Abstract
Objective
To determine whether genetic ancestry was associated with subclinical atherosclerosis measures after adjustment for traditional CVD risk factors, inflammatory marker, socioeconomic status (SES) and psychosocial factors in a large admixed African American population.
Approach and Results
Participants were drawn from Jackson Heart Study (JHS). Participant’s percent of European Ancestry (PEA) was estimated based on 1747 genetic markers using HAPMIX. Association of PEA with peripheral arterial disease (PAD) and common carotid intima media thickness (cCIMT) were investigated among 2168 participants and with coronary artery calcification (CAC >0) and abdominal aortic calcification (AAC >0) among 1139 participants. The associations were evaluated using multivariable regression models. Our results showed a 1 standard deviation increase in PEA was associated with a lower PAD prevalence after adjusting for age and gender [Prevalence ratio (PR) = 0. 90 (95% CI: 0.82, 0.99); p=0.036]. Adjustments for traditional CVD risk factors, SES, and psychosocial factors attenuated this association [PR=0.91 (0.82, 1.00); p=0.046]. There was also a non-linear association between PEA and CAC and AAC. The lowest PEA was associated with a lower CAC [PR=0.75 (0.58, 0.96); p=0.022] and a lower AAC [PR=0. 80 (0.67, 0.96); p=0.016] compared to the reference group (10th–90th percentile) after adjusting for traditional CVD risk factors, inflammatory marker, SES and psychosocial factors. However, we found no significant association between PEA and cCIMT.
Conclusions
Overall, our findings indicate that genetic ancestry was associated with subclinical atherosclerosis, suggesting unmeasured risk factors and/or interactions with genetic factors might contribute to the distribution of subclinical atherosclerosis among African Americans.
Keywords: African Americans, cardiovascular disease, epidemiology, genetic ancestry, subclinical atherosclerosis
Introduction
Subclinical atherosclerosis measures, including peripheral arterial disease (PAD), carotid intima media thickness (CIMT), coronary artery calcification (CAC) and abdominal aortic calcification (AAC) have been shown to be strong predictors of cardiovascular disease (CVD) events in numerous studies.1–3 Several studies have reported substantial racial/ethnic differences in prevalence of subclinical atherosclerosis between African Americans and European Americans. Despite a worse CVD risk factors profile compared with European Americans, African Americans have substantially lower prevalence of CAC and AAC.4–7 In contrast, African Americans have relatively higher mean of CIMT and prevalence of PAD than European Americans.8–11
Previous epidemiological studies have demonstrated that traditional CVD risk factors do not fully explain these differences.5, 9, 12 Other studies have also suggested that these race/ethnic differences are only partially explained by differences in socioeconomic status (SES), psychosocial factors as well as access to health care.13–17 These findings highlight the potentially important role of ancestry-related genetic factors in the development of subclinical atherosclerosis.5,12 However, the association of genetic ancestry with subclinical atherosclerosis is not fully understood probably because most of these studies relied on self-reported race/ethnicity or skin reflectance18, 19 which are measurements that do not take into account for genetic heterogeneity within each racial/ethnic group; ignoring such heterogeneity can conflate the contributions of genetic, SES, or environmental factors.20–22 This problem is particularly relevant in African Americans who are among the most genetic and culturally heterogeneous group in the US, with mixed ancestry mainly from West African and Europe.23 Recent advances in genomics research have allowed to precisely estimate individual genetic ancestry using ancestry informative markers (AIMs)23 or a large set of random markers.24 Genetic ancestry can be a useful tool in determining whether ancestry-related genetic factors or non-genetic factors driving the disparities in subclinical atherosclerosis. Furthermore, genetic ancestry associations can be used as a precursor to admixture mapping for uncovering genes that contribute to subclinical atherosclerosis.25
However, only a limited number of studies examined the association between genetic ancestry and subclinical atherosclerosis in African Americans and they reported mixed results.26–29 One study reported significant associations of European genetic ancestry with CAC and common CIMT (cCIMT) among self-reported African Americans.26 In contrast, another study which involved younger participants, found no significant association of African ancestry with CAC.28 Reiner et al also found that African ancestry was not significantly associated with cCIMT among elderly African Americans.27 Potential reasons for these mixed findings, in addition to age differences, include the use of relatively small sample sizes of African Americans and relatively small number of AIMs to accurately estimate genetic ancestry.26–28 Furthermore, only one study has investigated the association of genetic ancestry with PAD in African Americans, and reported an inverse but non-significant association29 and no study looked at the relationship between AAC and genetic ancestry. In addition, some of these studies have not accounted for a range of non-genetic risk factors, including SES and psychosocial factors. Therefore, the present study attempts to improve our understanding whether ancestry-related genetic factors play an important role in subclinical atherosclerosis by overcoming some of the limitations of previous studies, replicating earlier analysis with a large sample of African Americans whilst controlling for a wider-range of non-genetic risk factors, and examining for the first time the association of genetic ancestry and AAC.
In this study, we used the Jackson Heart Study (JHS) to determine whether genetic ancestry was associated with subclinical atherosclerosis measures after adjustment for traditional CVD risk factors, inflammatory marker as well as SES and psychosocial factors.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement
Results
Table 1 shows comparisons of baseline demographic and clinical characteristics of JHS participants across the percent of European ancestry (PEA) levels. Among the 2168 self-identified African Americans participants, the average age was 54, and 39% were male. On average the participants had a median of 16% European ancestry (IQR=12%–22%). Across the levels of PEA, participants were similar in gender; prevalence of diabetes; smoking; and levels of LDL, HDL, and triglycerides (all P > 0.05). However, those in the highest levels of PEA (>90% PEA) had lower prevalence of hypertension, and lower mean BMI, mean systolic blood pressure, mean hsCRP, mean cCIMT, and mean age (all P < 0.05). Furthermore, those in the highest levels of PEA had higher income and higher education compared with those in the lowest level of PEA (both P < 0.001), but none of the psychosocial variables were significant.
Table 1.
Characteristics of Participants by Levels of Percent of European Ancestry (PEA): The Jackson Heart Study (2000–2004)
| <10th Percentile |
10th–90th Percentile |
>90th Percentile |
||
|---|---|---|---|---|
|
|
||||
| Characteristics | n=202 | n=1749 | n=217 | *P value |
| Percent European Ancestry, median (IQR) (%) | 6.7 (5.7–7.8) | 15.6 (12.3–20.0) | 35.1 (31.5–41.6) | |
| Age, y, mean ± SD | 58.7±13.0 | 53.0±12.4 | 57.9±12.3 | <.0001 |
| Male, n (%) | 75 (37.1) | 665(38.0) | 91 (41.9) | 0.500 |
| CVD risk factors | ||||
| Current smoker, n (%) | 24 (11.9) | 240(13.7) | 22(10.1) | 0.287 |
| Hypertension, n (%) | 146 (72.28) | 1049 (60.0) | 130(59.1) | 0.003 |
| Diabetes, n (%) | 28 (13.9) | 263(15.0) | 32 (14.8) | 0.904 |
| Systolic BP, mm Hg, mean ± SD | 130.4±18.6 | 125.9±17.7 | 126.3±16.8 | 0.002 |
| Diastolic BP, mm Hg, mean ± SD | 79.1±10.3 | 79.6±10.5 | 77.8±9.5 | 0.056 |
| Fasting glucose, mg/dL, median (IQR)† | 92.0 (13.0) | 91.0(15.0) | 92.0(13.0) | 0.637 |
| HDL cholesterol, mg/dL, mean ± SD | 52.2±14.2 | 51.5±14.3 | 51.4±15.4 | 0.590 |
| LDL cholesterol, mg/dL, mean ± SD | 124.6±38.8 | 125.6±36.3 | 125.5±34.3 | 0.904 |
| Triglycerides, mg/dL, median (IQR)† | 85.0 (61.0) | 89.0 (59.0) | 95.0 (69.0) | 0.117 |
| Body mass index, kg/m2, mean ± SD | 31.6±7.1 | 31.7±7.1 | 30.1±6.4 | 0.005 |
| hsCRP, mg/dL, median (IQR)† | 0.24 (0.39) | 0.26 (0.46) | 0.21 (0.35) | 0.019 |
| Anti-hypertension med use, n (%) | 118(60.2) | 819(49.3) | 104(50.7) | 0.016 |
| SES & Psychosocial factors | ||||
| Education: ≥ Bachelor Degree, n (%) | 73 (36.1) | 717(41.0) | 119(54.8) | <0.001 |
| Income: ≥$40 000/y, n (%) | 83 (41.1) | 886 (50.7) | 138 (63.6) | <0.001 |
| Perceived Global Stress, mean ± SD | 1.1±0.98 | 1.2±0.99 | 1.2± 1.03 | 0.522 |
| Every day discrimination, mean ± SD | 2.0 ±1.1 | 2.0±0.97 | 2.0±0.91 | 0.785 |
| Life time discrimination, mean ± SD | 1.4±1.0 | 1.4±1.0 | 1.5±1.0 | 0.564 |
| Subclinical Atherosclerosis | ||||
| PAD, n (%) | 40 (19.8) | 258 (14.8) | 28 (12.9) | 0.107 |
| cCIMT, mm, median (IQR) † | 0.73 (0.21) | 0.69(0.22) | 0.71(0.21) | 0.009 |
| CAC, n (%) | 35(38.9) | 390(42.3) | 63(50.0) | 0.188 |
| AAC, n (%) | 49(54.4) | 550(59.5) | 75(59.5) | 0.636 |
P value by ANOVA, chi-square or Kruskal-Wallis as appropriate
Median (Interquartile range)
PEA: percent of European ancestry; PAD: peripheral arterial disease; cCIMT: common intima-media thickness; CAC: coronary artery calcium; AAC: abdominal aortic calcification: hsCRP: high sensitivity C – reactive protein;
Association between percent of European ancestry and PAD
Table 2 presents the prevalence ratio (PR) and 95% confidence interval (CI) for PAD per standard deviation (SD) increase in PEA. The restricted cubic spline (RCS) analysis indicated that the association between PEA and PAD was approximately linear (See Figure 1a). PEA was significantly and inversely related with PAD as shown in Table 2. One SD (9%) increase in PEA was associated with significantly lower PAD after adjusting for age and gender [Model1: PR = 0.90, 95% CI: (0.82, 0.99), p=0.036]. This association remained significant after adjusting for traditional CVD risk factors and inflammatory markers [Model 2: PR=0.90 (0.82, 0.99), p=0.024], but adjustment for SES and psychosocial factors alone attenuated the association which became non-significant [Model3: PR=0.92 (0.83, 1.01), p=0.082]. However, the association remained marginally significant in the fully adjusted model [Model 4: PR=0.91 (0.82, 1.00), p=0.046]. In sensitivity analyses, changing the Ankle Brachial Index (ABI) cut-point to <0.9, PAD was inversely associated with PEA in a similar pattern, but the associations were not-statistically significant (Supplementary Table 1).
Table 2.
Prevalence Ratio (PR) and 95% Confidence Interval (CI) of Peripheral Arterial Disease (PAD) per Standard Deviation Increase in Percent of European Ancestry (PEA) among African Americans: The Jackson Heart Study (2000–2004)
| PEA | PR (95% CI) | P value |
|---|---|---|
| Model1: Demographic | 0.90 (0.82, 0.99) | 0.036 |
| Model2: Traditional CVD factors | 0.90 (0.82, 0.99) | 0.024 |
| Model3: SES & Psychosocial factors | 0.92 (0.83, 1.01) | 0.082 |
| Model4: Fully adjusted | 0.91 (0.82, 1.00) | 0.046 |
PAD defined as ABI <=0.99
Standard deviation, 9.0%
Model1: Adjusted for demographic: age and gender;
Model2: Adjusted for model 1 and traditional CVD risk factors: BMI, current smoking, diabetes, hypertension, fasting plasma glucose, triglycerides, LDL cholesterol, HDL cholesterol, and hsCRP;
Model3: Adjusted for model 1 and SES & Psychosocial factors: household annual income, education, perceived global stress, and every day and lifetime discrimination;
Model4: Adjusted for all the covariates
PEA: percent of European ancestry; PAD: peripheral arterial disease; ABI: ankle-brachial index; hsCRP: high sensitivity C – reactive protein.
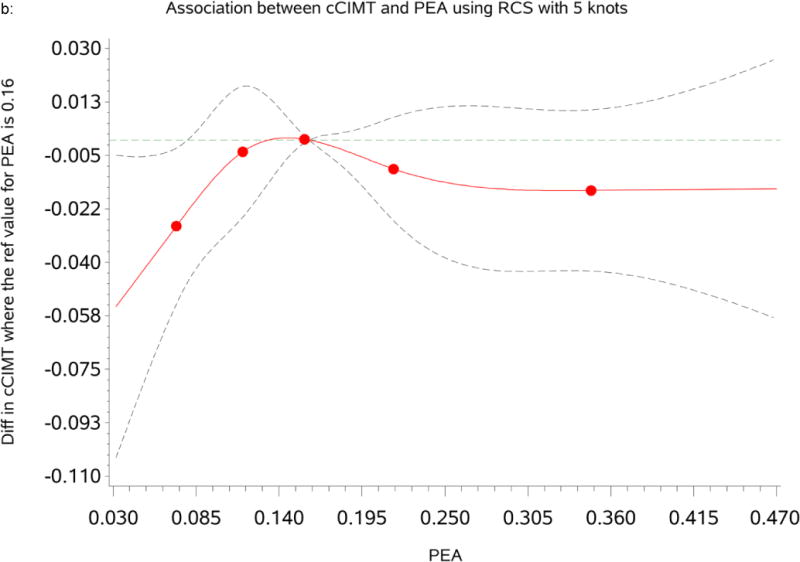
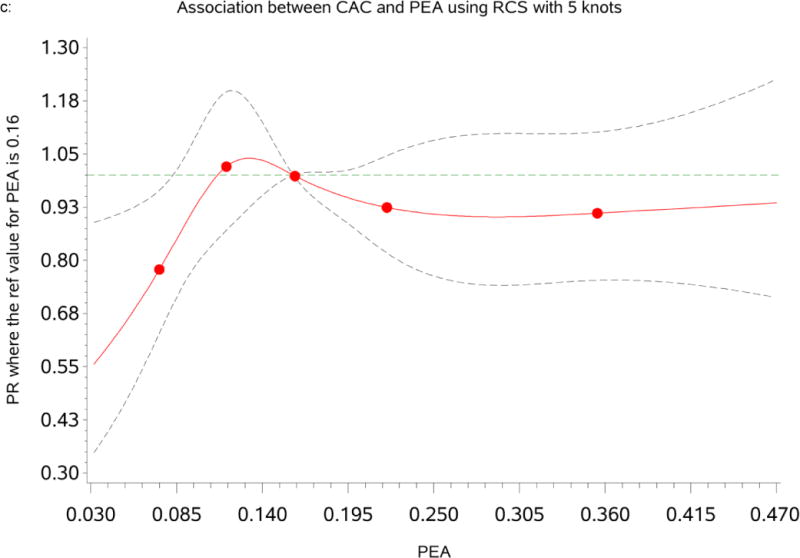
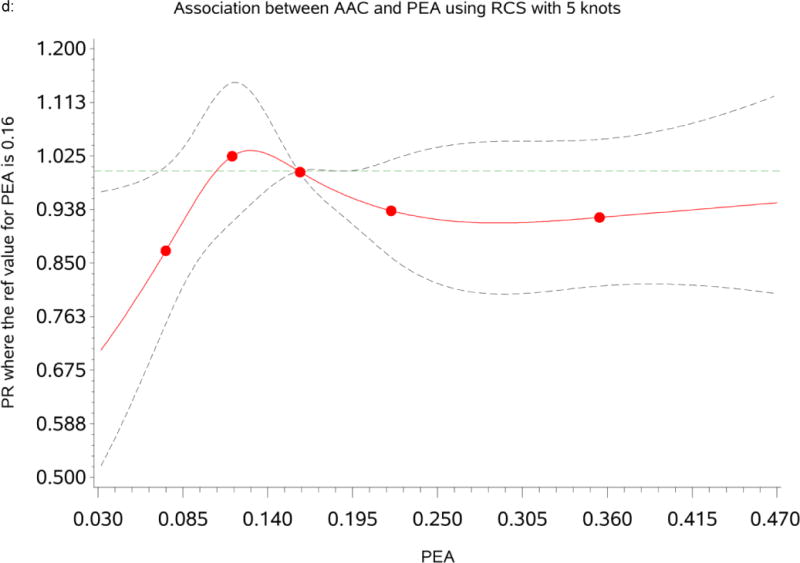
Figure 1.
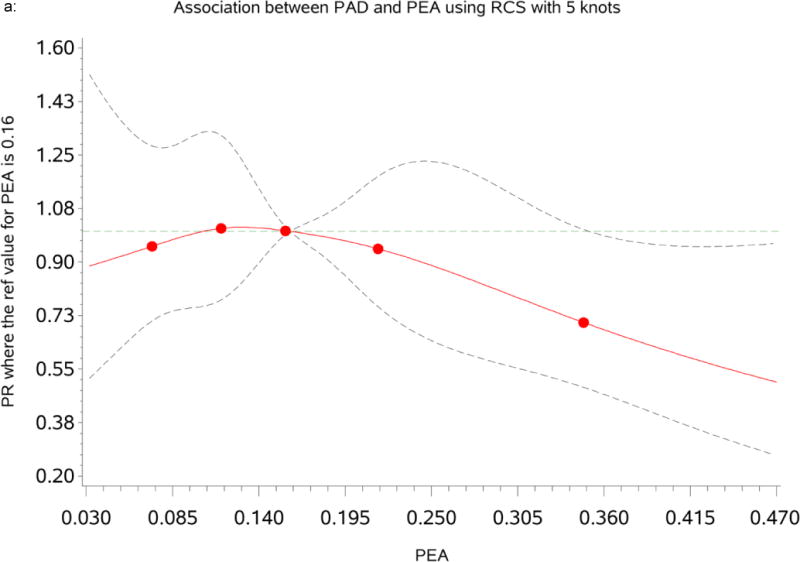
a: Dose-response relationship between percent of European ancestry (PEA) and Peripheral Arterial Disease (PAD). The Prevalence Ratios (PRs) were based on restricted cubic splines at knots of 5th, 35th, 65th and 95th percentiles. The reference was set at the 50th percentile (16%) of the European ancestry distribution. The solid line indicates the PR adjusted for age, sex, traditional CVD risk factors, inflammatory maker, socioeconomic status and psychosocial factors and the dashed lines represents its 95% confidence intervals.
b: Dose-response relationship between percent of European ancestry (PEA) and common Carotid Intima-Media Thickness (cCIMT). The mean differences were based on restricted cubic splines at knots of 5th, 35th, 65th and 95th percentiles. The reference was set at the 50th percentile (16%) of the European ancestry distribution. The solid line indicates the mean difference adjusted for age, sex, traditional CVD risk factors, inflammatory maker, socioeconomic status and psychosocial factors and the dashed lines represents its 95% confidence intervals.
c: Dose-response relationship between percent of European ancestry (PEA) and Coronary Artery Calcium (CAC). The Prevalence Ratios (PRs) were based on restricted cubic splines at knots of 5th, 35th, 65th and 95th percentiles. The reference was set at the 50th percentile (16%) of the European ancestry distribution. The solid line indicates the PR adjusted for age, sex, traditional CVD risk factors, inflammatory maker, socioeconomic status and psychosocial factors and the dashed lines represents its 95% confidence intervals.
d: Dose-response relationship between percent of European ancestry (PEA) and Abdominal Aortic Calcification (AAC.) The Prevalence Ratios (PRs) were based on restricted cubic splines at knots of 5th, 35th, 65th and 95th percentiles. The reference was set at the 50th percentile (16%) of the European ancestry distribution. The solid line indicates the PR adjusted for age, sex, traditional CVD risk factors, inflammatory maker, socioeconomic status and psychosocial factors and the dashed lines represents its 95% confidence intervals.
Association between percent of European ancestry and cCIMT
Table 3 summarizes the mean differences and Standard Error (SE) for cCIMT by categories of PEA. The RCS analysis indicated that the relationship between PEA and cCIMT was approximately non-linear as shown in Figure 1b. PEA was not significantly associated with cCIMT. In the model where we adjusted for age and gender (Model1), participants with the lowest (<10th percentile) and the highest PEA (>90th percentile) had a lower mean cCIMT [mean differences=−0.021 (SE=0. 014), p = 0.149 and −0.026 (SE=0.014), p = 0.118] compared to participants within the reference group (10th–90th percentile). However, the association remained non-significant after adjusting for traditional CVD risk factors, inflammatory marker, SES and psychosocial factors.
Table 3.
Mean Differences and Standard Error (SE) of Common Carotid Intima Media Thickness (cCIMT), and Prevalence Ratios (PRs) and 95% Confidence Interval (CI) of Coronary Artery Calcification (CAC) and Abdominal Aortic Calcification (AAC) by Categories of Percent of European Ancestry (PEA) among African Americans: the Jackson Heart Study (2000–2004)
| cCIMT | CAC | AAC | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| PEA | mean diff (SE) | P value | PRs (95% CIs) | P value | PRs (95 %CIs) | P value |
| Model1: Demographic | ||||||
| <10th percentile | −0.021(0.014) | 0.149 | 0.78(0.61, 0.99) | 0.041 | 0.80 (0.67, 0.96) | 0.015 |
| 10th–90th percentile | Reference | Reference | Reference | |||
| >90 percentile | −0.026 (0.014) | 0.118 | 0.93 (0.78, 1.12) | 0.463 | 0.84 (0.73, 0.96) | 0.010 |
| Model2: Traditional CVD factors | ||||||
| <10th percentile | −0.020(0.014) | 0.164 | 0.77(0.61,0.98) | 0.036 | 0.81 (0.68,0.96) | 0.021 |
| 10th–90th percentile | Reference | Reference | Reference | |||
| >90 percentile | −0.013(0.014) | 0.322 | 0.97 (0.82,1.16) | 0.7846 | 0.87 (0.77,0.99) | 0.040 |
| Model3: SES & Psychosocial factors | ||||||
| <10th percentile | −0.022 (0.014) | 0.128 | 0.76 (0.59, 0.98) | 0.033 | 0.80(0.67,0.96) | 0.015 |
| 10th–90th percentile | Reference | Reference | Reference | |||
| >90 percentile | −0.017(0.014) | 0.220 | 0.93 (0.78,1.13) | 0.393 | 0.85(0.74, 0.97) | 0.018 |
| Model4: Fully adjusted | ||||||
| <10th percentile | −0.020(0.014) | 0.152 | 0.75 (0.58,0.96) | 0.022 | 0.80 (0.67,0.96) | 0.016 |
| 10th–90th percentile | Reference | Reference | Reference | |||
| >90 percentile | −0.011(0.014) | 0.433 | 0.96 (0.80,1.15) | 0.657 | 0.88 (0.77,1.00) | 0.054 |
Model1: Adjusted for demographic: age and gender;
Model2: Adjusted for model 1 and traditional CVD risk factors: BMI, current smoking, diabetes, hypertension, fasting plasma glucose, triglycerides, LDL cholesterol, HDL cholesterol, and hsCRP;
Model3: Adjusted for model 1 and SES & Psychosocial factors: household annual income, education, perceived global stress, and every day and lifetime discrimination;
Model4: Adjusted for all the covariates
PEA: percent of European ancestry; cCIMT: common intima-media thickness; CAC: coronary artery calcium; AAC: abdominal aortic calcification: hsCRP: high sensitivity C – reactive protein;
Association between percent of European ancestry and CAC
Table 3 summarizes the prevalence ratio (PR) and 95% confidence interval for CAC and AAC prevalence by categories of PEA. The RCS analysis indicated that the associations of PEA with CAC and AAC were non-linear and fitted strongly at the lowest levels of PEA (see Figures 1c & d). We found a significant association between CAC and PEA; participants with the lowest level of PEA (<10th percentile) had significantly significant lower CAC prevalence [PR=0.78(0.61, 0.99), p=0.041] compared to participants within the reference group (10th–90th percentile) after adjusting for age and gender (Model1). Adjustment for traditional CVD risk factors and inflammation marker further strengthened the association of PEA with CAC [Model2: PR=0.77 (0.61, 0.98), p=0.036]. This association was also significant when we adjusted for SES and psychosocial risk factors [Model3: PR=0.76 (0.59, 0.98), p=0.033]. In the fully adjusted model, the association remained strong and significant [Model4: PR=0.76 (0.58, 0.96), p=0.022]. A similar pattern of association was observed with AAC (see Tables 3).
Discussion
In this study of a large sample of African Americans, we found evidence that genetic ancestry is associated with measures of subclinical atherosclerosis. We also found European ancestry was inversely associated with prevalence of PAD in African Americans. This association was attenuated, but remained marginally significant when we fully adjusted for traditional CVD risk factors, inflammatory marker, SES and psychosocial factors. We also identified significant non-linear associations of PEA with CAC and AAC, which shows that participants with the lowest European ancestry had a lower prevalence of CAC and AAC. Adjustment for traditional CVD risk factors, inflammatory marker, SES and psychosocial factors did not explain the observed associations. The apparent non-linear associations of PEA with CAC and AAC suggest that unmeasured risk factors and/or interaction with genetic factors could play a more important role than genetic effects in this sample of African Americans. However, we did not find significant association between genetic ancestry and cCIMT. Overall, our findings suggest that unmeasured risk factors and/or ancestry-related genetic differences may contribute to the distribution of subclinical atherosclerosis in African Americans.
Few prior studies have examined the associations between genetic ancestry and subclinical atherosclerosis in African Americans. Although non-significant, Allison et al. observed inverse association between European ancestry and PAD among 712 African Americans of the Multi-Ethnic Study of Atherosclerosis (MESA) study;29 in contrast, in our study, this association was marginally significant after adjusting for traditional CVD risk factors, inflammatory marker, SES and psychosocial factors. Our findings are also in line with previous studies that reported a significant association between European ancestry and CAC.26,30 For example, Wassel et al. reported that PEA was linearly, positively associated with prevalence of CAC among 712 African Americans in the MESA study, suggesting genetic components to CAC in African Americans.26 Unlike their findings, however, our results showed a dose–response relationship between PEA and CAC such that only participants with the lowest level of PEA had significantly lower CAC prevalence, indicating unknown risk factors and/or gene-environment interactions could play a more important role than genetic factors in this cohort. We did not observe a significant association between cCIMT and European ancestry in our study, which is similar to one study that found no association between African ancestry and cCIMT among elderly African Americans of the Cardiovascular Health Study (CHS).27 However, another study reported a linear, negative association between PEA and cCIMT independent of CVD risk factors and SES in African Americans of the MESA study, suggesting genetic influence on cCIMT.26 It is possible that differences in regional origin, sample size, estimation of ancestry, and methodological approaches in the measurement of cCIMT could have attributed to the discrepancies between these studies.26,27,31 Our study was also the first to look at PEA and AAC and displayed a similar pattern of association with those of CAC. There is no literature on genetic ancestry and AAC with which to compare our results, but our findings are in line with one study that reported a lower prevalence of AAC in African Americans compared to European Americans using self-reported race/ethnicity.7 In aggregate, the current and these previous studies provide evidence in support to the ancestry-related differences in the distribution of subclinical atherosclerosis within African Americans.
Explanations for the differences in associations between PEA and the different subclinical atherosclerosis measures remain unclear. However, these findings indicate that distinct pathophysiological processes or risk factors might be involved in different ethnic groups for the different atherosclerosis measures, which might explain some of the observed differences by genetic ancestry.5, 12, 18, 32–33 Evidence suggests that hypertension, diabetes, and obesity are more prevalent in African Americans or in those with low PEA and that they are strong and independent risk factors for PAD and cCIMT.12, 32–33 Our results showed that association between PEA and PAD remained significant after adjusting for these CVD risk factors and inflammatory marker. This association attenuated, but persisted after additional adjustment for SES and psychosocial factors. This suggests that unmeasured risk factors may play a role in the pathogenesis of PAD. A number of studies have found higher level of novel risk factors, such as Interleukin-6, fibrinogen, D-dimer, and homocysteine, were associated with increased odds of PAD among African Americans.12, 33–35 The same studies have also indicated that traditional or novel CVD risk factors do not entirely explain the high prevalence of PAD in African Americans, suggesting genetic components to the development of PAD.12, 32–35 In fact, a recent study found a novel region in chromosome 11 that was associated with high PAD in African Americans.36 Thus, it is probably the interplay of traditional CVD and novel risk factors, SES, and genetic factors could explain the differences in PAD among African Americans.
Although African Americans have worse CVD risk factors profiles, we observed significantly lower prevalence of CAC and AAC in African Americans with the lowest PEA even after adjustment for traditional CVD risk factors, inflammatory marker, SES and psychosocial factors. This finding is consistent with a large body of evidence reporting that standard CVD risk factors did not fully explain the ancestry-related differences in CAC and AAC.5, 26, 37 It is highly possible the observed differences in CAC and AAC could be due to non-genetic factors that were not accounted for in our study. Several previous studies have indicated that unmeasured risk factors, such as bone density, vitamin D, calcium metabolism, and other environmental factors, might play an important role in the development of CAC and AAC.5, 26, 37–40 Indeed, bone density has been found to be inversely related with CAC and African Americans have higher levels of bone density than whites.38 Other studies have also highlighted the association of lower vitamin D with CAC.5, 39, 40 Alternatively, it also possible that genetic factors may contribute to CAC in African Americans.26, 30, 41 For example, recent admixture mapping of CAC in African Americans identified several genomic regions in which excess European ancestry was associated with higher levels of CAC.41 Previous studies have also identified the 9p21 region was associated with CAC in predominantly European-derived populations.42, 43 Taken together, the apparent lower level of CAC and AAC in participants with the lowest PEA in our study indicates the importance of other unmeasured risk factors and/ or gene by environment interactions than genetic effects.26, 37, 41
Our study has some limitations which should be taken into consideration when interpreting the results. First, although we adjusted for several known risk factors of subclinical atherosclerosis, it is possible that residual confounding may have affected our results. Future research is needed to determine whether unmeasured risk factors and/or gene-environment interactions can help account for the observed associations in African Americans. Second, our sample came from a single site of Jackson, Mississippi may not be generalizable to the general population of African Americans across the US. Furthermore, the PEA in our study was skewed toward lower values and very few participants had 25% or more European ancestry, thus, we were unable examine the full range of PEA associations with subclinical atherosclerosis measures. Fourth, the time lag between evaluation of some of the baseline covariates (e.g. SES, current smoking and psychosocial factors) and measures of CAC and AAC in Exam 2, which could have influenced the associations of PEA with CAC and AAC. Finally, the cross-sectional design of our study limits our ability to draw conclusions whether genetic ancestry predicts subclinical atherosclerosis independent of non-genetic factors. Future studies are warranted to replicate our findings, perhaps using larger sample sizes of African Americans, with a full range of ancestry to overcome any chance findings might present in our analyses.
Despite these limitations, our study used a large well-characterized sample of African Americans and hence had greater statistical power than prior studies that examined the association of PEA with subclinical atherosclerosis. Moreover, PEA in our study was estimated based on a large number of AIMs, which minimized the errors associated with estimating genetic ancestry compared to prior studies. Our study also benefits from the state- of the-art measures of subclinical atherosclerosis and availability of a wide range of important confounding covariates for adjustment. Additionally, our study is also the first to show the association of AAC with genetic ancestry. Finally, our study significantly contributes to the limited body of literature on genetic ancestry and subclinical atherosclerosis among African Americans, which often used skin reflectance as a surrogate for genetic ancestry or not enough large number of AIMs to determine ancestry with precision.18, 19, 27 Furthermore, our findings provide background for pursing further studies on the role of genetic factors in subclinical atherosclerosis processes.
Conclusion
In conclusion, genetic ancestry was associated with subclinical atherosclerosis in a large sample of African Americans. We found significantly lower prevalence of CAC and AAC in participants with the lowest European ancestry, which appears not fully explained by traditional CVD risk factors, SES and psychosocial factors. We also found greater European ancestry was associated with lower prevalence of PAD although the association was attenuated after adjusting for SES and psychosocial factors. Our findings suggest that unmeasured risk factors and/or interacting with genetic determinants might play an important role to the distribution of subclinical atherosclerosis in African Americans. Future studies are needed to validate these findings and explore gene-environment interactions to better understand the complex biological mechanisms and to reduce ancestry-related disparities in subclinical atherosclerosis.
Supplementary Material
Significance.
African Americans are at greater risk for developing PAD and cCIMT, but at lower risk for developing CAC and AAC relative to European Americans. It is unclear whether genetic or non-genetic factors are driving these differences. African Americans are the most genetically heterogeneous group in the US, with varying proportion of African and European ancestry. We evaluated the associations between percent of European ancestry (PEA) and subclinical atherosclerosis in a large sample of admixed African Americans. Our results demonstrated that a higher PEA was associated with a lower PAD prevalence while the lowest European ancestry was associated with a lower CAC and AAC prevalence. Adjustment for the traditional and social CVD risk factors reduced the PEA-PAD associations whereas it further strengthened the associations of PEA with CAC and AAC. These findings suggest that unmeasured risk factors and their interactions with genetics might play an important role in the distribution of subclinical atherosclerosis in African Americans.
Acknowledgments
The authors thank the investigators, the staff, interns and the participants of the Jackson Heart Study for their long-term commitment and valuable contributions to the study. We also thank Drs. Jose Vargas and Amadou Gaye for their comments on the final draft of the manuscript.
Sources of Funding: This research is supported by supported by Intermural Program of National Human Genomics Institute (NHGRI), National Institutes of Health (NIH). The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities.
Nonstandard Abbreviations and Acronyms
- AAC
abdominal aortic calcification
- ABI
ankle-brachial index
- CAC
coronary artery calcium
- BMI
body mass index
- CI
confidence interval
- cCIMT
common carotid intima-media thickness
- CVD
cardiovascular disease
- hsCRP
high sensitivity C – reactive protein
- JHS
Jackson Heart Study
- LDL
low-density lipoprotein cholesterol
- HDL
high-density lipoprotein cholesterol
- PAD
peripheral arterial disease
- PEA
percent of European ancestry
- PR
prevalence ratio
- SD
standard deviation
- SE
standard error
- SES
socioeconomic status
Footnotes
Publisher's Disclaimer: Disclaimer:
The views expressed in this article are those of the authors and do not necessarily represent the views of the National Institutes of Health or the Department of Health and Human Services.
Disclosures
None
References
- 1.Greenland P, Bonow RO, Brundage BH, et al. Accf/aha 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: A report of the american college of cardiology foundation clinical expert consensus task force (accf/aha writing committee to update the 2000 expert consensus document on electron beam computed tomography) Circulation. 2007;115:402–426. doi: 10.1161/CIRCULATIONAHA..107.181425. [DOI] [PubMed] [Google Scholar]
- 2.Polak JF, Pencina MJ, Pencina KM, O’Donnell CJ, Wolf PA, D’Agostino RB. Carotid-wall intima–media thickness and cardiovascular events. The New England journal of medicine. 2011;365:213–221. doi: 10.1056/NEJMoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Criqui MH, Ninomiya JK, Wingard DL, Ji M, Fronek A. Progression of peripheral arterial disease predicts cardiovascular disease morbidity and mortality. Journal of the American College of Cardiology. 2008;52:1736–1742. doi: 10.1016/j.jacc.2008.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee TC, O’Malley PG, Feuerstein I, Taylor AJ. The prevalence and severity of coronary artery calcification on coronary artery computed tomography in black and white subjects. Journal of the American College of Cardiology. 2003;41:39–44. doi: 10.1016/s0735-1097(02)02618-9. [DOI] [PubMed] [Google Scholar]
- 5.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: The multi-ethnic study of atherosclerosis (mesa) Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 6.Budoff MJ, Nasir K, Mao S, Tseng PH, Chau A, Liu ST, Flores F, Blumenthal RS. Ethnic differences of the presence and severity of coronary atherosclerosis. Atherosclerosis. 2006;187:343–350. doi: 10.1016/j.atherosclerosis.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Allison MA, Budoff MJ, Nasir K, Wong ND, Detrano R, Kronmal R, Takasu J, Criqui MH. Ethnic-specific risks for atherosclerotic calcification of the thoracic and abdominal aorta (from the multi-ethnic study of atherosclerosis) The American journal of cardiology. 2009;104:812–817. doi: 10.1016/j.amjcard.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Agostino RB, Burke G, O’Leary D, Rewers M, Selby J, Savage PJ, Saad MF, Bergman RN, Howard G, Wagenknecht L, Haffner SM. Ethnic differences in carotid wall thickness. The insulin resistance atherosclerosis study. Stroke; a journal of cerebral circulation. 1996;27:1744–1749. doi: 10.1161/01.str.27.10.1744. [DOI] [PubMed] [Google Scholar]
- 9.Manolio TA, Arnold AM, Post W, Bertoni AG, Schreiner PJ, Sacco RL, Saad MF, Detrano RL, Szklo M. Ethnic differences in the relationship of carotid atherosclerosis to coronary calcification: The multi-ethnic study of atherosclerosis. Atherosclerosis. 2008;197:132–138. doi: 10.1016/j.atherosclerosis.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins TC, Petersen NJ, Suarez-Almazor M, Ashton CM. The prevalence of peripheral arterial disease in a racially diverse population. Archives of internal medicine. 2003;163:1469–1474. doi: 10.1001/archinte.163.12.1469. [DOI] [PubMed] [Google Scholar]
- 11.Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, Criqui MH. Ethnic-specific prevalence of peripheral arterial disease in the united states. American journal of preventive medicine. 2007;32:328–333. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Allison MA, Criqui MH, McClelland RL, Scott JM, McDermott MM, Liu K, Folsom AR, Bertoni AG, Sharrett AR, Homma S, Kori S. The effect of novel cardiovascular risk factors on the ethnic-specific odds for peripheral arterial disease in the multi-ethnic study of atherosclerosis (mesa) Journal of the American College of Cardiology. 2006;48:1190–1197. doi: 10.1016/j.jacc.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 13.Diez-Roux AV, Nieto FJ, Tyroler HA, Crum LD, Szklo M. Social inequalities and atherosclerosis. The atherosclerosis risk in communities study. American journal of epidemiology. 1995;141:960–972. doi: 10.1093/oxfordjournals.aje.a117363. [DOI] [PubMed] [Google Scholar]
- 14.Carson AP, Rose KM, Catellier DJ, Kaufman JS, Wyatt SB, Diez-Roux AV, Heiss G. Cumulative socioeconomic status across the life course and subclinical atherosclerosis. Annals of epidemiology. 2007;17:296–303. doi: 10.1016/j.annepidem.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Rosvall M, Ostergren PO, Hedblad B, Isacsson SO, Janzon L, Berglund G. Occupational status, educational level, and the prevalence of carotid atherosclerosis in a general population sample of middle-aged swedish men and women: Results from the malmö diet and cancer study. American journal of epidemiology. 2000;152:334–346. doi: 10.1093/aje/152.4.334. [DOI] [PubMed] [Google Scholar]
- 16.Troxel WM, Matthews KA, Bromberger JT, Sutton-Tyrrell K. Chronic stress burden, discrimination, and subclinical carotid artery disease in african american and caucasian women. Health psychology: official journal of the Division of Health Psychology, American Psychological Association. 2003;22:300–309. doi: 10.1037/0278-6133.22.3.300. [DOI] [PubMed] [Google Scholar]
- 17.Lewis TT, Everson-Rose SA, Powell LH, Matthews KA, Brown C, Karavolos K, Sutton-Tyrrell K, Jacobs E, Wesley D. Chronic exposure to everyday discrimination and coronary artery calcification in african-american women: The swan heart study. Psychosomatic medicine. 2006;68:362–368. doi: 10.1097/01.psy.0000221360.94700.16. [DOI] [PubMed] [Google Scholar]
- 18.Anand SS, Yusuf S, Vuksan V, Devanesen S, Teo KK, Montague PA, et al. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in canada: The study of health assessment and risk in ethnic groups (share) Lancet. 2000;356:279–284. doi: 10.1016/s0140-6736(00)02502-2. [DOI] [PubMed] [Google Scholar]
- 19.Shriver MD, Parra EJ, Dios S, Bonilla C, Norton H, Jovel C, et al. Skin pigmentation, biogeographical ancestry and admixture mapping. Hum Genet. 2003;112:387–399. doi: 10.1007/s00439-002-0896-y. [DOI] [PubMed] [Google Scholar]
- 20.Burchard EG, Ziv E, Coyle N, Gomez SL, Tang H, Karter AJ, Mountain JL. The importance of race and ethnic background in biomedical research and clinical practice. The New England journal of medicine. 2003;348:1170–1175. doi: 10.1056/NEJMsb025007. [DOI] [PubMed] [Google Scholar]
- 21.Shields AE, Fortun M, Hammonds EM, King PA, Lerman C, Rapp R, Sullivan PF. The use of race variables in genetic studies of complex traits and the goal of reducing health disparities: A transdisciplinary perspective. The American psychologist. 2005;60:77–103. doi: 10.1037/0003-066X.60.1.77. [DOI] [PubMed] [Google Scholar]
- 22.Risch N, Burchard E, Ziv E, Tang H. Categorization of humans in biomedical research: Genes, race and disease. Genome biology. 2002;3 doi: 10.1186/gb-2002-3-7-comment2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parra EJ, Marcini A, Akey J, Martinson J, Batzer MA, Cooper R, Forrester T, Allison DB, Deka R, Ferrell RE, Shriver MD. Estimating african american admixture proportions by use of population-specific alleles. American journal of human genetics. 1998;63:1839–1851. doi: 10.1086/302148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen G, Shriner D, Zhou J, Doumatey A, Huang H, Gerry NP, Herbert Alan, Christman Michael F, Chen Yuanxiu, Dunston Georgia M, Faruque Mezbah U, Rotimi Charles N, Adeyemo Adebowale. Development of admixture mapping panels for african americans from commercial high-density snp arrays. BMC genomics. 2010;11:417. doi: 10.1186/1471-2164-11-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKeigue PM. Mapping genes that underlie ethnic differences in disease risk: Methods for detecting linkage in admixed populations, by conditioning on parental admixture. Am J Hum Genet. 1998;63:241–251. doi: 10.1086/301908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wassel CL, Pankow JS, Peralta CA, Choudhry S, Seldin MF, Arnett DK. Genetic ancestry is associated with subclinical cardiovascular disease in african-americans and hispanics from the multi-ethnic study of atherosclerosis. Circulation. Cardiovascular genetics. 2009;2:629–636. doi: 10.1161/CIRCGENETICS.109.876243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reiner AP, Ziv E, Lind DL, Nievergelt CM, Schork NJ, Cummings SR, Phong A, Burchard EG, Harris TB, Psaty BM, Kwok PY. Population structure, admixture, and aging-related phenotypes in african american adults: The cardiovascular health study. American journal of human genetics. 2005;76:463–477. doi: 10.1086/428654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiner AP, Carlson CS, Ziv E, Iribarren C, Jaquish CE, Nickerson DA. Genetic ancestry, population sub-structure, and cardiovascular disease-related traits among african-american participants in the cardia study. Human genetics. 2007;121:565–575. doi: 10.1007/s00439-007-0350-2. [DOI] [PubMed] [Google Scholar]
- 29.Allison MA, Peralta CA, Wassel CL, Aboyans V, Arnett DK, Cushman M, Eng J, Ix J, Rich SS, Criqui MH. Genetic ancestry and lower extremity peripheral artery disease in the multi-ethnic study of atherosclerosis. Vascular medicine (London, England) 2010;15:351–359. doi: 10.1177/1358863X10375586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Divers J, Palmer ND, Lu L, et al. Admixture mapping of coronary artery calcified plaque in african americans with type 2 diabetes mellitus. Circulation. Cardiovascular genetics. 2013;6:97–105. doi: 10.1161/CIRCGENETICS.112.964114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the american society of echocardiography carotid intima-media thickness task force. Endorsed by the society for vascular medicine. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. quiz 189. [DOI] [PubMed] [Google Scholar]
- 32.Rampersaud E, Bielak LF, Parsa A, Shen H, Post W, Ryan KA, Donnelly P, Rumberger JA, Sheedly PF, II, Peyser PA, Shuldiner AR, Mitchell BD. The association of coronary artery calcification and carotid artery intima-media thickness with distinct, traditional coronary artery disease risk factors in asymptomatic adults. American Journal of Epidemiology. 2008;168:1016–1023. doi: 10.1093/aje/kwn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Criqui MH, Vargas V, Denenberg JO, Ho E, Allison M, Langer RD, Gamst A, Bundens WP, Fronek A. Ethnicity and peripheral arterial disease: the San Diego Population Study. Circulation. 2005;112:2703–2707. doi: 10.1161/CIRCULATIONAHA.105.546507. [DOI] [PubMed] [Google Scholar]
- 34.Ix JH, Allison MA, Denenberg JO, Cushman M, Criqui MH. Novel cardiovascular risk factors do not completely explain the higher prevalence of peripheral arterial disease among african americans. The san diego population study. J Am Coll Cardiol. 2008;51:2347–2354. doi: 10.1016/j.jacc.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 35.Wassel CL, Loomba R, Ix JH, Allison MA, Denenberg JO, Criqui MH. Family history of peripheral artery disease is associated with prevalence and severity of peripheral artery disease: The san diego population study. J Am Coll Cardiol. 2011;58:1386–1392. doi: 10.1016/j.jacc.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scherer ML, Nalls MA, Pawlikowska L, et al. Admixture mapping of ankle-arm index: Identification of a candidate locus associated with peripheral arterial disease. Journal of medical genetics. 2010;47:1–7. doi: 10.1136/jmg.2008.064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diez-Roux AV, Detrano R, Jackson S, Jacobs DR, Jr, Schreiner PJ, Shea S, Szklo M. Acculturation and socioeconomic position as predictors of coronary calcification in a multiethnic sample. Circulation. 2005;112:1557–1565. doi: 10.1161/CIRCULATIONAHA.104.530147. [DOI] [PubMed] [Google Scholar]
- 38.Looker AC, Melton LJ, Harris T, Borrud L, Shepherd J, McGowan J. Age, gender, and race/ethnic differences in total body and subregional bone density. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2009;20:1141–1149. doi: 10.1007/s00198-008-0809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doherty TM, Tang W, Dascalos S, Watson KE, Demer LL, Shavelle RM, et al. Ethnic origin and serum levels of 1alpha,25-dihydroxyvitamin d3 are independent predictors of coronary calcium mass measured by electron-beam computed tomography. Circulation. 1997;96:1477–1481. doi: 10.1161/01.cir.96.5.1477. [DOI] [PubMed] [Google Scholar]
- 40.Watson KE, Abrolat ML, Malone LL, Hoeg JM, Doherty T, Detrano R, Demer LL. Active serum vitamin d levels are inversely correlated with coronary calcification. Circulation. 1997;96:1755–1760. doi: 10.1161/01.cir.96.6.1755. [DOI] [PubMed] [Google Scholar]
- 41.Wojczynski MK, Li M, Bielak LF, et al. Genetics of coronary artery calcification among african americans, a meta-analysis. BMC medical genetics. 2013;14:75. doi: 10.1186/1471-2350-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen SN, Ballantyne CM, Gotto AM, Marian AJ. The 9p21 susceptibility locus for coronary artery disease and the severity of coronary atherosclerosis. BMC cardiovascular disorders. 2009;9:3. doi: 10.1186/1471-2261-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McPherson R. Chromosome 9p21 and coronary artery disease. The New England journal of medicine. 2010;362:1736–1737. doi: 10.1056/NEJMcibr1002359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


