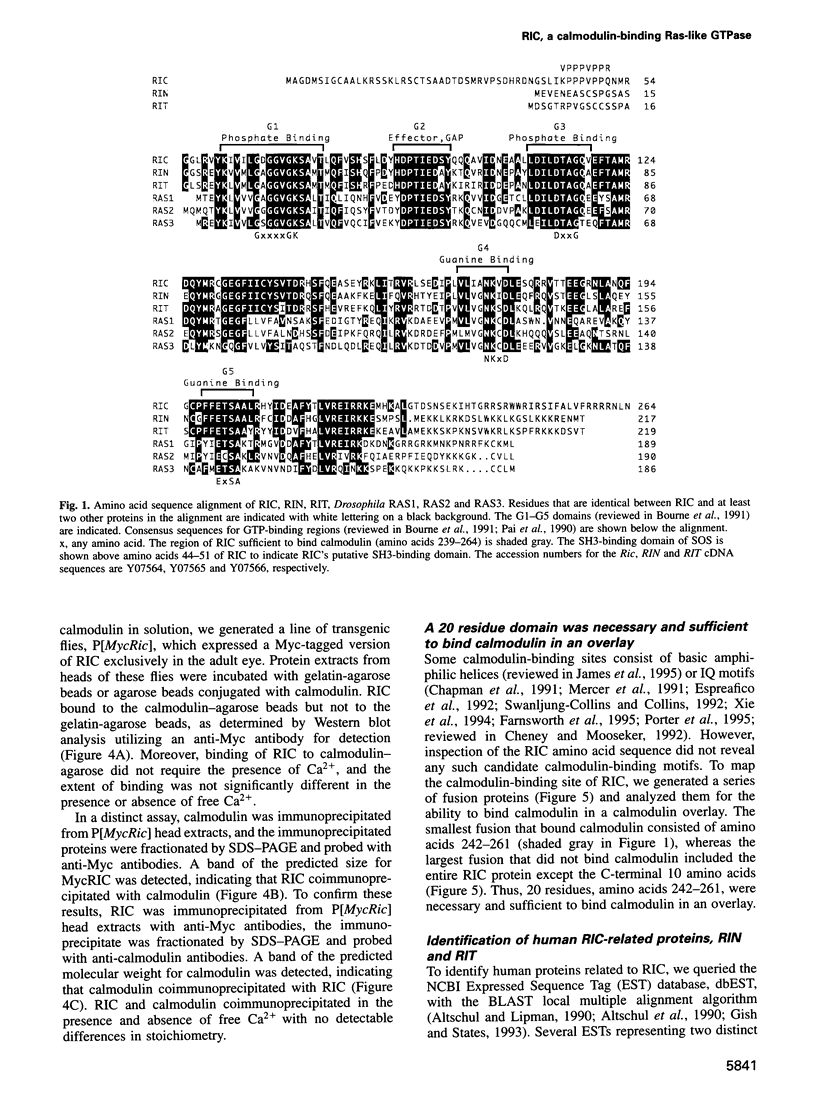
Abstract
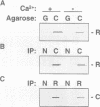
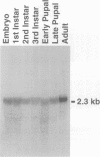
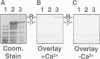
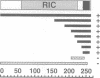
Neuronal activity dramatically increases the concentration of cytosolic Ca2+, which then serves as a second messenger to direct diverse cellular responses. Calmodulin is a primary mediator of Ca2+ signals in the nervous system. In a screen for calmodulin-binding proteins, we identified RIC, a protein related to the Ras subfamily of small GTPases. In addition to the ability to bind calmodulin, a number of unique features distinguished RIC from other Ras-like GTPases, including the absence of a signal for prenylation and a distinct effector (G2) domain. Furthermore, we describe two human proteins, RIN and RIT, which were 71% and 66% identical to RIC respectively, shared related G2 domains with RIC, and lacked prenylation signals, suggesting that the RIC family is conserved from flies to humans. While Ric and RIT were widely expressed, expression of RIN was confined to the neuron system.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajioka J. W., Smoller D. A., Jones R. W., Carulli J. P., Vellek A. E., Garza D., Link A. J., Duncan I. W., Hartl D. L. Drosophila genome project: one-hit coverage in yeast artificial chromosomes. Chromosoma. 1991 Sep;100(8):495–509. doi: 10.1007/BF00352200. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Lipman D. J. Protein database searches for multiple alignments. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5509–5513. doi: 10.1073/pnas.87.14.5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett D. E., Jr, Boguski M. S., Spencer F., Reeves R., Goebl M., Hieter P. Comparative genomics, genome cross-referencing and XREFdb. Trends Genet. 1995 Sep;11(9):372–373. doi: 10.1016/s0168-9525(00)89109-x. [DOI] [PubMed] [Google Scholar]
- Bear M. F., Malenka R. C. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994 Jun;4(3):389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Boguski M. S., McCormick F. Proteins regulating Ras and its relatives. Nature. 1993 Dec 16;366(6456):643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Nicoll R. Molecular machines integrate coincident synaptic signals. Cell. 1993 Jan;72 (Suppl):65–75. doi: 10.1016/s0092-8674(05)80029-7. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Cai H., Kiefel P., Yee J., Duncan I. A yeast artificial chromosome clone map of the Drosophila genome. Genetics. 1994 Apr;136(4):1385–1399. doi: 10.1093/genetics/136.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casale W. L., Mcconnell D. G., Wang S. Y., Lee Y. J., Linz J. E. Expression of a gene family in the dimorphic fungus Mucor racemosus which exhibits striking similarity to human ras genes. Mol Cell Biol. 1990 Dec;10(12):6654–6663. doi: 10.1128/mcb.10.12.6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavener D. R. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987 Feb 25;15(4):1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cen H., Papageorge A. G., Zippel R., Lowy D. R., Zhang K. Isolation of multiple mouse cDNAs with coding homology to Saccharomyces cerevisiae CDC25: identification of a region related to Bcr, Vav, Dbl and CDC24. EMBO J. 1992 Nov;11(11):4007–4015. doi: 10.1002/j.1460-2075.1992.tb05494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman E. R., Au D., Alexander K. A., Nicolson T. A., Storm D. R. Characterization of the calmodulin binding domain of neuromodulin. Functional significance of serine 41 and phenylalanine 42. J Biol Chem. 1991 Jan 5;266(1):207–213. [PubMed] [Google Scholar]
- Cheney R. E., Mooseker M. S. Unconventional myosins. Curr Opin Cell Biol. 1992 Feb;4(1):27–35. doi: 10.1016/0955-0674(92)90055-h. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Calcium: still center-stage in hypoxic-ischemic neuronal death. Trends Neurosci. 1995 Feb;18(2):58–60. [PubMed] [Google Scholar]
- Clapham D. E. Calcium signaling. Cell. 1995 Jan 27;80(2):259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- Clarke S. Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu Rev Biochem. 1992;61:355–386. doi: 10.1146/annurev.bi.61.070192.002035. [DOI] [PubMed] [Google Scholar]
- Cohen G. B., Ren R., Baltimore D. Modular binding domains in signal transduction proteins. Cell. 1995 Jan 27;80(2):237–248. doi: 10.1016/0092-8674(95)90406-9. [DOI] [PubMed] [Google Scholar]
- Cohen L., Mohr R., Chen Y. Y., Huang M., Kato R., Dorin D., Tamanoi F., Goga A., Afar D., Rosenberg N. Transcriptional activation of a ras-like gene (kir) by oncogenic tyrosine kinases. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12448–12452. doi: 10.1073/pnas.91.26.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Bliss T. V. Memories of NMDA receptors and LTP. Trends Neurosci. 1995 Feb;18(2):54–56. [PubMed] [Google Scholar]
- Dasgupta M., Honeycutt T., Blumenthal D. K. The gamma-subunit of skeletal muscle phosphorylase kinase contains two noncontiguous domains that act in concert to bind calmodulin. J Biol Chem. 1989 Oct 15;264(29):17156–17163. [PubMed] [Google Scholar]
- Emkey R., Freedman S., Feig L. A. Characterization of a GTPase-activating protein for the Ras-related Ral protein. J Biol Chem. 1991 May 25;266(15):9703–9706. [PubMed] [Google Scholar]
- Eshel Y., Shai Y., Vorherr T., Carafoli E., Salomon Y. Synthetic peptides corresponding to the calmodulin-binding domains of skeletal muscle myosin light chain kinase and human erythrocyte Ca2+ pump interact with and permeabilize liposomes and cell membranes. Biochemistry. 1993 Jul 6;32(26):6721–6728. doi: 10.1021/bi00077a027. [DOI] [PubMed] [Google Scholar]
- Espreafico E. M., Cheney R. E., Matteoli M., Nascimento A. A., De Camilli P. V., Larson R. E., Mooseker M. S. Primary structure and cellular localization of chicken brain myosin-V (p190), an unconventional myosin with calmodulin light chains. J Cell Biol. 1992 Dec;119(6):1541–1557. doi: 10.1083/jcb.119.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Matthews H. R. Calcium and the mechanism of light adaptation in vertebrate photoreceptors. Trends Neurosci. 1990 Sep;13(9):378–384. doi: 10.1016/0166-2236(90)90023-4. [DOI] [PubMed] [Google Scholar]
- Farnsworth C. L., Freshney N. W., Rosen L. B., Ghosh A., Greenberg M. E., Feig L. A. Calcium activation of Ras mediated by neuronal exchange factor Ras-GRF. Nature. 1995 Aug 10;376(6540):524–527. doi: 10.1038/376524a0. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S., Greenberg M. E. Ca(2+)-dependent routes to Ras: mechanisms for neuronal survival, differentiation, and plasticity? Neuron. 1996 Feb;16(2):233–236. doi: 10.1016/s0896-6273(00)80040-9. [DOI] [PubMed] [Google Scholar]
- Franklin J. L., Johnson E. M., Jr Elevated intracellular calcium blocks programmed neuronal death. Ann N Y Acad Sci. 1994 Dec 15;747:195–204. doi: 10.1111/j.1749-6632.1994.tb44410.x. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Greenberg M. E. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995 Apr 14;268(5208):239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- Gish W., States D. J. Identification of protein coding regions by database similarity search. Nat Genet. 1993 Mar;3(3):266–272. doi: 10.1038/ng0393-266. [DOI] [PubMed] [Google Scholar]
- Glomset J. A., Farnsworth C. C. Role of protein modification reactions in programming interactions between ras-related GTPases and cell membranes. Annu Rev Cell Biol. 1994;10:181–205. doi: 10.1146/annurev.cb.10.110194.001145. [DOI] [PubMed] [Google Scholar]
- Hall Z. W., Sanes J. R. Synaptic structure and development: the neuromuscular junction. Cell. 1993 Jan;72 (Suppl):99–121. doi: 10.1016/s0092-8674(05)80031-5. [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Paterson H., Marshall C. J. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990 Oct 5;63(1):133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Henikoff J. G. Performance evaluation of amino acid substitution matrices. Proteins. 1993 Sep;17(1):49–61. doi: 10.1002/prot.340170108. [DOI] [PubMed] [Google Scholar]
- James P., Vorherr T., Carafoli E. Calmodulin-binding domains: just two faced or multi-faceted? Trends Biochem Sci. 1995 Jan;20(1):38–42. doi: 10.1016/s0968-0004(00)88949-5. [DOI] [PubMed] [Google Scholar]
- Jaramillo F. Signal transduction in hair cells and its regulation by calcium. Neuron. 1995 Dec;15(6):1227–1230. doi: 10.1016/0896-6273(95)90003-9. [DOI] [PubMed] [Google Scholar]
- Jessell T. M., Kandel E. R. Synaptic transmission: a bidirectional and self-modifiable form of cell-cell communication. Cell. 1993 Jan;72 (Suppl):1–30. doi: 10.1016/s0092-8674(05)80025-x. [DOI] [PubMed] [Google Scholar]
- Kater S. B., Lipton S. A. Neurotransmitter regulation of neuronal outgrowth, plasticity and survival in the year 2001. Trends Neurosci. 1995 Feb;18(2):71–72. doi: 10.1016/0166-2236(95)93877-z. [DOI] [PubMed] [Google Scholar]
- Kater S. B., Mattson M. P., Cohan C., Connor J. Calcium regulation of the neuronal growth cone. Trends Neurosci. 1988 Jul;11(7):315–321. doi: 10.1016/0166-2236(88)90094-x. [DOI] [PubMed] [Google Scholar]
- Kater S. B., Mills L. R. Regulation of growth cone behavior by calcium. J Neurosci. 1991 Apr;11(4):891–899. doi: 10.1523/JNEUROSCI.11-04-00891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. B. Storage and release of neurotransmitters. Cell. 1993 Jan;72 (Suppl):43–53. doi: 10.1016/s0092-8674(05)80027-3. [DOI] [PubMed] [Google Scholar]
- Ladant D. Interaction of Bordetella pertussis adenylate cyclase with calmodulin. Identification of two separated calmodulin-binding domains. J Biol Chem. 1988 Feb 25;263(6):2612–2618. [PubMed] [Google Scholar]
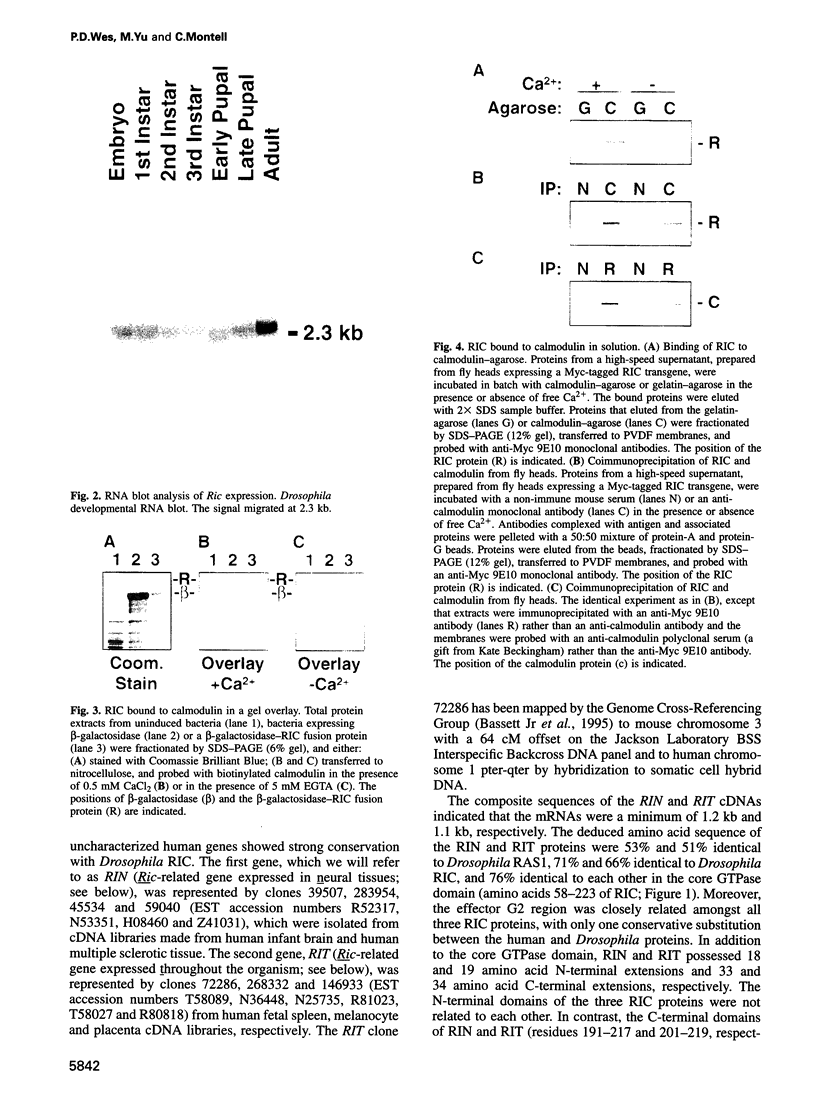
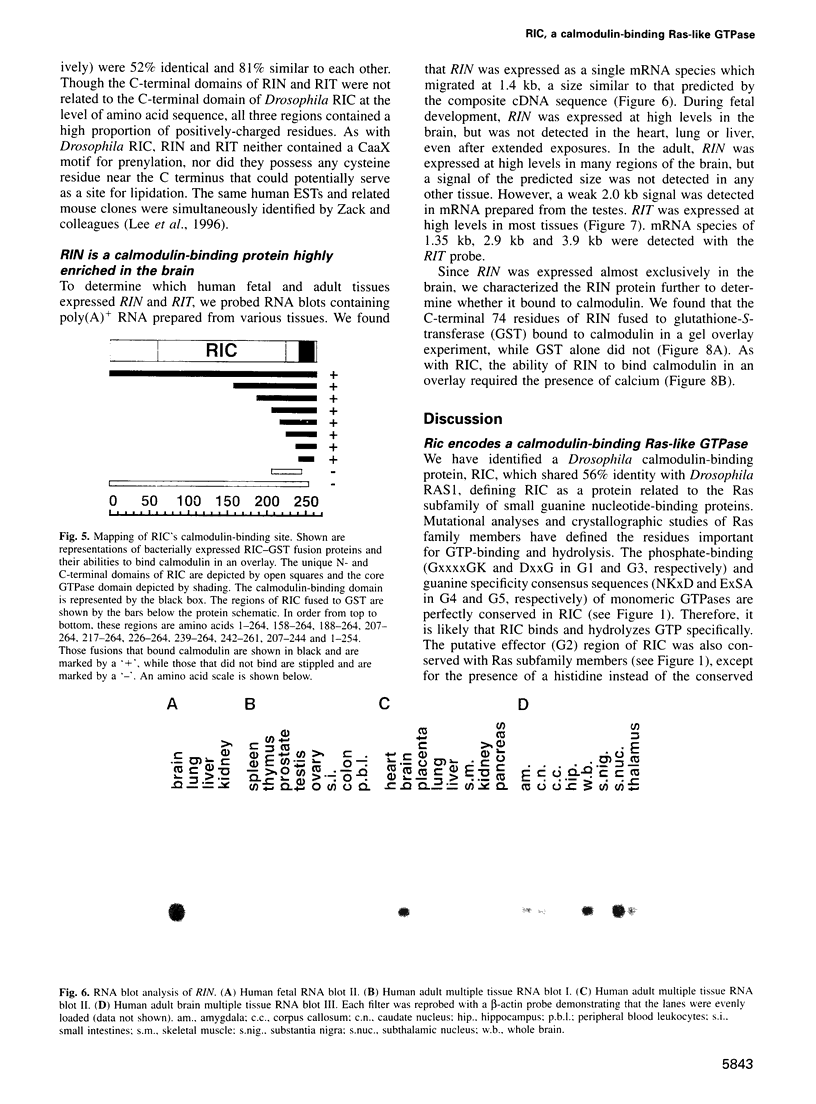
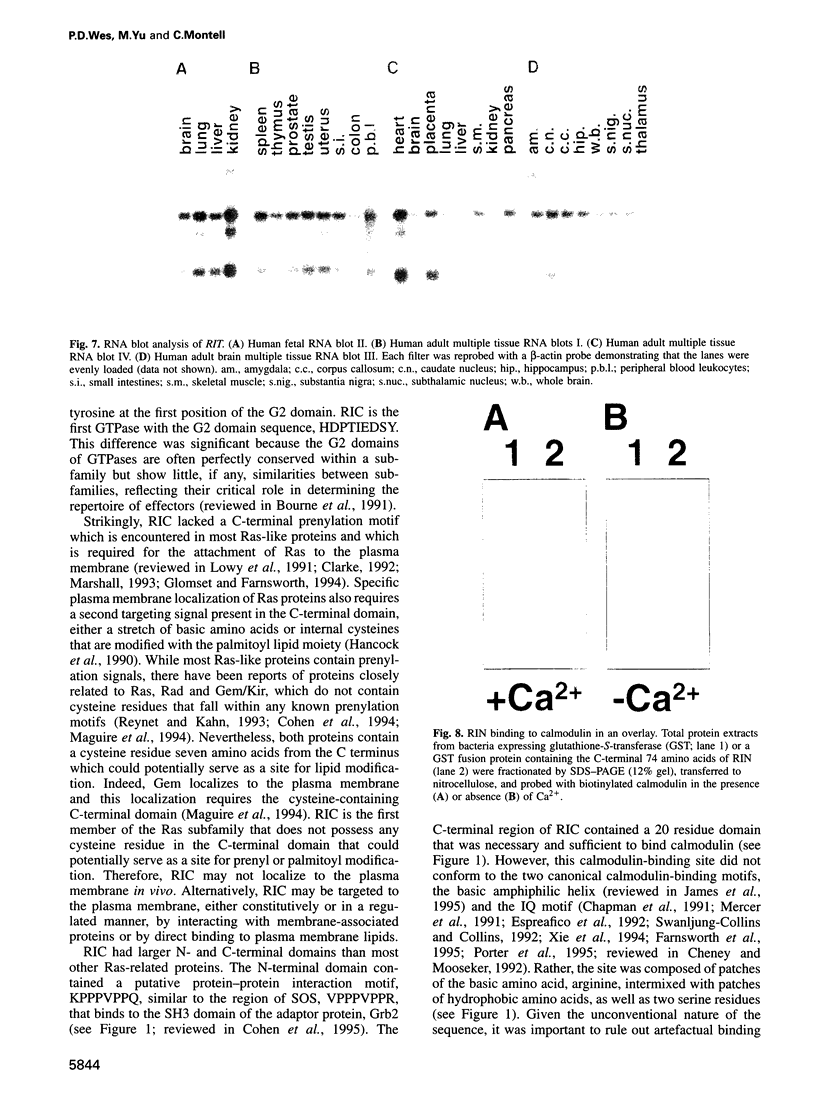
- Lee C. H., Della N. G., Chew C. E., Zack D. J. Rin, a neuron-specific and calmodulin-binding small G-protein, and Rit define a novel subfamily of ras proteins. J Neurosci. 1996 Nov 1;16(21):6784–6794. doi: 10.1523/JNEUROSCI.16-21-06784.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev S., Moreno H., Martinez R., Canoll P., Peles E., Musacchio J. M., Plowman G. D., Rudy B., Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995 Aug 31;376(6543):737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Zhang K., DeClue J. E., Willumsen B. M. Regulation of p21ras activity. Trends Genet. 1991 Nov-Dec;7(11-12):346–351. doi: 10.1016/0168-9525(91)90253-m. [DOI] [PubMed] [Google Scholar]
- Madueño E., Papagiannakis G., Rimmington G., Saunders R. D., Savakis C., Sidén-Kiamos I., Skavdis G., Spanos L., Trenear J., Adam P. A physical map of the X chromosome of Drosophila melanogaster: cosmid contigs and sequence tagged sites. Genetics. 1995 Apr;139(4):1631–1647. doi: 10.1093/genetics/139.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J., Santoro T., Jensen P., Siebenlist U., Yewdell J., Kelly K. Gem: an induced, immediate early protein belonging to the Ras family. Science. 1994 Jul 8;265(5169):241–244. doi: 10.1126/science.7912851. [DOI] [PubMed] [Google Scholar]
- Malenka R. C. Synaptic plasticity in the hippocampus: LTP and LTD. Cell. 1994 Aug 26;78(4):535–538. doi: 10.1016/0092-8674(94)90517-7. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Marshall C. J. Protein prenylation: a mediator of protein-protein interactions. Science. 1993 Mar 26;259(5103):1865–1866. doi: 10.1126/science.8456312. [DOI] [PubMed] [Google Scholar]
- McCombie W. R., Heiner C., Kelley J. M., Fitzgerald M. G., Gocayne J. D. Rapid and reliable fluorescent cycle sequencing of double-stranded templates. DNA Seq. 1992;2(5):289–296. doi: 10.3109/10425179209030961. [DOI] [PubMed] [Google Scholar]
- Mercer J. A., Seperack P. K., Strobel M. C., Copeland N. G., Jenkins N. A. Novel myosin heavy chain encoded by murine dilute coat colour locus. Nature. 1991 Feb 21;349(6311):709–713. doi: 10.1038/349709a0. [DOI] [PubMed] [Google Scholar]
- Mismer D., Rubin G. M. Analysis of the promoter of the ninaE opsin gene in Drosophila melanogaster. Genetics. 1987 Aug;116(4):565–578. doi: 10.1093/genetics/116.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil K. T., DeGrado W. F. How calmodulin binds its targets: sequence independent recognition of amphiphilic alpha-helices. Trends Biochem Sci. 1990 Feb;15(2):59–64. doi: 10.1016/0968-0004(90)90177-d. [DOI] [PubMed] [Google Scholar]
- Pai E. F., Krengel U., Petsko G. A., Goody R. S., Kabsch W., Wittinghofer A. Refined crystal structure of the triphosphate conformation of H-ras p21 at 1.35 A resolution: implications for the mechanism of GTP hydrolysis. EMBO J. 1990 Aug;9(8):2351–2359. doi: 10.1002/j.1460-2075.1990.tb07409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J. A., Hicks J. L., Williams D. S., Montell C. Differential localizations of and requirements for the two Drosophila ninaC kinase/myosins in photoreceptor cells. J Cell Biol. 1992 Feb;116(3):683–693. doi: 10.1083/jcb.116.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J. A., Minke B., Montell C. Calmodulin binding to Drosophila NinaC required for termination of phototransduction. EMBO J. 1995 Sep 15;14(18):4450–4459. doi: 10.1002/j.1460-2075.1995.tb00124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan R., Malicki D. M., Zuker C. S. Signal transduction in Drosophila photoreceptors. Annu Rev Neurosci. 1995;18:283–317. doi: 10.1146/annurev.ne.18.030195.001435. [DOI] [PubMed] [Google Scholar]
- Reynet C., Kahn C. R. Rad: a member of the Ras family overexpressed in muscle of type II diabetic humans. Science. 1993 Nov 26;262(5138):1441–1444. doi: 10.1126/science.8248782. [DOI] [PubMed] [Google Scholar]
- Ronnett G. V., Payne R. A tale of two senses. Neuron. 1995 Jul;15(1):11–16. doi: 10.1016/0896-6273(95)90058-6. [DOI] [PubMed] [Google Scholar]
- Rosen L. B., Ginty D. D., Weber M. J., Greenberg M. E. Membrane depolarization and calcium influx stimulate MEK and MAP kinase via activation of Ras. Neuron. 1994 Jun;12(6):1207–1221. doi: 10.1016/0896-6273(94)90438-3. [DOI] [PubMed] [Google Scholar]
- Rosen L. B., Greenberg M. E. Stimulation of growth factor receptor signal transduction by activation of voltage-sensitive calcium channels. Proc Natl Acad Sci U S A. 1996 Feb 6;93(3):1113–1118. doi: 10.1073/pnas.93.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Rusanescu G., Qi H., Thomas S. M., Brugge J. S., Halegoua S. Calcium influx induces neurite growth through a Src-Ras signaling cassette. Neuron. 1995 Dec;15(6):1415–1425. doi: 10.1016/0896-6273(95)90019-5. [DOI] [PubMed] [Google Scholar]
- Sharma R. K., Wang J. H. Purification and characterization of bovine lung calmodulin-dependent cyclic nucleotide phosphodiesterase. An enzyme containing calmodulin as a subunit. J Biol Chem. 1986 Oct 25;261(30):14160–14166. [PubMed] [Google Scholar]
- Shou C., Farnsworth C. L., Neel B. G., Feig L. A. Molecular cloning of cDNAs encoding a guanine-nucleotide-releasing factor for Ras p21. Nature. 1992 Jul 23;358(6384):351–354. doi: 10.1038/358351a0. [DOI] [PubMed] [Google Scholar]
- Sidén-Kiamos I., Saunders R. D., Spanos L., Majerus T., Treanear J., Savakis C., Louis C., Glover D. M., Ashburner M., Kafatos F. C. Towards a physical map of the Drosophila melanogaster genome: mapping of cosmid clones within defined genomic divisions. Nucleic Acids Res. 1990 Nov 11;18(21):6261–6270. doi: 10.1093/nar/18.21.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. M., Sanders J. Z., Kaiser R. J., Hughes P., Dodd C., Connell C. R., Heiner C., Kent S. B., Hood L. E. Fluorescence detection in automated DNA sequence analysis. Nature. 1986 Jun 12;321(6071):674–679. doi: 10.1038/321674a0. [DOI] [PubMed] [Google Scholar]
- Sonnenburg W. K., Seger D., Kwak K. S., Huang J., Charbonneau H., Beavo J. A. Identification of inhibitory and calmodulin-binding domains of the PDE1A1 and PDE1A2 calmodulin-stimulated cyclic nucleotide phosphodiesterases. J Biol Chem. 1995 Dec 29;270(52):30989–31000. doi: 10.1074/jbc.270.52.30989. [DOI] [PubMed] [Google Scholar]
- Spitzer N. C. Spontaneous Ca2+ spikes and waves in embryonic neurons: signaling systems for differentiation. Trends Neurosci. 1994 Mar;17(3):115–118. doi: 10.1016/0166-2236(94)90120-1. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Swanljung-Collins H., Collins J. H. Phosphorylation of brush border myosin I by protein kinase C is regulated by Ca(2+)-stimulated binding of myosin I to phosphatidylserine concerted with calmodulin dissociation. J Biol Chem. 1992 Feb 15;267(5):3445–3454. [PubMed] [Google Scholar]
- Urano T., Emkey R., Feig L. A. Ral-GTPases mediate a distinct downstream signaling pathway from Ras that facilitates cellular transformation. EMBO J. 1996 Feb 15;15(4):810–816. [PMC free article] [PubMed] [Google Scholar]
- Warr C. G., Kelly L. E. Identification and characterization of two distinct calmodulin-binding sites in the Trpl ion-channel protein of Drosophila melanogaster. Biochem J. 1996 Mar 1;314(Pt 2):497–503. doi: 10.1042/bj3140497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W., Schreiber S. S., Baudry M., Tocco G., Broek D. Localization of the cellular expression pattern of cdc25NEF and ras in the juvenile rat brain. Brain Res Mol Brain Res. 1993 Sep;19(4):339–344. doi: 10.1016/0169-328x(93)90136-d. [DOI] [PubMed] [Google Scholar]
- Xie X., Harrison D. H., Schlichting I., Sweet R. M., Kalabokis V. N., Szent-Györgyi A. G., Cohen C. Structure of the regulatory domain of scallop myosin at 2.8 A resolution. Nature. 1994 Mar 24;368(6469):306–312. doi: 10.1038/368306a0. [DOI] [PubMed] [Google Scholar]
- Zozulya S., Stryer L. Calcium-myristoyl protein switch. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11569–11573. doi: 10.1073/pnas.89.23.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker C. S., Cowman A. F., Rubin G. M. Isolation and structure of a rhodopsin gene from D. melanogaster. Cell. 1985 Apr;40(4):851–858. doi: 10.1016/0092-8674(85)90344-7. [DOI] [PubMed] [Google Scholar]