Abstract
The simplicity of C. elegans makes it an outstanding system to study the role of Wnt signaling in development. Many asymmetric cell divisions in C. elegans require the Wnt/β-catenin asymmetry pathway. Recent studies confirm that SYS-1 is a structurally and functionally divergent β-catenin, and implicate lipids and retrograde trafficking in maintenance of WRM-1/β-catenin asymmetry. Wnts also regulate short-range events such as spindle rotation and gastrulation, and a PCP-like pathway regulates asymmetric divisions. Long-range, cell non-autonomous Wnt signals regulate vulval induction. Both short-range and long-range Wnt signals are regulated by recycling of MIG-14/Wntless via the retromer complex. These studies indicate that C. elegans continues to be useful for identifying new, conserved mechanisms underlying Wnt signaling in metazoans.
Wnt signaling is a fundamental, conserved process during metazoan development. The bewildering array of responses of cells in embryos to Wnt signals makes it a daunting task to identify specific Wnt-dependent signaling inputs that influence differentiation. The simplicity of the C. elegans embryo and larva makes C. elegans an attractive model system for investigating the role of Wnt signaling in a variety of cellular contexts. Here we review recent progress in characterizing several different Wnt-dependent signaling pathways in the C. elegans embryo and larva. We have tried to avoid overlap with other recent reviews in what follows. For other recent reviews, please consult[1–3].
Expanding the canon: AXL-1 and the canonical Wnt pathway
As in Drosophila and vertebrates, some Wnt-dependent events in C. elegans use the canonical Wnt signaling pathway, including migration of the Q neuroblasts and establishment of the fate of vulval precursor cells (VPCs). As in other canonical Wnt signaling pathways, pry-1/Axin functions as a negative regulator of the pathway [4]. Oosterveen et al. [5] have identified another, divergent Axin-like protein, AXL-1, which like PRY-1, physically interacts with BAR-1/β-catenin, MIG-5/Dsh and DSH-2/ Dsh. Moreover, axl-1 mutants enhance the pry-1 null phenotype. axl-1 mutants display ectopic branching of the excretory cell, which is not enhanced by pry-1 loss of function, but can be rescued by excretory cell-specific expression of pry-1. These results indicate that while partially redundant, axl-1 and pry-1 may have functionally distinct roles.
Expanding the non-canon: new insights into Wnt/β-catenin asymmetry
Many asymmetric divisions during C. elegans development, including those of EMS, T blast cells and Z1/Z4 (somatic gonadal precursors) use a second Wnt signaling pathway, the Wnt/β-catenin asymmetry pathway (reviewed in [1,2,6,7]). Like canonical Wnt signaling, β-catenin function is required; however, unlike canonical signaling, a key role of β-catenin is to regulate asymmetric localization of the Tcf transcription factor POP-1, a process termed ‘POP-1 asymmetry’ [8]. In the posterior (distal) daughter cell Wnt signaling activates WRM-1/β-catenin and LIT-1/Nemo-like kinase. WRM-1 and LIT-1 show an asymmetric nuclear localization that is reciprocal to POP-1 [9•,10], consistent with their role in targeting POP-1 for nuclear export [11].
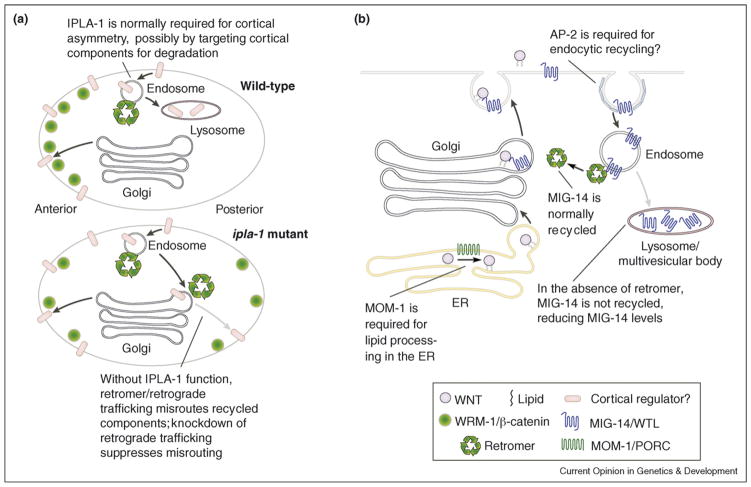
Although the mechanisms by which such asymmetries arise is still largely unknown (see [2] for extensive discussion), it must involve cortical asymmetries, which give rise to eventual nuclear asymmetries. Very recent work by Kanamori et al. [12•] shows that loss of function of the intracellular phospholipase A1, IPLA-1, results in cell autonomous defects in production of seam cells from T blast cells, spindle orientation defects and loss of cortical WRM-1 asymmetry. An ipla-1 suppressor screen identified mon-2, an ARF GEF-like protein and tbc-3, a Rab GAP, whose yeast homologues encode proteins involved in endosome-to-Golgi retrograde vesicle trafficking. In addition, knocking down the C. elegans homologues of the retromer complex, which is involved in retrograde transport of intracellular sorting receptor complexes in yeast and mammalian cells [13], suppresses ipla-1 mutants. These results suggest a model in which WRM-1 asymmetry is regulated by turnover of membrane components, whose steady-state levels are influenced by IPLA-1 and recycling endosomes (Figure 1A). Given the importance of recycling endosomes to Wntless trafficking (see below), these findings are intriguing.
Figure 1.
Retrograde trafficking affects Wnt signaling via multiple mechanisms in C. elegans. (A) WRM-1/β-catenin asymmetry is disrupted by loss of IPLA-1 function. In this hypothetical model, IPLA-1 is presumed to modify a lipid component that results in steady-state reduction in components at the cortex required for anterior localization of WRM-1. In ipla-1 mutants, misrouting of these components appears to require retrograde vesicle trafficking, since it is disrupted by knockdown of components of the retromer and retrograde trafficking machinery. (B) The retromer complex and AP-2 regulate MIG-14/ Wntless trafficking, which indirectly regulates Wnt secretion. MOM-1/Porcupine is required for an early step in Wnt ligand processing, presumably in the endoplasmic reticulum. Later steps in MIG-14 trafficking are affected by AP-2 and retromer. AP-2 knockdown presumably prevents endocytic reuptake of MIG-14, preventing its entry into recycling endosomes; the return of MIG-14 to the Golgi is regulated by retromer components. Their removal results in significant reduction, but not complete abrogation, of MIG-14 function, thereby reducing the amount of Wnt secretion. (A) is adapted from [12•]; (B) is adapted from [40].
Like other components of the Wnt/β-catenin asymmetry pathway, sys-1 is required for specification of distal cell fates in the somatic gonad, but it is not required for POP-1 asymmetry [14]. sys-1 encodes a protein with weak consensus armadillo repeats, and can bind to the β-catenin binding domain of POP-1 [15]. Several genetic experiments indicate that SYS-1 functions as a limiting coactivator for POP-1 [15,16•, 17•] (reviewed in [2]). SYS-1 has very recently been crystallized and shows a high degree of similarity to β-catenin at the tertiary level, confirming that it is indeed a divergent β-catenin [18•]. sys-1 function is required throughout development for Wnt/β-catenin asymmetry, including during endoderm formation [16•], and deletion alleles of sys-1, pop-1 and wrm-1 show similar embryonic arrest phenotypes (R King, T Walston and J Hardin, unpublished observations), suggesting that SYS-1 has widespread roles in anterior–posterior fate specification in the C. elegans embryo.
Expanding the non-canon: PCP-like signaling?
Evidence for other Wnt-dependent signaling pathways identified in vertebrates, such as the Wnt/Ca2+ [19] and planar cell polarity (PCP [20,21]) pathways is more equivocal. Disruption of calcium signaling, either through loss of function of plc-1/phospholipase C-ε or itr-1/inositol trisphosphate receptor, leads to defects during cell rearrangement in the embryonic epidermis [22,23], but how or when calcium signaling functions in regulating intercalation is unclear.
Perhaps even more enigmatic is whether C. elegans possesses a true PCP pathway. Asymmetric division of the male specific B cell requires lin-44/Wnt, lin-17/ frizzled [24]. While POP-1 shows asymmetric nuclear localization in the B cell daughters, this asymmetry does not require WRM-1 or LIT-1, but instead requires homologues of Wnt/planar cell polarity components. LIN-17/FZ and MIG-5/DSH both show asymmetric cortical localization reminiscent of Fz and Dsh in other situations involving PCP signaling [25]. Asymmetric division of the B cell requires signaling mediated through the DEP but not the DIX domain of MIG-5/Dsh, as well homologues of the PCP components Vang, Prickle, RhoA, Rock and Daam1, further implying that a PCP-like pathway functions to regulate asymmetric division in the B cell [24]. The PHA neuronal lineage, which requires dsh-2/Dsh [26], has similar genetic requirements (K Hinwing, S Lee, L Nykilchuk and N Hawkins, unpublished). Do such experiments demonstrate a bona fide PCP pathway in C. elegans? Not necessarily, since putative PCP components are not essential [24,25] (R King, T Walston and J Hardin, unpublished). On the contrary, C. elegans may be a system poised to identify other, previously unidentified PCP-like effectors.
Short-range signals: spindles, shrinking apices and synapses
Wnt signaling has long been known to regulate spindle rotation of the EMS and ABar blastomeres, a process that does not rely on POP-1/Tcf or new transcription (reviewed in [27]). Recent work by Zhang et al. suggests that Wnt signaling may function to localize dynactin in this process [28]. In wild-type embryos dynactin accumulates at the border between P2 and EMS, similar to DSH-2/Dsh and MOM-5/Fz. mom-5 knockdown results in reduced dynactin accumulation, and dynactin accumulation is abolished in mom-5; src-1 double knockdown embryos, consistent with the synergy between mom-5 and src-1 in regulating spindle rotation. Accumulation of dynactin at the P2-EMS border could recruit dynein and function to trap astral microtubules, placing torque on the attached centrosomes.
Wnt signaling also regulates an actomyosin-mediated event during gastrulation in C. elegans. MOM-2/Wnt and MOM-5/Fz are well known to be required for differentiation of the endodermal precursors, Ea and Ep, which apically constrict as they ingress. However, Lee et al. [29•] noticed that in some mom-2 and mom-5 mutant embryos in which endodermal fate appears normal, Ea and Ep fail to ingress. Such defects cannot be explained by a simple delay in the cell cycle, nor by loss of localized PAR or NMY-2/non-muscle myosin heavy chain, which are properly localized in the mutants. However, enrichment of phosphorylated regulatory myosin light chains at the apical surface of Ea and Ep is reduced in mom-5 mutants, which may lead to reduced apical constriction and gastrulation failure.
A third example of short-range Wnt signaling involves synapse formation. Wnts are known to positively influence synaptic connectivity in vertebrates and Drosophila (reviewed in [30]). Using SNB-1/synaptobrevin localization in the DA9 motor axon to monitor synapse formation in the posterior region of C. elegans hermaphrodites, Klassen and Shen [31••] show that local Wnt signaling influences the location of presynapses. In lin-44/Wnt, lin-44; egl-20/Wnt double mutants and lin-17/ Frizzled mutants, however, DA9 has ectopic presynapses in the tail, suggesting that Wnt signaling normally inhibits synapse formation. Consistent with this model, misexpression of lin-44 under control of the egl-20 promoter leads to changes in the location of the asynaptic region. How such local control of synaptogenesis is regulated is unclear. DSH-1/Dsh appears to be involved, but loss of function of pop-1, lit-1, wrm-1, bar-1 or pry-1 does not phenocopy loss of Wnt function. Further work will be needed in each of these three cases to identify how Wnts control local accumulation of subcellular components in specific regions of a single cell.
Long-range signals: sources and sinks during vulval induction
In addition to these examples of short-range Wnt-mediated signaling, there are several well-known cases of long-range Wnt signaling in C. elegans. Neuronal migration along the anterior–posterior axis has been extensively reviewed recently in this journal [3]. Another example involves the vulva. The ‘Pn.p cells’ are born in the L1 larva; five of these cells then fuse with the hyp7 syncytium (the ‘F’ fate) and another cell, P3.p, sometimes fuses with hyp7 in the L2 larva. The remaining (typically six) cells express the Hox gene lin-39 and become the vulval precursor cells (VPCs), which adopt different fates (1°, 2° or 3°) owing to EGF and Notch signaling (reviewed in [32]). Multiple Wnts and Frizzleds regulate lin-39 expression via bar-1/β-catenin, and hence VPC differentiation [33•].
Two recent studies have extended these findings. First, Myers and Greenwald examined Wnt regulation of the F fate in VPCs and found that CWN-1 and EGL-20 are involved in suppressing fusion of P4.p with hyp7 in L2 larvae. They went on to use tissue-specific promoters to rescue mig-14/Wntless function (and hence Wnt secretion) in various cells and found that expression of mig-14 in neurons, muscle or the epidermis can partially rescue P4.p fusion defects. This suggests that Wnt signaling may provide general competence-promoting functions during VPC specification; reduction of EGF signaling or ablation of the somatic gonadal primordium at the L1 stage synergizes with loss of multiple Wnt signals, suggesting both signals may promote VPC competence.
The second study focused on the ROR receptor tyrosine kinase, CAM-1, which had been shown previously to antagonize Wnt responses in neuronal cells through its CRD domain [34,35]. The simplest explanation for these data is that CAM-1 binds and sequesters Wnt ligands. In CAN neurons, genetic data suggest that CAM-1 activity is cell autonomous [36], but CAM-1 could also act cell non-autonomously to sequester Wnts. Green et al. examined this possibility in the context of VPC specification [37•]. cam-1 mutants synergize with lin-17/Frizzled and lin-18/ Ryk mutants, resulting in production of more than the usual 22 vulval cells. This phenotype can be ameliorated by reduction of egl-20/Wnt and cwn-1/Wnt function, suggesting that CAM-1 acts by inhibiting these Wnts, to which it can bind in vitro. Significantly, expression of cam-1 in neurons or muscle is sufficient to cause loss of 1° VPC induction, similar to loss of bar-1 and Wnt gene function. Taken together with the results of Myers and Greenwald, these results indicate that Wnt signaling is ‘buffered’ in the environment surrounding VPCs. The simplicity of the C. elegans larva may be an ideal place to dissect out the specific contribution of surrounding tissues in VPC specification in the future.
The long and the short of Wnt signaling: vesicular trafficking and MIG-14/Wntless
Production of both short-range and long-range Wnt signals requires correct processing of Wnts. Wnt processing is a complex subject, and has been reviewed extensively else-where(e.g.[38–40]). Very recently two groups working inC. elegans [41••,42••] along with simultaneous work in Drosophila (reviewed in [43]) provided new insights into Wnt secretion. The new work builds on two important recent results. First, two groups had shown that in the context of neuronal migration, the retromer complex regulates Wnt signaling in C. elegans [44••,45••]. Second, the Basler group identified a novel seven-pass membrane protein, Wntless (WLS), which is required for secretion of Wnts. The C. elegans wntless homologue corresponds to mutations originally identified through their role in endoderm induction (mom-3) and in neuronal migration (mig-14) [46••]. In wntless mutants, Wnts accumulate inside the cells that produce them, and secretion of Wnts is ultimately blocked.
Work by the Korswagen and Garriga labs now shows that retromer function is important in recycling MIG-14/WLS and that in the absence of retromer function, MIG-14 is targeted for lysosomal degradation [41••,42••]. First, the Garriga group shows that dpy-23 encodes the C. elegans homologue of the μ subunit of the AP-2 clathrin adaptor complex [41••]. They and the Korswagen group both show that loss of DPY-23 in Wnt producing cells results in phenotypes similar to Wnt loss of function. Both groups show that loss of retromer function also disrupts Wnt signaling. Significantly, the subcellular distribution of MIG-14::GFP is disrupted in dpy-23 and retromer loss of function backgrounds. In the former, MIG-14 accumulates at the plasma membrane, while in the latter MIG-14 accumulates in vesicular structures deeper in the cytoplasm, which the Korswagen group shows colocalize with lysosomal markers [42••]. Taken together, these results suggest that DPY-23, working at the plasma membrane, and retromer components, working at the level of recycling endosomes, are both required for recycling of MIG-14 (Figure 1B). MIG-14 presumably plays a modulatory role in this process, after initial lipid modifications to Wnts that occur in the endoplasmic reticulum, which are thought to be mediated by MOM-1/Porcupine (reviewed in [39,40]).
Conclusions
C. elegans continues to be a powerful model system to identify new regulators of Wnt signaling, and how these new regulators function. Along with new insights from other genetic model systems, results from C. elegans promise to continue to refine our understanding of how cells respond to multiple signaling inputs, and how they translate such signals into specific, molecular readouts to alter cell fate, motility and subcellular structure.
Acknowledgments
We apologize to many of our colleagues in advance for those instances in which we failed to discuss their work owing to space limitations. This work was supported by NSF grant IOB0518081 to JH and NIH Predoctoral Training Grant in Molecular Biosciences T32 GM07215 to RSK.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Eisenmann DM. Wnt signaling. WormBook. 2005:1–17. doi: 10.1895/wormbook.1.7.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizumoto K, Sawa H. Two betas or not two betas: regulation of asymmetric division by beta-catenin. Trends Cell Biol. 2007;17:465–473. doi: 10.1016/j.tcb.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Silhankova M, Korswagen HC. Migration of neuronal cells along the anterior–posterior body axis of C. elegans: Wnts are in control. Curr Opin Genet Dev. 2007;17:320–325. doi: 10.1016/j.gde.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Korswagen HC, Coudreuse DY, Betist MC, van de Water S, Zivkovic D, Clevers HC. The Axin-like protein PRY-1 is a negative regulator of a canonical Wnt pathway in C. elegans. Genes Dev. 2002;16:1291–1302. doi: 10.1101/gad.981802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oosterveen T, Coudreuse DY, Yang PT, Fraser E, Bergsma J, Dale TC, Korswagen HC. Two functionally distinct Axin-like proteins regulate canonical Wnt signaling in C. elegans. Dev Biol. 2007;308:438–448. doi: 10.1016/j.ydbio.2007.05.043. [DOI] [PubMed] [Google Scholar]
- 6.Herman MA. Control of cell polarity by noncanonical Wnt signaling in C. elegans. Semin Cell Dev Biol. 2002;13:233–241. doi: 10.1016/s1084-9521(02)00051-4. [DOI] [PubMed] [Google Scholar]
- 7.Korswagen HC. Canonical and non-canonical Wnt signaling pathways in Caenorhabditis elegans: variations on a common signaling theme. Bioessays. 2002;24:801–810. doi: 10.1002/bies.10145. [DOI] [PubMed] [Google Scholar]
- 8.Lin R, Hill RJ, Priess JR. POP-1 and anterior–posterior fate decisions in C. elegans embryos. Cell. 1998;92:229–239. doi: 10.1016/s0092-8674(00)80917-4. [DOI] [PubMed] [Google Scholar]
- 9•.Mizumoto K, Sawa H. Cortical beta-catenin and APC regulate asymmetric nuclear beta-catenin localization during asymmetric cell division in C. elegans. Dev Cell. 2007;12:287–299. doi: 10.1016/j.devcel.2007.01.004. This paper uses a membrane-targeted form of WRM-1/β-catenin to show that cortical WRM-1 inhibits Wnt signaling and nuclear localization of WRM-1 and that APR-1/APC regulates WRM-1 nuclear export. APR-1, PRY-1/Axin and Dishevelleds localize asymmetrically to the cortex, suggesting interplay between the cortex and the nucleus. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura K, Kim S, Ishidate T, Bei Y, Pang K, Shirayama M, Trzepacz C, Brownell DR, Mello CC. Wnt signaling drives WRM-1/beta-catenin asymmetries in early C. elegans embryos. Genes Dev. 2005;19:1749–1754. doi: 10.1101/gad.1323705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo MC, Gay F, Odom R, Shi Y, Lin R. Phosphorylation by the beta-catenin/MAPK complex promotes 14-3-3-mediated nuclear export of TCF/POP-1 in signal-responsive cells in C. elegans. Cell. 2004;117:95–106. doi: 10.1016/s0092-8674(04)00203-x. [DOI] [PubMed] [Google Scholar]
- 12•.Kanamori T, Inoue T, Sakamoto T, Gengyo-Ando K, Tsujimoto M, Mitani S, Sawa H, Aoki J, Arai H. beta-Catenin asymmetry is regulated by PLA(1) and retrograde traffic in C. elegans stem cell divisions. EMBO J. 2008 doi: 10.1038/emboj.2008.102. This paper shows that IPLA-1/intracellular phospholipase A regulates WRM-1 asymmetry in a retromer/retrograde trafficking dependent manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seaman MN. Recycle your receptors with retromer. Trends Cell Biol. 2005;15:68–75. doi: 10.1016/j.tcb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Siegfried KR, Kidd AR, 3rd, Chesney MA, Kimble J. The sys-1 and sys-3 genes cooperate with Wnt signaling to establish the proximal-distal axis of the Caenorhabditis elegans gonad. Genetics. 2004;166:171–186. doi: 10.1534/genetics.166.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kidd AR, 3rd, Miskowski JA, Siegfried KR, Sawa H, Kimble J. A beta-catenin identified by functional rather than sequence criteria and its role in Wnt/MAPK signaling. Cell. 2005;121:761–772. doi: 10.1016/j.cell.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 16•.Phillips BT, Kidd AR, 3rd, King R, Hardin J, Kimble J. Reciprocal asymmetry of SYS-1/beta-catenin and POP-1/TCF controls asymmetric divisions in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2007;104:3231–3236. doi: 10.1073/pnas.0611507104. This and the next reference show that SYS-1 has a nuclear localization reciprocal to POP-1/Tcf and that SYS-1 acts as a POP-1 coactivator in endoderm induction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17•.Huang S, Shetty P, Robertson SM, Lin R. Binary cell fate specification during C. elegans embryogenesis driven by reiterated reciprocal asymmetry of TCF POP-1 and its coactivator beta-catenin SYS-1. Development. 2007;134:2685–2695. doi: 10.1242/dev.008268. Same as reference [16•] [DOI] [PubMed] [Google Scholar]
- 18•.Liu J, Phillips BT, Amaya MF, Kimble J, Xu W. The C. elegans SYS-1 protein is a bona fide beta-catenin. Dev Cell. 2008;14:751–761. doi: 10.1016/j.devcel.2008.02.015. This paper uses X-ray crystallography to show that SYS-1 is structurally similar to classical β-catenins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signalling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 20.Montcouquiol M, Crenshaw EB, 3rd, Kelley MW. Noncanonical Wnt signaling and neural polarity. Annu Rev Neurosci. 2006;29:363–386. doi: 10.1146/annurev.neuro.29.051605.112933. [DOI] [PubMed] [Google Scholar]
- 21.Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129:1051–1063. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 22.Thomas-Virnig CL, Sims PA, Simske JS, Hardin J. The inositol 1,4,5-trisphosphate receptor regulates epidermal cell migration in Caenorhabditis elegans. Curr Biol. 2004;14:1882–1887. doi: 10.1016/j.cub.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Vazquez-Manrique RP, Nagy AI, Legg JC, Bales OA, Ly S, Baylis HA. Phospholipase C-epsilon regulates epidermal morphogenesis in Caenorhabditis elegans. PLoS Genet. 2008;4:e1000043. doi: 10.1371/journal.pgen.1000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu M, Herman MA. A novel noncanonical Wnt pathway is involved in the regulation of the asymmetric B cell division in C. elegans. Dev Biol. 2006;293:316–329. doi: 10.1016/j.ydbio.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 25.Wu M, Herman MA. Asymmetric localizations of LIN-17/Fz and MIG-5/Dsh are involved in the asymmetric B cell division in C. elegans. Dev Biol. 2007;303:650–662. doi: 10.1016/j.ydbio.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawkins NC, Ellis GC, Bowerman B, Garriga G. MOM-5 frizzled regulates the distribution of DSH-2 to control C. elegans asymmetric neuroblast divisions. Dev Biol. 2005;284:246–259. doi: 10.1016/j.ydbio.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Walston TD, Hardin J. Wnt-dependent spindle polarization in the early C. elegans embryo. Semin Cell Dev Biol. 2006;17:204–213. doi: 10.1016/j.semcdb.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Zhang H, Skop AR, White JG. Src and Wnt signaling regulate dynactin accumulation to the P2-EMS cell border in C. elegans embryos. J Cell Sci. 2008;121:155–161. doi: 10.1242/jcs.015966. [DOI] [PubMed] [Google Scholar]
- 29•.Lee JY, Marston DJ, Walston T, Hardin J, Halberstadt A, Goldstein B. Wnt/Frizzled signaling controls C. elegans gastrulation by activating actomyosin contractility. Curr Biol. 2006;16:1986–1997. doi: 10.1016/j.cub.2006.08.090. This paper indicates that Wnt signaling controls apical constriction of ingressing endodermal cells during gastrulation in C. elegans by regulating accumulation of myosin regulatory light chains at the apex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Speese SD, Budnik V. Wnts: up-and-coming at the synapse. Trends Neurosci. 2007;30:268–275. doi: 10.1016/j.tins.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31••.Klassen MP, Shen K. Wnt signaling positions neuromuscular connectivity by inhibiting synapse formation in C. elegans. Cell. 2007;130:704–716. doi: 10.1016/j.cell.2007.06.046. This paper shows that Wnt signaling exerts inhibitory effects on placement of presynaptic structures in identified C. elegans neurons. [DOI] [PubMed] [Google Scholar]
- 32.Sternberg PW. Vulval development. WormBook. 2005:1–28. doi: 10.1895/wormbook.1.6.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33•.Gleason JE, Szyleyko EA, Eisenmann DM. Multiple redundant Wnt signaling components function in two processes during C. elegans vulval development. Dev Biol. 2006;298:442–457. doi: 10.1016/j.ydbio.2006.06.050. This is the first paper to exhaustively examine redundant functions of Wnts in C. elegans. [DOI] [PubMed] [Google Scholar]
- 34.Forrester WC, Kim C, Garriga G. The Caenorhabditis elegans Ror RTK CAM-1 inhibits EGL-20/Wnt signaling in cell migration. Genetics. 2004;168:1951–1962. doi: 10.1534/genetics.104.031781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim C, Forrester WC. Functional analysis of the domains of the C. elegans Ror receptor tyrosine kinase CAM-1. Dev Biol. 2003;264:376–390. doi: 10.1016/j.ydbio.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Forrester WC, Dell M, Perens E, Garriga G. A C. elegans Ror receptor tyrosine kinase regulates cell motility and asymmetric cell division. Nature. 1999;400:881–885. doi: 10.1038/23722. [DOI] [PubMed] [Google Scholar]
- 37•.Green JL, Inoue T, Sternberg PW. The C. elegans ROR receptor tyrosine kinase, CAM-1, non-autonomously inhibits the Wnt pathway. Development. 2007;134:4053–4062. doi: 10.1242/dev.005363. This paper shows that CAM-1/Ror kinase acts cell non-autonomously to sequester Wnts in the posterior region of C. elegans, thereby influencing differentiation of vulval precursor cells (VPCs) [DOI] [PubMed] [Google Scholar]
- 38.Mikels AJ, Nusse R. Wnts as ligands: processing, secretion and reception. Oncogene. 2006;25:7461–7468. doi: 10.1038/sj.onc.1210053. [DOI] [PubMed] [Google Scholar]
- 39.Coudreuse D, Korswagen HC. The making of Wnt: new insights into Wnt maturation, sorting and secretion. Development. 2007;134:3–12. doi: 10.1242/dev.02699. [DOI] [PubMed] [Google Scholar]
- 40.Hausmann G, Banziger C, Basler K. Helping Wingless take flight: how Wnt proteins are secreted. Nat Rev Mol Cell Biol. 2007;8:331–336. doi: 10.1038/nrm2141. [DOI] [PubMed] [Google Scholar]
- 41••.Pan CL, Baum PD, Gu M, Jorgensen EM, Clark SG, Garriga G. C. elegans AP-2 and retromer control Wnt signaling by regulating mig-14/Wntless. Dev Cell. 2008;14:132–139. doi: 10.1016/j.devcel.2007.12.001. This paper and the companion paper in the next citation show that the AP-2 clathrin adaptor complex and retromer function are important for recycling MIG-14/Wntless, thereby regulating Wnt secretion in C. elegans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42••.Yang PT, Lorenowicz MJ, Silhankova M, Coudreuse DY, Betist MC, Korswagen HC. Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev Cell. 2008;14:140–147. doi: 10.1016/j.devcel.2007.12.004. See reference [41••] [DOI] [PubMed] [Google Scholar]
- 43.Eaton S. Retromer retrieves wntless. Dev Cell. 2008;14:4–6. doi: 10.1016/j.devcel.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 44••.Coudreuse DY, Roel G, Betist MC, Destree O, Korswagen HC. Wnt gradient formation requires retromer function in Wnt-producing cells. Science. 2006;312:921–924. doi: 10.1126/science.1124856. This and the next reference show that retromer function is required for Wnt signaling in C. elegans neurons. [DOI] [PubMed] [Google Scholar]
- 45••.Prasad BC, Clark SG. Wnt signaling establishes anteroposterior neuronal polarity and requires retromer in C. elegans. Development. 2006;133:1757–1766. doi: 10.1242/dev.02357. See reference [44••] [DOI] [PubMed] [Google Scholar]
- 46••.Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. In addition to being one of the first papers to describe Wntless in Drosophila, this paper also shows that mom-3/mig-14 is the wntless homologue in C. elegans and that MIG-14 is required for Wnt-dependent events in the early embryo. [DOI] [PubMed] [Google Scholar]