Abstract
Introduction
Atrial fibrillation and atrial flutter account for one third of hospitalizations due to arrhythmias, determining great social and economic impacts. In Brazil, data on hospital care of these patients is scarce.
Objective
To investigate the arrhythmia subtype of atrial fibrillation and flutter patients in the emergency setting and compare the clinical profile, thromboembolic risk and anticoagulants use.
Methods
Cross-sectional retrospective study, with data collection from medical records of every patient treated for atrial fibrillation and flutter in the emergency department of Instituto de Cardiologia do Rio Grande do Sul during the first trimester of 2012.
Results
We included 407 patients (356 had atrial fibrillation and 51 had flutter). Patients with paroxysmal atrial fibrillation were in average 5 years younger than those with persistent atrial fibrillation. Compared to paroxysmal atrial fibrillation patients, those with persistent atrial fibrillation and flutter had larger atrial diameter (48.6 ± 7.2 vs. 47.2 ± 6.2 vs. 42.3 ± 6.4; p < 0.01) and lower left ventricular ejection fraction (66.8 ± 11 vs. 53.9 ± 17 vs. 57.4 ± 16; p < 0.01). The prevalence of stroke and heart failure was higher in persistent atrial fibrillation and flutter patients. Those with paroxysmal atrial fibrillation and flutter had higher prevalence of CHADS2 score of zero when compared to those with persistent atrial fibrillation (27.8% vs. 18% vs. 4.9%; p < 0.01). The prevalence of anticoagulation in patients with CHA2DS2-Vasc ≤ 2 was 40%.
Conclusions
The population in our registry was similar in its comorbidities and demographic profile to those of North American and European registries. Despite the high thromboembolic risk, the use of anticoagulants was low, revealing difficulties for incorporating guideline recommendations. Public health strategies should be adopted in order to improve these rates.
Keywords: Arrhythmias, Cardiac, Atrial Fibrillation, Flutter Atrial, Anticoagulants/adverse effects, Stroke
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia in clinical practice, affecting around 1.5 million people in Brazil 1. The clinical presentation of AF can vary from an occasional finding in an asymptomatic patient to more severe presentations, such as stroke or acute heart failure (HF)1-3. The latest guidelines4-6 recommend classification of AF into paroxysmal (PaAF) and persistent or permanent (PeAF) and differentiation and characterization of atrial flutter events4.
AF is responsible for about one-third of all hospital admissions for heart rhythm disorders and is associated with increased morbidity and mortality in all its presentation forms. The incidence of AF has increased progressively in the last decades, relating closely to the aging of the population and increasing prevalence of risk factors such as hypertension, obesity, diabetes mellitus and sleep apnea2,3,7. As a result, the number of hospital admissions for AF is also increasing progressively. Patel et al.7 demonstrated an increase of 14.4% in the number of hospitalizations due to AF in the United States over 10 years, with an estimated cost of care exceeding U$ 6.7 billion a year, three-fourths of which are directed to hospital expenses. It is thus imperative to know the clinical characteristics of the patients who present to the emergency department with this pathology.
In Brazil, epidemiological data related to AF are scarce. The undergoing Brazilian Cardiovascular Record of Atrial Fibrillation (REgistro Brasileiro CArdiovascular de FibriLação AtriaL, RECALL)8 is seeking to define the characteristics of outpatients with AF. However, there are no specific data regarding the clinical characteristics of the patients who present to the emergency department with this pathology.
Recent studies have shown increased risk of development of AF after radiofrequency ablation of atrial flutter9,10. The risk appears to be greater with the practice of endurance sports11. Animal studies suggest that classical flutter (typical or atypical) requires a preceding period of AF, and that the organization (and maintenance) of the macro-reentrant circuit in flutter is dependent on a functional line of block between the inferior and superior vena cava. Thus, when cavotricuspid isthmus ablation is performed, the line of block is undone, preventing the "organization" of the circuit and causing AF to emerge and perpetuate. Furthermore, the use of antiarrhythmic drugs with sodium channel blocking properties (which affect atrial conduction - class IA, class IC and amiodarone) are associated with the "conversion" of AF into flutter12,13.
These observations corroborate the theories that atrial flutter and AF share similar pathophysiological mechanisms, in addition to having similar clinical impact, since both increase the risk of events and have an impact on the patient's quality of life9,12,13. Despite that, there is scant information about the epidemiology of atrial flutter. For this reason, the North American and European cardiology societies have requested, in a joint guideline of supraventricular arrhythmias, the inclusion of flutter in AF registries4.
The aim of our study was to describe and compare the clinical characteristics of patients with PaAF, PeAF and atrial flutter seen at the emergency department of a cardiology referral hospital.
Methods
Design
This is a cross-sectional, unicenter study that included all patients over the age of 18 years with atrial fibrillation and flutter seen at the emergency department of the Instituto de Cardiologia de Porto Alegre in the first trimester of 2012. The emergency department of this tertiary cardiology hospital serves annually over 40 thousand patients.
The study was submitted for approval to the Ethics and Research Committee of the Fundação Universitária do Instituto de Cardiologia.
Patients
Trained investigator and fellows collected from an electronic medical record system between January and May 2014 retrospective data associated with ICD I48 (atrial fibrillation or flutter).
The type of AF was registered by the physician during consultation. The patients were divided into three groups: PaAF, according to the classification of the AF guideline; PeAF, including patients with persistent and permanent fibrillation; and atrial flutter. We included patients with persistent and permanent fibrillation in a single group since these subtypes are often not distinguished during consultation.
We evaluated the following clinical variables retrieved from the medical records: gender, age, hypertension, previous stroke, diabetes mellitus, HF, prior myocardial infarction or known coronary artery disease, presence of aortic plaque, peripheral artery disease and use of anticoagulants. Calculation of the CHADS2 and CHA2DS2-VASc scores was based on the collected clinical information. The echocardiographic variables evaluated included atrial diameter (according to the criteria defined by the echocardiographer) and ejection fraction (estimated by the methods of Teicholz or Simpson). We evaluated the prescription of the following drugs: warfarin, phenprocoumon, dabigatran, rivaroxaban, amiodarone, sotalol, propafenone, beta-blockers, calcium channel blockers, digoxin, aspirin, clopidogrel, prasugrel, ticlopidine, statins, diuretics, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and antidiabetics. We also evaluated the international normalized ratio of prothrombin time (INR-PT) on the day of the consultation.
The information obtained was stored in a database developed especially for this purpose in a microcomputer, using the software MedCalc (BE, Netherlands).
Statistical analysis
The statistical analyses were processed with the software Statistical Package for the Social Sciences (SPSS), version 16.0 (SPSS, Inc., Chicago, Illinois). We prepared tables with absolute frequencies and percentages for sample characterization. Continuous variables were described as means and standard deviations, or medians and interquartile ranges, using the t-test for independent samples and analysis of variance (ANOVA) for their comparisons. Categorical variables were compared with the z-test. Variables were considered normal following observation of the measures of central tendency, kurtosis and asymmetry in frequency histograms. We considered p < 0.05 as statistically significant. Post-hoc analyses were performed using the Bonferroni test.
Results
In the first trimester of 2012, a total of 407 patients consulting at the emergency department were identified with ICD I48 as the main reason for the visit. Upon review of the medical records, AF was observed in 356 of these cases and atrial flutter in the remaining 51. The main clinical and demographic characteristics of the patients are shown in Table 1.
Table 1.
Clinical characteristics of the patients (n = 407) according to the type of atrial fibrillation (AF) or flutter
| Characteristic | AF | Flutter (n = 51) | p value | |
|---|---|---|---|---|
| Paroxysmal (n = 188) | Persistent (n = 168) | |||
| Age, years | 64 ± 15 | 69 ± 14* | 66 ± 14 | < 0.01 |
| Male gender, n (%) | 95 (50.5) | 82 (48.8) | 37 (72.5)†,‡ | 0.09 |
| Echocardiogram | ||||
| LVEF, % | 66.8 ± 11 | 57.4 ± 16* | 53.9 ± 17† | < 0.01 |
| LA, mm | 42.3 ± 6.4 | 48.6 ± 7.2* | 47.2 ± 6.2† | < 0.01 |
| Anticoagulation, n (%) | 40 (21.3) | 75 (44.6)* | 22 (43.1)† | < 0.01 |
| Comorbidities, n (%) | ||||
| HF | 37 (19.7) | 86 (51.2)* | 23 (45.1)† | < 0.01 |
| Hypertension | 106 (56.4) | 117 (69.6)* | 30 (58.8) | 0.03 |
| DM | 23 (12.2) | 29 (17.3) | 8 (15.7) | 0.4 |
| Stroke | 3 (1.6) | 18 (10.7)* | 5(9.8)† | < 0.01 |
| CHADS2 Score | < 0.01 | |||
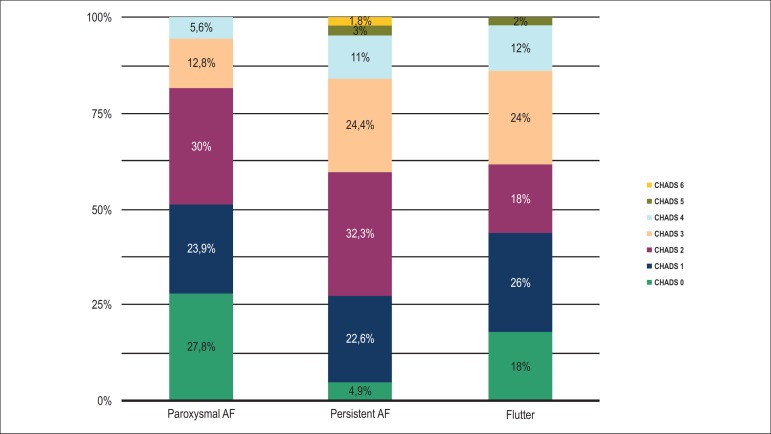
| 0 | 50 (27.8)* | 8 (4.9) | 9 (18)‡ | |
| 1 | 43 (23.9) | 37 (22.6) | 13 (26) | |
| 2 | 54 (30) | 53 (32.3) | 9 (18) | |
| 3 | 23 (12.8) | 40 (24.4)* | 12 (24) | |
| 4 | 10 (5.6) | 18 (11) | 6 (12) | |
| 5 | 0 | 5 (3) | 1 (2) | |
| 6 | 0 | 3 (1.8) | 0 | |
| Mean CHADS2 | 1.4 ± 1.2 | 2.3 ± 1.3* | 1.9 ± 1.4 | < 0.01 |
| Readmission, n (%) | 91 (48.4) | 80 (47.6) | 27 (52.9) | 0.8 |
Difference between the groups with persistent AF and paroxysmal AF;
difference between the groups with flutter and paroxysmal AF
difference between the groups with flutter and persistent AF. LVEF: left ventricular ejection fraction; LA: left atrium; HF: heart failure; DM: diabetes mellitus.
Mean age was higher in patients with PeAF compared with those with PaAF (69 ± 14 years vs. 64 ± 15 years; p < 0.01). On echocardiography, patients with PeAF and flutter, compared with those with PaAF, presented larger atrial diameter (48.6 ± 7.2 mm vs. 47.2 ± 6.2 mm vs. 42.3 ± 6.4 mm; p < 0.01) and lower mean ejection fraction (57.4 ± 16% vs. 53.9 ± 17% vs. 66.8 ± 11%; p < 0.01). There was no significant difference between PeAF and flutter regarding these two characteristics. The prevalence of HF and stroke was also higher in patients with PeAF and flutter compared with those with PaAF (HF: 51.2% vs. 45.1% vs. 19.7%, p < 0.01; stroke: 10.7% vs. 9.8% vs. 1.6%, p < 0.01). In contrast, the prevalence of hypertension was only increased in individuals with PeAF when compared with those with PaAF (69.6% vs. 56.4%; p = 0.03). The prevalence of diabetes mellitus did not differ significantly between the groups.
Figure 1 illustrates the prevalence of each risk category of the CHADS2 score in the subgroups with AF and flutter. The CHADS2 score also differed among the groups, with a higher prevalence of a score of zero among patients with PaAF and flutter compared with those with PeAF (27.8% vs. 18% vs. 4.9%; p < 0.01). Patients with PeAF presented more frequently with a CHADS2 score of 3 when compared with patients with PaAF (24.4% vs. 12.8%; p < 0.01), and the average score also differed significantly between these two groups (2.3 vs. 1.4; p < 0.01).
Figure 1.
Prevalence of each risk category of the CHADS2 score in paroxysmal atrial fibrillation, persistent atrial fibrillation and flutter.
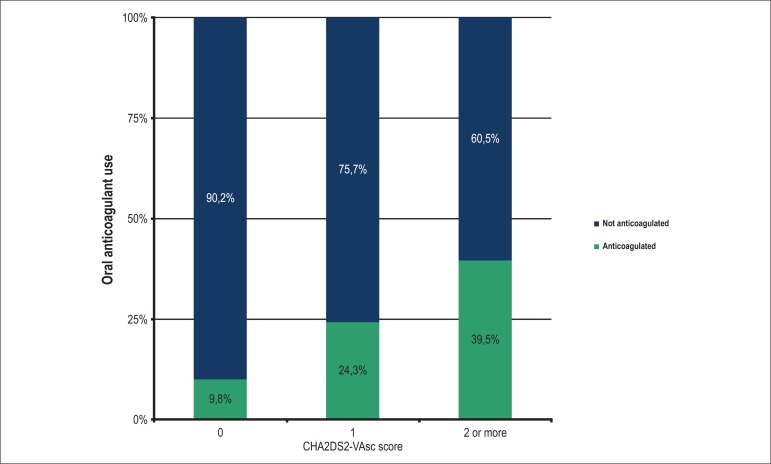
Table 2 shows the patients stratified according to the CHA2DS2-VASc score in all three groups. The prevalence of a zero score was higher in the PaAF group when compared with the PeAF group (15.5% vs. 3.6%; p < 0.01). Figure 2 shows the percentage of anticoagulated patients stratified into CHA2DS2-VASc scores of zero, 1 and ≥ 2. Patients with a score ≥ 2 were more often anticoagulated compared with those with a score of zero (40% vs. 10%; p < 0.01). Median INRs of the anticoagulated patients during the evaluation at the hospital or in the week before the evaluation were, respectively, 1.50 in the PaAF group, 1.63 in the PeAF group and 1.63 in the flutter group.
Table 2.
CHA2DS2-VASc score according to the type of atrial fibrillation
| Characteristic | Atrial fibrillation | Flutter (n = 51) | p value | |
|---|---|---|---|---|
| Paroxysmal (n = 188) | Persistent (n = 168) | |||
| CHA2DS2-VAsc | < 0.01 | |||
| 0 | 28 (15.5)* | 6 (3.6) | 6 (11.8) | |
| 1 | 39 (20.9) | 20 (12.0) | 11 (21.6) | |
| 2 | 27 (14.4) | 22 (13.2) | 9 (17.6) | |
| 3 | 41 (21.9) | 44 (26.3) | 8 (15.7) | |
| 4 | 32 (17.1) | 35 (21.0) | 10 (19.6) | |
| 5 | 13 (7.0) | 24 (14.4) | 7 (13.7) | |
| 6 | 4(2.1) | 9 (5.4) | 0 | |
| 7 | 2 (1.1) | 6 (3.6) | 0 | |
| 8 | 0 | 1 (0.6) | 0 | |
| 9 | 0 | 0 | 0 | |
Difference between the groups with persistent atrial fibrillation and paroxysmal atrial fibrillation.
Figure 2.
Prevalence of prescription of oral anticoagulants according to the CHA2DS2-VASc score.
Table 3 summarizes the drugs prescribed in each subgroup of patients. When we compared patients with PeAF and PaAF, those with PeAF used more frequently beta-blockers (68.9% vs. 50%; p < 0.01), digoxin (22.6% vs. 4.3%; p < 0.01), calcium channel blockers (19.9% vs. 8.5%; p = 0.03); diuretics (56% vs. 25%; p < 0.01) and angiotensin-converting enzyme inhibitors (41.7% vs. 21.8%; p < 0.01). Patients with flutter, compared with those with PaAF, used more frequently digoxin (15.7% versus 4.3%; p < 0.01) and warfarin (29.4% vs. 9.6%; p < 0.01).
Table 3.
Prevalences of use of each drug class according to the type of atrial fibrillation (AF) or flutter
| Drug | AF | Flutter(n = 51) | p value | |
|---|---|---|---|---|
| Paroxysmal (n = 188) | Persistent (n = 168) | |||
| Amiodarone | 25 (13.3) | 20 (11.9) | 9 (17.6) | 0.57 |
| Propafenone | 12 (6.4) | 5 (3) | 0 | 0.07 |
| Beta-blocker | 94(50) | 115 (68.9)* | 29 (56.9) | < 0.01 |
| Digoxin | 8 (4.3) | 38 (22.6)* | 8 (15.7)† | < 0.01 |
| CCB | 16 (8.5) | 32 (19.9)* | 3 (5.9) | 0.03 |
| Sotalol | 2 (1.1) | 3 (1.8) | 0 | 0.58 |
| Warfarin | 18 (9.6) | 47(28)† | 15 (29.4)† | < 0.01 |
| Phenprocoumon | 3 (1.6) | 12 (7.1)* | 4 (7.8) | 0.06 |
| Dabigatran | 6 (3.2) | 5 (3.0) | 0 | 0.44 |
| Rivaroxaban | 5 (2.7) | 3 (1.8) | 1 (2) | 0.82 |
| ACEi | 41 (21.8) | 70 (41.7)* | 16 (31.4) | < 0.01 |
| ARB | 31 (17) | 24 (14.3) | 4 (7.8) | 0.25 |
| Statins | 55 (29.3) | 50 (29.8) | 13 (25.5) | 0.83 |
| Diuretic | 47 (25) | 94 (56)* | 20 (39.2) | < 0.01 |
| Aspirin | 59 (31.4) | 62 (36.9) | 17 (33.3) | 0.54 |
| Clopidogrel | 13 (6.9) | 10 (6.0) | 1 (2.0) | 0.41 |
Difference between the groups with paroxysmal atrial fibrillation and persistent atrial fibrillation;
difference between the groups with flutter and paroxysmal atrial fibrillation. CCB: calcium channel blocker; ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker.
Discussion
Several AF registries have described the characteristics of outpatients presenting with this type of arrhythmia, as well as its treatment and prognosis over the years14-20. The registry GLORIA-AF21, which is currently in progress, aims to investigate the profile of patients with newly-diagnosed AF who are at risk of stroke. In Brazil, the RECALL8 is currently under way and aims to provide data on outpatients with AF in our environment.
AF subtypes differ in symptoms22, management15, prognosis and long-term complications23. Atrial flutter has a lower prevalence than AF and is associated with an increased incidence of thromboembolic events. As mentioned earlier, the physiopathology of AF and atrial flutter are likely to be interconnected and may represent a different spectrum of the same disease affecting the atrial conduction system12,13. Therefore, the registration of the characteristics of patients with flutter is fundamental for the expansion of this knowledge.
The prevalence of PaAF in our study population was comparable to that described in some registries15,17,24, but significantly higher than the prevalence described in others18-20,25. This may be due to the heterogeneous presentation of the disease and differences in classification and subtypes.
Unlike other studies that have reported a predominance of males (generally around 60%)14-20, in our population the prevalence was equal between genders in both groups with AF. In the flutter group, in turn, the prevalence of males was higher. The mean age of the patients in most reports is between 65 and 70 years, which is comparable to the mean age of our population14,16-19,23-26. Similar to reports from other registries14,21, our patients with PaAF were on average 5 years younger, which is compatible with the temporal progression of the disease to chronicity. In contrast, patients with atrial flutter were significantly older.
The ejection fraction estimated by echocardiogram was higher in the PaAF group compared with the PeAF group in our registry, which is not surprising, since these patients are older and have more comorbidities. The measurement of the left atrium also differed significantly and was around 6 mm greater in patients with PeAF. This is in line with the natural progression of atrial remodeling and increase in chamber diameter, which is directly proportional to the duration that the atrium remains in fibrillation - "atrial fibrillation begets atrial fibrillation". Nieuwlaat et al.15 have described similar ejection fractions among the groups, but greater mean atrial measurements in PeAF groups.
The most prevalent cardiovascular comorbidity in our population was hypertension. This is in line with the literature which shows prevalences ranging from 52 to 90%15,23,27, generally without differences among the groups. However, in our population the PaAF group had lower prevalence of hypertension (56.4% vs. 69.6%). We believe that this difference occurred due to the scenario of clinical emergency, which encompasses both patients with isolated AF who seek care (and who are usually not part of AF registries), as well as those who do not have regular health monitoring and therefore, may not have received a diagnosis for their comorbidities. The CHADS2 and CHA2DS2-VASc scores were lower in patients with PaAF, who also presented fewer comorbidities in other studies15,22,27. Vanassche et al.23 found similar CHA2DS2-VASc scores in the PaAF and PeAF groups (13% had a score of 0 to 1, 50% a score of 2 to 3, and 37% a score ≥ 4). However, patients with permanent AF presented higher scores than PaAF and PeAF(7%, 43% and 50%, respectively). Among the comorbidities that comprise the scores, it is worth mentioning the difference in prevalence of cerebral ischemic events between the groups (1.6% in the PaAF group versus 10% in the PeAF group), which has also been reported to a lesser degree by Vanassche, Nieuwlaat and Inoue15,23,27. This information is possibly associated with the difficulty of the health system to recognize the risk of thromboembolic events in these patients and to offer appropriate preventive treatment.
Oral anticoagulation was prescribed to 44.6% of the patients with PeAF and only 21.3% of the patients with PaAF, despite the fact that 72.5% of the patients presented with CHA2DS2-VASc ≥ 2. In the reviewed literature, the prescription of oral anticoagulation on hospital discharge was also lower in patients with PaAF (51 vs. 80%14, 55 vs. 74%22, 78 vs. 91%26). In a Swiss registry of outpatients with AF seen by cardiologists, prescription of anticoagulants reached 88% in patients with a CHADS2 score ≥ 1. However, 57% of the patients with a score of zero also received anticoagulants, which limits the interpretation of this information28. In our population, prescription of anticoagulants was low both in patients without an indication of receiving them for AF (10%) and in higher-risk patients with a CHA2DS2-VASc score ≥ 2 (40%). When this population was compared with patients at an anticoagulation clinic at our institution29, the INR showed lower median results and was below the therapeutic target in the three subgroups (1.50 in the PaAF group, 1.63 in the PeAF group and 1.63 in the flutter group). Possible explanations for this finding include (1) the presence of patients on anticoagulation for mechanical prosthetic heart valve at our outpatient clinic, a situation in which INR is recommended to be maintained at higher levels30, and (2) the possibility of the patients seeking the emergency service being more prone to misuse of anticoagulant medication. It is important to note that our institution is a tertiary referral center in cardiology and that the population that seeks the emergency service is most followed up in our clinics. Since the prescription of anticoagulants is a decision that involves multiple factors, including social factors, patients in whom anticoagulation is indicated are often referred to health centers for careful evaluation in a non-hospital environment.
The most frequently used drugs to control rhythm and frequency were beta-blockers, followed by digitalis and amiodarone. This same sequence also lead the preferences in other studies15,23,25. As expected, propafenone presented a trend for more frequent use in PaAF, whereas digoxin and calcium channel blockers were more commonly used in the PeAF group, which also had a higher prevalence of HF. Among previously used drugs, angiotensin-converting enzyme inhibitors and diuretics were also used more frequently in the PeAF group, probably due to the higher prevalence of comorbidities in this group. The prevalences of aspirin (~34%) and clopidogrel (~6.5%) use did not differ among the groups and was similar to those in other series15,27.
Limitations of our study include (1) lack of proper registration of the duration of the flutter, which did not occur with the AF. With that, the risk profile intermediate between PaAF and PeAF found in patients with flutter may be due to a heterogeneity of the patients in this group; (2) the possibility of a bias in the registry as a result of underreported data, which is characteristic of studies with data collected retrospectively from medical records; and (3) underestimation of the problem, since patients with AF may have received a different ICD as the main reason for admission. Unfortunately we did not have sufficient data to calculate the time in therapeutic INR, but we used as a substitute the available INR result from the last appointment to analyze the quality of the oral anticoagulation.
Conclusion
To the extent of our knowledge, this is the first Brazilian study to evaluate the population of patients presenting to the emergency with flutter or AF. The recognition of the clinical characteristics of flutter is recommended by the North American and European guidelines for management of supraventricular arrhythmias, since there are no accurate data on the magnitude of the increased risk of thromboembolic events associated with this pathology. Our patients with flutter often presented epidemiological profiles and comorbidities intermediate with those in patients with paroxysmal atrial fibrillation and permanent atrial fibrillation. We believe that these findings support the hypothesis that the two arrhythmias are interconnected by common pathophysiological mechanisms, since they occur in patients with similar profiles.
Patients with persistent atrial fibrillation in our registry had more comorbidities and worse echocardiographic parameters, which explains in part the greater prevalence of use of multiple medications.
Unlike other registries, the population in our study comprised exclusively of patients seeking the emergency service due to AF or atrial flutter. Considering that most direct and indirect expenses with AF are specifically associated with hospitalization, we believe that knowledge of the clinical characteristics of patients with AF in this environment should assist the implementation of measures to improve the care of patients in the public health system. The prevalence of anticoagulated patients was lower than expected and represents perhaps the first point to be addressed. Ways to address this issue include measures to facilitate the access to specialized anticoagulation clinics and improvement in the communication between the primary and tertiary sectors.
Footnotes
Author contributions
Conception and design of the research:Almeida ED, Guimarães RB, Stephan LS, Medeiros AK, Foltz K, Santanna RT, Pires LM, Kruse ML, Lima GG, Leiria TLL. Acquisition of data:Almeida ED, Guimarães RB, Stephan LS, Medeiros AK, Foltz K. Analysis and interpretation of the data: Almeida ED, Guimarães RB, Stephan LS, Leiria TLL. Statistical analysis: Almeida ED, Leiria TLL. Writing of the manuscript:Almeida ED, Guimarães RB, Stephan LS, Leiria TLL. Critical revision of the manuscript for intellectual content:Almeida ED, Guimarães RB, Stephan LS, Santanna RT, Pires LM, Kruse ML, Lima GG, Leiria TLL.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This article is part of the thesis of master submitted by Eduardo Dytz Almeida, from Programa de Pós Graduação em Ciências da Saúde da Fundação Universitaria de Cardiologia do Rio Grande du Sul.
References
- 1.Zimerman LI, Fenelon G, Martinelli M, Filho, Grupi C, Atié J, Lorga A, Filho, et al. Sociedade Brasileira de Cardiologia Diretrizes brasileiras de fibrilação atrial. Arq Bras Cardiol. 2009;92(6) supl 1:1–39. [Google Scholar]
- 2.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. Erratum in: Circulation. 2006;114(11):e498. [DOI] [PubMed] [Google Scholar]
- 3.Lip GYH, Tse HF, Lane DA. Atrial fibrillation. Lancet. 2012;379(9816):648–661. doi: 10.1016/S0140-6736(11)61514-6. [DOI] [PubMed] [Google Scholar]
- 4.Blomström-Lundqvist C, Scheinman MM, Aliot EM, Alpert JS, Calkins H, Camm AJ, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias. J Am Coll Cardiol. 2003;42(8):1493–1531. doi: 10.1016/j.jacc.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. European Heart Rhythm Association European Association for Cardio-Thoracic Surgery Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31(19):2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 6.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr, et al. ACC/AHA Task Force Members 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199–e267. doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel NJ, Deshmukh A, Pant S, Singh V, Patel N, Arora S, et al. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation. 2014;129(23):2371–2379. doi: 10.1161/CIRCULATIONAHA.114.008201. [DOI] [PubMed] [Google Scholar]
- 8.Figueiredo M, Mattos L, Lorga Fo A, Berwanger O. Registro Brasileiro Cardiovascular de Fibrilação Atrial - RECALL. São Paulo: Sociedade Brasileira de Cardiologia; SOBRAC; HCOR; 2012. [Google Scholar]
- 9.Chinitz JS, Gerstenfeld EP, Marchlinski FE, Callans DJ. Atrial fibrillation is common after ablation of isolated atrial flutter during long-term follow-up. Heart Rhythm. 2007;4(8):1029–1033. doi: 10.1016/j.hrthm.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Voight J, Akkaya M, Somasundaram P, Karim R, Valliani S, Kwon Y, et al. Risk of new-onset atrial fibrillation and stroke after radiofrequency ablation of isolated, typical atrial flutter. Heart Rhythm. 2014;11(11):1884–1889. doi: 10.1016/j.hrthm.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 11.Heidbüchel H, Anné W, Willems R, Adriaenssens B, Van de Werf F, Ector H. Endurance sports is a risk factor for atrial fibrillation after ablation for atrial flutter. Int J Cardiol. 2006;107(1):67–72. doi: 10.1016/j.ijcard.2005.02.043. [DOI] [PubMed] [Google Scholar]
- 12.Waldo AL. The interrelationship between atrial fibrillation and atrial flutter. Prog Cardiovasc Dis. 2005;48(1):41–56. doi: 10.1016/j.pcad.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Waldo AL, Feld GK. Inter-relationships of atrial fibrillation and atrial flutter mechanisms and clinical implications. J Am Coll Cardiol. 2008;51(8):779–786. doi: 10.1016/j.jacc.2007.08.066. [DOI] [PubMed] [Google Scholar]
- 14.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 15.Nieuwlaat R, Capucci A, Camm AJ, Olsson SB, Andresen D, Davies DW, et al. European Heart Survey Investigators Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2005;26(22):2422–2434. doi: 10.1093/eurheartj/ehi505. [DOI] [PubMed] [Google Scholar]
- 16.Tebbe U, Oeckinghaus R, Appel KF, Heuer H, Haake H, Eggers E, et al. AFFECT: a prospective, open-label, multicenter trial to evaluate the feasibility and safety of a short-term treatment with subcutaneous certoparin in patients with persistent non-valvular atrial fibrillation. Clin Res Cardiol. 2008;97(6):389–396. doi: 10.1007/s00392-008-0644-y. [DOI] [PubMed] [Google Scholar]
- 17.Le Heuzey J-Y, Breithardt G, Camm J, Crijns H, Dorian P, Kowey PR. The RecordAF study: design, baseline data, and profile of patients according to chosen treatment strategy for atrial fibrillation. Am J Cardiol. 2010;105(5):687–693. doi: 10.1016/j.amjcard.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Chiang CE, Naditch-Brûlé L, Murin J, Goethals M, Inoue H, O'Neill J, et al. Distribution and risk profile of paroxysmal, persistent and permanent atrial fibrillation in routine clinical practice insight from the real-life global survey evaluating patients with atrial fibrillation international registry. Circ Arrhythm Electrophysiol. 2012;5(4):632–639. doi: 10.1161/CIRCEP.112.970749. [DOI] [PubMed] [Google Scholar]
- 19.Zubaid M, Rashed WA, Alsheikh-Ali AA, Almahmeed W, Shehab A, Sulaiman K, et al. Gulf Survey of Atrial Fibrillation Events (Gulf SAFE): design and baseline characteristics of patients with atrial fibrillation in the Arab Middle East. Circ Cardiovasc Qual Outcomes. 2011;4(4):477–482. doi: 10.1161/CIRCOUTCOMES.110.959700. [DOI] [PubMed] [Google Scholar]
- 20.Kakkar AK, Mueller I, Bassand JP, Fitzmaurice DA, Goldhaber SZ, Goto S, et al. GARFIELD Registry Investigators Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the international, observational, prospective GARFIELD registry. PLoS One. 2013;8(5): doi: 10.1371/journal.pone.0063479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huisman MV, Lip GY, Diener HC, Dubner SJ, Halperin JL, Ma CS, et al. Design and rationale of Global Registry on Long-Term Oral Antithrombotic Treatment in Patients with Atrial Fibrillation: a global registry program on long-term oral antithrombotic treatment in patients with atrial fibrillation. Am Heart J. 2014;167(3):329–334. doi: 10.1016/j.ahj.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Nabauer M, Gerth A, Limbourg T, Schneider S, Oeff M, Kirchhof P, et al. The Registry of the German Competence NETwork on Atrial Fibrillation: patient characteristics and initial management. Europace. 2009;11(4):423–434. doi: 10.1093/europace/eun369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanassche T, Lauw MN, Eikelboom JW, Healey JS, Hart RG, Alings M, et al. Risk of ischaemic stroke according to pattern of atrial fibrillation: analysis of 6563 aspirin-treated patients in ACTIVE-A and AVERROES. Eur Heart J. 2015;36(5):281–288. doi: 10.1093/eurheartj/ehu307. [DOI] [PubMed] [Google Scholar]
- 24.Steinberg BA, Kim S, Fonarow GC, Thomas L, Ansell J, Kowey PR, et al. Drivers of hospitalization for patients with atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Am Heart J. 2014;167(5):735.e2–742.e2. doi: 10.1016/j.ahj.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lip GY, Laroche C, Ioachim PM, Rasmussen LH, Vitali-Serdoz L, Petrescu L, et al. Prognosis and treatment of atrial fibrillation patients by European cardiologists: one year follow-up of the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase (EORP-AF Pilot registry) Eur Heart J. 2014;1435(47):3365–3376. doi: 10.1093/eurheartj/ehu374. [DOI] [PubMed] [Google Scholar]
- 26.Lip GY, Laroche C, Dan GA, Santini M, Kalarus Z, Rasmussen LH, et al. "Real-world" antithrombotic treatment in atrial fibrillation: The EORP-AF pilot survey. Am J Med. 2014;127(6):519.e1–529.e1. doi: 10.1016/j.amjmed.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Inoue H, Atarashi H, Okumura K, Yamashita T, Kumagai N, Origasa H. Thromboembolic events in paroxysmal vs. permanent non-valvular atrial fibrillation: subanalysis of the J-RHYTHM Registry. Circ J. 2014;78(10):2388–2393. doi: 10.1253/circj.cj-14-0507. [DOI] [PubMed] [Google Scholar]
- 28.Meiltz A, Zimmermann M, Urban P, Bloch A, Association of Cardiologists of the Canton of Geneva Atrial fibrillation management by practice cardiologists: a prospective survey on the adherence to guidelines in the real world. Europace. 2008;10(6):674–680. doi: 10.1093/europace/eun086. [DOI] [PubMed] [Google Scholar]
- 29.Leiria TLL, Pellanda L, Miglioranza MH. Varfarina e femprocumona: experiência de um ambulatório de anticoagulação. Arq Bras Cardiol. 2010;94(1):41–45. doi: 10.1590/s0066-782x2010000100008. [DOI] [PubMed] [Google Scholar]
- 30.Leiria TL, Lopes RD, Williams JB, Katz JN, Kalil RA, Alexander JH. Antithrombotic therapies in patients with prosthetic heart valves: guidelines translated for the clinician. J Thromb Thrombolysis. 2011;31(4):514–522. doi: 10.1007/s11239-011-0574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]