Abstract
The first descriptions of muscle spindles with intrafusal fibres containing striated myofibrils and nervous elements were given approximately 150 years ago. It took, however, another 100 years to establish the presence of two types of intrafusal muscle fibres: nuclear bag and nuclear chain fibres. The present paper highlights primarily the contribution of Robert Banks in fibre typing of intrafusal fibres: the confirmation of the principle of two types of nuclear bag fibres in mammalian spindles and the variation in occurrence of a dense M-band along the fibres. Furthermore, this paper summarizes how studies from the Umeå University group (Laboratory of Muscle Biology in the Department of Integrative Medical Biology) on fibre typing and the structure and composition of M-bands have contributed to the current understanding of muscle spindle complexity in adult humans as well as to muscle spindle development and effects of ageing. The variable molecular composition of the intrafusal sarcomeres with respect to myosin heavy chains and M-band proteins gives new perspectives on the role of the intrafusal myofibrils as stretch-activated sensors influencing tension/stiffness and signalling to nuclei.
Keywords: cytoskeleton, M-band, M-protein, muscle spindle, myomesin, nuclear bag, nuclear chain, titin
Two intrafusal fibre types
The ‘New Anatomy of the Spindle’ that emerged about 1960, with the distinction of nuclear bag and nuclear chain fibres, plus the functional distinctiveness of primary and secondary endings, is illustrated in Fig.1 (taken from Matthews, 1964). It shows a larger fibre containing a bag of nuclei in the equatorial region and a smaller fibre with a chain of nuclei in the equatorial region. Group I and group II afferents simultaneously innervate both bag and chain fibres, whereas gamma motor nerves supply either bag or chain fibres. That same year, one of us (Lars-Eric Thornell) had the privilege of listening to Matthews’ lecture on this topic in Gothenburg, just before joining the Department of Anatomy at Umeå University, which was inaugurated in September 1965, headed by Professor Ebba Cedergren-Andersson. In ongoing ultrastructural research on frog muscle spindles, she demonstrated a unique organization of the contact area and the reticular zone between the afferent nerve terminals and the muscle spindle fibres (Karlsson et al. 1966; Andersson-Cedergren et al. 1973). Three-dimensional reconstructions revealed intrafusal fibre size variation but no accumulation of nuclei in bag fibres (Fig.2). These early studies highlighted the differences between mammalian and amphibian spindles, but were never published in full because of her early death in 1974.
Fig 1.
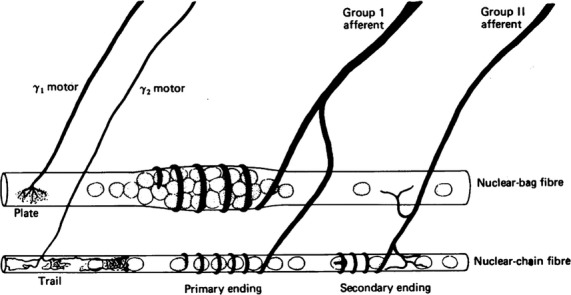
Simplified diagram of the central region of the muscle spindle illustrating its new anatomy as it came to be seen in the early 1960s. From Matthews (1964) with permission.
Fig 2.
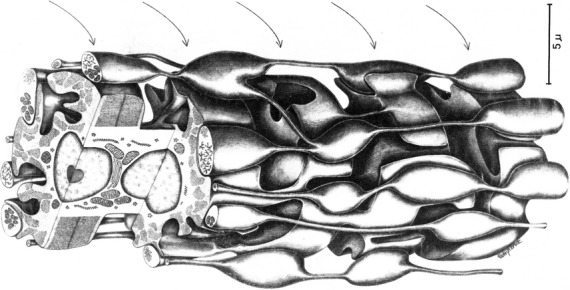
Three-dimensional reconstruction of a frog spindle intrafusal fibre at its reticular zone. The varicose swellings of sensory terminal bulbs are in close contact with the muscular processes that contain single sarcomeres. From Andersson-Cedergren et al. (1973) with permission.
Three intrafusal fibres
In subsequent years, enzyme-histochemical and ultrastructural studies suggested two types of bag fibres, but the issue was controversial for many years (see review by Boyd, 1981). Banks and colleagues made important contributions between 1975 and 1977 (e.g. Banks et al. 1975; Barker et al. 1976; Banks et al. 1977), which unified the field around the existence and nomenclature of three intrafusal fibre types. By combining histochemical and ultrastructural techniques (Fig.3), two types of nuclear bag fibres were shown to occur in the spindles from several mammalian species. They were named bag1 and bag2 following the nomenclature of Ovalle and Smith, who were the first to describe that bag fibres differed in staining reactions for myofibrillar ATPase (mATPase; Ovalle & Smith, 1972). By comparing previous classification schemes of intrafusal fibre types with the new terminology of bag1, bag2 and chain fibres (Table1), Banks and colleagues established what is still today ‘the gold standard’ for intrafusal fibre typing! Importantly, they also reported: (i) that variations in the histochemical profile along the length of individual intrafusal muscle fibres occurred; and (ii) that the bag fibres also showed regional differences in ultrastructure with regard to the presence or absence of an M-band in the middle of the myofibrillar A-band (Fig.3). These new morphological discoveries had a direct profound influence on the interpretation of observations from physiological studies. Previously, Matthews (1962) had found that the gamma axons could be divided into two functional types, ‘dynamic’ and ‘static’ (Matthews, 1962). Matthews (1964) proposed that the effects of activating dynamic gamma axons and static gamma axons could be explained, if they respectively innervated nuclear bag fibres and nuclear chain fibres with different viscoelastic properties (Matthews, 1964). However, now, given the new morphological background, work on isolated spindles with glycogen depletion demonstrated that the dynamic axons restricted themselves almost exclusively to the bag1 fibres previously named ‘dynamic nuclear bag fibres’ or ‘slow nuclear bag fibres’ described by Boyd (Boyd, 1976a,b; Boyd et al. 1977). Furthermore in cat spindles, the nuclear bag fibre controlled by static gamma axons was equivalent to the ‘fast nuclear bag fibre’. As the mATPase is related to contraction time, the differences in mATPase reactivity in histochemical stains became a meaningful parameter related to function. Thus, the contraction time of bag1 fibres was slower than that of bag2 fibres, which in turn was slower than that of chain fibres. Furthermore, localized contractions, increase in stiffness and differences in activation of the afferent innervation can be related to mechanical properties of the individual intrafusal fibre types (Matthews, 1981).
Fig 3.
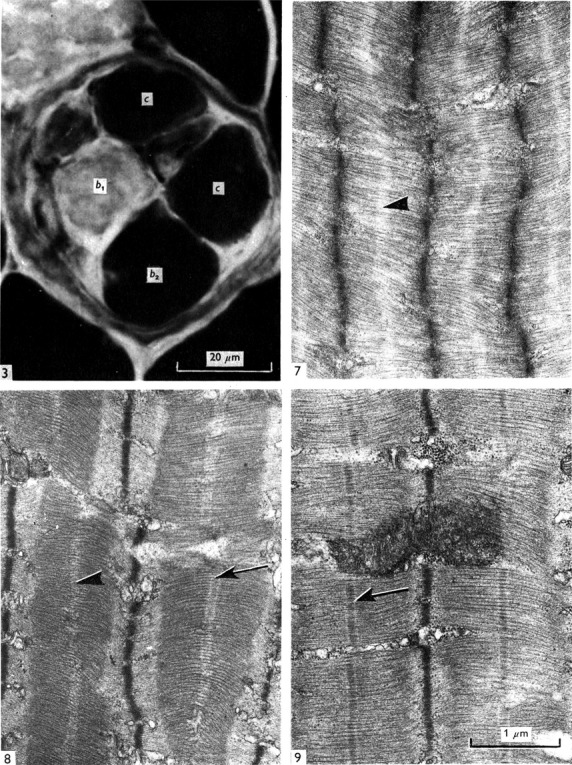
(3) Cross-section of a cat muscle spindle stained for mATPase following alkaline preincubation. The intensity is low in bag1 (b1) and high in bag2 (b2) and chain (c) fibres. (3:7–9) Electron micrographs of longitudinal sections of nuclear bag muscle fibres, showing the variation in structure in different regions. 3(7–8) illustrate the dM condition – (7) b2 A region: no line is present (arrowheaded); (8) b1 B region: a double M-line is present in some sarcomeres (arrowheaded). 3(9). The M-condition – an M-line is present in each sarcomere (arrowed).
Table 1.
Classifications of intrafusal muscle fibre types compared with present classification. For references to cited articles see Banks et al. (1977). From Banks et al. (1977) with permission
| Author, type of study and experimental animal | Original classification | Probable equivalent |
|---|---|---|
| Boyd (1977), morphology, cat | Nuclear bag | Bag1 |
| Bag2 | ||
| Nuclear chain | Chain | |
| Yellin (1977), histochemistry, rat | A | Bag2 |
| B | Bag1 | |
| C | Chain | |
| (One other type) | Bag1 | |
| Barker & Stacey (1977), histochemistry and morphology, rabbit | Nuclear bag | Bag2 |
| Nuclear chain | Chain | |
| Intermediate | Bag1 | |
| EM | Nuclear bag | Bag1 |
| Nuclear chain | Chain | |
| Intermediate | Bag2 | |
| Barker et al. (1977), histochemistry and EM, rabbit | Nuclear bag | Bag1 |
| Nuclear chain | Chain | |
| Intermediate | Bag2 | |
| Morphology | Nuclear bag | Bag2 |
| Nuclear chain | Chain | |
| Intermediate | Bag1 | |
| James (1977), histochemistry, rat; Banks (1977), histochemistry, rabbit | 1 | Chain |
| 2 | Bag2 | |
| 3 | Bag1 | |
| Ovalle & Smith (1972), histochemistry, monkey and cat | Bag1 | Bag1 |
| Bag2 | Bag2 | |
| Chain | Chain | |
| Milburn (1977), histochemistry and morphology rat | Typical bag | Bag2 |
| Intermediate bag | Bag1 | |
| Chain | Chain | |
| EM | Typical bag | Bag1 |
| Intermediate bag | Bag2 | |
| Chain | Chain | |
| Arendt & Asmussen (1977), histochemistry, rat, rabbit, cat, guinea-pig | 1 | Bag1 |
| 2 | ||
| 3 | Bag2 | |
| 4 | ||
| 5 | Chain | |
| 6 | ||
| Banks & James (1977), histochemistry, EM and morphology, rabbit | 1 | Chain |
| 2 | Bag2 | |
| 3 | Bag1 |
Another Banks’ contribution to muscle spindle science is the excellent review chapter: ‘The muscle spindle’ published in Myology, 1986 (updated versions in 1994 and in 2004; Banks & Barker, 2004), which set the standard for the subdivision of muscle spindles into A, B and C regions: the A and B regions are encapsulated, where the A region contains the equatorial region with a periaxial space, nuclear accumulation and the sensory innervation for all intrafusal fibres irrespective of type, the B regions are the encapsulated polar zones with motor innervation and the C regions are the extracapsular portions of the spindle.
Fibre typing of intrafusal fibres due to ‘new’ myosin isoforms in muscle spindles
The basis for ATPase reactions seen in intrafusal fibres became apparent after advances revealed the myosin heavy chain (MyHC) expression in these fibres. A fundamental paper by Schiaffino’s group (Bormioli et al. 1980) showed that two types of slow myosins were present in vertebrate muscle. In addition to the myosin present in slow-twitch fibres, another myosin was present in amphibian and chicken muscle fibres with slow-tonic properties, i.e. fibres that display multiple innervation and respond to stimulation with prolonged contractures. The latter had a limited distribution in mammals, with only a minority of fibres in extraocular muscles (EOMs) and the nuclear bag fibres of muscle spindles containing this myosin isoform. No immunohistochemical studies on human spindles were reported until Schiaffino et al. (1986) made a note that an antibody to foetal myosin stained nuclear chain fibres. During this period, in collaboration with Schiaffino, Butler-Browne, Virtanen and Whalen, the Umeå Group had ongoing research concerning human intrafusal fibre typing using antibodies against myofibrillar and cytoskeletal proteins developed in their and our laboratories (Eriksson et al. 1986). This work was greatly influenced by a symposium the following year in Prague, as described in the following section.
Immunocytochemical breakthrough in intrafusal fibre typing
The symposium ‘Mechanoreceptors: Development, Structure and Function’ in Prague in 1987 was a milestone in muscle spindle research for the Umeå group in several respects: (i) two immunohistochemical studies on human muscle spindles were reported; (ii) R. Banks and L.-E. Thornell had their first personal contact and important discussions; (iii) a fruitful collaboration on muscle spindles with Tomas Soukup, one of the organizers, was initiated; and (iv) Fatima Pedrosa joined the Umeå group, which speeded up the muscle spindle research (see below).
Under the title, ‘Myofibrillar and cytoskeletal proteins in human muscle spindles’, we presented data on the staining pattern of antibodies against: (i) embryonic, foetal/neonatal, fast-twitch, slow-twitch and slow-tonic MyHCs; and (ii) M-band proteins [MM-creatine kinase (MM-CK), M-protein and myomesin] and cytoskeletal proteins (desmin, vimentin, vinculin, fibronectin and spectrin) in bag1, bag2 and chain fibres vs. extrafusal fibres in the human masseter (Eriksson et al. 1988). In short, the main findings were: nuclear bag1 and nuclear bag2 fibres expressed predominantly slow-twitch and slow-tonic MyHCs (Fig.4). The bag2 fibres, in addition, contained embryonic and neonatal MyHCs. Nuclear chain fibres coexpressed embryonic, foetal and fast-twitch MyHCs. The bag2 and chain fibres contained all three M-band proteins (MM-CK, M-protein and myomesin), whereas the bag1 fibres contained only myomesin. All spindle fibres stained for desmin but lacked staining for vimentin. Our study showed that, with antibodies against slow-tonic and neonatal myosin and M-protein, a distinct classification of the intrafusal fibres in human muscle could be performed.
Fig 4.
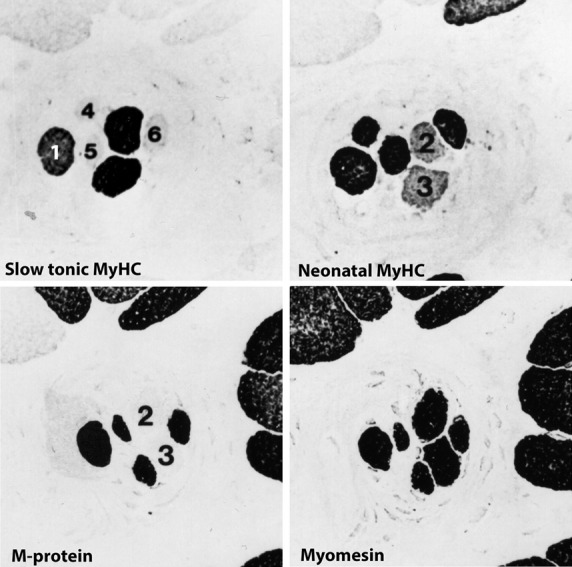
Serial cross-sections of human m. masseter. Anti-neonatal myosin stains the chain and the bag1 fibres, as well as some of the extrafusal fibres, a typical feature for the human masseter (Butler-Browne et al. 1988). Anti-slow-tonic myosin stains the bag1 fibre (1), bag2 fibres (2 and 3), whereas chain (4–6) and extrafusal fibres are unstained. The bag2 fibres lack staining for M-protein as well as some of the extrafusal fibres. Myomesin is present in all fibres.
In the other presentation, ‘Human muscle spindle development’, we extended our previous studies on the development of fibre types in human foetal muscle (Thornell et al. 1988). Serial sections stained for mATPase and antibodies against slow-twitch myosin, neonatal and fast myosins as well as antibodies against the three M-band proteins (MM-CK, M-protein and myomesin) were analysed (Fig.5). Already at 10 weeks of gestation we could separate three fibre types: primary myotubes were all stained for slow-twitch myosin, whereas secondary myotubes stained for neonatal myosin. Of special importance was that a subset of the primary myotubes was stained for an antibody raised against slow-tonic myosin. By 14 weeks of gestation, there were both primary and secondary myotubes stained with the antibodies against slow-tonic myosin, suggesting them to be primordia of bag fibres. In foetuses of 15–16 weeks of gestation, typical muscle spindles were observed. Whereas all three M-band proteins (MM-CK, M-protein and myomesin) were observed at 10–14 weeks gestation, some intrafusal fibres in each spindle appeared to lack staining for M-protein at later stages. Rowlerson at the same meeting reported on the early type-differentiation of intrafusal fibres and appearance of slow-tonic-reactive fibres, in cat, rat and human foetal muscle spindles (Rowlerson, 1988).
Fig 5.
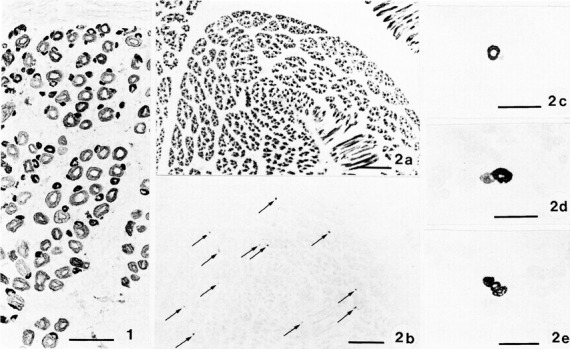
(1) Cross-section of the upper arm of a human foetus aged 10 weeks stained with antibodies against neonatal myosin. Note that there is a difference in staining of primary (large size) and secondary (small size) generation fibres; scale bar: 50 μm. (2) Serial cross-sections of limb muscle of human foetus aged 14 weeks stained with (2a) anti-slow-twitch myosin and (2b–e) anti-slow-tonic myosin. Note that all primary myotubes are stained in (2a), whereas in (2b) only scattered myotubes are stained (arrows). In (2c–e) slow-tonic-labelled myotubes are shown in higher magnification. Note that both primary and secondary generations of myotubes are labelled, whereas all other surrounding myotubes are completely unstained. Scale bars: 250 μm (2a–b); 50 μm (2c–e). From Thornell et al. (1988).
These studies raised a number of questions: Did the primary myotubes that express slow-tonic myosin arise from a special cell lineage or is sensory innervation regulating the genes of primary myotubes? Because innervation is also required for the nucleation and formation of the secondary generation myotubes, which are also considered to be derived from a separate lineage (Milburn, 1984), did sensory innervation likewise affect those cells formed close to the myotubes containing slow-tonic myosin? And, finally, what factors further regulated the diversification of the intrafusal fibres into types with different myosin isoform and M-band compositions?
Fatima Pedrosa set forth to unravel some of these questions. A series of publications on immunohistochemistry of rat muscle spindles (Pedrosa et al. 1989, 1990; Pedrosa & Thornell, 1990; Soukup et al. 1990; Pedrosa-Domellof et al. 1991) and her PhD thesis, ‘Expression of myosin heavy chain isoforms in rat muscle spindles: an immunocytochemical study’, set the stage for the characterization of the molecular composition of rat intrafusal types, how they develop and are influenced by altered nerve supply (Pedrosa-Domellöf, 1991). The main findings were:
Rat intrafusal fibres coexpressed several MyHC isoforms. The expression of some of these isoforms, namely of slow-tonic, neonatal and α-cardiac-like MyHC is muscle spindle-specific.
A MyHC isoform identical or closely related to α-cardiac MyHC was shown to be specifically expressed in rat nuclear bag fibres. Additional unidentified MyHC isoforms might be expressed in intrafusal fibres.
Each intrafusal type has a distinct pattern of MyHC expression with regional variation along the length of the fibres (Fig.6: 1–3). This characteristic pattern may be used for identification of the intrafusal fibre types.
Although enzyme histochemical analysis of the mATPase activity is still useful in distinguishing fibre types in the adult, it is not sensitive enough to detect differences in MyHC composition or to discriminate between mixtures of MyHC isoforms.
The use of antibodies against MyHC isoforms as molecular markers of muscle development is an excellent method for studying muscle spindle development by light microscopy.
During muscle spindle development, each intrafusal precursor has its own sequence and timing of expression of MyHC isoforms (Fig.7).
In order to clarify the exact origin of muscle spindle precursors, other markers will have to be discovered. At present, these data are compatible with the hypothesis that muscle spindles originate from distinct cell lineages.
The differentiation and maintenance of muscle spindles depend on the presence of sensory innervation from early development to the mature stage.
Motor innervation is involved in the regulation of MyHC expression along the length of the nuclear bag fibres. The typical regional variation in MyHC expression along the length of intrafusal fibres might reflect the existence of nuclear domains under the influence of both sensory and motor innervation.
Intrafusal fibres represent an attractive in situ model for investigating myogenesis, myofibrillogenesis and the mechanisms of regulation of MyHC expression. Further investigations, correlating the physiology of muscle spindles with the complexity of their contractile apparatus, are needed.
Fig 6.
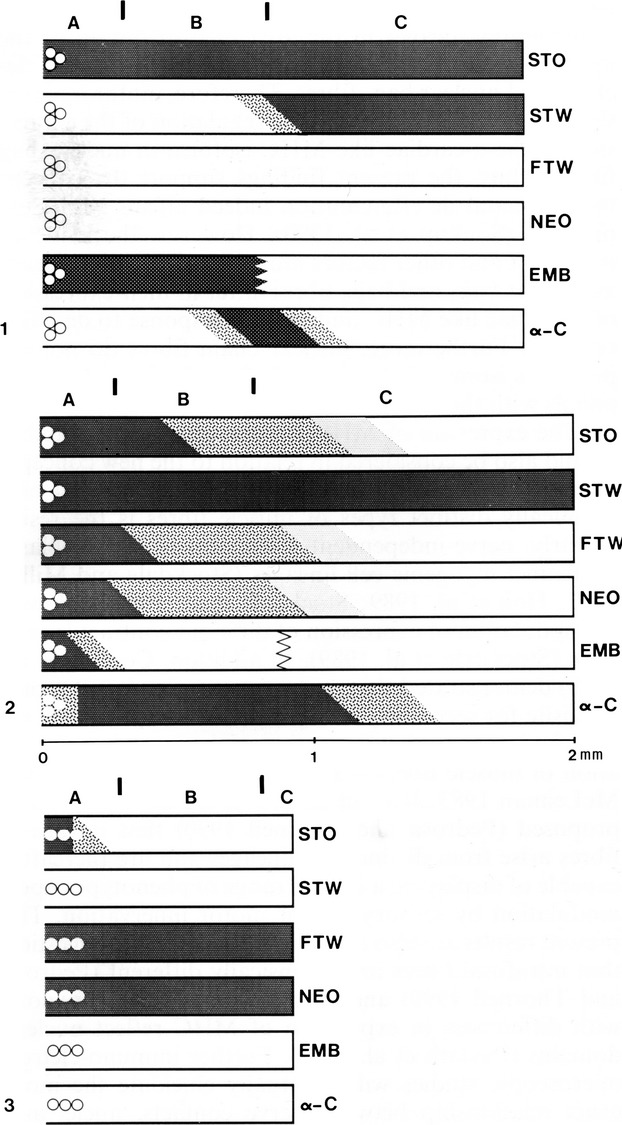
Schematic representation of the staining profile of adult soleus nuclear bag1 (1), bag2 (2) and chain (3) fibres with antibodies against slow-tonic (STO), slow-twitch (STW), fast-twitch (FTW), neonatal (NEO), embryonic (EMB) according to Maier et al. (1988) and α-cardiac MyHC (α-C). The circles represent the nuclei and each fibre is therefore depicted from the equator to one of the poles. Darker shades represent increasing staining, and the oblique limits between shades within a fibre represent a gradual transition in staining intensity. The zig-zag line within the bag fibres stained for embryonic MHC means that information is not available for the extracapsular portion of the fibre. The A, B and C spindle regions are marked at the top of each set of fibres, and the vertical bars represent, from left to right, the end of the periaxial space and the end of the capsule. Approximately drawn to scale only with respect to fibre length. From Pedrosa-Domellof et al. (1991).
Fig 7.
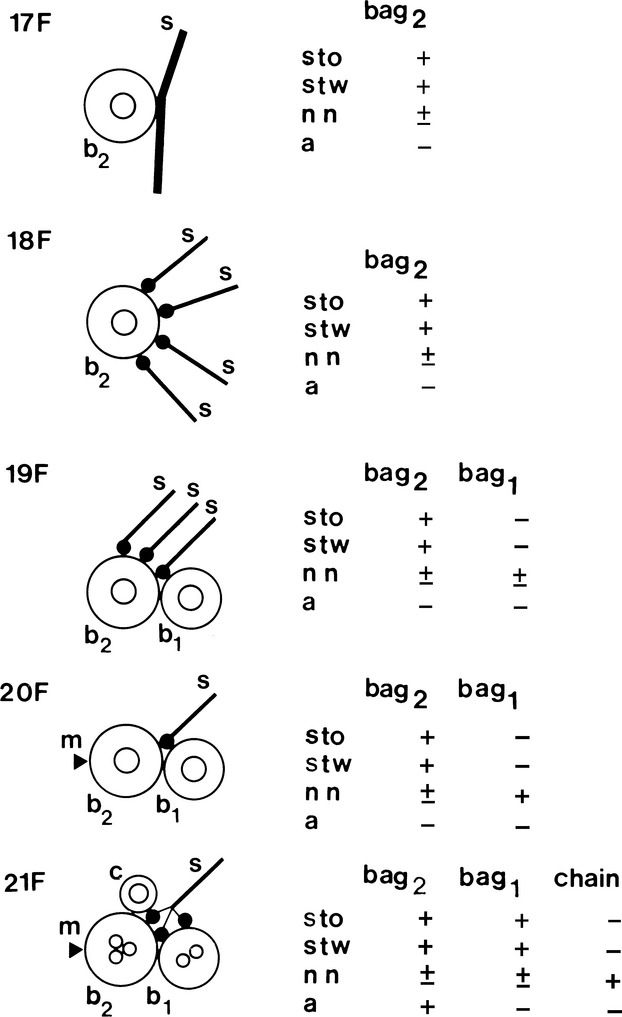
Simplified illustration of muscle spindle development showing the acquisition of sensory (s) and motor (m) innervation (based upon Kucera et al. 1989) and MyHC expression (based upon Pedrosa & Thornell, 1990). The earliest contacts between afferent terminals (s) and muscle cells, in rat soleus, are detectable on the 17th day of gestation (17F). Up till the 20th day in utero (20F), the nuclear bag2 myotubes (b2) are innervated by several afferents. Thereafter, each muscle spindle becomes innervated by a single primary afferent as in mature spindles, and the sensory endings are distributed to both immature nuclear bag2 and bag1 (b1) fibres and to the single intrafusal myoblasts that are the precursors of the nuclear chain fibres (c). Also by the 20th day in utero, the gamma motor innervation (arrowhead) reaches the muscle spindles initially innervating only the bag2 fibres. The nuclear bag1 and chain fibres acquire motor innervation later. Nuclear bag2 precursors are identifiable under light microscopy from the 17th day of gestation as the only primary myotubes expressing slow-tonic MyHC (sto), in addition to slow-twitch (stw) and neonatal (nn) MyHC. They acquire reactivity to anti-α-cardiac MyHC (a) by the 21st day of gestation. The future nuclear bag1 fibres are first seen at 19 days in utero as secondary myotubes in close apposition to the primary STO myotubes. Initially they express only neonatal MyHC and acquire reactivity to anti-slow-tonic and anti-slow-twitch MyHC 2 days later. Shortly before birth, the nuclear chain fibre precursors are identifiable as a 3rd myotube in each spindle strongly stained with anti-neonatal MyHC only. Three days after birth, the 2nd nuclear chain precursor arise and display a profile of reactivity identical to the 1st nuclear chain precursor. From Pedrosa et al. (1990).
For further reading and references on rat muscle spindles, see reviews by Zelená (1994), Soukup et al. (1995) and Soukup & Jirmanova (2000).
Immunohistochemical evaluation of intrafusal type development in human muscle spindles
In parallel to the progression of our immunohistochemical studies on human skeletal muscle development, where we could distinguish two generations of fibres early in development and the downregulation of embryonic and foetal MyHC upon fibre type specification of slow and fast MyHC-containing fibres (Thornell et al. 1984; Butler-Browne et al. 1990; Barbet et al. 1991), special focus was now put on the development of human muscle spindles. Undoubtedly, the first sign of human intrafusal fibre development was the divergence in staining for slow-tonic MyHC among the primary generation of myotubes. Whether the first generation of spindle myotubes/fibre developed from a separate lineage of myoblasts could not be settled. Therefore, an extensive study of human spindle development was performed and showed a much more diverse pattern of immunoreactivity to different MyHC antibodies than in rat muscle spindles (Pedrosa-Domellof & Thornell, 1994). Developing nuclear bag fibres were first identified at 10–11 weeks of gestation as single primary myotubes expressing slow-tonic and slow-twitch MyHCs, in addition to embryonic and foetal MyHCs. The secondary myotubes that formed in apposition to these primary myotubes initially expressed embryonic and foetal MyHCs only (10–11 weeks of gestation and even 14–16 weeks of gestation). Later, some of these secondary myotubes acquired slow-tonic and slow-twitch MyHCs, and were identified as nuclear bag fibres. The secondary myotubes that did not acquire slow-tonic, slow-twitch or α-cardiac MyHCs (16–20 weeks of gestation) developed into nuclear chain fibres. These results are summarized in Fig.8.
Fig 8.
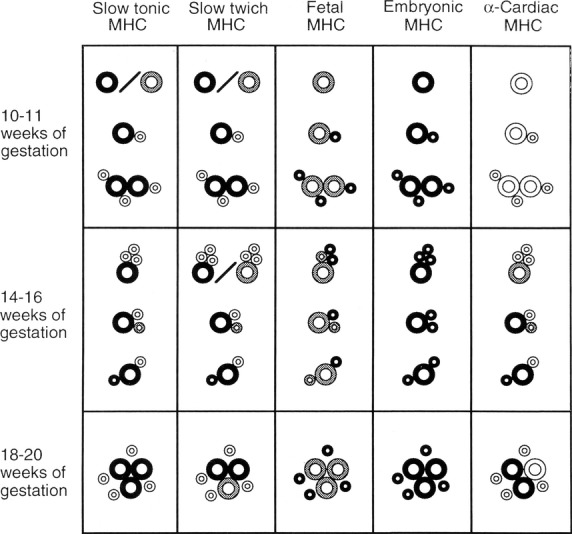
MyHC isoforms in developing human muscle spindles. Schematic representation of the pattern of MyHC expression in developing intrafusal fibres. Age in wG is shown at the left. The MyHC isoforms are specified at the top, and darker shades represent increasing levels of staining with the respective antibodies. Large circles denote first-generation myotubes and small circles denote second-generation myotubes. The myotubes expressing slow-tonic MyHCs are developing nuclear bag fibres. Muscle spindle development is asynchronous, and therefore at each given age muscle spindles containing different numbers of fibres were found and the level of reactivity of the fibres to each antibody varied. Developing nuclear bag fibres were first identified at 10–11 wG as single primary myotubes expressing slow-tonic and slow-twitch MyHCs, in addition to foetal and embryonic MyHCs. The secondary myotubes that formed in apposition to these primary myotubes initially expressed foetal and embryonic MyHCs only (10–11 wG and even 14–16 wG). Later, some of these secondary myotubes acquired slow-tonic and slow-twitch MyHCs, and therefore became nuclear bag fibres. The secondary myotubes that did not acquire slow-tonic, slow-twitch or α-cardiac MyHCs (16–20 wG) developed into nuclear chain fibres. Not drawn to scale. From Pedrosa-Domellof & Thornell (1994).
Human intrafusal fibre types on basis of MyHC composition
The summarized initial immunocytochemical analysis of MyHC isoforms in human spindles, in m. masseter, m. lumbricalis and m. biceps, was that nuclear bag1 and nuclear bag2 fibres expressed predominantly slow-twitch and slow-tonic MyHCs. Embryonic, foetal and α-cardiac MyHC staining may also be present to various degrees. Nuclear chain fibres coexpressed embryonic, foetal and fast-twitch MyHCs (Eriksson et al. 1988, 1994; Pedrosa et al. 1990). As different antibodies against the same MyHC yielded different staining patterns, a biochemical analysis of the MHC composition of intrafusal fibres was performed (Pedrosa-Domellof et al. 1993). Individual muscle spindles, as well as single extrafusal fibres, were isolated by microdissection and analysed for their MHC isoform pattern. At least four MyHC isoforms were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis in muscle spindles. A prominent band not seen in foetal or adult muscle fibres was suggested to represent a hitherto unidentified, spindle-specific MyHC isoform, MyHCif. The other three bands were identified as embryonic, neonatal/foetal and slow MyHCs. It was also verified that the relative concentrations of the MyHC isoforms differed along the length of a given muscle spindle (Pedrosa-Domellof et al. 1993).
Revised fibre typing on basis of completion of the human genome project
Eleven sarcomeric myosin heavy chain (MYH) genes were identified by the human genome project (Fig.9). The most ancient gene is named MYH16, and codes for a myosin expressed in jaw muscles of carnivores, primates and marsupials. It has been inversely related to the evolution of the human skull, as the gene in humans became silenced about 2.4 million years ago. The next modification was a duplication of genes that led to two additional ancestral genes, MYH15 and MYH14/7b (Rossi et al. 2010), and the two highly conserved gene clusters of so-called ‘cardiac’ and ‘skeletal’ MYH genes. In the human genome, the cluster of MYH6 and MYH7 is located in chromosome 14 and codes for the two ‘cardiac myosins’, α- and β-MYH. However, both genes are also expressed in skeletal muscle fibres. The β-MYH encodes for the slow-twitch MyHC in type I fibres. The ‘skeletal’ MYH cluster of six genes is located in human chromosome 17. The most ancient one of these, MYH13, codes for an isoform (MyHC-eom), expressed specifically in EOMs, and the next most ancient, MYH3, for embryonic myosin. For the four most recently eveloped MYH genes, one pair (MYH1and MYH2) code for the adult fast IIx and the adult fast IIa, the other pair (MYH4 and MYH8) for the adult fast IIb and the foetal/neonatal/perinatal MyHC, respectively. Some studies have shown that the question ‘IIb or not IIb’, related to the question of whether human IIB fibres in limb muscles identified on basis of mATPase staining actually express the MyHC-IIx isoform (Smerdu et al. 1994), has got some answers. Expression of MyHC-IIb mRNA has been observed in foetal human myotubes and in Duchenne Muscular Dystrophy skeletal muscle, but there was no compelling evidence for expression of the MyHC-IIb protein (Harrison et al. 2011). However, protein expression was observed in human laryngeal muscles (Andersen et al. 2000; Smerdu & Cvetko, 2013).
Fig 9.
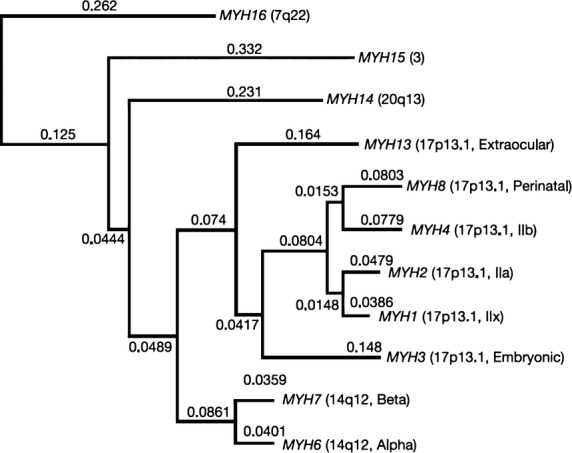
Phylogenetic reconstruction for all human sarcomeric myosin genes (heavy chain), showing early divergence of MYH16 from other MYH genes. Branch lengths shown are derived from a maximum likelihood analysis of the aligned cDNAs, beginning with the conserved proline at the head–rod junction. From Stedman et al. (2004) with permission.
It has become apparent that coexpression of two or more MyHC genes to various degrees is not exceptional (Schiaffino & Reggiani, 2011). Of the human extrafusal fibres, those in EOM, jaw and laryngeal muscles show the most complicated pattern of gene expression of MyHCs, for example, for EOM: MyHC-IIa, MyHC-I, MyHC-eom, MyHC-slow-tonic, MyHC-α-cardiac and MyHC-emb (Kjellgren et al. 2003); and for laryngeal, see Hoh (2005). Conversely, limb and trunk muscles utilize only the MYH7 gene to express slow-twitch MyHC, and only the most recently developed fast MYH genes to express the fast MyHC-IIa and MyHC-IIx proteins.
In human as well as in rat spindles, the MYH expression in the intrafusal fibres also shows a complicated pattern of MyHC expression. Likewise, their developmental pattern involves a number of MYH genes. The most ancestral genes as well as the so-called developmental MYH genes (embryonic and foetal/neonatal/perinatal gene product) and both the cardiac genes MYH-α and -β are expressed in bag and chain fibres, and with regional differences. The prominent MyHC band for the hitherto unidentified spindle-specific MyHCif isoform alluded to above (Pedrosa-Domellof et al. 1993; Liu et al. 2002) most likely reflects a slow-tonic MyHC isoform. Antibodies against the MYH7 gene product developed by Rossi and colleagues indeed stain human bag fibres (Thornell and Rossi, unpublished), and in Western blots of human EOM a band with mobility similar to that of the MyHCif is detected (Rossi et al. 2010).
Walro and Kucera speculated on why adult mammalian intrafusal fibres contain different MyHC isoforms (Walro & Kucera, 1999). They suggest that the intrafusal MyHCs are developmental isoforms, and that a slow-developmental isoform is expressed by prenatal precursors of both intrafusal and extrafusal fibres. In intrafusal fibres, the developmental isoforms then persist due to the afferent neurons arresting their maturational replacement by MyHCs needed for faster shortening velocities. However, based on antibody immunoreactivity, the current genomic MYH tree does not support their proposal (Schiaffino & Reggiani, 2011). Actually the antibody used, S46, recognizes both SM1 and SM2 in chicken muscle, where SM1 is the adult slow myosin isoform, SM2 the slow-tonic isoform and SM3 is the slow developmental isoform equivalent to the main chicken atrial MyHC (Page et al. 1992; Stockdale, 1997). The different MyHC isoforms are very similar, so there are limitations in immunohistochemistry as there can be cross-reactivity. Thus, the current antibodies are not necessarily as species-unspecific as many authors like to assume. In particular, a well-established pattern of reactivity in particular mammalian species may not extend to all species examined, while the developmental post-translational modifications of the epitope on a single MyHC gene product may further complicate interpretation. Some discrepancies between different research groups can thus be explained by the use of different antibodies (Pedrosa-Domellof et al. 1991; Smerdu & Soukup, 2008)
Consequences of improved typing of intrafusal fibres in human muscle
Our view is that each human muscle is unique with respect to its complement of fibre types, MyHC composition/coexpression, etc. (Fig.10). This also fits with the idea that muscle spindles vary in number in functionally diverse human muscles (Voss, 1971). In addition, our studies of samples from different muscles suggest that the content of intrafusal fibre types as well as their MyHC staining pattern also show differences between muscles. However, no systematic study on adult spindles had been undertaken. This has been addressed by Jing-Xia Liu who, as part of her PhD project, investigated the fibre content and MyHC composition of the muscle spindles in an ordinary limb muscle, the biceps brachii, compared with the deep neck muscles: the latter being chosen because they not only contain numerous muscle spindles, but they are also of great importance for body orientation (Cooper & Daniel, 1956). Furthermore, she also analysed the effect of ageing on these intrafusal fibre types. The use of a large battery of antibodies, staining of consecutive serial sections and biochemical analysis of antibody reactivity were performed. Her thesis: ‘Human muscle spindles; complex morphology and structural organization’ (Liu, 2004 can be downloaded from http://umu.divaportal.org/) unquestionably proved that each human muscle has a unique set of intrafusal fibres in their muscle spindles. In this, the main findings were:
Fig 10.
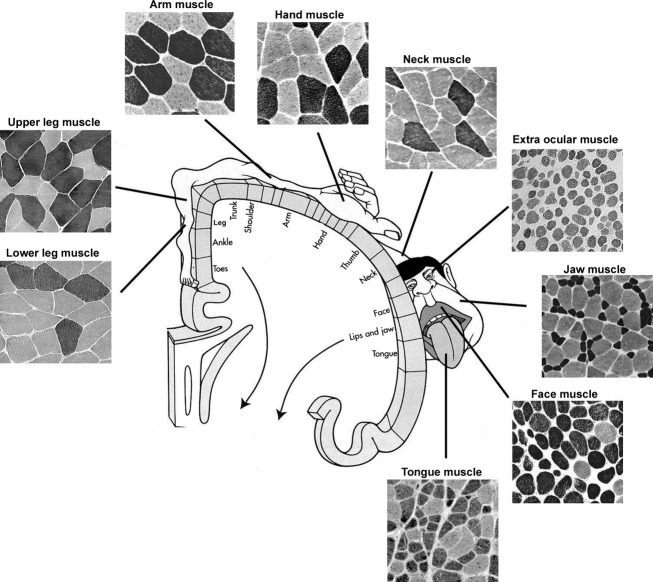
Human muscles are unique not only with regard to obvious differences such as origin, insertion, size and fibre pennation but also to fibre type composition, size of fibres, % of fibre types, MyHC content, coexpression of myosin isoforms, hybrid fibres (Thornell et al. 1984b; Butler-Browne et al. 1988; Stal et al. 1994; Kjellgren et al. 2003; Schiaffino & Reggiani, 2011). Schematic illustration of cross-sectioned human muscles stained for mATPase to display their muscle fibre types in relation to their cortical representation. Note that the jaw, face, tongue and hand regions have large representation in the cortex. Note the large variation in size of fibres and frequency of staining patterns.
Muscle spindles in m. biceps brachii (Liu et al. 2002). Four major MyHC isoforms (MyHC-I, -IIa, -α-cardiac and intrafusal slow-tonic) were detected in single muscle spindles. Immunoblots showed that often well-characterized antibodies actually had an affinity repertoire for several different MyHC isoforms. While a complex pattern of MyHC distribution in bag1, bag2 and chain fibres is apparent in regions A, B and C, there are undoubtedly distinct differences between the bag1 and bag2 fibres both with respect to enzyme-histochemical staining for mATPase and with the different antibodies. Thus, bag1 fibres stain for slow-tonic and α-cardiac in the equatorial region, whereas bag2 stain in addition for slow-twitch MyHC (two different antibodies). Nuclear chain fibres were unreactive with all these antibodies, but stained strongly with antibodies for embryonic, foetal and fast MyHCs (Fig.11). In the biceps brachii muscle, virtually each muscle spindle had its own unique number of bag1, bag2 and chain fibres.
Muscle spindles in deep neck muscles (Liu et al. 2003).The pattern of MyHC expression for bag1, bag2 and chain fibres was similar to that found in the biceps brachii, except fewer bag fibres were reactive for anti-α-cardiac MyHC antibody. Furthermore, in contrast to biceps muscle spindles, many neck spindles had the same combinations of numbers of bag1, bag2 and chain fibres. In addition, it was apparent that a number of the spindles lacked either bag1 or bag2 fibres, and that many fibres showed unusual staining features.
Potential age-related changes in human muscle spindle fibre types from biceps brachii muscle (Liu et al. 2005). The total number of intrafusal fibres per spindle was significantly lower in spindles of the elderly, due to fewer nuclear chain fibres. Nuclear chain fibres in old spindles were short and some showed novel expression of MyHC-α-cardiac. The expression of MyHC-α-cardiac in bag1 and bag2 fibres was much lower in the A region than in ‘adult’ spindles. The expression of MyHC-I was higher in nuclear bag1 fibres and that of foetal MyHC was lower in bag2 fibres, whereas the distribution of the remaining MyHC isoforms was generally not affected by aging.
Fig 11.
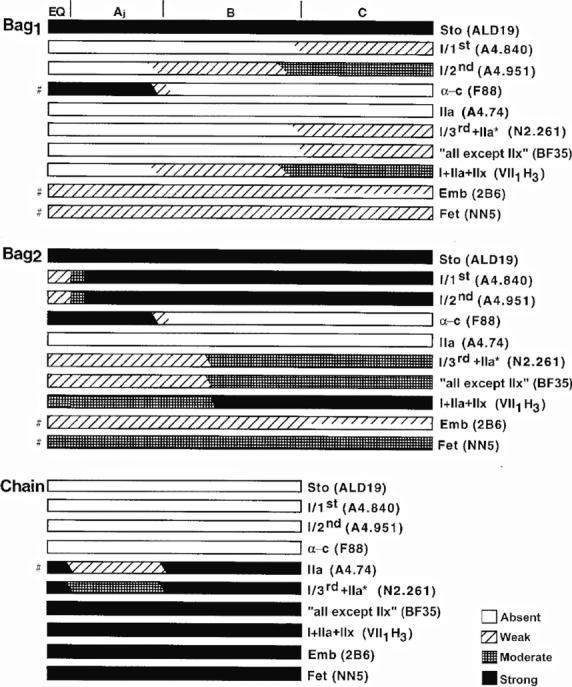
Schematic representation of the staining profiles of nuclear bag1, bag2, and chain fibres from adult human biceps muscle with the different MyHC antibodies. The A, B and C regions are marked at the top of the fibres, and the vertical bars represent, from left to right, the end of the periaxial space and the end of the capsule. The representation of staining profiles of chain fibres is lacking in the C region because few chain fibres extended into this region. The symbols ‘#’ on the left denote some transient variations with regard to the general staining profile, and which are described in detail in the Results. Not drawn to scale. From Liu et al. (2002).
Catharina Österlund has performed even more extensive intrafusal fibre typing in samples of human masseter and biceps brachii at a young age (3–7 years). A total of 624 spindles were detected in the masseter and, of these, 241 spindles were sectioned in the capsular region. One-hundred and ninety-eight were defined as single and 43 as compound spindles altogether, sampling a total of 2455 intrafusal fibres. In the biceps, 97 spindles were detected, of which 54 were sectioned in the capsular region and, of those, two were compound spindles, altogether containing 434 intrafusal fibres. Intrafusal fibres were first classified into four types of fibres: bag1, acid stable bag1 (AS-bag1; Eriksson & Thornell, 1990), bag2 and chain fibres on the basis of their mATPase staining intensity. Serial sections were in parallel incubated with 10 different antibodies against MyHC isoforms and together with an anti-laminin antibody to reveal all intrafusal fibres in each spindle. Muscle spindles were larger and more frequent in the masseter than in the biceps brachii, but the principal staining pattern for bag1, bag2 and chain fibres was as published for adult human spindles (Eriksson et al. 1994; Liu et al. 2002, 2003). Importantly, muscle spindles were morphologically mature already at a young age. Furthermore, the majority of intrafusal fibres contained a mixture of two–six MyHC isoforms as in adult spindles, resulting in a huge number of combinations of MyHC expression in individual fibres. The marked heterogeneity in staining patterns for the different antibodies to the individual intrafusal fibres and their regions is shown in Fig.11. Obvious differences can be seen between the intrafusal fibre reactivity in masseter vs. biceps spindles (Fig.12). Compare bag1 and AS-bag1 fibres with respect to staining for MyHC-α-cardiac (moderate–strong in masseter; weak–absent in biceps) and MyHC-I (stronger in masseter than in biceps; Österlund et al. 2011, 2013). Her PhD thesis: ‘Extra- and intrafusal muscle fibre type compositions of the human masseter at young age, in perspective of growth and functional maturation of the jaw-face motor system’ (Österlund, 2011) can be downloaded from http://umu.divaportal.org/.
Fig 12.

(a) Staining pattern for 10 mAbs of young masseter bag1, AS-bag1, bag2 and chain fibres in muscle spindle regions A, B and C, and (b) of young biceps in the same way. The proportion (%) of unstained (open circle) and weakly (grey), moderately (dark grey) and strongly (black) stained fibres are shown in the pie charts. Note the distinct differences in staining/reactivity for, e.g., A4951, A4840 and F88 in bag fibres of masseter vs. biceps. From Österlund et al. (2011).
On basis of mATPase staining and MyHC composition for intrafusal fibre typing, therefore, human muscle spindles proved to be more complex than anticipated. It is noteworthy that human spindles are far less stereotypical than spindles of small animals in terms of number of intrafusal fibres and their pattern of mATPase staining and MyHC expression. The clear differences shown between the biceps brachii muscle spindles, spindles in the deep neck and spindles in the masseter muscles suggest functional specialization among different human muscles in the control of movement.
Fibre typing on basis of ultrastructural variability in M-band appearance and differential composition
Chain fibres in rat, rabbit and cat spindles sampled from A, B and C regions always show a single prominent line in the middle of the A-band, a condition designated as ‘M’ (Barker et al. 1976; Banks et al. 1977). The other condition, ‘dM’, reflecting either the absence of an M-line or the presence of two faint lines, varied as follows: bag2 fibres, irrespective of the three species, showed dM for most of region A, switching to M as they approached the A/B border (Fig.13). Bag1 also differed between species such that in the cat, bag1 fibres show dM in region A, switching to M towards the polar end of region B, whereas in rabbit, a similar switch occurred in the middle of region C and in rat bag1 fibres the condition is dM throughout. Combining histochemical and ultrastructural analysis of human muscles spindles from intercostal muscle, Kucera and Dorovini-Zis proved their composition of bag1, bag2 and chain fibres to be analogus to animal spindles and that chain fibres had prominent M-bands, whereas M-band structure along the length of the bag fibres showed regional differences (Kucera & Dorovini-Zis, 1979). What significance has the variable M-band configuration on muscle spindle function? Banks et al. speculated that because the transition zone in rabbit and cat spindles occurred at a similar distance from the equator, it might be determined by developmental factors associated with the primary afferents. At that time knowledge on M-band structure and composition was in its infancy. The typical ultrastructural feature of the smallest contractile unit of the myofibril – the sarcomere – is the interdigitating set of thin actin filaments and thick myosin filaments. The former are attached to the Z-disc and the latter to the central M-band (Fig.14). With the improvement of biochemical and immunohistochemical methods, the components of the M-band were found to be a protein with a MW of 165 000 Dalton, named M-protein (Masaki & Takaiti, 1974), MM-CK (Turner et al. 1973; Wallimann et al. 1977a,b) and an 185 000-Dalton protein, myomesin (Grove et al. 1984). In parallel, it became evident that in negatively stained ultrathin cryosections, the M-band had distinct lines/striations crosslinking the thick filaments, forming M-bridges and M-filaments (Thornell & Sjöström, 1975). Interestingly, the lines showed a fibre type-related appearance in human limb skeletal muscle of 3, 3 + 2 or 5 lines (Sjostrom & Squire, 1977; Fig.14c). By combined immunocytochemistry and ultramicrotomy, ultrastructural localization of MM-CK to the M4-, M4′-lines in chicken breast muscle was revealed, whereas M-protein was interpreted to attach to the backbone of the thick filaments from M6 to M6′ or to form so-called M-filaments interlinking the M-bridges (Strehler et al. 1983). Furthermore, in collaboration with B.K. Grove, further details regarding M-band proteins relationships to fibre type development in chicken (Grove et al. 1987; Grove & Thornell, 1988) and rat were determined (Thornell et al. 1986; Carlsson & Thornell, 1987; Carlsson et al. 1990). In chicken muscle, the intrafusal fibres showed an aberrant staining pattern with regard to extrafusal fibre types (Grove & Thornell, 1988). By studying the M-band structure and composition of physiologically defined rat motor units in soleus, a slow muscle, and m. tibialis anterior, a fast muscle, it was possible to prove that the M-band structure and composition are fundamentally related to whether the fibre is innervated by a slow or a fast motorneuron in rat limb muscles (Thornell et al. 1987). Essential parameters, such as contraction time, Z-disc width and mitochondrial content of fast and slow fibres were relative and varied between muscles, whereas the M-band structure overrode the intragroup variability in contraction times of slow and fast units within and between the two muscles (Thornell et al. 1987). Nevertheless, the M-band ultrastructure (pattern of lines) is not constant either in slow and fast fibres of different species, nor between different muscles of the same species. Consequently, as expected, the composition of the M-band also differs (Thornell et al. 1990; Agarkova et al. 2004).
Fig 13.
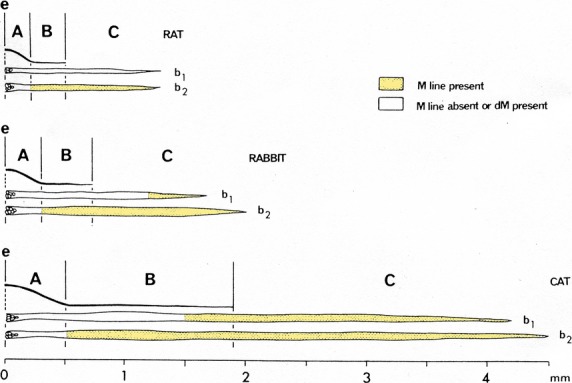
Variations in M-line condition of bag1 (b1) and bag2 (b2) fibres in regions A, B and C of the poles of typical rat, rabbit and cat spindles. (Muscle fibre width scale × 3 that used for length). e, equator. From Barker et al. (1976) with permission.
Fig 14.
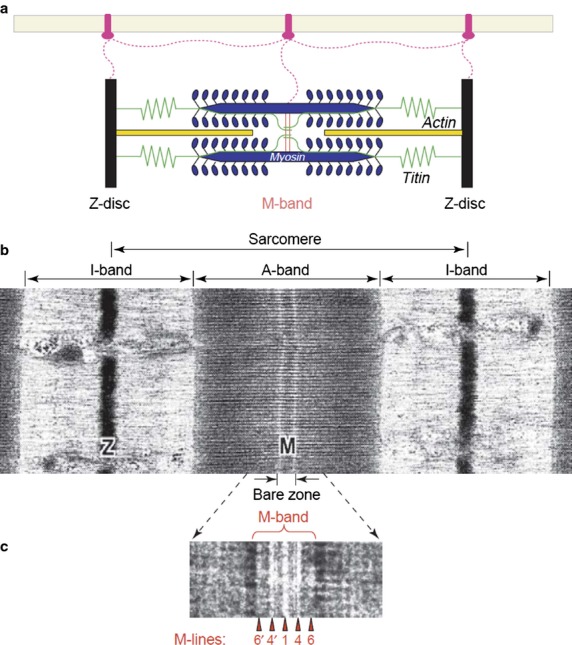
(a) Simplified schematic representation of sarcomeric structures. Color code: actin filaments, dark yellow; myosin filaments, blue; titin filaments, green. The transverse structures are the Z-disk (black) and the M-band (red). The extra-sarcomeric filaments (magenta) are anchored to transmembrane proteins in the sarcolemma. (b) Corresponding electron micrograph of a skeletal muscle sarcomere. The sarcomeric borders are delineated by the Z-discs (Z) in the middle of the I-band. The M-band (M) is seen as an electron-dense transverse band in the middle of the dark A-band and encompasses part of the lighter ‘bare’ zone, where neither myosin crossbridges nor thin filaments are present. (c) On this negatively stained cryosection (protein density appears white) of the higher-magnification view of human tibialis anterior muscle, the M-band breaks up into a series of several M-lines (arrowheads). The five strongest M-lines (or M-bridges) are designated M6′, M4′, M1, M4 and M6 (only numbers are shown). EM images are reproduced from Sjostrom & Squire (1977). Numbers give the distances in nm from the centre of the A-band, the M1-line. From Agarkova & Perriard (2005) with permission.
No detailed study on human limb muscle spindles where M-band structure has been related to M-band composition had been published until we presented the ultrastructure of three serially sectioned human lumbrical spindles (Thornell et al. 1995). The spindles were composed of two large bag fibres and varying numbers of chain fibres (Fig.15). The latter fibres always contained a dense M-band and showed immunolabelling for myomesin and M-protein. One bag fibre, interpreted as bag1 on the basis of double immunolabelling with anti M-protein and anti-slow-tonic MyHC, lacked M-bands in the equatorial and the main part of the polar regions. In the other bag fibre, the lack of an M-band varied between spindles. In one spindle a short segment of the equatorial region lacked a dense M-band, in a second spindle M-bands were seen along the whole length of the bag fibre, whereas in the third the equatorial region and a small part of one of the poles lacked dense M-bands (Fig.15). Typically for the sarcomeres lacking M-lines, the M-region and the borders of the A-band with the I-band were irregular [Figs16 (7) and 17(1)]. This strongly argues for a lack of register of the thick filaments within the A-band. In the fibres with dense M-bands, the A–I-band borders were in perfect register [Fig.17 (7 and 8)]. In negatively stained cryosections, where the proteins are embedded in the electron-dense stain, making the filaments appear in high contrast, there was a marked difference in appearance of the M-region between fibres showing no M-lines, 3 strong and 2 weaker or 5 equally strong lines (Fig.17).
Fig 15.
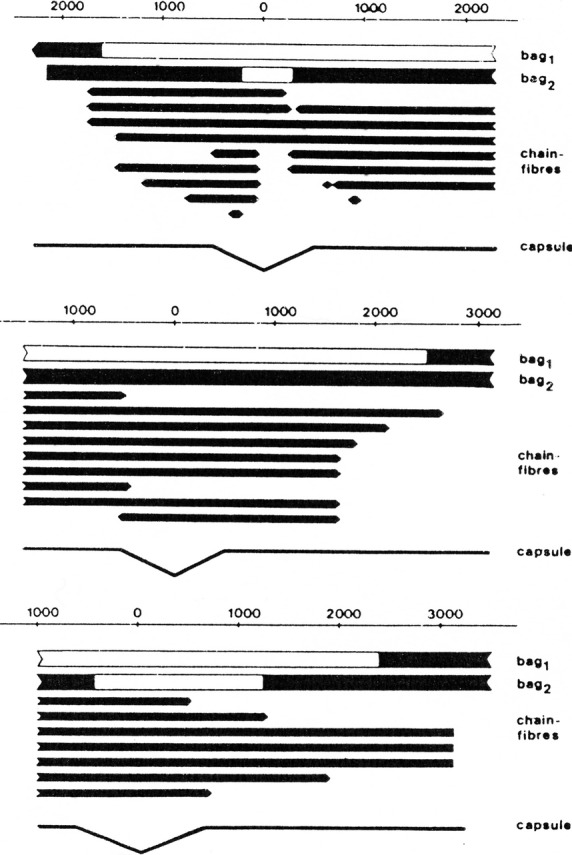
Schematic drawings depicting the organization of three serially sectioned human lumbrical spindles (1–3). Scale in millimeters, with centre at equator at zero. Two nuclear bag fibres with larger diameters together with variable numbers of nuclear chain fibres with small diameters were present in each spindle. Solid areas mark the regions of fibres where M-bands were present. Note the variable length of M-band absence inbetween the three bag1 fibres of the different spindles. Likewise, bag2 fibres showed marked differences – one fibre had a short segment without M-band, the second spindle had a bag2 fibre with presence of M-band along its whole length, whereas the third spindle had quite a long region showing no M-band. From Thornell et al. (1995).
Fig 16.
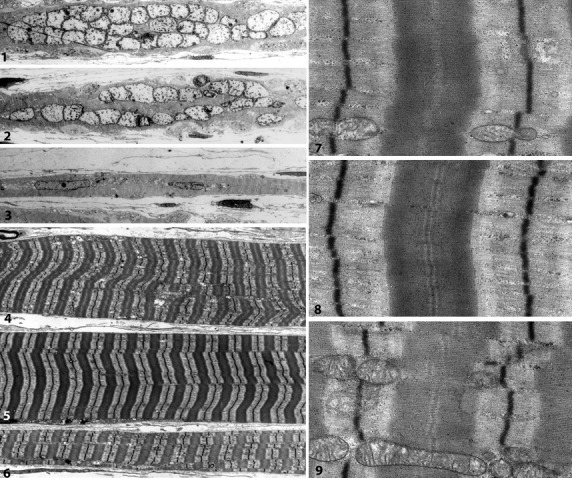
Electron microscopy of longitudinally sectioned intrafusal fibres of a human lumbrical muscle spindle. (1–3) Low magnification of the equatorial region of bag1 (1), bag2 (2) and chain (3) fibres. (4–6) Corresponding fibres in the polar region. Note the difference in fibre size and density/appearance of myofibrils. (7–9) A sarcomere in higher magnification from corresponding myofibrils and fibres in (4–6). The bag1 fibre in (7) lacks a dense M-band and shows an irregular border between the A- and I-band. In the bag2 fibre (8) and the chain fibre (9), a dense M-band is apparent in the centre of the A-band and the A/I-borders of each myofibril show a distinct shift in density (L Carlsson and LE Thornell, unpublished).
Fig 17.
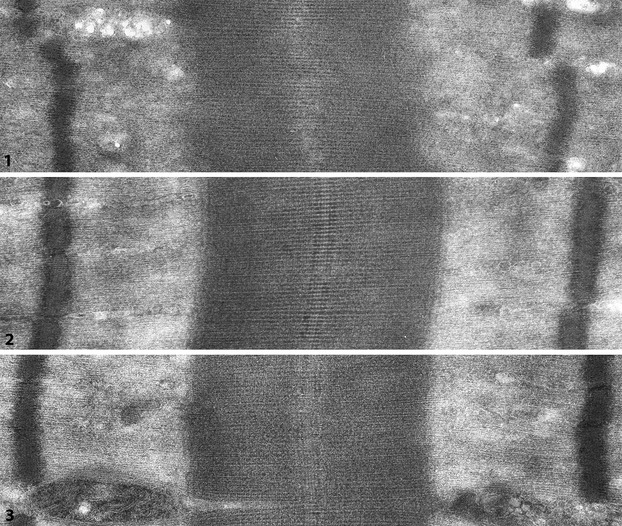
Longitudinally sectioned sarcomeres in ultrathin cryosections of intrafusal fibres from human lumbrical spindle. The sections are negatively stained with uranyl acetate, thereby proteins and filaments are seen in reversed contrast, i.e. structures/molecules are white against a dark background allowing high-resolution images. (1) Sarcomere of bag1 fibre – no M-bridges/striations appear between the thick filaments in the middle of the A-band (M-region) Note also the diffuse/irregular borders at the A/I-junction. (2 and 3) Sarcomeres with M-bridges between the thick filaments in the centre of the A-bands. The densities of the three central lines are most distinct, however, at places an additional two lines can be recognized (L Carlsson and LE Thornell, unpublished).
Molecular composition of the extra- and intra-sarcomeric cytoskeleton in relation to M-band function as a mechanical sensor with elastic properties
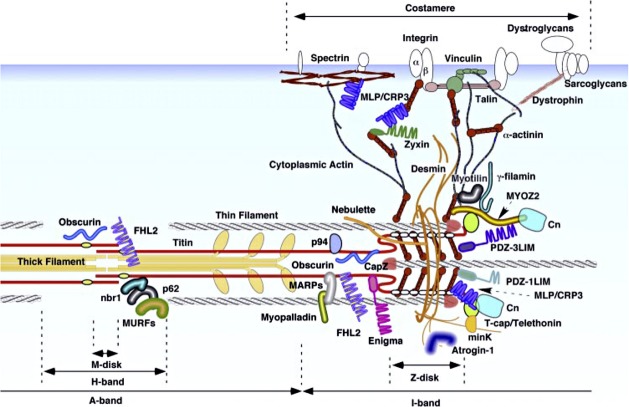
In parallel with the increased knowledge about the myofibrillar M-band, it became evident that muscle fibres, including intrafusal fibres (Eriksson et al. 1988), contained a cytoskeletal lattice of extra- and intra-sarcomeric filaments (Small et al. 1992). Intermediate filaments, composed of desmin, interlinked the myofibrillar Z-disc in cross-register, and anchored them to the sarcolemma, nuclei and mitochondria. Intra-sarcomeric filaments, composed of titin, spanned half a sarcomere from the Z-disc to the M-band. cDNA cloning determined that titin, myomesin and M-protein all belonged to a new family of proteins that are primarily composed of two motifs: immunoglobulin-like domains and fibronectin-like domains. The molecular layout of defined domains of titin, myomesin and M-protein resulted in the first molecular model of the M-band (Fig.18; Obermann et al. 1997). Since then a tremendous amount of detailed information has accumulated regarding the molecular organization of the extra- and intra-sarcomeric cytoskeleton (Fig.19), as well as on related genes, their sequences, disease-related mutations and isoforms. They function as a dynamic information switchboard that communicates between the contractile machinery and the nucleus then to central pathways controlling cell survival, protein breakdown, gene expression and extracellular signalling (Agarkova & Perriard, 2005; Lange et al. 2005; Schoenauer et al. 2008; Gautel, 2011a,b; Tskhovrebova & Trinick, 2012; Xiao & Grater, 2014). Extended molecular models of the M-band have been proposed (Fig.20), but none has so far been able to account for the variability in M-band appearance at the ultrastructural level. Nevertheless, the current knowledge implies that the M-band is ideally placed as a strain sensor (Tskhovrebova & Trinick, 2008, 2012; Gautel, 2011a,b; Linke & Hamdani, 2014). Furthermore, the realization that the M-band is elastic and serves a signalling function opens up also the possibility that M-band strain might also translate into modulation of metabolic activity, in addition to protein turnover and transcription regulation, and thereby regulate short-term adaptation of muscle to strain. A mechanosensitive signalling complex (interactome/signalsome) has been identified that interacts with an open conformation of titinkinase (TK), and by controlling both protein turnover and muscle gene transcription, seems to contribute to the adaptation of muscle in response to changes in mechanical strain (Gautel, 2011a,b; Linke & Hamdani, 2014). The intra-sarcomeric cytoskeleton of titin and its connection to the Z-disc, I-band and M-band is a hot spot of research, as seen from the number of publications and reviews recently published (e.g. Meyer & Wright, 2013; Linke & Hamdani, 2014; Chauveau et al. 2014; Kotter et al. 2014; Yin et al. 2015). Unfortunately, studies on the extra- and intra-sarcomeric cytoskeletal organization in muscle spindle fibres are lagging behind. Extrapolating the impact of the M-band signals on extrafusal fibres and cardiomyocytes to muscle spindle function, the special organization of the M-band in bag and chain fibres and along the length of bag fibres must have significance for the function of the different types of intrafusal fibres. At the moment one can only speculate how the detailed variations in MyHC expression in intrafusal fibres, in combination with M-band diversity in structure and composition, influence the special tasks of the muscle spindle. More precise information regarding the mechanism(s) ensuring the differential regulation of cytoskeletal proteins and myosin isoforms in the different regions and types of intrafusal fibres will serve as the backbone for further molecular modelling of neuronal interactions and mechanical factors for muscle spindle function.
Fig 18.
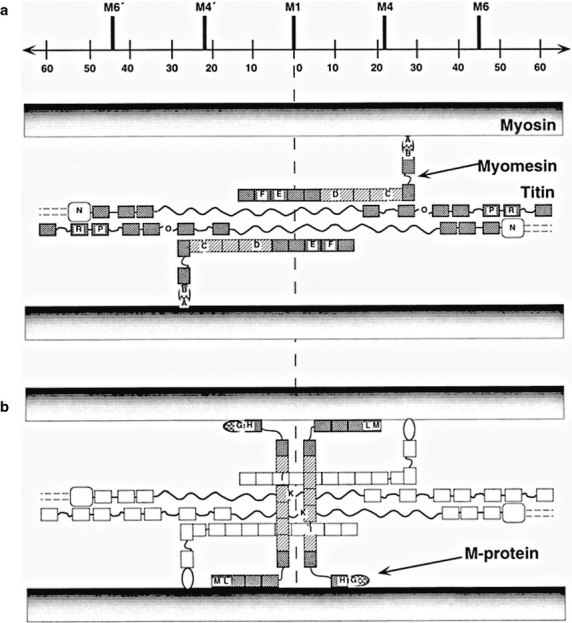
Schematic presentation of the arrangement of titin, myomesin and M-protein in the M-band compatible with the immunoelectronmicroscopical results (Obermann et al. 1996) At the top of the diagram, the positions of the most prominent M-band striations as defined by Sjostrom & Squire (1977) and a scale bar are indicated. (a) Summarized layout of titin and myomesin deduced from labelling data. Crosshatched and shaded boxes reflect Ig and Fn-domains, respectively. (b) In addition to structures shown in (a), the proposed location of M-protein around the M1-line is highlighted. From Obermann et al. (1996) with permission.
Fig 19.
Overview of cytoskeletal structure-associated proteins at the M-band and the Z-disk: for further details, see Hoshijima (2006). Reproduced with permission.
Fig 20.
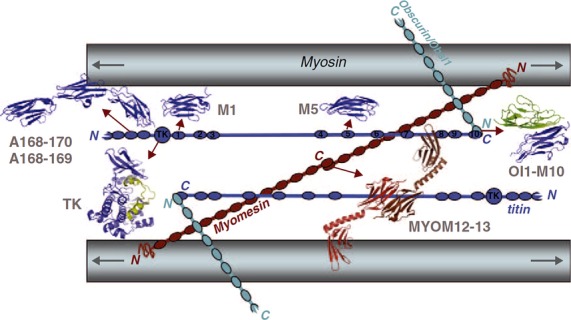
Schematic representation of the M-band formed by the bare zone of myosin filaments, titin, myomesin and obscurin/obsl1. Myosin filaments are crosslinked by antiparallel dimers of myomesin (MYOM12–13), which are linked in a ternary complex with obscurin/obsl1, which in turns binds the C-terminal domain of titin (OL1-M10). Structures for several individual and multi-domain titin domains have also been solved (A168–170, A168–A169, M1, M5). The catalytic Ser/Thr kinase domain of titin (TK) is situated at the M-band periphery. Although this model agrees with known interactions and ultrastructural locations of individual protein domains, alternative paths for titin are also compatible, and the conformation of large interdomain linkers (represented as simple lines) is yet unknown. Titin domains are shown in blue and are numbered; subunits of the myomesin dimer in shades of red. Arrows denote the bipolarity of the myosin filament and point towards the antiparallel motor domain arrays in the A-band. From Gautel (2011b) with permission.
However, one should bear in mind that for human muscles each has its own composition of muscle spindles, and each spindle has its special set of intrafusal fibres, indicating unique morphological and physiological characteristics. Structural complexity of the human muscle spindle system may fit well with its diverse functional roles in control of posture and locomotion, timing of locomotor phases, synergy formation, plasticity and motor learning, and to act as forward sensory models, i.e. they are affected both by the current state of their parent muscle and by efferent (fusimotor) control, and their discharges represent future kinematic states (Windhorst, 2007, 2008; Dimitriou & Edin, 2010; Dimitriou, 2014).
Acknowledgments
The authors thank Drs Anthea Rowlerson and Tomas Soukup for many helpful comments on the manuscript. Financial support from the Swedish Research Council (K2012-63x-20399-06-3; Stockholm, Sweden), Magnus Bergvall Foundation, Swedish National Centre for Research in Sports and Medical Faculty of Umeå University is acknowledged.
Conflict of interest
No conflict of interests to be declared.
References
- Agarkova I, Perriard JC. The M-band: an elastic web that crosslinks thick filaments in the center of the sarcomere. Trends Cell Biol. 2005;15:477–485. doi: 10.1016/j.tcb.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Agarkova I, Schoenauer R, Ehler E, et al. The molecular composition of the sarcomeric M-band correlates with muscle fiber type. Eur J Cell Biol. 2004;83:193–204. doi: 10.1078/0171-9335-00383. [DOI] [PubMed] [Google Scholar]
- Andersson-Cedergren E, Karlsson U, Sjöström M. Frog muscle spindle at different functional conditions. In: Kakulas BA, editor. Basic Research in Myology. Amsterdam: Excerpta Medica; 1973. pp. 32–40. [Google Scholar]
- Andersen JL, Weiss A, Sandri C, et al. The 2B myosin heavy chain gene is expressed in human skeletal muscle. J Physiol. 2000;539(Suppl):29P–30P. [Google Scholar]
- Banks RW, Barker D. The muscle spindle. In: Engel AndrewE., editor. Myology. 3rd. New York: McGraw-Hill; 2004. pp. 489–509. Clara Franzini-Armstrong. [Google Scholar]
- Banks RW, Barker D, Harker DW, et al. Correlation between ultrastructure and histochemistry of mammalian intrafusal fibres. J Physiol. 1975;252:16–17. [PMC free article] [PubMed] [Google Scholar]
- Banks RW, Harker DW, Stacey MJ. A study of mammalian intrafusal muscle fibres using a combined histochemical and ultrastructural technique. J Anat. 1977;123:783–796. [PMC free article] [PubMed] [Google Scholar]
- Barbet JP, Thornell LE, Butler-Browne GS. Immunocytochemical characterisation of two generations of fibers during the development of the human quadriceps muscle. Mech Dev. 1991;35:3–11. doi: 10.1016/0925-4773(91)90036-6. [DOI] [PubMed] [Google Scholar]
- Barker D, Banks RW, Harker DW, et al. Studies of the histochemistry, ultrastructure, motor innervation, and regeneration of mammalian intrafusal muscle fibres. Prog Brain Res. 1976;44:67–88. doi: 10.1016/s0079-6123(08)60724-4. [DOI] [PubMed] [Google Scholar]
- Bormioli SP, Sartore S, Vitadello M, et al. ‘Slow’ myosins in vertebrate skeletal muscle. An immunofluorescence study. J Cell Biol. 1980;85:672–681. doi: 10.1083/jcb.85.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd IA. The mechanical properties of dynamic nuclear bag fibres, static nuclear bag fibres and nuclear chain fibres in isolated cat muscle spindles. Prog Brain Res. 1976a;44:33–50. [PubMed] [Google Scholar]
- Boyd IA. The response of fast and slow nuclear bag fibres and nuclear chain fibres in isolated cat muscle spindles to fusimotor stimulation, and the effect of intrafusal contraction on the sensory endings. Q J Exp Physiol Cogn Med Sci. 1976b;61:203–254. doi: 10.1113/expphysiol.1976.sp002354. [DOI] [PubMed] [Google Scholar]
- Boyd IA. The muscle spindle controversy. Sci Prog. 1981;67:205–221. [PubMed] [Google Scholar]
- Boyd IA, Gladden MH, McWilliam PN, et al. Control of dynamic and static nuclear bag fibres and nuclear chain fibres by gamma and beta axons in isolated cat muscle spindels. J Physiol. 1977;265:133–162. doi: 10.1113/jphysiol.1977.sp011709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler-Browne GS, Barbet JP, Thornell LE. Myosin heavy and light chain expression during human skeletal muscle development and precocious muscle maturation induced by thyroid hormone. Anat Embryol. 1990;181:513–522. doi: 10.1007/BF00174624. [DOI] [PubMed] [Google Scholar]
- Butler-Browne GS, Eriksson PO, Laurent C, Thornell LE. Adult human masseter muscle fibers express myosin isozymes characteristic of development. Muscle Nerve. 1988;11:610–20. doi: 10.1002/mus.880110614. [DOI] [PubMed] [Google Scholar]
- Carlsson E, Thornell LE. Diversification of the myofibrillar M-band in rat skeletal muscle during postnatal development. Cell Tissue Res. 1987;248:169–180. doi: 10.1007/BF01239978. [DOI] [PubMed] [Google Scholar]
- Carlsson E, Grove BK, Wallimann T, et al. Myofibrillar M-band proteins in rat skeletal muscles during development. Histochemistry. 1990;95:27–35. doi: 10.1007/BF00737225. [DOI] [PubMed] [Google Scholar]
- Chauveau C, Rowell J, Ferreiro A. A rising titan: TTN review and mutation update. Hum Mutat. 2014;35:1046–1059. doi: 10.1002/humu.22611. [DOI] [PubMed] [Google Scholar]
- Cooper S, Daniel PM. Human muscle spindles. J Physiol. 1956;133:1–3. [PubMed] [Google Scholar]
- Dimitriou M. Human muscle spindle sensitivity reflects the balance of activity between antagonistic muscles. J Neurosci. 2014;34:13 644–13 655. doi: 10.1523/JNEUROSCI.2611-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriou M, Edin BB. Human muscle spindles act as forward sensory models. Curr Biol. 2010;20:1763–1767. doi: 10.1016/j.cub.2010.08.049. [DOI] [PubMed] [Google Scholar]
- Eriksson PO, Thornell LE. Variation in histochemical enzyme profile and diameter along human masseter intrafusal muscle fibers. Anat Rec. 1990;226:168–176. doi: 10.1002/ar.1092260206. [DOI] [PubMed] [Google Scholar]
- Eriksson PO, Butler-Browne G, Schiaffino S, et al. Distribution of myofibrillar and cytoskeletal proteins in human muscle spindles. Immunohistochemical studies. Muscle Nerve. 1986;9:225. [Google Scholar]
- Eriksson PO, Butler-Browne G, Fischman DA, et al. Myofibrillar and cytoskeletal proteins in human muscle spindles. In: Hník P, Soukup T, Vejsala R, Zelená J, editors. Mechanoreceptors, Development, Structure, and Function. New York: Plenum Press; 1988. pp. 273–274. [Google Scholar]
- Eriksson PO, Butler-Browne GS, Thornell LE. Immunohistochemical characterization of human masseter muscle spindles. Muscle Nerve. 1994;17:31–41. doi: 10.1002/mus.880170105. [DOI] [PubMed] [Google Scholar]
- Gautel M. Cytoskeletal protein kinases: titin and its relations in mechanosensing. Pflugers Arch. 2011a;462:119–134. doi: 10.1007/s00424-011-0946-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautel M. The sarcomeric cytoskeleton: who picks up the strain? Curr Opin Cell Biol. 2011b;23:39–46. doi: 10.1016/j.ceb.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Grove BK, Thornell LE. Noncoordinate expression of M band proteins in slow and fast embryonic chick muscles. Muscle Nerve. 1988;11:645–653. doi: 10.1002/mus.880110618. [DOI] [PubMed] [Google Scholar]
- Grove BK, Kurer V, Lehner C, et al. A new 185,000-dalton skeletal muscle protein detected by monoclonal antibodies. J Cell Biol. 1984;98:518–524. doi: 10.1083/jcb.98.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove BK, Holmbom B, Thornell LE. Myomesin and M protein: differential expression in embryonic fibers during pectoral muscle development. Differentiation. 1987;34:106–114. doi: 10.1111/j.1432-0436.1987.tb00056.x. [DOI] [PubMed] [Google Scholar]
- Harrison BC, Allen DL, Leinwand LA. IIb or not IIb? Regulation of myosin heavy chain gene expression in mice and men. Skelet Muscle. 2011;1:5. doi: 10.1186/2044-5040-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoh JF. Laryngeal muscle fibre types. Acta Physiol Scand. 2005;183:133–149. doi: 10.1111/j.1365-201X.2004.01402.x. [DOI] [PubMed] [Google Scholar]
- Hoshijima M. Mechanical stress-strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures. Am J Physiol Heart Circ Physiol. 2006;290:H1313–25. doi: 10.1152/ajpheart.00816.2005. . Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson U, Andersson-Cedergren E, Ottoson D. Cellular organisation of the frog muscle spindle as revealed by serial sections for electron microscopy. J Ultrastructure Research. 1966;14:1–35. doi: 10.1016/s0022-5320(66)80045-x. [DOI] [PubMed] [Google Scholar]
- Kjellgren D, Thornell LE, Andersen J, et al. Myosin heavy chain isoforms in human extraocular muscles. Invest Ophthalmol Vis Sci. 2003;44:1419–1425. doi: 10.1167/iovs.02-0638. [DOI] [PubMed] [Google Scholar]
- Kotter S, Andresen C, Kruger M. Titin: central player of hypertrophic signaling and sarcomeric protein quality control. Biol Chem. 2014;395:1341–1352. doi: 10.1515/hsz-2014-0178. [DOI] [PubMed] [Google Scholar]
- Kucera J, Dorovini-Zis K. Types of human intrafusal muscle fibers. Muscle Nerve. 1979;2:437–451. doi: 10.1002/mus.880020605. [DOI] [PubMed] [Google Scholar]
- Kucera J, Walro JM, Reichler J. Role of nerve and muscle factors in the development of rat muscle spindles. Am J Anat. 1989;186:144–60. doi: 10.1002/aja.1001860205. [DOI] [PubMed] [Google Scholar]
- Lange S, Himmel M, Auerbach D, et al. Dimerisation of myomesin: implications for the structure of the sarcomeric M-band. J Mol Biol. 2005;345:289–298. doi: 10.1016/j.jmb.2004.10.040. [DOI] [PubMed] [Google Scholar]
- Linke WA, Hamdani N. Gigantic business: titin properties and function through thick and thin. Circ Res. 2014;114:1052–1068. doi: 10.1161/CIRCRESAHA.114.301286. [DOI] [PubMed] [Google Scholar]
- Liu J-X. Umeå University Medical Dissertations New Series no 928. 2004. Human muscle spindles; complex morphology and structural organization. In: Umeå University.
- Liu JX, Eriksson PO, Thornell LE, et al. Myosin heavy chain composition of muscle spindles in human biceps brachii. J Histochem Cytochem. 2002;50:171–183. doi: 10.1177/002215540205000205. [DOI] [PubMed] [Google Scholar]
- Liu JX, Thornell LE, Pedrosa-Domellof F. Muscle spindles in the deep muscles of the human neck: a morphological and immunocytochemical study. J Histochem Cytochem. 2003;51:175–186. doi: 10.1177/002215540305100206. [DOI] [PubMed] [Google Scholar]
- Liu JX, Eriksson PO, Thornell LE, et al. Fiber content and myosin heavy chain composition of muscle spindles in aged human biceps brachii. J Histochem Cytochem. 2005;53:445–454. doi: 10.1369/jhc.4A6257.2005. [DOI] [PubMed] [Google Scholar]
- Maier A, Gambke B, Pette D. Immunohistochemical demonstration of embryonic myosin heavy chains in adult mammalian intrafusal fibers. Histochemistry. 1988;88:267–71. doi: 10.1007/BF00570283. [DOI] [PubMed] [Google Scholar]
- Masaki T, Takaiti O. M-protein. J Biochem. 1974;75:367–380. doi: 10.1093/oxfordjournals.jbchem.a130403. [DOI] [PubMed] [Google Scholar]
- Matthews PB. The differentiation of two types of fusimotor fibre by their effects on the dynamic response of muscle spindle primary endings. Q J Exp Physiol Cogn Med Sci. 1962;47:324–333. doi: 10.1113/expphysiol.1962.sp001616. [DOI] [PubMed] [Google Scholar]
- Matthews PB. Muscle spindles and their motor control. Physiol Rev. 1964;44:219–288. doi: 10.1152/physrev.1964.44.2.219. [DOI] [PubMed] [Google Scholar]
- Matthews PB. Evolving views on the internal operation and functional role of the muscle spindle. J Physiol. 1981;320:1–30. doi: 10.1113/jphysiol.1981.sp013931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer LC, Wright NT. Structure of giant muscle proteins. Front Physiol. 2013;4:368. doi: 10.3389/fphys.2013.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milburn A. Stages in the development of cat muscle spindles. J Embryol Exp Morphol. 1984;82:177–216. [PubMed] [Google Scholar]
- Obermann WM, Gautel M, Weber K, et al. Molecular structure of the sarcomeric M band: mapping of titin and myosin binding domains in myomesin and the identification of a potential regulatory phosphorylation site in myomesin. EMBO J. 1997;16:211–220. doi: 10.1093/emboj/16.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermann WM, Gautel M, Steiner F, van der Ven PF, Weber K, Furst DO. The structure of the sarcomeric M band: localization of defined domains of myomesin, M-protein, and the 250-kD carboxy-terminal region of titin by immunoelectron microscopy. J Cell Biol. 1996;134:1441–53. doi: 10.1083/jcb.134.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Österlund C. Umeå University Odontological Dissertations, Series No 120. 2011. Extra- and intrafusal muscle fibre type compositions of the human masseter at young age. In perspective of growth and functional maturation of the jaw-face motor system. In: Umeå University.
- Österlund C, Liu JX, Thornell LE, et al. Muscle spindle composition and distribution in human young masseter and biceps brachii muscles reveal early growth and maturation. Anat Rec (Hoboken) 2011;294:683–693. doi: 10.1002/ar.21347. [DOI] [PubMed] [Google Scholar]
- Österlund C, Liu JX, Thornell LE, et al. Intrafusal myosin heavy chain expression of human masseter and biceps muscles at young age shows fundamental similarities but also marked differences. Histochem Cell Biol. 2013;139:895–907. doi: 10.1007/s00418-012-1072-7. [DOI] [PubMed] [Google Scholar]
- Ovalle WK, Smith RS. Histochemical identification of three types of intrafusal muscle fibers in the cat and monkey based on the myosin ATPase reaction. Can J Physiol Pharmacol. 1972;50:195–202. doi: 10.1139/y72-030. [DOI] [PubMed] [Google Scholar]
- Page S, Miller JB, DiMario JX, et al. Developmentally regulated expression of three slow isoforms of myosin heavy chain: diversity among the first fibers to form in avian muscle. Dev Biol. 1992;154:118–128. doi: 10.1016/0012-1606(92)90053-j. [DOI] [PubMed] [Google Scholar]
- Pedrosa F, Thornell LE. Expression of myosin heavy chain isoforms in developing rat muscle spindles. Histochemistry. 1990;94:231–244. doi: 10.1007/BF00266623. [DOI] [PubMed] [Google Scholar]
- Pedrosa F, Butler-Browne GS, Dhoot GK, et al. Diversity in expression of myosin heavy chain isoforms and M-band proteins in rat muscle spindles. Histochemistry. 1989;92:185–194. doi: 10.1007/BF00500917. [DOI] [PubMed] [Google Scholar]
- Pedrosa F, Soukup T, Thornell LE. Expression of an alpha cardiac-like myosin heavy chain in muscle spindle fibres. Histochemistry. 1990;95:105–113. doi: 10.1007/BF00266582. [DOI] [PubMed] [Google Scholar]
- Pedrosa-Domellöf F. Umeå University Medical Dissertations New Series No 304. 1991. Expression of myosin heavy chain isoforms in rat muscle spindles: an immunocytochemical study. In: Umeå University.
- Pedrosa-Domellof F, Thornell LE. Expression of myosin heavy chain isoforms in developing human muscle spindles. J Histochem Cytochem. 1994;42:77–88. doi: 10.1177/42.1.8263326. [DOI] [PubMed] [Google Scholar]
- Pedrosa-Domellof F, Soukup T, Thornell LE. Rat muscle spindle immunocytochemistry revisited. Histochemistry. 1991;96:327–338. doi: 10.1007/BF00271354. [DOI] [PubMed] [Google Scholar]
- Pedrosa-Domellof F, Gohlsch B, Thornell LE, et al. Electrophoretically defined myosin heavy chain patterns of single human muscle spindles. FEBS Lett. 1993;335:239–242. doi: 10.1016/0014-5793(93)80737-f. [DOI] [PubMed] [Google Scholar]
- Rossi AC, Mammucari C, Argentini C, et al. Two novel/ancient myosins in mammalian skeletal muscles: MYH14/7b and MYH15 are expressed in extraocular muscles and muscle spindles. J Physiol. 2010;588:353–364. doi: 10.1113/jphysiol.2009.181008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlerson A. Early type-differentiation of intrafusal fibers. In: Hník P, Soukup T, Vejsala R, Zelená J, editors. Mechanoreceptors, Development, Structure, and Function. New York: Plenum Press; 1988. pp. 45–50. [Google Scholar]
- Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91:1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Gorza L, Dones I, et al. Fetal myosin immunoreactivity in human dystrophic muscle. Muscle Nerve. 1986;9:51–58. doi: 10.1002/mus.880090108. [DOI] [PubMed] [Google Scholar]
- Schoenauer R, Lange S, Hirschy A, et al. Myomesin 3, a novel structural component of the M-band in striated muscle. J Mol Biol. 2008;376:338–351. doi: 10.1016/j.jmb.2007.11.048. [DOI] [PubMed] [Google Scholar]
- Sjostrom M, Squire JM. Fine structure of the A-band in cryo-sections. The structure of the A-band of human skeletal muscle fibres from ultra-thin cryo-sections negatively stained. J Mol Biol. 1977;109:49–68. doi: 10.1016/s0022-2836(77)80045-4. [DOI] [PubMed] [Google Scholar]
- Small JV, Furst DO, Thornell LE. The cytoskeletal lattice of muscle cells. Eur J Biochem. 1992;208:559–572. doi: 10.1111/j.1432-1033.1992.tb17220.x. [DOI] [PubMed] [Google Scholar]
- Smerdu V, Cvetko E. Myosin heavy chain-2b transcripts and isoform are expressed in human laryngeal muscles. Cells Tissues Organs. 2013;198:75–86. doi: 10.1159/000351293. [DOI] [PubMed] [Google Scholar]
- Smerdu V, Soukup T. Demonstration of myosin heavy chain isoforms in rat and humans: the specificity of seven available monoclonal antibodies used in immunohistochemical and immunoblotting methods. Eur J Histochem. 2008;52:179–190. doi: 10.4081/1210. [DOI] [PubMed] [Google Scholar]
- Smerdu V, Karsch-Mizrachi I, Campione M, et al. Type IIx myosin heavy chain transcripts are expressed in type IIb fibers of human skeletal muscle. Am J Physiol. 1994;267:C1723–C1728. doi: 10.1152/ajpcell.1994.267.6.C1723. [DOI] [PubMed] [Google Scholar]
- Soukup T, Jirmanova I. Regulation of myosin expression in developing and regenerating extrafusal and intrafusal muscle fibers with special emphasis on the role of thyroid hormones. Physiol Res. 2000;49:617–633. [PubMed] [Google Scholar]
- Soukup T, Pedrosa F, Thornell LE. Influence of neonatal motor denervation on expression of myosin heavy chain isoforms in rat muscle spindles. Histochemistry. 1990;94:245–256. doi: 10.1007/BF00266624. [DOI] [PubMed] [Google Scholar]
- Soukup T, Pedrosa-Domellof F, Thornell LE. Expression of myosin heavy chain isoforms and myogenesis of intrafusal fibres in rat muscle spindles. Microsc Res Tech. 1995;30:390–407. doi: 10.1002/jemt.1070300506. [DOI] [PubMed] [Google Scholar]
- Stal P, Eriksson PO, Schiaffino S, Butler-Browne GS, Thornell LE. Differences in myosin composition between human oro-facial, masticatory and limb muscles: enzyme-, immunohisto- and biochemical studies. J Muscle Res Cell Motil. 1994;15:517–34. doi: 10.1007/BF00121158. [DOI] [PubMed] [Google Scholar]
- Stockdale FE. Mechanisms of formation of muscle fiber types. Cell Struct Funct. 1997;22:37–43. doi: 10.1247/csf.22.37. [DOI] [PubMed] [Google Scholar]
- Stedman HH, Kozyak BW, Nelson A, et al. Myosin gene mutation correlates with anatomical changes in the human lineage. Nature. 2004;428:415–8. doi: 10.1038/nature02358. [DOI] [PubMed] [Google Scholar]
- Strehler EE, Carlsson E, Eppenberger HM, et al. Ultrastructural localization of M-band proteins in chicken breast muscle as revealed by combined immunocytochemistry and ultramicrotomy. J Mol Biol. 1983;166:141–158. doi: 10.1016/s0022-2836(83)80003-5. [DOI] [PubMed] [Google Scholar]
- Thornell LE, Sjöström M. The myofibrillar M-band in the cryo-section-analysis of section thickness. J Microsc. 1975;104:263–269. doi: 10.1111/j.1365-2818.1975.tb04024.x. [DOI] [PubMed] [Google Scholar]
- Thornell LE, Billeter R, Butler-Browne GS, et al. Development of fiber types in human fetal muscle. An immunocytochemical study. J Neurol Sci. 1984;66:107–115. doi: 10.1016/0022-510x(84)90146-1. [DOI] [PubMed] [Google Scholar]
- Thornell LE, Billeter R, Eriksson PO, Ringqvist M. Heterogeneous distribution of myosin in human masticatory muscle fibres as shown by immunocytochemistry. Arch Oral Biol. 1984b;29:1–5. doi: 10.1016/0003-9969(84)90034-7. [DOI] [PubMed] [Google Scholar]
- Thornell LE, Butler-Browne GS, Carlsson E, et al. Cryoultramicrotomy and immunocytochemistry in the analysis of muscle fine structure. Scan Electron Microsc. 1986;1986:1407–1418. (Pt 4) [PubMed] [Google Scholar]
- Thornell LE, Carlsson E, Kugelberg E, et al. Myofibrillar M-band structure and composition of physiologically defined rat motor units. Am J Physiol. 1987;253:C456–C468. doi: 10.1152/ajpcell.1987.253.3.C456. [DOI] [PubMed] [Google Scholar]
- Thornell LE, Eriksson PO, Fischman DA, et al. Human muscle spindle development. In: Hník P, Soukup T, Vejsala R, Zelená J, editors. Mechanoreceptors, Development, Structure, and Function. New York: Plenum Press; 1988. pp. 39–44. [Google Scholar]
- Thornell LE, Carlsson E, Pedrosa F. M-band structure and composition in relation to fiber types. In: Dirk Pette., editor. The Dynamic State of Muscle Fibers. University of Michigan: Walter de Gruyter; 1990. pp. 369–383. [Google Scholar]
- Thornell LE, Carlsson L, Fridén J. Ultrastructure of bag and chain fibres and 3-D reconstruction of the sensory innervation in human lumbrical spindles. In: Anthony Taylor, Margaret H, Rade Durbaba., editors. In Alpha and Gamma Motor Systems. New York: Plenum Press; 1995. pp. 222–229. [Google Scholar]
- Tskhovrebova L, Trinick J. Giant proteins: sensing tension with titin kinase. Curr Biol. 2008;18:R1141–R1142. doi: 10.1016/j.cub.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Tskhovrebova L, Trinick J. Making muscle elastic: the structural basis of myomesin stretching. PLoS Biol. 2012;10:e1001264. doi: 10.1371/journal.pbio.1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DC, Wallimann T, Eppenberger HM. A protein that binds specifically to the M-line of skeletal muscle is identified as the muscle form of creatine kinase. Proc Natl Acad Sci USA. 1973;70:702–705. doi: 10.1073/pnas.70.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss H. Tabelle der abosluten und relativen Muskelspindelzahlen der menschlichen Skelettmuskulatur. Anat Anz. 1971;129:562–572. [PubMed] [Google Scholar]
- Wallimann T, Kuhn HJ, Pelloni G, et al. Localization of creatine kinase isoenzymes in myofibrils. II. Chicken heart muscle. J Cell Biol. 1977a;75:318–325. doi: 10.1083/jcb.75.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallimann T, Turner DC, Eppenberger HM. Localization of creatine kinase isoenzymes in myofibrils. I. Chicken skeletal muscle. J Cell Biol. 1977b;75:297–317. doi: 10.1083/jcb.75.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walro JM, Kucera J. Why adult mammalian intrafusal and extrafusal fibers contain different myosin heavy-chain isoforms. Trends Neurosci. 1999;22:180–184. doi: 10.1016/s0166-2236(98)01339-3. [DOI] [PubMed] [Google Scholar]
- Windhorst U. Muscle proprioceptive feedback and spinal networks. Brain Res Bull. 2007;73:155–202. doi: 10.1016/j.brainresbull.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Windhorst U. Muscle spindles are multi-functional. Brain Res Bull. 2008;75:507–508. doi: 10.1016/j.brainresbull.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Xiao S, Grater F. Molecular basis of the mechanical hierarchy in myomesin dimers for sarcomere integrity. Biophys J. 2014;107:965–973. doi: 10.1016/j.bpj.2014.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Ren J, Guo W. Sarcomeric protein isoform transitions in cardiac muscle: a journey to heart failure. Biochim Biophys Acta. 2015;1852:47–52. doi: 10.1016/j.bbadis.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelená J. Nerves and Mechanoreceptors. London: Chapman & Hall; 1994. [Google Scholar]