activation under alcohol in the whole-brain conjunction analysis: RIFG/insula (B), and the occipito-temporal cortex (C). Regional analyses: Correlation between alcohol-induced impairment of inhibitory control (SSRTalcohol-SSRTplacebo; >0: impaired; <0: improved) and alcohol effects on regional inhibition-related brain responses in the RIFG/insula (D), and the occipitotemporal cortex (E). Error bars represent the standard error of the mean. Locations are given in MNI-space. Abbreviations: mm3 = cubic millimeter, ms = milliseconds, occ/temp = occipito-temporal cortex, r = Pearson correlation coefficient, RIFG = right inferior frontal gyrus, SSRT = stop-signal reaction time, * = p <.025 (α-level corrected for multiple testing, p=.05/[number of tests=2]).
Association of alcohol effects on regional inhibition-related brain responses and inhibitory control
For regional analyses, we extracted mean brain responses in the RIFG/Insula and occipito-temporal cortex (see Figure 4BC), and collapsed alcohol effects across stopping conditions ([StopInhibit+StopFail]alcohol – [StopInhibit+StopFail]placebo). Alcohol effects on inhibition-related brain responses in the RIFG/insula (Figure 4D; Pearson's r(40)=-.35, p=.024), and the occipito-temporal cortex (Figure 4E; Pearson's r(40)=-.33, p=.032) correlated negatively with alcohol-induced impaired inhibitory control. The negative correlation indicates larger activation decreases with worsened inhibitory control under alcohol. The correlation within the occipito-temporal cortex did not survive correction of the α-level for multiple testing (number of tests=2).
Furthermore, global and local (RIFG/insula, occipito-temporal cortex) cerebral perfusion measured before the SST increased significantly under alcohol (supplement, Table S3). Path analyses for the RIFG/insula, and occipito-temporal cortex showed that perfusion alcohol effects neither significantly influenced alcohol effects on inhibition-related BOLD responses, nor on inhibitory control (supplement Table S4, Figure S2). As indicated by brain-behavior correlations, only alcohol effects on inhibition-related BOLD responses in the RIFG/Insula and the occipito-temporal cortex significantly mediated alcohol-induced impairment of inhibitory control (Table S4).
Alcohol-induced impairment of inhibitory control and free-access alcohol consumption
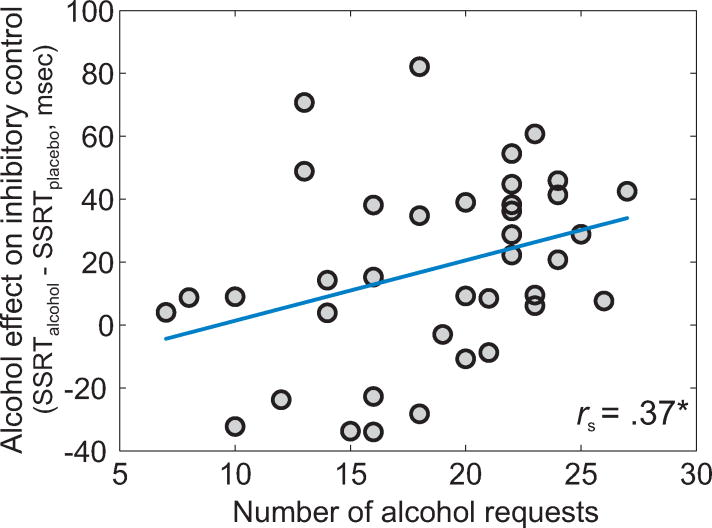
Alcohol-induced impairment of inhibitory control during fMRI alcohol clamping correlated positively with NoAR of free-access ASA (Spearman's r(36)=.37, p=.02; Figure 5). Thus, larger impairment of inhibitory control under alcohol was linked to more alcohol requests in the free-access experiment.
Figure 5.
Correlation between alcohol-induced impairment of inhibitory control at 0.6 g/kg during fMRI alcohol clamping and number of alcohol requests during free-access ASA (n=38, valid free-access data). Abbreviations: ms = milliseconds, rs = Spearman correlation coefficient, SSRT = stop-signal reaction time, * = p <.05.
Discussion
In the present study, moderate alcohol intoxication impaired inhibitory control indicated by longer SSRTs, and decreased inhibition-related brain responses in inhibition-related right fronto-temporal areas. Importantly, participants with pronounced impaired inhibitory control under alcohol showed a greater blunting of inhibition-related brain responses in the RIFG/insula and the right occipito-temporal cortex, and consumed more alcohol during free-access ASA.
Alcohol decreased inhibition-related brain responses in the RIFG/insula and the occipitotemporal cortex, two areas belonging to the right ventral fronto-parietal attention network (49; 50). The cluster encompassing the RIFG and anterior insula matches the brain area that was active during inhibitory control across SST and Go/Nogo tasks (19; 23). Recent evidence suggests that during inhibitory control the RIFG/insula might subserve cognitive processes that precede actual motor inhibition: the attentional capture of infrequent stop-signals (26; 27; 49; 50), and subsequent updating of action plans from response execution to inhibition (especially linked to the pars opercularis 23; 28). The anterior insula has not only been linked to detecting salient events (23; 51), and maintenance of task set (52), but also to error-monitoring (compare Figure 3; 53-55). We assume that during inhibitory control the RIFG and anterior insula are co-activated (cf. also, 26) and mediate attention and updating processes. However, precise functional localization within the RIFG/Insula in inhibitory control is still debated (19; 22; 23; 26; 28). Activation of the occipito-temporal cortex during inhibitory control likely reflects visual attention processes triggered by the visual modality of stop-signals (cf, 22).
Larger decreases of inhibition-related brain responses in the RIFG/insula and the occipitotemporal cortex under alcohol were linked to more pronounced alcohol-induced impairment of inhibitory control. Previous studies related decreased activation of the RIFG with low inhibitory control in general (21; 29-31), and in anterior RIFG across placebo and alcohol conditions (18). While alcohol decreased inhibition-related activation in the anterior portion of the RIFG in FHN people only, a higher risk for alcoholism in FHP participants was linked to less neural reactivity to alcohol (18). Compared to this population-specific alcohol effect in anterior RIFG, we observed a robust main effect of alcohol in the RIFG/Insula, a crucial area for inhibitory control (19; 23). Specifically, alcohol-induced impairment of inhibitory control, depicting the difference of “alcohol-placebo”, was linked to decreased inhibition-related brain responses in the RIFG/insula under alcohol compared to placebo. Noteworthy, FHA neither affected behavioral, nor neural alcohol effects in our study, which could be explained by a younger sample and the fact that we did not match our participants on FHA.
In the current study, alcohol effects on inhibition-related brain responses did not differ between stopping conditions. This gives rise to the assumption that during inhibitory control alcohol acts on the attentional capture of stop-signals and subsequent initiation of motor inhibition (23; 26-28), processes present in both conditions that precede motor inhibition. We do not assume that alcohol impaired the attention capacity in general, since the mean Go RT was not affected by alcohol (cf, 6). Our results underline that during inhibitory control alcohol affects cognitive control processes indicated by decreased brain responses in prefrontal areas (compare also, 15; 16; 18), and not directly motor inhibition associated with subcortical areas and the pre-SMA (26; 27).
Furthermore, impaired inhibitory control under alcohol predicted free-access alcohol consumption with people who were more impaired under alcohol, consuming also more alcohol. Importantly, low inhibitory control under alcohol was the only significant predictor of free-access alcohol consumption in a “post-hoc backward regression analysis” (see supplement) with the predictors “SSRTplacebo”, subjective perceptions of alcohol, and saccadic latency (variables significantly affected by alcohol (e.g., 56-58); supplement, “Level of alcohol intoxication”: Tables S5/S6). This finding corroborates that low inhibitory control under alcohol might be a specific mechanism underlying dysfunctional drinking (12-14). One might assume that the neural correlates of impaired inhibitory control under alcohol would also predict free-access alcohol consumption. This was however not the case and could possibly be explained by the fact that alcohol affected fronto-temporal, and not stopping-related pre-motor and subcortical areas. To further explore if alcohol also affects stopping-related areas and if this would specifically interact with free-access alcohol consumption, future studies might use SST versions delineating attention and updating processes from motor inhibition itself (26; 28). Possibly, higher alcohol doses would affect the subcortical motor inhibition system. Furthermore, participants showed high alcohol consumption in the laboratory (maximum BrAC∼0.9 g/kg), and in real life (Table S1, supplement) although not meeting the criteria for alcohol use disorders. This might have reduced the bandwidth of alcohol consumption levels required to establish a correlation between two independently acquired measures.
It is a limitation of this and other pharmacological BOLD-fMRI studies that group differences in baseline perfusion or metabolic activity may cause alterations of BOLD responses that are not due to task-related neural activity (42; 59; 60). In our data, we observed lower average task-related BOLD responses with higher baseline perfusion. Such effects could be responsible for the observed alcohol effects on regional BOLD responses in the RIFG/insula and occipito-temporal cortex, and on inhibitory control. However, path analyses showed that only alcohol effects on regional BOLD responses were significantly linked to alcohol-induced impairment of inhibitory control (Figure S2/Table S4). Increased perfusion under alcohol neither significantly influenced alcohol effects on regional BOLD responses (RIFG/Insula, occipito-temporal cortex), nor on inhibitory control. We conclude that alcohol effects on regional BOLD responses are likely due to changes in task-related neural activity, though we cannot completely disentangle vascular effects from task-related changes of neural activity with our methodology. To explore this, one might use quantitative or calibrated BOLD imaging (59; 61), which would allow to estimate changes in the metabolic rate of oxygen. For reasons of experimental complexity, we did not measure heart rate, respiration, and blood pressure that might also have contributed to disentangle cardiovascular from neural alcohol effects.
Conclusion
Alcohol affects the attentional capture of stop-signals and subsequent updating of action plans from response execution to inhibition, cognitive processes that precede motor inhibition, indicated by decreased brain responses in the RIFG/insula and occipito-temporal cortex. Still, precise functional localization within the RIFG/insula is debated. Under alcohol, diminished attentional capture of stop-signals might slow down initiation of motor inhibition which might impair inhibitory control via functional connections between the RIFG/insula and the pre-SMA (26; 27). In turn, alcohol-induced impairment of inhibitory control may enhance free-access alcohol consumption. We suggest that these processes interact and form a self-enhancing loop in which more alcohol consumption leads to lower inhibitory control and vice versa. Young adults with low inhibitory control under alcohol might consume more alcohol which might promote alcohol-related problems in the future (36; 37).
At the age of 21, we will test in the same participants whether alcohol-related impairment of inhibitory control and attenuated inhibition-related brain responses can predict free-access alcohol consumption that may have escalated in some and went back to normal in others. Further, we will study whether behavioral and neural correlates of alcohol-related impairment of inhibitory control are linked to other dysfunctional behaviors such as alcohol-induced aggression, or risk taking.
Supplementary Material
Acknowledgments
This research was done in collaboration with Prof. Sean O'Connor (Indiana University School of Medicine) and was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA, grant # U01AA017900), the Bundesministerium für Bildung und Forschung (BMBF grant # 01EV0711), and the Deutsche Forschungsgemeinschaft (DFG FOR 1617 (grant # SM 80/7-1, ZI 1119/4-1) and SFB 940). We thank Victor Vitvitsky for adjusting the CAIS software to the German settings, the staff at the Neuroimaging Centre Dresden for managing MRI recordings, Marie-Charlott Randhan, and Christine Markert for help during data acquisition, Dr. Inge Mick, Alexandra Markovic, and Christian Seipt for recruitment and data acquisition of the free-access alcohol self-administration part, Dr. Nils B. Kroemer for statistical advice, and the Center for Information Services and High Performance Computing (ZIH) at TU Dresden for cluster-computing time.
Footnotes
Clinical Trials: Trial name: “Neural Mechanisms Underlying Alcohol Induced Disinhibition” URL: http://www.clinicaltrials.gov/ct2/show/NCT01097213?term=Neural+Mechanisms+Underlying+Alcohol+Induced+Disinhibition&rank=1 Registration number: NCT01097213
Financial disclosures: The authors declare that over the past three years USZ has received compensation from sächsische Landesärztekammer, Gewerkschaft Erziehung und Wissenschaft, Park-Krankenhaus Leipzig, ABW Wissenschaftsverlag, Servier, Janssen, and Lundbeck. All other authors report no biomedical financial interests or potential conflicts of interest.
Online document: World Health Organization (2010). Global strategy to reduce the harmful use of alcohol. http://www.who.int/substance_abuse/alcstratenglishfinal.pdf.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference list
- 1.Corte CM, Sommers MS. Alcohol and risky behaviors. Annu Rev Nurs Res. 2005;23:327–360. [PubMed] [Google Scholar]
- 2.Halpern-Felsher BL, Millstein SG, Ellen JM. Relationship of alcohol use and risky sexual behavior: a review and analysis of findings. J Adolesc Health. 1996;19:331–336. doi: 10.1016/S1054-139X(96)00024-9. [DOI] [PubMed] [Google Scholar]
- 3.Gilman JM, Smith AR, Ramchandani VA, Momenan R, Hommer DW. The effect of intravenous alcohol on the neural correlates of risky decision making in healthy social drinkers. Addict Biol. 2012;17:465–478. doi: 10.1111/j.1369-1600.2011.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giancola PR, Parrott DJ. Further evidence for the validity of the Taylor Aggression Paradigm. Aggress Behav. 2008;34:214–229. doi: 10.1002/ab.20235. [DOI] [PubMed] [Google Scholar]
- 5.Hoaken PN, Pihl RO. The effects of alcohol intoxication on aggressive responses in men and women. Alcohol Alcohol. 2000;35:471–477. doi: 10.1093/alcalc/35.5.471. [DOI] [PubMed] [Google Scholar]
- 6.de Wit H, Crean J, Richards JB. Effects of d-amphetamine and ethanol on a measure of behavioral inhibition in humans. Behav Neurosci. 2000;114:830–837. doi: 10.1037//0735-7044.114.4.830. [DOI] [PubMed] [Google Scholar]
- 7.Loeber S, Duka T. Acute alcohol impairs conditioning of a behavioural reward-seeking response and inhibitory control processes--implications for addictive disorders. Addiction. 2009;104:2013–2022. doi: 10.1111/j.1360-0443.2009.02718.x. [DOI] [PubMed] [Google Scholar]
- 8.Mulvihill LE, Skilling TA, Vogel-Sprott M. Alcohol and the ability to inhibit behavior in men and women. J Stud Alcohol. 1997;58:600–605. doi: 10.15288/jsa.1997.58.600. [DOI] [PubMed] [Google Scholar]
- 9.Feola TW, de Wit H, Richards JB. Effects of d-amphetamine and alcohol on a measure of behavioral inhibition in rats. Behavioral Neuroscience. 2000;114:838–848. doi: 10.1037/0735-7044.114.4.838. [DOI] [PubMed] [Google Scholar]
- 10.Marczinski CA, Abroms BD, Van Selst M, Fillmore MT. Alcohol-induced impairment of behavioral control: differential effects on engaging vs. disengaging responses. Psychopharmacology (Berl) 2005;182:452–459. doi: 10.1007/s00213-005-0116-2. [DOI] [PubMed] [Google Scholar]
- 11.Fillmore MT, Weafer J. Alcohol impairment of behavior in men and women. Addiction. 2004;99:1237–1246. doi: 10.1111/j.1360-0443.2004.00805.x. [DOI] [PubMed] [Google Scholar]
- 12.Field M, Wiers RW, Christiansen P, Fillmore MT, Verster JC. Acute alcohol effects on inhibitory control and implicit cognition: implications for loss of control over drinking. Alcohol Clin Exp Res. 2010;34:1346–1352. doi: 10.1111/j.1530-0277.2010.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weafer J, Fillmore MT. Individual differences in acute alcohol impairment of inhibitory control predict ad libitum alcohol consumption. Psychopharmacology (Berl) 2008;201:315–324. doi: 10.1007/s00213-008-1284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marczinski CA, Combs SW, Fillmore MT. Increased sensitivity to the disinhibiting effects of alcohol in binge drinkers. Psychol Addict Behav. 2007;21:346–354. doi: 10.1037/0893-164X.21.3.346. [DOI] [PubMed] [Google Scholar]
- 15.Anderson BM, Stevens MC, Meda SA, Jordan K, Calhoun VD, Pearlson GD. Functional imaging of cognitive control during acute alcohol intoxication. Alcohol Clin Exp Res. 2011;35:156–165. doi: 10.1111/j.1530-0277.2010.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marinkovic K, Rickenbacher E, Azma S, Artsy E. Acute alcohol intoxication impairs top-down regulation of stroop incongruity as revealed by blood oxygen level-dependent functional magnetic resonance imaging. Hum Brain Mapp. 2012;33:319–333. doi: 10.1002/hbm.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuckit MA, Tapert S, Matthews SC, Paulus MP, Tolentino NJ, Smith TL, et al. fMRI Differences Between Subjects with Low and High Responses to Alcohol During a Stop Signal Task. Alcohol Clin Exp Res. 2012;36:130–140. doi: 10.1111/j.1530-0277.2011.01590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kareken DA, Dzemidzic M, Wetherill L, Eiler W, Oberlin BG, Harezlak J, et al. Family history of alcoholism interacts with alcohol to affect brain regions involved in behavioral inhibition. Psychopharmacology (Berl) 2013;228:335–345. doi: 10.1007/s00213-013-3038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56:1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- 20.Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boehler CN, Appelbaum LG, Krebs RM, Hopf JM, Woldorff MG. Pinning down response inhibition in the brain--conjunction analyses of the Stop-signal task. Neuroimage. 2010;52:1621–1632. doi: 10.1016/j.neuroimage.2010.04.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy BJ, Wagner AD. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann N Y Acad Sci. 2011;1224:40–62. doi: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- 25.Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychological Science. 1997;8:60–64. [Google Scholar]
- 26.Sharp DJ, Bonnelle V, De B X, Beckmann CF, James SG, Patel MC, et al. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc Natl Acad Sci U S A. 2010;107:6106–6111. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duann JR, Ide JS, Luo X, Li CS. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J Neurosci. 2009;29:10171–10179. doi: 10.1523/JNEUROSCI.1300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verbruggen F, Aron AR, Stevens MA, Chambers CD. Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. Proc Natl Acad Sci U S A. 2010;107:13966–13971. doi: 10.1073/pnas.1001957107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whelan R, Conrod PJ, Poline JB, Lourdusamy A, Banaschewski T, Barker GJ, et al. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat Neurosci. 2012;15:920–U153. doi: 10.1038/nn.3092. [DOI] [PubMed] [Google Scholar]
- 30.Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am J Psychiatry. 2005;162:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- 31.Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum Brain Mapp. 2007;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chamberlain SR, Hampshire A, Muller U, Rubia K, Del Campo N, Craig K, et al. Atomoxetine modulates right inferior frontal activation during inhibitory control: a pharmacological functional magnetic resonance imaging study. Biol Psychiatry. 2009;65:550–555. doi: 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 33.Jacobson L, Javitt DC, Lavidor M. Activation of Inhibition: Diminishing Impulsive Behavior by Direct Current Stimulation over the Inferior Frontal Gyrus. J Cogn Neurosci. 2011;23:3380–3387. doi: 10.1162/jocn_a_00020. [DOI] [PubMed] [Google Scholar]
- 34.Palmer RH, Young SE, Hopfer CJ, Corley RP, Stallings MC, Crowley TJ, et al. Developmental epidemiology of drug use and abuse in adolescence and young adulthood: Evidence of generalized risk. Drug Alcohol Depend. 2009;102:78–87. doi: 10.1016/j.drugalcdep.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grant JD, Scherrer JF, Lynskey MT, Lyons MJ, Eisen SA, Tsuang MT, et al. Adolescent alcohol use is a risk factor for adult alcohol and drug dependence: evidence from a twin design. Psychological Medicine. 2006;36:109–118. doi: 10.1017/S0033291705006045. [DOI] [PubMed] [Google Scholar]
- 36.McCambridge J, McAlaney J, Rowe R. Adult consequences of late adolescent alcohol consumption: a systematic review of cohort studies. PLoS Med. 2011;8:e1000413. doi: 10.1371/journal.pmed.1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiesner M, Weichold K, Silbereisen RK. Trajectories of alcohol use among adolescent boys and girls: identification, validation, and sociodemographic characteristics. Psychol Addict Behav. 2007;21:62–75. doi: 10.1037/0893-164X.21.1.62. [DOI] [PubMed] [Google Scholar]
- 38.Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Dev Psychol. 1999;35:205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- 39.Gunther M, Oshio K, Feinberg DA. Single-shot 3D imaging techniques improve arterial spin labeling perfusion measurements. Magn Reson Med. 2005;54:491–498. doi: 10.1002/mrm.20580. [DOI] [PubMed] [Google Scholar]
- 40.Khalili-Mahani N, van Osch MJ, Baerends E, Soeter RP, de Kam M, Zoethout RW, et al. Pseudocontinuous arterial spin labeling reveals dissociable effects of morphine and alcohol on regional cerebral blood flow. J Cereb Blood Flow Metab. 2011;31:1321–1333. doi: 10.1038/jcbfm.2010.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volkow ND, Mullani N, Gould L, Adler SS, Guynn RW, Overall JE, et al. Effects of acute alcohol intoxication on cerebral blood flow measured with PET. Psychiatry Res. 1988;24:201–209. doi: 10.1016/0165-1781(88)90063-7. [DOI] [PubMed] [Google Scholar]
- 42.Wise RG, Tracey I. The role of fMRI in drug discovery. J Magn Reson Imaging. 2006;23:862–876. doi: 10.1002/jmri.20584. [DOI] [PubMed] [Google Scholar]
- 43.Zimmermann US, Mick I, Laucht M, Vitvitskiy V, Plawecki MH, Mann KF, et al. Offspring of parents with an alcohol use disorder prefer higher levels of brain alcohol exposure in experiments involving computer-assisted self-infusion of ethanol (CASE) Psychopharmacology (Berl) 2009;202:689–697. doi: 10.1007/s00213-008-1349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Connor S, Morzorati S, Christian J, Li TK. Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcohol Clin Exp Res. 1998;22:202–210. [PubMed] [Google Scholar]
- 45.Zimmermann US, Mick I, Vitvitskyi V, Plawecki MH, Mann KF, O'Connor S. Development and pilot validation of computer-assisted self-infusion of ethanol (CASE): a new method to study alcohol self-administration in humans. Alcohol Clin Exp Res. 2008;32:1321–1328. doi: 10.1111/j.1530-0277.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ridderinkhof KR, Band GPH, Logan GD. A study of adaptive behavior: effects of age and irrelevant information on the ability to inhibit one's actions. Acta Psychol. 1999;101:315–337. [Google Scholar]
- 47.Verbruggen F, Logan GD. Models of response inhibition in the stop-signal and stop-change paradigms. Neurosci Biobehav Rev. 2009;33:647–661. doi: 10.1016/j.neubiorev.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers' self-reports of drinking behavior. Behav Res Ther. 1979;17:157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- 49.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 50.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hester R, Fassbender C, Garavan H. Individual differences in error processing: A review and reanalysis of three event-related fMRI studies using the GO/NOGO task. Cerebral Cortex. 2004;14:986–994. doi: 10.1093/cercor/bhh059. [DOI] [PubMed] [Google Scholar]
- 54.Ullsperger M, von Cramon DY. Neuroimaging of performance monitoring: Error detection and beyond. Cortex. 2004;40:593–604. doi: 10.1016/s0010-9452(08)70155-2. [DOI] [PubMed] [Google Scholar]
- 55.Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Brain network dynamics during error commission. Hum Brain Mapp. 2009;30:24–37. doi: 10.1002/hbm.20478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68:389–399. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jantti V, Lang AH, Keskinen E, Lehtinen I, Pakkanen A. Acute effects of intravenously given alcohol on saccadic eye movements and subjective evaluations of intoxication. Psychopharmacology. 1983;79:251–255. doi: 10.1007/BF00427822. [DOI] [PubMed] [Google Scholar]
- 58.Ramchandani VA, O'Connor S, Blekher T, Kareken D, Morzorati S, Nurnberger J, Jr, et al. A preliminary study of acute responses to clamped alcohol concentration and family history of alcoholism. Alcohol Clin Exp Res. 1999;23:1320–1330. [PubMed] [Google Scholar]
- 59.Leontiev O, Buxton RB. Reproducibility of BOLD, perfusion, and CMRO2 measurements with calibrated-BOLD fMRI. Neuroimage. 2007;35:175–184. doi: 10.1016/j.neuroimage.2006.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: the deoxyhemoglobin dilution model. Magn Reson Med. 1999;42:849–863. doi: 10.1002/(sici)1522-2594(199911)42:5<849::aid-mrm4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 61.Pike GB. Quantitative functional MRI: Concepts, issues and future challenges. Neuroimage. 2012;62:1234–1240. doi: 10.1016/j.neuroimage.2011.10.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.