Abstract
Gender differences in tryptophan (TRP) breakdown in obese individuals have been previously reported. This could be both contributory to, as well as a consequence of, gender differences in mood changes among obese people. To exclude the potential effect of depression on TRP breakdown and its levels in obesity, we replicated analyses in psychiatrically healthy individuals. In 1000 participants, plasma kynurenine (KYN), TRP, and the KYN/TRP ratio were compared between overweight/obese and normal-weight individuals using analysis of covariance, with adjustment for age and gender. Bivariate post hoc tests were also conducted. There were no significant relationships between KYN, TRP, or the KYN/TRP ratio and overall overweight/obese status. However, a significant gender by weight category interaction was identified for TRP only, with overweight/obese women having lower TRP than overweight/obese men (p = 0.02). No gender differences in TRP were found in non-obese participants. Our study in psychiatrically healthy individuals suggested that lower TRP levels in obese women were not secondary to depression, strengthening the possibility that TRP levels could mediate depression in vulnerable women. Thus experimental manipulations of TRP levels could be used to advance theoretical knowledge, prevention, and clinical control of depression in obese women.
Keywords: gender differences, obesity, tryptophan, women
Introduction
Obesity is a major medical problem, which has reached epidemic proportions in developed countries including the United States, affecting both adults and children [1,2]. Insidiously, obesity is related to sequelae such as hypertension, type 2 diabetes mellitus, cardiovascular and cerebrovascular ischemic disease, and metabolic syndrome [3]. In addition, obesity has been linked to depression in adults [4–6] and shame in adolescents [7]. The relationship between obesity and depression is likely bidirectional, with obesity contributing to depression and vice versa [8]. Both obesity [9–11] and depression [12,13] have also been linked with inflammation and activation of the immune system.
Following the onset of adolescence, the prevalence of depression is about 1.5–3 times higher in women than in men [14]. Obesity is considered to be a result of increased caloric intake (intake of all constituents of food, including carbohydrates, lipids, and proteins) and decreased physical activity. Increased protein intake is associated with the availability of amino acids such as the essential amino acid tryptophan (TRP), a substrate for the production of serotonin and melatonin, both of which are involved in the regulation of satiety and caloric intake. However, morbidly obese individuals have been found to have low, rather than high, TRP levels [15]. This finding may be explained by the conversion of TRP to kynurenine (KYN), with tryptophan 2,3-dioxygenase (TDO) and indolamine 2,3-dioxygenase (IDO) acting as the rate-limiting enzymes of this pathway [16]. Pro-inflammatory cytokines, potentially induced by inflammatory pathways, may result in IDO activation [17] while corticosteroids may activate TDO [18].
In a recent study, Mangge et al. [19] found that, in comparison to normal-weight controls, overweight and obese adults between 18 and 65 years of age had elevated levels of KYN and an elevated KYN/TRP ratio (a marker of IDO activation). The most markedly significant increase was noted in adults who had metabolic syndrome. They also found that overweight/obese women had significantly lower TRP levels than overweight/obese men. However, there was no significant difference in TRP levels between overweight/obese individuals and normal-weight individuals.
Carpenter et al. [20] showed that obese women had 37% higher odds of having had depression in the past year than women who were not obese. In contrast, obese men had 37% lower odds of having had depression in the past year than normal-weight men. Individuals with depression have lower plasma TRP levels [21] and, hence, lower central nervous system (CNS) serotonin levels [22,23], which are associated with depression [24]. Experimental TRP depletion studies confirm the depressogenic role of lowering TRP [25–31]. To further explore the proposed bidirectional link with depression and both obesity and inflammation, we examine, to our knowledge for the first time, the relationship between obesity, KYN, TRP, and the KYN/TRP ratio in patients free of history of depression or other common psychiatric conditions.
Materials and methods
Participants
Male and female adults over the age of 18 from Munich, Germany, were randomly selected from the Munich City registry and invited via mail to participate in the study. This is a secondary analysis. The primary investigation was focused on the genetics of schizophrenia, with recruitment started in 1998. Medical and psychiatric histories were obtained from responders via telephone, and those who screened negative became a part of the control branch of the case-control study and were invited to a clinic where they were administered the Structured Interview for DSM-IV (SCID I and II) to confirm the absence of primary lifetime psychiatric and personality disorders. These individuals were also identified as having a negative history of suicide attempts. History of neurological (neurological examination) and psychiatric disorders in first-degree relatives (Family History Assessment Module of the SCID) were also ruled out. Participants over the age of 60 years underwent a Mini Mental State Examination (MMSE) to screen for cognitive dysfunction. All participants gave written informed consent, and the study was conducted with approval from the local Ethics Committee of Ludwig Maximilians University in Germany, within the parameters of the ethical standards of the Declaration of Helsinki (1964).
The weight and height of the participants were obtained by self-report. For those that could not recall their height and weight, measurements were taken.
Plasma samples were obtained to measure KYN and TRP levels by high-performance liquid chromatography as described by Widner et al. [32]. An estimation of the TRP breakdown rate was provided by the calculated KYN/TRP ratio.
Statistical analysis
Self-reported or measured weight and height were used to calculate body mass indices (BMIs) and categorize participants as normal-weight (BMI ≤ 24.9) versus overweight/obese (25 ≤ BMI ≤ 29.9 for overweight and BMI ≥ 30 for obese). The means and standard deviations of KYN and TRP were calculated. Analysis of covariance was performed to determine the relationship between weight groups and KYN, TRP, and KYN/TRP, with adjustments for age and gender, to check for significant effects and interactions. If an interaction was significant, post hoc t-tests were performed. Criterion α was set at 0.05, two tailed.
Results
Demographics
Our sample consisted of 1000 healthy adults between the ages of 20 years (minimum) and 74 years (maximum) from Munich, Germany. There were 490 men (49%) and 510 women (51%) in the sample (χ2 = 0.40; df = 1; p = 0.53). Average age (mean±SD) was 53.6 (±15.8) years overall; for men, the mean age was 56.1 (±15.0) years and for women it was 51.1 (±16.1) years (t = 5.00; df = 998; p<0.0001).
Of the 1000 individuals in our sample, data on BMI were available for 997 individuals (489 men and 508 women). Mean BMI was 24.78 kg/m2 with a standard deviation of 3.87. Men had a mean (±SD) BMI of 25.38 (±3.20) kg/m2, whereas women had a mean BMI of 24.20 (±4.34) kg/m2 (t = 4.88, df = 995, p<0.0001). BMI was positively correlated with age (r = 0.27, p<0.0001). Overall, 575 participants (57.67%) had a BMI ≤ 24.9 (defined as normal weight); of these, nine participants had a BMI between 16.30 and 17.99 (underweight). Four hundred twenty-two participants (42.33%) had a BMI ≥ 25, thereby classified as overweight/obese (χ2 = 23.48, df = 1, p<0.0001); of the latter, 346 (34.70%) had a BMI between 25 and 29.9 (overweight) and 76 participants (7.64%) had a BMI ≥ 30 (obese).
Of 489 women, 252 (51.53%) were overweight/obese, and of 508 men, 170 (33.46%) were overweight/obese; of 422 overweight/obese participants, 59.72% were men and 40.28% were women (χ2 = 33.33, df = 1, p<0.0001). Obese status (BMI ≥ 30) was independent of gender (χ2 = 0.61, df = 1, p = 0.43). χ2 analysis found significant gender differences between normal-weight, overweight, and obese groups [χ2 = 41.65, df = 2, p<0.0001].
As expected, individuals who were overweight/obese were significantly older than individuals who were not Overweight/obese (57.96±13.33 vs. 50.27±16.64 years; t = −7.82, df = 995, p<0.0001). Similarly, obese (BMI ≥ 30) individuals were significantly older than those who were not obese (60.54±11.77 vs. 52.95±15.94 years; t = −4.06, df = 995, p<0.0001). There were significant differences in ages between the obese, overweight, and normal-weight adults (F(2,994) = 32, p<0.0001).
Kynurenine, tryptophan, and overweight/obese status
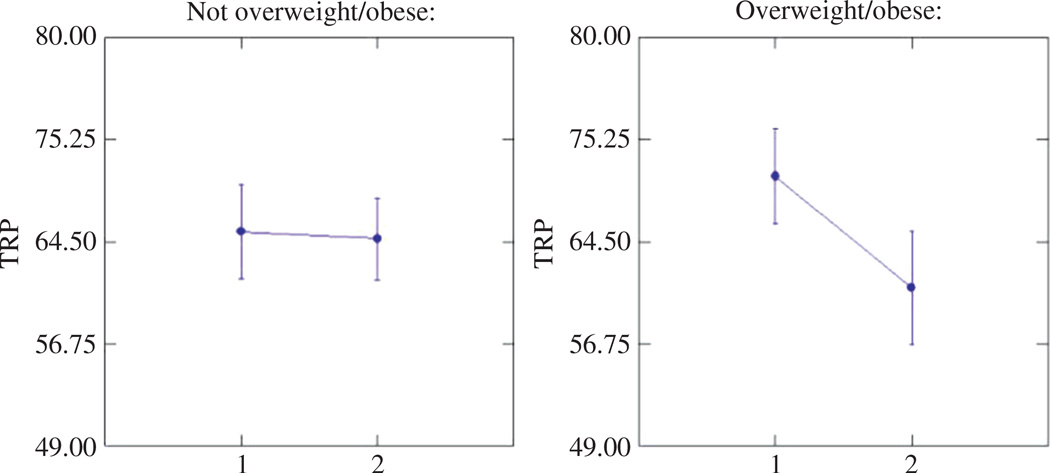
Data on KYN, TRP, and KYN/TRP were available for 976 subjects; of these, three subjects did not have data on BMI and weight status (overweight/obese or not). Therefore, data on 973 participants were used for the analysis of weight status and KYN, TRP, and KYN/TRP. Of the subjects excluded for lack of KYN and TRP data, 12 were overweight/ obese and two were obese. Mean (±SD) level for KYN was 2.39±1.21 µmol/L; for TRP, 65.43±28.2 µmol/L; and for KYN/TRP, 40.9±27.31. The relationship between KYN [F(1968) = 1.43, p = 0.23] and KYN/TRP [F(1968) = 1.15, p = 0.28] and overweight/obesity was not significant after adjustment for age and gender. Similarly, the relationship between TRP and overweight/obesity in the full sample was not significant [F(1,968) = 0.02, p = 0.90] after adjustment for age and gender, but there was a significant interaction (Figure 1) between gender and weight status [F(1,968) = 4.53, p = 0.03]. A post hoc Tukey test showed that there was a significant difference in TRP levels between overweight/obese women and overweight/obese men (p = 0.02), with overweight/obese women having lower levels of TRP. There was no significant difference in TRP between women and men who were not overweight/obese.
Figure 1.
Levels of tryptophan by gender and weight status (overweight/obese vs. not overweight/obese); analysis of covariance adjusted by age (1 male, 2 female).
Discussion
Our study is the first study conducted on the relationship between obesity and KYN, TRP, and KYN/TRP in healthy individuals who were screened for neurological and psychiatric illness, including mood, psychotic, and personality disorders. We found that, in contrast to previous reports, in psychiatrically healthy individuals there was no significant relationship between overweight/obese status and levels of KYN and KYN/TRP. Furthermore, as expected based on prior research, we found that overweight/ obese women had significantly lower levels of TRP than overweight/obese men.
A study by Mangge et al. [19] found a relationship between overweight/obese status and elevated levels of KYN and KYN/TRP, the latter likely associated with IDO (rather than with TDO) activation because higher KYN/TRP in obese individuals has been previously documented to be also associated with signs of immune activation [15]. In the study by Mangge et al. [19], participants were excluded if they were on medications or hormone replacement, and psychopathology, including personality disorders, was not screened for using a gold standard measure. The elevated KYN/TRP was considered to be the result of inflammation associated with obesity. It has been shown in previous studies that IDO activation and elevated levels of kynurenine pathway metabolites are associated with various psychiatric disorders, including depression [12,13] and schizophrenia (reviewed in Sublette and Postolache [33]). Contrasting our results with previous studies suggests that the elevation in KYN and KYN/TRP ratio associated with IDO or TDO activation may be the result of psychopathology or of unmeasured causes of psychopathology and not necessarily due to inflammation or cortisol elevation associated with obesity. Increased cortisol in overweight/obese individuals would also explain higher KYN/TRP and KYN with lower TRP.
Furthermore, we found lower levels of TRP in women who were overweight or obese in comparison to men who were overweight or obese. There was no significant difference in TRP between normal-weight men and women. This finding is not necessarily associated with obesity, as lower TRP (and KYN) levels in women as compared with men have been reported to be independent of obesity, potentially in association with higher rates of depression in women (e.g., Widner et al. [32])
Carpenter et al. [20] reported that obese women have 37% higher odds of having had depression over the past year compared to normal-weight women, whereas obese men have equally lower odds of having had depression over the past year compared to normal-weight men. Tryptophan depletion decreases CNS TRP availability and has been associated with depression [22,23,26–29], potentially moderated by certain serotonin transporter gene polymorphisms [30,31]. Furthermore, tryptophan depletion and decreased CNS serotonin availability have also been associated with impulsivity in both animal and human studies [34]. Impulsivity itself is associated with rapid discernment of high-calorie foods [35] and increased responsiveness to delectable food items [36]. By excluding patients with mood disorders, our study firmly establishes that the lower tryptophan in obese women is nota consequence of depression.
Also, our results suggest that, while decreased TRP may lead to depression in women, an additional predisposition for depression is necessary, as the obese women participating in this study had no history or symptoms of depression. It should also be noted that the individuals in our sample were, in a sense, “hypernormal” as they did not have a family history of depression, raising the possibility that they may have some genetic or environmental protective factors and possibly explaining why in our study obese women with low TRP did not have depression. It appears that lower TRP alone precipitates depression only in conditions of additional vulnerabilities, such as genetic or developmental factors.
Lower TRP could also be one reason for obesity development when it is accompanied with lower brain serotonin that leads to an increased intake of food rich in calories [37–39]. It should be noted that the findings of Mangge et al. [19] of elevated KYN/TRP would proscribe against TRP repletion in obese women, as it would result in increased KYN and its metabolite quinolinic acid in glial cells. Quinolinic acid, through its agonist action on N-methyl-d-aspartate receptors, is neurotoxic and could worsen preexisting depression and trigger syndromal depression in vulnerable individuals. Therefore, a trial of TRP repletion could only be justified for low TRP without elevation in KYN or KYN/TRP ratio, as we are now reporting in our study.
Limitations of our study include the self-reported weight and height of many participants and lack of measurement regarding certain confounders related to obesity, inflammation, and serotonin function, such as sleep (e.g., sleep apnea). Regarding self-reported weight, the majority of the evidence supports individuals’ ability to accurately self-report their weight [40,41]. Nonetheless, there is also evidence that women may under- or overreport their weight [42]. However, a particular strength of our study is the use of a structured psychiatric evaluation (using SCID I and II) to rule out psychopathology in participants, and the use of the Family History Assessment Module to rule out psychopathology in first-degree relatives of participants. We thus were able to minimize, if not exclude, the effect of depression and other mental illness on inflammation, IDO, KYN, and TRP.
Conclusion
Our finding, that overweight/obese women had lower TRP levels than overweight/obese men suggests that low TRP, thus low CNS serotonin synthesis, may contribute to either vulnerability to depression in obese women or vulnerability to obesity in depressed women. Excluding patients with mental illness leads us to conclude that low TRP itself is not a consequence of depression in obese women. The finding that low TRP in our sample of psychiatrically “super-healthy” obese women was not associated with depressed mood are consistent with previous findings that suggest that low TRP alone is not a sufficient cause of depression. Additional experimental paradigms or clinical trials using TRP depletion or supplementation could advance our understanding of molecular interactions between metabolic and affective dysregulation.
Acknowledgments
The AFSP Distinguished Investigator Award (PI Postolache, co-PI Rujescu). Dr. Postolache and Dr. Rujescu equally contributed and share senior authorship. The study was also funded in part by the Mid-Atlantic Nutrition Obesity Research Center Pilot NORC grant (PI Postolache), a subgrant of the parent grant P30 DK072488. Additional support for the writing of this manuscript was provided by the Rocky Mountain Mental Illness Research Education and Clinical Center (MIRECC, Denver, CO, USA) and by the Veterans Integrated Service Network 5, MIRECC (Baltimore, MD, USA). The views, opinions, and findings contained in this article belong to the authors and should not be construed as an official position of the NIH, American Foundation for Suicide Prevention, or Department of Veterans Affairs. We are grateful for the excellent administrative support from Mr. Francis Iyoriobhe and Ms. Winny Mwaura as well as the help given by Drs. Aamar Sleemi and Dipika Vaswani with database and project management. We are thankful to Mrs. Meghan Barnhart, Ms. Heidi Hand, and Dr. Mohyuddin for editorial support on the final stage of the manuscript.
Footnotes
Conflicts of interest: Dr. Sergio Rovner received speaking honoraria from Takeda Pharmaceuticals USA, Inc. (Deerfield, IL, USA) (maker of Contrave), and Vivus, Inc. (Mountain View, CA, USA) (maker of Qsymia). The other authors declare that they have no conflict of interest related to the publication of this paper.
Contributor Information
Uttam K. Raheja, Mood and Anxiety Program, Department of Psychiatry, University of Maryland School of Medicine, Baltimore, MD, USA; and Psychiatry Residency Training Program, Saint Elizabeth’s Hospital, Washington, DC, USA
Dietmar Fuchs, Division of Biological Chemistry, Biocenter, Innsbruck Medical University, Innrain 80, 6020 Innsbruck, Austria.
Ina Giegling, Department of Psychiatry, Martin-Luther-Universität Halle-Wittenberg, Halle, Germany.
Lisa A. Brenner, Rocky Mountain Mental Illness Research Education and Clinical Center (MIRECC), Denver, CO, USA; and Anschutz Medical Center, University of Colorado, Aurora, CO, USA
Sergio F. Rovner, Department of Family Medicine, Texas Tech University, El Paso, TX, USA; and Frontier Medical Group, El Paso, TX, USA
Iqra Mohyuddin, Mood and Anxiety Program, Department of Psychiatry, University of Maryland School of Medicine, Baltimore, MD, USA.
Daniel Weghuber, Department of Pediatrics, Paracelsus Medical University, Salzburg, Austria; and Obesity Research Unit, Paracelsus Medical University, Salzburg, Austria.
Harald Mangge, Research Unit on Lifestyle and Inflammation-Associated Risk Biomarkers, Clinical Institute for Medical and Chemical Laboratory Diagnosis, Medical University of Graz, Graz, Austria.
Dan Rujescu, Department of Psychiatry, Martin-Luther-Universität Halle-Wittenberg, Halle, Germany.
References
- 1.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes. 2006;1:11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among us adults, 1999–2010. J Am Med Assoc. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 3.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583–2589. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 4.Stunkard AJ, Faith MS, Allison KC. Depression and obesity. Biol Psychiatry. 2003;54:330–337. doi: 10.1016/s0006-3223(03)00608-5. [DOI] [PubMed] [Google Scholar]
- 5.Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 6.Preiss K, Brennan L, Clarke D. A systematic review of variables associated with the relationship between obesity and depression. Obes Rev. 2013;14:906–918. doi: 10.1111/obr.12052. [DOI] [PubMed] [Google Scholar]
- 7.Sjoberg RL, Nilsson KW, Leppert J. Obesity, shame, and depression in school-aged children: a population-based study. Pediatrics. 2005;116:e389–e392. doi: 10.1542/peds.2005-0170. [DOI] [PubMed] [Google Scholar]
- 8.Cizza G. Major depressive disorder is a risk factor for low bone mass, central obesity, and other medical conditions. Dialogues Clin Neurosci. 2011;13:73–87. doi: 10.31887/DCNS.2011.13.1/gcizza. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandacher G, Hoeller E, Fuchs D, Weiss HG. Chronic immune activation underlies morbid obesity: Is IDO a key player? Curr Drug Metab. 2007;8:289–295. doi: 10.2174/138920007780362590. [DOI] [PubMed] [Google Scholar]
- 10.Miller AA, Spencer SJ. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav Immun. 2014;42:10–21. doi: 10.1016/j.bbi.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Castanon N, Lasselin J, Capuron L. Neuropsychiatric comorbidity in obesity: role of inflammatory processes. Front Endocrinol (Lausanne) 2014;5:74. doi: 10.3389/fendo.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dantzer R, O’Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrino. 2011;36:426–436. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCR-R) J Am Med Assoc. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 15.Brandacher G, Winkler C, Aigner F, Schwelberger H, Schroecksnadel K, Margreiter R, et al. Bariatric surgery cannot prevent tryptophan depletion due to chronic immune activation in morbidly obese patients. Obes Surg. 2006;16:541–548. doi: 10.1381/096089206776945066. [DOI] [PubMed] [Google Scholar]
- 16.Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol. 2003;81:247–265. doi: 10.1046/j.1440-1711.2003.t01-1-01177.x. [DOI] [PubMed] [Google Scholar]
- 17.Kwidzinski E, Bunse J, Aktas O, Richter D, Mutlu L, Zipp F, et al. Indolamine 2,3-dioxygenase is expressed in the cns and down-regulates autoimmune inflammation. FASEB J. 2005;19:1347–1349. doi: 10.1096/fj.04-3228fje. [DOI] [PubMed] [Google Scholar]
- 18.Salter M, Pogson CI. The role of tryptophan 2,3-dioxygenase in the hormonal control of tryptophan metabolism in isolated rat liver cells. Effects of glucocorticoids and experimental diabetes. Biochem J. 1985;229:499–504. doi: 10.1042/bj2290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mangge H, Summers KL, Meinitzer A, Zelzer S, Almer G, Prassl R, et al. Obesity-related dysregulation of the tryptophan-kynurenine metabolism: role of age and parameters of the metabolic syndrome. Obesity (Silver Spring) 2014;22:195–201. doi: 10.1002/oby.20491. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter KM, Hasin DS, Allison DB, Faith MS. Relationships between obesity and DSM-IV major depressive disorder, suicide ideation, and suicide attempts: results from a general population study. Am J Public Health. 2000;90:251–257. doi: 10.2105/ajph.90.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogawa S, Fujii T, Koga N, Hori H, Teraishi T, Hattori K, et al. Plasma l-tryptophan concentration in major depressive disorder: new data and meta-analysis. J Clin Psychiatry. 2014;75:e906–e915. doi: 10.4088/JCP.13r08908. [DOI] [PubMed] [Google Scholar]
- 22.Moreno FA, Parkinson D, Palmer C, Castro WL, Misiaszek J, El Khoury A, et al. CSF neurochemicals during tryptophan depletion in individuals with remitted depression and healthy controls. Eur Neuropsychopharmacol. 2010;20:18–24. doi: 10.1016/j.euroneuro.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delgado PL. The tryptophan depletion challenge test in medical research: unresolved issues and broader implications for the use of physiological challenges to investigate and categorize disease. Biol Psychiatry. 2011;69:718–719. doi: 10.1016/j.biopsych.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Owens MJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem. 1994;40:288–295. [PubMed] [Google Scholar]
- 25.Bell C, Abrams J, Nutt D. Tryptophan depletion and its implications for psychiatry. Br J Psychiatry. 2001;178:399–405. doi: 10.1192/bjp.178.5.399. [DOI] [PubMed] [Google Scholar]
- 26.Delgado PL, Price LH, Miller HL, Salomon RM, Aghajanian GK, Heninger GR, et al. Serotonin and the neurobiology of depression. Effects of tryptophan depletion in drug-free depressed patients. Arch Gen Psychiatry. 1994;51:865–874. doi: 10.1001/archpsyc.1994.03950110025005. [DOI] [PubMed] [Google Scholar]
- 27.Delgado PL, Miller HL, Salomon RM, Licinio J, Krystal JH, Moreno FA, et al. Tryptophan-depletion challenge in depressed patients treated with desipramine or fluoxetine: implications for the role of serotonin in the mechanism of antidepressant action. Biol Psychiatry. 1999;46:212–220. doi: 10.1016/s0006-3223(99)00014-1. [DOI] [PubMed] [Google Scholar]
- 28.Moreno FA, Heninger GR, McGahuey CA, Delgado PL. Tryptophan depletion and risk of depression relapse: a prospective study of tryptophan depletion as a potential predictor of depressive episodes. Biol Psychiatry. 2000;48:327–329. doi: 10.1016/s0006-3223(00)00893-3. [DOI] [PubMed] [Google Scholar]
- 29.Moreno FA, McGavin C, Malan TP, Gelenberg AJ, Heninger GR, Mathe AA, et al. Tryptophan depletion selectively reduces CSF 5-HT metabolites in healthy young men: results from single lumbar puncture sampling technique. Int J Neuropsychopharmacol. 2000;3:277–283. doi: 10.1017/S1461145700002133. [DOI] [PubMed] [Google Scholar]
- 30.Neumeister A, Konstantinidis A, Stastny J, Schwarz MJ, Vitouch O, Willeit M, et al. Association between serotonin transporter gene promoter polymorphism (5HTTLPR) and behavioral responses to tryptophan depletion in healthy women with and without family history of depression. Arch Gen Psychiatry. 2002;59:613–620. doi: 10.1001/archpsyc.59.7.613. [DOI] [PubMed] [Google Scholar]
- 31.Neumeister A, Hu XZ, Luckenbaugh DA, Schwarz M, Nugent AC, Bonne O, et al. Differential effects of 5-HTTLPR genotypes on the behavioral and neural responses to tryptophan depletion in patients with major depression and controls. Arch Gen Psychiatry. 2006;63:978–986. doi: 10.1001/archpsyc.63.9.978. [DOI] [PubMed] [Google Scholar]
- 32.Widner B, Werner ER, Schennach H, Wachter H, Fuchs D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin Chem. 1997;43:2424–2426. [PubMed] [Google Scholar]
- 33.Sublette ME, Postolache TT. Neuroinflammation and depression: the role of indoleamine 2,3-dioxygenase (IDO) as a molecular pathway. Psychosom Med. 2012;74:668–672. doi: 10.1097/PSY.0b013e318268de9f. [DOI] [PubMed] [Google Scholar]
- 34.Worbe Y, Savulich G, Voon V, Fernandez-Egea E, Robbins TW. Serotonin depletion induces ‘waiting impulsivity’ on the human four-choice serial reaction time task: crossspecies translational significance. Neuropsychopharmacol. 2014;39:1519–1526. doi: 10.1038/npp.2013.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bongers P, van de Giessen E, Roefs A, Nederkoorn C, Booij J, van den Brink W, et al. Being impulsive and obese increases susceptibility to speeded detection of high-calorie foods. Health Psychol. 2014 Nov 3; doi: 10.1037/hea0000167. [Epub ahead of print] PMID: 25365413. [DOI] [PubMed] [Google Scholar]
- 36.Houben K, Nederkoorn C, Jansen A. Eating on impulse: the relation between overweight and food-specific inhibitory control. Obesity (Silver Spring) 2014;22:E6–E8. doi: 10.1002/oby.20670. [DOI] [PubMed] [Google Scholar]
- 37.Fernstrom JD, Wurtman RJ. Brain serotonin content: physiological dependence on plasma tryptophan levels. Science. 1971;173:149–152. doi: 10.1126/science.173.3992.149. [DOI] [PubMed] [Google Scholar]
- 38.Fernstrom JD, Wurtman RJ. Brain serotonin content: increase following ingestion of carbohydrate diet. Science. 1971;174:1023–1025. doi: 10.1126/science.174.4013.1023. [DOI] [PubMed] [Google Scholar]
- 39.Wurtman RJ, Wurtman JJ. Carbohydrate craving, obesity and brain serotonin. Appetite. 1986;7(Suppl):99–103. doi: 10.1016/s0195-6663(86)80055-1. [DOI] [PubMed] [Google Scholar]
- 40.Stunkard AJ, Albaum JM. The accuracy of self-reported weights. Am J Clin Nutr. 1981;34:1593–1599. doi: 10.1093/ajcn/34.8.1593. [DOI] [PubMed] [Google Scholar]
- 41.Bowman RL, Delucia JL. Accuracy of self-reported weight: a meta-analysis. Behav Ther. 1992;23:637–655. [Google Scholar]
- 42.Engstrom JL, Paterson SA, Doherty A, Trabulsi M, Speer KL. Accuracy of self-reported height and weight in women: an integrative review of the literature. J Midwifery Womens Health. 2003;48:338–345. doi: 10.1016/s1526-9523(03)00281-2. [DOI] [PubMed] [Google Scholar]