Abstract
The relative contribution of central and peripheral mechanisms to the generation and maintenance of allograft tolerance is of considerable interest. Here, we present new evidence that regulatory T cells (Foxp3+) maintain skin and heart allograft tolerance in mixed hematopoietic chimeric mice. Transient depletion of both donor- and recipient-derived Foxp3+ cells was necessary and sufficient to induce decisive rejection of long-accepted skin and heart allografts. In contrast, stable hematopoietic chimerism remained, and there was no detectable induction of donor-specific reactivity to hematopoietic cells. Foxp3+ cell depletion did not result in the rejection of skin grafts of only MHC-disparate donors (B6.C-H2d/bByJ), indicating that MHC antigens were not the target in the graft. We conclude that two different mechanisms of tolerance are present in mixed chimeras. Hematopoietic chimerism, resistant to Foxp3+ depletion, is probably due to deletional tolerance to MHC antigens, as supported by previous studies. In contrast, regulatory tolerance mechanisms involving Foxp3+ cells are required to control reactivity against non-MHC antigens not present on hematopoietic lineages.
Introduction
Tolerance of allografts is defined as a state in which donor-specific immune unresponsiveness permits the unimpeded survival of allogeneic transplants. One promising strategy toward this goal depends upon the establishment of mixed chimerism whereby both donor and recipient hematopoietic cells coexist in a stable equilibrium without evidence of graft-versus-host reactions (1–4). This strategy has been investigated extensively both in rodents and in large animals (5,6). Protocols resulting in transient mixed chimerism have been successfully applied in experiments with nonhuman primates and, more recently, in clinical studies with kidney transplants that have been accepted in selected patients who consequently are completely free from long-term immunosuppression (7,8).
Central clonal deletion has been shown to be an important feature of both the induction and the maintenance phases of hematopoietic chimerism in several systems (9–15). There is also evidence that peripheral mechanisms, especially those involving regulatory cells, can be involved, although the nature of the regulation has not been identified (14–16). In nonhuman primates, peripheral regulatory mechanisms can be particularly important, as allotransplants have been shown to survive without immunosuppression even after the loss of detectable donor chimerism (7,17). Similar results have been found in humans (8). To date, none of the experiments implicating Foxp3+ regulatory T cells (Tregs) have explored the consequences of their selective depletion from tolerant animals.
In a previous study, we appraised the role of Foxp3+ cells in the tolerance induced spontaneously by transplanted kidneys between certain MHC-incompatible mouse strains, without the involvement of hematopoietic cell chime-rism (18). By employing the C57/BL6.Foxp3DTR mice, a “knock-in” strain in which Foxp3+ cells are selectively susceptible to destruction by diphtheria toxin (DT) as diphtheria toxin receptor (DTR) is associated with Foxp3 expression (C57/BL6.Foxp3DTR) (19), we investigated the individual role of donor- and recipient-derived Foxp3+ Tregs to the maintenance of allograft tolerance in mixed chimeras.
Materials and Methods
Mice
Foxp3DTR (H-2b) mice were a kind gift from Dr. Alexander Rudensky (Memorial Sloan Kettering Cancer Center) and bred in our facility as previously described (18). The B6, DBA/2 (H-2d), C3H (H-2k) and B6.C-H2d/bByJ strains were purchased from Jackson Laboratories (Bar Harbor, ME). Bone marrow (BM) was obtained from F1 mice; male mice were hemizygous for Foxp3DTR (Foxp3DTR/y) and female mice were heterozygous for Foxp3DTR (Foxp3DTR/WT) (Figure S1). All mice were maintained under pathogen-free conditions in filter-top cages throughout the experiments with an automatic water system and were cared for according to the methods approved by the American Association for the Accreditation of Laboratory Animal Care. All animal experiments were approved by the Center for Comparative Medicine's at Massachusetts General Hospital.
Mixed allogeneic chimerism regimen and DT treatment
Mixed allogeneic chimeras were prepared by a dose-modified protocol previously described (14). Briefly, 8- to 12-week-old recipients (B6.Foxp3DTR or B6) were treated with a nonmyeloablative dose of total body irradiation (3 Gy) followed by injection of 20–30 × 106 unseparated BM cells from sex-matched F1 mice described above. Recipients were also treated with anti-CD4 mAb (GK1.5, 100 μg) and anti-CD8 mAb (2.43, 100 μg) a day before BM transplant and with anti-CD154 mAb (MR1, 500 μg) on day 0 (Figure 1). All therapeutic mAbs were purchased from BioXCell (West Lebanon, NH). Recipients were treated with two consecutive doses of DT (50 μg/kg) at 28 days postheart transplantation following the protocol in Figure 1.
Figure 1. Schematic representation of the experimental design.
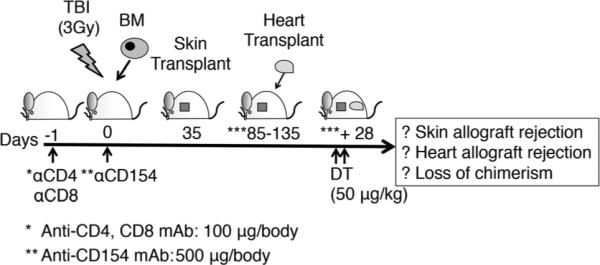
Recipients were treated with both anti-CD4 (100 μg/body) and anti-CD8 (100 μg/body) mAb a day before bone marrow transplantation (BMT). On the day of BMT, they were treated with anti-CD154 mAb (500 μg/body) and 3 Gy total body irradiation. Recipients that developed chimerism 35 days post-BMT received sex-matched allogeneic skin grafts from DBA/2 mice. Recipients that accepted DBA/2 skin graft for more than 50 days were then selected to receive allogeneic hearts from sex-matched DBA/2 donors. The specificity of the tolerance was confirmed in selected recipients (n = 6) by challenging them with C3H third-party skin grafts. These C3H grafts were rejected over 10–14 days by recipients that had accepted DBA/2 skin grafts. Mice that accepted DBA/2 skin grafts for 50–100 days were subsequently transplanted with sex-matched DBA/2 heart allografts. Then, 4-week postheart transplant, recipients that accepted the heart allografts were treated with two consecutive doses of DT (50 μg/kg) at 28 days postheart transplantation.
Skin transplantation
Full-thickness trunk skin allografts were transplanted to the dorsal thoracic wall of recipients and monitored by daily visual inspection. Skin allografts were determined to be rejected when less than 10% of the graft remained viable.
Heterotopic heart transplantation
Transplantation of mouse heart allografts was performed according to our previously described microsurgical technique (20). The survival of grafts was monitored daily by abdominal palpation (scoring 1–3) until the cessation of cardiac contraction.
Histological and immunopathological assessment
Transplanted cardiac grafts were removed when contraction completely ceased or on the scheduled day and cross-sectioned into two parts (base and apex). The base was fixed in 10% formalin and embedded in paraffin. The apex was embedded and frozen in OCT compound (Sakura Finetek USA Inc., Torrance, CA) and stored at −20°C. Accepted skin grafts were also removed on the scheduled day. Paraffin sections were further processed for the staining of CD3 (Polyclonal DAKO; DAKO, Carpinteria, CA), Foxp3 (JK-16S; eBioscience, San Diego, CA) and B220 (RA3-6B2; BD Pharmingen, San Jose, CA). Frozen samples were stained for CD8 (Santa Cruz Biotechnology, Santa Cruz, CA), C4d (16D2; Abcam Inc., Cambridge, MA), CD4 (GK 1.5) or Ly49g2 (4D11), the latter two from BD Pharmingen (21). C4d stains included a positive control. Slides were scanned by Aperio Scanscope CS2, and positive-stained cells were counted by Aperio Imagescope software (Aperio ePathology Solutions, Vista, CA).
Flow cytometric analysis of multilineage chimerism in lymphocytes
Cells were stained with PE-conjugated anti-H-2Dd mAb (34-5-8S; BD Pharmingen) to distinguish donor cells from recipient cells in combination with four different types of fluorescent staining for the following specific lineage markers: PerCP-Cy5.5-conjugated anti-CD8 (53-6.7), APC-conjugated anti-CD19 (MB19-1) (eBioscience); anti-CD49b (Dx5), APC-Cy7-cinjugated anti-CD4 (RM4-5) or PE-Cy7-conjugated anti-CD3 (145-2C11) (BioLegend, San Diego, CA). Intracellular staining was performed by permeabilization of cell membranes (Foxp3-staining buffer set; eBioscience), followed by staining with APC-conjugated anti-Foxp3 (FJK-16s; eBioscience). Isotype-matched immunoglobulin staining served as negative controls. All samples were analyzed on FACSVerse™ (BD Biosciences) with FlowJo software (Tree Star Data Analysis Software, Ashland, OR).
Antibody assays
Sera, collected on the day mice were euthanized, were tested for the presence of donor-specific antibodies (DSA). Binding was assessed using flow cytometry (18). Thymocytes or splenocytes were used as target cells, and FITC-conjugated anti-mouse IgG (Rat polyclonal antibody; eBioscience) were used as a secondary antibodies; in the splenocyte assays, CD19 antibody was used to select the B cells. For a negative control, cells were incubated with sera from naïve B6.Foxp3DTR mice. The positive control was serum from nonchimeric B6 recipients that had rejected DBA/2 cardiac allografts.
Direct enzyme-linked immunospot assay
The enzyme-linked immunospot (ELISPOT) assays for interferon gamma (IFN-γ) were performed as previously described (18). Spots were counted on a computer-assisted ELISPOT reader (ImmunoSpot S5 Core Analyzer; Cellular Technologies, Ltd, Cleveland, OH).
Statistical analysis
Results are given as the mean s.e.m. Variables among groups were compared using Student's t-test or analysis of variance (ANOVA), with p < 0.05 considered significant. Allograft survival curves were constructed by the Kaplan and Meier method and comparisons were performed using the log-rank test. Analyses were performed with Prism ver.5 (GraphPad Software Inc., La Jolla, CA).
Results
Effect of donor and recipient Foxp3+ cell depletion on tolerance of skin and heart allografts
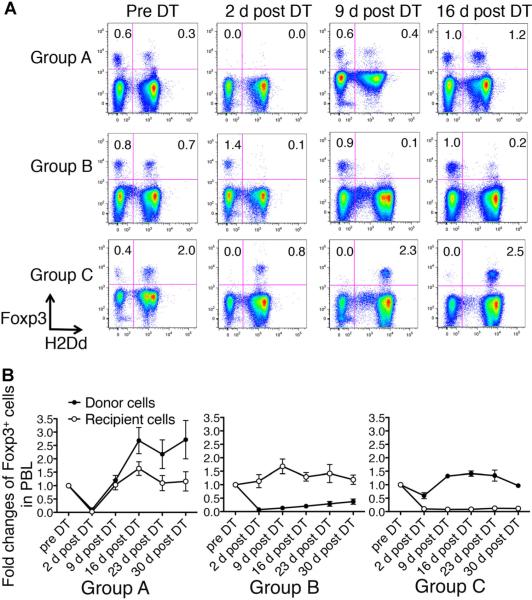
To assess Foxp3+ cell depletion following DT treatment, we stained peripheral blood lymphocytes with anti-Foxp3 mAb and analyzed them by flow cytometry at several time points (Figure 2A and B). The majority of donor and recipient Foxp3+ cells were depleted following DT administration. By 9 days post-DT, Group A mice began to regenerate Foxp3+ cells, with donor-derived Foxp3+ cells gradually increasing to twice their baseline level. The phenomenon of the transient depletion of Foxp3+ cells in the blood followed by repopulation at higher levels than before depletion was also seen in previously published results using B6.Foxp3DTR (19). Similar results were observed in lymph node, spleen and thymus. We further showed that there was no evidence of autoimmunity by histology (18).
Figure 2. Analyses of donor and recipient Foxp3+ cell depletion in the three groups after two doses of diphtheria toxin (DT).
(A) Peripheral blood lymphocytes (PBL) were stained with anti-H-2Dd (donor MHC class I) mAb and anti-Foxp3 mAb and analyzed by flow cytometry. Numbers represent the percentage of each subset in PBL. Both donor and recipient-derived Foxp3+ cells were depleted in Group A, while only recipient-derived Foxp3+ cells were retained in Group B and only donor-derived Foxp3+ cells were retained in Group C 2 days after DT treatment. (B) Fold changes of recipient (open circles) and donor (closed circles) Foxp3+ cells over 30 days post-DT. The percentage of Foxp3+ cells in PBL represents the value at each time point following DT treatment divided by the base line value obtained prior to DT treatment (baseline) for each recipient. Donor and recipient Foxp3+ cells in Group A recovered to greater than baseline levels at 16–30 days post-DT. In contrast, donor-derived Foxp3+ cells in Group B and recipient-derived Foxp3+ cells in Group C showed little evidence of recovery at 30 days post-DT. Inset shows the percentage of events in PBL in the upper two quadrants. Results represent mean values ± s.e.m. of 9, 5 and 5 recipients in Groups A, B and C, respectively.
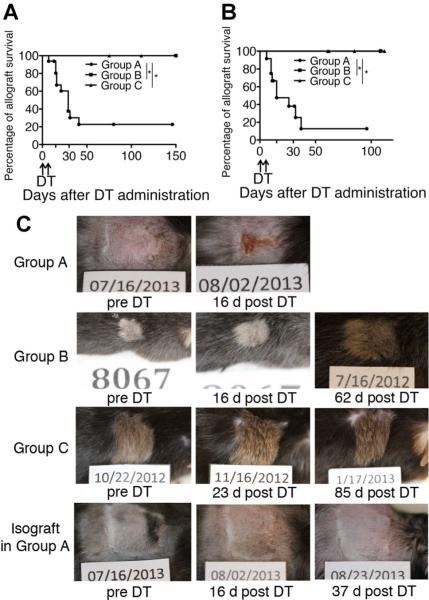
Allograft tolerance in mixed chimeras was abrogated when both donor and recipient Foxp3+ cells were depleted. Approximately 90% of skin allografts and 80% of cardiac allografts in Group A were rejected by 35 days post-DT (Figure 3A and B). Skin isografts (B6 donor) in Group A mice remained intact even after DT treatment (n = 3). Figure 3C shows the time course regarding the appearance of representative skin allografts and an isograft following DT. The treatment was well tolerated in the majority of the recipients.
Figure 3. Heart and skin allografts were rejected only when both donor and recipient Foxp3+ cells were depleted.
(A) Cardiac allograft survival in each group (Group A, n = 12; Group B, n = 6; Group C, n = 5) was assessed by trans-abdominal palpation (scoring 0–3) and defined as rejected when cardiac contraction completely stopped. All cardiac allograft recipients were treated with diphtheria toxin (DT) weeks after grafts had been accepted. Most heart grafts in Group A were rejected over 10–40 days post-DT, while all grafts in Groups B and C were accepted even after DT treatment. (B) Skin allograft survival in each group (Group A, n = 12; Group B, n = 6; Group c, n = 5) was monitored by daily visual inspection and defined as rejected when less than 10% of the graft remained viable. Recipients were treated with DT after skin grafts had been accepted for at least 50 days. Most skin grafts in Group A were rejected over 10–35 days post-DT, while all grafts in Groups B and C remained intact even after DT treatment. (C) Morphological changes of skin allografts (DBA/2) and isografts (B6) before and after DT treatment. *p < 0.001 (log-rank test).
Selective depletion of either donor or recipient Foxp3+ cells
Because Foxp3 is an X chromosome-encoded gene, selective depletion of donor and recipient Foxp3+ cell populations is possible. Thus, two additional groups were established (Figure S1 and Table 1). To deplete only donor Foxp3+ cells (Group B), recipient WT-B6 male mice (Foxp3WT/y) received BM from F1 male mice (Foxp3DTR/y). To deplete recipient and retain donor Foxp3+ cells (Group C), recipient B6.Foxp3DTR female mice (Foxp3DTR/DTR) received BM from F1 female mice (Foxp3DTR/WT). These groups were treated as in Group A with sequential skin/heart transplantation, and DT treatment. To exclude the influence of minor antigens coded by Y-linked genes, we used female DBA/2 as donors of skin/heart allografts for the recipients in Group C.
Table 1.
Study design for Foxp3+ cell depletion
| Group | Recipient | BM donor | Skin/heart donor | DT treatment |
|---|---|---|---|---|
| A | B6.Foxp3DTR/Y | F1.Foxp3DTR/Y | DBA/2 male | Depletion of all Foxp3+ cells |
| B | B6.WT/Y | F1.Foxp3DTR/Y | DBA/2 male | Depletion of all donor Foxp3+ cells |
| C | B6.Foxp3DTR/DTR | F1.Foxp3DTR/WT | DBA/2 female | Depletion of all recipient Foxp3+ cells and about 50% of donor Foxp3+ cells |
All donor-derived Foxp3+ cells in Group B and all recipient-derived Foxp3+ cells in Group C were eliminated following DT treatment (Figure 2A and B). Of note, approximately half of donor-derived Foxp3+ cells in Group C mice were retained after DT treatment. This was expected due to random X-chromosome inactivation (22), since approximately 50% of donor Foxp3+ cells in Group C mice (Foxp3DTR/WT) do not express DTR (Figure S1 and Table 1). In Group B mice, donor Foxp3+ cells were regenerated relatively slowly compared with those in the Group A. This population eventually recovered to baseline levels by 8 weeks post-DT (data not shown). In contrast, recovery of the depleted recipient Foxp3+ cells could not be detected in the blood or spleen for as long as 300 days post-DT in Group C mice. This did not affect the state of mixed chimerism nor evoke deleterious immune responses to recipients.
Effect of selective Foxp3+ cell depletion on skin and heart allografts
In contrast to Group A, skin and heart allografts in Groups B and C mice survived long-term despite DT treatment (Figure 3A–C). This suggests that either donor or recipient Foxp3+ cells are sufficient to maintain allograft tolerance in mixed chimeras.
Foxp3+ cells are not necessary for the maintenance of hematopoietic chimerism
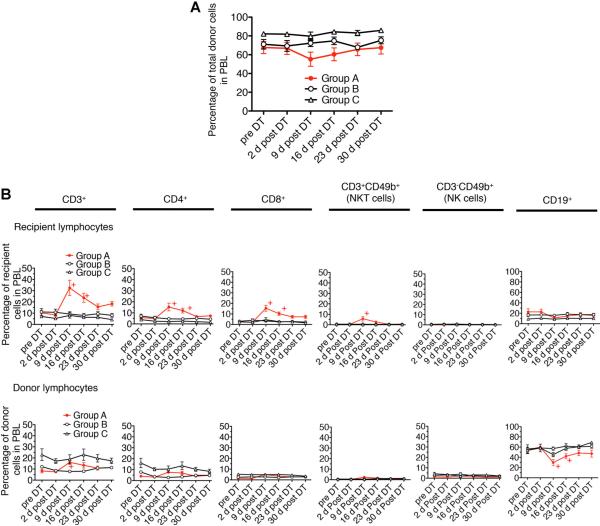
To investigate whether Foxp3+ cell depletion affected the state of mixed chimerism, we stained blood lymphocytes with mAb to donor MHC class I antigen (H-2Dd). The overall level of donor chimerism was not affected by Foxp3+ cell depletion (Figure 4A). While the percentage of donor lymphocytes in Group A decreased slightly 9 days post-DT, the differences between groups was statistically insignifi-cant by two-way repeated measures ANOVA (p = 0.116). The percentage of donor lymphocytes promptly recovered to the baseline level by 23 days post-DT. The percentage of donor lymphocytes in Group B or C mice was not affected by DT treatment, ruling out the possibility of nonspecific effects of DT. Thus, depletion of both donor and recipient Foxp3+ cells, which results in the rejection of long-accepted allografts, was insufficient to diminish the circulating levels of donor lymphocytes.
Figure 4. Peripheral blood lymphocyte (PBL) levels of donor chimerism were preserved after Foxp3+ cell depletion.
(A) PBL were stained with anti H-2Dd (donor MHC class I) mAb and plotted over 30 days after diphtheria toxin (DT) treatment. The difference between three groups is statistically insignificant by analysis of variance (p = 0.116). (B) Analysis of lymphocyte subpopulations after DT treatment shows a relative increase in recipient-derived T cells (CD4+, CD8+ and NKT cells) and a corresponding relative decrease in donor-derived B cells (CD19+ cells). PBL were stained with anti-CD3, CD4, CD8, CD49b (Dx5) and CD19 mAb. The graph in A shows the percentage of donor cells in PBL following DT treatment. The graph in B shows the percentage of recipient or donor-derived subpopulations in the whole peripheral blood lymphocytes. +p < 0.05 compared with data in Group A at pre-DT (t-test). Results represent mean values ± s.e.m. of 12, 6 and 5 recipients in Groups A, B and C, respectively.
We assessed whether specific lymphocyte subsets were affected by Foxp3+ cell depletion (Figures 4B and S2A–F) in Group A recipients. A majority of circulating donor cells were CD19+ (50–60%; Table S1). Transient expansion of T cells including CD3+, CD4+, CD8+, peaking by day 9 post-DT, was observed in both donor and recipient-derived population in the Group A mice, with increases of two- to tenfold of baseline levels. The percentage of CD8+ T cell increased to threefold of baseline levels and remained above baseline levels 4 weeks post-DT. In contrast, the percentages of both donor and recipient CD19+ cells were reduced by about half 9 days post-DT in Group A. However, these levels subsequently recovered by 23 days post-DT. Although CD3−CD49b+ cells (natural killer [NK] cells) also transiently expanded (Figure S2E and F), the change was not statistically significant due to the small number of cells represented in the overall population. All lymphocyte subsets in Groups B and C showed no significant change. These results demonstrate that depletion of both donor and recipient Foxp3+ cells transiently altered the homeostatic balance among circulating lymphocyte subsets, but did not diminish the overall level of chimerism. We found no evidence of a compensatory change in the frequency of CD4+Foxp3− CD49b+LAG-3+ cells, another Treg phenotype reported by Gagliani et al (23).
Mechanism of rejection induced by Foxp3+ cell depletion
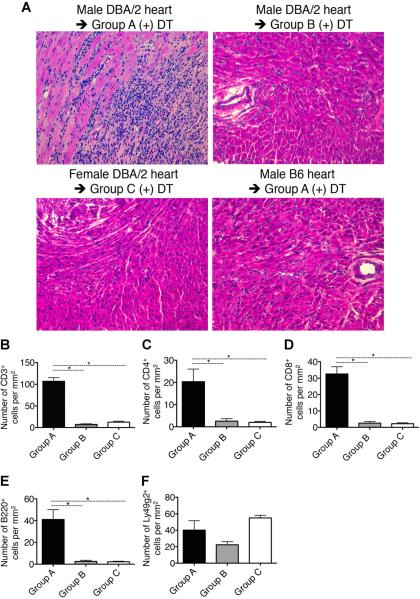
Histologic examination of rejected heart allografts in Group A showed widespread interstitial infiltrates of mononuclear cells along with destruction of myocytes (Figure 5A). Cardiac allografts transplanted in Groups B and C showed intact cardiac myocytes with little mononuclear cell infiltration. Recipients of B6 heart isografts treated with DT (serving as controls for Group A with n = 5 for each control group) showed no clinical or histologic evidence of rejection. These findings argue against any influence of nonalloimmune inflammatory response evoked by DT. Immunohistochemical studies of rejected hearts in Group A revealed multifocal infiltration of T lymphocytes (CD3+, CD4+, CD8+), B cells (B220+) and NK cells (Ly49g2+) (Figures 5B–F and S3A–C). However, none of the rejected cardiac allografts in Group A showed evidence of antibody-mediated rejection as judged by the absence of C4d deposition in capillaries (data not shown). In Groups B and C, the long-term accepted allografts showed a small number of infiltrating CD4+, CD8+ or B220+ cells without myocyte damage, although NK cells (Figures 5F and S3C) and Foxp3+ cells were present (see below).
Figure 5. Histology of representative heart grafts from each group.
(A) Hematoxylin and eosin (H&E) image of the DBA/2 allograft from Group A shows severe rejection with extensive mononuclear cell infiltration, while those of heart allografts from Groups B and C as well as a B6 isograft in Group A show scant inflammation and no rejection after DT treatment. (B–F) The number of specific infiltrates in the DBA/2 allografts in each group. Significant infiltration of T cells (CD3+, CD4+ and CD8+) and B cells (B220+) is observed in the DBA/2 heart allografts in Group A compared to that in the allografts in Groups B and C. Natural killer cells (Ly49g2+) infiltrated with similar levels in the DBA/2 allografts in each group (F). Results represent mean values ± s.e.m. of 12, 6 and 5 recipients in Groups A, B and C, respectively. *p < 0.05 (t-test). The magnification of all photos is 200×. Samples of allografts from Groups A, B and C and isograft from Group A were excised at 32, 307, 86 and 40 days post-DT, respectively. The basal portions including proximal coronary arteries were serially sectioned into 4 μm.
We tested for DSA in Group A mice that rejected skin and heart allografts following Foxp3+ cell depletion. No circulating DSA to DBA/2 thymocytes could be detected by flow cytometry (Figure 6A). We also did not detect DSA to DBA/2 splenocytes, including B cell targets. These findings argue that the acute rejection observed was T cell–mediated and independent of classes I and II MHC antibodies.
Figure 6. Mixed chimera recipients that rejected DBA/2 allografts following Foxp3+ cell depletion did not develop donor-specific antibodies (DSA) nor did they develop T cell reactivity to donor antigens on hematopoietic cells.
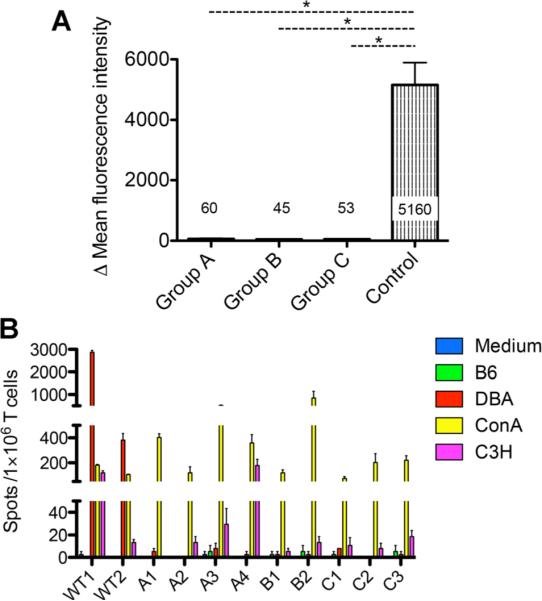
(A) DSA were not detected after allograft rejection following Foxp3+ cell depletion. DBA/2 thymocytes were incubated with serum from recipients in each group or with serum from naïve B6 mice and subsequently stained with anti-mouse IgG secondary antibody. The graphs show the average delta mean fluorescence intensity values from histograms of anti-mouse IgG between thymocytes incubated with each recipient serum and those incubated with naïve B6 serum. Results represent mean values ± s.e.m. of 12, 6 and 5 recipients in Groups A, B and C, respectively. *p < 0.05 (t-test). (B) Enzyme-linked immunospot assays to measure recipient T cell reactivity to donor (DBA/2), self (B6) or third-party (C3H). WT1 and WT2 represent nonchimeric WT B6 recipients of DBA/2 allografts, sacrificed 6 weeks after transplant. A1-4, B1-2 and C1-3 represent recipients in Groups A, B and C, respectively, sacrificed 6 weeks after diphtheria toxin treatment. Little or no reactivity to donor splenocytes (DBA/2) was detected in Groups A, B or C, while Group A recipients had rejected skin and heart allografts. All recipients had strong responses to Con A and variable responses to third-party (C3H). In contrast, control WT recipients that had rejected DBA/2 cardiac allografts had strong responses to DBA/2 splenocytes. Results represent mean values of triplicates ± s.e.m. of interferon gamma producing clones/106 recipient T cells.
We next investigated whether T cell reactivity to donor hematopoietic cells could be detected in Group A mice. ELISPOT assays were performed to test for the presence of IFN-g producing donor-reactive T cells. Splenic T cells were isolated at 6 weeks post-DT, when Foxp3+ cell populations fully recovered, and were co-cultured with several types of stimulators, specifically splenocytes from DBA, B6 (syngeneic) and C3H (third-party). No donor-reactive memory T cell responders could be detected in recipients that rejected both skin and heart allografts (Figure 6B). As a control, donor-specific reactivity was observed in splenic T cells isolated from nonchimeric B6 recipients that had rejected DBA/2 cardiac allografts.
Skin allograft rejection following Foxp3+ cell depletion in mixed chimeras is dependent on non-MHC alloantigens
Since hematopoietic chimerism is maintained during the course of skin/heart allograft rejection, we hypothesized that alloantigens responsible for the rejection of skin/heart allografts may be non-MHC antigens. To test this hypothesis, we employed a congeneic strain, B6.C-H2d/bByJ, as a donor of skin allografts, differing from recipient B6 only in the expression of MHC (H2d). We transplanted skin allografts of DBA/2 and B6.C-H2d/bByJ on the same recipients (n = 4), confirmed allograft tolerance over 50 days, and treated the recipients with two consecutive doses of DT. While all DBA/2 allografts were rejected within 15 days post-DT, B6.C-H2d/bByJ allografts were accepted for over 50 days post-DT, without any signs of rejection (Figure 7). As a control, B6.C-H2d/bByJ skin allografts on nonchimeric B6 recipient were rejected with a mean survival time of 14 days (n = 4), a rate similar to previously published results (24). These data support the hypothesis that effector lymphocytes reacted to non-MHC allo-antigens in the DBA/2 skin and the tolerance of MHC alloantigens in the skin is independent of peripheral regulation by Foxp3+ cells.
Figure 7. Skin grafts of only MHC-mismatched strain were not rejected following Foxp3+ cell depletion.
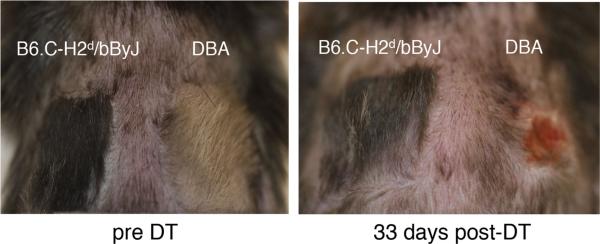
Morphological changes of skin allografts of DBA/2 (fully mismatched) and B6.C-H2d/bByJ (only MHC-mismatched) before and after diphtheria toxin (DT) treatment. Allograft tolerance was confirmed over 50 days before DT treatment. Shown are representative images among four Group A recipients which were transplanted both DBA/2 and B6.C-H2d/bByJ grafts. All DBA/2 allografts were rejected within 15 days post-DT. All B6.C-H2d/bByJ grafts were accepted for the observational period, over 50 days post-DT.
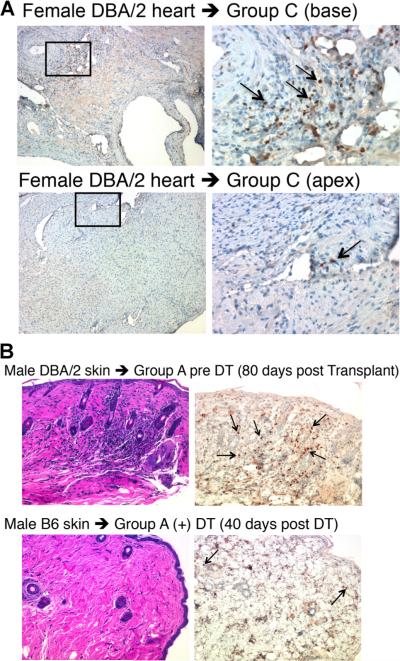
Foxp3+ cells infiltrate allografts in tolerant mixed chimeras
We sought evidence for Foxp3+ cell infiltration was present in accepted allografts in the recipients in Group C. At 200 days or more post-DT, Foxp3+ cells were detected in areas around the coronary artery and the thoracic aorta; however, there were few Foxp3+ cells in myocardium (Figure 8A). Hematoxylin and eosin stainings of long-accepted skin grafts show the preservation of architecture, even though infiltrated mononuclear cells could be detected in some areas (Figure 8B). Immunohistochemical staining demonstrates that among the infiltrates, many are CD3+ cells (data not shown) with a substantial number of Foxp3+ cells (Figure 8B). This phenomenon is reminiscent of aggregated lymphocyte nodules made up of similar cell populations that we previously observed in spontaneous accepted kidney allografts (18). Foxp3+ cells were marginally detectable in isografts transplanted in mice in Group A (Figure 8B).
Figure 8. Foxp3+ cell infiltration in DBA/2 heart and skin allografts.
(A) DBA/2 cardiac allograft from Group C mice was stained with antibodies to Foxp3. The areas highlighted in the small boxes in the left panels (100×) are shown in the right panels at a magnification of 400×. (B) Shown are representative images of accepted DBA/2 skin allografts and isografts after diphtheria toxin (DT) treatment. Hematoxylin and eosin staining and immunohistochemical for Foxp3 staining are of the same area. The magnification is 200×. The arrows indicate Foxp3+ cells.
Discussion
We have demonstrated that peripheral regulation by Foxp3+ cells plays an important role in maintaining tolerance of tissue and organ allografts in established mixed chimeras. Tolerance of skin and heart allografts was abrogated when both donor and recipient Foxp3+ cells were depleted. However, retention of either donor or recipient Foxp3+ cells was sufficient to maintain allograft tolerance. The different graft survival outcome cannot be attributed to differences in strain combinations. In all cases, the MHC and non-MHC incompatibility were the same, and therefore would not be expected to contribute to differences in graft survival. Our findings demonstrate that mixed chimeras have substantial numbers of T effector lymphocytes that escape central deletion and are able to reject grafts. They mount an acute cellular rejection response once both donor and recipient Foxp3+ cells are depleted. There was no evidence of antibody participation in the process. Combined with data from our previous study of Foxp3+ cell depletion in kidney allograft tolerance (18), these findings demonstrate that for skin, heart and kidney allografts, tolerance in the maintenance phase heavily depends on the regulatory mechanisms by Foxp3+ cells.
A notable finding in this study is that transient depletion of Foxp3+ cells, while resulting in the rejection of long-accepted allografts, did not affect the state of donor hematopoietic mixed chimerism in our recipients. This suggests that the maintenance of hematopoietic chime-rism is independent of peripheral regulation by Foxp3+ cells. Our data are consistent with a previous report that showed that regulatory mechanisms are unnecessary for maintaining the state of mixed chimerism by means of the depletion of CD4+CD25+ cells (25). These results are compatible with central deletion of donor MHC-reactive T cells, which was further manifested by the in vitro lack of reactivity to donor lymphocytes in a direct ELISPOT assay. In a recent study in which mixed hematopoietic chimerism (DBA/2 to B6) was induced with radiation and adaptive transfer of Foxp3+ cells from a B6.Foxp3DTR strain after culture with F1 lymphocytes (26), they showed chimerism persisted following depletion of both adaptively transferred Tregs and recipient Tregs. Skin allografts transplanted after Tregs depletion acutely rejected while chimerism was maintained. There are two major differences between this system and ours. First, donor BM-derived Foxp3+ cells were insufficient to accept skin allografts. One might expect the donor and recipient cells to be equally effective regulatory cells, since they are both educated in the thymus. In their system in contrast to ours, donor BM-derived Tregs represented a small fraction of the total pool of CD4+ cells. Second, depletion of Tregs was performed before skin transplant, unlike our data where Foxp3+ cells were depleted in recipients who long accepted allografts.
A central question is the specificity of the T cells mediating skin and heart graft rejection. As mentioned previously, we find no evidence of T helper (Th)1 reactivity to MHC antigens, as judged by ELISPOT analysis of their IFN-γ response to donor spleen cells. This is one measure of T cell reactivity, and would not, for example, exclude cytotoxic reactivity or Th2 responses. However, the negative ELISPOT assay contrasts with the consistently strong reactivity in untreated control B6 recipients that had rejected DBA/2 skin grafts. In addition, no donor-specific MHC class I or II antibody was detectable by flow cytometry, as might be expected with Th2 responses.
The most definitive evidence that non-MHC alloantigens are the T cell targets in the grafts comes from the results of the B6.C skin grafts. These grafts express donor MHC (H-2d) and B6 non-MHC and were not rejected after depletion of Foxp3+ cells. DBA/2 grafts that bear donor non-MHC antigens were rejected under the same conditions. This offers an explanation to the simultaneous “dual” tolerance mechanisms observed in the same recipient, whereby once accepted skin/heart allografts were rejected after Foxp3+ cell depletion but donor hematopoietic chimerism is maintained. The nature of the target antigens was not identified, although the H-Y antigen can be excluded, since we used gender-matched recipients (27). These non-MHC antigens are apparently lacking on hematopoietic cells, evidenced by ELISPOT assays, and may or may not be skin specific (28,29). We have also observed that in a small minority of recipients, heart or skin allografts continued to be accepted even after Foxp3+ cell depletion (Table S2). This heterogeneity may be due to faster recovery of Foxp3+ cells in some recipients, thus inhibiting the immunogenic response in these animals.
An unexpected finding was that the kinetics of Foxp3+ cell-reconstitution post-DT treatment differed in the three groups. When both donor and recipient Foxp3+ cells were depleted, they returned to pretreatment levels or slightly higher by 16 days. In contrast, the affected donor-derived Foxp3+ cells recovered slowly (Group B) and affected recipient-derived Foxp3+ cells did not recover as long as the animals were followed (Group C, up to 300 days post-DT). We suspect this phenomenon is related to homeostatic proliferation. Foxp3+ cells are subject to homeostatic proliferation, a process dependent on IL-2, dendritic cells, IL-7 and other factors (30–32). The sensor and the threshold for a deficiency in Foxp3+ cells are unknown, but we postulate that the maintenance of substantial numbers of Foxp3+ cells in these two groups suppresses the homeostatic response. While we do not know the mechanism, we can conclude that it did not affect the results; in fact, the persistence of only donor or recipient Foxp3+ cells shows that either can maintain tolerance long term. How either donor or recipient Tregs can function in maintaining tolerance is currently being investigated. One plausible mechanism is linked suppression, where donor or recipient Tregs reactivity to one antigen could be sufficient to inhibit nearby T effectors to another antigen (33). And whether induced Tregs and/or natural Tregs play a role requires further works. Analysis of selective demethylation of the conserved element within the Foxp3 locus named TSDR (Treg-specific demethylated region) would provide definitive evidence but it is beyond the scope of this study.
Passenger Tregs transferred with the skin or heart allografts may also play a role in graft acceptance. These cells would not carry the DTR gene and therefore would remain after DT treatment. Since Group A rejected the skin and heart after DT, the putative passenger Tregs are insufficient to maintain graft tolerance. Furthermore, in Group B, the donor Tregs did not fully recover, yet the grafts continued to be accepted.
In summary, this study demonstrates that Foxp3+ cells play a critical role in the maintenance of skin/heart allograft tolerance established in mixed chimeras but are not necessary for the tolerance of donor hematopoietic cells. Apparently other potential regulatory cells are insufficient to maintain tolerance without Foxp3+ cells. Thus, two distinct mechanisms of tolerance, deletion in the thymus and peripheral regulation, can co-exist in the same recipient for different forms of donor allografts. Peripheral tolerance mechanisms are required to control reactivity against non-MHC alloantigens.
Supplementary Material
Acknowledgments
This work was supported by NIH grants RO1 AI081734 (RBC) and RO1 HL071932 (JCM) and a grant from the Roche Organ Transplant Research Foundation (JCM). We thank Dr. David H. Sachs for helpful discussions and review of the manuscript.
Abbreviations
- ANOVA
analysis of variance
- BM
bone marrow
- DSA
donor-specific antibodies
- DT
diphtheria toxin
- DTR
diphtheria toxin receptor
- ELISPOT
enzyme-linked immunospot
- H&E
hematoxylin and eosin
- IFN-γ
interferon gamma
- NK
natural killer
- Th
T helper
- Treg
regulatory T cell
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
References
- 1.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med. 1989;169:493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomita Y, Sachs DH, Khan A, Sykes M. Additional monoclonal antibody (mAB) injections can replace thymic irradiation to allow induction of mixed chimerism and tolerance in mice receiving bone marrow transplantation after conditioning with anti-T cell mABs and 3-Gy whole body irradiation. Transplantation. 1996;61:469–477. doi: 10.1097/00007890-199602150-00027. [DOI] [PubMed] [Google Scholar]
- 3.Wekerle T, Sayegh MH, Ito H, et al. Anti-CD154 or CTLA4Ig obviates the need for thymic irradiation in a non-myeloablative conditioning regimen for the induction of mixed hematopoietic chimerism and tolerance. Transplantation. 1999;68:1348–1355. doi: 10.1097/00007890-199911150-00022. [DOI] [PubMed] [Google Scholar]
- 4.Wekerle T, Kurtz J, Ito H, et al. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nat Med. 2000;6:464–469. doi: 10.1038/74731. [DOI] [PubMed] [Google Scholar]
- 5.Wekerle T, Sykes M. Mixed chimerism as an approach for the induction of transplantation tolerance. Transplantation. 1999;68:459–467. doi: 10.1097/00007890-199908270-00001. [DOI] [PubMed] [Google Scholar]
- 6.Wekerle T, Sykes M. Mixed chimerism and transplantation tolerance. Annu Rev Med. 2001;52:353–370. doi: 10.1146/annurev.med.52.1.353. [DOI] [PubMed] [Google Scholar]
- 7.Kawai T, Cosimi AB, Colvin RB, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59:256–262. [PubMed] [Google Scholar]
- 8.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomita Y, Khan A, Sykes M. Role of intrathymic clonal deletion and peripheral anergy in transplantation tolerance induced by bone marrow transplantation in mice conditioned with a nonmyeloablative regimen. J Immunol. 1994;153:1087–1098. [PubMed] [Google Scholar]
- 10.Manilay JO, Pearson DA, Sergio JJ, Swenson KG, Sykes M. Intrathymic deletion of alloreactive T cells in mixed bone marrow chimeras prepared with a nonmyeloablative conditioning regimen. Transplantation. 1998;66:96–102. doi: 10.1097/00007890-199807150-00015. [DOI] [PubMed] [Google Scholar]
- 11.Colson YL, Lange J, Fowler K, Ildstad ST. Mechanism for cotolerance in nonlethally conditioned mixed chimeras: Negative selection of the Vbeta T-cell receptor repertoire by both host and donor bone marrow-derived cells. Blood. 1996;88:4601–4610. [PubMed] [Google Scholar]
- 12.Zhang QW, Mayumi H, Umesue M, Tomita Y, Nomoto K, Yasui H. Fractionated dosing of cyclophosphamide for establishing long-lasting skin allograft survival, stable mixed chimerism, and intrathymic clonal deletion in mice primed with allogeneic spleen cells. Transplantation. 1997;63:1667–1673. doi: 10.1097/00007890-199706150-00022. [DOI] [PubMed] [Google Scholar]
- 13.Eto M, Kong YY, Uozumi J, Naito S, Nomoto K. Importance of intrathymic mixed chimerism for the maintenance of skin allograft tolerance across fully allogeneic antigens in mice. Immunology. 1999;96:440–446. doi: 10.1046/j.1365-2567.1999.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito H, Kurtz J, Shaffer J, Sykes M. CD4 T cell-mediated alloresistance to fully MHC-mismatched allogeneic bone marrow engraftment is dependent on CD40-CD40 ligand interactions, and lasting T cell tolerance is induced by bone marrow transplantation with initial blockade of this pathway. J Immunol. 2001;166:2970–2981. doi: 10.4049/jimmunol.166.5.2970. [DOI] [PubMed] [Google Scholar]
- 15.Kurtz J, Shaffer J, Lie A, Anosova N, Benichou G, Sykes M. Mechanisms of early peripheral CD4 T-cell tolerance induction by anti-CD154 monoclonal antibody and allogeneic bone marrow transplantation: Evidence for anergy and deletion but not regulatory cells. Blood. 2004;103:4336–4343. doi: 10.1182/blood-2003-08-2642. [DOI] [PubMed] [Google Scholar]
- 16.Domenig C, Sanchez-Fueyo A, Kurtz J, et al. Roles of deletion and regulation in creating mixed chimerism and allograft tolerance using a nonlymphoablative irradiation-free protocol. J Immunol. 2005;175:51–60. doi: 10.4049/jimmunol.175.1.51. [DOI] [PubMed] [Google Scholar]
- 17.Kawai T, Sogawa H, Boskovic S, et al. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant. 2004;4:1391–1398. doi: 10.1111/j.1600-6143.2004.00523.x. [DOI] [PubMed] [Google Scholar]
- 18.Miyajima M, Chase CM, Alessandrini A, et al. Early acceptance of renal allografts in mice is dependent on foxp3(+) cells. Am J Pathol. 2011;178:1635–1645. doi: 10.1016/j.ajpath.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 20.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16:343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Uehara S, Chase CM, Cornell LD, Madsen JC, Russell PS, Colvin RB. Chronic cardiac transplant arteriopathy in mice: Relationship of alloantibody. C4d deposition and neointimal fibrosis. Am J Transplant. 2007;7:57–65. doi: 10.1111/j.1600-6143.2006.01599.x. [DOI] [PubMed] [Google Scholar]
- 22.Lyon MF. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. 1961;190:372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- 23.Gagliani N, Magnani CF, Huber S, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med. 2013;19:739–746. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
- 24.Andersson G, Illigens BM, Johnson KW, et al. Nonmyeloablative conditioning is sufficient to allow engraftment of EGFP-expressing bone marrow and subsequent acceptance of EGFP-transgenic skin grafts in mice. Blood. 2003;101:4305–4312. doi: 10.1182/blood-2002-06-1649. [DOI] [PubMed] [Google Scholar]
- 25.Bigenzahn S, Blaha P, Koporc Z, et al. The role of non-deletional tolerance mechanisms in a murine model of mixed chimerism with costimulation blockade. Am J Transplant. 2005;5:1237–1247. doi: 10.1111/j.1600-6143.2005.00862.x. [DOI] [PubMed] [Google Scholar]
- 26.Pasquet L, Douet JY, Sparwasser T, Romagnoli P, van Meerwijk JP. Long-term prevention of chronic allograft rejection by regulatory T-cell immunotherapy involves host Foxp3-expressing T cells. Blood. 2013;121:4303–4310. doi: 10.1182/blood-2012-08-452037. [DOI] [PubMed] [Google Scholar]
- 27.Simpson E, Scott D, Chandler P. The male-specific histocompatibility antigen, H-Y: A history of transplantation, immune response genes, sex determination and expression cloning. Ann Rev Immunol. 1997;15:39–61. doi: 10.1146/annurev.immunol.15.1.39. [DOI] [PubMed] [Google Scholar]
- 28.Silverman MS, Chin PH. Differences in the rejection of skin transplants and blood cells of donor marrow origin by radiation-induced chimeras. Ann N Y Acad Sci. 1962;99:542–549. doi: 10.1111/j.1749-6632.1962.tb45335.x. [DOI] [PubMed] [Google Scholar]
- 29.Boyse EA, Lance EM, Carswell EA, Cooper S, Old LJ. Rejection of skin allografts by radiation chimaeras: Selective gene action in the specification of cell surface structure. Nature. 1970;227:901–903. doi: 10.1038/227901a0. [DOI] [PubMed] [Google Scholar]
- 30.Zelenay S, Lopes-Carvalho T, Caramalho I, Moraes-Fontes MF, Rebelo M, Demengeot J. Foxp3+ CD25-CD4 T cells constitute a reservoir of committed regulatory cells that regain CD25 expression upon homeostatic expansion. Proc Natl Acad Sci U S A. 2005;102:4091–4096. doi: 10.1073/pnas.0408679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suffner J, Hochweller K, Kuhnle MC, et al. Dendritic cells support homeostatic expansion of Foxp3+ regulatory T cells in Foxp3. LuciDTR mice. J Immunol. 2010;184:1810–1820. doi: 10.4049/jimmunol.0902420. [DOI] [PubMed] [Google Scholar]
- 33.Wise MP, Bemelman F, Cobbold SP, Waldmann H. Linked suppression of skin graft rejection can operate through indirect recognition. J Immunol. 1998;161:5813–5816. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.