Abstract
Breast cancer-related lymphedema (LE) is a progressive, chronic disease that affects millions of cancer survivors and primarily results from surgical lymphatic vessel/node removal and radiation therapy. Patient education and support for importance of early detection is essential in helping health care providers detect lymphedema early, when there is the best chance to prevent progression. Improved imaging and surgical techniques have decreased the incidence of LE; however, effective risk-reduction and treatment have historically lacked the level of evidence necessary to standardize effective treatment. The purpose of this article is to report an extensive review of literature, including highlighted multidisciplinary studies within the past three years, in order to update best practice guidelines in assessment, risk reduction, management, and surveillance for post-breast cancer lymphedema.
Keywords: Breast cancer, Lymphedema, Practices, Risk reduction, Management, Surveillance, Lymphatic, Combined decongestive therapy, Complementary, Manual lymphatic drainage, Exercise, Compression garment, Intermittent pneumatic compression pump, Limb volume
INTRODUCTION
Secondary lymphedema (LE) is a chronic, progressive, and debilitating condition estimated to impact over 11.4 million American cancer survivors who are at risk for developing LE in their lifetimes [1,2] LE is the abnormal accumulation of lymph in the interstitial spaces leading to persistent swelling of the affected regions, resulting in an array of symptoms and sequelae [2]. Breast cancer survivors are at lifetime risk for developing LE, with an occurrence rate of 41% to 94% within 57 months depending on the measurement tools and defining criteria; however, it can present with an onset ranging from early in the post-operative period to beyond 30 years post-treatment [2, 3,4]. Among breast cancer survivors, the most common causes of secondary LE are lymphatic vessel/node removal and radiation treatment [4]. LE symptoms have been shown to adversely impact survivorship following breast cancer treatment [5].
While the definition of LE has not been standardized, a common definition is swelling of the affected limb which is greater than baseline (pre-operative) measures or compared to the unaffected limb [6]. Multiple longitudinal studies provide evidence that limb volume and its appearance may fluctuate over time with LE emerging as transient, chronic, mild, or severe [3,4]. However, presence of initial transient swelling in the early post-operative phase has been associated with later LE development [7]. Identification of swelling during the post-operative phase provides an opportunity for early intervention which may theoretically preempt the progression to a chronic edematous state.
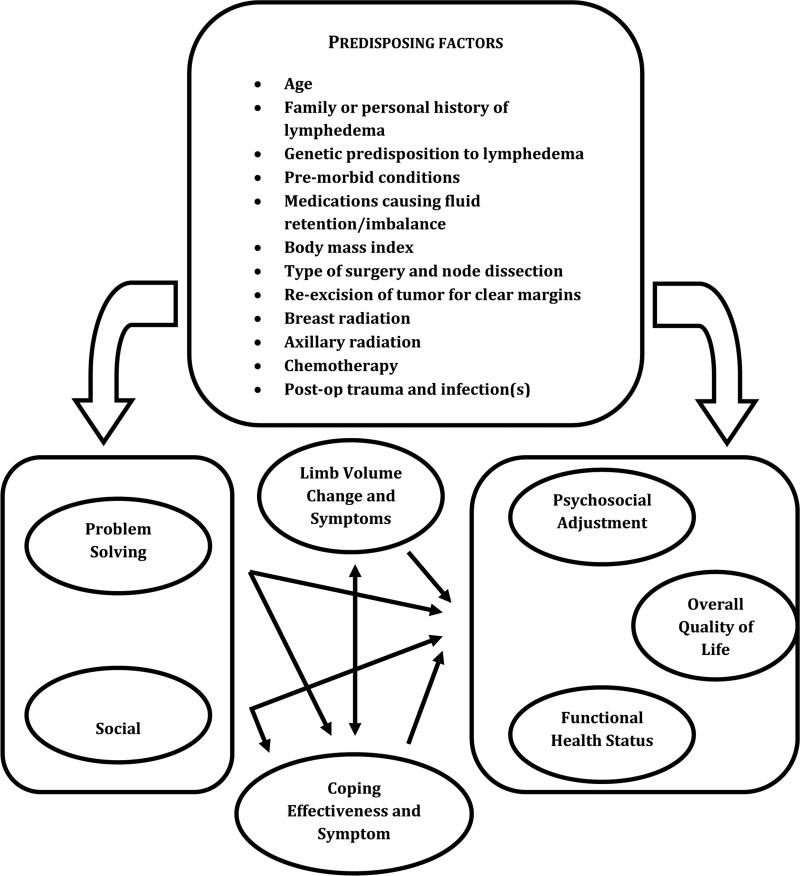
With an aging population of breast cancer survivors, education should include LE risk-reducing and rehabilitative strategies with management support [8]. According to the theoretical model of elderly cancer survivorship by Bellury et al. [9], age alone is a risk factor for cancer diagnosis and treatment-related sequelae, such as LE. Risk factors for breast cancer survivorship have been categorized as: 1) personal-modifiable (e.g., BMI, physical activity), 2) personal-nonmodifiable (e.g., age, gender), 3) age-specific (e.g., cognition), 4) cancer-specific (e.g., treatment type, metastasis), and 5) baseline health status (e.g., frailty) [9]. Figure 1 offers a conceptual model of the biopsychosocial factors influencing post-breast cancer LE [8].
Figure 1.
Conceptual model of biopsychosocial factors influencing post–breast cancer lymphedema. Adapted from Armer et al. [8]. Rehabilitation concepts among aging survivors living with and at risk for lymphedema: A framework for assessment, enhancing strengths, and minimizing vulnerability. Top Geriatr Rehabil 2012;28(4):260-8.
DIAGNOSIS and ASSESSMENT
History and Examination
Detection and management of LE builds from a clinical assessment to include cancer history, including primary and adjuvant cancer treatment(s); assessment of the anatomy and function of the lymphatic system, as well as physical manifestations; and LE staging determination [6]. In a patient presenting with a swollen upper extremity, clinical, lymphatic, and venous system evaluation (with appropriate imaging) should be conducted. Consideration of duplex ultrasonography to rule out deep venous thrombosis and computed tomographic scanning to rule out cancer recurrence (which is known to precipitate and exacerbate LE) may be warranted [6, 8].
Imaging
A number of imaging modalities are available to assess the anatomy and function of the lymphatic system. Lymphoscinitigraphy (LS) is the ‘gold standard’ for LE assessment [6]. Lymphoscinitigraphy utilizes a nuclear tracer, 99m-technetium, which is injected intradermally into the hand for lymphatic system uptake. Tracer imaging allows depiction of lymphatic anatomy and flow and can highlight pathologic changes due to lymphatic obstruction [10, 11]. Direct contrast lymphography has largely been replaced by lymphoscintigraphy as the imaging method of choice due to the complexity of the procedure and associated risk of pulmonary embolism [5]. Despite this, lymphography may be useful for pre-operative assessment of complex lymphatic anatomy [5]. Indocyanine Green (ICG) infrared fluorescent imaging is also used for imaging the lymphatic system in select research centers in the United States [12-16]. Magnetic Resonance Imaging (MRI) using gadolinium tracers has also been used outside the United States; however, it lacks FDA approval for intradermal injection of gadolinium [6, 17]. Ultrasound can be useful to assess venous system changes and define tissue spaces, as well as fluid accumulation [6].
Assessment Tools
Objective limb volume (LV) measurement is used to detect increased swelling and to monitor changes over time [6]. The exact method is not as important as using a standard, reproducible method consistently over time [6]. Water displacement remains the ‘gold standard’ for LV measure; however, this method is limited due to its cumbersome nature, hygienic concerns, and difficult implementation [18, 19].
Circumference tape measurement at designated landmarks, using a nonstretch tape measure is the most common method for assessing LV change. It is not prohibited by limb mobility and size and is reproducible if done correctly using a standardized protocol [6, 20]. Both limbs are measured at identified anatomical landmarks in order to monitor for changes over time [6]. Circumference measurement is inexpensive, but time-consuming, and requires rigorous training to achieve reliable and accurate measures [18].
Perometry (Juzo, Cuyahoga Falls, OH) uses infrared light and opto-electronic sensors to calculate LV from the three-dimensional silhouette of the limb [18, 21]. Perometry is efficient in time and hygienic, allowing measurement even of limbs with wounds since no equipment touches the skin of the arm, but the instrument is costly, particularly for smaller clinics.
In a longitudinal study of breast cancer survivors, LV change from pre-operative baseline through 57 months post-surgery was assessed using four commonly cited diagnostic criteria (2 cm circumferential change, 200 mL perometry limb volume change, 10% perometry limb volume change, and signs/symptoms) [3]. Investigators reported that the 2 cm criteria resulted in the highest incidence of LE, followed by the 200mL perometry criteria, with 10% limb volume change by perometry, and symptom report, resulting in the lowest incidence [3, 22].
Fluid content can be measured using bioelectrical impedance spectroscopy (BIS) which measures extracellular fluid by resistance to a small electrical current [23-25]. BIS appears to be more sensitive than traditional diagnostic methods in potentially detecting early LE changes before physically measurable changes in LV are observed [6, 26].
Tissue texture is an important assessment characteristic that should be assessed in LE patients due to the skin's increased susceptibility to injury and infection [6]. With LE progression, the limb tissues develop a fatty and fibrotic quality and subsequently become resistant to compression [6, 27]. Tonometry has been used to measure the degree of tissue compressibility and its correlation with limb swelling; however, standard protocols have not been established and reliability remains an issue [23, 27].
Classification
Historically, there has not been an international consensus on the definition of LE or the staging parameters used to reflect LE severity [6]. In 2009, the International Society of Lymphology (ISL) published an updated Consensus documenting the Clinical Staging and Severity System based on objective physical examination findings (see Table 1) [28].
Table 1.
Clinical Staging and Severity According to the International Society of Lymphology Consensus Document. Data reproduced with permission of Lymphology [28]: International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema. Consensus document of the International Society of Lymphology. Lymphology.2009, 40:49-57.
| Clinical Stage | Description |
|---|---|
| 0 | A subclinical stage where swelling is not seen despite underlying changes in the lymphatic system |
| I | The initial stage of swelling which can be transient and where simple elevation can alleviate swelling |
| II | Swelling is constant and pitting without resolution using elevation |
| III | The tissue has become hard and fibrotic with associated skin changes |
| Severity | based on volume differences between affected and contralateral limb in unilateral presentation |
| mild= <20% increase; moderate=20-38% increase, severe=>38% increase |
RISK REDUCTION
Absolute prevention is not yet fully possible in breast cancer-related LE; however, advances in cancer diagnostic imaging and surgical techniques have reduced the risk of LE and newer tools/techniques have allowed earlier detection of subclinical LE. In addition, early implementation of risk-reduction strategies may improve outcomes by preventing progression and sequelae of LE [29].
Methods to reduce development of LE are largely operative or physical. Operative methods include the following:
Sentinel Lymph Node Biopsy (SLNB) vs. Axillary Lymph Node Dissection (ALND)
Earlier breast cancer detection has allowed for less invasive surgical procedures to assess and treat the regional lymph nodes [6]. In a systematic review, Sanghani et al. [30] reported ALND does not offer any survival benefit compared to no dissection in patients with negative sentinel lymph nodes. The American College of Surgeons Oncology Group (ACOSOG) Z0011 trial (n=813) reported there is no benefit to performing ALND in patients with clinical T1-T2 breast cancer with 1 or 2 positive (microscopic disease) SLNs who are treated with breast conservation, whole breast irradiation, and systemic therapy. At a median of 6.3 years follow-up, SLNB alone remained equivalent to ALND [31].
Axillary Reverse Mapping
A newer technique has been proposed to identify and make a distinction between the breast- and arm-draining lymphatic channels and nodes. This technique avoids disruption of lymphatic vessels draining the arm at the time of SLND or ALND. While this technique is promising for reducing LE incidence, long-term follow-up studies have not been conducted and there has been controversy regarding the oncologic safety of this procedure [6, 32, 33].
Lymphatic-Venous Anastomosis (LVA)
This operative technique uses blue dye injection into the upper arm to identify lymphatic channels at the time of ALND in order to create connections between these channels and the venous system in an attempt to preserve lymphatic drainage of the affected limb [34]. Data from a one-year follow-up study show that patients who underwent ALND and LVA did not develop LE as evidenced through LS [35]. Longer follow-up and randomized control trials (RCTs) are needed to determine the true effectiveness in preventing LE.
Physical Methods
Early detection and intervention remain the primary strategy for reducing the incidence of chronic LE [6, 29]. Because there is a lower incidence of LE associated with contemporary surgical techniques, it is anticipated that breast cancer-related LE incidence will decrease further as more is learned from lymphatic preservation research with breast cancer treatment. Physical risk-reduction recommendations should take LE incidence rates into consideration and recognize the benefit-risk ratio when proposing particular risk-reduction regimens to patients [6].
Promising findings have emerged from selected studies suggesting noninvasive approaches to reducing the occurrence of LE in at-risk survivors [36]. Stout Gergich et al. [37] reported in an early pilot study that LE incidence may successfully be reduced by using accurate assessment techniques and early intervention with compression garments. Recent data show that early physiotherapy after breast cancer surgery, including ALND, may be effective in reducing LE risk for at least one year [38]. Post-operative swelling has been associated with later LE development [7]. Awareness of an initial episode of transient swelling provides an opportunity for early intervention which may theoretically preempt progression to a chronic state. Finally, a growing body of evidence suggests that exercise does not exacerbate or trigger secondary LE [39, 40].
MANAGEMENT
Complete Decongestive Therapy (CDT), also called complex lymphatic therapy, has long been the standard of care in the treatment of acute and long-term management of breast cancer-related LE [4, 41-44]. The primary components of CDT recognized by the Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) in 2009 include: 1) manual lymphatic drainage (MLD); 2) compression bandaging (CB) and/or garments (CG); 3) exercise; 4) skin care; and 5) sequential pumps. CDT is usually initiated as two phases, intensive CDT, indicated for moderate to severe LE, and modified CDT, for mild or moderate LE [42].
In a systematic review of literature published from 2004 to 2011 regarding the evidence for CDT therapy, reviewers recruited by the American Lymphedema Framework Project (ALFP) rated 26 eligible studies [41]. They found that CDT as a bundled intervention, as well as MLD and CB as individual components, was effective in reducing lymphedema [8]. Despite the limited number of RCTs, CDT continues to be viewed as effective in reducing LE, although the relative contribution of each of the individual components influencing the efficacy of CDT is not well understood [8]. CDT should be administered by a specialty-trained therapist with the goal of reducing swelling and fibrosis in the affected area. Patient training in life-long LE self-management practices should also be included [4, 41, 43, 45].
Following intensive CDT, self-maintenance may include self-MLD, daytime compression garments, night-time bandaging, exercise, skin care, and nutrition. Wearing properly-fitted compression garments offers management for swelling, as well as preservation of skin integrity [41-43]. Frail patients with LE may not tolerate high levels of compression; modification of compression bandaging with an alternative non-elastic compression device (e.g., Velcro® closure) should be considered [8]. Although the level of evidence in the literature regarding compression garment use during exercise is ‘moderate’ [41], experts recommend the use of a compression garment during exercise [43].
Manual lymphatic drainage involves light, lymphatic massage to move lymph fluid from the extremity to a more central (proximal) region of the body where lymphatic flow is not impaired [41]. Induced muscle contractions increase lymphatic and vascular flow throughout the body and thereby reduce lymphatic stasis and LE symptoms [4, 41, 46]. Survivors have varied levels of physical and cognitive limitations; therefore, individualized, supervised exercise programs should be developed by a multi-disciplinary rehabilitation team in order to create a safe, appropriate regimen [47].
Patient education regarding proper skin care management is an essential component of CDT which reduces the risk of exacerbating LE due to inflammation and infection. Self-maintenance instruction should include hygiene, moisturizing, sunscreen, and avoidance of constriction from blood pressure cuffs or tourniquet application [43, 48]. Additional patient education should also include nutritional information and strategies to promote optimal weight management as another means of preventing LE development and progression [48, 49].
Few recommendations exist in the literature for LE management in the palliative care setting. Beck et al. [50] conducted a systematic review of the published literature from 2004 through 2011 regarding the evidence of LE management in palliative care and summarized five eligible studies using the Oncology Nursing Society (ONS) Putting Evidence into Practice (PEP) guidelines [51]. They concluded that CDT, MLD, and compression bandaging are categorized as “effectiveness is not established,” but note that no adverse findings were reported [50]. Based on these findings, it is suggested that CDT, MLD, and compression bandaging offer a potential benefit to LE symptom control and improved quality of life for LE patients receiving palliative care for symptom management in advanced disease, such as recurrent breast cancer.
Surgical Management
Cormier et al. [52] conducted a systematic review of the literature from 2004 to 2010 pertaining to the surgical treatment of LE. Twenty identified studies met the inclusion criteria and were categorized as excisional/debulking, lymphatic reconstruction, or tissue transfer. Excisional procedures remove fibrofatty tissue that has formed secondary to sustained lymphatic fluid stasis. Procedures include debulking, liposuction, and amputation and should be considered only when standard LE treatment, such as CDT, has failed. Lymphatic reconstruction is a microsurgical technique for the reconstruction or bypassing of obstructed lymphatic channels which is performed to improve lymphatic drainage. These procedures can include anastomoses from the lymph vessels to veins, lymph nodes to veins, or distal to proximal lymphatics using lymphaticovenular anastomosis (LVA). Tissue transfer procedures involve transferring lymph tissue into a congested area with anastomosis of lymphatic vessels in order to reestablish lymphatic flow. The largest LE volume reduction was associated with excisional procedures (91.1%), followed by lymphatic reconstruction (52.9%), and lastly, tissue transfer procedures (45.6%) [52].
It is noted that the majority of these surgical procedures will require lifelong compression garment use to maintain post-operative results [52]. The findings regarding surgical procedures are difficult to generalize due to lack of high-level evidence and the need for surgical vs. non-surgical studies with larger sample sizes. While no adverse events were reported in studies reviewed, patient teaching should include awareness that these surgical procedures can be associated with significant risks, as with any surgical procedure, including infection, delayed wound healing, stricture/occlusion of newly created anastomoses [52].
Intermittent Pneumatic Compression (IPC) Therapy
Several early systematic reviews exist pertaining to the use of IPC therapy for LE management. Previously, in a review by Moseley et al. [53], IPC was identified as the most likely therapeutic modality to facilitate large volume reductions in the treatment of LE. Conversely, Rinehart-Ayres et al. [54] reported there is no evidence to suggest that use of an IPC pump offers more benefit than arm care and hygiene practices, nor does evidence support one type of IPC pump regimen over another. However, a recent study reported lymphatic function improvement in four of six subjects by NIR fluorescence imaging after using an advanced programmable IPC with a segmented sleeve and calibrated gradient processor versus a less advanced older version device [12].
Due to the lack of consensus regarding the recommended IPC treatment parameters or frequency, Feldman et al. [55] conducted a systematic review of peer-reviewed studies from 2004 to 2010. Findings indicate that although IPC devices are reportedly well-tolerated in low to moderate pressure ranges and appear to be safe for home use, there were no clear guidelines for compression levels and frequency emerging from the literature. They concluded that IPC may be appropriate as part of a supervised multi-modality approach for home-based LE management in select patients.
Exercise
Schmitz et al. [56] report that at six years post-diagnosis, 57% of survivors (n=287) experienced one or more late effects of breast cancer treatment amenable to rehabilitative intervention (exercise). Furthermore, a systematic review by Kwan et al. [57] of published studies from 2004 to 2011 of exercise and LE care concluded that breast cancer survivors may safely engage in an instructed, supervised exercise regimen throughout the survivorship continuum, including during treatment. They highlighted six RCTs [19, 58-62] and one cross-over study [63] that offer “highly-likely-be-effective” evidence that resistance exercise poses minimal risk in the development or exacerbation of upper extremity LE [57]. The Physical Activity and Lymphedema (PAL) studies by Schmitz et al. [57-59] were cited as landmark studies demonstrating that slow, progressive resistance exercise with weight-lifting is likely to be effective in reducing risk of LE symptoms and progression, and in increasing overall strength in survivors status post-ALND. Preliminary studies examining aerobic and resistance combination exercises also report no increase in LE and appear safe [39, 64, 65].
In the review by Kwan et al. [57], the reported range of LE incidence among intervention participants was: 13% at 2 years [19], 17% at 10 to 38 months [60], 30% at 18 months [39], and 17% at 2-6 years post-diagnosis [58]. It should be noted that higher rates of LE occurred in control groups rather than in the intervention groups [19, 58, 61, 62]. Rehabilitative and exercise interventions have shown benefit to breast cancer survivors with LE; however, programs must be structured according to the abilities of each patient with close monitoring by qualified therapists to ensure safety and standardization [66,67]. Additional research is needed to offer recommendations regarding compression garment use during exercise [57].
Complementary and Alternative Medicine (CAM)
Complementary and alternative medicine (CAM) is widely used as a means to achieve health and well-being. Breast cancer patients use CAM more often than patients with other types of cancer due to treatment side effects and problems continuing after treatment [68]. Wanchai et al. [68] found that among Thai survivors, CAM information is obtained through peers and only one-third of Thai breast cancer survivors report sharing information about CAM usage with their physicians due to their fear of a negative response.
Studies to evaluate the effectiveness of CAM in reducing LE are usually based on self-report and results are variable [69]. A growing body of literature offers evidence that the practice of mindfulness-based stress reduction (MBSR) improves quality of life and reduces psychological distress [70]. The use of aqua lymphatic therapy to manage LE has also been reported with favorable results. Investigators noted a higher adherence rate (78%) compared to a less-than-30% adherence to compression bandages/garments, self-massage, and special exercise [71]. Recent pilot data suggest that yoga, acupuncture/moxibustion, and Tai Chi breathing with arm exercises appear safe [72-75]; however, additional large-scale studies are needed to determine the role of CAM therapy in LE symptom management.
Psychosocial and Economic Considerations
It is well-established that recurrence of breast cancer is the greatest fear among survivors, followed by development of LE [6, 76]. Investigators from the United Kingdom found that at least 78% of patients with LE reported lost work time, with 9% suffering a negative job status outcome [77]. Shih et al. [78] reported that two-year medical insurance claim costs were nearly double for US breast cancer survivors with LE ($23,164), compared to those without LE ($14,875). Patients with LE were twice as likely to have lymphangitis or cellulitis, contributing to a more advanced condition and compounding medical costs [78]. These findings demonstrate financial issues for cancer survivors with LE extend beyond insurance coverage and rehabilitation costs [8, 78, 79].
Data suggest that breast cancer survivors experience chronic psychological distress associated with symptoms beginning at pre-diagnosis (biopsy), extending throughout the post-treatment period [79]. Survivors with LE often report poor physical function, physical self-image, and quality of life, and social isolation leading to persistent psychological distress [80-82]. Fu et al. [81] conducted a systematic review of 23 published studies from 2004 to 2011 on the psychosocial impact of LE and reported that poor psychosocial and social well-being are prevailing findings among survivors with LE.
SURVEILLANCE
Surveillance for LE signs and symptoms starts with obtaining a focused patient history regarding swelling of the at-risk limb and other areas of the body potentially affected by cancer treatment [8]. Regular anthropometric and symptom assessment beginning pre-operatively through the post-operative period and in three-month intervals thereafter for at least the first year post treatment provide the best opportunity to monitor for LE [8]. Early detection provides the best chance to prevent progression [6]. Routine LV measures during follow-up visits in a busy clinical practice setting are feasible, efficient, [38, 83] and should be included in standard surveillance practices [84]. Triage for further assessment is recommended when symptoms (e.g. sensation of heaviness and/or observed swelling) or girth and/or volume measures increase.
Awareness of predisposing factors such as high BMI, weight gain after breast cancer treatment, post-operative swelling, post-operative seroma, infection, and LE family history (Figure 1) can be used to guide individualized education and support in developing LE risk-reduction behaviors. Regardless of the method used to assess LV, it is important that the selected approach be consistently applied at regular intervals, such as pre- and post-operatively, quarterly (for 12 months), semi-annually (for 1-3 years), and then annually, thereafter. Such an assessment timeline, accompanied by education on self-monitoring, provides optimal opportunity for early detection and intervention.
Stout et al. [85] suggest that goals for a prospective surveillance model for breast cancer survivors should include the following components: 1) promotion of monitoring for functional and physical impairments commonly associated with breast-cancer treatment; 2) the provision of education about early signs and symptoms of LE and the importance of early detection; 3) referral for rehabilitation and exercise interventions once physical limitations are identified; and 4) promotion and support of physical activity, exercise, nutrition, and weight-management behaviors throughout the survivorship continuum. These recommendations are supported by Schmitz et al. [67] and Binkley et al. [86] who also advocate a multidisciplinary prospective surveillance approach in the management and treatment of adverse effects of breast cancer treatment.
An additional component of an effective surveillance program is the involvement of researchers in developing minimum data sets (MDS) in order to create, organize, and disseminate up-to-date clinical research data and measure patient outcomes nationally and worldwide. Under the oversight of Chi-Ren Shyu, PhD, Director of the University of Missouri Informatics Institute, and with National Library of Medicine funding, an internet-based information technology system has been designed and tested as a platform for the collection and transfer of data which will be used to update Best Practices with new research at regular intervals [87].
Surveillance programs should include a patient-related component with pre-operative assessment and measures on all breast cancer patients requiring surgical treatment, education and supportive regimens tailored to each patient relevant to prevention and early detection of LE, ongoing monitoring and assessment for physical impairment throughout the breast cancer continuum, referral to resources and implementation of self-care management regimens for patients who have developed breast cancer-related LE, and opportunities for clinical trials and programs to engage psychosocial and social well-being. A research component of the surveillance plan should include support for ongoing development of the MDS system. Minimally at each visit, the clinicians should assess for symptoms of heaviness and/or observed swelling. Self-report of symptoms is sufficient to trigger referral for further assessment by an expert in this area.
CONCLUSION
These guidelines are provided using the latest information available from published reports and experts in the field. These recommendations are synergistic with the new multidisciplinary model proposed by the National Accreditation Program for Breast Centers (NAPBC) for early detection of physical impairments with goals to: 1) promote monitoring for common post- treatment physical impairments; 2) introduce rehabilitation and exercise interventions when issues are identified; and 3) encourage and support physical activity throughout breast cancer patients' diagnosis, treatment, recovery and survivorship [88].
Despite the authors’ desire to define clear, agreed-upon practices, data are still limited in many areas due to the lack of large replicated trials. Diagnosis and assessment methods are available which should be utilized in standardized fashion to follow patients and their treatment [89]. Newer detection methods and increased survivorship attention are likely to shorten the time to initial diagnosis and thereby improve patient outcomes. In addition, continued improvement in cancer diagnosis and treatments are also likely to reduce incidence. Finally, a good treatment armament exists for patients with LE. Hopefully, an increase in the number of high-level studies will move research forward in determining the best treatment(s) for each patient, leading to optimal quality of life.
Footnotes
Disclosure J.M. Armer declares that she has no conflict of interest.
J.M. Hulett declares that she has no conflict of interest.
M. Bernas declares that he has no conflict of interest.
P. Ostby declares that she has no conflict of interest.
B.R. Stewart declares that he has no conflict of interest.
Janice N. Cormier declares that she has no conflict of interest.
Contributor Information
Jane M. Armer, Sinclair School of Nursing Director, Nursing Research, Ellis Fischel Cancer Center Director, American Lymphedema Framework Project DC 116.05 Suite 415 EFCC University of Missouri-Columbia Columbia, MO 62011.
Jennifer M. Hulett, Sinclair School of Nursing University of Missouri-Columbia Jmh5f3@mail.missouri.edu.
Michael Bernas, Department of Surgery University of Arizona michaelb@u.arizona.edu.
Pam Ostby, Sinclair School of Nursing University of Missouri-Columbia Plo7c9@mail.missouri.edu.
Bob R. Stewart, College of Education and Sinclair School of Nursing University of Missouri-Columbia stewartb@missouri.edu.
Janice N. Cormier, Departments of Surgical Oncology and Biostatistics The University of Texas MD Anderson Cancer Center Houston, TX jcormier@mdanderson.org.
References
Papers of particular interest, published recently, have been highlighted as:
*Of importance
**Of major importance
- 1.American Cancer Society . The global economic cost of cancer. American Cancer Society; Atlanta: 2010. [February 22, 2013]. Available at http://www.cancer.org/acs/groups/content/@internationalaffairs/documents/document/acspc-026203.pdf. [Google Scholar]
- 2.Rockson SG, Rivera KK. Estimating the population burden of lymphedema. Ann New York Academy of Sciences. 2008;1131:147–54. doi: 10.1196/annals.1413.014. [DOI] [PubMed] [Google Scholar]
- 3*.Armer JM, Stewart BR. Post-breast cancer lymphedema: Incidence increases from 12 to 30 to 60 months. Lymphology. 2010;43:118–27. [This study followed survivors’ limb volume change from pre-surgery through 57 months post-surgery using the four most commonly cited diagnostic criteria (2 cm circumferential change, 200 mL perometry LVC, 10% perometry LVC, and signs/symptoms) and found that the 2 cm criteria remains the most liberal definition of LE, followed by the 200mL perometry criteria.] [PMC free article] [PubMed] [Google Scholar]
- 4.Földi M, Földi E, Kubik S. Textbook of lymphology: For physicians and lymphedema therapists. Urban and Fisher; San Francisco, CA: 2003. [Google Scholar]
- 5.Hayes S, Di Sipio T, Rye S, et al. Prevalence and prognostic significance of secondary lymphedema following breast cancer. Lymphat Res Biol. 2011;9(3):135–41. doi: 10.1089/lrb.2011.0007. [DOI] [PubMed] [Google Scholar]
- 6**.Bernas M, Askew R, Armer J, Cormier J. Lymphedema: How do we diagnose and reduce the risk of this dreaded complication of breast cancer treatment? Cur Breast Cancer Rep. 2010;2(1):53–8. [This article highlights current practices in LE risk reduction practices methods of diagnosis and assessment. Recommendations include identifying patients with early swelling for referral to LE treatment specialists.] [Google Scholar]
- 7.Mahamaneerat WK, Shyu C-R, Stewart BR, Armer JM. Breast cancer treatment, bmi, post-op swelling/lymphoedema. J Lymphoedema. 2008;3(2):38–44. [PMC free article] [PubMed] [Google Scholar]
- 8**.Armer JM, Stewart BR, Wanchai A, et al. Rehabilitation concepts among aging survivors living with and at risk for lymphedema: A framework for assessment, enhancing strengths, and minimizing vulnerability. Top Geriatr Rehabil. 2012;28(4):260–8. [This article discusses the complexity of issues facing rehabilitative outcomes in detection and management of post-treatment LE in an aging breast cancer survivor population. With improved cancer detection and treatment, the number of older cancer survivors is predicted to increase, making imperative attention to a survivorship rehabilitation-focused program of surveillance.] [Google Scholar]
- 9.Bellury LM, Ellington L, Beck SL, et al. Elderly cancer survivorship: An integrative review and conceptual framework. Eur J Oncol Nurs. 2011;15:233–42. doi: 10.1016/j.ejon.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Modi S, Stanton AWB, Mortimer PS, Levick JR. Clinical assessment of human lymph flow using removal rate constants of interstitial macromolecules: A critical review of lymphoscintigraphy. Lymphat Res Biol. 2007;5(3):183–202. doi: 10.1089/lrb.2007.5306. [DOI] [PubMed] [Google Scholar]
- 11.Witte CL. Advances in imaging of lymph flow disorders. Radio Graphics. 2000;20:1697–719. doi: 10.1148/radiographics.20.6.g00nv141697. [DOI] [PubMed] [Google Scholar]
- 12.Adams KE, Rasmussen JC, Darne C, et al. Direct evidence of lymphatic function improvement after advanced pneumatic compression device treatment of lymphedema. Biomedical Optics Express. 2010;1:114–25. doi: 10.1364/BOE.1.000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unno N, Nishiyama M, Suzuki M, et al. Quantitative lymph imaging for assessment of lymph function using indocyanine green fluorescence lymphography. Eur J Endovasc Surg. 2008;36(2):230–6. doi: 10.1016/j.ejvs.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Rasmussen JC, Tan IC, Marshall MV, et al. Lymphatic imaging in humans with near-infrared fluorescence. Cur Opin Biotech. 2009;20(1):74–82. doi: 10.1016/j.copbio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasmussen JC, Kwon S, Sevick-Muraca EM, Cormier JN. The role of lymphatics in cancer as assessed by near-infrared fluorescence imaging. Ann of Biomed Engineer. 2012;40(2):408–21. doi: 10.1007/s10439-011-0476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giacalone G, Belgrado JP, Bourgeois P, et al. A new dynamic imaging tool to study lymphoedema and associated treatments. The Euorpean J Lymphology. 2011;22(62):10–4. [Google Scholar]
- 17.Liu NF, Lu Q, Jiang ZH, et al. Anatomic and functional evaluation of the lymphatics and lymph nodes in diagnosis of lymphatic circulation disorders with contrast magnetic resonance lymphangiography. J Vascular Surgery. 2009;49(4):980–7. doi: 10.1016/j.jvs.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 18.Armer JM. The problem of post-breast cancer lymphedema: Impact and measurement issues. Cancer investigation. 2005;23(1):76–83. [PubMed] [Google Scholar]
- 19.Sagen A, Karesen R, Risberg MA. Physical activity for the affected limb and arm lymphedema after breast cancer surgery. A prospective, randomized controlled trial with two years follow-up. Acta Oncol. 2009;48(8):1102–10. doi: 10.3109/02841860903061683. [DOI] [PubMed] [Google Scholar]
- 20.Mayrovitz HN. Limb volume estimates based on limb elliptical vs. Circular cross section models. Lymphology. 2003;36(3):140–3. [PubMed] [Google Scholar]
- 21.Stanton AW, Northfield JW, Holroyd B, et al. Validation of an optoelectronic limb volumeter (perometer). Lymphology. 1997;30(2):77–97. [PubMed] [Google Scholar]
- 22.Armer JM, Stewart BR, Shook RP. 30-month post-breast cancer treatment lymphoedema. J Lymphoedema. 2009;4(1):14–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Moseley A, Piller N. Reliability of bioimpedance spectroscopy and tonometry after breast conserving cancer treatment. Lymphat Res Biol. 2008;6(2):85–7. doi: 10.1089/lrb.2008.1002. [DOI] [PubMed] [Google Scholar]
- 24.Smoot BJ, Wong JF, Dodd MJ. Comparison of diagnostic accuracy of clinical measures of breast cancer-related lymphedema: Area under the curve. Archives of Physical Medicine and Rehabilitation. 2011;92(4):603–10. doi: 10.1016/j.apmr.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornish BH, Chapman M, Hirst C, et al. Early diagnosis of lymphedema using multiple frequency bioimpedance. Lymphology. 2001;34(1):2–11. [PubMed] [Google Scholar]
- 26.Mayrovitz HN, Brown-Cross D, Mayrovitz BL, Golla AH. Lymphedema: Role of truncal clearance as a therapy component. Home Health Care Management Practice. 2009;21(5):325–37. [Google Scholar]
- 27.Bagheri S, Ohlin K, Olsson G, Brorson H. Tissue tonometry before and after liposuction of arm lymphedema following breast cancer. Lymph Res Biol. 2005;3(2):66–80. doi: 10.1089/lrb.2005.3.66. [DOI] [PubMed] [Google Scholar]
- 28.International Society of Lymphology The diagnosis and treatment of peripheral lymphedema: 2009 consensus document of the International Society of Lymphology. Lymphology. 2009;42:51–60. [PubMed] [Google Scholar]
- 29.Shah C, Arthur D, Riutta J, et al. Breast-cancer related lymphedema: A review of procedure-specific incidence rates, clinical assessment aids, treatment paradigms, and risk reduction. The Breast Journal. 2012;18(4):357–61. doi: 10.1111/j.1524-4741.2012.01252.x. [DOI] [PubMed] [Google Scholar]
- 30.Sanghani M, Balk EM, Cady B. Impact of axillary lymph node dissection on breast cancer outcome in clinically node negative patients: A systematic review and meta-analysis. Cancer. 2009;115(8):1613–20. doi: 10.1002/cncr.24174. [DOI] [PubMed] [Google Scholar]
- 31.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: A randomized clinical trial. JAMA. 2011;305(6):569–75. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boneti C, Korourian S, Bland K, et al. Axillary reverse mapping: Mapping and preserving arm lymphatics may be important in preventing lymphedema during sentinel lymph node biopsy. J American College of Surgeons. 2008;206(5):1038–42. doi: 10.1016/j.jamcollsurg.2007.12.022. discussion 42-44. [DOI] [PubMed] [Google Scholar]
- 33.Klimberg VS. A new concept toward the prevention of lymphedema: Axillary reverse mapping. J Surg Oncol. 2008;97(7):563–4. doi: 10.1002/jso.20905. [DOI] [PubMed] [Google Scholar]
- 34.Boccardo FM, Ansaldi F, Bellini C, et al. Prospective evaluation of a prevention protocol for lymphedema following surgery for breast cancer. Lymphology. 2009;42(1):1–9. [PubMed] [Google Scholar]
- 35.Boccardo F, Casabona F, De Cian F, et al. Lymphedema microsurgical preventive healing approach: A new technique for primary prevention of arm lymphedema after mastectomy. Ann Surg Oncol. 2009;16(3):703–8. doi: 10.1245/s10434-008-0270-y. [DOI] [PubMed] [Google Scholar]
- 36.Box RC, Reul-Hirche HM, Bullock-Saxton JE, Furnival CM. Physiotherapy after breast cancer surgery: Results of a randomised controlled study to minimise lymphoedema. Breast Cancer Res Treat. 2002;75(1):51–64. doi: 10.1023/a:1016591121762. [DOI] [PubMed] [Google Scholar]
- 37.Stout Gergich NL, Pfalzer LA, Mcgarvey C, et al. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer. 2008;112(12):2809–19. doi: 10.1002/cncr.23494. [DOI] [PubMed] [Google Scholar]
- 38.Torres Lacomba M, Yuste Sanchez MJ, Zapico Goni A, et al. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: Randomised, single blinded, clinical trial. BMJ. 2010;340:b5396. doi: 10.1136/bmj.b5396. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayes SC, Reul-Hirche H, Turner J. Exercise and secondary lymphedema: Safety, potential benefits, and research issues. Med Sci Sports Exerc. 2009;41(3):483–9. doi: 10.1249/MSS.0b013e31818b98fb. [DOI] [PubMed] [Google Scholar]
- 40.Schmitz KH. Balancing lymphedema risk: Exercise versus deconditioning for breast cancer survivors. Exercise and Sport Sciences Reviews. 2010;38(1):17–24. doi: 10.1097/JES.0b013e3181c5cd5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41**.Lasinski BB, Mckillip Thrift K, Squire D, et al. A systematic review of the evidence for complete decongestive therapy in the treatment of lymphedema from 2004 to 2011. PM&R. 2012;4(8):580–601. doi: 10.1016/j.pmrj.2012.05.003. [In a review of 26 articles published between 2004 to 2011, complete decongestive therapy as a bundled intervention was found to decrease limb swelling associated with lymphedema, as do individual components of manual lymphatic drainage and compression bandaging.] [DOI] [PubMed] [Google Scholar]
- 42.Moseley AM, Sherrington C, Elkins MR, et al. Indexing of randomised controlled trials of physiotherapy interventions: A comparison of AMED, CENTRAL, CINAHL, EMBASE, hooked on evidence, PEDro, PsycINFO and PubMed. Physiotherapy. 2009;95(3):151–6. doi: 10.1016/j.physio.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Position statement of the National Lymphedema Network [March 21, 2012];Topic: The diagnosis and treatment of lymphedema. Available at http://www.lymphnet.org/pdfDocs/nlntreatment.pdf.
- 44.Lymphoedema Framework . Best Practice for the Management of Lymphoedema. MEP Ltd; London: 2006. [Google Scholar]
- 45*.Ridner SH, Fu MR, Wanchai A, et al. Self-management of lymphedema: A systematic review of the literature from 2004 to 2011. Nurs Res. 2012;61(4):291–9. doi: 10.1097/NNR.0b013e31824f82b2. [Combined decongestive therapy and full body exercise as self-management actions were given a “likely to be effective” rating using the Oncology Nursing Society (ONS) Putting Evidence into Practice (PEP) criteria.] [DOI] [PubMed] [Google Scholar]
- 46.Schmitz KH, Troxel AB, Cheville A, et al. Physical activity and lymphedema (the PAL trial): Assessing the safety of progressive strength training in breast cancer survivors. Contemp Clin Trials. 2009;30(3):233–45. doi: 10.1016/j.cct.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morello E, Sandri R, Monfardini S. Enough rehabilitation for our elderly cancer patients? Eur J Cancer. 2008;44(16):2338–9. doi: 10.1016/j.ejca.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 48.American Cancer Society (ACS) [January 8, 2013];Lymphedema: What every woman with breast cancer should know. 2013 Available at http://www.cancer.org/acs/groups/cid/documents/webcontent/002876-pdf.pdf.
- 49.Thiadens SRJ. [January 2, 2013];18 steps to prevention revised: Lymphedema risk-reduction practices. Available at http://lymphnet.org/lymphedemaFAQs/riskReduction/riskReduction.htm.
- 50*.Beck M, Wanchai A, Stewart BR, et al. Palliative care for cancer-related lymphedema: A systematic review. J Pallitive Med. 2012;15(7):821–7. doi: 10.1089/jpm.2011.0494. [This systematic review of palliative care LE management and concluded that CDT, MLD, and compression bandaging are categorized as “effectiveness not established,” but note that no adverse findings were reported. Based on these findings, CDT, MLD, and compression bandaging offer a potential benefit to LE symptom control and improved quality of life for LE patients receiving palliative care.] [DOI] [PubMed] [Google Scholar]
- 51.Mitchell S, Friese C. [January 2, 2013];ONS PEP (Putting Evidence into Practice) weight of evidence classification schema: Decision rules for summative evaluation of a body of evidence. 2011 Available at http://www.ons.org/Research/media/ons/docs/research/outcomes/weightofevidence-table.pdf.
- 52**.Cormier JN, Rourke L, Crosby M, et al. The surgical treatment of lymphedema: A systematic review of the contemporary literature (2004-2010). Ann Surg Oncol. 2012;19(2):642–51. doi: 10.1245/s10434-011-2017-4. [Surgical techniques have been shown to benefit select patients with LE symptoms, but not without continued use of CDT and compression for the majority of the patients in the study. Studies with non-surgical vs. surgical interventions are needed to evaluate efficacy as well as consideration of risk. Conservative therapies remain the first-line treatment. Risks of surgery must be weighed with benefits in survivor outcomes; compression garments must still be worn long-term post-operatively.] [DOI] [PubMed] [Google Scholar]
- 53.Moseley AL, Carati CJ, Piller NB. A systematic review of common conservative therapies for arm lymphoedema secondary to breast cancer treatment. Ann Oncol. 2007;18:639–46. doi: 10.1093/annonc/mdl182. [DOI] [PubMed] [Google Scholar]
- 54.Rinehart-Ayres M, Fish K, Lapp K, Borwn CN. Use of compression pumps for treatment of upper extremity lymphedema following treatment for breastcancer: A systematic review. Rehab Oncol. 2010;28:10–8. [Google Scholar]
- 55.Feldman JL, Stout NL, Wanchai A, et al. Intermittent pneumatic compression therapy: A systematic review. Lymphology. 2012;45(1):13–25. [PubMed] [Google Scholar]
- 56*.Schmitz KH, Speck RM, Rye SA, et al. Prevalence of breast cancer treatment sequelae over 6 years of follow-up: The pulling through study. Cancer. 2012;118(8 Suppl):2217–25. doi: 10.1002/cncr.27474. [At six years post-diagnosis, a majority of survivors experience one or more late effects of breast cancer treatment amenable to rehabilitative intervention. Data support a multidisciplinary prospective surveillance approach in the management and treatment of adverse effects of breast cancer treatment.] [DOI] [PubMed] [Google Scholar]
- 57**.Kwan ML, Cohn JC, Armer JM, et al. Exercise in patients with lymphedema: A systematic review of the contemporary literature. J Cancer Surviv. 2011;5(4):320–36. doi: 10.1007/s11764-011-0203-9. [This systematic review of the literature concludes that supervised slow, progressive resistance exercise programs appear safe for breast cancer survivors at-risk for secondary LE under proper supervision. Combination aerobic and resistance programs appear safe, but require more rigorous study.] [DOI] [PubMed] [Google Scholar]
- 58*.Schmitz KH, Ahmed RL, Troxel AB, et al. Weight lifting for women at risk for breast cancer-related lymphedema: A randomized trial. JAMA. 2010;304(24):2699–705. doi: 10.1001/jama.2010.1837. [In breast cancer survivors at-risk for LE, exercise in a supervised progressive weight lifting program did not increase the incidence of LE when compared to no exercise.] [DOI] [PubMed] [Google Scholar]
- 59*.Schmitz KH, Ahmed RL, Troxel A, et al. Weight lifting in women with breast-cancer-related lymphedema. New Engl J Med. 2009;361(7):664–73. doi: 10.1056/NEJMoa0810118. [The PALS trial showed that in breast-cancer survivors with LE supervised slowly progressive weight lifting had no significant effect on limb edema. Those exercising experienced a decreased incidence of LE exacerbation, reduced symptoms, and increased strength.] [DOI] [PubMed] [Google Scholar]
- 60.Ahmed RL, Schmitz KH, Prizment AE, Folsom AR. Risk factors for lymphedema in breast cancer survivors, the Iowa Women's Health Study. Breast Cancer Res Treat. 2011;130(3):981–91. doi: 10.1007/s10549-011-1667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kilbreath SL, Refshauge KM, Beith JM, et al. Progressive resistance training and stretching following surgery for breast cancer: study protocol for a randomised controlled trial. BMC Cancer. 2006;6:273. doi: 10.1186/1471-2407-6-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Irdesel J, Kahraman CS. Effectiveness of exercise and compression garments in the treatment of breast cancer related lymphedema. Turk J Phys Med Rehab. 2007;53(1):16–21. [Google Scholar]
- 63.Sander AP. A safe and effective upper extremity resistive exercise program for woman post breast cancer treatment. Rehab Oncol. 2008;26(3):3–10. [Google Scholar]
- 64.Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J Clin Oncol. 2007;25(28):4396–404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 65.Portela ALM, Santaella CLC, Gomez CC, Burch A. Feasibility of an exercise program for Puerto Rican women who are breast cancer survivors. Rehab Oncol. 2008;26(2):20–31. doi: 10.1901/jaba.2008.26-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kilbreath S, Refshauge K, Beith J, Lee M. Resistance and stretching shoulder exercises early following axillary surgery for breast cancer. Rehab Oncol. 2006;24(2):9–14. [Google Scholar]
- 67.Schmitz KH, Stout NL, Andrews K, et al. Prospective evaluation of physical rehabilitation needs in breast cancer survivors: A call to action. Cancer. 2012;118(8 Suppl):2187–90. doi: 10.1002/cncr.27471. [DOI] [PubMed] [Google Scholar]
- 68.Wanchai A, Armer JM, Stewart BR. Performance care practices in complementary and alternative medicine by thai breast cancer survivors: An ethnonursing study. Nursing & Health Sciences. 2012;14(3):339–44. doi: 10.1111/j.1442-2018.2012.00730.x. [DOI] [PubMed] [Google Scholar]
- 69.Finnane A, Liu Y, Battistutta D, et al. Lymphedema after breast or gynecological cancer: Use and effectiveness of mainstream and complementary therapies. Journal of Alternative & Complementary Medicine. 2011;17(9):867–9. doi: 10.1089/acm.2010.0456. [DOI] [PubMed] [Google Scholar]
- 70.Matchim Y, Armer JM, Stewart BR. Effects of mindfulness-based stress reduction (MBSR) on health among breast cancer survivors. Western Journal of Nursing Research. 2011;33(8):996–1016. doi: 10.1177/0193945910385363. [DOI] [PubMed] [Google Scholar]
- 71.Tidhar D, Katz-Leurer M. Aqua lymphatic therapy in women who suffer from breast cancer treatment-related lymphedema: A randomized controlled study. Supportive Care Cancer. 2010;18(3):383–92. doi: 10.1007/s00520-009-0669-4. [DOI] [PubMed] [Google Scholar]
- 72.Arinaga Y. Holistic management of lymphoedema in japan: Two contrasting cases. J Lymphoedema. 2012;7(2):40–2. [Google Scholar]
- 73.Cassileth BR, Van Zee KJ, Chan Y, et al. A safety and efficacy pilot study of acupuncture for the treatment of chronic lymphoedema. Acupuncture in Medicine. 2011;29(3):170–2. doi: 10.1136/aim.2011.004069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Valois BA, Young TE, Melsome E. Assessing the feasibility of using acupuncture and moxibustion to improve quality of life for cancer survivors with upper body lymphoedema. Eur J Oncol Nurs. 2012;16(3):301–9. doi: 10.1016/j.ejon.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 75.Douglass J, Immink M, Piller N, Ullah S. Yoga for women with breast cancer-related lymphoedema: A preliminary 6-month study. J Lymphoedema. 2012;7(2):30–38. [Google Scholar]
- 76.Disa JJ, Petrek J. Rehabilitation after treatment for cancer of the breast. In: Devita VT Jr., Hellman S, Rosenberg SA 6th, editors. Cancer Principles and Practice of Oncology. Lippincott, Williams, & Wilkins; Philadelphia: 2001. pp. 1717–26. [Google Scholar]
- 77.Moffatt CJ, Franks PJ, Doherty DC, et al. Lymphoedema: An underestimated health problem. QJM. 2003;96(10):731–8. doi: 10.1093/qjmed/hcg126. [DOI] [PubMed] [Google Scholar]
- 78.Shih YC, Xu Y, Cormier JN, et al. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: A 2-year follow-up study. J Clin Oncol. 2009;27(12):2007–14. doi: 10.1200/JCO.2008.18.3517. [DOI] [PubMed] [Google Scholar]
- 79.Witek-Janusek L, Gabram S, Mathews HL. Psychologic stress, reduced nk cell activity, and cytokine dysregulation in women experiencing diagnostic breast biopsy. Psychoneuroendocrinology. 2007;32(1):22–35. doi: 10.1016/j.psyneuen.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ridner SH. The psycho-social impact of lymphedema. Lymphat Res Biol. 2009;7(2):109–12. doi: 10.1089/lrb.2009.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fu MR, Ridner SH, Hu SH, et al. Psychosocial impact of lymphedema: A systematic review of literature from 2004 to 2011. Psycho-Oncology. 2012 doi: 10.1002/pon.3201. first published online: 9 Oct 2012 DOI: 10.1002/pon.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cormier JN, Ross MI, Gershenwald JE. Prospective assessment of the reliability, validity, and sensitivity to change of the functional assessment of cancer therapy-melanoma questionnaire. Cancer. 2008;112(10):2249–57. doi: 10.1002/cncr.23424. [DOI] [PubMed] [Google Scholar]
- 83.Johansson K, Branje E. Arm lymphoedema in a cohort of breast cancer survivors 10 years after diagnosis. Acta Oncol. 2010;49:166–73. doi: 10.3109/02841860903483676. [DOI] [PubMed] [Google Scholar]
- 84**.Hayes SC, Johansson K, Stout NL, et al. Upper-body morbidity after breast cancer: Incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer. 2012;118(8 Suppl):2237–49. doi: 10.1002/cncr.27467. [This paper reviewed the incidence of upper-body morbidity (arm and breast symptoms, impairments, and lymphedema), methods for diagnosis, and prevention and treatment strategies, as well as the evidence base for integration of prospective surveillance for upper-body morbidity within standard clinical care of women with breast cancer. There exists evidence in support of integrating regular surveillance for upper-body morbidity into the routine clinical care provided to women with breast cancer, with early diagnosis potentially contributing to more effective management and prevention of progression of these conditions.] [DOI] [PubMed] [Google Scholar]
- 85**.Stout NL, Binkley JM, Schmitz KH, et al. A prospective surveillance model for rehabilitation for women with breast cancer. Cancer. 2012;118(8 Suppl):2191–200. doi: 10.1002/cncr.27476. [The goals of a prospective surveillance model include: 1) promotion of surveillance of common physical and functional limitations associated with breast-cancer treatment; 2) education for early identification of physical impairments and functional limitation; 3) referral for rehabilitation and exercise interventions once physical limitations are identified; and 4) promotion and support of physical activity, exercise, nutrition, and weight-management behaviors throughout the survivorship continuum.] [DOI] [PubMed] [Google Scholar]
- 86*.Binkley JM, Harris SR, Levangie PK, et al. Patient perspectives on breast cancer treatment side effects and the prospective surveillance model for physical rehabilitation for women with breast cancer. Cancer. 2012;118(8 Suppl):2207–16. doi: 10.1002/cncr.27469. [The prospective surveillance model of rehabilitation serves the needs of breast cancer survivors by providing education and information about treatment side effects, reducing the incidence and burden of side effects through early identification and treatment, and enhancing access to timely rehabilitation. Exercise integration into the model benefits patients at every phase of survivorship. Application of the model can meet the needs of survivors for information, guidance, and intervention—thus addressing, and potentially improving, overall quality of life for individuals treated for breast cancer.] [DOI] [PubMed] [Google Scholar]
- 87.Reneker J, Armer J, Stewart B, Shyu CR. Development of a minimum data set to assist in international collaborative lymphedema studies.. Lymphology; 23rd International Congress of Lymphology proceedings; 2013. in press. [Google Scholar]
- 88. [January 13, 2013];National Accreditation Program for Breast Centers (NAPBC) components. Available at http://napbc-breast.org/standards/standards.html.
- 89**.Bernas M. Assessment and risk reduction in lymphedema. Seminars in Oncology Nursing. 2013;29(1):12–9. doi: 10.1016/j.soncn.2012.11.003. [Risk-reduction strategies and methods for the assessment, diagnosis, and treatment of lymphedema represent a major focus in oncology. Operative and physical methods to reduce the risk of lymphedema and an emphasis on early detection have shown promising results post-breast cancer surgery and/or radiation therapy. Standardization of reproducible methods utilizing currently available and reliable tools are necessary to determine the most effective means to care for patients at risk for the development of lymphedema.] [DOI] [PubMed] [Google Scholar]