Abstract
Precise fluorescence-guided surgery (FGS) for pancreatic cancer has the potential to greatly improve the outcome in this recalcitrant disease. In order to achieve this goal, we have used genetic reporters to color code cancer and stroma cells in a patient-derived orthotopic xenograft (PDOX) model. The telomerase-dependent green fluorescent protein (GFP) containing adenovirus OBP401 was used to label the cancer cells of the pancreatic cancer PDOX. The PDOX was previously grown in a red fluorescent protein (RFP) transgenic mouse that stably labeled the PDOX stroma cells bright red. The color-coded PDOX model enabled FGS to completely resect the pancreatic tumors including stroma. Dual-colored FGS significantly prevented local recurrence, which bright-light surgery (BLS) or single color could not. FGS, with color-coded cancer and stroma cells has important potential for improving the outcome of recalcitrant cancer.
Keywords: fluorescence-guided surgery (FGS), patient-derived orthotopic xenograft (PDOX), adenovirus OBP-401, green fluorescent protein, GFP, red fluorescent protein, RFP, pancreatic cancer, stroma, color-coded fluorescence imaging
Introduction
Accurate visualization of primary tumor margins and metastatic deposits at the time of surgery is critical to determine the success of a cancer operation. In order to achieve this goal, there is currently intense interest in labeling of tumors for fluorescence-guided surgery (FGS). In the clinic, sentinel lymph nodes were labeled by the near-infrared (NIR) fluorescing dye indocyanine.1 However, indocyanine does not specifically label tumor cells. 5-Aminolevulinic acid, a precursor of hemoglobin, can selectively label malignant glioma. Porphyrin fluorescence in the brain tumor can then be visualized with a fluorescence neurosurgical microscope.2 FGS resulted in a higher 6-month progression-free survival rates than did those who had bright-light surgery (BLS).2 Folate conjugated to fluorescein isothiocyanate (FITC) targeted the folate receptor-α (FR-α), which is often overexpressed in ovarian cancers. FGS, with a real-time multispectral intraoperative fluorescence imaging system, enabled tumors less than 1 mm in diameter to be resected. However, FR-α may not generally overexpressed in cancer.3
In other studies, monoclonal antibodies against cancer antigen 19-9 (CA19-9) or carcino-embryonic antigen (CEA), were conjugated to a green fluorophore and delivered intravenously into nude mice with orthotopic human pancreatic or colon tumors.4 The fluorescent antibodies adhered to the CEA or CA19-9 antigens of CEA- or CA19-9–expressing cancer cells, respectively, making the tumors become fluorescent and visible and resectable under fluorescence guidance,4 which in subsequent studies resulted in reduced recurrence and increased survival compared to BLS.3,5-12
Kishimoto et al.13 selectively labeled tumors with green fluorescent protein (GFP) using a telomerase-dependent adenovirus (OBP-401) that expresses the gfp gene only in cancer cells. The labeled tumors could then be resected under fluorescence guidance. OBP-401 GFP-labeled tumors that recurred after fluorescence-guided surgery maintained GFP expression.14 Thus, imaging cancer recurrence and metastasis is also possible with OBP-401 GFP labeling, which is not possible with non-genetic probes such as fluorescent antibodies.3
OBP-401 could selectively label soft-tissue sarcoma (STS) with GFP in an orthotopic nude-mouse model. OBP-401-based FGS resulted in superior resection of STS compared to BLS. OBP-401 based-FGS improved disease free survival as well as preserved muscle function compared with BLS.15
Glioblastoma multiforme (GBM) is one of the most invasive of cancers and is not totally resectable using standard BLS or current FGS. OBP-401 infection brightly and selectively labeled GBM with GFP for effective FGS in orthotopic nude-mouse models without recurrence.16
It is also possible to selectively label the stroma of a tumor. Pancreatic-cancer patient-derived orthotopic xenografts (PDOX) tumors were passaged orthotopically in transgenic nude mice ubiquitously expressing red fluorescent protein (RFP). The primary patient tumors acquired RFP-expressing stroma. The RFP-expressing stroma included cancer-associated fibroblasts (CAFs) and tumor-associated macrophages (TAMs). The RFP stroma persisted in the tumors after passage.17 It was possible to image the brightly fluorescent tumors noninvasively longitudinally as they progressed after passage to nontransgenic nude mice.18
In the present study, we demonstrate a novel approach to FGS, with cancer cells labeled with GFP by GFP-401 and stroma labeled with red fluorescence protein (RFP) by previous growth in RFP transgenic mice. Dual-color FGS was more effective than BLS or single-color FGS to prevent recurrence in a pancreatic cancer patient-derived orthotopic xenograft (PDOX) nude-mouse model.
Materials and Methods
GFP-expressing telomerase-specific adenovirus
Adenovirus OBP-401 contains a promoter element of the human telomerase reverse transcriptase (hTERT) gene which drives the expression of E1A and E1B genes linked to an internal ribosome entry site. OBP-401 also contains the GFP gene which is driven by the CMV promoter. The virus only replicates in cancer cells, labeling them with the GFP gene.13
Mice
Athymic nude nu/nu mice or transgenic RFP nu/nu mice 19 (AntiCancer, Inc., San Diego, CA, USA) (5 weeks old), were kept in a barrier facility under HEPA filtration. Mice were fed with autoclaved laboratory rodent diet (Tecklad LM-485, Western Research Products). All animal studies were conducted in accordance with the principals and procedures outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals under Assurance no. A3873-01.
Establishment of patient pancreatic cancer
Pancreatic cancer tumor tissue from a single patient was originally obtained at surgery at the MD Anderson Cancer Center and cut into 3-mm3 fragments and transplanted subcutaneously in NOD/SCID mice.20,21
Patient-derived orthotopic xenograft (PDOX®)
The fluorescent PDOX (fPDOX) model was passaged, using surgical orthotopic implantation (SOI) 22 of tumor previously grown in the NOD/SCID mice, in transgenic RFP nude mice.17,18,19 A small 6-to-10 mm transverse incision was made on the left flank of the RFP nude mouse through the skin and peritoneum. The tail of the pancreas was exposed through this incision, and a single 1-mm3 tumor fragment was sutured to the tail of the pancreas using 8-0 nylon surgical sutures (Ethilon; Ethicon, Inc., NJ). On completion, the tail of the pancreas was returned to the abdomen and the incision was closed in one layer using 6-0 nylon surgical sutures (Ethilon).22-26
OBP-401 labeling of the RFP-expressing PDOX
The PDOX tumor was exposed in the peritoneal cavity during laparatomy. OBP-401 (1 × 108 PFU/tumor) was then injected into the exposed tumor.
Imaging
PDOX tumors, labeled with genetic reporters, were imaged with a laser-scanning microscope (IV100; Olympus, Tokyo, Japan) 27 or a confocal laser-scanning microscope (FV1000; Olympus) 28 or an OV-100 Olympus Small Animal Imaging System (Olympus Corp.).29
Fluorescence-guided surgery
Mice were given a ketamine mixture (10 μl ketamine HCl, 7.6 μl xylazine, 2.4 μl acepromazine maleate, and 10 μl PBS) (i.m.) before fluorescence-guided surgery. .The Dino-Lite mobile imaging system was used for imaging in live mice. The portable system has seven filtered (wavelength) blue LEDs for excitation lighting; a 510 nm emission filter; a white LED switched by software, and a 1.3 megapixel sensor. This digital camera can magnify from ×30 up to ×200 and can readily take both pictures and videos of magnified green, fluorescent objects. The camera's dimensions are 10.5 × 3.2 cm and the weight is only 105 g. This all-in-one compact digital camera makes the Dino-Lite imaging system easily transportable and thereby suitable for FGS.30
All animal procedures were performed under anesthesia using s.c. administration of a ketamine mixture (see above). OBP-401 GFP-labeled cancer cells and/or RFP stromal cells in the PDOX were imaged with the Dino-Lite hand-held fluorescence scope to determine the resection area and margin. A 20 mm incision was made at the lateral midline of the abdomen. The pancreatic tumor was then exposed. Either under bright light or fluorescence tumor was resected using a scalp and foreceps. Fluorescence imaging with the Dino-Lite was performed to visualize residual GFP cancer cells or RFP stroma. The resected area of the pancreas was closed with a 6-0 suture. After surgery, the abdominal wall was closed with 6-0 suture.
Statistical analysis
PASW Statistics 18.0 (SPSS, Inc) was used for all statistical analyses. Data are shown as mean ± SD. For comparison between two groups, significant differences were determined using Student's t-test. P values of < 0.05 were considered significant.
Results and Discussion
FGS of OBP-401-GFP
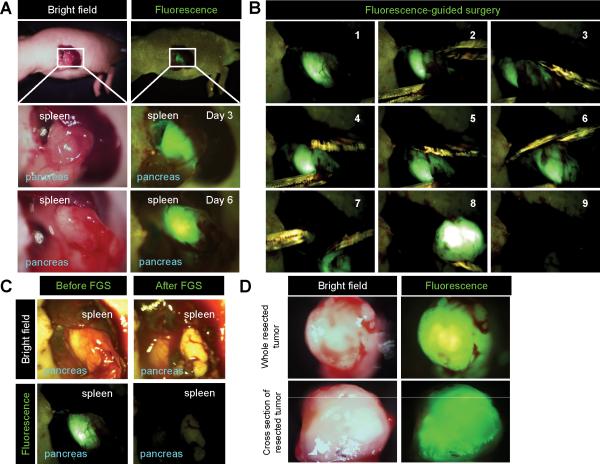
Time-lapse imaging showed that pancreatic cancer cells in the PDOX model were labeled by OB-401 three days after OBP-401 infection. GFP fluorescence remained sufficiently strong to use FGS 5 days after OBP-401 infection (Fig. 1A). The margin between the tumor and pancreas was very unclear under bright-light (Fig. 1A). In contrast, OBP-401 made the tumor margin much clearer than under bright-light (Fig. 1A). Using the Dino-Lite fluorescence digital camera system, the margin between tumor and normal was visualized sufficiently clearly for FGS of the pancreatic tumor (Fig. 1B, 1C). Whole-tumor and cross-section imaging demonstrated that OBP-401 labeled the whole tumor (Fig. 1D).
Figure 1. OBP-401 based FGS of a pancreatic cancer PDOX.
A. Representative intravital images of a pancreatic cancer patient-derived orthotopic xenograft (PDOX) labeled with OBP-401-GFP three days after infection (upper and middle panels) and five days after infection (lower panels). B. FGS of GFP labeled PDOX was performed with a Dino-Lite hand-held fluorescence scope. C. Representative intravital images before and after FGS. D. Representative images of resected tumor image (upper) and cross section (lower). Images were acquired with the OV100 fluorescence microscope.
FGS detects and resects residual cancer cells in OBP-401-GFP-labeled PDOX after BLS
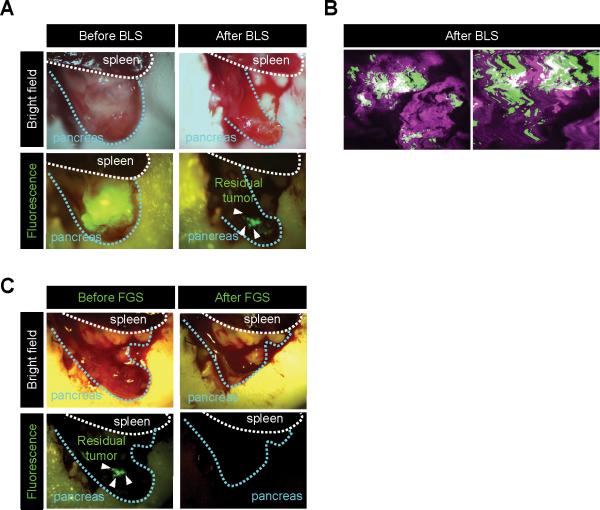
PDOX tumors were initially resected by BLS 5 days after injection of OBP-401 (Fig. 2A). After BLS, confocal laser microscopy detected residual GFP-expressing cancer (Fig. 2B) at the single cell level. The residual cancer cells were then resected by FGS (Fig. 2C).
Figure 2. OBP-401 based FGS for residual cancer cells in PDOX under BLS.
A. Representative intravital images of GFP-labeled PDOX five days after infection and before BLS. Bright-field images (upper panels) and fluorescence images (lower panels). B. Representative images of residual cancer cells. Images were acquired with a confocal laser scanning microscope FV1000 (Olympus). Low-magnification image (left panel) and high-magnification image (right panel). C. Representative intravital images before and after FGS of GFP-labeled residual tumor in PDOX after BLS. After BLS, FGS was performed using the Dino-Lite hand-held fluorescence scope.
BLS of PDOX labeled with RFP stroma
Pancreatic cancer PDOX, previously grown in RFP transgenic nude mice in order to acquire RFP stroma cells, were orthotopically transplanted to non-colored nude mice.17,18,31 The RFP-expressing stroma cells were readily imaged in the PDOX (Fig. 3A). Residual RFP-expressing stromal cells were readily visualized after BLS (Fig. 3A, 3B).
Figure 3. BLS of PDOX labeled with RFP stroma cells.
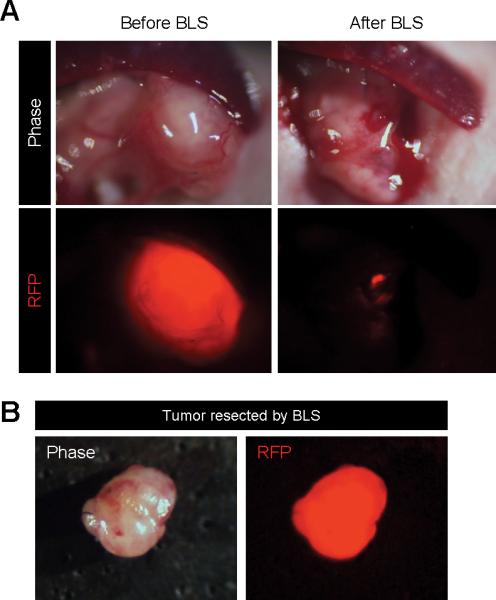
A. Representative intravital images of PDOX labeled with RFP stroma cells before and after BLS. B. Representative images of tumor resected by BLS.
OBP-401-GFP labeling of PDOX with RFP stromal cells
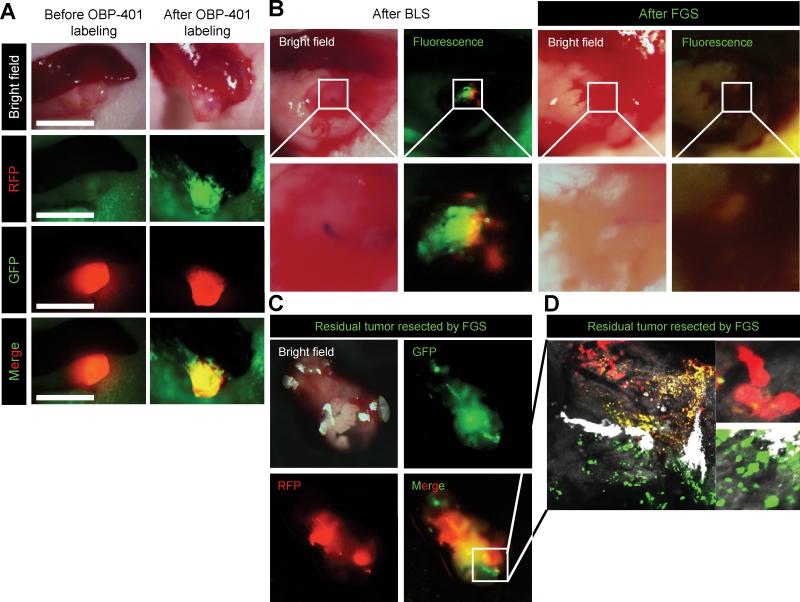
Pancreatic cancer PDOX with RFP-expressing stroma were labeled with GFP in their cancer cells by administration of OBP-401 (Fig 4A). The pancreatic cancer PDOX with RFP-expressing stroma and GFP-labeled cancer cells was incompletely resected by BLS (Fig. 4B). Fluorescence imaging demonstrated that there were residual GFP-expressing cancer cells and RFP-expressing stroma cells after BLS (Fig. 4B).
Figure 4. FGS of residual GFP cancer cells and RFP stroma cells in PDOX remaining after BLS.
A. Representative intravital images of PDOX labeled with RFP stroma cells, acquired by a passage in an RFP transgenic mouse before and after OBP-401 labeling. Bright-field images (left panel) and fluorescence images (right panel). Images were acquired with the OV100. B. Representative intravital images of residual GFP cancer cells and RFP stroma cells after BLS and FGS after BLS were acquired with the FV1000 confocal laser microscope. Low-magnification images (upper panels) and high-magnification images (lower panels) images obtained with the OV100. C. Representative images of a dual-color tumor resected by FGS. D. High-magnification image from 2C of resected two-color tumor. Image was acquired with the FV1000.
FGS completely resects pancreatic cancer PDOX with GFP-expressing cancer cells and RFP-expressing stroma cells
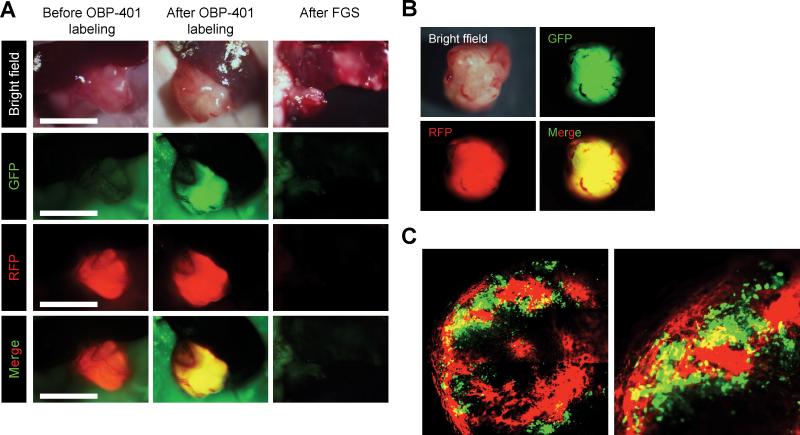
GFP and RFP imaging showed that cancer and stromal cells in the color-coded labeled PDOX invaded beyond the tumor capsule (Fig. 4C, 4D). However, FGS enabled complete resection of the PDOX with GFP-expressing cancer cells and RFP-expressing stroma cells (Fig. 4B, Fig. 5, Supplementary Movie 1).
Figure 5. FGS of PDOX with GFP-labeled cancer cells and RFP-labeled stroma.
A. Representative intravital images of a PDOX with RFP stroma before labeling with OBP-401 (left panels); after 0BP-401-GFP labeling (middle panels); and after FGS (right panels). Images were acquired with the OV100 before FGS and after FGS. B. Representative images of resected dual-color tumor after FGS. Images were acquired with the OV100 fluorescence imaging system. C. Representative image of a cross section of the resected dual-color tumor. Image was acquired with the FV1000 confocal laser microscope.
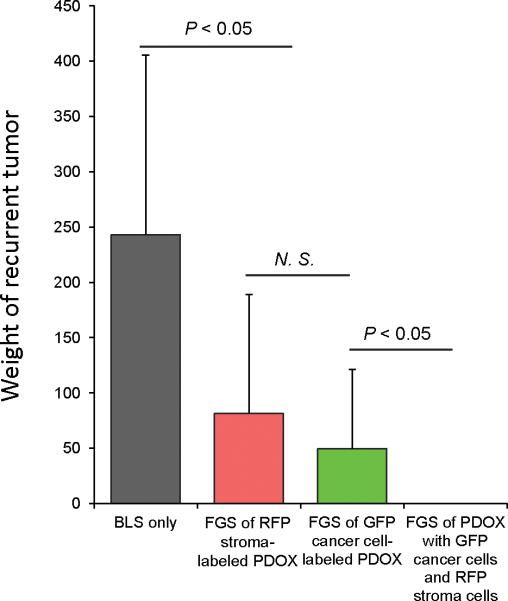
Color-coded FGS enables recurrence-free surgery
Two months after BLS, eight of ten mice had local recurrences (Table 1). Five of twelve mice which received FGS of RFP stroma-labeled PDOX had local recurrences (Table 1). Four of eleven mice which received FGS of GFP-labeled PDOX had local recurrences (Table 1). In contrast, there was no local recurrence in nine mice which received FGS of the color-coded PDOX with GFP-labeled cancer cells and RFP stroma cells (Fig. 6, Table 1). These data demonstrated that dual-color FGS resulted in complete resection and prevented local recurrence of the pancreatic cancer PDOX, which BLS or single-color FGS failed to do.
TABLE 1.
Comparison of local recurrence after indicated surgery methods.
| Surgical method | Positive | Negative |
|---|---|---|
| BLS only | 10 | 2 |
| Single-color FGS of RFP stroma-labeled PDOX | 5 | 7 |
| Single-color FGS of GFP cancer cells-labeled PDOX | 4 | 7 |
| Dual-color FGS of PDOX with GFP cancer cells and RFP stroma cells | 0 | 9 |
Figure 6. Comparison of the extent of tumor recurrence after different modalities of surgery.
Tumors were resected by either BLS or FGS with tumors labeled either with RFP stroma only, GFP cancer cells only, or with both RFP-labeled stroma and GFP-labeled cancer cells. Please see Materials and Methods for details.
OBP-401 selectively labeled cancer cells in the pancreatic cancer PDOX with RFP-expressing stroma. The double-labeled PDOX enabled precise visualization of color-coded cancer and stromal cells after BLS and improved the efficacy of FGS.
Tumor-specific antibodies conjugated with fluorophores can only access the tumor from the outside and cannot label all the cancer cells in the central part of the PDOX. The present study demonstrated that OBP-401 infection labels cancer cells with GFP within the tumor and this signal, along with the RFP-expressing stromal cells, remained sufficiently strong to perform FGS 5 days after infection.
Future experiments will develop reporter gene vectors to target the stromal cells in order to apply color-coded cancer cell-stromal cell labeling for FGS in the clinic.
Supplementary Material
Supplementary Movie 1. FGS of tumor with GFP-labeled cancer cells and RFP-labeled stroma cells.
Acknowledgements
This study was supported in part by the National Cancer Institute grant CA142669. This study was also supported in part by grants-in-Aid from the Ministry of Education, Science and Culture, Japan, and grants from the Ministry of Health and Welfare, Japan.
Abbreviations
- PDOX
patient-derived orthotopic xenograft
- GFP
green fluorescent protein
- RFP
red fluorescent protein
- FGS
fluorescent-guided surgery
- BLS
bright light surgery
Footnotes
Author Contributions
SY, YH, and AM performed experiments. SY and RMH wrote the paper. SY, YH, AM, HK, AS, SM, MT, MY, MHGK, JBF, YR, HT, SK, TF, MB and RMH analyzed the data.
Dedication
This paper is dedicated to the memory of A. R. Moossa, MD.
Competing Financial Interest
Y. Urata is President & CEO of Oncolys BioPharma, Inc., the manufacturer of OBP-401 (Telomescan). H. Tazawa and T. Fujiwara are consultants of Oncolys BioPharma, Inc. There are no other competing financial interests.
References
- 1.Troyan SL, Kianzad V, Gibbs-Strauss SL, Gioux S, Matsui A, Oketokoun R, et al. The FLARE intraoperative near-infrared fluorescence imaging system: A first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol. 2009;16:2943–2952. doi: 10.1245/s10434-009-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 3.Bouvet M, Hoffman RM. Glowing tumors make for better detection and resection. Sci Transl Med. 2011;3:110sf10. doi: 10.1126/scitranslmed.3003375. [DOI] [PubMed] [Google Scholar]
- 4.McElroy M, Kaushal S, Luiken GA, Talamini MA, Moossa AR, Hoffman RM, et al. Imaging of primary and metastatic pancreatic cancer using a fluorophore-conjugated anti-CA19-9 antibody for surgical navigation. World J Surg. 2008;32:1057–1066. doi: 10.1007/s00268-007-9452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metildi CA, Kaushal S, Lee C, Hardamon CR, Snyder CS, Luiken GA, et al. An LED light source and novel fluorophore combinations improve fluorescence laparoscopic detection of metastatic pancreatic cancer in orthotopic mouse models. J Am Coll Surg. 2012;214:997–1007. doi: 10.1016/j.jamcollsurg.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metildi CA, Kaushal S, Hardamon CR, Snyder CS, Pu M, Messer KS, et al. Fluorescence-guided surgery allows for more complete resection of pancreatic cancer, resulting in longer disease-free survival compared with standard surgery in orthotopic mouse models. J Am Coll Surg. 2012;215:126–136. doi: 10.1016/j.jamcollsurg.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metildi CA, Kaushal S, Snyder CS, Hoffman RM, Bouvet M. Fluorescence-guided surgery of human colon cancer increases complete resection resulting in cures in an orthotopic nude mouse model. J Surg Res. 2013;179:87–93. doi: 10.1016/j.jss.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Metildi CA, Hoffman RM, Bouvet M. Fluorescence-guided surgery and fluorescence laporascopy for gastrointestinal cancers in clinically-relevant mouse models. Gastroenterol Res Pract. 2013 doi: 10.1155/2013/290634. Article ID 290634, 8 pages, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metildi CA, Tang CM, Kaushal S, Leonard SY, Magistri P, Tran Cao HS, et al. In vivo fluorescence imaging of gastrointestinal stromal tumors using fluorophore-conjugated anti-KIT antibody. Ann Surg Oncol. Suppl. 2013;3:693–700. doi: 10.1245/s10434-013-3172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metildi CA, Kaushal S, Pu M, Messer KA, Luiken GA, Moossa AR, et al. Fluorescence-guided surgery with a fluorophore-conjugated antibody to carcinoembryonic antigen (CEA), that highlights the tumor, improves surgical resection and increases survival in orthotopic mouse models of human pancreatic cancer. Ann Surg Oncol. 2014;21:1405–1411. doi: 10.1245/s10434-014-3495-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metildi CA, Kaushal S, Luiken GA, Talamini MA, Hoffman RM Bouvet M. Fluorescently-labeled chimeric anti-CEA antibody improves detection and resection of human colon cancer in a patient-derived orthotopic xenograft (PDOX) nude mouse model. J Surg Oncol. 2014;109:451–458. doi: 10.1002/jso.23507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metildi CA, Kaushal S, Luiken GA, Hoffman RM, Bouvet M. Advantages of fluorescence-guided laparoscopic surgery of pancreatic cancer labeled with fluorescent anti-carcinoembryonic antigen antibodies in an orthotopic mouse model. J Am Coll Surg. 2014;219:132–141. doi: 10.1016/j.jamcollsurg.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kishimoto H, Zhao M, Hayashi K, Urata Y, Tanaka N, Fujiwara T, et al. In vivo internal tumor illumination by telomerase-dependent adenoviral GFP for precise surgical navigation. Proc Natl Acad Sci USA. 2009;106:14514–14517. doi: 10.1073/pnas.0906388106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kishimoto H, Aki R, Urata Y, Bouvet M, Momiyama M, Tanaka N, et al. Tumor-selective, adenoviral-mediated GFP genetic labeling of human cancer in the live mouse reports future recurrence after resection. Cell Cycle. 2011;10:2737–2741. doi: 10.4161/cc.10.16.16756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yano S, Miwa S, Kishimoto H, Uehara F, Tazawa H, Toneri M, et al. Targeting tumors with a killer-reporter adenovirus for curative fluorescence-guided surgery of soft-tissue sarcoma. Oncotarget. doi: 10.18632/oncotarget.3811. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yano S, Miwa S, Kishimoto H, Toneri M, Hiroshima Y, Yamamoto M, et al. Curative fluorescence-guided surgery of highly-invasive cancer selectively labeled with a killer-reporter adenovirus. Molecular Therapy. doi: 10.1038/mt.2015.63. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suetsugu A, Katz M, Fleming J, Truty M, Thomas R, Moriwaki H, et al. Multi-color palette of fluorescent proteins for imaging the tumor microenvironment of orthotopic tumorgraft mouse models of clinical pancreatic cancer specimens. J Cell Biochem. 2012;113:2290–2295. doi: 10.1002/jcb.24099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suetsugu A, Katz M, Fleming J, Truty M, Thomas R, Saji S, et al. Non-invasive fluorescent-protein imaging of orthotopic pancreatic-cancer-patient tumorgraft progression in nude mice. Anticancer Res. 2012;32:3063–3068. [PubMed] [Google Scholar]
- 19.Yang M, Reynoso J, Bouvet M, Hoffman RM. A transgenic red fluorescent protein-expressing nude mouse for color-coded imaging of the tumor microenvironment. J Cell Biochem. 2009;106:279–284. doi: 10.1002/jcb.21999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim MP, Evans DB, Wang H, Abbruzzese JL, Fleming JB, Gallick GE. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat Protol. 2009;4:1670–1680. doi: 10.1038/nprot.2009.171. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim MP, Truty MJ, Choi W, Kang Y, Chopin-Lally X, Gallick GE, Wang H, McConkey DJ, Hwang R, Logsdon C, Abbruzzesse J, Fleming JB. Molecular profiling of direct xenograft tumors established from human pancreatic adenocarcinoma after neoadjuvant therapy. Ann Surg Oncol. Suppl. 2012;3:S395–403. doi: 10.1245/s10434-011-1839-4. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructedorthotopically from histologically intact patient specimens. Proc Natl Acad Sci USA. 1992;89:5645–5649. doi: 10.1073/pnas.89.12.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffman RM. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic. Investigational New Drugs. 1999;17:343–359. doi: 10.1023/a:1006326203858. [DOI] [PubMed] [Google Scholar]
- 24.Bouvet M, Yang M, Nardin S, Wang X, Jiang P, Baranov E, et al. Chronologically-specific metastatic targeting of human pancreatic tumors in orthotopic models. Clin Exp Metastasis. 2000;18:213–218. doi: 10.1023/a:1006767405609. [DOI] [PubMed] [Google Scholar]
- 25.Bouvet M, Wang J-W, Nardin SR, Nassirpour R, Yang M, Baranov E, et al. Real-time optical imaging of primary tumor growth and multiple metastatic events in a pancreatic cancer orthotopic model. Cancer Res. 2002;62:1534–1540. [PubMed] [Google Scholar]
- 26.Katz MH, Takimoto S, Spivack D, Moossa AR, Hoffman RM, Bouvet M. A novel red fluorescent protein orthotopic pancreatic cancer model for the preclinical evaluation of chemotherapeutics. J Surg Res. 2003;113:151–160. doi: 10.1016/s0022-4804(03)00234-8. [DOI] [PubMed] [Google Scholar]
- 27.Yang M, Jiang P, Hoffman RM. Whole-body subcellular multicolor imaging of tumor-host interaction and drug response in real time. Cancer Res. 2007;67:5195–5200. doi: 10.1158/0008-5472.CAN-06-4590. [DOI] [PubMed] [Google Scholar]
- 28.Uchugonova A, Zhao M, Weinigel M, Zhang Y, Bouvet M, Hoffman RM, et al. Multiphoton tomography visualizes collagen fibers in the tumor microenvironment that maintain cancer-cell anchorage and shape. J Cell Biochem. 2013;114:99–102. doi: 10.1002/jcb.24305. [DOI] [PubMed] [Google Scholar]
- 29.Yamauchi K, Yang M, Jiang P, Xu M, Yamamoto N, Tsuchiya H, et al. Development of real-time subcellular dynamic multicolor imaging of cancer cell trafficking in live mice with a variable-magnification whole-mouse imaging system. Cancer Res. 2006;66:4208–4214. doi: 10.1158/0008-5472.CAN-05-3927. [DOI] [PubMed] [Google Scholar]
- 30.Hiroshima Y, Maawy A, Sato S, Murakami T, Uehara F, Miwa S, et al. Hand-held high-resolution fluorescence imaging system for fluorescence-guided surgery of patient and cell-line pancreatic tumors growing orthotopically in nude mice. J Surg Res. 2014;187:510–517. doi: 10.1016/j.jss.2013.11.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suetsugu A, Katz M, Fleming J, Truty M, Thomas R, Saji S, et al. Imageable fluorescent metastasis resulting in transgenic GFP mice orthotopically implanted with human-patient primary pancreatic cancer specimens. Anticancer Res. 2012;32:1175–1180. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Movie 1. FGS of tumor with GFP-labeled cancer cells and RFP-labeled stroma cells.