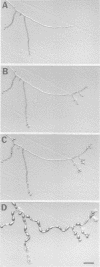
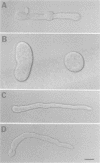
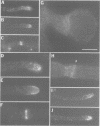
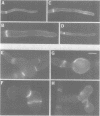
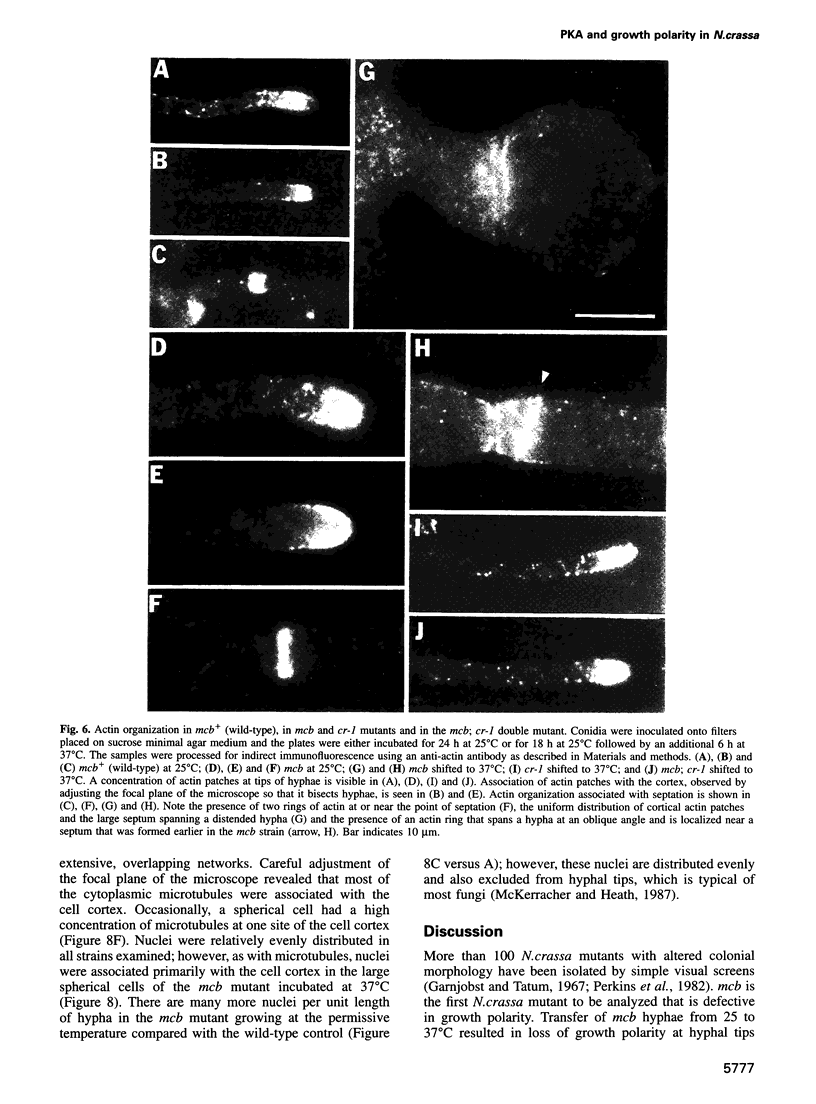
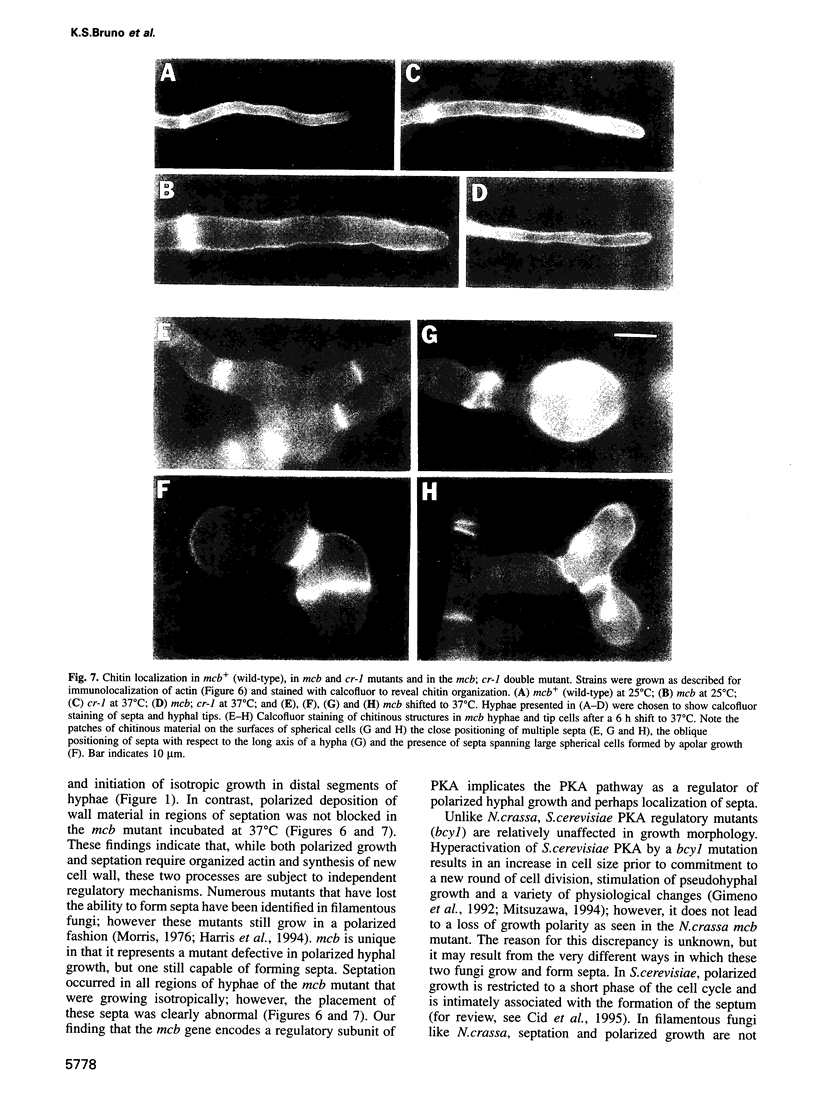
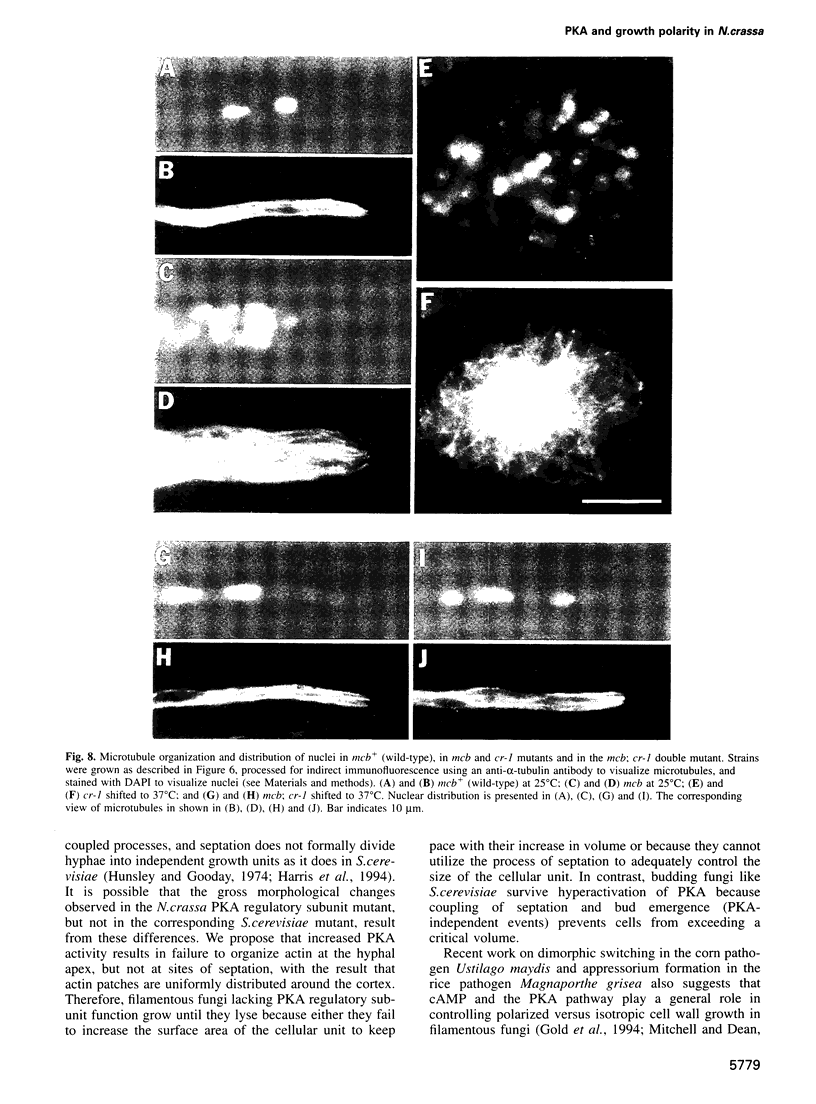
Abstract
In filamentous fungi, growth polarity (i.e. hyphal extension) and formation of septa require polarized deposition of new cell wall material. To explore this process, we analyzed a conditional Neurospora crassa mutant, mcb, which showed a complete loss of growth polarity when incubated at the restrictive temperature. Cloning and DNA sequence analysis of the mcb gene revealed that it encodes a regulatory subunit of cAMP-dependent protein kinase (PKA). Unexpectedly, the mcb mutant still formed septa when grown at the restrictive temperature, indicating that polarized deposition of wall material during septation is a process that is, at least in part, independent of polarized deposition during hyphal tip extension. However, septa formed in the mcb mutant growing at the restrictive temperature are mislocalized. Both polarized growth and septation are actin-dependent processes, and a concentration of actin patches is observed at growing hyphal tips and sites where septa are being formed. In the mcb mutant growing at the restrictive temperature, actin patches are uniformly distributed over the cell cortex; however, actin patches are still concentrated at sites of septation. Our results suggest that the PKA pathway regulates hyphal growth polarity, possibly through organizing actin patches at the cell cortex.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A. E., Johnson D. I., Longnecker R. M., Sloat B. F., Pringle J. R. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J Cell Biol. 1990 Jul;111(1):131–142. doi: 10.1083/jcb.111.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams A. E., Pringle J. R. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J Cell Biol. 1984 Mar;98(3):934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Barja F., Thi B. N., Turian G. Localization of actin and characterization of its isoforms in the hyphae of Neurospora crassa. FEMS Microbiol Lett. 1991 Jan 1;61(1):19–24. doi: 10.1016/0378-1097(91)90007-w. [DOI] [PubMed] [Google Scholar]
- Berlin V., Yanofsky C. Isolation and characterization of genes differentially expressed during conidiation of Neurospora crassa. Mol Cell Biol. 1985 Apr;5(4):849–855. doi: 10.1128/mcb.5.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A., Drees B., Harsay E., Schott D., Wang T. What are the basic functions of microfilaments? Insights from studies in budding yeast. J Cell Biol. 1994 Aug;126(4):821–825. doi: 10.1083/jcb.126.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F., Nurse P. How fission yeast fission in the middle. Cell. 1996 Jan 26;84(2):191–194. doi: 10.1016/s0092-8674(00)80973-3. [DOI] [PubMed] [Google Scholar]
- Chant J. Cell polarity in yeast. Trends Genet. 1994 Sep;10(9):328–333. doi: 10.1016/0168-9525(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Chant J. Septin scaffolds and cleavage planes in Saccharomyces. Cell. 1996 Jan 26;84(2):187–190. doi: 10.1016/s0092-8674(00)80972-1. [DOI] [PubMed] [Google Scholar]
- Cid V. J., Durán A., del Rey F., Snyder M. P., Nombela C., Sánchez M. Molecular basis of cell integrity and morphogenesis in Saccharomyces cerevisiae. Microbiol Rev. 1995 Sep;59(3):345–386. doi: 10.1128/mr.59.3.345-386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARKEN M. A. Applications of fluorescent brighteners in biological techniques. Science. 1961 May 26;133(3465):1704–1705. doi: 10.1126/science.133.3465.1704. [DOI] [PubMed] [Google Scholar]
- DeVoti J., Seydoux G., Beach D., McLeod M. Interaction between ran1+ protein kinase and cAMP dependent protein kinase as negative regulators of fission yeast meiosis. EMBO J. 1991 Dec;10(12):3759–3768. doi: 10.1002/j.1460-2075.1991.tb04945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin D. G., Nelson W. J. Origins of cell polarity. Cell. 1996 Feb 9;84(3):335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- Garnjobst L., Tatum E. L. A survey of new morphological mutants in Neurospora crassa. Genetics. 1967 Nov;57(3):579–604. doi: 10.1093/genetics/57.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno C. J., Ljungdahl P. O., Styles C. A., Fink G. R. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell. 1992 Mar 20;68(6):1077–1090. doi: 10.1016/0092-8674(92)90079-r. [DOI] [PubMed] [Google Scholar]
- Gold S., Duncan G., Barrett K., Kronstad J. cAMP regulates morphogenesis in the fungal pathogen Ustilago maydis. Genes Dev. 1994 Dec 1;8(23):2805–2816. doi: 10.1101/gad.8.23.2805. [DOI] [PubMed] [Google Scholar]
- Grove S. N., Bracker C. E. Protoplasmic organization of hyphal tips among fungi: vesicles and Spitzenkörper. J Bacteriol. 1970 Nov;104(2):989–1009. doi: 10.1128/jb.104.2.989-1009.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding R. W., Melles S. Genetic Analysis of Phototropism of Neurospora crassa Perithecial Beaks Using White Collar and Albino Mutants. Plant Physiol. 1983 Aug;72(4):996–1000. doi: 10.1104/pp.72.4.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M. To shape a cell: an inquiry into the causes of morphogenesis of microorganisms. Microbiol Rev. 1990 Dec;54(4):381–431. doi: 10.1128/mr.54.4.381-431.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S. D., Morrell J. L., Hamer J. E. Identification and characterization of Aspergillus nidulans mutants defective in cytokinesis. Genetics. 1994 Feb;136(2):517–532. doi: 10.1093/genetics/136.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. J. Ultrastructural analysis of hyphal tip cell growth in fungi: Spitzenkörper, cytoskeleton and endomembranes after freeze-substitution. J Cell Sci. 1981 Apr;48:89–103. doi: 10.1242/jcs.48.1.89. [DOI] [PubMed] [Google Scholar]
- Hunsley D., Gooday G. W. The structure and development of septa in Neurospora crassa. Protoplasma. 1974;82(1):125–146. doi: 10.1007/BF01276876. [DOI] [PubMed] [Google Scholar]
- Innocenti F. D., Pohl U., Russo V. E. Photoinduction of protoperithecia in Neurospora crassa by blue light. Photochem Photobiol. 1983 Jan;37(1):49–51. doi: 10.1111/j.1751-1097.1983.tb04432.x. [DOI] [PubMed] [Google Scholar]
- Johnson T. E. Analysis of pattern formation in Neurospora perithecial development using genetic mosaics. Dev Biol. 1976 Nov;54(1):23–36. doi: 10.1016/0012-1606(76)90283-9. [DOI] [PubMed] [Google Scholar]
- Kore-eda S., Murayama T., Uno I. Isolation and characterization of the adenylate cyclase structural gene of Neurospora crassa. Jpn J Genet. 1991 Jun;66(3):317–334. doi: 10.1266/jjg.66.317. [DOI] [PubMed] [Google Scholar]
- Lauter F. R., Russo V. E., Yanofsky C. Developmental and light regulation of eas, the structural gene for the rodlet protein of Neurospora. Genes Dev. 1992 Dec;6(12A):2373–2381. doi: 10.1101/gad.6.12a.2373. [DOI] [PubMed] [Google Scholar]
- Maeda H., Ishida N. Specificity of binding of hexopyranosyl polysaccharides with fluorescent brightener. J Biochem. 1967 Aug;62(2):276–278. doi: 10.1093/oxfordjournals.jbchem.a128660. [DOI] [PubMed] [Google Scholar]
- Maheshwari R. Microcycle conidiation and its genetic basis in Neurospora crassa. J Gen Microbiol. 1991 Sep;137(9):2103–2115. doi: 10.1099/00221287-137-9-2103. [DOI] [PubMed] [Google Scholar]
- Metzenberg R. L., Stevens J. N., Selker E. U., Morzycka-Wroblewska E. Identification and chromosomal distribution of 5S rRNA genes in Neurospora crassa. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2067–2071. doi: 10.1073/pnas.82.7.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell T. K., Dean R. A. The cAMP-dependent protein kinase catalytic subunit is required for appressorium formation and pathogenesis by the rice blast pathogen Magnaporthe grisea. Plant Cell. 1995 Nov;7(11):1869–1878. doi: 10.1105/tpc.7.11.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuzawa H. Increases in cell size at START caused by hyperactivation of the cAMP pathway in Saccharomyces cerevisiae. Mol Gen Genet. 1994 Apr;243(2):158–165. doi: 10.1007/BF00280312. [DOI] [PubMed] [Google Scholar]
- Morris N. R. Mitotic mutants of Aspergillus nidulans. Genet Res. 1975 Dec;26(3):237–254. doi: 10.1017/s0016672300016049. [DOI] [PubMed] [Google Scholar]
- Nurse P. Fission yeast morphogenesis--posing the problems. Mol Biol Cell. 1994 Jun;5(6):613–616. doi: 10.1091/mbc.5.6.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins D. D., Radford A., Newmeyer D., Björkman M. Chromosomal loci of Neurospora crassa. Microbiol Rev. 1982 Dec;46(4):426–570. doi: 10.1128/mr.46.4.426-570.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju N. B. Meiosis and ascospore genesis in Neurospora. Eur J Cell Biol. 1980 Dec;23(1):208–223. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M. L., Yanofsky C. A morphological and genetic analysis of conidiophore development in Neurospora crassa. Genes Dev. 1989 Apr;3(4):559–571. doi: 10.1101/gad.3.4.559. [DOI] [PubMed] [Google Scholar]
- Taylor S. S., Buechler J. A., Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- Terenzi H. F., Flawia M. M., Tellez-Inon M. T., Torres H. N. Control of Neurospora crassa morphology by cyclic adenosine 3', 5'-monophosphate and dibutyryl cyclic adenosine 3', 5'-monophosphate. J Bacteriol. 1976 Apr;126(1):91–99. doi: 10.1128/jb.126.1.91-99.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- That T. C., Rossier C., Barja F., Turian G., Roos U. P. Induction of multiple germ tubes in Neurospora crassa by antitubulin agents. Eur J Cell Biol. 1988 Apr;46(1):68–79. [PubMed] [Google Scholar]
- Tinsley J. H., Minke P. F., Bruno K. S., Plamann M. p150Glued, the largest subunit of the dynactin complex, is nonessential in Neurospora but required for nuclear distribution. Mol Biol Cell. 1996 May;7(5):731–742. doi: 10.1091/mbc.7.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde F., Mata J., Nurse P. Fission yeast cell morphogenesis: identification of new genes and analysis of their role during the cell cycle. J Cell Biol. 1995 Dec;131(6 Pt 1):1529–1538. doi: 10.1083/jcb.131.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer S. J., Yanofsky C. Efficient cloning of genes of Neurospora crassa. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4869–4873. doi: 10.1073/pnas.83.13.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden O., Plamann M., Ebbole D. J., Yanofsky C. cot-1, a gene required for hyphal elongation in Neurospora crassa, encodes a protein kinase. EMBO J. 1992 Jun;11(6):2159–2166. doi: 10.1002/j.1460-2075.1992.tb05275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Kuret J., Cameron S., Levin L., Johnson K. E. Purification and characterization of C1, the catalytic subunit of Saccharomyces cerevisiae cAMP-dependent protein kinase encoded by TPK1. J Biol Chem. 1988 Jul 5;263(19):9142–9148. [PubMed] [Google Scholar]