Abstract
Although roles for both sterol carrier protein-2/sterol carrier protein-x (SCP-2/SCP-x) and liver fatty acid binding protein (L-FABP) have been proposed in hepatic lipid accumulation, individually ablating these genes has been complicated by concomitant alterations in the other gene product(s). For example, ablating SCP2/SCP-x induces upregulation of L-FABP in female mice. Therefore, the impact of ablating SCP-2/SCP-x (DKO) or L-FABP (LKO) individually or both together (TKO) was examined in female mice. Loss of SCP-2/SCP-x (DKO, TKO) more so than loss of L-FABP alone (LKO) increased hepatic total lipid and total cholesterol content, especially cholesteryl ester. Hepatic accumulation of nonesterified long chain fatty acids (LCFA) and phospholipids occurred only in DKO and TKO mice. Loss of SCP-2/SCP-x (DKO, TKO) increased serum total lipid primarily by increasing triglycerides. Altered hepatic level of proteins involved in cholesterol uptake, efflux, and/or secretion was observed, but did not compensate for the loss of L-FABP, SCP-2/SCP-x or both. However, synergistic responses were not seen with the combinatorial knock out animals—suggesting that inhibiting SCP-2/SCP-x is more correlative with hepatic dysfunction than L-FABP. The DKO- and TKO-induced hepatic accumulation of cholesterol and long chain fatty acids shared significant phenotypic similarities with non-alcoholic fatty liver disease (NAFLD).
Keywords: L-FABP, SCP-2, SCP-x, gene ablation
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) occurs with high frequency, ranging from 11-46% of the US population [1-5]. While NAFLD is generally thought to be due to hepatic triglyceride (TG) accumulation, the hepatic lipids of NAFLD are not well characterized since most studies focused only on indirect determinations [2;3;6-8]. However, recent lipidomic studies indicate that NAFLD livers also exhibit increased accumulation of: i) free or esterified cholesterol—especially with progression to nonalcoholic steatohepatitis (NASH) [9-11]; and ii) unesterified long chain free fatty acids (LCFA) [12;13]. While these data suggested contributions of altered cholesterol as well as LCFA metabolism in NAFLD, relatively little is known regarding the individual roles of the soluble sterol carrier proteins (SCP-2, SCP-x) and liver fatty acid binding protein (L-FABP) in hepatic lipid accumulation.
Sterol carrier protein-2 (SCP-2) and sterol carrier protein-x (SCP-x) are coded through alternate transcription sites by a single gene, SCP-2/SCP-x [14]. While neither SCP-2 nor L-FABP have intrinsic enzymatic activity, in vitro studies show that both SCP-2 and L-FABP: i) bind cholesterol [15-18]; ii) bind LCFA-CoA [19-22]; iii) enhance LCFA-CoA transacylation to cholesterol by microsomal ACAT-2, the rate limiting enzymes in cholesteryl ester synthesis in vitro [23-26] and in cultured fibroblasts overexpressing the respective proteins [27;28]; iii) enhance LCFA-CoA transacylation to glycerol-3-phosphate by microsomal glycerol-3-phosphate acyltransferase (GPAT/GPAM), the rate limiting enzyme in glyceride (phospholipid, triglyceride) synthesis [29-31]. Conversely, both SCP-2 and L-FABP also stimulate carnitine palmitoyl acyl transferase I (CPT1)-mediated LCFA-CoA transacylation in the outer mitochondrial membrane to facilitate LCFA β-oxidation [32]. Further, both in vitro and cultured cell studies have shown that SCP-x is the only known peroxisomal 3-keto-thiolase enzyme capable of oxidizing cholesterol’s branched side chain to form bile acids in the liver [33-35].
Liver fatty acid binding protein (L-FABP) is thought to promote an early adaptive response to hepatocyte stress by partitioning potentially lipotoxic long chain fatty acids (LCFAs) into stable triglyceride stores [36]. Murine L-FABP stimulates microsomal GPAT/GPAM, the rate-limiting step leading to phosphatidic acid, which is the hepatic precursor of triglycerides [29;30;37-42]. L-FABP is upregulated in human NAFLD and in NAFLD animal models [43-46], while murine L-FABP ablation decreases hepatic TG accumulation [16;47-51]. By binding with oxidized and reactive LCFA species L-FABP initially prevents LCFA lipotoxicity [52-59], but becomes depleted as NAFLD progresses to NASH [45;53-57]. Finally, a human L-FABP T94A single nucleotide polymorphism (SNP) variant is associated with NAFLD [10]. This variant occurs with a high minor allele frequency (26-38%)—one of the highest incidence among all FABPs (MAF for 1000 genomes in NCBI dbSNP database; ALFRED) [10;60-65].
Although SCP-2/SCP-x and L-FABP genes have been individually ablated, interpretation of phenotype has been complicated by concomitant upregulation [66-68] or downregulation [69] of liver fatty acid binding protein (L-FABP). To better resolve the impact of these proteins on hepatic lipid accumulation, studies were undertaken comparing female mice singly ablated in L-FABP (LKO), singly ablated in SCP-2/SCP-x (DKO), or ablated in both L-FABP and SCP-2/SCP-x (TKO). The data suggest unique roles of SCP-2/SCP-x and L-FABP, wherein SCP-2/SCP-x had a much greater impact on hepatic total lipid accumulation, especially cholesterol and phospholipid.
EXPERIMENTAL PROCEDURES
Materials
Protein was determined with Protein Assay Kit I (Cat # 500-0001, bovine gamma globulin) obtained from Bio-Rad (Hercules, CA). Diagnostic kits for cholesterol E (total cholesterol, TC), free cholesterol E (free cholesterol, C), nonesterified fatty acid-HR (NEFA), phospholipids C (PL), triglyceride M (TG), glucose, and high density lipoprotein cholesterol (HDLC), were purchased from Wako Diagnostics (Richmond, VA). Diagnostic kits for apolipoprotein AI (APO AI), apolipoprotein B (APO B), and glycated serum protein (GSP) were obtained from Diazyme Labs (Poway, CA). Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined using kits purchased from Stanbio Lab (Boerne, TX). Rabbit or goat polyclonal antibody to mouse ABCA1 (sc-5490), ABCG1 (sc-11150), APO AI (sc-23606), APO AII (sc-23609), APO B (sc-11795), β-actin (sc-47778), LDL receptor (LDLR, sc-11826), or SRB1 (sc-32342) was purchased from Santa Cruz Biotechnology (Dallas, TX). Rabbit polyclonal antibody directed against mouse ACAT-2 (ab66259) or COX4 (ab16056) was obtained from Abcam (Cambridge, MA). Rabbit polyclonal antibody to mouse PPARα (PA1-822A) was purchased from Pierce Antibody (Rockford, IL). Mouse monoclonal antibody against mouse GAPDH (MAB374) was obtained from Millipore (Billerica, MA). Rabbit polyclonal antibody against recombinant rat L-FABP, mouse SCP-2, mouse SCP-x, or mouse ACBP was produced as previously described [70]. Alkaline phosphatase-conjugated goat polyclonal antibody to rabbit IgG (product # A3687) and rabbit polyclonal antibody to goat IgG (product # A4187) were obtained from Sigma-Aldrich (St. Louis, MO). Alkaline phosphatase-conjugated rabbit polyclonal antibody against mouse IgG (product # ab6729-1) was purchased from Abcam (Cambridge, MA). One-Step PCR Master Mix reagent kit and gene-specific assays for Mttp (Mm00435015_ml), Abcg5 (Mm01226965_m1), and Abcg8 (Mm00445977_m1) were obtained from Applied Biosystems (Foster City, CA). Recombinant L-FABP, SCP-2, and SCP-x were purified as described [19;70;71]. All reagents and solvents were the highest grade commercially available.
Animals
Female (6 weeks old, 20-30 g) inbred C57BL/6NCr wild-type (WT) mice were obtained from the National Cancer Institute (Frederick Cancer Research and Development Center, Frederick, MD). L-FABP gene-ablated (null, LKO) mice were generated by our laboratory as described previously [72]. SCP-2/SCP-x null (DKO) mice were generated as previously described [73]. L-FABP/SCP-2/SCP-x null (TKO) mice were generated as previously described [74]. All null mouse strains were backcrossed to the C57BL/6NCr background to ≥10 generations. Mice were kept in a temperature-controlled facility (T = 25 °C), 12 h light/dark cycle with ad libitum access to water and food (Research Diets D11243, phytol/phytoestrogen-free, 5 g% fat, Research Diets, New Brunswick, NJ). The phytol/phytoestrogen free control diet was chosen because of the presence of variable levels of dietary phytol and phytoestrogen in standard rodent diets [67;75;76]. Phytanic acid and pristanic acid, hepatic metabolites of dietary phytol, are the most potent known naturally-occurring ligand activators of PPARα, which dramatically alter both fatty acid and cholesterol metabolism by inducing transcription of LCFA β-oxidative enzymes, apolipoproteins A1 and A2, as well as L-FABP and SCP-2 [77-81]. Estrogenic effects of variable levels of dietary phytoestrogens can in turn alter lipid metabolism [75;76]. Analysis of serum parameters of liver damage indicated minor, if any, changes in the concentrations of glycated serum protein (GSP), alanine amino transferase (ALT), or aspartate amino transferase (AST) in any of the mouse strains examined, which suggested little liver hepatotoxicity as a result of the loss of L-FABP, SCP-2/SCP-x, or both (data not shown). All animal protocols were Institutional Animal Care and Use Committee (Texas A&M University) approved, mice were monitored quarterly for known rodent pathogens, and were determined to be pathogen-free.
Animal Euthanasia and Tissue Collection
Prior to euthanasia, mice were 12-hr fasted, weighed, anesthetized with Avertin, and blood collected via cardiac puncture into a 1.5-mL polypropylene microtube. Freshly collected blood was immediately processed to serum, volume of serum measured, flash-frozen on dry ice, and the serum stored at -80 °C. Mice were euthanized by cervical dislocation while anesthetized. The liver was collected, blotted dry, weighed, flash-frozen with phosphate buffered saline (0.5 mL, pH 7.4) on dry ice, and stored at -80 °C.
Analysis of liver lipids
A small portion of mouse liver (~0.1 g tissue) was minced, placed in a 1.5-mL microcentrifuge tube, and homogenized in 0.5 mL of PBS (pH 7.4) for 5 min on ice with a motor-driven pestle at 2000 rpm (Tekmar Co, Cincinnati, OH). The crude homogenate was further processed by sonication on ice with a Fisher Scientific Sonic Dismembrator 550 equipped with micro-tip (Fisher Scientific, Pittsburgh, PA) set at 4, total processing time 5 min, on-time 15.0 sec, and off-time 15.0 sec. The final homogenate was centrifuged at 600 × g and 4 °C for 10 min to remove insoluble debris. Aliquots of homogenate and protein standard were placed in a Costar 96-well assay plate (Corning, Corning, NY) for determining protein content by Bradford protein micro-assay (Bio-Rad, Hercules, CA) as measured by a BioTek Synergy 2 micro-plate reader (BioTek Instruments, Winooski, VT). Lipid class composition was determined by two independent techniques.
First, lipids were solvent-extracted from liver homogenate protein as described [72]. Briefly, lipids were extracted from liver homogenates using the solvent system n-hexane/2-propanol (3:2, vol/vol). Aliquots of liver lipids as well as cholesterol (C), cholesteryl ester (CE), triglyceride (TG), and non-esterified free fatty acid (NEFA) standards were spotted onto Silica Gel G thin layer chromatography (TLC) plates, resolved in the solvent system petroleum ether/diethyl ether/methanol/acetic acid (90:7:2:0.5, vol/vol), and quantified by sulfuric acid charring assay as described [82;83]. Total phospholipid content was determined by digesting the aqueous phospholipid fraction from above as well as phospholipid standards in deionized water and perchloric acid at 180 °C for 1 hr followed by the addition of ascorbic acid and ammonium molybdate [84]. The samples were heated in a boiling water bath for 5 min, cooled, and the sample absorbance was determined at 660 and 797 nm. Protein concentration was determined utilizing the BioRad modified Bradford assay from the dried protein extract residue after digesting overnight in 0.2 M KOH. All lipid samples were stored at -20 °C under N2 in order to minimize oxidation. All glassware was washed with sulfuric acid/chromate and rinsed extensively with deionized water prior to use.
Second, total cholesterol (TC, i.e. cholesterol + esterified cholesterol), C, TG, NEFA, and PL were directly quantified in the liver homogenates with Wako diagnostic kits as per the manufacturer’s instructions. Cholesteryl ester (CE) was determined by subtracting free cholesterol (C) from total cholesterol (TC). Sample volumes were adjusted to allow aliquots of homogenate and lipid standards to be placed in Costar 96-well assay plates (Corning, Corning, NY) for detection with the respective assay kits and detection by a BioTek Synergy 2 micro-plate reader (BioTek Instruments, Winooski, VT). Liver homogenate protein concentration was determined utilizing the BioRad modified Bradford assay. Our laboratory has found no significant differences in liver lipid class quantified (nmol lipid/mg protein) between the two lipid analysis procedures (data not shown).
Analysis of serum lipids
Serum lipids (TC, C, TG, NEFA, and PL) were also quantified by solvent extraction/thin-layer chromatography and by the Wako diagnostic kits as described above for liver lipids. Aliquots of serum and lipid standards were placed in Costar 96-well assay plates (Corning, Corning, NY) for determining lipid class content with the Wako diagnostic kits for total cholesterol (TC, i.e. cholesterol + esterified cholesterol), free cholesterol (C), HDL cholesterol (HDLC), triglyceride (TG), non-esterified free fatty acid (NEFA), and phospholipid (PL) as per the manufacturer’s instructions. Lipids detected by the respective assay kits were quantified with a BioTek Synergy 2 micro-plate reader (BioTek Instruments, Winooski, VT). Again, our laboratory has found no significant differences in serum lipid quantification (mmol lipid/L serum) between the two lipid analysis procedures (data not shown). Serum non-HDL cholesterol (non-HDLC) was calculated by subtracting the HDL cholesterol concentration from the total cholesterol concentration.
Western blotting of key proteins in hepatic lipid metabolism
Liver homogenates were assayed for protein as described [85;86]. Aliquots of liver homogenate were subjected to SDS-PAGE gel electrophoresis and western blotting as described [85;86]. SDS-PAGE gel-loading control proteins (COX4, GAPDH, or β-Actin) were used that were appropriate for the protein of interest (primary antibody cross-reactivity, protein molecular size). L-FABP, SCP-2, and SCP-x were quantified (ng protein/μg liver homogenate protein) by comparison of band intensity to a series of pure protein standards co-electrophoresed with liver homogenate samples. Loss of L-FABP, SCP-2, and/or SCP-x had no effect on liver levels of any of the control proteins used (data not shown). Protein quantification data are shown in relative units with WT protein level = 1.0 except as noted above for L-FABP, SCP-2, and SCP-x.
QrtPCR of liver mRNAs
Liver total RNA was isolated and purified by RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer’s standard protocol and stored at -80°C. Nucleic acid quality and concentration were assayed with a NanoDrop 1000 Spectrophotometer (Thermo Scientific, Waltham, MA). QrtPCR expression was determined with a ABI PRISM 7000 (Applied Biosystems®, Foster City, CA) using TaqMan® RNA-to-CT™ 1-Step PCR Master Mix Reagent kit (cat # 4309169), gene-specific TaqMan PCR probes and primers, and a thermal cycler protocol as follows: 48°C for 30 min, 95°C for 10min, 95°C for 0.15 min and 60°C for 1.0 min, repeated a total of 60 cycles. Specific probe and primer TaqMan® gene expression assays were from Life Technologies™ (Carlsbad, CA) to determine hepatic mRNA levels of microsomal triglyceride transport protein (Mttp), Abcg5, and Abcg8. Sample reactions (20μL total volume each) were performed in duplicate on 96 well optical reaction plates (Applied Biosystems®, Foster City, CA). The threshold cycle from each well was established by ABI Prism 7000 SDS software (Applied Biosystems®, Foster City, CA) and QrtPCR data were normalized to the housekeeping gene 18S RNA (cat # 4310893E) for mRNA expression of Mttp, Abcg5, and Abcg8 and made relative to the control mouse group (female WT mice on control diet = 1) for final calculations.
Statistics
Statistical analysis was one-way analysis of variance (ANOVA) combined with the Newman-Keuls multiple-comparisons post-test (GraphPad Prism Version 3.03, San Diego, CA). All data passed Bartlett’s test for equal variances and are expressed as means ± standard error of the mean (n = number of mice = 8 per group). Graphical analysis was accomplished using SigmaPlot 2002 for Windows Version 8.02 (SPSS, Chicago, IL). Statistical differences (P < 0.05) were indicated by labeling data with different lower-case letters.
RESULTS
Ablating SCP-2/SCP-x had highest impact on hepatic lipid accumulation
Hepatic lipid mass differed significantly among liver homogenates of WT, LKO, DKO, and TKO female mice. Loss of SCP-2/SCP-x (DKO) induced hepatic accumulation of total lipid (Table 1) more than loss of L-FABP (LKO). The DKO increased total hepatic lipid concentration 2-fold, while the LKO increased total lipid concentration by 20%. Ablating L-FABP in SCP-2/SCP-x null mice (TKO) did not further exacerbate hepatic total lipid accumulation.
Table 1. Hepatic lipid content in WT, LKO, DKO, and TKO mice.
Liver lipid concentrations (nmol lipid/mg liver homogenate protein) were determined as described in Methods.
| Lipid | WT nmol/mg | LKO nmol/mg | DKO nmol/mg | TKO nmol/mg |
|---|---|---|---|---|
| Total Lipid | 189±7a | 229±5b | 370±30c | 380±20c |
| C | 22±1a | 32±2b | 33±4b | 45±4c |
| CE | 20±1a | 28±1b | 61±9c | 53±5c |
| NEFA | 28±2a | 19±1b | 90±5c | 93±7c |
| PL | 32±1a | 82±4b | 89±6b | 86±7b |
| TG | 87±7a | 74±3a | 90±10a | 99±6a |
C, cholesterol; CE, cholesteryl ester; NEFA, nonesterified fatty acid; PL phospholipid; TG, triglyceride. Total lipid concentrations were obtained by adding the individual lipid concentrations. Values = means ± SEM (n = 8). Statistically different values (P < 0.05, ANOVA) within a row (lipid class) are denoted by a different lower-case letter (a, b, c).
Lipid resolution into separate lipid classes revealed that SCP-2/SCP-x ablation (DKO, TKO) altered hepatic lipid class composition much more than L-FABP ablation (LKO). DKO increased hepatic total cholesterol (TC, 2.1-fold), especially cholesteryl ester (CE, 3.1-fold), much more than L-FABP ablation (LKO) (Table 1). Total cholesterol increased in the order TKO, DKO > LKO. Non-esterified cholesterol (C) was increased in the order TKO > DKO, LKO while cholesteryl ester (CE) increased in the order TKO, DKO > LKO.
Loss of SCP-2/SCP-x (DKO) alone, but not loss of L-FABP (LKO) alone, increased hepatic accumulation of non-esterified fatty acids (NEFA, 3.2-fold, Table 1). The LKO actually decreased hepatic NEFA levels by 32%, consistent with decreased hepatic uptake of serum fatty acid observed earlier [72]. Ablating both genes (TKO) did not further increase NEFA levels above that observed in DKO livers.
DKO, LKO, and TKO all similarly increased hepatic phospholipid accumulation nearly 3-fold (Table 1). In contrast, hepatic triglyceride levels were unaffected in all KO mice (LKO, DKO, TKO, Table 1).
These data indicate that SCP-2/SCP-x has a larger role in hepatic accumulation of cholesterol, both free and more so esterified, than L-FABP. While SCP-2/SCP-x and L-FABP have opposite roles in hepatic accumulation of NEFA, loss of either or both similarly induced phospholipid accumulation.
Ablating either or both genes modestly affected serum lipid content and class composition
Analysis of serum lipid levels in female mice revealed small but significant differential effects of SCP-2/SCP-x and L-FABP gene ablation. DKO and TKO, but not LKO, mice had increased serum total lipid concentration (Table 2).
Table 2. Serum lipid content in WT, LKO, DKO, and TKO mice.
Serum lipid concentrations (mmol lipid/L serum) were determined as described in Methods.
| Lipid | WT mmol/L | LKO mmol/L | DKO mmol/L | TKO mmol/L |
|---|---|---|---|---|
| Total Lipid | 5.3±0.1a | 5.3±0.1a | 7.6±0.8b | 6.6±0.2b |
| C | 0.28±0.05a | 0.13±0.02b | 0.34±0.08a | 0.5±0.1a |
| CE | 0.96±0.03a | 0.79±0.02b | 1.0±0.1ab | 0.7±0.1ab |
| NEFA | 1.3±0.1a | 1.0±0.2a | 1.2±0.2a | 2.0±0.1b |
| PL | 2.40±0.04a | 1.87±0.03b | 2.7±0.2a | 1.78±0.03b |
| TG | 0.63±0.06a | 1.5±0.1b | 2.3±0.4b | 1.6±0.2b |
C, cholesterol; CE, cholesteryl ester; NEFA, nonesterified fatty acid; PL phospholipid; TG, triglyceride. Total lipid concentrations were obtained by adding the individual lipid concentrations. Values represent means ± SEM (n = 8). Statistically different values (P < 0.05, ANOVA) within a row (lipid class) are denoted by a different lower-case letter (a, b).
Examination of individual serum lipid classes showed that LKO females had small but significant decreases in three major lipid classes (C, CE, and PL), while serum NEFA levels were unaffected, and serum TG content was increased by 2.4-fold (Table 2). By contrast, DKO increased serum TG by 3.6-fold without significantly altering the levels of other lipid classes. Loss of both genes (TKO) increased serum NEFA (1.5-fold) and TG (2.5-fold), decreased serum PL by 26%, and had no effect on serum content of either C or CE (Table 2).
Ablating SCP-2/SCP-x, L-FABP, or both differentially affected serum lipoprotein content and/or apolipoprotein composition
The alterations in serum lipid and/or lipid class composition suggested that DKO, LKO and/or TKO altered serum lipoprotein distribution and/or apolipoprotein content. The major lipoprotein class in fasted mouse serum is high-density lipoprotein (HDL, rich in cholesterol as well as apolipoprotein AI). The non-high-density lipoprotein concentration in fasted mouse serum is much lower than the HDL concentration and consists primarily of low-density and very low-density lipoprotein (LDL, VLDL), both rich in cholesterol, triglyceride and cholesteryl ester as well as apolipoprotein B [69].
Ablations of these genes differentially altered serum HDL cholesterol (HDLC) and non-HDL-cholesterol (non-HDLC). The TKO, but not DKO or LKO, significantly decreased the serum content of APO A1 by 38% (Table 3). In contrast, serum HDLC was unaffected in TKO mice; however, loss of L-FABP (LKO) resulted in a 27% decrease in serum HDLC concentration relative to WT (Table 3).
Table 3. Serum levels of APO AI, HDL cholesterol, APO B, and non-HDL cholesterol.
Serum concentrations (mg protein/dL serum) of apolipoprotein AI (APO AI), high density lipoprotein cholesterol (HDLC, mmol C/L serum), apolipoprotein B (APO B, mg protein/dL serum), and non-high density lipoprotein cholesterol (non-HDLC, mmol C/L serum) were determined as described in Methods.
| Component | WT | LKO | DKO | TKO |
|---|---|---|---|---|
| APO AI, mg/dL | 82±6a | 90±10a | 80±5a | 51±2b |
| HDLC, mmol/L | 1.05±0.03a | 0.76±0.04b | 1.2±0.2a | 1.2±0.1a |
| APO B, mg/dL | 26±3a | 21±2a | 45±4b | 18±1a |
| non-HDLC, mmol/L | 0.15±0.01a | 0.16±0.02a | 0.14±0.02a | 0.05±0.01b |
Values represent means ± SEM (n = 8). Statistically different values (P < 0.05, ANOVA) within a row are denoted by a different lower-case letter (a, b).
With respect to the impact on the non-HDL fraction, the DKO significantly increased APO B content (1.7-fold, Table 3). While neither LKO nor TKO affected serum APO B concentration, only TKO decreased serum non-HDLC by 68% (Table 3).
Impact of ablating SCP-2/SCP-x, L-FABP, or both on proteins involved in cholesterol uptake
Analysis of key proteins involved in hepatic cholesterol uptake from HDL and LDL revealed significant differences among the different gene-ablated female mice.
Exogenous cholesterol/cholesteryl ester can be taken up by from HDL via the non-lysosomal scavenger receptor B1 (SRB1). The DKO, LKO, and TKO each differentially impacted the level of basolateral membrane proteins involved in HDL-mediated hepatic cholesterol homeostasis (Fig. 1). Ablation of either L-FABP (LKO) or SCP-2/SCP-x (DKO) had no effect on hepatic protein levels of the HDL receptor SRB1 (Fig 1A), however, absence of both L-FABP and SCP-2/SCP-x (TKO) resulted in a 40% decrease in SRB1 protein (Fig 1A).
Figure 1. Hepatic levels of key proteins involved in cholesterol uptake, transport, and efflux are altered in L-FABP/SCP-2/SCP-x gene-ablated female mice.
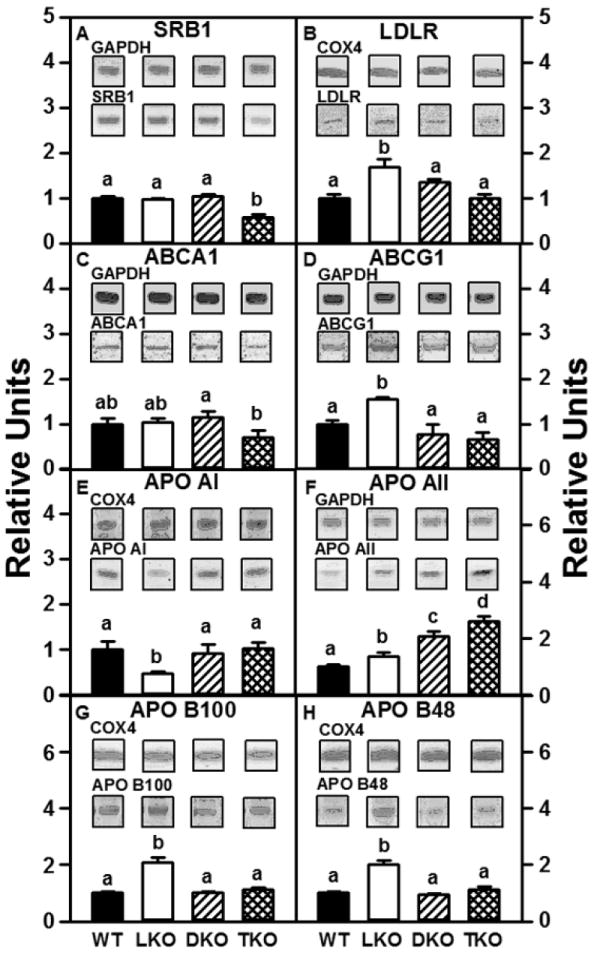
Aliquots of liver homogenate proteins were examined by SDS-PAGE and subsequent western blot analysis to determine levels of SRB1 (panel A), LDLR (panel B), ABCA1 (panel C), ABCG1 (panel D), APO AI (panel E), APO AII (panel F), APO B100 (panel G), and APO B48 (panel H). Insets show representative western blots of the respective protein (lower blot) and the gel-loading control protein (GAPDH or COX4, upper blot). Relative concentration values (WT = 1) represent means ± SEM (n = 8). Statistically different values (P < 0.05, ANOVA) within a panel are denoted by a different lower-case letter (a, b, c, d).
In addition, exogenous cholesterol/cholesteryl ester can also be taken up by the mouse hepatocyte via the LDL receptor (LDLR) and into lysosomes for cholesterol release and utilization. The effect of ablating L-FABP, SCP-2/SCP-x, or both on hepatic protein levels of LDLR was examined. The absence of L-FABP (LKO) increased hepatic protein levels of LDLR by 1.7-fold (Fig 1B). However, absence of SCP-2/SCP-x in presence of L-FABP (DKO) or in the absence of L-FABP (TKO) had no effect on hepatic levels of LDLR protein (Fig 1B).
Impact of ablating SCP-2/SCP-x, L-FABP, or both on proteins involved in cholesterol efflux
While the SRB1-receptor mediated pathway can also operate in reverse to mediate cholesterol efflux to HDL, the ATP-binding cassette transporters ABCA1 and ABCG1 also mediate vectorial cholesterol efflux across the basolateral hepatocyte membrane.
DKO, LKO, and TKO each differentially impacted the level of basolateral membrane proteins involved in HDL-mediated hepatic cholesterol homeostasis (Fig. 1). The rate-limiting step in hepatic HDL formation involves the efflux of cholesterol/phospholipid across the hepatocyte basolateral membrane via ABCA1 to APO A1 in nascent HDL. Neither loss of L-FABP (LKO) or SCP-2/SCP-x (DKO) affected hepatic levels of ABCA1 protein (Fig 1C); however, ablation of both L-FABP and SCP-2/SCP-x (TKO) resulted in a 30% reduction in hepatic ABCA1 protein (Fig 1C). While cholesterol is also exported across the basolateral membrane via ABCG1 to more mature HDL, ABCG1 protein levels were unaffected by the loss of SCP-2/SCP-x (DKO, TKO, Fig 1D). However, L-FABP gene ablation (LKO) increased hepatic levels of ABCG1 protein by 1.6-fold (Fig 1D).
Hepatic apolipoprotein components of HDL (APO AI, AII) were differentially affected by L-FABP and/or SCP-2/SCP-x gene ablation. Loss of SCP-2/SCP-x (DKO, TKO) had no effect on hepatic levels of APO AI protein (Fig 1E); however, loss of only L-FABP (LKO) resulted in a 50% decrease in APO AI protein (Fig 1E). By contrast, APO AII protein levels were increased in all gene-ablated mice; the greatest effect was observed in TKO mice (Fig 1F).
Impact of ablating SCP-2/SCP-x, L-FABP, or both on proteins involved in cholesterol secretion
Cholesterol (especially cholesteryl ester) and triglyceride can be secreted from hepatocytes via nascent very low density lipoproteins (VLDL). Production and secretion of neutral lipids as nascent VLDL into serum requires the concerted action of three proteins: i) L-FABP (and/or SCP-2), which facilitates fatty acid uptake and esterification to cholesteryl ester and triglyceride [23;24;29-31;87;88]; ii) microsomal triglyceride transfer protein (MTP) which loads triglyceride and cholesteryl ester onto APO B [89]; and iii) apolipoprotein B (APO B), the major apolipoprotein constituent of nascent VLDL (as well as from recycled endocytosed LDL) [89]. Mouse liver produces both APO B100 and APO B48 via post-transcriptional mRNA editing and both forms of APO B are found in mouse hepatic VLDL [90;91]. The absence of L-FABP (LKO) increased hepatic protein levels of APO B100 and APO B48 by 1.7- to 2-fold, respectively (Fig 1 G, H). However, the absence of SCP-2/SCP-x in the presence of L-FABP (DKO) or in the absence of L-FABP (TKO) had no effect on hepatic levels of APO B100 or APO B48 protein (Fig 1 G, H).
Hepatic microsomal triglyceride transfer protein (MTP) is a key protein involved in the production of VLDL via the transfer of triglyceride to apolipoprotein B100 to form the pre-VLDL particle. Loss of L-FABP and/or SCP-2/SCP-x function differentially affected the levels of Mttp mRNA. Absence of SCP-2/SCP-x alone (DKO) or combined with ablation of L-FABP (TKO) had no effect on hepatic levels of Mttp mRNA (Table 4). In contrast, the loss of L-FABP (LKO) resulted in a 56% decrease in the level of Mttp mRNA in female mice (Table 4). DKO, LKO, and TKO also differentially affected the level of mRNAs for canalicular membrane proteins involved in hepatic biliary secretion of cholesterol, ABCG5/G8. Neither the loss of L-FABP (LKO) nor the loss of SCP-2/SCP-x (DKO) affected hepatic levels of Abcg5 and Abcg8 mRNA (Table 4). In contrast, the absence of both L-FABP and SCP-2/SCP-x increased mRNA levels of both Abcg5 and Abcg8 by 2.3-fold and 2.7-fold, respectively (Table 4).
Table 4. mRNA levels of key proteins involved in hepatic cholesterol secretion in WT, LKO, DKO, and TKO mice.
Quantitative rtPCR (qrtPCR) of liver mRNA was accomplished as described in Methods.
| Component | WT Relative Value | LKO Relative Value | DKO Relative Value | TKO Relative Value |
|---|---|---|---|---|
| Mttp | 1.0±0.1a | 0.44±0.04b | 1.2±0.1a | 1.2±0.2a |
| Abcg5 | 1.0±0.1a | 1.2±0.4a | 1.1±0.2a | 2.3±0.4b |
| Abcg8 | 1.0±0.2a | 1.2±0.2a | 1.1±0.2a | 2.7±0.6b |
Levels of mRNA are shown as relative values (WT = 1) and represent means ± SEM (n = 8). Statistically different values (P < 0.05, ANOVA) within a row are denoted by a different lower-case letter (a, b).
Effect of gene ablation on liver proteins involved in intracellular uptake, trafficking, and microsomal esterification of cholesterol, long chain fatty acid or long chain fatty acyl CoA
Hepatocytes express several proteins in cholesterol, LCFA and LCFA-CoA trafficking and targeting: i) L-FABP and SCP-2 both bind cholesterol, LCFA, and LCFA-CoA; ii) acyl CoA binding protein (ACBP), which binds only LCFA-CoA; iii) peroxisome proliferator activated receptor-α (PPARα), which regulates expression of L-FABP, SCP-2, and ACBP; iv) acyl CoA acyl transferase-2 (ACAT-2), which catalyzes the fatty acyl esterification of cholesterol.
LKO mice did not produce L-FABP (Fig 2C); however, there was no compensatory alteration in protein levels of SCP-2, ACBP, ACAT-2, or PPARα (Fig 2A, D, E, F). LKO mice were associated with a significant reduction in levels of SCP-x protein (Fig 2B).
Figure 2. Effect of ablating SCP-2/SCP-x, L-FABP, or both on expression of key soluble intracellular proteins involved in cholesterol binding, transport, and metabolism in female mice.
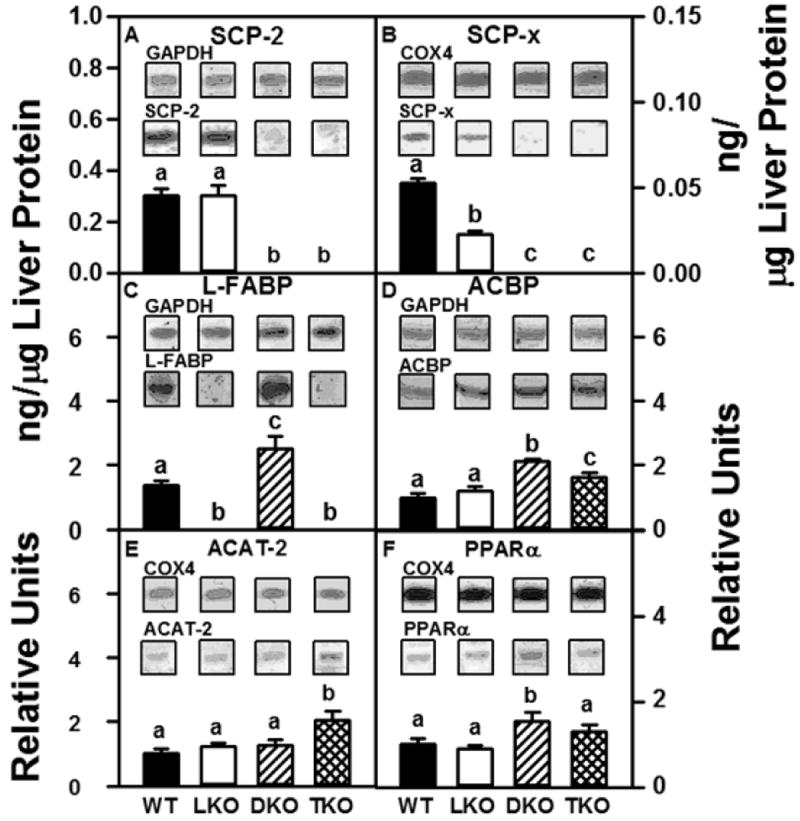
Aliquots of liver homogenate proteins were examined by SDS-PAGE and subsequent western blot analysis as described in Methods to determine levels of SCP-2 (panel A), SCP-x (panel B), L-FABP (panel C), ACBP (panel D), ACAT-2 (panel E), and PPARα (panel F). Insets show representative western blots of the respective protein (lower blot) and the gel-loading control protein (GAPDH or COX4, upper blot). SCP-2, SCP-x, and L-FABP were quantified (ng protein/μg liver homogenate protein) as described in Methods. Relative concentration values for ACBP, ACAT-2, and PPARα protein are shown with WT = 1. Values represent means ± SEM (n = 8). Statistically different values (P < 0.05, ANOVA) within a panel are denoted by a different lower-case letter (a, b, c).
As expected, DKO mice had no detectable SCP-2 (Fig 2A) or SCP-x (Fig 2B). The absence of SCP-2/SCP-x (DKO) was associated with increased levels of L-FABP, ACBP, and PPARα (Fig 2C, D, F). Loss of SCP-2/SCP-x did not affect hepatic levels of ACAT-2 protein (Fig 2 E)
Finally, TKO mice were associated with the absence of SCP-2 and SCP-x (Fig 2A, B) as well as L-FABP (Fig 2C). Loss of SCP-2/SCP-x and L-FABP resulted in increased ACBP and ACAT-2 protein (Fig 2 D, E); however, PPARα protein levels were unchanged (Fig 2 F).
DISCUSSION
Several hepatic gene products are involved in hepatic uptake, intracellular trafficking, and targeting of cholesterol and fatty acids including: i) SCP-2 and SCP-x derived by alternate transcription sites from a single SCP-2/SCP-x gene [14]. Human genetic variants in the SCP-2/SCP-x gene are associated with lipid metabolic abnormalities [92]; ii) L-FABP [93]. Human genetic variants in L-FABP gene are also associated with lipid metabolic abnormalities [10;11;22;60-63;94-97]; iii) acyl CoA binding protein (ACBP) which exclusively binds LCFA-CoA and facilitates ACAT-2-mediated esterification in the endoplasmic reticulum [26;98-100]. While studies with individually ablated SCP-2/SCP-x or L-FABP genes have been informative, impact on phenotype interpretations of many SCP-2/SCP-x gene-ablated mouse studies have been complicated by concomitant upregulation of L-FABP [66-68]. Thus the current study was undertaken with gene-targeted mice to examine the impact of ablating not only SCP-2/SCP-x (DKO) or L-FABP (LKO) individually, but also ablating both (TKO) on hepatic cholesterol metabolism in female mice. The data provided several new insights into the roles of SCP-2/SCP-x and L-FABP in hepatic lipid, especially cholesterol and cholesteryl ester accumulation in female mice.
First, loss of L-FABP (LKO) alone exerted the least effect on hepatic total lipid accumulation (Table 5). L-FABP is the major hepatocyte cytosolic protein that binds cholesterol [16;96;101] as well as LCFA and LCFA-CoA [21;102;103]. While loss of L-FABP would be expected to decrease hepatic uptake of cholesterol and LCFA, this appeared to be more than counteracted by decreased expression of other proteins involved in VLDL (TG, CE) secretion and HDL (C) efflux. Unaltered expression of SCP-2 and ACBP appeared sufficient for facilitating LCFA-CoA transacylation to cholesteryl-esters and glycerides in the endoplasmic reticulum. Unlike loss of SCP-2/SCP-x (DKO, TKO), LKO decreased hepatic total non-esterified fatty acid levels (Table 5, NEFA). This was consistent with reduced hepatic uptake of LCFA from serum along with reduced cytosolic LCFA binding capacity of LKO mice [72]
Table 5. Major significant changes in hepatic lipid phenotype between L-FABP, SCP-2/SCP-x, and L-FABP/SCP-2/SCP-x knockout mice.
| Parameter | LKO nmol/mg | DKO nmol/mg | TKO nmol/mg |
|---|---|---|---|
| Liver Total Lipid | + | +++ | +++ |
| Liver Total Cholesterol | + | ++ | ++ |
| Liver Total Phospholipid | ++ | ++ | ++ |
| Liver Total NEFA | - | +++ | +++ |
| Liver L-FABP | ND | ++ | ND |
The symbols +, ++, and +++ indicate the extent of significant increase relative to WT. Conversely, the symbol - indicates the significant decrease relative to WT.
ND, not detectable
Second, loss of SCP-2/SCP-x (DKO, TKO) induced much higher, but similar, hepatic total lipid accumulation (Table 5). Accumulated lipid was enriched not only with cholesterol and phospholipid, but even more so cholesteryl ester than in LKO. While loss of SCP-2/SCP-x L-FABP would be expected to decrease hepatic uptake of cholesterol and decrease endoplasmic reticulum formation of cholesteryl esters (via ACAT-2) and glycerides, this appeared to be counteracted in part by compensatory: i) increased levels of L-FABP and ACBP, which stimulate microsomal ACAT-2 mediated cholesterol esterification [24;26;99;100] and glyceride formation [30;31;37;39;104]; ii) increased levels of hepatic L-FABP to increase APO B loading consistent with increased appearance of APO B in serum. Finally, loss of SCP-2/SCP-x (DKO, TKO) increased hepatic total non-esterified fatty acid levels (Table 5, NEFA). This was consistent with the concomitant upregulation of L-FABP, a cholesterol [15;101;105;106] and LCFA/LCFA-CoA [19;105;107] binding protein that is already 6-8-fold more prevalent than SCP-2 in livers of WT control-fed mice [93;108]. L-FABP overexpression enhances [109-111] while L-FABP deletion [47;72] inhibits the uptake of LCFA.
In summary, both SCP-2/SCP-x and L-FABP gene products play significant roles in hepatic lipid accumulation—especially free cholesterol, esterified cholesterol, and phospholipid. The data suggested a potentially more selective role for L-FABP in regulating not only cholesterol and cholesteryl esters but also triglycerides, while SCP-2 may play a more selective role toward regulating cholesterol and cholesteryl esters, and both impacted phospholipid levels. The highest hepatic lipid accumulation was observed upon loss of SCP-2/SCP-x (DKO). For the most part, however, the impact of combinatorial knock out (SCP2/SCP-x and L-FABP) on hepatic lipid function was not significantly different from the effects of the individual SCP-2/SCP-x knockout experiments. While concomitant upregulation of L-FABP may have compensated in part for loss of SCP-2/SCP-x, the same hepatic phenotype was observed upon loss of both SCP-2/SCP-x and L-FABP (TKO). Overall hepatic lipid phenotype synergistic responses were not seen with the combinatorial knock out animals. This would suggest an alternate hypothesis, and possibly a more likely scenario, that SCP-2/SCP-x is more correlative than L-FABP with hepatic dysfunction in female mice.
Highlights.
L-FABP and/or SCP-2/SCP-x gene ablation in female mice
SCP-2/SCP-x gene ablation: increased hepatic lipid accumulation
Significant phenotypic similarities to non-alcoholic fatty liver disease
Acknowledgments
Funding Information: This work was supported in part by the United States Public Health Service, National Institutes of Health Grants DK41402 (F.S. and A.B.K.) and DK70965 (B.P.A).
Abbreviations
- ABCA1
G1, G5, G8 ATP-binding cassette transporter A1, G1, G5, G8
- ACAT-2
acyl-CoA cholesterol acyltransferase-2
- APO AI
AII, B, apolipoprotein AI, AII, B
- BA
bile acid
- C
cholesterol
- CE
cholesteryl ester
- CoA
Coenzyme A
- DKO
SCP-2/SCP-x double null mouse
- ER
endoplasmic reticulum
- HDL
high density lipoprotein
- HDLC
high density lipoprotein cholesterol
- LCFA
long chain fatty acid
- LD
lipid droplet
- LDL
low density lipoprotein
- L-FABP
liver fatty acid binding protein or FABP1
- LKO
L-FABP null mouse
- Lyso
lysosome
- Mito
mitochondrion
- MTP
microsomal triglyceride transfer protein
- non-HDLC
non-high density lipoprotein cholesterol
- Nuc
nucleus
- Per/BA Synth
peroxisome/bile acid synthesis
- PL
phospholipid
- PM
plasma membrane
- PPARα
-β/δ, or -γ, peroxisome proliferator-activated receptor alpha, beta/delta, or gamma
- QrtPCR
quantitative real-time polymerase chain reaction
- SCP-2
sterol carrier protein-2
- SCP-x
sterol carrier protein-x/peroxisomal thiolase 2
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SRB1
scavenger receptor class B member 1
- SREBP
sterol regulatory element binding protein
- TG
triglyceride
- TKO
L-FABP/SCP-2/SCP-x triple null mouse
- WT
wild-type C57BL/6NCr mouse
Footnotes
AUTHOR CONTRIBUTIONS
Author Contributions: G.G.M., planned experiments, performed experiments, analyzed data, wrote the paper; B.P.A., planned experiments, performed experiments; K.K.L., performed experiments, analyzed data; D.L., planned experiments, performed experiments, analyzed data; F.S., planned experiments, wrote the paper; A.B.K., planned experiments, wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non-alcoholic liver disease. J Gastroenterology. 2013;48:434–441. doi: 10.1007/s00535-013-0758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gariani K, Philippe J, Jornayvaz FR. Non-alcoholic liver disease and insulin resistance: from bench to bedside. Diabetes and Metabolism. 2013;39:16–26. doi: 10.1016/j.diabet.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Tailleux A, Wouters K, Staels B. Role of PPARs in NAFLD: potential therapeutic targets. Biochim Biophys Acta. 2012;1821:809–818. doi: 10.1016/j.bbalip.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Paz-Filho G, Mastronardi CA, Parker BJ, Khan A, Inserra A, Matthaei KI, Erhart-Bornstein M, Bornstein S, Wong M-L, Licinio J. Molecular pathways involved in the improvement of nonalcoholic fatty liver disease. J Mol Endocrinol. 2013;51:167–179. doi: 10.1530/JME-13-0072. [DOI] [PubMed] [Google Scholar]
- 5.Vluggens A, Reddy JK. Nuclear receptors and transcription factors in the development of fatty liver disease. Curr Drug Metabolism. 2012;13:1422–1435. doi: 10.2174/138920012803762710. [DOI] [PubMed] [Google Scholar]
- 6.Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sergeant C, Contos MJ, Sanyal AJ. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 7.Jensen-Urstad APL, emenkovich CF. Fatty acid synthase and liver triglyceride metabolism: housekeeper or messenger? Biochim Biophys Acta. 2012;1821:747–753. doi: 10.1016/j.bbalip.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quiroga AD, Lehner R. Liver triacylglycerol lipases. Biochim Biophys Acta. 2012;1821:762–769. doi: 10.1016/j.bbalip.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Puri P, Wiest MM, Cheung O, Mirshahi F, Sargeant C, Min H-K, Contos MJ, Sterling RK, Fuchs M, Zhou H, Watkins SM, Sanyal AJ. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology. 2009;50:1827–1838. doi: 10.1002/hep.23229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng X-E, Wu YL, Lu Q-Q, Ju Z-J, Lin X. Two genetic variants in FABP1 and susceptibility to non-alcoholic fatty liver disease in a Chinese population. Gene. 2012;500:54–58. doi: 10.1016/j.gene.2012.03.050. [DOI] [PubMed] [Google Scholar]
- 11.McIntosh AL, Huang H, Storey SM, Landrock K, Landrock D, Petrescu AD, Gupta S, Atshaves BP, Kier AB, Schroeder F. Human FABP1 T94A variant impacts fatty acid metabolism and PPARa activation in cultured human female hepatocytes. Am J Physiol Gastrointest and Liver Phys. 2014;307:G164–G176. doi: 10.1152/ajpgi.00369.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldstein AE, Werneburg NW, Canbay A, et al. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–194. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 13.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis summary of an AASLD single topic conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 14.Gallegos AM, Atshaves BP, Storey SM, Starodub O, Petrescu AD, Huang H, McIntosh A, Martin G, Chao H, Kier AB, Schroeder F. Gene structure, intracellular localization, and functional roles of sterol carrier protein-2. Prog Lipid Res. 2001;40:498–563. doi: 10.1016/s0163-7827(01)00015-7. [DOI] [PubMed] [Google Scholar]
- 15.Colles SM, Woodford JK, Moncecchi D, Myers-Payne SC, McLean LR, Billheimer JT, Schroeder F. Cholesterol interactions with recombinant human sterol carrier protein-2. Lipids. 1995;30:795–804. doi: 10.1007/BF02533954. [DOI] [PubMed] [Google Scholar]
- 16.Martin GG, Atshaves BP, Huang H, McIntosh AL, Williams BW, Pai P-J, Russell DH, Kier AB, Schroeder F. Hepatic phenotype of liver fatty acid binding protein (L-FABP) gene ablated mice. Am J Physiol. 2009;297:G1053–G1065. doi: 10.1152/ajpgi.00116.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin GG, Atshaves BP, Landrock KK, Landrock D, Storey SM, Howles PN, Kier AB, Schroeder F. Ablating L-FABP in SCP-2/SCP-x null mice impairs bile acid metabolism and biliary HDL-cholesterol secretion. Am J Physiol Gastrointest and Liver Phys. 2014;307:G1130–G1143. doi: 10.1152/ajpgi.00209.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang H, McIntosh AL, Martin GG, Landrock KK, Landrock D, Storey SM, Gupta S, Atshaves BP, Kier AB, Schroeder F. Human L-FABP T94A variant enhances cholesterol uptake. Biochim Biophys Acta. 2015;1851:946–955. doi: 10.1016/j.bbalip.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frolov A, Cho TH, Billheimer JT, Schroeder F. Sterol carrier protein-2, a new fatty acyl coenzyme A-binding protein. J Biol Chem. 1996;271:31878–31884. doi: 10.1074/jbc.271.50.31878. [DOI] [PubMed] [Google Scholar]
- 20.Hubbell T, Behnke WD, Woodford JK, Schroeder F. Recombinant liver fatty acid binding protein interactions with fatty Acyl-Coenzyme A. Biochemistry. 1994;33:3327–3334. doi: 10.1021/bi00177a025. [DOI] [PubMed] [Google Scholar]
- 21.Frolov A, Cho TH, Murphy EJ, Schroeder F. Isoforms of rat liver fatty acid binding protein differ in structure and affinity for fatty acids and fatty acyl CoAs. Biochemistry. 1997;36:6545–6555. doi: 10.1021/bi970205t. [DOI] [PubMed] [Google Scholar]
- 22.Huang H, McIntosh AL, Martin GG, Landrock K, Landrock D, Gupta S, Atshaves BP, Kier AB, Schroeder F. Structural and functional interaction of fatty acids with human liver fatty acid binding protein (L-FABP) T94A variant. FEBS J. 2014;281:2266–2283. doi: 10.1111/febs.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gavey KL, Noland BJ, Scallen TJ. The participation of sterol carrier protein2 in the conversion of cholesterol to cholesterol ester by rat liver microsomes. J Biol Chem. 1981;256:2993–2999. [PubMed] [Google Scholar]
- 24.Nemecz G, Schroeder F. Selective binding of cholesterol by recombinant fatty acid-binding proteins. J Biol Chem. 1991;266:17180–17186. [PubMed] [Google Scholar]
- 25.Chao H, Billheimer JT, Kier AB, Schroeder F. Microsomal long chain fatty acyl CoA transacylation: differential effect of SCP-2. Biochim Biophys Acta. 1999;1439:371–383. doi: 10.1016/s1388-1981(99)00109-2. [DOI] [PubMed] [Google Scholar]
- 26.Chao H, Zhou M, McIntosh A, Schroeder F, Kier AB. Acyl CoA binding protein and cholesterol differentially alter fatty acyl CoA utilization by microsomal acyl CoA: cholesterol transferase. J Lipid Res. 2003;44:72–83. doi: 10.1194/jlr.m200191-jlr200. [DOI] [PubMed] [Google Scholar]
- 27.Murphy EJ, Schroeder F. Sterol carrier protein-2 mediated cholesterol esterification in transfected L-cell fibroblasts. Biochimica et Biophysica Acta. 1997;1345:283–292. doi: 10.1016/s0005-2760(97)00003-9. [DOI] [PubMed] [Google Scholar]
- 28.Jefferson JR, Slotte JP, Nemecz G, Pastuszyn A, Scallen TJ, Schroeder F. Intracellular sterol distribution in transfected mouse L-cell fibroblasts expressing rat liver fatty acid binding protein. J Biol Chem. 1991;266:5486–5496. [PubMed] [Google Scholar]
- 29.Bordewick U, Heese M, Borchers T, Robenek H, Spener F. Compartmentation of hepatic fatty-acid-binding protein in liver cells and its effect on microsomal phosphatidic acid biosynthesis. Biol Chem Hoppe-Seyler. 1989;370:229–238. doi: 10.1515/bchm3.1989.370.1.229. [DOI] [PubMed] [Google Scholar]
- 30.Jolly CA, Hubbell T, Behnke WD, Schroeder F. Fatty acid binding protein: Stimulation of microsomal phosphatidic acid formation. Arch Biochem Biophys. 1997;341:112–121. doi: 10.1006/abbi.1997.9957. [DOI] [PubMed] [Google Scholar]
- 31.Jolly CA, Murphy EJ, Schroeder F. Differential influence of rat liver fatty acid binding protein isoforms on phospholipid fatty acid composition: phosphatidic acid biosynthesis and phospholipid fatty acid remodeling. Biochim Biophys Acta. 1998;1390:258–268. doi: 10.1016/s0005-2760(97)00186-0. [DOI] [PubMed] [Google Scholar]
- 32.Hostetler HA, Lupas D, Tan Y, Dai J, Kelzer MS, Martin GG, Woldegiorgis G, Kier AB, Schroeder F. Acyl-CoA binding proteins interact with the acyl-CoA binding domain of mitochondrial carnitine palmitoyltransferase I. Mol Cell Biochem. 2011;355:135–148. doi: 10.1007/s11010-011-0847-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawata S, Imai Y, Inada M, Inui M, Kakimoto H, Fukuda K, Maeda Y, Tarui S. Modulation of cholesterol 7-a hydroxylase activity by nsLTP in human liver - possibe altered regulation of its cytosolic level in patients with gallstones. Clin Chim Acta. 1991;197:201–208. doi: 10.1016/0009-8981(91)90140-8. [DOI] [PubMed] [Google Scholar]
- 34.Seedorf U, Brysch P, Engel T, Schrage K, Assmann G. Sterol carrier protein X is peroxisomal 3-oxoacyl coenzyme A thiolase with intrinsic sterol carrier and lipid transfer activity. J Biol Chem. 1994;269:21277–21283. [PubMed] [Google Scholar]
- 35.Wanders RJA, Denis S, Wouters F, Wirtz KWA, Seedorf U. Sterol carrier Protein X (SCPx) is a peroxisomal branched-chain b-ketothiolase specifically reacting with 3-Oxo-pristanoyl-CoA: A new, unique role for SCPx in branched-chain fatty acid metabolism in peroxisomes. Biochem Biophys Res Commun. 1997;236:565–569. doi: 10.1006/bbrc.1997.7007. [DOI] [PubMed] [Google Scholar]
- 36.Anstee QM, Daly AK, Day CP. Genetic modifiers of non-alcoholic fatty liver disease progression. Biochim Biophys Acta. 2011;1812:1557–1566. doi: 10.1016/j.bbadis.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Jolly CA, Hubbell T, Behnke WD, Schroeder F. Fatty acid binding protein: stimulation of microsomal phosphatidic acid formation. Arch Biochem Biophys. 1997;341:112–121. doi: 10.1006/abbi.1997.9957. [DOI] [PubMed] [Google Scholar]
- 38.Schroeder F, Jolly CA, Cho TH, Frolov AA. Fatty acid binding protein isoforms: structure and function. Chem Phys Lipids. 1998;92:1–25. doi: 10.1016/s0009-3084(98)00003-6. [DOI] [PubMed] [Google Scholar]
- 39.Gossett RE, Edmondson RD, Jolly CA, Cho TH, Russell DH, Knudsen J, Kier AB, Schroeder F. Structure and function of normal and transformed murine acyl CoA binding proteins. Arch Biochem Biophys. 1998;350:201–213. doi: 10.1006/abbi.1997.0521. [DOI] [PubMed] [Google Scholar]
- 40.Mishkin S, Turcotte R. Stimulation of monoacylglycerophosphate formation by Z protein. Biochem Biophys Res Commun. 1974;60:376–381. doi: 10.1016/0006-291x(74)90215-0. [DOI] [PubMed] [Google Scholar]
- 41.Burnett DA, Lysenko N, Manning JA, Ockner RK. Utilization of long chain fatty acids by rat liver: studies of the role of fatty acid binding protein. Gastroenterology. 1979;77:241–249. [PubMed] [Google Scholar]
- 42.Jamdar SC. Hepatic lipid metabolism: effect of spermine, albumin, and Z protein on microsomal lipid formation. Arch Biochem Biophys. 1979;195:81–94. doi: 10.1016/0003-9861(79)90329-1. [DOI] [PubMed] [Google Scholar]
- 43.Yang SY, He XY, Schulz H. Fatty acid oxidation in rat brain is limited by the low activity of 3-ketoacyl-coenzyme A thiolase. J Biol Chem. 1987;262:13027–13032. [PubMed] [Google Scholar]
- 44.Higuchi N, Kato M, Tanaka M, et al. Effects of insulin resistance and hepatic lipid accumulation on hepatic mRNA expression levels of apoB, MTP, and L-FABP in non-alcoholic fatty liver disease. Exp and Ther Med. 2011;2:1077–1081. doi: 10.3892/etm.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charlton M, Viker K, Krishnan A, Sanderson S, Veldt B, Kaalsbeek AJ, Kendrick M, Thompson G, Que F, Swain J, Sarr M. Differential expression of lumican and fatty acid binding protein-1: new insights into the histologic spectrum of nonalcoholic fatty liver disease. Hepatology. 2009;49:1375–1384. doi: 10.1002/hep.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baumgardner JN, Shankar K, Hennings L, Badger TM, Ronis MJJ. A new model for nonalcoholic steatohepatitis in the rat utilizing total enteral nutrition to overfeed a high-polyunsaturated fat diet. Am J Physiol Gastrointest and Liver Phys. 2007;294:G27–G38. doi: 10.1152/ajpgi.00296.2007. [DOI] [PubMed] [Google Scholar]
- 47.Newberry EP, Xie Y, Kennedy S, Buhman KK, Luo J, Gross RW, Davidson NO. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid binding protein gene. J Biol Chem. 2003;278:51664–51672. doi: 10.1074/jbc.M309377200. [DOI] [PubMed] [Google Scholar]
- 48.Newberry EP, Kennedy SM, Xie Y, Sternard BT, Luo J, Davidson NO. Diet-induced obesity and hepatic steatosis in L-FABP-/- mice is abrogated with SF, but not PUFA, feeding and attenuated after cholesterol supplementation. Am J Physiol Gastrointest and Liver Phys. 2008;294:G307–G314. doi: 10.1152/ajpgi.00377.2007. [DOI] [PubMed] [Google Scholar]
- 49.Newberry EP, Kennedy SM, Xie Y, Luo J, Stanley SE, Semenkovich CF, Crooke RM, Graham MJ, Davidson NO. Altered hepatic triglyceride content after partial hepatectomy without impaired liver regeneration in multiple muring genetic models. Hepatology. 2008;48:1097–1105. doi: 10.1002/hep.22473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie Y, Newberry EP, Kennedy SM, Luo J, Davidson NO. Increased susceptibility to diet-induced gallstones in liver fatty acid binding protein knockout mice. J Lipid Res. 2009;50:977–987. doi: 10.1194/jlr.M800645-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen A, Tang Y, Davis V, Hsu F-F, Kennedy SM, Song H, Turk J, Brunt EM, Newberry EP, Davidson NO. L-FABP modulates murine stellate cell activation and diet induced nonalcoholic fatty liver disease. Hepatology. 2013;57:2202–2212. doi: 10.1002/hep.26318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guzman C, Benet M, Pisonero-Vaquero S, Moya M, Garcia-Mediavilla MV, Martinez-Chantar ML, Gonzalez-Gallego J, Castell JV, Sanchez-Campos S, Jover R. The human liver fatty acid binding protein (FABP1) gene is activated by FOXA1 and PPARa; and repressed by C/EBPa: implicaiton in FABP1 down-regulation in nonalcoholic liver disease. Biochim Biophys Acta. 2013;1831:803–818. doi: 10.1016/j.bbalip.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 53.Rajaraman G, Wang GQ, Yan J, Jiang P, Gong Y, Burczynski FJ. Role of cytosolic liver fatty acid binding protein in hepatocellular oxidative stress: effect of dexamethasone and clofibrate treatment. Mol Cell Biochem. 2007;295:27–34. doi: 10.1007/s11010-006-9268-6. [DOI] [PubMed] [Google Scholar]
- 54.Wang G, Gong Y, Anderson J, Sun D, Minuk G, Robertes MS, Burczynski FJ. Antioxidative function of L-FABP in L-FABP stably transfected Chang liver cells. Hepatology. 2005;42:871–879. doi: 10.1002/hep.20857. [DOI] [PubMed] [Google Scholar]
- 55.Wang G, Shen H, Rajaraman G, Roberts MS, Gong Y, Jiang P, Burczynski FJ. Expression and antioxidant function of liver fatty acid binding protein in normal and bile-duct ligated rats. Eur J Pharm. 2007;560:61–68. doi: 10.1016/j.ejphar.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 56.Yan J, Gong Y, She Y-M, Wang G, Roberts MS, Burczynski FJ. Molecular mechanism of recombinant liver fatty acid binding protein’s antioxidant activity. J Lipid Res. 2009;50:2445–2454. doi: 10.1194/jlr.M900177-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan J, Gong Y, She YM, Wang G, Robertes MS, Burczynski FJ. Molecular mechanism of recombinant L-FABP’s antioxidant activity. J Lipid Res. 2010;50:2445–2454. doi: 10.1194/jlr.M900177-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smathers RL, Fritz KS, Galligan JJ, Shearn CT, Reigan P, Marks MJ, Petersen DR. Characterizationof 4-HNE modified L-FABP reveals alterations in structural and functional dynamics. PPAR Research. 2012;7:e38459. doi: 10.1371/journal.pone.0038459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fan W, Chen K, Zheng G, Wang W, Teng A, Liu A, Ming D, Yan P. Role of liver fatty acid binding protein in hepatocellular injury: Effect of CrPic treatment. J Inorganic Biochem. 2013;124:46–53. doi: 10.1016/j.jinorgbio.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 60.Robitaille J, Brouillette C, Lemieux S, Perusse L, Gaudet D, Vohl M-C. Plasma concentrations of apolipoprotein B are modulated by a gene-diet interaction effect between the L-FABP T94A polymorphism and dietary fat intake in French-Canadian men. Mol Gen and Metab. 2004;82:296–303. doi: 10.1016/j.ymgme.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 61.Fisher E, Weikert C, Klapper M, Lindner I, Mohlig M, Spranger J, Boeing H, Schrezenmeir J, Doring F. L-FABP T94A is associated with fasting triglycerides and LDL-cholesterol in women. Mol Gen and Metab. 2007;91:278–284. doi: 10.1016/j.ymgme.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 62.Weikert MO, Loeffelholz Cv, Roden M, Chandramouli V, Brehm A, Nowotny P, Osterhoff MA, Isken F, Spranger J, Landau BR, Pfeiffer A, Mohlig M. A Thr94Ala mutation in human liver fatty acid binding protein contributes to reduced hepatic glycogenolysis and blunted elevation of plasma glucsoe levels in lipid-exposed subjects. Am J Physiol Endocrinol Metab. 2007;293:E1078–E1084. doi: 10.1152/ajpendo.00337.2007. [DOI] [PubMed] [Google Scholar]
- 63.Yamada Y, Kato K, Oguri M, Yoshida T, Yokoi K, Watanabe S, Metoki N, Yoshida H, Satoh K, Ichihara S, Aoyagi Y, Yasunaga A, Park H, Tanaka M, Nozawa Y. Association of genetic variants with atherothrombotic cerebral infarction in Japanese individuals with metabolic syndrome. Int J Mol Med. 2008;21:801–808. [PubMed] [Google Scholar]
- 64.Bu L, Salto LM, De Leon KJ, De Leon M. Polymorphisms in fatty acid binding protein 5 show association with type 2 diabetes. Diabetes Res Clin Prac. 2011;92:82–91. doi: 10.1016/j.diabres.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mansego ML, Martinez F, Martinez-Larrad MT, Zabena C, Rojo G, Morcillo S, Soriguer F, Martin-Escudero JC, Serrano-Rios M, Redon J, Chaves FJ. Common variants of the liver fatty acid binding protein gene influence the risk of Type 2 Diabetes and insulin resistance in Spanish population. PLoS ONE. 2012;7:e31853. doi: 10.1371/journal.pone.0031853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fuchs M, Hafer A, Muench C, Kannenberg F, Teichmann S, Scheibner J, Stange EF, Seedorf U. Disruption of the sterol carrier protein 2 gene in mice impairs biliary lipid and hepatic cholesterol metabolism. J Biol Chem. 2001;276:48058–48065. doi: 10.1074/jbc.M106732200. [DOI] [PubMed] [Google Scholar]
- 67.Seedorf U, Raabe M, Ellinghaus P, Kannenberg F, Fobker M, Engel T, Denis S, Wouters F, Wirtz KWA, Wanders RJA, Maeda N, Assmann G. Defective peroxisomal catabolism of branched fatty acyl coenzyme A in mice lacking the sterol carrier protein-2/sterol carrier protein-x gene function. Genes and Development. 1998;12:1189–1201. doi: 10.1101/gad.12.8.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Atshaves BP, McIntosh AL, Landrock D, Payne HR, Mackie J, Maeda N, Ball JM, Schroeder F, Kier AB. Effect of SCP-x gene ablation on branched-chain fatty acid metabolism. Am J Physiol. 2007;292:939–951. doi: 10.1152/ajpgi.00308.2006. [DOI] [PubMed] [Google Scholar]
- 69.Atshaves BP, McIntosh AL, Martin GG, Landrock D, Payne HR, Bhuvanendran S, Landrock K, Lyuksyutova OI, Johnson JD, Macfarlane RD, Kier AB, Schroeder F. Overexpression of sterol carrier protein-2 differentially alters hepatic cholesterol accumulation in cholesterol-fed mice. J Lipid Res. 2009;50:1429–1447. doi: 10.1194/jlr.M900020-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Atshaves BP, Petrescu A, Starodub O, Roths J, Kier AB, Schroeder F. Expression and Intracellular Processing of the 58 kDa Sterol Carrier Protein 2/3-Oxoacyl-CoA Thiolase in Transfected Mouse L-cell Fibroblasts. J Lipid Res. 1999;40:610–622. [PubMed] [Google Scholar]
- 71.Jolly CA, Wilton DA, Schroeder F. Microsomal fatty acyl CoA transacylation and hydrolysis: fatty acyl CoA species dependent modulation by liver fatty acyl CoA binding proteins. Biochim Biophys Acta. 2000;1483:185–197. doi: 10.1016/s1388-1981(99)00170-5. [DOI] [PubMed] [Google Scholar]
- 72.Martin GG, Danneberg H, Kumar LS, Atshaves BP, Erol E, Bader M, Schroeder F, Binas B. Decreased liver fatty acid binding capacity and altered liver lipid distribution in mice lacking the liver fatty acid binding protein (L-FABP) gene. J Biol Chem. 2003;278:21429–21438. doi: 10.1074/jbc.M300287200. [DOI] [PubMed] [Google Scholar]
- 73.Atshaves BP, McIntosh AL, Payne HR, Gallegos AM, Landrock K, Maeda N, Kier AB, Schroeder F. Sterol carrier protein-2/sterol carrier protein-x gene ablation alters lipid raft domains in primary cultured mouse hepatocytes. J Lipid Res. 2007;48:2193–2211. doi: 10.1194/jlr.M700102-JLR200. [DOI] [PubMed] [Google Scholar]
- 74.Storey SM, Atshaves BP, McIntosh AL, Landrock KK, Martin GG, Huang H, Johnson JD, Macfarlane RD, Kier AB, Schroeder F. Effect of sterol carrier protein-2 gene ablation on HDL-mediated cholesterol efflux from primary cultured mouse hepatocytes. Am J Physiol. 2010;299:244–254. doi: 10.1152/ajpgi.00446.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thigpen JE, Setchell KD, Ahlmark KB, Kocklear J, Spahr T, Caviness GF, Goelz MF, Haseman JK, Newbold RR, Forsythe DB. Phytoestrogen content of purified, open- and closed-formula laboratory animal diets. Lab An Science. 1999;49:530–536. [PubMed] [Google Scholar]
- 76.Thigpen JE, Setchell KD, Goelz MF, Forsythe DB. The phytoestrogen content of rodent diets. Envron Health Persp. 1999;107:A182–A183. doi: 10.1289/ehp.107-1566530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adida A, Spener F. Intracellular lipid binding proteins and nuclear receptors inolved in branched-chain fatty acid signaling. Prost Leukot Essen Fatty Acids. 2002;67:91–98. doi: 10.1054/plef.2002.0404. [DOI] [PubMed] [Google Scholar]
- 78.Ellinghaus P, Wolfrum C, Assmann G, Spener F, Seedorf U. Phytanic acid activates the peroxisome proliferator-activated receptor alpha (PPARalpha) in sterol carrier protein-2-/sterol carrier protein x-deficient mice. J Biol Chem. 1999;274:2766–2772. doi: 10.1074/jbc.274.5.2766. [DOI] [PubMed] [Google Scholar]
- 79.Hanhoff T, Benjamin S, Borchers T, Spener F. Branched-chain fatty acids as activators of peroxisome proliferators. Eur J Lip Sci Technol. 2005;107:716–729. [Google Scholar]
- 80.Wolfrum C, Ellinghaus P, Fobker M, Seedorf U, Assmann G, Borchers T, Spener F. Phytanic acid is ligand and transcriptional activator of murine liver fatty acid binding protein. J Lipid Res. 1999;40:708–714. [PubMed] [Google Scholar]
- 81.Atshaves BP, Payne HR, McIntosh AL, Tichy SE, Russell D, Kier AB, Schroeder F. Sexually dimorphic metabolism of branched chain lipids in C57BL/6J mice. J Lipid Res. 2004;45:812–830. doi: 10.1194/jlr.M300408-JLR200. [DOI] [PubMed] [Google Scholar]
- 82.Atshaves BP, Storey S, McIntosh AL, Petrescu AD, Lyuksyutova OI, Greenberg AS, Schroeder F. Sterol carrier protein-2 expression modulates protein and lipid composition of lipid droplets. J Biol Chem. 2001;276:25324–25335. doi: 10.1074/jbc.M100560200. [DOI] [PubMed] [Google Scholar]
- 83.Marzo A, Ghirardi P, Sardini D, Meroni G. Simplified measurement of monoglycerides, diglycerides, triglycerides, and free fatty acids in biological samples. Clin Chem. 1971;17:145–147. [PubMed] [Google Scholar]
- 84.Murphy EJ, Stiles T, Schroeder F. Sterol carrier protein-2 expression alters phospholipid content and fatty acid composition in L-cell fibroblasts. J Lipid Res. 2000;41:788–796. [PubMed] [Google Scholar]
- 85.Atshaves BP, McIntosh AL, Lyuksyutova OI, Zipfel WR, Webb WW, Schroeder F. Liver fatty acid binding protein gene ablation inhibits branched-chain fatty acid metabolism in cultured primary hepatocytes. J Biol Chem. 2004;279:30954–30965. doi: 10.1074/jbc.M313571200. [DOI] [PubMed] [Google Scholar]
- 86.McIntosh AL, Atshaves BP, Hostetler HA, Huang H, Davis J, Lyuksyutova OI, Landrock D, Kier AB, Schroeder F. Liver type fatty acid binding protein (L-FABP) gene ablation reduces nuclear ligand distribution and peroxisome proliferator activated receptor-alpha activity in cultured primary hepatocytes. Arch Biochem Biophys. 2009;485:160–173. doi: 10.1016/j.abb.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Starodub O, Jolly CA, Atshaves BP, Roths JB, Murphy EJ, Kier AB, Schroeder F. Sterol carrier protein-2 immunolocalization in endoplasmic reticulum and stimulation of phospholipid formation. Am J Physiol. 2000;279:C1259–C1269. doi: 10.1152/ajpcell.2000.279.4.C1259. [DOI] [PubMed] [Google Scholar]
- 88.Moncecchi DM, Nemecz G, Schroeder F, Scallen TJ. The participation of sterol carrier protein-2 (SCP-2) in cholesterol metabolism. In: Patterson GW, Nes WD, editors. Physiology and Biochemistry of Sterols. American Oil Chemists’ Society Press; Champaign, IL: 1991. pp. 1–27. [Google Scholar]
- 89.Sato R, Miyamoto W, Inoue J, Terada T, Imanaka T, Maeda M. SREBP negatively regulates MTTP gene transcription. J Biol Chem. 1999;274:24714–24720. doi: 10.1074/jbc.274.35.24714. [DOI] [PubMed] [Google Scholar]
- 90.Hinsdale ME, Sullivan PM, Mezdour H, Maeda N. Apo B-48 and Apo B-100 differentially influence the expression of type-III hyperlipoproteinemia in apoE-2 mice. Diabetes. 2002;43:1520–1528. doi: 10.1194/jlr.m200103-jlr200. [DOI] [PubMed] [Google Scholar]
- 91.Wiegman CH, Bandsma HJ, Ouwens M, van der Sluijs FH, Havinga R, Boes T, Reijngoud D-J, Romjin JA, Kuipers F. Hepatic VLDL production in ob/ob mice is not stimulated by massive de novo lipogenesis but is less sensitive to suppressive effects of insulin. Diabetes. 2003;52:1081–1089. doi: 10.2337/diabetes.52.5.1081. [DOI] [PubMed] [Google Scholar]
- 92.Ferdinandusse S, Kostopoulos P, Denis S, Rusch R, Overmars H, Dillman U, Reith W, Haas D, Wanders RJA, Duran M, Marziniak M. Mutations in the gene encoding peroxisomal sterol carrier protein-x (SCP-x) cause leukencephalopathy with dystonia and motor neuropathy. Am J Hum Genet. 2006;78:1046–1052. doi: 10.1086/503921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Atshaves BP, Martin GG, Hostetler HA, McIntosh AL, Kier AB, Schroeder F. Liver fatty acid binding protein (L-FABP) and Dietary Obesity. Journal of Nutritional Biochemisty. 2010;21:1015–1032. doi: 10.1016/j.jnutbio.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brouillette C, Bose Y, Perusse L, Gaudet D, Vohl M-C. Effect of liver fatty acid binding protein (FABP) T94A missense mutation on plasma lipoprotein responsiveness to treatment with fenofibrate. J Hum Gen. 2004;49:424–432. doi: 10.1007/s10038-004-0171-2. [DOI] [PubMed] [Google Scholar]
- 95.Tian Y, Li H, Wang S, Yan J, hen Z, i Z, eng H, Zhou H, Ouyang D-S. Association of L-FABP T94A, MTP I128T polymorphisms and hyperlipidemia in Chinese subjects. Lipids. 2015;50:275–282. doi: 10.1007/s11745-015-3990-3. [DOI] [PubMed] [Google Scholar]
- 96.Huang H, McIntosh AL, Martin GG, Landrock KK, Landrock D, Storey SM, Gupta S, Atshaves BP, Kier AB, Schroeder F. Human L-FABP T94A variant enhances cholesterol uptake. Biochim Biophys Acta. 2015;1851:946–955. doi: 10.1016/j.bbalip.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Martin GG, McIntosh AL, Huang H, Gupta S, Atshaves BP, Kier AB, Schroeder F. Human liver fatty acid binding protein (L-FABP) T94A variant alters structure, stability, and interaction with fibrates. Biochemistry. 2013;52:9347–9357. doi: 10.1021/bi401014k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Frolov AA, Schroeder F. Acyl coenzyme A binding protein: conformational sensitivity to long chain fatty acyl-CoA. J Biol Chem. 1998;273:11049–11055. doi: 10.1074/jbc.273.18.11049. [DOI] [PubMed] [Google Scholar]
- 99.Chao H, Schroeder F, Kier AB. Acyl CoA binding protein (ACBP) In: Creighton TE, editor. Wiley Encyclopedia of Molecular Medicine. J. Wiley & Sons, Inc.; New York, N.Y: 2002. pp. 44–47. [Google Scholar]
- 100.Chao H, Martin G, Russell WK, Waghela SD, Russell DH, Schroeder F, Kier AB. Membrane charge and curvature determine interaction with acyl CoA binding protein (ACBP) and fatty acyl CoA targeting. Biochemistry. 2002;41:10540–10553. doi: 10.1021/bi0259498. [DOI] [PubMed] [Google Scholar]
- 101.Martin GG, Hostetler HA, McIntosh AL, Tichy SE, Williams BJ, Russell DH, Berg JM, Spencer TA, Ball JA, Kier AB, Schroeder F. Structure and function of the sterol carrier protein-2 (SCP-2) N-terminal pre-sequence. Biochem. 2008;47:5915–5934. doi: 10.1021/bi800251e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Richieri GV, Ogata RT, Kleinfeld AM. Equilibrium constants for the binding of fatty acids with fatty acid binding proteins from adipocyte, intestine, heart, and liver measured with the fluorescent probe ADIFAB. J Biol Chem. 1994;269:23918–23930. [PubMed] [Google Scholar]
- 103.Rolf B, Oudenampsen-Kruger E, Borchers T, Faergeman NJ, Knudsen J, Lezius A, Spener F. Analysis of the ligand binding properties of recombinant bovine liver-type fatty acid binding protein. Biochim Biophys Acta. 1995;1259:245–253. doi: 10.1016/0005-2760(95)00170-0. [DOI] [PubMed] [Google Scholar]
- 104.Kannan L, Knudsen J, Jolly CA. Aging and acyl CoA binding protein alter mitochondrial glycerol-3-phosphate acyltransferase activity. Biochim Biophys Acta. 2003;1631:12–16. doi: 10.1016/s1388-1981(02)00367-0. [DOI] [PubMed] [Google Scholar]
- 105.Stolowich NJ, Frolov A, Petrescu AD, Scott AI, Billheimer JT, Schroeder F. Holo-sterol carrier protein-2: 13C-NMR investigation of cholesterol and fatty acid binding sites. J Biol Chem. 1999;274:35425–35433. doi: 10.1074/jbc.274.50.35425. [DOI] [PubMed] [Google Scholar]
- 106.Stolowich NJ, Petrescu AD, Huang H, Martin G, Scott AI, Schroeder F. Sterol carrier protein-2: structure reveals function. Cell Mol Life Sci. 2002;59:193–212. doi: 10.1007/s00018-002-8416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stolowich NJ, Frolov A, Atshaves BP, Murphy E, Jolly CA, Billheimer JT, Scott AI, Schroeder F. The sterol carrier protein-2 fatty acid binding site: an NMR, Circular Dichroic, and Fluorescence spectroscopic determination. Biochemistry. 1997;36:1719–1729. doi: 10.1021/bi962317a. [DOI] [PubMed] [Google Scholar]
- 108.Schroeder F, Frolov A, Schoer J, Gallegos A, Atshaves BP, Stolowich NJ, Scott AI, Kier AB. Intracellular sterol binding proteins, cholesterol transport and membrane domains. In: Chang TY, Freeman DA, editors. Intracellular Cholesterol Trafficking. Kluwer Academic Publishers; Boston: 1998. pp. 213–234. [Google Scholar]
- 109.Murphy EJ, Prows DR, Jefferson JR, Schroeder F. Liver fatty acid binding protein expression in transfected fibroblasts stimulates fatty acid uptake and metabolism. Biochim Biophys Acta. 1996;1301:191–198. doi: 10.1016/0005-2760(96)00024-0. [DOI] [PubMed] [Google Scholar]
- 110.Prows DR, Murphy EJ, Schroeder F. Intestinal and liver fatty acid binding proteins differentially affect fatty acid uptake and esterification in L-Cells. Lipids. 1995;30:907–910. doi: 10.1007/BF02537481. [DOI] [PubMed] [Google Scholar]
- 111.Murphy EJ. L-FABP and I-FABP expression increase NBD-stearate uptake and cytoplasmic diffusion in L-cells. Am J Physiol. 1998;275:G244–G249. doi: 10.1152/ajpgi.1998.275.2.G244. [DOI] [PubMed] [Google Scholar]


