Abstract
During mouse development, core planar cell polarity (PCP) proteins become polarized in the epidermal plane to guide angling/morphogenesis of hair follicles. How PCP is established is poorly understood. Here, we identify a key role for Wdr1 (also known as Aip1), an F-actin-binding protein that enhances cofilin/destrin-mediated F-actin disassembly. We show that cofilin and destrin function redundantly in developing epidermis, but their combined depletion perturbs cell adhesion, cytokinesis, apicobasal polarity and PCP. Although Wdr1 depletion accentuates single-loss-of-cofilin/destrin phenotypes, alone it resembles core PCP mutations. Seeking a mechanism, we find that Wdr1 and cofilin/destrin-mediated actomyosin remodelling are essential for generating or maintaining cortical tension within the developing epidermal sheet and driving the cell shape and planar orientation changes that accompany establishment of PCP in mammalian epidermis. Our findings suggest intriguing evolutionary parallels but mechanistic modifications to the distal wing hinge-mediated mechanical forces that drive cell shape change and orient PCP in the Drosophila wing disc.
PCP, the collective polarization of cells within a tissue plane, is an evolutionarily conserved hallmark of epithelial tissues1–3. Mouse skin development affords an excellent model to study the molecular mechanisms underlying this process in mammals. Epidermal cells use PCP as early as embryonic day 14.5 (E14.5), when core PCP proteins become asymmetrically localized along the anterior–posterior faces of basal layer cells4. When conserved PCP components Fzd6 (frizzled-6), Celsr1 (flamingo) and Vangl2 (strabismus) are mutated, perturbations arise in anterior–posterior distributions of other PCP components leading to misangling of emerging hair follicles4,5.
How PCP is established is still unfolding. In Drosophila, contraction of the developing wing hinge exposes wing-blade epithelial cells to anisotropic tension along the proximal–distal axis, which in turn, leads to oriented elongation, rearrangement and divisions that realign cell polarization along this axis6. Moreover, hinge severing eliminates these forces and alters tissue dynamics and PCP alignment. Whether mechanical forces function in PCP-driven events in other organisms/tissues and if so, by what molecular mechanisms, are unexplored questions in developmental biology7.
Although not investigated in the context of mammalian PCP, pronounced changes in shape accompany the ability of mouse epidermal cells to maintain spindle orientation. Indeed, if progenitors within the innermost, basal layer cannot round up as they enter mitosis, spindle orientation defects arise8. In vivo, epidermal mitotic rounding is regulated by the actin-regulated transcription factor SRF (ref. 8). In vitro, Wdr1 is also involved8–10. Studies from other systems suggest that the effects of Wdr1 depend on its physical interaction with F-actin, as well as its association with members of the cofilin family of actin-binding proteins11–17. However, the mechanisms by which Wdr1 exerts its physiological roles in mammalian systems in vivo are poorly understood. In the mouse, its loss is lethal18, whereas in yeast, it has no obvious phenotype17.
In the present study, we show that, unexpectedly, Wdr1 depletion in embryonic mouse epidermis results in a striking PCP phenotype. In pursuing a mechanism, we discovered that, like the Drosophila wing disc, mouse epidermal basal cells change their shape and orientation during PCP establishment. Combining laser ablation with video microscopy, we further show that coincident with the timing of PCP, cells within the developing epidermis are under tension. Finally, we show that Wdr1 is an important mediator of epidermal tension through its ability to promote cofilin-mediated actin severing, without which PCP cannot be established. Overall, our findings unravel important insights into the physiological roles of Wdr1-mediated actin dynamics and mechanical/geometrical cues in PCP.
RESULTS
Cytoskeletal and PCP phenotypes in Wdr1-deficient skin
To study the role of Wdr1 in vivo, we injected the amniotic sacs of E9.5 embryos in utero with high-titre lentivirus harbouring Wdr1 or scramble short hairpin RNAs (shRNAs) and an H2B–GFP reporter gene19 (Fig. 1a). Western blot and phalloidin (F-actin) staining of Wdr1-depleted basal cells revealed a striking increase in F-actin but not overall actin levels, consistent with the notion that Wdr1 functions as an enhancer of cofilin's F-actin severing activity (Fig. 1a–c). In control tissue, much of the F-actin was cortical and co-localized with E-cadherin. Without Wdr1, the belt of intense F-actin staining extended internally from the cell borders. Adhesion between neighbouring epidermal cells and adherence to their underlying basement membrane seemed to be normal, as judged by ultrastructural analysis (Supplementary Fig. 1). Wdr1-deficient epidermis also exhibited normal patterns of immunolabelling for adhesion proteins (β6 integrin, E-cadherin, ZO1), basement membrane protein (laminin-5) and apicobasal polarity markers (Par3 and centrosomes; Fig. 1c and Supplementary Fig. 2).
Figure 1.
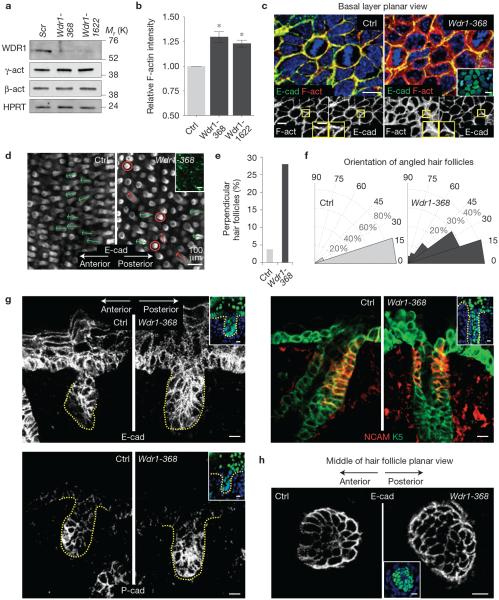
Depletion of Wdr1 alters the cytoskeleton and yields a PCP phenotype. (a) Immunoblot of primary mouse keratinocytes infected with Scr, Wdr1-368 or Wdr1-1622 shRNAs and probed with Wdr1, β-actin or γ-actin and HPRT (loading control) antibodies. (b) FACS analysis of F-actin (phalloidin) relative intensity in control versus Wdr1-368 and control versus Wdr1-1622-depleted primary mouse keratinocytes. Error bars represent s.d. for n=3 independent experiments per sample. P =0.032, control versus Wdr1-368; P =0.022, control versus Wdr1-1622 (independent, unpaired t-tests). Asterisks indicate statistical significance at P <0.05. (c) Whole-mount immunofluorescence of E15.5 embryos labelled for E-cadherin (E-cad), F-actin (F-act) and 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI, in blue) and imaged in the mid-plane of the basal layer. (d) Whole-mount anti-E-cadherin immunofluorescence of E18.5 backskin in control and Wdr1 KD imaged in a plane parallel to the skin surface but near the base of downgrowing hair follicles. Circles denote perpendicular orientations; arrows denote hair follicle angling (green, normal; red, perturbed). (e,f) Quantifications of data shown in d. Control, n = 325; Wdr1, n = 412 hair follicles from 3 embryos per condition. (g) Immunofluorescence of control and Wdr1-KD hair follicles stained for E-cadherin (E-cad) and P-cadherin (P-cad) and co-labelled for NCAM and keratin 5 (K5). (h) Whole-mount anti-E-cadherin immunofluorescence of control and Wdr1-KD hair follicles imaged through a plane at the bottom of the hair follicle. Yellow outlines and inset in c show digital magnification (×2) of F-actin/E-cadherin staining. Insets in c, g and h show that the region imaged was transduced (H2B−GFP+ nuclei). Scale bars, 10 μm (c,g,h) and 100μm (d). Uncropped images of blots are shown in Supplementary Fig. 6.
Probing deeper into the consequences of Wdr1 deficiency, we turned to the hair follicle. Instead of the characteristic anterior–posterior angling of their control counterparts, many of the follicles in Wdr1-368-transduced embryos grew straight downward (Fig. 1d). Quantifications revealed that in control backskins, <4% of hair follicles grew straight downward, whereas most hair follicles were oriented along the anterior–posterior axis (Fig. 1e,f). In contrast, nearly 30% of hair follicles pointed straight downwards in Wdr1-depleted epidermis, and for those that were angled, their orientation was broader than normal. Also lost were the anterior–posterior asymmetries of E-cadherin and P-cadherin expression at the follicle tip, and of NCAM along the posterior side of the follicle shaft (Fig. 1g).
Whole-mount immunofluorescence microscopy also revealed a loss of the typical elongation of anterior hair follicle cells relative to their posterior counterparts (Fig. 1h). These hair follicle defects are hallmarks of looptail and crash embryos4, which harbour mutations in the core PCP genes, Vangl2 and Celsr1 (refs 20,21). Together, these data show that Wdr1 depletion in skin results in most if not all typical PCP abnormalities, including loss of molecular and cell shape asymmetry of the basal epidermal cells at the juncture of hair follicle downgrowths, as well as randomization of follicle orientation within the developing hair coat.
Wdr1-depleted epidermis fails to establish PCP
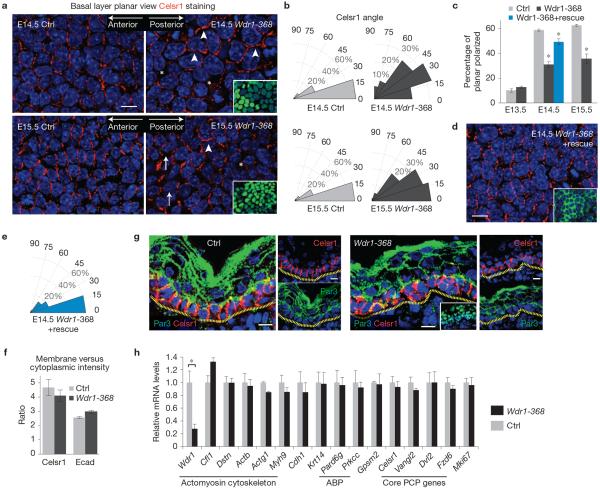
To determine whether Wdr1 activity affects the localization of core PCP components, we analysed the distribution of Celsr1 between E13.5 and E15.5. Before PCP establishment (E13.5), this atypical cadherin was largely unpolarized in both control and Wdr1-deficient epidermis. By E14.5, however, Celsr1 polarization in control basal cells had jumped from <10 to 60%, and the typical angle of polarity was largely along the anterior–posterior axis (Fig. 2a–c). In contrast, Celsr1 distribution in the basal layer cells of Wdr1-deficient epidermis was noticeably perturbed. In some cells, its membrane localization was diminished (asterisk) whereas in others it was concentrated along the dorso-ventral axis (arrowhead). Quantifications showed that <30% of cells showed Celsr1 polarization, and those that did exhibited a broader angle of polarity than control cells (Fig. 2b,c). These defects were not attributable to a developmental delay in PCP, as perturbations were still evident at E15.5 (Fig. 2a). Moreover, the overall ratio between membrane and cytoplasmic intensities of both Celsr1 and E-cadherin was not altered (Fig. 2f), suggesting that these proteins maintain their cortical localization.
Figure 2.
Wdr1-depleted epidermis fails to establish PCP. (a) Whole-mount immunofluorescence of E14.5 and E15.5 backskins from control and Wdr1-KD embryos labelled for Celsr1 and DAPI and imaged in the mid-plane of the basal layer. Arrowheads indicate normally planar polarization, arrows indicate misoriented polarity, asterisks indicate absence of polarity. (b) Histograms of the orientation of Celsr1 relative to the anterior–posterior axis. Measurements are pooled from 3 embryos. E14.5 control, n = 197 cells; E14.5 Wdr1-368, n = 192 cells; E15.5 control, n = 145 cells; E15.5 Wdr1-368, n = 157 cells. (c) Quantification of the percentage of planar-polarized cells from n = 3 embryos per condition. P = 0.29 (Wdr1 versus Ctrl, E13.5), 0.00012 (Wdr1 versus Ctrl, E14.5), 0.0103 (Wdr1+rescue versus Ctrl), 0.026 (Wdr1 versus Ctrl, E15.5), ANOVA followed by Tukey's HSD test. Asterisks indicate statistical significance at P < 0.05. (d) Rescue data showing that the Wdr1-depletion phenotype can be compensated for by Wdr1–GFP. (e) Quantification of data from d, n = 201 cells pooled from 3 embryos. (f) Quantifications of membrane versus cytoplasmic immunofluorescence intensities of Celsr1 and E-cadherin in E14.5 basal epidermis. There is no significant loss of Celsr1 at the membrane in Wdr1-KD epidermis (P = 0.4315, unpaired t-test, n = 8 frames of ~200 cells from 4 embryos for Wdr1 KD, n = 5 frames from 2 embryos for Ctrl). (g) Sagittal views of 10 μm sections of Ctrl and Wdr1-KD E16.5 backskins immunolabelled for Celsr1 and Par3. Dotted lines denote dermal–epidermal border. (h) qPCR of mRNAs from FACS-purified, E14.5 basal epidermal progenitors from Wdr1-368- and scramble- (Ctrl) transduced E14.5 embryos, n = 3 embryos per condition. A statistically significant change was detected only in Wdr1 mRNA levels (1 ± 0.18 versus 0.28±0.07, P = 0.0002, unpaired t-test). ABP, apicobasal polarity. Insets in a,d show that the region imaged was transduced (H2B−GFP+ nuclei). Error bars indicate mean ± s.e.m. Asterisks in c and h indicate statistical significance at P < 0.05, scale bars, 10 μm.
The PCP defects seen with Wdr1-368 were recapitulated in embryos transduced with a second Wdr1 shRNA (Wdr1-1622; Supplementary Fig. 3). Furthermore, a hairpin-resistant complementary DNA encoding a Wdr1–GFP protein rescued the Wdr1-knockdown (KD) PCP defects (Fig. 2c–e and Supplementary Fig. 4). These results validated the specificity of our findings, and underscored the importance of Wdr1 in the process.
Given the known link between cofilin family activity, adhesion and apicobasal polarity13,22, we examined E-cadherin and Par3 immunolocalization (Fig. 2g). Importantly, the apical polarization of Par3 was maintained in Wdr1-KD epidermis, indicating that loss of Wdr1 does not result in a general loss of tissue polarity. Furthermore, by quantitative PCR (qPCR), levels of messenger RNAs encoding E-cadherin, PCP proteins and key apicobasal polarity and cytoskeletal genes were normal (Fig. 2h). Together, these results suggest that without Wdr1, the epidermis cannot establish PCP, even though it still expresses its core constituents and maintains apicobasal polarity.
Wdr1 cooperates with destrin/cofilin to establish planar and apicobasal polarity
Previous in vitro studies have demonstrated that Wdr1 is a potent enhancer of cofilin-mediated actin severing10,23,24. We thus explored the function of cofilin/destrin in establishing epidermal PCP and their relationship to Wdr1. In control epidermis, cofilin, destrin and Wdr1–GFP were all enriched at the periphery of basal cells (Fig. 3a). Their immunofluorescence patterns were not obviously perturbed in Wdr1-KD epidermis, suggesting that their general localization does not depend on Wdr1 in the skin (Supplementary Fig. 5a).
Figure 3.
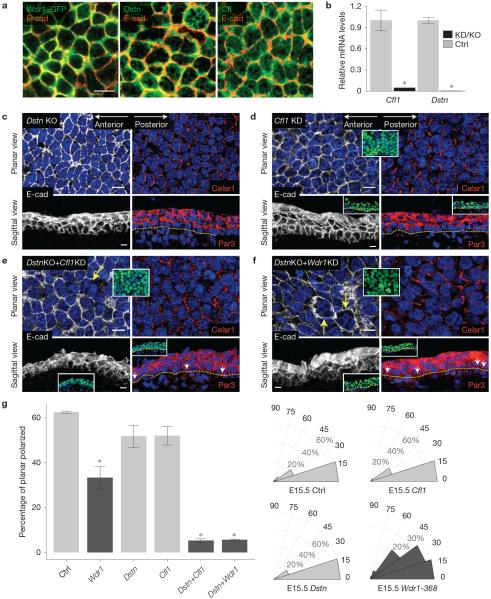
Molecular genetic analyses illuminate roles for the Wdr1-cofilin/destrin pathway in apicobasal and planar cell polarity. (a) Whole-mount immunofluorescence of basal layer cells in E15.5 backskin co-labelled for E-cadherin and WDR1–GFP or destrin (Dstn) or cofilin (Cfl1) and imaged within the mid-plane of the basal layer. (b) qPCR of mRNAs from control and Cfl1KD/Dstn-null (KD/KO) primary mouse keratinocytes, n = 3 samples per condition. P = 0.0026 (Cfl1 levels, Ctrl versus KD); P = 0.009 (Dstn levels in Ctrl versus KO), unpaired t-test. (c–f) Whole-mount immunofluorescence of E15.5 backskins co-labelled for E-cadherin and Celsr1. Sagittal views are of 10 μm sections of E15.5 backskin immunolabelled for E-cadherin and Par3. Data are for the following genetic manipulations: Dstn KO (c), Cfl1 KD (d), DstnKO+Cfl1KD (e), DstnKO+Wdr1-368 (f). (g) Quantifications of the data shown in c–f. For the percentage of planar-polarized cells, P = 0.0014 (Wdr1 versus Ctrl), 0.6487 (Dstn versus Ctrl), 0.5766 (Cfl1 versus Ctrl), 3 × 10−7 (Cfl1+Dstn versus Ctrl), 4 × 10−7 (Wdr1+Dstn versus Ctrl), ANOVA followed by Tukey's HSD test, n = 3 embryos per condition. For histograms, n = 91 cells (Ctrl); n = 135 cells (Cfl1); n = 104 cells (Dstn); n = 82 cells (Wdr1-368). Yellow arrows denote bi/multinucleated cells, occasionally noted in the double mutants; white arrows denote abnormal Par3 localization along the basal surface of progenitor cells. Error bars indicate mean ± s.e.m. Asterisks indicate statistical significance at P < 0.05. Scale bars, 10 μm.
We next addressed whether epidermal loss of Cfl1 and/or Dstn mimics the Wdr1-KD phenotype. To do so, we transduced E9.5 embryos in utero with shRNAs targeting Cfl1 (Fig. 3b), and compared them with embryos harbouring a Dstn null allele (Dstncorn1; refs 25,26). We examined E-cadherin and Par3 immunolocalization as indicators of apical polarization and adhesion. In E15.5 backskins lacking one of the two cofilins, E-cadherin localization remained membranous, and the apical polarization of Par3 was maintained (Fig. 3c,d). In these single mutants, the number of cells exhibiting Celsr1 anterior–posterior enrichment decreased subtly from 63% in control E15.5 epidermis to 52% and 55% respectively (Fig. 3c,d,g). However, a long-range Celsr1 labelling pattern was established in these mutants.
To address the possibility that Cfl1 and Dstn function redundantly in the skin, we knocked down Cfl1 in Dstncorn1 embryos. In sharp contrast to single depletions, removal of both proteins resulted in defects in adhesion, apicobasal polarity, cytokinesis and PCP (Fig. 3e). Par3 localization within the basal layer was no longer excluded from the basal membrane. The typical PCP patterning of Celsr1 was also grossly disrupted, with more than a tenfold decrease in the number of polarized cells (Fig. 3e,g).
In contrast to double cofilin/destrin deficiency, Wdr1 deficiency affected planar polarity but not apicobasal polarity (Figs 2a–g and 3e). However, knockdown of Wdr1 in Dstncorn1 mice (DstnKO+Wdr1) yielded a phenotype similar to those of cofilin/destrin double mutants, where both apicobasal and planar polarity were grossly perturbed (Fig. 3f,g). The effects of destrin, cofilin and Wdr1 on PCP were quantified and are presented in Fig. 3g. These data underscore the impact of Wdr1 on cofilin/destrin function. They further suggest that when Wdr1 is absent, the F-actin-severing activity imparted by one of the cofilin/destrin pair is not sufficiently high to compensate for loss of the other.
If Wdr1 functions primarily to enhance cofilin/destrin activity, then overexpressing a constitutively active mutant of cofilin (CflS3A–GFP; ref. 27) might be expected to compensate for the loss of Wdr1. Although purified CflS3A does not exhibit enhanced enzymatic activity relative to wild-type cofilin28, its overexpression in cells is expected to increase F-actin severing by increasing the pool of cofilin capable of binding and severing actin29.
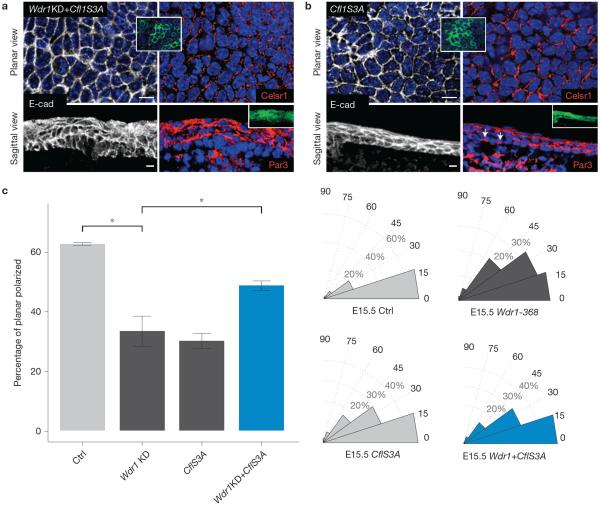
Indeed, CflS3A–GFP restored Celsr1 localization in Wdr1-KD embryos to the anterior–posterior pattern typical of PCP (Fig. 4a,c). This was particularly intriguing, given that in wild-type embryos, constitutively active cofilin caused subtle perturbations in both apicobasal and planar cell polarity (Fig. 4b,c). Supplementary Table 1 summarizes the phenotypic analyses of the data from Figs 3 and 4. Together, these data suggest that Wdr1 is a critical enhancer of cofilin activity, which in turn must be tightly balanced for proper PCP establishment.
Figure 4.
Rescue of the planar polarity defects of Wdr1 mutants by expression of constitutively active cofilin. (a,b) Whole-mount immunofluorescence of E15.5 backskins co-labelled for E-cadherin and Celsr1 or sagittal views labelled with E-cadherin and Par3. (a) Wdr1-368+Cfl1S3A overexpression. (b) Cfl1S3A overexpression. (c) Quantification of data shown in a,b. For the percentage of planar-polarized cells, P = 0.00142 (Wdr1 versus Ctrl), 0.00207 (Cfl1S3A versus Ctrl), 0.247 (Wdr1+Cfl1S3A versus Ctrl), 0.0479 (Wdr1 versus Wdr1+CflS3A), ANOVA followed by Tukey's HSD test, n = 3 embryos per condition. For histograms, n = 91 cells (Ctrl); n = 82 cells (Wdr1-368); n = 131 cells (Cfl1S3A); n = 176 cells (Wdr1KD+Cfl1S3A). Error bars indicate mean ± s.e.m. Asterisks indicate statistical significance at P < 0.05. Scale bars, 10 μm.
Wdr1 is required for efficient F-actin severing in keratinocytes
How Wdr1 enhances cofilin activity is not fully understood. Several potential mechanisms have been proposed, including inhibiting the elongation of ADP–actin filaments with capping protein23 and enhancing the lateral displacement of cofilin to promote actin severing10. It is nevertheless well established that Wdr1 has no catalytic activity of its own and that it exerts its role by interacting with both actin filaments and cofilins.
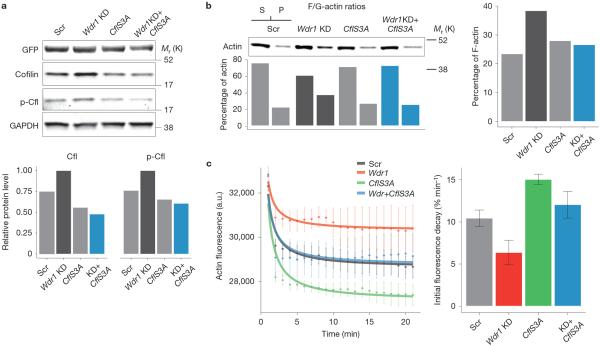
To dig deeper into the link between the cytoskeletal and PCP phenotypes of Wdr1-depleted epidermis and cofilin-mediated actin severing, we first examined endogenous levels of active (unphosphorylated) and inactive (phosphorylated) cofilin in lysates of cells expressing control and Wdr1 shRNAs, as well as cells overexpressing CflS3A–GFP in both a control and Wdr1-depleted background. Wdr1-depleted cells exhibited a ~1.3-fold increase in endogenous cofilin levels (Fig. 5a and Supplementary Figs 5c and 6a). This was surprising, because phalloidin staining in sagittal sections had indicated an increase in F-actin (Fig. 1c). Indeed, western blot analysis in Wdr1-depleted keratinocytes showed a ~2-fold increase in F-actin content (Fig. 5b and Supplementary Fig. 6b). Moreover, F-actin levels were restored to near wild-type levels when CflS3A–GFP was overexpressed in Wdr1-KD cells. These results are consistent with previous in vitro studies suggesting that Wdr1 regulates actin severing not by modulating the pool of cofilin capable of binding actin through phosphorylation, but rather as a direct enhancer of cofilin-mediated actin severing10,23,24. Supporting this, the ability of Wdr1-depleted cell lysates to sever exogenous, pyrene-labelled F-actin was reduced in comparison with scramble-shRNA controls, and could be rescued by CflS3A–GFP overexpression (Fig. 5c).
Figure 5.
Wdr1 is required for efficient F-actin severing in keratinocytes. (a) Western blots illustrating endogenous levels of active (unphosphorylated) and inactive (phosphorylated) cofilin in Wdr1-368-, CflS3A–GFP- and Wdr1+CflS3A–GFP-expressing keratinocytes. Note that histone H2B–GFP was contained within the same vectors as Scramble and Wdr1-368 shRNAs, but not CflS3A–GFP. Quantifications of bands, normalized for GAPDH, are provided below. (b) Loss of Wdr1 is associated with an increase in F-actin, and normal F-actin levels are restored when CflS3A–GFP is expressed in Wdr1-KD keratinocytes. F-actin (pellet), G-actin (supernatant) and total actin levels were determined by quantifying western blots and normalizing against control proteins (see Supplementary Fig. 6). (c) Pyrene F-actin severing assay illustrating that Wdr1 is required for efficient F-actin severing. The rate of fluorescence quenching can be restored in Wdr1-KD keratinocytes by overexpression of CflS3A–GFP. Rates are determined by a linear fit to the initial part of the plot. Data represent the mean ± s.e.m. from n = 4 independent measurements. Uncropped images of blots are shown in Supplementary Fig. 6.
Wdr1 does not affect trafficking of PCP proteins
In contemplating how Wdr1 might affect PCP, we were intrigued by a recent report that depletion of Cfl1 in looptail (Vangl2) mutant mice accentuates PCP defects within the node and midline cells of early post-gastrulation (E9.5) mouse embryos30. In that case, Cfl1 depletion seemed to exacerbate the PCP phenotype by perturbing the planar-polarized trafficking of Rab11+ vesicles to the apical membrane.
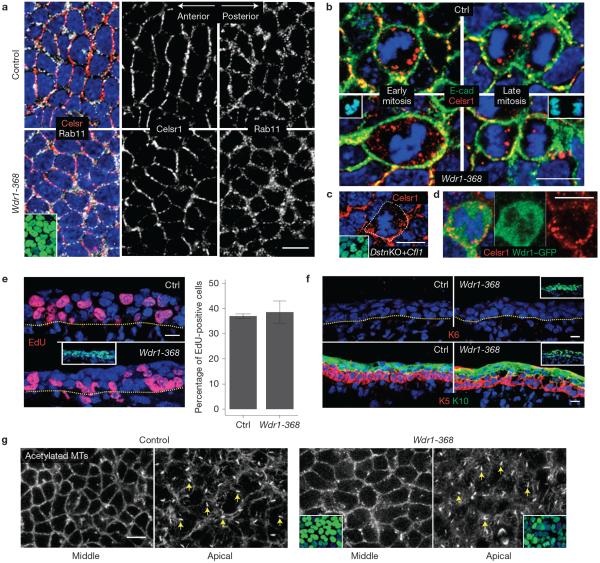
In the epidermis, Rab11 is involved in the recycling of PCP proteins during mitosis31. In E14.5 Wdr1-depleted backskin, however, both Rab11 localization and Celsr1 mitotic internalization seemed to be unperturbed (Fig. 6a,b). This was also true under destrin/cofilin-deficient conditions (Fig. 6c). Furthermore, mitotic epidermal cells of Wdr1–GFP-transduced embryos revealed no obvious signs of Wdr1 internalization into Celsr1-containing vesicles (Fig. 6d).
Figure 6.
Wdr1 does not affect trafficking of PCP proteins, epidermal differentiation, or proliferation. (a) Whole-mount immunofluorescence of E14.5–E15.5 backskins immunolabelled for Celsr1 and Rab11, imaged in the mid-plane of the basal layer. No overt defects in the recycling of Celsr1 to the membrane in Wdr1-KD epidermis are observed. (b,c) Immunofluorescence analysis of mitotic cells reveals no obvious defects in the mitotic internalization of Celsr1 in Wdr1 KD (b), DstnKO+Cfl1KD (c). (d) In Wdr1–GFP-overexpressing epidermis, Wdr1 does not internalize with Celsr1, suggesting that its role in PCP is separate from Celsr1 endocytosis. (e) Left, control and Wdr1-368-KD embryos, transduced at E9.5, were pulsed for 2 h with EdU at E14.5 and then imaged in 10 μm sagittal sections. Right, quantification of EdU+ cells, revealing no proliferation defects in Wdr1-KD epidermis (P = 0.602, unpaired t-test, n = 3 embryos per condition; error bars represent s.d.). (f) Immunofluorescence labelling for keratin 6 (K6), a marker induced on hyperproliferation, or co-labelling for basal marker keratin 5 (K5) and differentiation marker keratin 10 (K10) in 10 μm sagittal sections, revealing no defects in differentiation. (g) Whole-mount immunofluorescence of backskins labelled for acetylated microtubules (MTs), a marker of primary cilia, and imaged in the mid-plane (left) and apical plane (right) of the basal layer. Results are representative of embryos collected from multiple separate litters. Insets indicate transduced cells (H2B−GFP+ nuclei) except for b, where they depict mitoses (DAPI in blue). Dotted lines denote the dermal–epidermal border. Scale bars, 10 μm.
We also pursued possible parallels to loss-of-function defects in flare, the Drosophila orthologue of Wdr1. Flare mutants exhibit a complex phenotype involving cell proliferation, viability and PCP defects32. Despite similarities in PCP defects, Wdr1 KD differed from Flare in showing seemingly unperturbed cell proliferation, viability and differentiation in the developing mouse epidermis (Fig. 6e,f). Moreover, Wdr1 KD did not overtly perturb the microtubule cytoskeleton, which has been reported to affect PCP in Drosophila by regulating the delivery of core PCP proteins33. Wdr1 KD also showed no alterations in the primary cilium, some of whose components have been genetically linked to PCP defects34 (Fig. 6g).
Overall, these results suggest that destrin/cofilin and/or Wdr1 may affect PCP differently in different organisms, tissues and/or developmental windows. Our findings suggest that Wdr1 and destrin/cofilin do not exhibit the typical behaviour of core PCP components (anterior–posterior localization/polarized recycling), but rather act as mediators of PCP. We therefore returned our focus to the loss of PCP-mediated asymmetries in Wdr1-depleted hair follicles (Fig. 1e) and the aberrations in mitotic rounding of basal epidermal cells (Fig. 1c and Supplementary Fig. 9). These defects in cell shape were of particular interest given that in the Drosophila wing disc, global cell shape dynamics are coupled to PCP reorientation6.
Cell shape dynamics are regulated by Wdr1 and coincide with PCP establishment
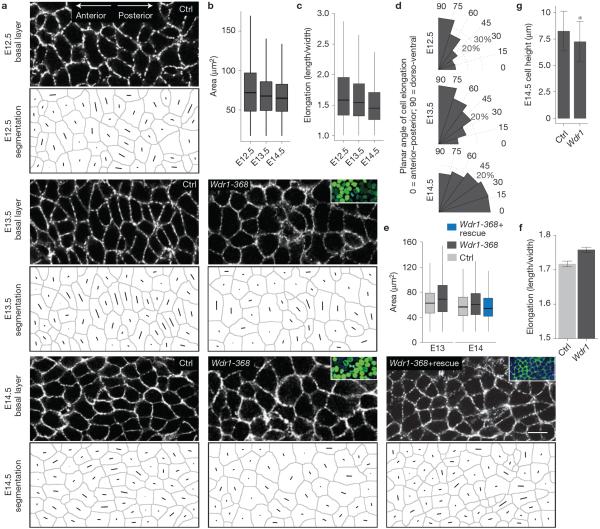
Although developmental cell shape changes have not been previously described within the plane of the mouse epidermal sheet, we wondered whether they might have been hitherto overlooked. To examine this, we used E-cadherin immunolabelling to delineate the epidermal cell borders of control and Wdr1-depleted embryos during the period when PCP is established. Surprisingly, automated analyses of cell shape revealed that from E12.5 (non-polarized epidermis) to E13.5 (partially polarized) to E14.5 (polarized)4, normal basal cells indeed underwent a striking change in both their shape and orientation (Fig. 7a–d). During this process, cells decreased their surface area (from 59.9±0.8 to 51.7±0.8 μm−2) and became less elongated (from 0.44±0.006 to 0.37±0.006). Moreover, cells underwent a reorientation from the dorso-ventral to anterior–posterior axis by E14.5.
Figure 7.
Cell shape dynamics are regulated by Wdr1 and coincide with PCP establishment. (a) Representative whole-mount anti-E-cadherin immunofluorescence images of E12.5, 13.5 and 14.5 control backskins, imaged through the centre plane of cells within the basal layer. Each cell's shape and a line through its axis of elongation were computer-generated for quantifications. (b–d) Quantifications of data from a, n = 3,238 cells (E12); n = 2,984 cells (E13); n = 2,425 cells (E14) pooled from at least 4 embryos per condition. (b) For cell area, P = 3.9 × 10−6 (E12.5 versus E13.5); P = 6.7 × 10−6 (E12.5 versus E14.5); P = 0.0 (E13.5 versus E14.5). (c) For cell elongation, p = 0.0163 (E12.5 versus E13.5); P = 0.0 (E12.5 versus E14.5); P = 0.0 (E13.5 versus E14.5). (d) Histograms of cell orientation relative to the anterior–posterior axis. (e,f) Analogous experiments and analyses as in a–c except for Ctrl versus Wdr1-368 and Ctrl versus Wdr1-368+ rescue backskins, n = 3,695 (E13 scramble); n = 3,085 (E13 Wdr1 KD); n = 3,683 (E14 Ctrl); n = 4,316 (E14 Wdr1 KD); n = 2,565 (Wdr1KD+rescue) pooled from at least 3 embryos. For cell area in e, P = 2.2 × 10−16 (E13.5 Ctrl versus Wdr1-368); P = 6.6 × 10−14 (E14.5 Ctrl versus Wdr1-368); P = 0.127 (E14.5 Ctrl versus Wdr1-368), ANOVA followed by Tukey's HSD test. (f) Cell elongation for E14.5 Ctrl versus Wdr1-368 basal epidermal cells, P = 0.0008156 (unpaired t-test). (g) E14.5 basal progenitor cell heights were calculated from 10 μm sections, P = 0.0007 for Ctrl versus Wdr1 KD (unpaired t-test). Insets in a denote transduced cells (H2B−GFP+). Error bars represent mean ± s.e.m. For box plots in b, c and e, line: median, box: 50% range, whiskers: 1.5 × inter-quartile range. Asterisks indicate statistical significance at P < 0.05. Scale bar, 10 μm.
In Wdr1-KD epidermis, these shape changes did not take place, and cells failed to reorient as in wild-type embryos (Fig. 7a,e–g). At E14.5, Wdr1-depleted cells were also less columnar than their wild-type counterparts (Fig. 7g). Notably, a hairpin-resistant cDNA encoding a Wdr1–GFP protein rescued the Wdr1-KD cell area defects (Fig. 7a,e), underscoring the specificity of Wdr1 in effecting these cell shape changes.
In the Drosophila wing disc, external mechanical forces from the distant wing hinge drive both tissue dynamics and PCP alignment6. In mammalian epidermis, there is no apparent analogy for the forces exerted by the wing hinge. However, Wdr1-depleted mouse epidermis did exhibit defects in F-actin content and organization (Fig. 1), which is known to affect myosin II activity35 and therefore a cell's ability to generate tension. Moreover, Wdr1 is essential for the assembly of contractile actin networks in the Caenorhabditis elegans myoepithelial sheath36, the ability of the mouse heart to contract37, and to recruit myosin II to the contractile ring during cytokinesis10. This prompted us to wonder whether Wdr1 might also regulate tension within the mammalian epidermis.
To test this, we added a membrane GFP marker38 to our lentiviral vectors, and repeated transductions as before.
Wdr1 activity is essential for cell ability to generate/maintain cortical tension
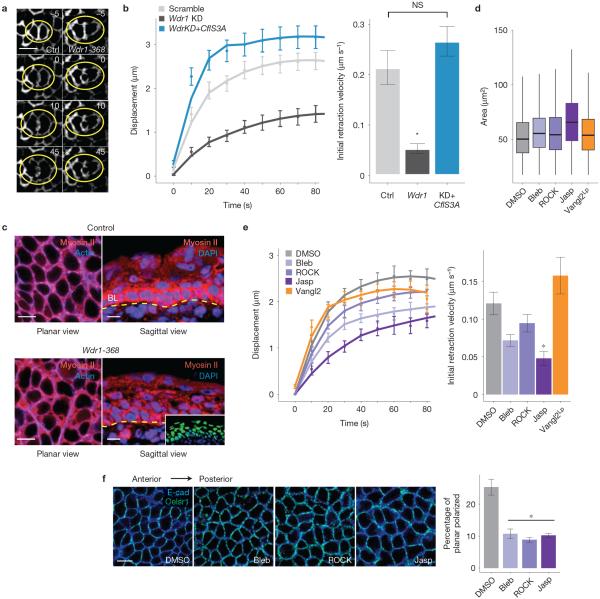
At E14.5, embryos were subjected to laser ablation and imaging ex vivo to estimate tension in the epidermis (Fig. 8a). In scramble-shRNA control skin, laser ablation of membrane and cortical cytoskeleton39–41 at a basal cell–cell interface yielded a mean initial retraction velocity (a quantity proportional to tension) of 0.214 μm s−1 versus 0.0534 μm s−1 in Wdr1-deficient epidermis (Fig. 8b). Similar results were obtained when cells were ablated along the anterior–posterior or dorsal–ventral axes.
Figure 8.
Wdr1 activity is essential for the cell to generate/maintain cortical tension. (a,b) E9.5 embryos were infected with shRNAs in a membrane-targeted GFP expression vector and subjected to laser ablation at E14.5. (a) Representative example of laser ablation in control and Wdr1-368 epidermis. Numbers represent time in seconds before/after ablation. (b) Quantifications of data shown in a, n = 15 (scramble); n = 13 (Wdr1 KD); n = 15 (Wdr1 KD+CflS3A) ablations per condition. Mean initial retraction velocity: Ctrl, 0.214 μm s−1; Wdr1-368, 0.0564 μm s−1, Wdr1-368+CflS3A, 0.266 μm s−1. P = 6.1 × 10−5 (Ctrl versus Wdr1-368), P = 0.314 (Ctrl versus Wdr1-368+CflS3A), ANOVA followed by Tukey's HSD test. (c) Myosin II immunolabelling of E14.5 embryos mosaically expressing Wdr1-368. Planar and sagittal views are indicated for untransduced and transduced regions of epidermis. Dotted line, dermo-epidermal border; BL, basal layer. (d,e) Wild-type mGFP+ embryos at E14.5 were treated with DMSO, jasplakinolide, Y27632 or blebbistatin. All embryos were subjected to cell shape analysis and laser ablation. Data for Vangl2-KD embryos are shown for comparison. (d) Quantification of cell area, n = 2,466 (DMSO); n = 2,764 (Bleb); n = 3,678 (ROCK); n = 514 (Jasp); n = 6,365 (Vangl2Lp), pooled from at least 3 embryos. (e) Mean initial retraction velocities: DMSO, 0.121 μm s−1; Bleb, 0.0716 μm s−1; ROCK, 0.0946 μm s−1; Jasp, 0.0486 μm s−1; and Vangl2Lp, 0.158 μm s−1, P = 0.0563, 0.492, 0.00269 and 0.492, respectively, ANOVA followed by Tukey's HSD test. n = 21 (DMSO); n = 10 (Bleb); n = 11 (ROCK); n = 11 (Jasp); n = 14 (Vangl2 KD). (f) Celsr1 immunolabelling in whole mounts from E14.5 embryos treated with DMSO, jasplakinolide, Y27632 or blebbistatin. The number of planar-polarized cells is reduced following inhibition of tension in the epidermis. DMSO, 25.3%; Bleb, 10.70%; ROCK, 8.86%; Jasp, 10.20%, n = 4 embryos per condition. P = 0.00028, 0.00013 and 0.00022, respectively (ANOVA followed by Tukey's HSD test). Error bars indicate mean ± s.e.m., asterisks indicate statistical significance at P < 0.05. NS, not significant. Scale bars, 10 μm.
To explore the link between Wdr1, actin severing, cortical tension and the establishment of PCP, we investigated whether CflS3A–GFP, which rescues actin severing in Wdr1-depleted keratinocytes, can also rescue the cortical tension defects of Wdr1-KD epidermis. In contrast to Wdr1 KD alone, Wdr1KD+CflS3A–GFP epidermis showed an initial retraction velocity of laser-ablated cell junctions that was essentially equivalent to scramble-shRNA epidermis (0.266 μm s−1 versus 0.214 μm s−1, Fig. 8b). These findings imply a relationship between the regulation of actin severing and tension by Wdr1 and its role in establishing PCP.
Consistent with this notion, loss of Wdr1 resulted in a striking reduction in the localization of myosin II at the cortex of basal epidermal cells (Fig. 8c). Compared with adjacent, untransduced regions (Fig. 8c, left), which exhibited tight localization of myosin II at the cortex, the myosin IIA network of Wdr1-KD cells was more diffuse and extended into the cell interior (Fig. 8c, right).
If the tension defects we see in Wdr1-depleted epidermis reflect perturbations in actomyosin-derived tension, then altering myosin II function10 should generate similar defects. Although expression of multiple myosin II genes in embryonic epidermis precluded genetic analysis, we repeated our assays in E14.5 embryos exposed to either blebbistatin, which inhibits myosin II motor activity42, or Y27632, which inhibits Rho kinase43. Indeed, these inhibitors resulted in modest increases in planar cell area and boundary tension defects similar to the loss of Wdr1 (Fig. 8d,e).
Given the increase in F-actin we observed in the absence of Wdr1, we repeated our assays in E14.5 embryos exposed to jasplakinolide, a drug that stabilizes F-actin and leads to its ectopic accumulation44. Both cell area and boundary tension defects were nearly identical to those of Wdr1-KD embryos (Fig. 8d,e), illustrating that increasing F-actin levels in the epidermis leads to a reduction in cortical tension. Importantly, each of these pharmacological perturbations resulted in a disruption in planar polarity compared with dimethylsulphoxide (DMSO)-treated embryos, with Celsr1 acquiring a more uniform, punctate distribution at the cortex (Fig. 8f).
Importantly, the striking defects in cell shape and epidermal tension were not observed in E14.5 Vangl2 (looptail) mutant embryos, suggesting that Wdr1-mediated changes in cortical tension, organization of the actomyosin cortex, and epidermal architecture function upstream of PCP establishment (Fig. 8d,e and Supplementary Fig. 3e).
DISCUSSION
During development, cells and tissues undergo structural, mechanical and geometrical remodelling that can be coupled to cell fate determination, differentiation and proliferation45,46. These fascinating mechanisms have been explored predominantly in cultured cells and invertebrate model organisms, and are still poorly understood in mammalian systems in vivo. Our study has unearthed pronounced changes in the shape and orientation of epidermal cells that occur within developing mouse skin. Moreover, we showed that the timing of these dynamic changes not only coincides with PCP establishment, but is also physiologically relevant to the process, as reflected by the striking defects in PCP that occur when the shape changes are perturbed.
Our results further unveiled Wdr1 as a major regulator of cell shape dynamics in the skin. Our in vitro and in vivo data showed that depletion of Wdr1 increases F-actin content and perturbs the actin cytoskeleton. Interestingly, although loss of Wdr1 correlates with an increase in endogenous cofilin levels, it ultimately results in a decreased ability to sever F-actin. To understand this seemingly paradoxical result, it is important to note that, although phosphorylation by LIMK diminishes cofilin's activity by abrogating its ability to bind F-actin47,48, actin severing can be regulated by other means than cofilin phosphorylation49. Even when it is bound, cofilin does not necessarily sever F-actin. In fact, dephosphorylated (active) cofilin can form stable F-actin–cofilin structures, as previously reported in several cell types.
The means by which Wdr1 enhances cofilin-mediated actin severing is a matter of debate. According to one view, Wdr1 and cofilin compete for the same binding site on F-actin, where the role of Wdr1 is to reduce cofilin binding to within an optimal range for severing10. Alternatively, Wdr1 could cooperate with cofilin to sever actin filaments rather than compete for the same binding site24. Our finding that overexpression of constitutively active cofilin rescues actin severing in Wdr1-deficient cells is compatible with either model, as high levels of active cofilin are likely to override the requirement for Wdr1 either to competitively bind actin or to cooperate with cofilin to promote severing.
The broader polarity and adhesion defects of combined cofilin/destrin deficiency in the skin suggest a special importance of Wdr1 in epidermal cell shape dynamics associated with PCP. The architecture of actin networks affects myosin II activity35, and cofilin competes with myosin II for the F-actin binding site50. In line with this, our laser ablation studies demonstrated a significant decrease in boundary tension on loss of Wdr1, suggesting that actomyosin-based tension was reduced. Moreover, as treating embryos with jasplakinolide revealed, increasing F-actin content within the developing embryonic epidermis perturbed rather than enhanced actomyosin tension and probably represented an extreme case of the Wdr1 loss-of-function phenotype. Whether these effects are due to competitive binding of either cofilin or jasplakinolide at the expense of myosin II or more generally to organization of the actin cortex remains to be determined.
In closing, the link we unearthed between mammalian epidermal cell shape, actin severing, tension and PCP in some ways resembles Drosophila wing disc development6. However, in contrast to the fly wing disc, intrinsic forces seem to be at the root of this connection in the mouse epidermis. Our additional finding that PCP is linked to Wdr1, cofilins and actin dynamics is interesting in light of the recent surfacing of cofilin (Cfl1) in a genetic screen for PCP mutants in the early mouse embryo30. However, in contrast to the role for cofilins in orchestrating polarized vesicle trafficking30, our data indicate that cells within the developing epidermis are under considerable mechanical tension at the time when PCP is established, and rely on a Wdr1/cofilin-based mechanism to govern the actomyosin dynamics that sculpt cell shapes and orient PCP. These findings unveil diversities in the complex relationship between the cells' mechanical and geometrical cues and tissue development.
METHODS
Mouse lines and lentivirus production and injection
The following mouse strains were used: CD1 (Charles River Laboratories) and Dstncorn1 (Jackson Laboratories). With the exception of Dstn KO and DstnKO/Cfl1KD embryos, all experiments were performed on E14.5–E18.5 embryos obtained by mating wild-type CD1 males to CD1 females. Dstn KO and DstnKO/Cfl1KD embryos were obtained by mating homozygous Dstncorn1 males to homozygous females and injecting with a scrambled-sequence control shRNA or shRNAs against Cfl1. Lentiviral shRNAs were obtained from the TRC-1 Library (Sigma). Production and injection of shRNA lentivirus was performed as described previously19. Briefly, females at E9.5 of gestation were anaesthetized with isoflurane (Hospira) and the sacs of up to eight embryos per litter were injected with 0.25–1 μl of lentivirus (~109 cfu). The Rockefeller University Animal Care and Use Committee approved animal experimentation protocols used in the study.
Epidermal keratinocytes were isolated and cultured from dispase-treated skins of wild-type CD-1 mice. For in vitro infections, cells were plated at 1×105 cells per 6-well plate, infected with >109 cfu lentivirus in the presence of Polybrene. Forty-eight hours after infection, cells were selected with 1 mg ml−1 puromycin (Sigma).
The hairpin sequences used were as follows: Wdr1-368 (TRCN0000108912): 5′-GCTGGGAAGATCAAGGACATT-3′; Wdr1-1622 (TRCN0000108914): 5′-GATGGCTATTCGGAGAATAAT-3′; Cfl1 (TRCN0000071694): 5′-CGCAAGTCTTCAACACCAGAA-3′; Vangl2-1738 (TRCN0000124572): 5′-GTTCTGCATTACCCACGACAT-3′.
Immunofluorescence and western blotting
For tissue analyses, skins were frozen and embedded in OCT compound. For immunoreactions, skin sections (10 μM) were blocked with PBS, 0.3% Triton X-100, 1% bovine serum albumin, 5% normal goat serum, 5% normal donkey serum, or MOM Basic kit (Vector Labs). Primary antibodies were incubated for 1 h at room temperature or overnight at 4 °C. Secondary antibodies were incubated at room temperature for 1 h. For whole-mount immunofluorescence microscopy, embryos were fixed for 1 h in 4% formaldehyde, and primary antibodies were incubated overnight at 4 °C.
Immunoblot analyses were performed as previously described8. Briefly, proteins were extracted in RIPA buffer. Samples were run on 4–12% gradient gels, transferred to nitrocellulose, and blotted overnight with the indicated antibodies. Blots were scanned on an Odyssey CLx imager (LiCor) and quantified by fluorescence intensity.
Antibodies
Primary antibodies used were: Wdr1 (G-13, Santa Cruz), 1:100; Destrin (ab11072, abcam), 1:100; cofilin (ab42824, abcam), 1:5,000; HPRT (ab10479, abcam), 1:5,000; GAPDH (ab8245, abcam), 1:5,000; GFP (ab13970), 1:2,000; myosin II (PRB-440P, Covance), 1:200; pericentrin (PRB-432C, Covance), 1:1,000; E-cadherin (ECCD-1, M. Takeichi, RIKEN, Japan), 1:500; CD104 (β4 integrin, 553745 BD-Pharmingen), 1:500; P-cadherin (P-cad, Zymed), 1:1,000; Keratin14 (PRB-155P, Covance), 1:1,000; keratin 10 (PRB-159P, Covance), 1:1,000; Celsr1 (Fuchs lab), 1:250; keratin 6 (Rb 415, Millipore), 1:500; PAR3 (07-330, Millipore), 1:100; phospho-S3-Cofilin (77G2, Cell Signaling), 1:5,000; Rab11 (D4F5, Cell Signaling), 1:500; β-actin (AC-15, Sigma), 1:5,000; γ-actin (2-2.1.14.17, Sigma), 1:5,000; α-tubulin (DM1A, Sigma), 1:1,000; acetylated tubulin (6-11B-1, Sigma), 1:1,000. Secondary antibodies conjugated to FITC, rhodamine (Jackson Immunoresearch), or Alexa Fluor 488/546/647 (Invitrogen) were diluted 1:1,000 (sagittal sections) or 1:200 (whole mount), and samples were incubated 1 h–overnight. F-actin was labelled with phalloidin–Alexa Fluor 546/647 (Invitrogen).
Microscopy and image processing
Images were acquired on a Zeiss LSM780 laser-scanning confocal microscope with ×63/1.4 and ×40/1.4 W objectives. Basic image adjustments were performed in Fiji (ImageJ) and Adobe Photoshop CS5. Figures were assembled in Adobe Illustrator CS5.
Quantification of PCP and hair follicle orientation
Whole-mount samples from backskins were processed, labelled for Celsr1 and imaged as described above. Confocal analyses and data collection were conducted at a single horizontal plane through the middle of cells within the basal layer (~3–4 μm above the basement membrane). Background fluorescence was subtracted and a PCP-polarized cell was defined as a basal layer cell flanked by two distinct and opposing lateral domains of Celsr1. To determine the angle of polarity, the `straight line' tool in ImageJ was used to draw a line between the centres of the two opposing Celsr1 domains.
To determine the hair follicle orientation, whole-mount samples from backskins were labelled for E-cadherin and imaged at low magnification in Z-stacks from the plane of the epidermis to the tips of hair follicles. Z-stacks allowed a clear observation of hair follicle angularity. Hair follicles were defined as `perpendicular' if their angularity was 90° to the basal epidermal plane; for angled hair follicles, the degree of angling was measured by using the ImageJ `straight line' tool to draw a line between the base of the hair follicle and its tip.
Quantification of F-actin content and actin-severing assays
For measurements of F/G-actin ratios, cells were lysed in F-actin stabilization buffer (50 mM PIPES at pH 6.9, 50 mM NaCl, 5 mM MgCl2, 5 mM EGTA, 5% glycerol, 0.1% NP-40, 0.1% Triton X-100, 0.1% Tween-20, 0.1% beta-mercaptoethanol, 1 mM ATP, protease and phosphatase inhibitor cocktails) at 37°. F-actin was pelleted by ultracentrifugation at 150,000g, reconstituted in an equal volume of ice-cold 10 μM cytochalasin D, and incubated on ice for 1 h. Actin in supernatant and pellets was quantified by western blot using G-actin standards (Cytoskeleton).
For actin-severing assays, cells were lysed in buffer containing 50 mM KCl, 2 mM MgCl2, 0.5 mM ATP, 2 mM Tris, pH 8.0, 1 mM EGTA, and 1% Triton X-100 and dialysed (Slide-A-Lyzer 10K, Pierce) to remove detergent. Pyrene-labelled F-actin (Cytoskeleton) was added to cell lysates at a final concentration of 3.8 μM. Pyrene fluorescence was monitored for 1 h using a POLARstar Optima fluorescence plate reader (BMG Labtechnologies) using 355 nm excitation and 405 nm emission filters. Cytochalasin D and jasplakinolide-treated lysates were used as negative and positive controls.
Quantitative analysis of cell area and shape
Measurement of cell area and elongation was performed using custom MATLAB scripts. Confocal images of basal layer epidermis were filtered using a two-dimensional band-pass filter, and cells were segmented on the basis of cortical E-cadherin staining using a watershed algorithm. Cell elongation is defined as the ratio of major and minor axes of automatically segmented cells. For each set of measurements, >2,000 cells were measured from >3 embryos.
Laser ablation
Laser ablation of the cell cortex was performed by targeting an ~7 μm2 circular region on a Zeiss LSM 510 NLO system using a Ti:sapphire laser (Chameleon Ultra, Coherent Scientific) tuned to 800 nm. Laser power and dwell time were calibrated per experiment, but were typically performed between 80–100% transmission using scan speed 6 and 50–75 repetitions (~90–140 μs dwell time).
Pharmacological inhibition of tension
Tension was inhibited in live E14.5 embryos by treating them with 50 μM blebbistatin, 0.5 μM jasplakinolide, 10 μM Y-27632, or an equal volume of DMSO for 6 h in Defined Keratinocyte Medium (Life) with 1.5 mM CaCl2. Embryos were subjected to laser ablation studies or fixed for whole-mount immunostaining.
Electron microscopy
For correlative light and electron microscopy, head skin was carefully dissected out from E16.5 embryos and cut into smaller samples. Samples were fixed in 4% PFA, 0.05% glutaraldehyde in sodium cacodylate buffer for ~4 h. After washing with PBS, samples were permeabilized and blocked in a solution containing 1% BSA, 0.2% Triton X-100, NH4Cl 50 mM and incubated overnight in primary antibody (GFP Rabbit IgG Polyclonal Antibody Fraction, Invitrogen, diluted 1:200 in the same solution). Samples were washed and incubated in biotinylated goat anti-rabbit IgG antibody (Vector Laboratories) diluted 1:200 and then processed with the Vectastain ABC reagent (Vector laboratories), using DAB as an enzyme substrate.
For, transmission electron microscopy, samples were fixed in 1% glutaraldehyde in 0.1 M cacodylate buffer, followed by osmium tetroxide fixation and processed for electron microscopy as previously described8. Sections (1 μm) were stained for toluidine blue and examined for the presence of cells immunolabelled for GFP. An ultrathin section (70 nm) immediately consecutive to the semithin section was obtained from selected areas from wild-type and WDR1-368 skin respectively. Ultrathin sections were examined at the electron microscope. The area containing exactly the same cells as the semithin section was photographed and analysed at high magnification.
Fluorescence-activated cell sorting
FACS purification of basal epidermal cells from CD1 E14.5 scramble-shRNA; PGK–H2B–GFP-infected mice (Ctrl) or Wdr1-shRNA; PGK–H2B–GFP (KD) was performed on a FACS Vantage SE system equipped with FACS DiVa software (BD Biosciences). Cells were gated for single events and viability and then sorted according to α6 integrin–PE expression and GFP.
For quantification of F-actin levels, primary mouse keratinocytes were infected with Scramble or WDR1-KD virus, selected with puromycin, trypsinized, fixed and labelled with phalloidin–Alexa Fluor 647.
Semi-quantitative RT-PCR
Equal amounts of RNAs were added to a reverse-transcriptase reaction mix (SuperScript VILO, Invitrogen), and semi-quantitative PCR was conducted with a LightCycler system (Roche Diagnostics). Reactions were performed using the indicated primers and template mixed with the LightCycle DNA master SYBR Green kit and run for 50 cycles. Specificity of the reactions was determined by subsequent melting curve analysis. LightCycle analysis software was used to remove background fluorescence (noise band). The number of cycles needed to reach the crossing point for each sample was used to calculate the amount of each product using the 2–CP method. Levels of PCR product were expressed as a function of Ppib and/or HPRT expression. Sequences of primers used were as follows: Wdr1, forward: 5′-TGGAGCGGGGCGTCTCTA-3′, reverse: 5′-AATCCGCTGGGTGCATACTTG-3′; Cfl1, forward: 5′-ATGCACCCCTCAAGAGCAAAAT-3′, reverse: 5′-AGGGGGTGGGAGGGATGTT-3′; Actb, forward: 5′-CGGCCAGGTCATCACTATTGG-3′, reverse: 5′-AGGGGCCGGACTCATCGTA-3′; Actg1, forward: 5′-CCCAAAGCTAACAGAGAGAAGATGACG-3′, reverse: 5′-GTGGTAAAGCTGTAGCCCCGTTCA-3′; Myh9, forward: 5′-CCTGCCATAAGGGAACCTAATCAC-3′, reverse: 5′-GCGCTCTGGTGCCTCTCCTA-3′; Krt14, forward: 5′-CGCCGCCCCTGGTGTGG-3′, reverse: 5′-ATCTGGCGGTTGGTGGAGGTCA-3′; Cdh1, forward: 5′-GATGATGCCCCCAACACTCC-3′, reverse: 5′-CTCTCGAGCGGTATAAGATGTGATTT-3′; Pard6g, forward: 5′-CAAGCCTGGGAAGTTTGAAGATTT-3′, reverse: 5′-TGCGGCATGCTGATGTTGA-3′; Prkcc, forward: 5′-CAGCGACAGAGAAAACTTCCTGAA-3′, reverse: 5′-TCCCGCCATCATCTCAAACATA-3′; Gpsm2, forward: 5′-TCTGCTGCAAAGAGATCCAAACA-3′, reverse: 5′-TCATGGGCAGGTACAAAAAGTCC-3′; Celsr1, forward: 5′-GGCAGTCATGACCTTGGACTA-3′, reverse: 5′-AGCTGATTCCCAATCTGCAC-3′; Vangl2, forward: 5′-CCAGCCGCTTCTACAATGTC-3′, reverse: 5′-TCTCCAGGATCCACACTGC-3′; Dvl2, forward: 5′-ACTTCACCCTCCCTCGAAA-3′, reverse: 5′-GAGGAGCCAGGGTAAGCAG-3′; Fzd6, forward: 5′-TTAAGCGAAACCGCAAGC-3′, reverse: 5′-TTGGAAATGACCTTCAGCCTA-3′; Ki67, forward: 5′-CCCAGCTCGTCTCCACCACTAGAG-3′, reverse: 5′-TCTGTGTGTTTCTGGTTTGCCTTAC-3′; HPRT, forward: 5′-GATCAGTCAACGGGGGACATAAA-3′, reverse: 5′-CTTGCGCTCATCTTAGGCTTTGT-3′; Ppib, forward: 5′-GTGAGCGCTTCCCAGATGAGA-3′, reverse: 5′-TGCCGGAGTCGACAATGATG-3′.
Quantification of cell proliferation
E14.5 Scr- or Wdr1-shRNA-infected embryos were pulsed with 5 μl g−1 5-ethynyl-2′-deoxyuridine (EdU) (Click-iT EdU Cell Proliferation Assays, Life Technologies) for 2 h. Samples were frozen in OCT, sectioned (10 μm) and processed according to the manufacturer's instructions. The ratio between EdU+/GFP+ (for WDR1- or Scr-infected) cells and nuclei number (DAPI+) was calculated.
Statistics
Quantitative data were statistically analysed (mean and s.e.m.) and compared using a paired or n unpaired t-test (two experimental groups), or ANOVA followed by Tukey's honest significant difference test (multiple groups) in Microsoft Excel or the R statistical environment. Sample sizes and the specific tests performed are indicated in the figure legends. No statistical method was used to predetermine sample size, experiments were not randomized, and investigators were not blinded to allocation during experiments and outcome assessments.
Supplementary Material
ACKNOWLEDGEMENTS
We thank D. Devenport, S. Williams, S. Beronja, A. R. Folgueras, D. Schramek, I. Matos and E. Ezratty for intellectual input; D. Oristian and A. Aldeguer as mouse specialists; Comparative Bioscience Center (AAALAC accredited) for care of mice in accordance with National Institutes of Health (NIH) guidelines; Bioimaging Center (A. North, director) for advice; Flow Cytometry facility (S. Mazel, director) for FACS sorting. Cfl–GFP was a generous gift from J. Condeelis (Albert Einstein college of Medicine, New York, USA); E.F. is an Investigator of the Howard Hughes Medical Institute. This research was supported by a grant from the NIH (R37-AR27883, E.F.), a Starr Stem Cell Postdoctoral Fellowship (C.L.) and a Genetics Training Grant by the NIH (E.H.).
Footnotes
Supplementary Information is available in the online version of the paper
AUTHOR CONTRIBUTIONS C.L. and E.F. conceived the study. C.L., E.H. and E.F. designed the experiments. C.L. and E.H. carried out the experiments and analysed the data. H.A.P. performed the ultrastructural analyses (Supplementary Fig. 1S). S.C. made the Wdr1-rescue construct, C.L. and N.S. performed the in utero injections. C.L. and M.N. prepared high-titre viruses. C.L., E.H. and E.F. wrote the paper. All authors provided intellectual input, vetted and approved the final manuscript.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
References
- 1.Eaton S, Jülicher F. Cell flow and tissue polarity patterns. Curr. Opin. Genet. Dev. 2011;21:747–752. doi: 10.1016/j.gde.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McNeill H. Planar cell polarity: keeping hairs straight is not so simple. Cold Spring Harb. Perspect. Biol. 2010;2:a003376. doi: 10.1101/cshperspect.a003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat. Cell Biol. 2008;10:1257–1268. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo N, Hawkins C, Nathans J. Frizzled6 controls hair patterning in mice. Proc. Natl Acad. Sci. USA. 2004;101:9277–9281. doi: 10.1073/pnas.0402802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aigouy B, et al. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell. 2010;142:773–786. doi: 10.1016/j.cell.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 7.Matis M, Axelrod JD. Regulation of PCP by the Fat signaling pathway. Genes Dev. 2013;27:2207–2220. doi: 10.1101/gad.228098.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luxenburg C, Pasolli HA, Williams SE, Fuchs E. Developmental roles for Srf, cortical cytoskeleton and cell shape in epidermal spindle orientation. Nat. Cell Biol. 2011;13:203–214. doi: 10.1038/ncb2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujibuchi T, et al. AIP1/WDR1 supports mitotic cell rounding. Biochem. Biophys. Res. Commun. 2005;327:268–275. doi: 10.1016/j.bbrc.2004.11.156. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Courtemanche N, Pollard TD. Aip1 promotes actin filament severing by cofilin and regulates the constriction of the cytokinetic contractile ring. J. Biol. Chem. 2014;290:2289–2300. doi: 10.1074/jbc.M114.612978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aizawa H, et al. Hyperosmotic stress-induced reorganization of actin bundles in Dictyostelium cells over-expressing cofilin. Genes Cells. 1999;4:311–324. doi: 10.1046/j.1365-2443.1999.00262.x. [DOI] [PubMed] [Google Scholar]
- 12.Augustine RC, Pattavina KA, Tuzel E, Vidali L, Bezanilla M. Actin interacting protein1 and actin depolymerizing factor drive rapid actin dynamics in Physcomitrella patens. Plant Cell. 2011;23:3696–3710. doi: 10.1105/tpc.111.090753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu D, et al. AIP1 acts with cofilin to control actin dynamics during epithelial morphogenesis. Development. 2012;139:3561–3571. doi: 10.1242/dev.079491. [DOI] [PubMed] [Google Scholar]
- 14.Lin M-C, Galletta BJ, Sept D, Cooper JA. Overlapping and distinct functions for cofilin, coronin and Aip1 in actin dynamics in vivo. J. Cell Sci. 2010;123:1329–1342. doi: 10.1242/jcs.065698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohri K, Ono S. Actin filament disassembling activity of Caenorhabditis elegans actin-interacting protein 1 (UNC-78) is dependent on filament binding by a specific ADF/cofilin isoform. J. Cell Sci. 2003;116:4107–4118. doi: 10.1242/jcs.00717. [DOI] [PubMed] [Google Scholar]
- 16.Okada K, Obinata T, Abe H. XAIP1: a Xenopus homologue of yeast actin interacting protein 1 (AIP1), which induces disassembly of actin filaments cooperatively with ADF/cofilin family proteins. J. Cell Sci. 1999;112(Pt 10):1553–1565. doi: 10.1242/jcs.112.10.1553. [DOI] [PubMed] [Google Scholar]
- 17.Rodal AA, Tetreault JW, Lappalainen P, Drubin DG, Amberg DC. Aip1p interacts with cofilin to disassemble actin filaments. J. Cell Biol. 1999;145:1251–1264. doi: 10.1083/jcb.145.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kile BT, et al. Mutations in the cofilin partner Aip1/Wdr1 cause autoinflammatory disease and macrothrombocytopenia. Blood. 2007;110:2371–2380. doi: 10.1182/blood-2006-10-055087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beronja S, Livshits G, Williams S, Fuchs E. Rapid functional dissection of genetic networks via tissue-specific transduction and RNAi in mouse embryos. Nat. Med. 2010;16:821–827. doi: 10.1038/nm.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curtin JA, et al. Mutation of celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr. Biol. 2003;13:1129–1133. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- 21.Kibar Z, et al. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat. Genet. 2001;28:251–255. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Macara IG. Par-3 mediates the inhibition of LIM kinase 2 to regulate cofilin phosphorylation and tight junction assembly. J. Cell Biol. 2006;172:671–678. doi: 10.1083/jcb.200510061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michelot A, et al. Actin filament elongation in Arp2/3-derived networks is controlled by three distinct mechanisms. Dev. Cell. 2013;24:182–195. doi: 10.1016/j.devcel.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nadkarni AV, Brieher WM. Aip1 destabilizes cofilin-saturated actin filaments by severing and accelerating monomer dissociation from ends. Curr. Biol. 2014;24:2749–2757. doi: 10.1016/j.cub.2014.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeda S, et al. Aberrant actin cytoskeleton leads to accelerated proliferation of corneal epithelial cells in mice deficient for destrin (actin depolymerizing factor) Hum. Mol. Genet. 2003;12:1029–1037. doi: 10.1093/hmg/ddg112. [DOI] [PubMed] [Google Scholar]
- 26.Smith RS, et al. Corn1: a mouse model for corneal surface disease and neovascularization. Invest. Ophthalmol. Vis. Sci. 1996;37:397–404. [PubMed] [Google Scholar]
- 27.Agnew BJ, Minamide LS, Bamburg JR. Reactivation of phosphorylated actin depolymerizing factor and identification of the regulatory site. J. Biol. Chem. 1995;270:17582–17587. doi: 10.1074/jbc.270.29.17582. [DOI] [PubMed] [Google Scholar]
- 28.Nagaoka R, Abe H, Obinata T. Site-directed mutagenesis of the phosphorylation site of cofilin: its role in cofilin-actin interaction and cytoplasmic localization. Cell. Motil. Cytoskeleton. 1996;35:200–209. doi: 10.1002/(SICI)1097-0169(1996)35:3<200::AID-CM3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 29.Kiuchi T, Ohashi K, Kurita S, Mizuno K. Cofilin promotes stimulus-induced lamellipodium formation by generating an abundant supply of actin monomers. J. Cell Biol. 2007;177:465–476. doi: 10.1083/jcb.200610005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahaffey JP, Grego-Bessa J, Liem KF, Anderson KV. Cofilin and Vangl2 cooperate in the initiation of planar cell polarity in the mouse embryo. Development. 2013;140:1262–1271. doi: 10.1242/dev.085316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Devenport D, Oristian D, Heller E, Fuchs E. Mitotic internalization of planar cell polarity proteins preserves tissue polarity. Nat. Cell Biol. 2011;13:893–902. doi: 10.1038/ncb2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren N, Charlton J, Adler PN. The flare gene, which encodes the AIP1 protein of Drosophila, functions to regulate F-actin disassembly in pupal epidermal cells. Genetics. 2007;176:2223–2234. doi: 10.1534/genetics.107.072959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimada Y, Yonemura S, Ohkura H, Strutt D, Uemura T. Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Dev. Cell. 2006;10:209–222. doi: 10.1016/j.devcel.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 34.Ross AJ, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat. Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 35.Reymann AC, et al. Actin network architecture can determine myosin motor activity. Science. 2012;336:1310–1314. doi: 10.1126/science.1221708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ono K, Ono S. Two actin-interacting protein 1 isoforms function redundantly in the somatic gonad and are essential for reproduction in Caenorhabditis elegans. Cytoskeleton (Hoboken) 2014;71:36–45. doi: 10.1002/cm.21152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan B, et al. A cardiomyocyte-specific Wdr1 knockout demonstrates essential functional roles for actin disassembly during myocardial growth and maintenance in mice. Am. J. Pathol. 2014;184:1967–1980. doi: 10.1016/j.ajpath.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Rhee JM, et al. In vivo imaging and differential localization of lipidmodified GFP-variant fusions in embryonic stem cells and mice. Genesis. 2006;44:202–218. doi: 10.1002/dvg.20203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hutson MS, et al. Forces for morphogenesis investigated with laser microsurgery and quantitative modeling. Science. 2003;300:145–149. doi: 10.1126/science.1079552. [DOI] [PubMed] [Google Scholar]
- 40.Farhadifar R, Röper J-C, Aigouy B, Eaton S, Jülicher F. The influence of cell mechanics, cell–cell interactions, and proliferation on epithelial packing. Curr. Biol. 2007;17:2095–2104. doi: 10.1016/j.cub.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez-Gonzalez R, Simoes SDEM, Röper JC, Eaton S, Zallen JA. Myosin II dynamics are regulated by tension in intercalating cells. Dev. Cell. 2009;17:736–743. doi: 10.1016/j.devcel.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Straight AF, et al. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 43.Ishizaki T, et al. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol. Pharmacol. 2000;57:976–983. [PubMed] [Google Scholar]
- 44.Bubb MR, Senderowicz AM, Sausville EA, Duncan KL, Korn ED. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J. Biol. Chem. 1994;269:14869–14871. [PubMed] [Google Scholar]
- 45.Heisenberg CP, Bellaïche Y. Forces in tissue morphogenesis and patterning. Cell. 2013;153:948–962. doi: 10.1016/j.cell.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 46.Guillot C, Lecuit T. Mechanics of epithelial tissue homeostasis and morphogenesis. Science. 2013;340:1185–1189. doi: 10.1126/science.1235249. [DOI] [PubMed] [Google Scholar]
- 47.Arber S, et al. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- 48.Yang N, et al. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- 49.Moriyama K, Yahara I. The actin-severing activity of cofilin is exerted by the interplay of three distinct sites on cofilin and essential for cell viability. Biochem. J. 2002;365:147–155. doi: 10.1042/bj20020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiggan O, Shaw AE, DeLuca JG, Bamburg JR. ADF/cofilin regulates actomyosin assembly through competitive inhibition of myosin II binding to F-actin. Dev. Cell. 2012;22:530–543. doi: 10.1016/j.devcel.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.