Abstract
Introduction
The involvement of dopaminergic neurons in the ventral tegmental area (VTA) in Parkinson’s disease (PD) has not been universally recognized by neuroscientists and neurologists. Here, we conduct a review of previous research documenting dopaminergic neuronal loss in both the substantia nigra pars compacta (SNpc) and VTA and add three new post-mortem PD cases to the literature.
Methods
PD and control brains were sectioned, stained for tyrosine hydroxylase, and cells in the SNpc and VTA were counted.
Results
Based on the review, we report two main results: 1) the VTA does degenerate in PD, and 2) the VTA degenerates less than the SNpc.
Conclusion
Inconsistent clinical information about these cases limits our ability to interpret how the VTA contributes to PD symptoms. However, our data in combination with prior PD neuropathological cases in the literature unequivocally establish that the VTA is involved in PD, and could be relevant for future investigation of non-motor symptoms in PD.
Keywords: Ventral tegmental area, Parkinson’s disease
INTRODUCTION
Parkinson’s disease (PD) is a progressive neurodegenerative disorder that causes motor disabilities and cognitive dysfunction due to the degeneration and loss of dopaminergic neurons in the midbrain. There is no debate as to whether the loss of neurons in the substantia nigra pars compacta (SNpc) is directly causative of the motor symptoms of PD [1]. While the ventral tegmental area (VTA) has long been suggested to be involved in PD [2], some authors have stated that the VTA is relatively spared [3–6] and some textbooks of neurology, neuroscience, and movement disorders state that the VTA is “affected little or not at all” [7,8]. Our motivation for this study was to review the existing literature and investigate the degree to which the VTA is involved in PD.
Previous research has provided several theories explaining this relative sparing, such as the variety of neurons found in the VTA [3,4], lower expression of the dopamine transporter [5], differences in calcium channel expression, as well as in levels of cytosolic dopamine and the presence of α-synuclein [6,9]. Here, we conduct a review of eight previous neuropathological studies in humans that directly quantified dopamine neurons in both the SNpc and VTA and add three new PD cases to the literature. We conclude, unequivocally, that the VTA degenerates in PD. This information could be helpful in understanding the pathophysiology of non-motor symptoms of Parkinson’s disease. This line of evidence is consistent with widespread degeneration of ascending projection systems in PD including cholinergic, noradrenergic, serotonergic, and dopaminergic nuclei [10].
The VTA extends laterally from the midline (0 mm) to 4 mm and from 4 mm caudal to the mammillary bodies to 9 mm [11]. It contains dopamine neurons that project mainly to the ventral striatum and prefrontal cortex, with some projections to the amygdala. The VTA integrates information from a variety of cortical, brainstem, and peripheral centers, and contains a diversity of dopaminergic, GABAergic, and glutamatergic neurons, while the SNpc does not express glutamatergic neurons [3,4,12,13]. Due to the diversity of neurons, the VTA responds to local and distant neuromodulators [5]. The VTA has been implicated in a variety of behaviors and psychopathological states, including depression, anxiety, drug addiction, feeding, reward processing, and executive function [14]. Crucially, many PD patients have non-motor symptoms that include disorders associated with the VTA. For instance, 25% of PD patients have anxiety and/or depression [15] and nearly 30% of PD patients have executive dysfunction [16]. We present evidence from 43 previous PD cases and three new ones to explicitly test the hypothesis that the VTA is extensively involved in PD.
METHODS
Literature review
The following key words were used to collect published journal articles on the degeneration of the VTA in PD: ventral tegmental area, Parkinson’s disease, dopamine, and degeneration. The articles were then analyzed for content of dopaminergic degeneration in the VTA and SNpc.
Collection
Seven perfusion-fixed (4% formaldehyde) human midbrains (from superior colliculus to cerebral peduncles) were collected. Brain blocks were post-fixed for at least one week in formaldehyde. For cryoprotection, midbrains were maintained in 30% sucrose. Midbrains were sectioned on a sliding microtome at 40 µm. All procedures complied with the University of Iowa Deeded Body Program guidelines [17].
Staining
Sections were blocked at room temperature for 1 hour in 0.1% Triton-X with normal horse serum (10 drops/ 30 mL) and then washed three times with 0.1 M PBS. Sections were then incubated in the primary antibody, rabbit anti-tyrosine hydroxylase (TH) (Abcam, 1:2000), on a shaker at 4°C for 48 hours. Following three washes with 1XPBS, sections were incubated in the secondary antibody, biotinylated anti-rabbit IgG (made in horse) (1:200), at room temperature for 2 hours. For DAB staining, the ABC mix kit (Vector) was used. Sections were mounted on subbed slides, subsequently dehydrated, and cover-slipped with Permount for imaging and storage [18,19].
Imaging and counting
Images were uploaded using Adobe Photoshop for TH positive cell counts. The SNpc and VTA were outlined using previously described parameters [11,20]. Cells positive for TH were counted in the SNpc and VTA and the average dopaminergic degeneration was calculated by comparing the mean number of TH positive cells in the PD group to the control group. Cell counts were compared via a t-test.
RESULTS
We reviewed eight studies in which TH expression was quantified in the VTA and SNpc. Though all of the previous studies reported here aimed to quantify TH differences between PD and controls, not all used the same methods. For instance, most studies used haematoxylin-eosin staining to label neuromelanin [11,21,22], which Bogerts had established as an appropriate marker for catecholaminergic neurons [23]. One only characterized pigmented neurons [20], another used immunostaining for specific proteins such as TH [24]. Only one of the studies reported here used radioenzymatic methods to quantify TH activity [25].
Of these studies, Bogerts et al. reported the lowest average VTA dopamine loss, as measured by TH expression, at 40 percent (3,100 cell in PD vs. 5,155 cells in controls) [21]. Additionally, in 1984 Javoy-Agid et al. reported the highest average loss at 77 percent (6,235 cells in PD vs 27,440 cells in controls) [26]. Across all reported studies, the VTA had significantly less cell loss compared to the SNpc (p<0.05). Unfortunately, not all studies reported clinical data on the PD cases. Of those that did, the widest range of disease duration was from months to 37 years [21]. Bogerts et al., Hirsch et al., and German et al. reported mean disease durations of 11, 7, and 14 years, respectively [11,21,22]. Additionally, of the studies providing patient symptoms, two stated that all cases exhibited tremor, rigidity, and akinesia/hypokinesia [21,22], while another reported that approximately half of the cases presented with these symptoms [24]. Interestingly, only one study mentioned cognitive symptoms and reported that two cases showed intellectual impairment while one case suffered from hallucinations [22].
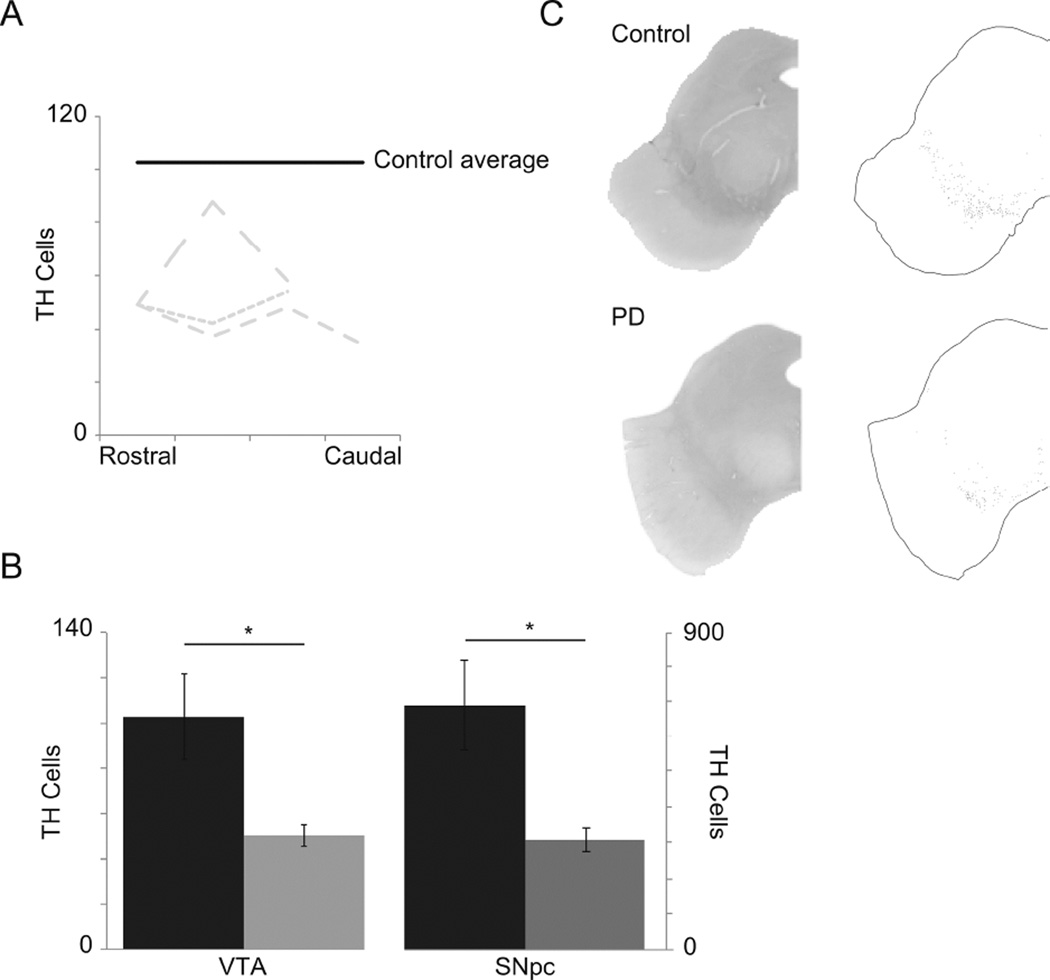
In our cases, we characterized the VTA based on Uhl et al. [20] and German et al. [11], identified the VTA in all possible sections, and counted the TH-positive cells (Figure 1A). We report that, on average, PD brains showed a 49 percent decrease in TH-positive cells in the VTA when compared to controls (p< 0.01), and a 41 percent decrease in the SNpc (p< 0.01) (Figure 1B). These differences are notable in the images (Figure 1C). Due to the fact that the cases used in this study were obtained from the Deeded Body Program at the University of Iowa, no clinical data was available from donors.
Figure 1.
A. Mean number of TH positive cells for controls (black line) and individual case TH positive cell counts from rostral to caudal (grey dashed lines). B. Average TH positive cell counts in the VTA and SNpc of controls (black) and PD cases (grey). C. Representative images of staining (left) for TH of a Control (top) and PD (bottom) cases and the respective cell labeling (right).
DISCUSSION
We report that both in previous literature and in our new data, the VTA consistently degenerates in PD. Our new cases indicate a 49 percent decrease, on average, of TH positive cells (Table 1), which falls in the 40–77 percent range reported in previous studies [21,26]. These data clearly and unequivocally establish that the VTA is involved in PD. We used TH as a marker of dopamine neurons; however, it should be noted that this enzyme is also found in other catecholaminergic neurons. Furthermore, there is a diversity of cell types in the VTA; thus, future work is required to account for this diversity to better study the differential dopamine loss in the VTA. Our review of the literature suggests that the VTA does appear to degenerate less than the SNpc. Since very little clinical data was provided in the literature, it is unclear if these differences can be accounted for by the clinical heterogeneity of the disease.
Table 1.
Literature Data and New Cases.
| Author, Year | Number of cases | |||
|---|---|---|---|---|
| Control | PD | Average decrease in SN (%) |
Average decrease in VTA (%) |
|
| Javoy-Agid & Agid, 1980 | 13 | 7 | 70 | 57 |
| Bogerts et al., 1983 | 9 | 5 | 65 | 40 |
| Javoy-Agid et al., 1984 | 2 | 2 | NA | 77 |
| Uhl et al., 1985 | 4 | 4 | NA | 55 |
| Waters et al., 1988 | 15 | 12 | 57 | 45 |
| Hirsch et al., 1988 | 3 | 4 | 77 | 48 |
| German et al., 1989 | 3 | 5 | 73 | 58 |
| Graybiel et al., 1990 | 3 | 4 | 88 | 48 |
| Present study | 3 | 3 | 41 | 49 |
| Total: | 55 | 47 | 67 | 53 |
The average decrease of SN and VTA dopaminergic cells was calculated in each paper by comparing the number of SN and VTA dopaminergic cells in PD brains and healthy controls. NA: not available.
Two purported reasons for the differential loss of VTA dopamine neurons in PD have been proposed. First, Reyes et al. recently reported lower levels of calbindin in the SNpc when compared to the VTA in both mice and humans [27], consistent with previous studies [28]. They also reported that more neurons in the VTA co-express TH and calbindin than in the SNpc [27]. In cultured dopaminergic midbrain neurons from rats, higher levels of calbindin resulted in lower vesicular release, thus, VTA dopaminergic neurons release lower levels of dopamine [9]. This may result in fewer reactive oxygen species and less neurotoxicity. Secondly, SNpc neurons express Cav1.3 channels while those in the VTA express hyperpolarization-activated, cyclic nucleotide-regulated cation channels [29,30]. In vivo, blocking Cav1.3 channels decreases the levels of cytosolic dopamine following exposure to L-Dopa in SNpc neurons but not in VTA neurons, suggesting that the SNpc is more vulnerable to degeneration because of cellular mechanisms that increase intracellular dopamine [6]. However, most neurons in the central nervous system express Cav1.3 channels, including degenerating populations in the basal forebrain and locus coeruleus; thus, this reason alone cannot explain the difference.
Executive and cognitive functions have consistently been localized to the prefrontal cortex. , thus deficits in behaviors requiring executive function show abnormal activity in this area [31–33]. Moreover, lesions to the ventral midbrain have been associated with dysexecutive syndrome and dementia. Two lesion studies support the role of the VTA in executive function [34,35]. In these two case studies, patients presented with behavioral changes representative of dysexecutive syndrome and magnetic resonance imaging demonstrated, in both cases, a lesion to the VTA [34,35]. These case studies provide evidence for the importance of the VTA in cognition.
Notably, PD patients are clinically heterogeneous. Over 80% develop non-motor symptoms affecting mood, cognition, and sleep [15,36]. Though several studies have reported improvements in cognition in PD patients while on dopamine replacement therapy (e.g. levodopa) when compared to off treatment [37,38], these results are not consistent [39], supporting the notion that PD is a complex disease. In the literature reported here, only one study provided information on non-motor symptoms: two patients had intellectual impairment and only one subject presented with hallucinations [22]. Goetz et al. and Forsaa et al. identified the development of psychotic symptoms as a risk factor for mortality, as those patients with psychotic symptoms died at earlier ages following PD diagnosis [40,41]. Similarly, PD patients demonstrating executive impairments exhibited lower prefrontal cortex activity as measured by fMRI during a working memory task [42]. An idea that grows out of this insight is that VTA dopamine loss contributes to non-motor symptoms such as depression [43]. Many of the PD patients in pathological studies in the literature, in this and previous studies, die before non-motor symptoms are routinely investigated, thus rendering it impossible to assess the significance of VTA cell loss in PD. Neuroimaging studies, however have shed some light on this question by documenting abnormalities in mesocortical and mesolimbic circuits. For instance, Frosini et al. demonstrated decreased dopaminergic innervation of the anterior cingulate cortex in PD patients with depression as compared to PD patients without depression, measured by dopamine transporter density [43]. Moreover, extensive animal work has indicated that dopamine dysfunction in mesocortical and mesolimbic pathways can consistently impair behaviors that are impaired in PD patients [44].
One specific hypothesis that grows out of this review is that PD patients with anxiety, depression, and executive dysfunction have substantial VTA/medial nigral dopamine loss. However, this hypothesis is made with caution as the dopaminergic system is not the only one affected during disease progression [10]. It has been reported in PD patients that dopamine levels are decreased by 70–90% [45,46], serotonin levels by 60% [46], norepinephrine levels by 80% [47], and acetylcholine levels by 70% [48]. Although these systems have been implicated in the non-motor symptoms of PD, it is possible that they also play a role in the emergence of certain motor symptoms, such as those that do not respond to dopamine replacement therapy. Additionally, some of these systems have also been demonstrated to degenerate with aging, specifically the dopaminergic system. Histological and imaging studies in humans and animal models of aging demonstrate decreases in dopamine in several nuclei [49–52]. However, on average the extent of dopaminergic degeneration is negligible when compared to that seen in PD [49]. Therefore, aging alone cannot explain the decreases in dopaminergic neurons in the VTA found in the present study and the literature.
Our patient samples and the studies we reviewed here did not reliably investigate the many nuclei containing the varying neurotransmitters. Our conclusions are limited because our patients and previous studies did not examine the level of Lewy bodies, tau, or other toxic proteins in the midbrain or cortex which have been previously associated with PD [53–55]. Unfortunately, detailed clinical information was not available for the present or past studies. Future work will explore these issues and will help establish the significance of cell loss in the VTA by combining detailed clinical phenotyping over the disease course with emerging structural, neurochemical, and pathological markers.
Highlights.
We ran a literature review on degeneration of the VTA in PD and added new cases.
The literature unequivocally demonstrates that the VTA is involved in PD.
Our new cases support previous ones with significant degeneration in the VTA.
ACKNOWLEDGMENTS
The authors thank the donors and their families. This study was funded by the University of Iowa Pharmacological Sciences Training Grant (5T32GM067795-11) and Dr. Narayanan’s R01 (NS089470). These funding sources did not partake in study design, data collection and analysis, or the drafting and submission of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davie CA. A review of Parkinson’s disease. Br Med Bull. 2008;86:109–127. doi: 10.1093/bmb/ldn013. [DOI] [PubMed] [Google Scholar]
- 2.Jellinger KA. Pathology of Parkinson’s disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol Spons Int Soc Neurochem World Fed Neurol Res Groups Neurochem Cerebrospinal Fluid. 1991;14:153–197. doi: 10.1007/BF03159935. [DOI] [PubMed] [Google Scholar]
- 3.Hur EE, Zaborszky L. Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: a combined retrograde tracing in situ hybridization study [corrected] J Comp Neurol. 2005;483:351–373. doi: 10.1002/cne.20444. [DOI] [PubMed] [Google Scholar]
- 4.Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152:1024–1031. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lammel S, Hetzel A, Häckel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, et al. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62:218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ropper AH. Adams and Victor’s principles of neurology. Tenth edition. New York: McGraw-Hill Education Medical; 2014. [Google Scholar]
- 8.Marsden CD, Donaldson I, editors. Marsden’s book of movement disorders. Oxford: Oxford University Press; 2012. [Google Scholar]
- 9.Pan P-Y, Ryan TA. Calbindin controls release probability in ventral tegmental area dopamine neurons. Nat Neurosci. 2012;15:813–815. doi: 10.1038/nn.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narayanan NS, Rodnitzky RL, Uc EY. Prefrontal dopamine signaling and cognitive symptoms of Parkinson’s disease. Rev Neurosci. 2013;24:267–278. doi: 10.1515/revneuro-2013-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.German DC, Manaye K, Smith WK, Woodward DJ, Saper CB. Midbrain dopaminergic cell loss in Parkinson’s disease: computer visualization. Ann Neurol. 1989;26:507–514. doi: 10.1002/ana.410260403. [DOI] [PubMed] [Google Scholar]
- 12.Nagai T, McGeer PL, McGeer EG. Distribution of GABA-T-intensive neurons in the rat forebrain and midbrain. J Comp Neurol. 1983;218:220–238. doi: 10.1002/cne.902180209. [DOI] [PubMed] [Google Scholar]
- 13.Narayanan NS, Guarnieri DJ, DiLeone RJ. Metabolic hormones, dopamine circuits, and feeding. Front Neuroendocrinol. 2010;31:104–112. doi: 10.1016/j.yfrne.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cereb Cortex N Y N 1991. 1998;8:321–345. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- 15.Aarsland D, Bronnick K, Williams-Gray C, Weintraub D, Marder K, Kulisevsky J, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology. 2010;75:1062–1069. doi: 10.1212/WNL.0b013e3181f39d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elgh E, Domellöf M, Linder J, Edström M, Stenlund H, Forsgren L. Cognitive function in early Parkinson’s disease: a population-based study. Eur J Neurol Off J Eur Fed Neurol Soc. 2009;16:1278–1284. doi: 10.1111/j.1468-1331.2009.02707.x. [DOI] [PubMed] [Google Scholar]
- 17.Feekes JA, Cassell MD. The vascular supply of the functional compartments of the human striatum. Brain. 2006;129:2189–2201. doi: 10.1093/brain/awl158. [DOI] [PubMed] [Google Scholar]
- 18.Barnett EM, Cassell MD, Perlman S. Two neurotropic viruses, herpes simplex virus type 1 and mouse hepatitis virus, spread along different neural pathways from the main olfactory bulb. Neuroscience. 1993;57:1007–1025. doi: 10.1016/0306-4522(93)90045-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freedman LJ, Cassell MD. Distribution of dopaminergic fibers in the central division of the extended amygdala of the rat. Brain Res. 1994;633:243–252. doi: 10.1016/0006-8993(94)91545-8. [DOI] [PubMed] [Google Scholar]
- 20.Uhl GR, Hedreen JC, Price DL. Parkinson’s disease: loss of neurons from the ventral tegmental area contralateral to therapeutic surgical lesions. Neurology. 1985;35:1215–1218. doi: 10.1212/wnl.35.8.1215. [DOI] [PubMed] [Google Scholar]
- 21.Bogerts B, Häntsch J, Herzer M. A morphometric study of the dopamine-containing cell groups in the mesencephalon of normals, Parkinson patients, and schizophrenics. Biol Psychiatry. 1983;18:951–969. [PubMed] [Google Scholar]
- 22.Hirsch E, Graybiel AM, Agid YA. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature. 1988;334:345–348. doi: 10.1038/334345a0. [DOI] [PubMed] [Google Scholar]
- 23.Bogerts B. A brainstem atlas of catecholaminergic neurons in man, using melanin as a natural marker. J Comp Neurol. 1981;197:63–80. doi: 10.1002/cne.901970106. [DOI] [PubMed] [Google Scholar]
- 24.Waters CM, Peck R, Rossor M, Reynolds GP, Hunt SP. Immunocytochemical studies on the basal ganglia and substantia nigra in Parkinson’s disease and Huntington’s chorea. Neuroscience. 1988;25:419–438. doi: 10.1016/0306-4522(88)90249-7. [DOI] [PubMed] [Google Scholar]
- 25.Javoy-Agid F, Agid Y. Is the mesocortical dopaminergic system involved in Parkinson disease? Neurology. 1980;30:1326–1330. doi: 10.1212/wnl.30.12.1326. [DOI] [PubMed] [Google Scholar]
- 26.Javoy-Agid F, Ruberg M, Taquet H, Bokobza B, Agid Y, Gaspar P, et al. Biochemical neuropathology of Parkinson’s disease. Adv Neurol. 1984;40:189–198. [PubMed] [Google Scholar]
- 27.Reyes S, Fu Y, Double K, Thompson L, Kirik D, Paxinos G, et al. GIRK2 expression in dopamine neurons of the substantia nigra and ventral tegmental area. J Comp Neurol. 2012;520:2591–2607. doi: 10.1002/cne.23051. [DOI] [PubMed] [Google Scholar]
- 28.Chung CY, Seo H, Sonntag KC, Brooks A, Lin L, Isacson O. Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Hum Mol Genet. 2005;14:1709–1725. doi: 10.1093/hmg/ddi178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nedergaard S, Flatman JA, Engberg I. Nifedipine- and omega-conotoxin-sensitive Ca2+ conductances in guinea-pig substantia nigra pars compacta neurones. J Physiol. 1993;466:727–747. [PMC free article] [PubMed] [Google Scholar]
- 30.Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, et al. “Rejuvenation” protects neurons in mouse models of Parkinson’s disease. Nature. 2007;447:1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- 31.Coull JT, Cheng R-K, Meck WH. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2011;36:3–25. doi: 10.1038/npp.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- 33.Dolan RJ, Bench CJ, Liddle PF, Friston KJ, Frith CD, Grasby PM, et al. Dorsolateral prefrontal cortex dysfunction in the major psychoses; symptom or disease specificity? J Neurol Neurosurg Psychiatry. 1993;56:1290–1294. doi: 10.1136/jnnp.56.12.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adair JC, Williamson DJ, Schwartz RL, Heilman KM. Ventral tegmental area injury and frontal lobe disorder. Neurology. 1996;46:842–843. [PubMed] [Google Scholar]
- 35.Nishio Y, Ishii K, Kazui H, Hosokai Y, Mori E. Frontal-lobe syndrome and psychosis after damage to the brainstem dopaminergic nuclei. J Neurol Sci. 2007;260:271–274. doi: 10.1016/j.jns.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 36.Hely MA, Reid WGJ, Adena MA, Halliday GM, Morris JGL. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord Off J Mov Disord Soc. 2008;23:837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 37.Jahanshahi M, Jones CRG, Zijlmans J, Katzenschlager R, Lee L, Quinn N, et al. Dopaminergic modulation of striato-frontal connectivity during motor timing in Parkinson’s disease. Brain. 2010;133:727–745. doi: 10.1093/brain/awq012. [DOI] [PubMed] [Google Scholar]
- 38.Cools R, Stefanova E, Barker RA, Robbins TW, Owen AM. Dopaminergic modulation of high-level cognition in Parkinson’s disease: the role of the prefrontal cortex revealed by PET. Brain J Neurol. 2002;125:584–594. doi: 10.1093/brain/awf052. [DOI] [PubMed] [Google Scholar]
- 39.Harrington DL, Castillo GN, Greenberg PA, Song DD, Lessig S, Lee RR, et al. Neurobehavioral mechanisms of temporal processing deficits in Parkinson’s disease. PloS One. 2011;6:e17461. doi: 10.1371/journal.pone.0017461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goetz CG, Fan W, Leurgans S, Bernard B, Stebbins GT. The malignant course of “benign hallucinations” in Parkinson disease. Arch Neurol. 2006;63:713–716. doi: 10.1001/archneur.63.5.713. [DOI] [PubMed] [Google Scholar]
- 41.Forsaa EB, Larsen JP, Wentzel-Larsen T, Alves G. What predicts mortality in Parkinson disease?: a prospective population-based long-term study. Neurology. 2010;75:1270–1276. doi: 10.1212/WNL.0b013e3181f61311. [DOI] [PubMed] [Google Scholar]
- 42.Lewis SJG, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci Off J Soc Neurosci. 2003;23:6351–6356. doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frosini D, Unti E, Guidoccio F, Del Gamba C, Puccini G, Volterrani D, et al. Mesolimbic dopaminergic dysfunction in Parkinson’s disease depression: evidence from a 123I-FP-CIT SPECT investigation. J Neural Transm Vienna Austria. 1996:2015. doi: 10.1007/s00702-015-1370-z. [DOI] [PubMed] [Google Scholar]
- 44.Parker KL, Alberico SL, Miller AD, Narayanan NS. Prefrontal D1 dopamine signaling is necessary for temporal expectation during reaction time performance. Neuroscience. 2013;255:246–254. doi: 10.1016/j.neuroscience.2013.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hornykiewicz O. Biochemical aspects of Parkinson’s disease. Neurology. 1998;51:S2–S9. doi: 10.1212/wnl.51.2_suppl_2.s2. [DOI] [PubMed] [Google Scholar]
- 46.Kish SJ, Tong J, Hornykiewicz O, Rajput A, Chang L-J, Guttman M, et al. Preferential loss of serotonin markers in caudate versus putamen in Parkinson’s disease. Brain J Neurol. 2008;131:120–131. doi: 10.1093/brain/awm239. [DOI] [PubMed] [Google Scholar]
- 47.Scatton B, Javoy-Agid F, Rouquier L, Dubois B, Agid Y. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson’s disease. Brain Res. 1983;275:321–328. doi: 10.1016/0006-8993(83)90993-9. [DOI] [PubMed] [Google Scholar]
- 48.Arendt T, Bigl V, Arendt A, Tennstedt A. Loss of neurons in the nucleus basalis of Meynert in Alzheimer’s disease, paralysis agitans and Korsakoff’s Disease. Acta Neuropathol (Berl) 1983;61:101–108. doi: 10.1007/BF00697388. [DOI] [PubMed] [Google Scholar]
- 49.Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain J Neurol. 1991;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 50.Rinne JO, Lönnberg P, Marjamäki P. Age-dependent decline in human brain dopamine D1 and D2 receptors. Brain Res. 1990;508:349–352. doi: 10.1016/0006-8993(90)90423-9. [DOI] [PubMed] [Google Scholar]
- 51.Salvatore MF, Apparsundaram S, Gerhardt GA. Decreased plasma membrane expression of striatal dopamine transporter in aging. Neurobiol Aging. 2003;24:1147–1154. doi: 10.1016/s0197-4580(03)00129-5. [DOI] [PubMed] [Google Scholar]
- 52.Kanaan NM, Kordower JH, Collier TJ. Age-related changes in dopamine transporters and accumulation of 3- nitrotyrosine in rhesus monkey midbrain dopamine neurons: relevance in selective neuronal vulnerability to degeneration. Eur J Neurosci. 2008;27:3205–3215. doi: 10.1111/j.1460-9568.2008.06307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 54.Braak E, Sandmann-Keil D, Rüb U, Gai WP, de Vos RA, Steur EN, et al. alpha-synuclein immunopositive Parkinson’s disease-related inclusion bodies in lower brain stem nuclei. Acta Neuropathol (Berl) 2001;101:195–201. doi: 10.1007/s004010000247. [DOI] [PubMed] [Google Scholar]
- 55.Irwin DJ, White MT, Toledo JB, Xie SX, Robinson JL, Van Deerlin V, et al. Neuropathologic substrates of Parkinson disease dementia. Ann Neurol. 2012;72:587–598. doi: 10.1002/ana.23659. [DOI] [PMC free article] [PubMed] [Google Scholar]