Abstract
Signals from sensory receptors in muscles and skin enter the central nervous system (CNS), where they contribute to kinaesthesia and the generation of motor commands. Many lines of evidence indicate that sensory input from skin receptors, muscle spindles and Golgi tendon organs play the predominant role in this regard. Yet in spite of over 100 years of research on this topic, some quite fundamental questions remain unresolved. How does the CNS choose to use the ability to control muscle spindle sensitivity during voluntary movements? Do spinal reflexes contribute usefully to load compensation, given that the feedback gain must be quite low to avoid instability? To what extent do signals from skin stretch receptors contribute? This article provides a brief review of various theories, past and present, that address these questions. To what extent has the knowledge gained resulted in clinical applications? Muscles paralyzed as a result of spinal cord injury or stroke can be activated by electrical stimulation delivered by neuroprostheses. In practice, at most two or three sensors can be deployed on the human body, providing only a small fraction of the information supplied by the tens of thousands of sensory receptors in animals. Most of the neuroprostheses developed so far do not provide continuous feedback control. Instead, they switch from one state to another when signals from their one or two sensors meet pre-set thresholds (finite state control). The inherent springiness of electrically activated muscle provides a crucial form of feedback control that helps smooth the resulting movements. In spite of the dissimilarities, parallels can be found between feedback control in neuroprostheses and in animals and this can provide surprising insights in both directions.
Keywords: feedback control of movement, muscle receptors, muscle spindles, neuroprostheses, sensorimotor control, tendon organs
Introduction
Several reviews have described in detail the anatomy and physiology of sensory receptors implicated in the control of bodily movement (Matthews, 1972; Hulliger, 1984), the sense of movement (kinaesthesia) (Proske & Gandevia, 2009, 2012) and the underlying neuronal mechanism [proprioception: sensing the body’s own movements (Sherrington, 1907)]. This article reviews the nature of the sensory signals, the ways in which they might be used by the central nervous system (CNS) and the application of this knowledge to neuroprosthetic devices.
The mammalian body contains thousands of sensory receptors. The human upper limb contains about 4000 muscle spindles, 2500 Golgi tendon organs and a few hundred joint receptors (Barker et al. 1962; Voss, 1971; Hulliger, 1984). Sensory receptors in skin are even more numerous and varied. For example, the surface of the human hand alone has about 17 000 myelinated cutaneous afferents (Johansson & Vallbo, 1979).
Sensory receptors involved in motor control
Muscle spindles
The mean number of spindles in a muscle is roughly 38 * (cube root of mass in grams) (Banks & Stacey, 1988). Thus a 125-g muscle contains about 190 spindles. Spindles are attached at each end to the surrounding extrafusal muscle fibres that produce the forces for bodily movement. Much smaller intrafusal muscle fibres within the muscle spindle don’t contribute measurably to these forces, their role being to control the stretch-sensitivity and baseline activity (bias or offset) of the spindle’s afferents. They do this by pulling on the central non-contractile region of the spindle that contains the stretch-sensitive spindle primary (group Ia) afferents and secondary (group II) afferents. Intrafusal muscle fibres are activated by fusimotor neurons, also called γ-motoneurons by virtue of their conduction velocity. Some α-motoneurons send branches to intrafusal muscle and these are called β-motoneurons. Details of intrafusal and fusimotor subdivisions and their effect on spindle afferent firing are summarized in Fig.1 and in the associated animation (angeltear.com/spindle/spindle.html). Briefly, static fusimotor neurons (γs-motoneurons) increase the firing rate of both spindle primary (group Ia) and secondary (group II) afferents in the absence of a length change of the receptor-bearing muscle (biasing action). This can keep Ia and II afferents firing during rapid muscle shortening. The sensitivity of Ia and II afferents to muscle length changes is increased by γd and γs action, respectively.
Fig 1.
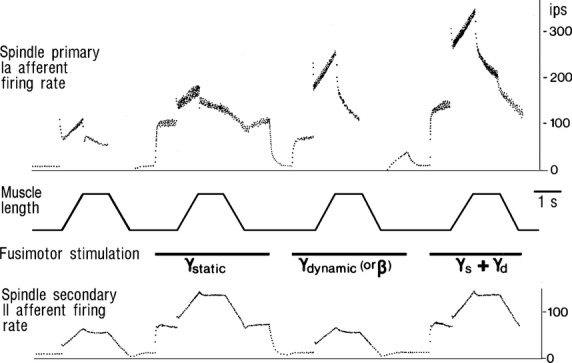
Firing rate responses of group Ia and II spindle afferents to trapezoidal length changes with and without concomitant fusimotor stimulation. The firing rates are typical of displacements of about 10% of muscle rest-length and velocities of 0.05 rest length per second. Horizontal bars indicate periods of fusimotor firing. Note the increase in Ia stretch responses with γd stimulation and the biasing effect of γs stimulation (ips: impulses per second) (Prochazka, 1996).
There has been much debate on the nature of fusimotor activity during voluntary movement. The main barrier has been the difficulty in recording from the small axons of γ-motoneurons. This is less of a problem in anaesthetized or decerebrate animals. Four main hypotheses have emerged. The first was the follow-up length servo hypothesis (Rossi, 1927; Merton, 1953), which proposed that in a voluntary muscle contraction, γ-motoneurons are activated first, increasing spindle afferent firing, which in turn reflexly activates α-motoneurons (Fig.2A). This theory was replaced by the servo-assistance hypothesis (Matthews, 1970; Fig.2B), which proposed that γ-motoneurons were co-activated with α-motoneurons, keeping spindle afferents firing during muscle shortening. It was further suggested that the biasing action of γ drive might exactly compensate for the shortening, except when this was impeded (Phillips, 1969). In this view, spindle afferents were misalignment detectors, sensing a disparity between expected and actual muscle length changes. Early recordings from spindle afferents in awake humans showed that in isometric contractions, spindle afferents did indeed increase their firing rates, which supported α–γ co-activation and the servo-assistance hypothesis (Vallbo, 1970), though it was arguably at odds with the idea of misalignment detection given that the subjects did not expect the contracting muscle to shorten.
Fig 2.
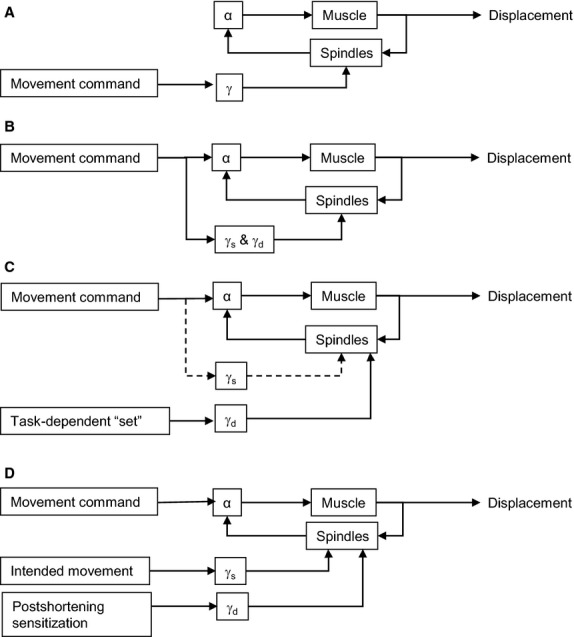
Four hypotheses of fusimotor control. (A) Follow-up length servo, (B) servo-assistance, (C) fusimotor set, (D) γ s: ‘temporal template’ of intended movement, γd: sensitizes Ia afferents to stretch following muscle shortening.
The plot thickened when it became possible in the 1970s to record from muscle afferents in freely moving animals. It was quickly noticed that spindle afferents did not fire steadily during unobstructed movements, as they should do if they were misalignment detectors. Rather, their firing rates were deeply modulated. In some cases the firing rates were closely related to the time course of muscle length and velocity (Cody & Taylor, 1973; Cody et al. 1975; Goodwin & Luschei, 1975). In other cases, increased spindle afferent firing during muscle shortening suggested strong α-linked γs activity (Loeb, 1981). In movements that were novel or very difficult, such as walking on a narrow beam, large increases in Ia stretch-sensitivity were observed in cats implanted with dorsal root electrodes. This led to the hypothesis of behaviour-related fusimotor set, whereby γ-motoneurons were activated independently of γ-motoneurons (Prochazka et al. 1985; Fig.2C). α–γ independence was subsequently supported in some studies (Roll & Vedel, 1982; Ribot et al. 1986) and refuted in others (Aniss et al. 1990). Evidence was adduced for specific combinations in flexor and extensor muscles: α–γs co-activation with independent γd activity (Gottlieb & Taylor, 1983; Taylor et al. 1993) and α–γd co-activation with independent γs activity (Perret & Buser, 1972; Cabelguen, 1981; Murphy et al. 1984; Bessou et al. 1986; Greer & Stein, 1990; Murphy & Martin, 1993). In an elegant set of studies in walking decerebrate cats Ellaway et al. (2015), presumed γs motoneurons fired throughout the step cycle, weakly modulated roughly in time with the phasic bursts of α-activity in the muscle they innervated, whereas γd motoneurons fired at high rates during α-activity and fell silent otherwise. Although this could have been interpreted as α–γd co-activation with partial independence of γs activity, other considerations, including the behaviour of spindle group II afferents, led to the hypothesis that γs firing was a temporal template of the expected movement (Fig.2D), while γd firing sensitized Ia afferents to muscle stretch following shortening (Ellaway et al. 2002; Taylor et al. 2006).
As mentioned, early spindle recordings in humans performing isometric contractions supported α–γ co-activation. Subsequent data collected during unimpeded movement indicated that spindle afferents were affected more by muscle length changes than by presumed α-linked γ-activity. To add to the confusion, Ia firing recently recorded during hand movements in humans performing keyboard tasks was not only correlated with muscle velocity and acceleration, but also with future movement (Dimitriou & Edin, 2008). It was proposed that fusimotor drive reflected internal predictive models (Wolpert & Miall, 1996; Dimitriou & Edin, 2010), an idea similar to that of the ‘temporal template of expected movement’ (Taylor et al. 2000). The most recent human work has refuted this idea, and instead has not only supported α–γ co-activation and a velocity-signalling role for Ia afferents, but also suggested a concomitant inhibition of antagonist γ-motoneurons (Dimitriou, 2014). Figure3, modified from that study, shows the responses of finger extensor Ia afferents in seven subjects performing voluntary flexion–extension movements of the fingers in the presence or absence of steady, externally imposed torques resisting or assisting extension. Torque-resisting extension elicited a compensatory increase in voluntary extensor EMG activity, particularly during the muscle shortening phases. This was correlated with an increase in bias (offset) and stretch-sensitivity of the extensor Ia responses compared with those in unloaded movements, which was interpreted by Dimitriou as evidence for α–γ linkage. Because the Ia firing rate was well correlated with muscle velocity throughout the cycle, with no obvious increase during the phasic increase in extensor EMG during muscle shortening, this could also have been interpreted as evidence of a tonic increase in fusimotor drive throughout the cycle, related to overall effort. Movements in the presence of imposed extensor torque were associated with elevated flexor EMG activity and this was correlated with a reduction in the extensor Ia responses in the early part of muscle stretch, suggesting reciprocal inhibition of extensor fusimotor drive. Interestingly, if we assume that the CNS decodes movement by taking into account Ia signals from antagonist and agonist muscle pairs, the reciprocal fusimotor effects proposed by Dimitriou would tend to cancel each other out.
Fig 3.
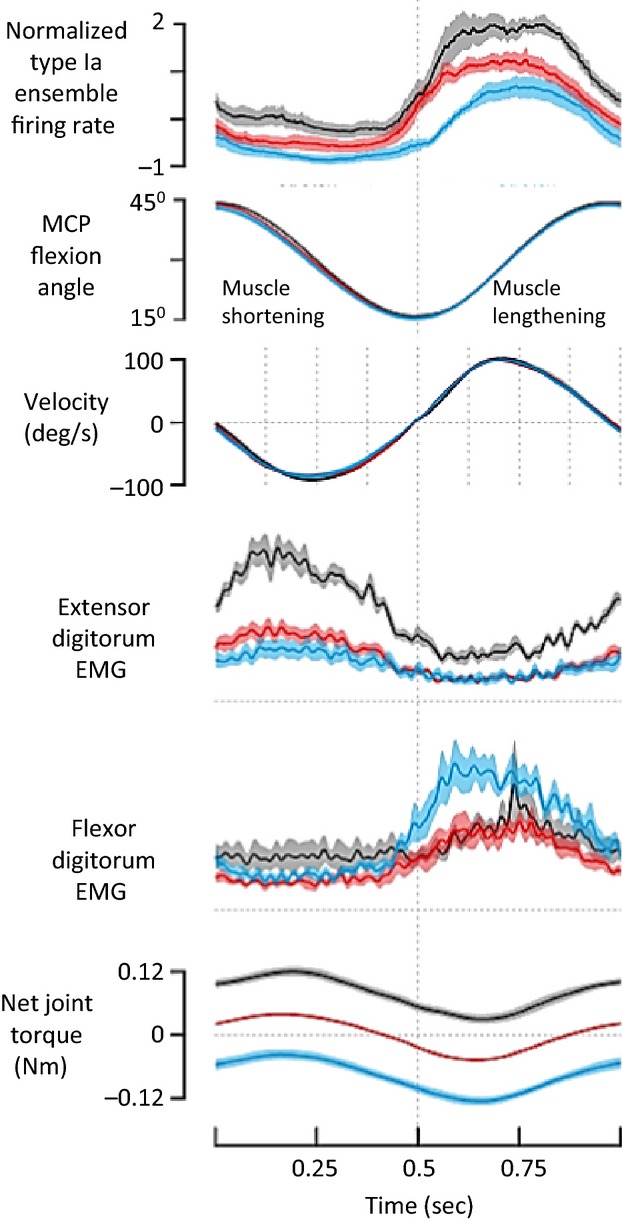
Mean normalized firing rates of nine extensor digitorum Ia afferents in seven subjects performing voluntary finger movement without loading (red) or in the presence of steady imposed flexor torque (black) or extensor torque (blue). Imposed flexor torque required a compensatory increase in extensor EMG activity, particularly during muscle shortening. This was correlated with an increase in the offset and stretch-sensitivity of extensor Ia firing compared with unloaded movements, consistent with elevated extensor fusimotor drive. Movements in the presence of imposed extensor torque (blue) required compensatory increases in flexor EMG. The associated reduction in extensor Ia firing in response to muscle stretch suggested reduced extensor fusimotor drive. Ia firing was well correlated with muscle velocity in all cases. Shaded areas represent +1 SEM. Modified with permission from Dimitriou (2014).
So what can we conclude about the role of the fusimotor system? First, there is much evidence that a component of γ-activity is linked in time and strength to the activation of α-motoneurons (Wilson et al. 1997). β-motoneurons are α-motoneurons with branches innervating intrafusal muscle fibres, so β-fusimotor drive would also be linked to α-activity. Secondly, there is a separate component of γ-activity that is independent of α-activity. On this view, some γ-motoneurons fire tonically throughout movement sequences, their firing rate being modulated in relation to behavioural set or to an internal model of the expected movement trajectory.
What is the final effect of the interaction of fusimotor drive and muscle length changes on muscle spindle firing? The simplest view, and the one favoured by the author and several other groups, is that spindle group Ia and II afferents are essentially velocity and length detectors whose sensitivity and bias are adjusted by fusimotor action according to the demands of the motor task. One caveat to this simple view is that tendons of phasically active muscles can absorb a significant portion of the length change occurring between the origin and insertion of the muscle, distorting the relationship between muscle length (or velocity) and spindle afferent firing (Griffiths, 1991; Maganaris & Paul, 1999; Lichtwark & Wilson, 2008; Magnusson et al. 2008). Nonetheless, the time course of limb joint angles during normal locomotion has been inferred reasonably accurately from the firing rates of a handful of afferents recorded with an implanted multi-electrode array. Analysis showed that it was the spindle afferents that provided the most information in this regard (Weber et al. 2006, 2007). From the perspective of mimicking muscle spindle feedback in a neuroprosthesis, the closest practical embodiments are length gauges or Hall effect sensors signalling joint rotation, whose signals are conditioned with variable-gain filters (see below) (Prochazka & Wiles, 1983; Prochazka et al. 1997a; Johnson et al. 1999).
Golgi tendon organs
Fortunately, there is much more agreement on the signalling properties of ensembles of Ib afferents from Golgi tendon organs. Unlike spindles, Golgi tendon organs do not have a mechanism to modulate their sensitivity. They respond to force actively generated by the motor units they monitor (Houk & Henneman, 1967; Goslow et al. 1973; Stephens & Stuart, 1974; Stephens et al. 1975). When the firing rates of several Ib afferents recorded during normal movements were summed, the net firing rate was closely related to whole-muscle force (Prochazka & Gorassini, 1998) as predicted in an earlier study (Crago et al. 1982). In a neuroprosthesis, the closest approximation to an ensemble of Golgi tendon organs would be a buckle transducer implanted on a tendon (Walmsley et al. 1978). This would be invasive and of limited lifespan, at least with current technology. Practical, though less direct, equivalents include force or pressure sensors attached externally to a limb, for example in the heel of a shoe (Burridge et al. 2001) or in the fingertips of a glove (Su et al. 2012).
Receptors in joints and skin
Early work suggested that joint receptors signalled joint angle over the full range of motion (Boyd & Roberts, 1953). This was challenged in studies suggesting that joint receptors only fired at the extremes of joint angle and not in the mid-range (Burgess & Clark, 1969; Tracey, 1979). Subsequent work showed that at least some joint afferents signalled the full range of motion (Godwin-Austen, 1969; Zalkind, 1971; Carli et al. 1979; Ferrell, 1980; Lund & Matthews, 1981; Ferrell et al. 1987), but it is possible that these afferents were from muscle receptors near the joints (McIntyre et al. 1978; Clark et al. 1985; Gregory et al. 1989). Loading of the joint capsule may be necessary to enable mid-range responsiveness (Grigg & Greenspan, 1977). Most joint afferents have weak polysynaptic connections with α-motoneurons (Johansson et al. 1991), their role possibly being to inhibit α-motoneurons when joints are damaged (Iles et al. 1990).
There are several kinds of skin receptors which respond to rapid hair deflection or skin stretch (Willis & Coggeshall, 1991). The skin receptors best suited to signal position are slowly adapting type II receptors which respond to stretching of the skin over and around a joint (Horch et al. 1977; Edin & Johansson, 1995; Edin, 2001, 2004). Type I cutaneous receptors respond more locally, fire less regularly and adapt more rapidly. My colleagues and I have recently been experimenting with artificial skin stretch sensors to control stimulation in a foot-drop neuroprosthesis (see below).
The role of sensory input to the CNS from muscle and skin receptors
Kinaesthesia
Muscle and skin afferents both contribute to kinaesthesia (Goodwin et al. 1972; McCloskey et al. 1983; Collins & Prochazka, 1996; Collins et al. 2005). This topic is covered in detail in a recent review (Proske & Gandevia, 2012) and in the article by Proske in this volume of the journal. The following section will focus on the role of muscle and skin sensory feedback in the control of movement.
Stretch reflexes
Active muscles resist length changes through their intrinsic stiffness, providing the equivalent of negative length feedback (Partridge, 1967). Stretching a muscle causes an increase in firing of its muscle spindle Ia afferents, which monosynaptically excite homonymous α-motoneurons, activating the muscle to resist the increase in length (Marsden et al. 1976; Matthews, 1986). This is also equivalent to negative length feedback. Golgi tendon organ Ib afferents respond to the increase in force caused by muscle stretch, particularly when the muscle is active. They reflexly activate interneurons that inhibit homonymous α-motoneurons, thereby resisting changes in force. This is equivalent to negative force feedback (Houk et al. 1970). Concomitant length and force feedback results in spring-like behaviour, the stiffness of which depends on the ratio of length to force feedback gains (Prochazka & Yakovenko, 2002).
Studies have indicated that the loop gain of negative force feedback may in general be quite low (Houk et al. 1970; Hoffer & Andreassen, 1981). Furthermore, during locomotion in the cat, homonymous Ib reflex action has been found to become excitatory (Conway et al. 1987; Pearson & Collins, 1993), which is equivalent to positive force feedback (Prochazka et al. 1997b; Geyer et al. 2003; Grey et al. 2007). In closed-loop control systems, positive feedback with a loop gain exceeding one is unstable. However, as muscles shorten, the increment in force they generate for a given increment in activation declines. Thus even if the loop gain of positive force feedback initially exceeds unity, as the muscle shortens, loop gain returns to unity and instability is avoided (Prochazka et al. 1997b). This makes positive force feedback mediated by Ib afferents a suitable mechanism to compensate for weight-bearing in the stance phase of locomotion.
Intrinsic muscle stiffness increases with increasing muscle activation, including activation resulting from reflex responses to sensory input. Intrinsic muscle stiffness also depends on biomechanical factors such as muscle length and velocity (Bennett, 1993, 1994; Bennett et al. 1994). In a classical study in the decerebrate cat, the force response to stretch of electrically activated muscles in the absence of stretch reflexes was compared with the response of naturally activated muscles with intact stretch reflexes (Nichols & Houk, 1976). In the electrically activated muscles, force rose rapidly for the first 50 ms but then suddenly declined. In contrast, in the reflexly active muscles, force increased throughout the stretch. It was concluded that reflex action compensated for the yielding that occurred with steady activation alone, thereby linearizing the response and increasing the net stiffness. In later human experiments, electrically activated muscles did not yield nearly as much and so the linearizing effect of stretch reflexes was not as marked (Sinkjaer et al. 1988). At medium activation levels, stretch reflexes increased muscle stiffness by up to 60%, in accordance with the decerebrate experiments, but surprisingly, at both low and high levels of activation the stretch reflex contributions dropped to zero.
It was originally assumed that the short-latency monosynaptic stretch reflex mediated by Ia afferents was important in controlling load-bearing during locomotion (Capaday & Stein, 1986). Sinkjaer and colleagues challenged this, instead proposing that spindle group II afferents were the main contributors (Sinkjaer et al. 2000; Grey et al. 2001). This has in turn been challenged in experiments which implicated short-latency positive force feedback mediated by Ib afferents (Grey et al. 2007; af Klint et al. 2010). In the cat, large, rapid, perturbations are required to elicit short-latency stretch reflexes during locomotion (Gorassini et al. 1994; Gritsenko et al. 2001). This led to some doubt as to the importance of short-latency stretch reflexes in load-bearing (Prochazka et al. 2002), but other work suggested that longer latency Ib-mediated stretch reflexes may contribute up to 35% of muscle activation during cat locomotion (Stein et al. 2000; Donelan & Pearson, 2004; Donelan et al. 2009).
Neuromechanical modelling has been employed to help clarify these partly contradictory results (Prochazka et al. 2002; Yakovenko et al. 2004; Ekeberg & Pearson, 2005; Prochazka & Yakovenko, 2007a; Stienen et al. 2007; Markin et al. 2010). Mathematical models of muscle afferent responses to muscle length and force variations were used (Prochazka, 2015). In one such model of locomotion, the phasic activation of four hindlimb muscle groups was produced by a locomotor central pattern generator (CPG; Prochazka et al. 2002; Yakovenko et al. 2004). In the absence of stretch reflexes, and with levels of muscle activation adequate to support body weight, the model produced several stable step cycles before becoming unco-ordinated and collapsing. Adding stretch reflexes, including positive force feedback mediated by Ib afferents, resulted in a slight increase in body height during the stance phases. When the CPG-generated muscle activation levels were set slightly too low to support the body, without stretch reflexes the model quickly collapsed. With stretch reflexes, stable steps were restored.
General conclusions on the role of stretch reflexes
Taking all of the evidence together, stretch reflexes clearly modulate muscle stiffness and this can play a linearizing and stabilizing role during movement. Short-latency, spinally mediated reflex responses to Ia input may play a lesser role than short-latency reflex responses to group II and Ib input. Supra-spinally-mediated long-latency responses to Ia, II and Ib input can play a more significant role, but in the words of a recent study: ‘Neural activity occurring within the period normally ascribed to the long-latency stretch reflex is highly adaptable to current task demands and possibly should be considered more intelligent than “reflexive”’ (Shemmell et al. 2010).
Phase-switching
In the absence of sensory input, the transitions between the stance and swing phases of the locomotor step cycle would rely entirely on a pre-set temporal sequence of muscle activations generated by the locomotor CPG. It has been proposed that the timing of the switch between stance and swing phases of locomotion is triggered by finite state rules of the type ‘IF in stance phase AND leg is extended AND unloaded AND contralateral leg is loaded THEN switch to swing’ (Tomovic & McGhee, 1966; Cruse, 1990; Tomovic et al. 1990; Prochazka, 1993). When such rules were used to fine-tune the timing of phase transitions generated by the CPG, locomotion became much more stable (Yakovenko et al. 2004; Prochazka & Yakovenko, 2007a,b). Similar results were obtained by Rybak and colleagues, who concluded that afferent feedback adjusts CPG operation to the kinematics and dynamics of the limb providing stable locomotion (Markin et al. 2010). In another modelling study, there was no CPG at all; instead, phase-switching was controlled entirely by IF–THEN rules (Ekeberg & Pearson, 2005).
Clinical applications
Feedback control of stereotyped movements
Because of the practical difficulty of deploying numerous sensors on the human body in assistive devices designed for the rigours of daily use, it has not generally been possible to mimic the biological control mechanisms discussed above in neuroprostheses. It is interesting that in the few cases where analogous mechanisms of control were implemented, these were developed without specific reference to the corresponding biological mechanisms. A good example of this is the control of the stance to swing phase transition of hemiplegic gait with functional electrical stimulation (FES). The control method in most cases is to use under-heel switches or pressure transducers to detect the decline in force that occurs at the end of the stance phase of gait and to use this to trigger stimulation of the common peroneal nerve, which activates the muscles to dorsiflex the foot during the ensuing swing (Liberson et al. 1961). This corresponds to the unloading part of the finite state rule referred to above. In animals, Golgi tendon organs of the foot plantarflexor muscles may provide the sensory input relevant to this rule. Input from the skin of the sole of the foot may also contribute, as suggested by the locomotor deficits experienced by people with peripheral sensory denervation due to diabetes or chemotherapy.
Vodovnik and colleagues, who were among the pioneers of FES in the 1960s, explored the possibility of proportional feedback control of movements about the human elbow joint (Crochetiere et al. 1967; Vodovnik et al. 1967). Although the results were promising, the approach was not tested clinically, possibly because the motor points of biceps and triceps brachii shift under the skin as the elbow flexes and extends. This limits the range of movement over which reliable control can be achieved via surface electrodes. Furthermore, deriving a voluntary command signal was problematic. The only closed-loop FES devices to have been tested in participants going about their daily lives were a foot-drop stimulator (Prochazka & Wiles, 1983) and a tremor-suppressing system (Javidan et al. 1992; Prochazka et al. 1992). In the former case the signal from a length sensor spanning the front of the ankle was used to control the level of stimulation of the ankle dorsiflexor muscles that lift the foot. The system was modelled on the stretch reflex, the length gauge taking the place of spindles in the ankle dorsiflexor muscles and the stimulator taking the place of α-motoneurons activating those muscles. This provided proportional negative feedback control of length. The command input to the controller was set to maintain an ankle angle of about 90° during the locomotor step cycle, minimizing the foot-drop that occurs in hemiplegia at the onset of swing and for the brief period at the onset of stance between heel-strike and toe-down. The system worked well in a number of hemiplegic people who went about their daily lives wearing the device. However, the location and attachments of the sensor on the lower leg and the shoe made it vulnerable to incorrect donning, dislodgement and mechanical inputs from clothing. Small shifts in the stimulating electrodes could cause foot inversion and eversion that were not detected by the sensor. For these reasons, under-heel switches were deemed to be more reliable and convenient, and the length feedback system was not pursued further.
In the 1990s a third mode of control was developed, namely the use of an accelerometer to monitor tilting of the lower leg. During locomotion, at the end of the stance phase, the lower leg tilts forward with respect to the vertical as the leg extends. In the Walkaide foot-drop stimulator, when the level of tilt reaches a pre-set threshold value, stimulation is delivered to the common peroneal nerve, activating the ankle dorsiflexor muscles to lift the foot during the ensuing swing phase (Everaert et al. 2013). This corresponds to the extension part of the finite state rule above. In terms of a direct biological equivalent, although there are no sensory receptors that monitor limb tilt, it has been shown that limb joint angles can be derived from signals from muscle and skin receptors and in principle this could be done by neural networks within the CNS (Weber et al. 2007). The Walkaide functions well in many people with hemiplegia, but not all users have enough range and reproducibility of leg tilt for the control method to work reliably (Gunther Gallagher, 2011). An under-heel switch is therefore provided as an alternative.
In relation to the probable contribution of cutaneous receptors to proprioception, the present author and his colleagues Michel Gauthier and Jacques Bobet recently experimented with the use of a skin stretch sensor to control foot-drop stimulation. The idea was essentially the same as in the system described above involving a length sensor spanning the ankle joint, namely to use continuous proportional feedback control of stimulation to resist increases in ankle angle exceeding 90°. As discussed earlier, signals from cutaneous stretch receptors around limb joints correlate well with joint displacement, so it was posited that an artificial sensor that mimicked these receptors would provide usable control signals. This was confirmed in experiments in three normal individuals in whom skin stretch was monitored with sensors stuck to the skin over the Achilles tendon, about 15 cm proximal to the sole of the foot. A detailed report of these and related experiments is in preparation. Regarding the feasibility of this approach, sensors in the form of stretchable membranes have recently been developed for long-term monitoring of biological variables, so the requisite technology already exists (Hu et al. 2011).
Voluntary command signals
Restoring voluntary control of movements of the paralyzed upper limb is an important goal. People with tetraplegia rate this as their primary concern (Anderson, 2004, 2009). As mentioned above, it is a challenge to derive voluntary command signals corresponding to descending drive from the brain, particularly for hand movements. One approach is to monitor the activity of muscles that remain under voluntary control, or the movements they produce. This has been implemented in several upper limb neuroprostheses that have been used in exercise training and activities of daily life. For example, in the implanted Freehand system, signals from a shoulder movement sensor were used to control FES-evoked hand opening and grasp (Smith et al. 1987; Keith et al. 1989). Electromyograms from proximal muscles were used in a second-generation version of this device (Kilgore et al. 2008; Memberg et al. 2014). Wrist movements were used in a surface stimulator (Prochazka et al. 1997a) and a third version of the Freehand system (Peckham et al. 2002). Tooth-clicks monitored by a wireless earpiece were used in a recent surface hand stimulator (Kowalczewski et al. 2011) and an implanted version of this device (Gan et al. 2012). Finally, head movements, also monitored by a wireless earpiece, are used to control hand grasp and release in a surface stimulator currently being developed commercially by the author and colleagues. The shoulder and wrist-controlled versions of the Freehand system enabled users to generate a number of hand movements that allowed simple activities of daily life. The EMG-controlled version allowed proportional control of shoulder, elbow and hand movements with visual feedback (Memberg et al. 2014), though weight-support was required for functional tasks. In the other cases the signals simply provided a trigger to switch from one state to the next in a sequence, a biological function proposed above for input from Ia and Ib afferents. To enable a variety of graded, co-ordinated hand movements, several degrees of freedom are required of the control signals. Brain machine interfaces could in principle provide such signals, but currently this technology has only been used to control robot arms and hands (Collinger et al. 2013). Less invasive sensor systems that combine signals derived from 3-D head movements, tooth-clicks and voice commands, are being developed in the author’s laboratory.
Concluding remarks
Sensory input from many thousands of sensory receptors contributes to the control of mammalian movement and to kinaesthesia. The nervous system is able to modulate the sensitivity of the received sensory information at synapses within the CNS, and in the case of muscle spindles, within the receptor itself, by activating intrafusal muscle fibres via fusimotor neurons. In voluntary movements, fusimotor action seems to have at least two components, one proportional to the activity of α-motoneurons and the other being in some way related to motor task or predicted movement. The details of this second component are still disputed. In the author’s view, spindle afferents are predominantly muscle length and velocity detectors whose sensitivity and bias are adjusted by fusimotor action according to the demands of the motor task. A component of fusimotor action linked to muscle contraction helps maintain spindle afferent signalling during muscle shortening and subsequent lengthening. Stretch reflexes mediated by muscle spindles and Golgi tendon organs contribute to load compensation and to higher level control, as exemplified by the switching between phases of a cyclical movement such as locomotion. In this article we have discussed the extent to which these biological mechanisms have been mimicked in neuroprosthetic devices. The main obstacle has been the enormous mismatch between the numbers of sensory receptors in animals compared with the handful of artificial sensors that can be attached to, or implanted in, the body in devices robust and convenient enough to be used by people in activities of daily life. Nonetheless, some analogous feedback control mechanisms do exist. They have provided insights into the way nervous systems have solved the problem of movement control. As technology advances it will be possible to deploy more sensors and this is likely to lead to neuroprostheses that are increasingly biomimetic. A clear understanding of the neurophysiology of motor control, and in particular the sensory receptors Robert Banks has studied in such detail, will then become increasingly important.
Acknowledgments
This work was supported by the Canadian Institutes for Health Research and Alberta Innovates Health Solutions.
References
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Anderson KD. Consideration of user priorities when developing neural prosthetics. J Neural Eng. 2009;6:055003. doi: 10.1088/1741-2560/6/5/055003. [DOI] [PubMed] [Google Scholar]
- Aniss AM, Diener HC, Hore J, et al. Behavior of human muscle receptors when reliant on proprioceptive feedback during standing. J Neurophysiol. 1990;64:661–670. doi: 10.1152/jn.1990.64.2.661. [DOI] [PubMed] [Google Scholar]
- Banks RW, Stacey MJ. Quantitative studies on mammalian muscle spindles and their sensory innervation. In: Hnik P, Soukup T, Vejsada R, Zelena J, editors. Mechanoreceptors: Development, Structure and Function. London: Plenum; 1988. pp. 263–269. [Google Scholar]
- Barker D, Ip MC, Adal MN. A correlation between the receptor population of the cat’s soleus muscle and the afferent fibre diameter spectrum of the nerve supplying it. In: Barker D, editor. Symposium on Muscle Receptors. Hong Kong: Hong Kong University Press; 1962. pp. 257–261. [Google Scholar]
- Bennett DJ. Torques generated at the human elbow joint in response to constant position errors imposed during voluntary movements. Exp Brain Res. 1993;95:488–498. doi: 10.1007/BF00227142. [DOI] [PubMed] [Google Scholar]
- Bennett DJ. Stretch reflex responses in the human elbow joint during a voluntary movement. J Physiol. 1994;474:339–351. doi: 10.1113/jphysiol.1994.sp020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DJ, Gorassini M, Prochazka A. Catching a ball: contributions of intrinsic muscle stiffness, reflexes, and higher order responses. Can J Physiol Pharmacol. 1994;72:525–534. doi: 10.1139/y94-076. [DOI] [PubMed] [Google Scholar]
- Bessou P, Cabelguen JM, Joffroy M, et al. Efferent and afferent activity in a gastrocnemius nerve branch during locomotion in the thalamic cat. Exp Brain Res. 1986;64:553–568. doi: 10.1007/BF00340493. [DOI] [PubMed] [Google Scholar]
- Boyd IA, Roberts TDM. Proprioceptive discharges from stretch receptors in the knee joint of the cat. J Physiol. 1953;122:38–58. doi: 10.1113/jphysiol.1953.sp004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PR, Clark FJ. Characteristics of knee joint receptors in the cat. J Physiol. 1969;203:317–335. doi: 10.1113/jphysiol.1969.sp008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge JH, Wood DE, Taylor PN, et al. Indices to describe different muscle activation patterns, identified during treadmill walking, in people with spastic drop-foot. Med Eng Phys. 2001;23:427–434. doi: 10.1016/s1350-4533(01)00061-3. [DOI] [PubMed] [Google Scholar]
- Cabelguen JM. Static and dynamic fusimotor controls in various hindlimb muscles during locomotor activity in the decorticate cat. Brain Res. 1981;213:83–97. doi: 10.1016/0006-8993(81)91249-x. [DOI] [PubMed] [Google Scholar]
- Capaday C, Stein RB. Amplitude modulation of the soleus H-reflex in the human during walking and standing. J Neurosci. 1986;6:1308–1313. doi: 10.1523/JNEUROSCI.06-05-01308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carli G, Farabollini F, Fontani G, et al. Slowly adapting receptors in cat hip joint. J Neurophysiol. 1979;42:767–778. doi: 10.1152/jn.1979.42.3.767. [DOI] [PubMed] [Google Scholar]
- Clark FJ, Burgess RC, Chapin JW, et al. Role of intramuscular receptors in the awareness of limb position. J Neurophysiol. 1985;54:1529–1540. doi: 10.1152/jn.1985.54.6.1529. [DOI] [PubMed] [Google Scholar]
- Cody FW, Taylor A. The behaviour of spindles in the jaw-closing muscles during eating and drinking in the cat. J Physiol. 1973;231:49P–50P. [PubMed] [Google Scholar]
- Cody FW, Harrison LM, Taylor A. Analysis of activity of muscle spindles of the jaw-closing muscles during normal movements in the cat. J Physiol. 1975;253:565–582. doi: 10.1113/jphysiol.1975.sp011207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinger JL, Wodlinger B, Downey JE, et al. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet. 2013;381:557–564. doi: 10.1016/S0140-6736(12)61816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Prochazka A. Movement illusions evoked by ensemble cutaneous input from the dorsum of the human hand. J Physiol. 1996;496(Pt 3):857–871. doi: 10.1113/jphysiol.1996.sp021733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Refshauge KM, Todd G, et al. Cutaneous receptors contribute to kinesthesia at the index finger, elbow, and knee. J Neurophysiol. 2005;94:1699–1706. doi: 10.1152/jn.00191.2005. [DOI] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cat. Exp Brain Res. 1987;68:643–656. doi: 10.1007/BF00249807. [DOI] [PubMed] [Google Scholar]
- Crago PE, Houk JC, Rymer WZ. Sampling of total muscle force by tendon organs. J Neurophysiol. 1982;47:1069–1083. doi: 10.1152/jn.1982.47.6.1069. [DOI] [PubMed] [Google Scholar]
- Crochetiere WJ, Vodovnik L, Reswick JB. Electrical stimulation of skeletal muscle – a study of muscle as an actuator. Med Biol Eng. 1967;5:111–125. doi: 10.1007/BF02474499. [DOI] [PubMed] [Google Scholar]
- Cruse H. What mechanisms coordinate leg movement in walking arthropods? Trends Neurosci. 1990;13:15–21. doi: 10.1016/0166-2236(90)90057-h. [DOI] [PubMed] [Google Scholar]
- Dimitriou M. Human muscle spindle sensitivity reflects the balance of activity between antagonistic muscles. J Neurosci. 2014;34:13644–13655. doi: 10.1523/JNEUROSCI.2611-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriou M, Edin BB. Discharges in human muscle spindle afferents during a key-pressing task. J Physiol. 2008;586:5455–5470. doi: 10.1113/jphysiol.2008.160036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriou M, Edin BB. Human muscle spindles act as forward sensory models. Curr Biol. 2010;20:1763–1767. doi: 10.1016/j.cub.2010.08.049. [DOI] [PubMed] [Google Scholar]
- Donelan JM, Pearson KG. Contribution of force feedback to ankle extensor activity in decerebrate walking cats. J Neurophysiol. 2004;92:2093–2104. doi: 10.1152/jn.00325.2004. [DOI] [PubMed] [Google Scholar]
- Donelan JM, McVea DA, Pearson KG. Force regulation of ankle extensor muscle activity in freely walking cats. J Neurophysiol. 2009;101:360–371. doi: 10.1152/jn.90918.2008. [DOI] [PubMed] [Google Scholar]
- Edin BB. Cutaneous afferents provide information about knee joint movements in humans. J Physiol. 2001;531:289–297. doi: 10.1111/j.1469-7793.2001.0289j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin BB. Quantitative analyses of dynamic strain sensitivity in human skin mechanoreceptors. J Neurophysiol. 2004;92:3233–3243. doi: 10.1152/jn.00628.2004. [DOI] [PubMed] [Google Scholar]
- Edin BB, Johansson N. Skin strain patterns provide kinaesthetic information to the human central nervous system. J Physiol. 1995;487:243–251. doi: 10.1113/jphysiol.1995.sp020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekeberg O, Pearson K. Computer simulation of stepping in the hind legs of the cat: an examination of mechanisms regulating the stance-to-swing transition. J Neurophysiol. 2005;94:4256–4268. doi: 10.1152/jn.00065.2005. [DOI] [PubMed] [Google Scholar]
- Ellaway P, Taylor A, Durbaba R, et al. Role of the fusimotor system in locomotion. Adv Exp Med Biol. 2002;508:335–342. doi: 10.1007/978-1-4615-0713-0_39. [DOI] [PubMed] [Google Scholar]
- Ellaway P, Taylor A, Durbaba R. Muscle spindle and fusimotor activity in locomotion. Journal of Anatomy. 2015 doi: 10.1111/joa.12299. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaert DG, Stein RB, Abrams GM, et al. Effect of a foot-drop stimulator and ankle-foot orthosis on walking performance after stroke: a multicenter randomized controlled trial. Neurorehabil Neural Repair. 2013;27:579–591. doi: 10.1177/1545968313481278. [DOI] [PubMed] [Google Scholar]
- Ferrell WR. The adequacy of stretch receptors in the cat knee joint for signalling joint angle throughout a full range of movement. J Physiol. 1980;299:85–100. doi: 10.1113/jphysiol.1980.sp013112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell WR, Gandevia SC, McCloskey DI. The role of joint receptors in human kinaesthesia when intramuscular receptors cannot contribute. J Physiol. 1987;386:63–71. doi: 10.1113/jphysiol.1987.sp016522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan LS, Ravid E, Kowalczewski JA, et al. First permanent implant of nerve stimulation leads activated by surface electrodes, enabling hand grasp and release: the stimulus router neuroprosthesis. Neurorehabil Neural Repair. 2012;26:335–343. doi: 10.1177/1545968311420443. [DOI] [PubMed] [Google Scholar]
- Geyer H, Seyfarth A, Blickhan R. Positive force feedback in bouncing gaits? Proc Biol Sci. 2003;270:2173–2183. doi: 10.1098/rspb.2003.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin-Austen RB. The mechanoreceptors of the costo-vertebral joints. J Physiol. 1969;202:737–753. doi: 10.1113/jphysiol.1969.sp008838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, Luschei ES. Discharge of spindle afferents from jaw-closing muscles during chewing in alert monkeys. J Neurophysiol. 1975;38:560–571. doi: 10.1152/jn.1975.38.3.560. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Matthews PB. Proprioceptive illusions induced by muscle vibration: contribution by muscle spindles to perception? Science. 1972;175:1382–1384. doi: 10.1126/science.175.4028.1382. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Prochazka A, Hiebert GW, et al. Corrective responses to loss of ground support during walking. I. Intact cats. J Neurophysiol. 1994;71:603–610. doi: 10.1152/jn.1994.71.2.603. [DOI] [PubMed] [Google Scholar]
- Goslow GE, Jr, Stauffer EK, Nemeth WC, et al. The cat step cycle; responses of muscle spindles and tendon organs to passive stretch within the locomotor range. Brain Res. 1973;60:35–54. doi: 10.1016/0006-8993(73)90849-4. [DOI] [PubMed] [Google Scholar]
- Gottlieb S, Taylor A. Interpretation of fusimotor activity in cat masseter nerve during reflex jaw movements. J Physiol. 1983;345:423–438. doi: 10.1113/jphysiol.1983.sp014986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Stein RB. Fusimotor control of muscle spindle sensitivity during respiration in the cat. J Physiol. 1990;422:245–264. doi: 10.1113/jphysiol.1990.sp017982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory JE, McIntyre AK, Proske U. Tendon organ afferents in the knee joint nerve of the cat. Neurosci Lett. 1989;103:287–292. doi: 10.1016/0304-3940(89)90114-6. [DOI] [PubMed] [Google Scholar]
- Grey MJ, Ladouceur M, Andersen JB, et al. Group II muscle afferents probably contribute to the medium latency soleus stretch reflex during walking in humans. J Physiol. 2001;534:925–933. doi: 10.1111/j.1469-7793.2001.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey MJ, Nielsen JB, Mazzaro N, et al. Positive force feedback in human walking. J Physiol. 2007;581:99–105. doi: 10.1113/jphysiol.2007.130088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RI. Shortening of muscle fibres during stretch of the active cat medial gastrocnemius muscle: the role of tendon compliance. J Physiol. 1991;436:219–236. doi: 10.1113/jphysiol.1991.sp018547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg P, Greenspan BJ. Response of primate joint afferent neurons to mechanical stimulation of knee joint. J Neurophysiol. 1977;40:1–8. doi: 10.1152/jn.1977.40.1.1. [DOI] [PubMed] [Google Scholar]
- Gritsenko V, Mushahwar V, Prochazka A. Adaptive changes in locomotor control after partial denervation of triceps surae muscles in the cat. J Physiol. 2001;533:299–311. doi: 10.1111/j.1469-7793.2001.0299b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther Gallagher MB. 2011. p. 199. Marquette University Comparison of an ankle-foot-orthosis and neuroprosthesis during level and non-level walking for individuals post-stroke http://epublications.marquette.edu/theses_open/115/ (In Graduate Studies)
- Hoffer JA, Andreassen S. Regulation of soleus muscle stiffness in premammillary cats: intrinsic and reflex components. J Neurophysiol. 1981;45:267–285. doi: 10.1152/jn.1981.45.2.267. [DOI] [PubMed] [Google Scholar]
- Horch KW, Tuckett RP, Burgess PR. A key to the classification of cutaneous mechanoreceptors. J Investig Dermatol. 1977;69:75–82. doi: 10.1111/1523-1747.ep12497887. [DOI] [PubMed] [Google Scholar]
- Houk J, Henneman E. Responses of Golgi tendon organs to active contractions of the soleus muscle of the cat. J Neurophysiol. 1967;30:466–481. doi: 10.1152/jn.1967.30.3.466. [DOI] [PubMed] [Google Scholar]
- Houk JC, Singer JJ, Goldman MR. An evaluation of length and force feedback to soleus muscles of decerebrate cats. J Neurophysiol. 1970;33:784–811. doi: 10.1152/jn.1970.33.6.784. [DOI] [PubMed] [Google Scholar]
- Hu X, Krull P, de Graff B, et al. Stretchable inorganic-semiconductor electronic systems. Adv Mater. 2011;23:2933–2936. doi: 10.1002/adma.201100144. [DOI] [PubMed] [Google Scholar]
- Hulliger M. The mammalian muscle spindle and its central control. [Review] Rev Physiol Biochem Pharmacol. 1984;101:1–110. doi: 10.1007/BFb0027694. [DOI] [PubMed] [Google Scholar]
- Iles JF, Stokes M, Young A. Reflex actions of knee joint afferents during contraction of the human quadriceps. Clin Physiol. 1990;10:489–500. doi: 10.1111/j.1475-097x.1990.tb00828.x. [DOI] [PubMed] [Google Scholar]
- Javidan M, Elek J, Prochazka A. Attenuation of pathological tremors by functional electrical stimulation. II: clinical evaluation. Ann Biomed Eng. 1992;20:225–236. doi: 10.1007/BF02368522. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Vallbo AB. Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J Physiol. 1979;286:283–300. doi: 10.1113/jphysiol.1979.sp012619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson H, Sjolander P, Sojka P. Receptors in the knee joint ligaments and their role in the biomechanics of the joint. Crit Rev Biomed Eng. 1991;18:341–368. [PubMed] [Google Scholar]
- Johnson MW, Peckham PH, Bhadra N, et al. Implantable transducer for two-degree of freedom joint angle sensing. IEEE Trans Rehabil Eng. 1999;7:349–359. doi: 10.1109/86.788471. [DOI] [PubMed] [Google Scholar]
- Keith MW, Peckham PH, Thrope GB, et al. Implantable functional neuromuscular stimulation in the tetraplegic hand. J Hand Surg Am. 1989;14:524–530. doi: 10.1016/s0363-5023(89)80017-6. [DOI] [PubMed] [Google Scholar]
- Kilgore KL, Hoyen HA, Bryden AM, et al. An implanted upper-extremity neuroprosthesis using myoelectric control. J Hand Surg Am. 2008;33:539–550. doi: 10.1016/j.jhsa.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- af Klint R, Mazzaro N, Nielsen JB, et al. Load rather than length sensitive feedback contributes to soleus muscle activity during human treadmill walking. J Neurophysiol. 2010;103:2747–2756. doi: 10.1152/jn.00547.2009. [DOI] [PubMed] [Google Scholar]
- Kowalczewski J, Chong SL, Galea M, et al. In-home tele-rehabilitation improves tetraplegic hand function. Neurorehabil Neural Repair. 2011;25:412–422. doi: 10.1177/1545968310394869. [DOI] [PubMed] [Google Scholar]
- Liberson WT, Holmquest HJ, Scott D, et al. Functional electrotherapy: stimulation of the peroneal nerve synchronized with the swing phase of the gait of hemiplegic patients. Arch Phys Med Rehabil. 1961;42:101–105. [PubMed] [Google Scholar]
- Lichtwark GA, Wilson AM. Optimal muscle fascicle length and tendon stiffness for maximising gastrocnemius efficiency during human walking and running. J Theor Biol. 2008;252:662–673. doi: 10.1016/j.jtbi.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Loeb GE. Somatosensory unit input to the spinal cord during normal walking. Can J Physiol Pharmacol. 1981;59:627–635. doi: 10.1139/y81-097. [DOI] [PubMed] [Google Scholar]
- Lund JP, Matthews B. Responses of temporomandibular joint afferents recorded in the Gasserian ganglion of the rabbit to passive movements of the mandible. In: Kawamura Y, editor. Oral-facial Sensory and Motor Functions. Tokyo: Quintessence; 1981. pp. 153–160. [Google Scholar]
- Maganaris CN, Paul JP. In vivo human tendon mechanical properties. J Physiol. 1999;521(Pt 1):307–313. doi: 10.1111/j.1469-7793.1999.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson SP, Narici MV, Maganaris CN, et al. Human tendon behaviour and adaptation, in vivo. J Physiol. 2008;586:71–81. doi: 10.1113/jphysiol.2007.139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markin SN, Klishko AN, Shevtsova NA, et al. Afferent control of locomotor CPG: insights from a simple neuromechanical model. Ann N Y Acad Sci. 2010;1198:21–34. doi: 10.1111/j.1749-6632.2010.05435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB. Servo action in the human thumb. J Physiol. 1976;257:1–44. doi: 10.1113/jphysiol.1976.sp011354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PBC. The origin and functional significance of the stretch reflex. In: Andersen P, Jansen JKS, editors. Excitatory Synaptic Mechanisms. Oslo: Universitetsforlaget; 1970. pp. 301–315. [Google Scholar]
- Matthews PBC. Mammalian Muscle Receptors and Their Central Actions. London: Edward Arnold; 1972. [Google Scholar]
- Matthews PBC. What are the afferents of origin of the human stretch reflex, and is it a purely spinal reaction? Prog Brain Res. 1986;64:55–66. doi: 10.1016/s0079-6123(08)63400-7. [DOI] [PubMed] [Google Scholar]
- McCloskey DI, Cross MJ, Honner R, et al. Sensory effects of pulling or vibrating exposed tendons in man. Brain. 1983;106(Pt 1):21–37. doi: 10.1093/brain/106.1.21. [DOI] [PubMed] [Google Scholar]
- McIntyre AK, Proske U, Tracey DJ. Afferent fibres from muscle receptors in the posterior nerve of the cat’s knee joint. Exp Brain Res. 1978;33:415–424. doi: 10.1007/BF00235563. [DOI] [PubMed] [Google Scholar]
- Memberg WD, Polasek KH, Hart RL, et al. Implanted neuroprosthesis for restoring arm and hand function in people with high level tetraplegia. Arch Phys Med Rehabil. 2014;95:1201–1211. doi: 10.1016/j.apmr.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merton PA. Speculations on the servo-control of movement. In: Wolstenholme GEW, editor. The Spinal Cord. London: Churchill; 1953. pp. 247–255. [Google Scholar]
- Murphy PR, Martin HA. Fusimotor discharge patterns during rhythmic movements. Trends Neurosci. 1993;16:273–278. doi: 10.1016/0166-2236(93)90181-k. [DOI] [PubMed] [Google Scholar]
- Murphy PR, Stein RB, Taylor J. Phasic and tonic modulation of impulse rates in gamma-motoneurons during locomotion in premammillary cats. J Neurophysiol. 1984;52:228–243. doi: 10.1152/jn.1984.52.2.228. [DOI] [PubMed] [Google Scholar]
- Nichols TR, Houk JC. Improvement in linearity and regulation of stiffness that results from actions of stretch reflex. J Neurophysiol. 1976;39:119–142. doi: 10.1152/jn.1976.39.1.119. [DOI] [PubMed] [Google Scholar]
- Partridge LD. Intrinsic feedback factors producing inertial compensation in muscle. Biophys J. 1967;7:853–863. doi: 10.1016/S0006-3495(67)86625-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KG, Collins DF. Reversal of the influence of group Ib afferents from plantaris on activity in medial gastrocnemius muscle during locomotor activity. J Neurophysiol. 1993;70:1009–1017. doi: 10.1152/jn.1993.70.3.1009. [DOI] [PubMed] [Google Scholar]
- Peckham PH, Kilgore KL, Keith MW, et al. An advanced neuroprosthesis for restoration of hand and upper arm control using an implantable controller. J Hand Surg Am. 2002;27:265–276. doi: 10.1053/jhsu.2002.30919. [DOI] [PubMed] [Google Scholar]
- Perret C, Buser P. Static and dynamic fusimotor activity during locomotor movements in the cat. Brain Res. 1972;40:165–169. doi: 10.1016/0006-8993(72)90123-0. [DOI] [PubMed] [Google Scholar]
- Phillips CG. The Ferrier lecture, 1968. Motor apparatus of the baboon’s hand. Proc Biol Sci. 1969;173:141–174. doi: 10.1098/rspb.1969.0044. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Comparison of natural and artificial control of movement. IEEE Trans Rehabil Eng. 1993;1:7–17. [Google Scholar]
- Prochazka A. Proprioceptive feedback and movement regulation. In: Rowell L, Sheperd JT, editors. Handbook of Physiology. Section 12. Exercise: Regulation and Integration of Multiple Systems. New York: American Physiological Society; 1996. pp. 89–127. [Google Scholar]
- Prochazka A. Proprioceptor models. In: Jaeger D, Jung R, editors. Encyclopedia of Computational Neuroscience. Berlin: Springer-Verlag; 2015. www.springerreference.com ) [Google Scholar]
- Prochazka A, Gorassini M. Ensemble firing of muscle afferents recorded during normal locomotion in cats. J Physiol. 1998;507(Pt 1):293–304. doi: 10.1111/j.1469-7793.1998.293bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A, Wiles CM. Electrical stimulation of paretic leg muscles in man, allowing feedback controlled movements to be generated from the wrist. J Physiol. 1983;343:20P. [Google Scholar]
- Prochazka A, Yakovenko S. Locomotor control: from spring-like reactions of muscles to neural prediction. In: Nelson RJ, editor. The Somatosensory System: Deciphering The Brain’s Own Body Image. Boca Raton: CRC Press; 2002. pp. 141–181. [Google Scholar]
- Prochazka A, Yakovenko S. The neuromechanical tuning hypothesis. In: Cisek P, Drew T, Kalaska J, editors. Progress in Brain Research, Computational Neuroscience: Theoretical Insights into Brain Function. New York: Elsevier; 2007a. pp. 255–265. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Yakovenko S. Predictive and reactive tuning of the locomotor CPG. Integr Comp Biol. 2007b;47:474–481. doi: 10.1093/icb/icm065. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Hulliger M, Zangger P, et al. ‘Fusimotor set’: new evidence for alpha-independent control of gamma-motoneurones during movement in the awake cat. Brain Res. 1985;339:136–140. doi: 10.1016/0006-8993(85)90632-8. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Elek J, Javidan M. Attenuation of pathological tremors by functional electrical stimulation. I: Method. Ann Biomed Eng. 1992;20:205–224. doi: 10.1007/BF02368521. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Gauthier M, Wieler M, et al. The bionic glove: an electrical stimulator garment that provides controlled grasp and hand opening in quadriplegia. Arch Phys Med Rehabil. 1997a;78:608–614. doi: 10.1016/s0003-9993(97)90426-3. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Gillard D, Bennett DJ. Implications of positive feedback in the control of movement. J Neurophysiol. 1997b;77:3237–3251. doi: 10.1152/jn.1997.77.6.3237. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Gritsenko V, Yakovenko S. Sensory control of locomotion: reflexes versus higher-level control. Adv Exp Med Biol. 2002;508:357–367. doi: 10.1007/978-1-4615-0713-0_41. [DOI] [PubMed] [Google Scholar]
- Proske U, Gandevia SC. The kinaesthetic senses. J Physiol. 2009;587:4139–4146. doi: 10.1113/jphysiol.2009.175372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol Rev. 2012;92:1651–1697. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- Ribot E, Roll JP, Vedel JP. Efferent discharges recorded from single skeletomotor and fusimotor fibres in man. J Physiol. 1986;375:251–268. doi: 10.1113/jphysiol.1986.sp016115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JP, Vedel JP. Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res. 1982;47:177–190. doi: 10.1007/BF00239377. [DOI] [PubMed] [Google Scholar]
- Rossi G. Asimmetrie toniche posturali ed asimmetrie motorie. Aggiorn Fisiol. 1927;25:146–157. [Google Scholar]
- Shemmell J, Krutky MA, Perreault EJ. Stretch sensitive reflexes as an adaptive mechanism for maintaining limb stability. Clin Neurophysiol. 2010;121:1680–1689. doi: 10.1016/j.clinph.2010.02.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington CS. On the proprioceptive system, especially in its reflex aspects. Brain. 1907;29:467–482. [Google Scholar]
- Sinkjaer T, Toft E, Andreassen S, et al. Muscle stiffness in human ankle dorsiflexors: intrinsic and reflex components. J Neurophysiol. 1988;60:1110–1121. doi: 10.1152/jn.1988.60.3.1110. [DOI] [PubMed] [Google Scholar]
- Sinkjaer T, Andersen JB, Ladouceur M, et al. Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. J Physiol. 2000;523:817–827. doi: 10.1111/j.1469-7793.2000.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B, Peckham PH, Keith MW, et al. An externally powered, multichannel, implantable stimulator for versatile control of paralyzed muscle. IEEE Trans Biomed Eng. 1987;34:499–508. doi: 10.1109/tbme.1987.325979. [DOI] [PubMed] [Google Scholar]
- Stein RB, Misiaszek JE, Pearson KG. Functional role of muscle reflexes for force generation in the decerebrate walking cat. J Physiol. 2000;525:781–791. doi: 10.1111/j.1469-7793.2000.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens JA, Stuart DG. Proceedings: the responses of Golgi tendon organs to contractions of single motor units in a mixed mammalian muscle. J Physiol. 1974;242:62P–63P. [PubMed] [Google Scholar]
- Stephens JA, Reinking RM, Stuart DG. Tendon organs of cat medial gastrocnemius: responses to active and passive forces as a function of muscle length. J Neurophysiol. 1975;38:1217–1231. doi: 10.1152/jn.1975.38.5.1217. [DOI] [PubMed] [Google Scholar]
- Stienen AH, Schouten AC, Schuurmans J, et al. Analysis of reflex modulation with a biologically realistic neural network. J Comput Neurosci. 2007;23:333–348. doi: 10.1007/s10827-007-0037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Fishel JA, Yamamoto T, et al. Use of tactile feedback to control exploratory movements to characterize object compliance. Front Neurorobot. 2012;6:7. doi: 10.3389/fnbot.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Donga R, Juch PJ. Fusimotor effects of midbrain stimulation on jaw muscle spindles of the anaesthetized cat. Exp Brain Res. 1993;93:37–45. doi: 10.1007/BF00227778. [DOI] [PubMed] [Google Scholar]
- Taylor A, Durbaba R, Ellaway PH, et al. Patterns of fusimotor activity during locomotion in the decerebrate cat deduced from recordings from hindlimb muscle spindles. J Physiol. 2000;522:515–532. doi: 10.1111/j.1469-7793.2000.t01-3-00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Durbaba R, Ellaway PH, et al. Static and dynamic gamma-motor output to ankle flexor muscles during locomotion in the decerebrate cat. J Physiol. 2006;571:711–723. doi: 10.1113/jphysiol.2005.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomovic R, McGhee R. A finite state approach to the synthesis of control systems. IEEE Trans Hum Fact. 1966;7:122–128. [Google Scholar]
- Tomovic R, Anastasijevic R, Vuco J, et al. The study of locomotion by finite state models. Biol Cybern. 1990;63:271–276. doi: 10.1007/BF00203450. [DOI] [PubMed] [Google Scholar]
- Tracey DJ. Characteristics of wrist joint receptors in the cat. Exp Brain Res. 1979;34:165–176. doi: 10.1007/BF00238349. [DOI] [PubMed] [Google Scholar]
- Vallbo AB. Response patterns of human muscle spindle endings to isometric voluntary contractions. Acta Physiol Scand. 1970;80:43A. doi: 10.1111/j.1748-1716.1970.tb04866.x. [DOI] [PubMed] [Google Scholar]
- Vodovnik L, Crochetiere WJ, Reswick JB. Control of a skeletal joint by electrical stimulation of antagonists. Med Biol Eng. 1967;5:97–109. doi: 10.1007/BF02474498. [DOI] [PubMed] [Google Scholar]
- Voss H. Tabelle der absoluten und relativen Muskelspindelzahlen der menschlichen Skelettmuskulatur. Anat Anz. 1971;129:5562–5572. [PubMed] [Google Scholar]
- Walmsley B, Hodgson JA, Burke RE. Forces produced by medial gastrocnemius and soleus muscles during locomotion in freely moving cats. J Neurophysiol. 1978;41:1203–1216. doi: 10.1152/jn.1978.41.5.1203. [DOI] [PubMed] [Google Scholar]
- Weber DJ, Stein RB, Everaert DG, et al. Decoding sensory feedback from firing rates of afferent ensembles recorded in cat dorsal root ganglia in normal locomotion. IEEE Trans Neural Syst Rehabil Eng. 2006;14:240–243. doi: 10.1109/TNSRE.2006.875575. [DOI] [PubMed] [Google Scholar]
- Weber DJ, Stein RB, Everaert DG, et al. Limb-state feedback from ensembles of simultaneously recorded dorsal root ganglion neurons. J Neural Eng. 2007;4:S168–S180. doi: 10.1088/1741-2560/4/3/S04. [DOI] [PubMed] [Google Scholar]
- Willis WD, Coggeshalll RE. Sensory Mechanisms of the Spinal Cord. New York: Plenum; 1991. [Google Scholar]
- Wilson LR, Gandevia SC, Burke D. Discharge of human muscle spindle afferents innervating ankle dorsiflexors during target isometric contractions. J Physiol. 1997;504:221–232. doi: 10.1111/j.1469-7793.1997.221bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC. Forward models for physiological motor control. Neural Netw. 1996;9:1265–1279. doi: 10.1016/s0893-6080(96)00035-4. [DOI] [PubMed] [Google Scholar]
- Yakovenko S, Gritsenko V, Prochazka A. Contribution of stretch reflexes to locomotor control: a modeling study. Biol Cybern. 2004;90:146–155. doi: 10.1007/s00422-003-0449-z. [DOI] [PubMed] [Google Scholar]
- Zalkind VI. Method for an adequate stimulation of receptors of the cat carpo-radialis joint. Sechenov Physiol J USSR. 1971;57:1123–1127. [Google Scholar]


