Abstract
Purpose of review
When it comes to tolerance induction, kidney allografts behave differently from heart allografts which behave differently from lung allografts. Here, we examine how and why different organ allografts respond differently to the same tolerance induction protocol.
Recent findings
Allograft tolerance has been achieved in experimental and clinical kidney transplantation. However, inducing tolerance in experimental recipients of heart and lung allografts has proven to be more challenging. New protocols being developed in nonhuman primates based on mixed chimerism and co-transplantation of tolerogenic organs may provide mechanistic insights to help overcome these challenges.
Summary
Tolerance induction protocols that are successful in patients transplanted with “tolerance-prone” organs such as kidneys and livers will most likely not succeed in recipients of “tolerance-resistant” organs such as hearts and lungs. Separate clinical trials using more robust tolerance protocols will be required to achieve tolerance in heart and lung recipients.
Keywords: Transplantation, tolerance, heart, lung, kidney, organ-specificity
INTRODUCTION
Induction of immune tolerance is the ultimate goal in the field of organ transplantation. Achieving a state of tolerance would lead to indefinite graft survival without the need for chronic immunosuppression and, would theoretically prevent chronic rejection. Tolerance of kidney allografts has been achieved in non-human primates (NHPs)(1-3) and in humans (4-7) by using a combination of nonmyeloablative conditioning and donor bone marrow transplantation that results in mixed chimerism. However, mixed chimerism protocols that achieve long-term tolerance of kidney allografts in NHPs fail to induce tolerance in heart recipients (8).
The reasons for this organ-specific difference are not clear. However, it is clear that all transplanted organs are not created equal. Not only does the strength of the immune response to a particular organ vary with the organ transplanted but the nature of response itself, rejection versus tolerance, varies from organ to organ. In most experimental transplant models, kidney and liver allografts evoke a weaker rejection response than heart and lung allografts. Moreover, kidney and liver allografts can actively participate in the induction and maintenance of tolerance and thus, can be considered “tolerance-prone” organs. The same cannot be said for heart and lung allografts which are, for the most part, “tolerance-resistant.” Finally, kidney and liver allografts also possess the unique ability to confer unresponsiveness upon co-transplanted, tolerance-resistant organs like hearts.
Mechanisms underlying these organ-specific differences are unclear but understanding them could contribute to the development of strategies that extend tolerance to recipients of tolerance-resistant organs. Here, we review organ-specific differences in tolerance induction, focusing on the dissimilarities between tolerogenic kidney allografts and the more stringent heart and lung allografts.
Organ-specific differences in spontaneous tolerance
Different organ allografts have different propensities to be spontaneously accepted without any treatment. Murine skin, hearts, intestines, lungs and hepatocytes are largely rejected when transplanted across MHC barriers (9-13). In contrast, kidneys and livers are commonly accepted across the same MHC barriers (10,14,15). Zhang et al. (10) compared liver, kidney and heart transplantation in three different MHC disparate mouse strain combinations without treatment. The majority of liver allografts (57-72%) in each strain combination were spontaneously accepted long term, whereas hearts transplanted across identical histocompatibility barriers all rejected in less than 10 days. The pattern of kidney allograft rejection was mixed with 20% to 50% organs surviving long term (10). Our results (13,16) and others (17-19) in mice support the fact that kidneys have a much greater propensity for spontaneous acceptance compared to hearts transplanted across the same MHC barrier.
Spontaneously accepted kidney allografts show prominent peri-arterial lymphoid sheaths containing nodules of CD3+Foxp3+ T cells, CD4+ T cells, DCs, B cells and indoleamine-pyrrole 2,3-dioxygenase positive cells (16). These regulatory T cell-rich organized lymphoid structures, which we termed “TOLs”, were distinct from tertiary lymphoid organs (TLOs) found in chronic inflammation in that they lacked high endothelial venules (20). Similar structures have been identified in tolerant pig and nonhuman primate kidney allografts (Farkash, et al., unpublished data). We have also shown that Foxp3+ regulatory T cells (Tregs) are necessary to maintain unresponsiveness in spontaneously accepted MHC mismatched mouse kidney allografts (16). Treg depletion in long term surviving kidney allograft recipients triggered disintegration of TOLs and acute cellular rejection (16).
Liver allografts are also spontaneously accepted across certain mouse and rat strain combinations (10,21-24). Like kidney allografts, however, depletion of Tregs (using an anti-CD25 antibody) induces acute liver rejection (22).
Among higher-order animals, swine is the only species in which spontaneous tolerance has been reported after liver (25) or kidney (26) transplantation. Currently, there are no specific agents to deplete Tregs in swine, however, other studies suggest that Tregs play an important role in this effect (27).
Although a somewhat different phenomenon, spontaneous graft acceptance after cessation of immunosuppression (operational tolerance) has been observed in human kidney and liver recipients. Examination of the natural history of 27 kidney transplant patients rendered operationally tolerant after withdrawing immunosuppression found that 70% maintained stable graft function for an average of 9 years after transplantation (28). In adult liver transplantation, 8% to 33% of patients who withdraw from immunosuppression exhibit operational tolerance (29-32), although in the pediatric population the incidence is much higher (60%)(33). In contrast, there exist only anecdotal cases of operational tolerance in a lung recipient and heart recipients (34).
Organ-specific effects in calcineurin inhibitor-based tolerance protocols
Perhaps the most compelling evidence for the organ-specificity in tolerance induction comes from studies of heart, kidney and lung transplantation in MHC inbred miniature swine. When porcine recipients were transplanted with MHC class I disparate hearts and treated with 12 days of cyclosporine (CyA), they all rejected within 55 days and exhibited cardiac allograft vasculopathy (CAV) (35). In contrast, when swine were transplanted with class I disparate kidneys allografts and treated with the same course of CyA, they all became tolerant to donor antigen and maintained excellent renal function long-term, in some instances for over 2 years (36). The survival of lungs transplanted in an analogous setting was in between that of hearts and kidneys with graft survival ranging from 67 to >605 days and two thirds developing obliterative bronchiolitis (OB) (37).
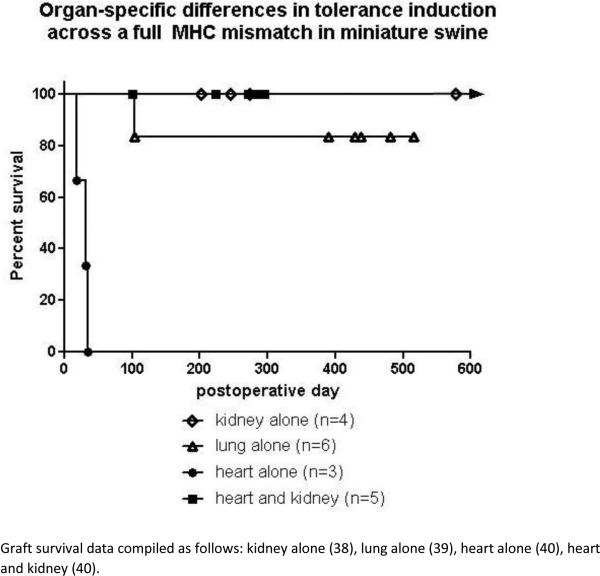
A similar hierarchy was observed when organs were transplanted across a full MHC barrier and FK506 was substituted for CyA in the swine model. Long term tolerance was easily induced in renal allografts (38), whereas lung survival ranged from 100 to >500 days (39) and hearts only survived 18-35 days (40)(Fig 1).
Figure 1.
Graft survival data compiled as follows: kidney alone (38), lung alone (39), heart alone (40), heart and kidney (40).
Thus, clear differences exist between organs that dramatically affect the ability of a particular treatment protocol to induce tolerance of that organ. Kidneys have a greater propensity for tolerance induction than lungs which have a greater propensity than hearts, at least in swine. These observations emphasize that tolerance induction protocols are not necessarily transferable between organs and therefore, that preclinical and clinical testing of tolerance protocols for human transplantation must proceed in an organ-specific manner (41).
Even more striking than the apparent hierarchy in organ tolerogenicity, is the observation that tolerance-prone organs are able to confer unresponsiveness upon tolerance-resistant organs procured from the same donor. Using MHC inbred miniature swine, we have shown that instead of rejecting their hearts, recipients co-transplanted with hearts and kidneys from the same class I disparate (42) or fully MHC mismatched (40) donor developed stable tolerance. The result was the long-term, rejection-free survival of both kidney and heart allografts (Fig 1) with no alloantibody formation or evidence of CAV. Neither heart nor kidney allograft survival was affected by the placement of donor-specific and third party skin grafts despite the return of anti-donor CML reactivity, which suggests the effects of ongoing T cell regulation (43,44). We have termed this phenomenon kidney-induced cardiac allograft tolerance (KICAT).
We found that the donor kidney must remain in the recipient for over 8 days to achieve KICAT (45) and that a radiosensitive cell population within the donor kidney appeared necessary for the development of KICAT (46). We also found that the host thymus played a critical role during the induction phase of KICAT (47) as did MHC matching of the heart and kidney allografts (48). We have examined the phenotypes of graft infiltrating lymphocytes (GILs) retrieved from isolated kidneys transplanted across a class I barrier which accepted versus rejected (49). The number of cells expressing the phenotypic markers of Tregs was substantially higher in acceptor than in rejector transplants at all time points. We went on to show that CD25+ T cells in peripheral blood leukocytes from heart/kidney recipients but not isolated heart recipients could suppress the anti-donor response of naïve-matched T cells in co-culture CML assays (50). Based on these results and others (20-22), we hypothesize that cells or cell products intrinsic to kidney, but not heart, allografts promote the activation/expansion of host Tregs which induce tolerance of heart grafts (reviewed in (27)).
Of note, a similar effect has been described in human recipients of liver transplants (25,51-54). Recipients of combined heart-liver transplants require less immunosuppression than recipients of isolated hearts or kidneys (55) and despite the lower immunosuppression, rejection rates for heart and liver are low (13% and 10%, respectively) (56,57). In combined liver/lung transplantation, acute rejection of the lung graft was observed at comparatively low rates (58,59) although definitive conclusions about a potential liver-protective effect cannot be drawn from these small series. Several clinical studies have examined outcomes between combined heart/kidney transplants versus heart alone transplants. Although there does not appear to be any difference in patient survival, some groups report decreased rejection episodes and incidence of CAV in heart/kidney recipients (60-64). Chronic immunosuppression in these patients probably masks any tolerogenic effects of the kidney or liver allograft.
Organ-specific effects in mixed chimerism-based tolerance protocols
We have extended the study of KICAT to cynomolgus monkeys using a mixed chimerism tolerance induction strategy instead of a short course of CyA or FK506. Tolerance of isolated MHC disparate kidneys allografts in cynomolgus monkeys has been previously reported from our laboratory using mixed chimerism-based protocols that included T cell depletion, nonmyeloablative total body irradiation, thymic irradiation, donor bone marrow infusion and a short course of calcineurin inhibitor (1-3). However, the same mixed chimerism protocol that induced tolerance of kidneys failed to induce tolerance of isolated hearts (8). The majority of heart recipients developed anti-donor cellular and humoral immunity, resulting in severe rejection with CAV by day 175 (8). Recipients cotransplanted with heart and kidney allografts, however, became tolerant of both organs (Tonsho, M. manuscript submitted). Every heart/kidney recipient that successfully completed its conditioning regimen became tolerant. To our knowledge, these heart/kidney recipients represent the first nonhuman primates to become tolerant of cardiac allografts.
Potential mechanisms underlying organ-specific differences in tolerance induction
Why different organs exhibit different thresholds for tolerance induction is unknown. However, factors intrinsic to the organ as well as extrinsic factors such as exposure to the external environment in the case of lung and intestine, likely play a role. We hypothesize that the tolerogencity of a particular organ is a function of on how internal and external factors combine to promote the activation of host regulatory mechanisms and/or the inactivation of alloaggressive mechanisms, thereby shifting the balance of the overall immune response away from rejection and towards tolerance. Similar mechanisms have been put forth to explain the phenomenon of “immune priviledge” (65).
In terms of external factors, exposure to environmental antigens could contribute to the tolerance-resistance of lung and intestinal allografts. Stimulation of toll-like receptors (TLRs) by microorganisms could activate the innate and adaptive immune systems, thereby promoting rejection. This was suggested by studies showing that TLR signaling interferes with the immunomodulatory milieu established by T regulatory cells (66). Clinically, respiratory infections predispose lung grafts to immune-mediated failure (67) and infection with Pseudomonas aeruginosa results in neutrophilic graft infiltration that breaks established costimulation blockade-mediated lung allograft acceptance in mice (68). Low molecular weight hyaluronan, a damage-associated molecular pattern that is associated with chronic lung rejection in humans, breaks costimulation blockade-mediated acceptance of mouse pulmonary allografts (69). This abrogation of acceptance is dependent on neutrophilic graft infiltration and signaling via TLR2/TLR4 as well as MyD88 in the recipients.
Other important external factors include donor brain death (BD) and ischemia/reperfusion injury (IRI) which result in the elaboration of proinflammatory cytokines and chemokines leading to augmented graft antigenicity and increased susceptibility to host alloimmune responses. Indeed, there are many examples of how the deleterious effects of BD (70) and IRI (71) accelerate acute rejection in experimental models and negatively affect long-term graft survival in humans (72). However, while the effects of BD and IRI on organ quality have been studied extensively, investigations into the effect of brain death and ischemia on tolerance induction are extremely limited. In a rat kidney transplant model, brain death was shown to interfere with the beneficial effects of anti-CD4 mAb therapy on the long term graft function (73). Severe IRI abrogated costimulatory blockade-mediated induction of long-term acceptance of lung allografts in a mouse model (74). This was linked to neutrophilic graft infiltration that resulted in activation of donor antigen presenting cells and induction of Th1 responses. This observation is of potential clinical relevance as IRI-mediated primary graft dysfunction is a recognized risk factor for the development of chronic lung rejection in humans (75). To our knowledge, the only large animal study to address this question is our earlier report in MHC inbred miniature swine showing that following traumatic brain death, proinflammatory cytokines were released systemically in the donor's serum and up-regulated in the donor's tissue. Furthermore, transplanting lung allografts procured from these brain dead donors resulted in the failure of a tolerance induction protocol that was otherwise successful when allografts were retrieved form non-brain dead donors (76). However, in contrast to lung recipients, brain death and ischemia did not prevent tolerance induction in analogously treated heart/kidney recipients (Michel S. manuscript submitted). This finding suggests that the effects of donor brain death and ischemia on tolerance induction also vary with the organ transplanted.
The striking difference in tolerogenicity between kidney and heart allografts combined with our finding that regulatory mechanisms play an important role in spontaneous tolerance and KICAT suggest that cells or cell products intrinsic to kidney but not heart allografts promote the activation and/or expansion of host Tregs which in turn, mediate tolerance of heart (and kidney) grafts. While the identity of those subpopulations is not clear, there are at least two cell populations present in kidneys capable of down-regulating alloimmune responses: 1) plasmacytoid dendritic cells (pDCs), capable of promoting the generation of Tregs and inducing tolerance to hearts in mice (77-79) and 2) renal tubular epithelial cells (RTECs), capable of inducing T cell unresponsiveness to self- and allo-antigens in mice and humans (80-85).
We have recently demonstrated that CD11c+ cells isolated from naïve, untransplanted kidneys (from DBA/2 and BALB/c mice) contain a subpopulation of PDCA-1+B220+pDCs not found in hearts from the same animals (Alessandrini et al., unpublished data). The presence of PDCA-1+B220+pDCs in DBA/2 kidneys but not hearts may explain why DBA/2 kidneys are spontaneously accepted when transplanted into B6 WT recipients, whereas DBA/2 hearts are rejected (17,18).
Alternatively, it has been previously shown that Foxp3+ T cells are enriched in the tubules of mouse (86) and human (87) renal allografts. Frasca and colleagues have shown that IFNγ-treated human RTECs induce allospecific tolerance via a class II pathway (80). IFNγ induces PD-L1 and IDO on RTECs (88,89), and PD-L1 can induce the generation of allogeneic Foxp3+ Tregs (90). Finally, RTECs are known to produce and activate TGFβ (91), which is an inducer of Foxp3+ Treg (92) and tolerogenic pDC (93) generation. High levels of TGFβ have been documented in accepted DBA/2 kidneys (17,18).
Recently, erythropoietin (EPO) was shown to inhibit T cell immunity in vitro, providing another possibly mechanism to explain the protective effects of kidney allografts (94).
Like kidney allografts, liver allografts are spontaneously accepted across certain mouse and rat strain combinations (10,21-24). The tolerogenic properties of livers suggest that, as in kidneys, cells or cell products intrinsic to the organ are important. Three cell populations present in liver have been shown capable of suppressing an alloimmune response; 1) pDCs, located within periportal areas and around central veins (95), 2) liver sinusoidal endothelial cells (LSECs) that generate anti-inflammatory cytokines (96) and diminish the survival of CD8+ T cells (97-99) and 3) hepatic stellate cells (HSCs) that can induce Tregs (100,101).
Hepatic dendritic cells are less immunogenic than splenic dendritic cells and only 5% of splenic DCs are made up of pDCs while 19% of the liver DC population is made up of pDCs (102). Furthermore, circulating pDCs were increased relative to myeloid DCs (mDCs) in operationally tolerant pediatric liver allograft recipients as compared to patients maintained on chronic immunosuppression (103).
LSECs secreting IL-10 (96) are able to induce CD8+ T cell tolerance rather than immunity via cross-presentation of antigen to CD8+ T cells (97,98). LSEC-primed, naïve CD8+ T cells are initially induced to proliferate, to release cytokines, such as IL-2 and IFNγ, but eventually begin to secrete low levels of these cytokines and exhibit low cytotoxicity (97). The induction of CD8+ T cell tolerance has been correlated with and has been shown to be dependent on the induction of PD-L1 by LSECs (104).
Finally, HSCs store vitamin A and can produce TGFβ in response to inflammation and injury (104,105). Vitamin A-derived retinoic acid and TGFβ have been shown to participate in the conversion of CD4+ T cells to Tregs (106,107). Furthermore, activated HSCs express PD-L1 (108). Importantly, HSCs can confer unresponsiveness and long term survival to islet allografts by inducing Tregs (100) and myeloid-derived suppressor cells (109,110).
It has been the prevailing view that memory T cell infiltration into allografts is a barrier to tolerance induction and attempts have been made to eliminate this cell population (111). In fact, clinical immunosuppression protocols depleting T cells indiscriminately in the peri-operative period that have been successful in kidney transplantation have been adapted to lung transplant patients (112). However, we have recently shown that graft infiltration by central memory CD8+ T cells is critical to induce costimulatory blockade-mediated tolerance after lung transplantation in the mouse (113). Induction of tolerance was dependent on production of IFN-γ by central memory CD8+ T cells within the graft, which results in production of nitric oxide. One factor that needs to be considered when developing lung-specific tolerance protocols is that propagation of immune responses that lead to rejection or tolerance of lungs can occur in the graft itself (114,115). This is in contrast to other organs such as hearts where such responses depend on cellular trafficking to secondary lymphoid tissues (116,117).
Lymphoid neogenesis, the development of organized tertiary lymphoid tissue, has been observed at chronic sites of inflammation, including transplanted grafts. These structures play a role in generating local cellular and humoral immune responses. For example, the Lakkis group has shown that tertiary lymphoid organs are associated with graft rejection in murine heart transplants (20). In contrast, we have observed the induction of organized lymphoid structures in mouse lung allografts that are accepted long term after treatment with perioperative costimulatory blockade (114). These structures are rich in CD11c+ cells that express PD-L1 as well as Foxp3+ cells. In contrast to the “TOLs” observed in spontaneously accepted kidney allografts, high endothelial venules are induced in the tertiary lymphoid structures in accepted lung allografts.
Conclusion
Tolerance protocols already in use for clinical kidney transplantation will require modifications before becoming applicable to more stringent organs such as heart and lung. In fact, some approaches that have been developed for kidney transplants may be deleterious for thoracic organs (113). Modifications may include administration of immunomodulatory cellular therapy, blunting heterologous immunity, or donor-organ preconditioning. Studies focusing on the mechanisms of tolerance induction, and the differences between tolerance-prone versus tolerance-resistant organs, may provide further insight into ways to optimize immunosuppression-free survival in heart and lung transplant recipients.
Key points.
Attaining tolerance of heart and lung allografts is more difficult than of liver and kidney allografts, reflecting organ-specificity in tolerance induction.
The mechanisms underlying organ-specificity in tolerance are unknown but may be based on cells or cell products resident in tolerance-prone but not tolerance-resistant organs.
Understanding the mechanisms underlying organ-specific alloimmune responses could contribute to the success of strategies aimed at extending tolerance to recipients of all tolerance-resistant organs.
Separate clinical trials using more robust tolerance protocols will be required to achieve tolerance in heart and lung recipients.
Acknowledgements
We would like to thank Dr. Gilles Benichou for critical review of the manuscript.
Financial support and sponsorship
This work was supported in part by grants from the National Heart, Lung, and Blood Institute (P01HL18646, R01HL113931, R01HL094601) and the National Institute of Allergy and Infectious Disease (U01AI94374) of National Institutes of Health, and from the Thoracic Surgery Foundation for Research & Education. Dr. Madariaga is an Edward D. Churchill Surgical Research Fellow, Massachusetts General Hospital, recipient of a fellowship from the International Heart and Lung Transplantation Society and was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number F32HL117540. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest
None declared
Reference List
- 1.Kawai T, Sogawa H, Boskovic S, et al. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant. 2004;4:1391–1398. doi: 10.1111/j.1600-6143.2004.00523.x. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T, Cosimi AB, Colvin RB, et al. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59:256–262. [PubMed] [Google Scholar]
- 3.Kawai T, Poncelet A, Sachs DH, et al. Long-term outcome and alloantibody production in a non-myeloablative regimen for induction of renal allograft tolerance. Transplantation. 1999;68:1767–1775. doi: 10.1097/00007890-199912150-00022. [DOI] [PubMed] [Google Scholar]
- 4.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Kawai T, Sachs DH, Sprangers B, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant. 2014;14:1599–1611. doi: 10.1111/ajt.12731. [This paper provides long-term follow-up of HLA-mismatched patients who underwent combined kidney and bone marrow transplantation, resulting in transient mixed chimerism. This protocol resulted in the first successful demonstration of tolerance in 7 of 10 patients for at least 4 years. In comparison to a matched cohort of living donor kidney recipients, tolerant patients had significantly fewer postoperative complications.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6*.Leventhal JR, Elliott MJ, Yolcu ES, et al. Immune reconstitution/immunocompetence in recipients of kidney plus hematopoietic stem/facilitating cell transplants. Transplantation. 2015;99:288–298. doi: 10.1097/TP.0000000000000605. [This paper provides long-term follow-up of HLA-mismatched patients who underwent combined kidney and facilitating cell-enriched hematopoietic stem cell transplantation, demonstrating successful tolerance in 12 of 19 patients who achieved stable donor chimerism.] [DOI] [PubMed] [Google Scholar]
- 7*.Scandling JD, Busque S, Shizuru JA, et al. Chimerism, Graft Survival, and Withdrawal of Immunosuppressive Drugs in HLA Matched and Mismatched Patients After Living Donor Kidney and Hematopoietic Cell Transplantation. Am J Transplant. 2015;15:695–704. doi: 10.1111/ajt.13091. [This paper provides long-term follow-up of HLA-matched patients who underwent combined living donor kidney and enriched CD34(+) hematopoietic cell transplants, resulting in transient mixed chimerism. This protocol resulted in successful tolerance induction in 16 of 22 HLA-matched patients to date. Recently, HLA-mismatched patients underwent the same protocol with results pending.] [DOI] [PubMed] [Google Scholar]
- 8.Kawai T, Cosimi AB, Wee SL, et al. Effect of mixed hematopoietic chimerism on cardiac allograft survival in cynomolgus monkeys. Transplantation. 2002;73:1757–1764. doi: 10.1097/00007890-200206150-00011. [DOI] [PubMed] [Google Scholar]
- 9.Milton AD, Fabre JW. Massive induction of donor-type class I and class II major histocompatibility complex antigens in rejecting cardiac allografts in the rat. J Exp Med. 1985;161:98–112. doi: 10.1084/jem.161.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z, Zhu L, Quan D, et al. Pattern of liver, kidney, heart, and intestine allograft rejection in different mouse strain combinations. Transplantation. 1996;62:1267–1272. doi: 10.1097/00007890-199611150-00016. [DOI] [PubMed] [Google Scholar]
- 11.Bumgardner GL, Li J, Heininger MB, et al. In vivo immune response to allogeneic hepatocytes. Transplant Proc. 1997;29:2059–2060. doi: 10.1016/s0041-1345(97)00230-3. [DOI] [PubMed] [Google Scholar]
- 12.Gelman AE, Okazaki M, Lai J, et al. CD4+ T lymphocytes are not necessary for the acute rejection of vascularized mouse lung transplants. J Immunol. 2008;180:4754–4762. doi: 10.4049/jimmunol.180.7.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madsen JC, Morris PJ, Wood KJ. Immunogenetics of Heart Transplantation in Rodents. Transplantation Reviews. 1997;11:141–150. [Google Scholar]
- 14.Russell PS, Chase CM, Colvin RB, Plate JM. Kidney transplants in mice. An analysis of the immune status of mice bearing long-term, H-2 incompatible transplants. J Exp Med. 1978;147:1449–1468. doi: 10.1084/jem.147.5.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bickerstaff AA, Wang JJ, Pelletier RP, Orosz CG. The graft helps to define the character of the alloimmune response. Transpl Immunol. 2002;9:137–141. doi: 10.1016/s0966-3274(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 16.Miyajima M, Chase CM, Alessandrini A, et al. Early acceptance of renal allografts in mice is dependent on foxp3(+) cells. Am J Pathol. 2011;178:1635–1645. doi: 10.1016/j.ajpath.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bickerstaff AA, Wang JJ, Pelletier RP, Orosz CG. Murine renal allografts: spontaneous acceptance is associated with regulated T cell-mediated immunity. J Immunol. 2001;167:4821–4827. doi: 10.4049/jimmunol.167.9.4821. [DOI] [PubMed] [Google Scholar]
- 18.Cook CH, Bickerstaff AA, Wang JJ, et al. Spontaneous renal allograft acceptance associated with “regulatory” dendritic cells and IDO. J Immunol. 2008;180:3103–3112. doi: 10.4049/jimmunol.180.5.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C, Cordoba S, Hu M, et al. Spontaneous acceptance of mouse kidney allografts is associated with increased Foxp3 expression and differences in the B and T cell compartments. Transpl Immunol. 2011;24:149–156. doi: 10.1016/j.trim.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Baddoura FK, Nasr IW, Wrobel B, Li Q, Ruddle NH, Lakkis FG. Lymphoid neogenesis in murine cardiac allografts undergoing chronic rejection. Am J Transplant. 2005;5:510–516. doi: 10.1111/j.1600-6143.2004.00714.x. [DOI] [PubMed] [Google Scholar]
- 21.Dahmen U, Qian S, Rao AS, et al. Split tolerance induced by orthotopic liver transplantation in mice. Transplantation. 1994;58:1–8. doi: 10.1097/00007890-199407000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Kuhr CS, Zheng XX, et al. New insights into mechanisms of spontaneous liver transplant tolerance: the role of Foxp3-expressing CD25+CD4+ regulatory T cells. Am J Transplant. 2008;8:1639–1651. doi: 10.1111/j.1600-6143.2008.02300.x. [DOI] [PubMed] [Google Scholar]
- 23.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19:916–924. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sriwatanawongsa V, Davies HS, Calne RY. The essential roles of parenchymal tissues and passenger leukocytes in the tolerance induced by liver grafting in rats. Nat Med. 1995;1:428–432. doi: 10.1038/nm0595-428. [DOI] [PubMed] [Google Scholar]
- 25.Calne RY, Sells RA, Pena JR. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472–476. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 26.Pescovitz MD, Thistlethwiate JRJ, Auchincloss H, Jr, et al. Effect of class II antigen matching on renal allograft survival in miniature swine. J Exp Med. 1984;160:1495–1508. doi: 10.1084/jem.160.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mezrich J, Yamada K, Sachs DH, Madsen JC. Regulatory T cells generated by the kidney may mediate the beneficial immune effects of combining kidney with heart transplantation. Surgery. 2004;135:473–478. doi: 10.1016/j.surg.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Brouard S, Pallier A, Renaudin K, et al. The natural history of clinical operational tolerance after kidney transplantation through twenty-seven cases. Am J Transplant. 2012;12:3296–3307. doi: 10.1111/j.1600-6143.2012.04249.x. [DOI] [PubMed] [Google Scholar]
- 29.Liu XQ, Hu ZQ, Pei YF, Tao R. Clinical operational tolerance in liver transplantation: state-of-the-art perspective and future prospects. Hepatobiliary Pancreat Dis Int. 2013;12:12–33. doi: 10.1016/s1499-3872(13)60002-8. [DOI] [PubMed] [Google Scholar]
- 30.Alex BG, Bertolino PD, Bowen DG, McCaughan GW. Tolerance in liver transplantation. Best Pract Res Clin Gastroenterol. 2012;26:73–84. doi: 10.1016/j.bpg.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Assy N, Adams PC, Myers P, Simon V, Ghent CN. A randomised controlled trial of total immunosuppression withdrawal in stable liver transplant recipients. Gut. 2007;56:304–306. doi: 10.1136/gut.2006.107862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramos HC, Reyes J, Abu-Elmagd K, et al. Weaning of immunosuppression in long-term liver transplant recipients. Transplantation. 1995;59:212–217. doi: 10.1097/00007890-199501270-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng S, Ekong UD, Lobritto SJ, et al. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA. 2012;307:283–293. doi: 10.1001/jama.2011.2014. [DOI] [PubMed] [Google Scholar]
- 34.Chandrasekharan D, Issa F, Wood KJ. Achieving operational tolerance in transplantation: how can lessons from the clinic inform research directions? Transpl Int. 2013;26:576–589. doi: 10.1111/tri.12081. [DOI] [PubMed] [Google Scholar]
- 35.Madsen JC, Sachs DH, Fallon JT, Weissman NJ. Cardiac allograft vasculopathy in partially inbred miniature swine.I.Time course, pathology, and dependence on immune mechanisms. J Thorac Cardiovasc Surg. 1996;111:1230–1239. doi: 10.1016/s0022-5223(96)70226-x. [DOI] [PubMed] [Google Scholar]
- 36.Rosengard BR, Ojikutu CA, Guzzetta PC, et al. Induction of specific tolerance to class I disparate renal allografts in miniature swine with cyclosporine. Transplantation. 1992;54:490–497. doi: 10.1097/00007890-199209000-00020. [DOI] [PubMed] [Google Scholar]
- 37.Allan JS, Wain JC, Schwarze ML, et al. Modeling chronic lung allograft rejection in miniature swine. Transplantation. 2002;73:447–453. doi: 10.1097/00007890-200202150-00020. [DOI] [PubMed] [Google Scholar]
- 38.Utsugi R, Barth RN, Lee RS, et al. Induction of transplantation tolerance with a short course of tacrolimus (FK506): I. Rapid and stable tolerance to two-haplotype fully mhc-mismatched kidney allografts in miniature swine. Transplantation. 2001;71:1368–1379. doi: 10.1097/00007890-200105270-00003. [DOI] [PubMed] [Google Scholar]
- 39.Shoji T, Sahara H, Muniappan A, et al. An MHC class II disparity raises the threshold for tolerance induction in pulmonary allografts in miniature swine. Transplant Proc. 2006;38:3268–3270. doi: 10.1016/j.transproceed.2006.10.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madariaga ML, Michel SG, Tasaki M, et al. Induction of cardiac allograft tolerance across a full MHC barrier in miniature swine by donor kidney cotransplantation. Am J Transplant. 2013;13:2558–2566. doi: 10.1111/ajt.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Massicot-Fisher J, Noel P, Madsen JC. Recommendations of the NHLBI heart and lung tolerance working group. Transplantation. 2001;72:1467–1470. doi: 10.1097/00007890-200110270-00028. [DOI] [PubMed] [Google Scholar]
- 42.Madsen JC, Yamada K, Allan JS, et al. Transplantation tolerance prevents cardiac allograft vasculopathy in major histocompatibility complex class I-disparate miniature swine. Transplantation. 1998;65:304–313. doi: 10.1097/00007890-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 43.Mezrich J, Yamada K, Sachs DH, Madsen JC. Regulatory T cells generated by the kidney may mediate the beneficial immune effects of combining kidney with heart transplantation. Surgery. 2004;135:473–478. doi: 10.1016/j.surg.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Mezrich JD, Kesselheim JA, Johnston DR, Yamada K, Sachs DH, Madsen JC. The role of regulatory cells in miniature swine rendered tolerant to cardiac allografts by donor kidney cotransplantation. Am J Transplant. 2003;3:1107–1115. doi: 10.1046/j.1600-6143.2003.00202.x. [DOI] [PubMed] [Google Scholar]
- 45.Mezrich JD, Benjamin LC, Sachs JA, et al. Role of the thymus and kidney graft in the maintenance of tolerance to heart grafts in miniature swine. Transplantation. 2005;79:1663–1673. doi: 10.1097/01.tp.0000160679.04441.b7. [DOI] [PubMed] [Google Scholar]
- 46.Mezrich JD, Yamada K, Lee RS, et al. Induction of tolerance to heart transplants by simultaneous cotransplantation of donor kidneys may depend on a radiation-sensitive renal-cell population. Transplantation. 2003;76:625–631. doi: 10.1097/01.TP.0000079926.80833.42. [DOI] [PubMed] [Google Scholar]
- 47.Yamada K, Choo JK, Allan JS, et al. The effect of thymectomy on tolerance induction and cardiac allograft vasculopathy in a miniature swine heart/kidney transplantation model. Transplantation. 1999;68:485–491. doi: 10.1097/00007890-199908270-00007. [DOI] [PubMed] [Google Scholar]
- 48.Madariaga ML, Michel SG, La Muraglia GM, et al. Kidney-induced Cardiac Allograft Tolerance in Miniature Swine is Dependent on SLA-Matching of Donor Cardiac and Renal Parenchyma. American Journal of Transplantation. doi: 10.1111/ajt.13131. In Press. 3-25-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giangrande I, Yamada K, Arn S, Lorf T, Sachs DH, LeGuern C. Selective increase in CD4-positive graft-infiltrating mononuclear cells among the infiltrates in class I disparate kidney grafts undergoing rejection. Transplantation. 1997;63:722–728. doi: 10.1097/00007890-199703150-00019. [DOI] [PubMed] [Google Scholar]
- 50.Mezrich JD, Kesselheim JA, Johnston DR, Yamada K, Sachs DH, Madsen JC. The role of regulatory cells in miniature Swine rendered tolerant to cardiac allografts by donor kidney cotransplantation. Am J Transplant. 2003;3:1107–1115. doi: 10.1046/j.1600-6143.2003.00202.x. [DOI] [PubMed] [Google Scholar]
- 51.Calne R, Davies H. Organ graft tolerance: the liver effect. Lancet. 1994;343:67–68. doi: 10.1016/s0140-6736(94)90809-5. [DOI] [PubMed] [Google Scholar]
- 52.Rasmussen A, Davies HF, Jamieson NV, Evans DB, Calne RY. Combined transplantation of liver and kidney from the same donor protects the kidney from rejection and improves kidney graft survival. Transplantation. 1995;59:919–921. [PubMed] [Google Scholar]
- 53.Praseedom RK, McNeil KD, Watson CJ, et al. Combined transplantation of the heart, lung, and liver. Lancet. 2001;358:812–813. doi: 10.1016/S0140-6736(01)06003-2. [DOI] [PubMed] [Google Scholar]
- 54.Hart AJ, Smellie WA, Calne RY. Fate of kidney allografts from cadavers whose livers were also transplanted. Lancet. 1971;1:103–105. doi: 10.1016/s0140-6736(71)90839-7. [DOI] [PubMed] [Google Scholar]
- 55.Te HS, Anderson AS, Millis JM, Jeevanandam V, Jensen DM. Current state of combined heart-liver transplantation in the United States. J Heart Lung Transplant. 2008;27:753–759. doi: 10.1016/j.healun.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 56.Topilsky Y, Raichlin E, Hasin T, et al. Combined heart and liver transplant attenuates cardiac allograft vasculopathy compared with isolated heart transplantation. Transplantation. 2013;95:859–865. doi: 10.1097/TP.0b013e31827eef7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raichlin E, Daly RC, Rosen CB, et al. Combined heart and liver transplantation: a single-center experience. Transplantation. 2009;88:219–225. doi: 10.1097/TP.0b013e3181ac60db. [DOI] [PubMed] [Google Scholar]
- 58.Barshes NR, DiBardino DJ, McKenzie ED, et al. Combined lung and liver transplantation: the United States experience. Transplantation. 2005;80:1161–1167. doi: 10.1097/01.tp.0000165717.23652.09. [DOI] [PubMed] [Google Scholar]
- 59.Couetil JP, Houssin DP, Soubrane O, et al. Combined lung and liver transplantation in patients with cystic fibrosis. A 4 1/2-year experience. J Thorac Cardiovasc Surg. 1995;110:1415–1422. doi: 10.1016/s0022-5223(95)70064-1. [DOI] [PubMed] [Google Scholar]
- 60.Czer LS, Ruzza A, Vespignani R, et al. Survival and allograft rejection rates after combined heart and kidney transplantation in comparison with heart transplantation alone. Transplant Proc. 2011;43:3869–3876. doi: 10.1016/j.transproceed.2011.08.095. [DOI] [PubMed] [Google Scholar]
- 61.Raichlin E, Kushwaha SS, Daly RC, et al. Combined heart and kidney transplantation provides an excellent survival and decreases risk of cardiac cellular rejection and coronary allograft vasculopathy. Transplant Proc. 2011;43:1871–1876. doi: 10.1016/j.transproceed.2011.01.190. [DOI] [PubMed] [Google Scholar]
- 62.Hermsen JL, Nath DS, del Rio AM, et al. Combined heart-kidney transplantation: the University of Wisconsin experience. J Heart Lung Transplant. 2007;26:1119–1126. doi: 10.1016/j.healun.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 63.Narula J, Bennett LE, DiSalvo TG, Hosenpud JD, Semigran MJ, Dec GW. Outcomes in recipients of combined heart-kidney transplantation. Multi-organ, same-donor transplant study of the ISHLT/UNOS scientific registry. Transplantation. 1997;63:861–867. doi: 10.1097/00007890-199703270-00012. [DOI] [PubMed] [Google Scholar]
- 64.Luckraz H, Parameshwar J, Charman SC, Firth J, Wallwork J, Large S. Short- and long-term outcomes of combined cardiac and renal transplantation with allografts from a single donor. J Heart Lung Transplant. 2003;22:1318–1322. doi: 10.1016/s1053-2498(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 65.Cobbold SP, Adams E, Graca L, et al. Immune privilege induced by regulatory T cells in transplantation tolerance. Immunol Rev. 2006;213:239–255. doi: 10.1111/j.1600-065X.2006.00428.x. [DOI] [PubMed] [Google Scholar]
- 66.Porrett PM, Yuan X, LaRosa DF, et al. Mechanisms underlying blockade of allograft acceptance by TLR ligands. J Immunol. 2008;181:1692–1699. doi: 10.4049/jimmunol.181.3.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Witt CA, Meyers BF, Hachem RR. Pulmonary infections following lung transplantation. Thorac Surg Clin. 2012;22:403–412. doi: 10.1016/j.thorsurg.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamamoto S, Nava RG, Zhu J, et al. Cutting edge: Pseudomonas aeruginosa abolishes established lung transplant tolerance by stimulating B7 expression on neutrophils. J Immunol. 2012;189:4221–4225. doi: 10.4049/jimmunol.1201683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Todd JL, Wang X, Sugimoto S, et al. Hyaluronan contributes to bronchiolitis obliterans syndrome and stimulates lung allograft rejection through activation of innate immunity. Am J Respir Crit Care Med. 2014;189:556–566. doi: 10.1164/rccm.201308-1481OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilhelm MJ, Pratschke J, Beato F, et al. Activation of the heart by donor brain death accelerates acute rejection after transplantation. Circulation. 2000;102:2426–2433. doi: 10.1161/01.cir.102.19.2426. [DOI] [PubMed] [Google Scholar]
- 71.Slegtenhorst BR, Dor FJ, Rodriguez H, Voskuil FJ, Tullius SG. Ischemia/reperfusion Injury and its Consequences on Immunity and Inflammation. Curr Transplant Rep. 2014;1:147–154. doi: 10.1007/s40472-014-0017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemiareperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10:79–89. doi: 10.1038/nrgastro.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Francuski M, Reutzel-Selke A, Weiss S, et al. Donor brain death significantly interferes with tolerance induction protocols. Transpl Int. 2009;22:482–493. doi: 10.1111/j.1432-2277.2008.00776.x. [DOI] [PubMed] [Google Scholar]
- 74.Kreisel D, Sugimoto S, Zhu J, et al. Emergency granulopoiesis promotes neutrophildendritic cell encounters that prevent mouse lung allograft acceptance. Blood. 2011;118:6172–6182. doi: 10.1182/blood-2011-04-347823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Daud SA, Yusen RD, Meyers BF, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175:507–513. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 76.Meltzer AJ, Veillette GR, Aoyama A, et al. Donor Brain Death Inhibits Tolerance Induction in Miniature Swine Recipients of Fully MHC-Disparate Pulmonary Allografts. Am J Transplant. 2012;12:1290–1295. doi: 10.1111/j.1600-6143.2011.03949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ochando JC, Homma C, Yang Y, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 78.Abe M, Wang Z, de CA, Thomson AW. Plasmacytoid dendritic cell precursors induce allogeneic T-cell hyporesponsiveness and prolong heart graft survival. Am J Transplant. 2005;5:1808–1819. doi: 10.1111/j.1600-6143.2005.00954.x. [DOI] [PubMed] [Google Scholar]
- 79.Gehrie E, Van der Touw W, Bromberg JS, Ochando JC. Plasmacytoid dendritic cells in tolerance. Methods Mol Biol. 2011;677:127–147. doi: 10.1007/978-1-60761-869-0_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frasca L, Marelli-Berg F, Imami N, et al. Interferon-gamma-treated renal tubular epithelial cells induce allospecific tolerance. Kidney Int. 1998;53:679–689. doi: 10.1046/j.1523-1755.1998.00800.x. [DOI] [PubMed] [Google Scholar]
- 81.Deckers JGM, Boonstr JG, Van der Kooij SW, Daha MR, van der Woude FJ. Tissue-specific characteristics of cytotoxic graft-infiltrating T cells during renal allograft rejection. Transplantation. 1997;64:178–181. doi: 10.1097/00007890-199707150-00034. [DOI] [PubMed] [Google Scholar]
- 82.Hadley GA, Rostapshova EA, Bartlett ST. Dominance of tissue-restricted cytotoxic T lymphocytes in the response to allogeneic renal epithelial cell lines. Transplantation. 1996;62:75–83. doi: 10.1097/00007890-199607150-00016. [DOI] [PubMed] [Google Scholar]
- 83.Hagerty DT, Allen PM. Processing and presentation of self and foreign antigens by the renal proximal tubule. J Immunol. 1992;148:2324–2330. [PubMed] [Google Scholar]
- 84.Kirby JA, Rajasekar MR, Lin Y, Proud G, Taylor RM. Interaction between T lymphocytes and kidney epithelial cells during renal allograft rejection. Kidney Int Suppl. 1993;39:S124–S128. [PubMed] [Google Scholar]
- 85.Singer GG, Yokoyama H, Bloom RD, Jevnikar AM, Nabavi N, Kelley VR. Stimulated renal tubular epithelial cells induce anergy in CD4+ T cells. Kidney Int. 1993;44:1030–1035. doi: 10.1038/ki.1993.345. [DOI] [PubMed] [Google Scholar]
- 86.Brown K, Moxham V, Karegli J, Phillips R, Sacks SH, Wong W. Ultra-localization of Foxp3+ T cells within renal allografts shows infiltration of tubules mimicking rejection. Am J Pathol. 2007;171:1915–1922. doi: 10.2353/ajpath.2007.070396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Veronese F, Rotman S, Smith RN, et al. Pathological and clinical correlates of FOXP3+ cells in renal allografts during acute rejection. Am J Transplant. 2007;7:914–922. doi: 10.1111/j.1600-6143.2006.01704.x. [DOI] [PubMed] [Google Scholar]
- 88.Schoop R, Wahl P, Le HM, Heemann U, Wang M, Wuthrich RP. Suppressed T-cell activation by IFN-gamma-induced expression of PD-L1 on renal tubular epithelial cells. Nephrol Dial Transplant. 2004;19:2713–2720. doi: 10.1093/ndt/gfh423. [DOI] [PubMed] [Google Scholar]
- 89.Mohib K, Guan Q, Diao H, Du C, Jevnikar AM. Proapoptotic activity of indoleamine 2,3-dioxygenase expressed in renal tubular epithelial cells. Am J Physiol Renal Physiol. 2007;293:F801–F812. doi: 10.1152/ajprenal.00044.2007. [DOI] [PubMed] [Google Scholar]
- 90.Krupnick AS, Gelman AE, Barchet W, et al. Murine vascular endothelium activates and induces the generation of allogeneic CD4+25+Foxp3+ regulatory T cells. J Immunol. 2005;175:6265–6270. doi: 10.4049/jimmunol.175.10.6265. [DOI] [PubMed] [Google Scholar]
- 91.Robertson H, Wong WK, Burt AD, Mohamed MA, Talbot D, Kirby JA. Relationship between TGFbeta(1), intratubular CD103 positive T cells and acute renal allograft rejection. Transplant Proc. 2001;33:1159. doi: 10.1016/s0041-1345(00)02441-6. [DOI] [PubMed] [Google Scholar]
- 92.Zheng SG. The Critical Role of TGF-beta1 in the Development of Induced Foxp3+ Regulatory T Cells. Int J Clin Exp Med. 2008;1:192–202. [PMC free article] [PubMed] [Google Scholar]
- 93.Pallotta MT, Orabona C, Volpi C, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12:870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 94.Cravedi P, Manrique J, Hanlon KE, et al. Immunosuppressive effects of erythropoietin on human alloreactive T cells. J Am Soc Nephrol. 2014;25:2003–2015. doi: 10.1681/ASN.2013090945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sumpter TL, Abe M, Tokita D, Thomson AW. Dendritic cells, the liver, and transplantation. Hepatology. 2007;46:2021–2031. doi: 10.1002/hep.21974. [DOI] [PubMed] [Google Scholar]
- 96.Knolle P, Schlaak J, Uhrig A, Kempf P, Meyer zum Buschenfelde KH, Gerken G. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J Hepatol. 1995;22:226–229. doi: 10.1016/0168-8278(95)80433-1. [DOI] [PubMed] [Google Scholar]
- 97.Limmer A, Ohl J, Kurts C, et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med. 2000;6:1348–1354. doi: 10.1038/82161. [DOI] [PubMed] [Google Scholar]
- 98.von ON, Schurich A, Hegenbarth S, et al. Systemic antigen cross-presented by liver sinusoidal endothelial cells induces liver-specific CD8 T-cell retention and tolerization. Hepatology. 2009;49:1664–1672. doi: 10.1002/hep.22795. [DOI] [PubMed] [Google Scholar]
- 99.Bowen DG, Zen M, Holz L, Davis T, McCaughan GW, Bertolino P. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J Clin Invest. 2004;114:701–712. doi: 10.1172/JCI21593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang HR, Chou HS, Gu X, et al. Mechanistic insights into immunomodulation by hepatic stellate cells in mice: a critical role of interferon-gamma signaling. Hepatology. 2009;50:1981–1991. doi: 10.1002/hep.23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schildberg FA, Sharpe AH, Turley SJ. Hepatic immune regulation by stromal cells. Curr Opin Immunol. 2015;32C:1–6. doi: 10.1016/j.coi.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 102.Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004;172:1009–1017. doi: 10.4049/jimmunol.172.2.1009. [DOI] [PubMed] [Google Scholar]
- 103.Mazariegos GV, Zahorchak AF, Reyes J, Chapman H, Zeevi A, Thomson AW. Dendritic cell subset ratio in tolerant, weaning and non-tolerant liver recipients is not affected by extent of immunosuppression. Am J Transplant. 2005;5:314–322. doi: 10.1111/j.1600-6143.2004.00672.x. [DOI] [PubMed] [Google Scholar]
- 104.Diehl L, Schurich A, Grochtmann R, Hegenbarth S, Chen L, Knolle PA. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T cell tolerance. Hepatology. 2008;47:296–305. doi: 10.1002/hep.21965. [DOI] [PubMed] [Google Scholar]
- 105.Tiegs G, Lohse AW. Immune tolerance: what is unique about the liver. J Autoimmun. 2010;34:1–6. doi: 10.1016/j.jaut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 106.Xiao S, Jin H, Korn T, et al. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008;181:2277–2284. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu SM, Lee DH, Sullivan JM, et al. Differential IL-21 signaling in APCs leads to disparate Th17 differentiation in diabetes-susceptible NOD and diabetes-resistant NOD.Idd3 mice. J Clin Invest. 2011;121:4303–4310. doi: 10.1172/JCI46187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yu MC, Chen CH, Liang X, et al. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology. 2004;40:1312–1321. doi: 10.1002/hep.20488. [DOI] [PubMed] [Google Scholar]
- 109.Chou HS, Hsieh CC, Yang HR, et al. Hepatic stellate cells regulate immune response by way of induction of myeloid suppressor cells in mice. Hepatology. 2011;53:1007–1019. doi: 10.1002/hep.24162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chou HS, Hsieh CC, Charles R, et al. Myeloid-Derived Suppressor Cells Protect Islet Transplants By B7-H1 Mediated Enhancement of T Regulatory Cells. Transplantation. 2011 doi: 10.1097/TP.0b013e31823ffd39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Koyama I, Nadazdin O, Boskovic S, et al. Depletion of CD8 memory T cells for induction of tolerance of a previously transplanted kidney allograft. Am J Transplant. 2007;7:1055–1061. doi: 10.1111/j.1600-6143.2006.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sweet SC. Induction therapy in lung transplantation. Transpl Int. 2013;26:696–703. doi: 10.1111/tri.12115. [DOI] [PubMed] [Google Scholar]
- 113**.Krupnick AS, Lin X, Li W, et al. Central memory CD8+ T lymphocytes mediate lung allograft acceptance. J Clin Invest. 2014;124:1130–1143. doi: 10.1172/JCI71359. [CD8+ memory T cells were commonly viewed as active promotors of alloreactivity that were associated with graft rejection. This paper provides evidence for an alternate role of CD8+ memory T cells particular to lung allografts that leads to downregulation of alloimmunity and increased graft acceptance.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li W, Bribriesco AC, Nava RG, et al. Lung transplant acceptance is facilitated by early events in the graft and is associated with lymphoid neogenesis. Mucosal Immunol. 2012;5:544–554. doi: 10.1038/mi.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gelman AE, Li W, Richardson SB, et al. Cutting edge: Acute lung allograft rejection is independent of secondary lymphoid organs. J Immunol. 2009;182:3969–3973. doi: 10.4049/jimmunol.0803514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lakkis FG, Arakelov A, Konieczny BT, Inoue Y. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med. 2000;6:686–688. doi: 10.1038/76267. [DOI] [PubMed] [Google Scholar]
- 117.Zhang N, Schroppel B, Lal G, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]