Abstract
Mechanotransduction by proprioceptive sensory organs is poorly understood. Evidence was recently shown that muscle spindle and hair follicle primary afferents (lanceolates) constantly release glutamate from synaptic-like vesicles (SLVs) within the terminals. The secreted glutamate activates a highly unusual metabotropic glutamate receptor (mGluR) to modulate the firing rate (spindles) and SLV recycling (lanceolates). This receptor has yet to be isolated and sequenced. To further investigate this receptor’s pharmacology, ligands selective for classical mGluRs have been recently characterised for their ability to alter stretch-evoked spindle firing and SLV endocytosis in these different endings. Here, it is described how the results of these screens facilitated the development of novel compounds to be used in the process of isolating and sequencing of this non-canonical mGluR. This study shows how the compounds were tested for their ability to alter stretch-evoked afferent firing in muscle spindles and SLV endocytosis in the lanceolate endings of hair follicles to ensure they maintained their ability to bind to the receptor. For the development of novel compounds, kainate was chosen as the parent ligand due to its potency and ease of chemical modification. Novel kainate derivatives were then synthesised and tested to find potent analogues suitable for ‘click-chemistry’, an established technique for relatively quick, cheap, stereospecific and high-yield chemical modifications (Angewandte Chemie (International ed. in English), 40, 2001, pp2004). Of the novel kainate analogues developed, unfortunately ZCZ49 and ZCZ50 lost the ability to produce a significant change in spindle stretch-evoked firing. However, ZCZ90 was as potent as kainate, increasing firing by a similar margin at 1 μm (n = 8; P < 0.001). The addition of either a biotin or a fluorescein side group to ZCZ90, using the click-chemistry technique, did not affect the potency and hence these compounds will be used in further studies of the receptor. As well as the development of these compounds, the study found not only many similarities, but also some key differences between the two types of primary mechanosensory endings investigated. These differences must be taken into account in further study. However, they also present an intriguing opportunity for these receptors to be targeted selectively to modulate ending sensitivity as treatments for muscle spasm in multiple sclerosis and spinal cord injury, and possibly even baroreceptor firing to treat hypertension.
Keywords: mGluR, glutamate, glutamate receptor, muscle spindle, lanceolate, PCCG-13
Introduction
Despite clearly not being presynaptic, all mechanosensory terminals investigated by electron microscopy thus far have been shown to contain a population of vesicles (Merrillees, 1960; Landon, 1972; Akoev et al. 1988; Zelena, 1994). These vesicles have a clear lumen and are 50 nm in diameter, just like synaptic vesicles. Studies have shown that they also share many other similarities with synaptic vesicles, which has led to them being termed synaptic-like vesicles (SLVs; Bewick et al. 2005). These similarities include the presence of several typically synaptic proteins, such as syntaxin 1A (Aguado et al. 1999), synapsin I and synaptophysin (De Camilli et al. 1988), and the presence of neurexin and/or latrophilin (Queiroz & Duchen, 1982). Despite their presence being known since the 1960s (Cauna, 1966), the function of these SLVs remained a mystery until investigated by Bewick et al. (2005) (also see Bewick article in this volume). Using rat lumbrical muscle spindles as the mechanosensory model, Bewick et al. (2005) firstly noted that even at rest muscle spindles take up the vesicle-marking dye FM1-43 (Fig.1). This dye was first developed for optical monitoring of synaptic vesicle recycling at synaptic endings. Unlike synaptic endings, FM1-43 staining in muscle spindles occurred even when action potential generation was blocked by tetrodotoxin (Bewick & Betz, unpublished observations). Stretching the muscle increased uptake of the dye, suggesting an important link between stretch-induced mechanotransduction and SLV recycling.
Fig 1.
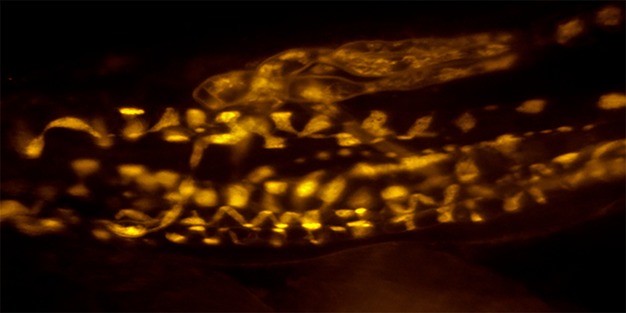
Muscle spindle stained with FM1-43. A muscle spindle at rest will spontaneously take up the dye FM1-43, which has been shown to reflect vesicle endocytosis. Photo courtesy of Dr Anna Simon. Scale bar: 50 μm.
Immunogold labelling showed that, as with the central presynaptic ending of spindle primary afferents, the sensory terminals of muscle spindles contain glutamate (Bewick et al. 2005). It was found that bathing lumbrical muscles in exogenous glutamate increased muscle spindle afferent firing rate during the static phase of ramp-and-hold stretches. Ultimately, this led to the hypothesis that SLVs release glutamate upon stretch, which activates a glutamate receptor, and in turn increases afferent firing. Preliminary experiments attempted to identify the receptor involved. Very interestingly, neither broad spectrum antagonists of ionotropic glutamate receptors (iGluRs) nor antagonists of metabotropic glutamate receptors (mGluRs), alone or in combination, abolished the excitation exogenous glutamate caused on stretch-induced afferent firing. Given these results, the group tested the effect of RS-DHPG, shown previously to antagonise a less well-known, and so far unsequenced, receptor: the phospholipase D-coupled mGluR (the PLD-mGluR; Pellegrini-Giampietro et al. 1996). The first evidence for the existence of this glutamate receptor coupled to PLD was presented by a study that found PLD activation in hippocampal slices caused by the mGluR agonist ACPD (Boss & Conn, 1992). The pharmacology of this PLD activation suggested an unknown glutamate receptor, as iGluR antagonists were unable to attenuate it, and it was also activated by the mGluR antagonist (l)-2-amino-3-phosphonopropionic acid (Boss & Conn, 1992), a response counter to that expected if activating a mGluR from the three known groups. Giving further evidence of the presence of a novel mGluR with a unique pharmacology, Pellegrini-Giampietro et al. (1996) found the rank order of potency for PLD activation in the hippocampus did not match any currently sequenced mGluR. Until the work of Bewick et al. (2005), this pharmacology had not been seen outside the hippocampus. This included the atypical inhibition by the classical group I mGluR agonist, RS-DHPG. This same response was found in spindles, as RS-DHPG totally inhibited the exogenous glutamate-induced increase in excitability. A specific antagonist PCCG-13 was later developed for this PLD-mGluR by the same group (Albani-Torregrossa et al. 1999). This was found to totally attenuate the effect of exogenous glutamate on stretch-induced afferent firing. Not only that, this highly selective antagonist inhibited firing when applied alone, and could even reversibly abolish firing completely if applied for prolonged periods (6–10 h). This suggests three things. First, that there is tonic endogenous glutamate secretion to activate this receptor; second, that without tonic activation of the PLD-mGluR by this endogenous glutamate, the spindle terminal loses its ability to respond to stimuli; and third, that the PLD-mGluR is the only glutamate receptor present in muscle spindles.
Given that SLVs are present in all primary mechanosensory nerve terminals, our group subsequently sought another preparation to examine their properties. This would be used to test both the ubiquity of this SLV/glutamate system and make studying the SLV recycling process easier. Prof. Clarke Slater (Newcastle University) suggested the lanceolate endings of rodent hair follicles – particularly the mouse ear skin. He had found they labelled with FM1-43, a fluorescent amphipathic membrane dye used to study vesicle membrane recycling (Betz & Bewick, 1992), in only 30 min, which is much quicker than the 2 h for spindle afferents. This labelling was also Ca2+ dependent. The term ‘lanceolate’ ending comes from the shape of the somatosensory terminals surrounding individual hair shafts that form the mechanosensory ending. Each terminal runs parallel to the long axis of the hair follicle, surrounded by processes from a glial Schwann cell, with the terminals forming a ‘palisade’ ring of endings round the hair shaft (as seen in fig. 3.1 in Halata, 1993). These endings are rapidly adapting and therefore, along with their anatomy, differ from muscle spindles. Because of these differences, their FM1-43 labelling was very interesting, as it supported the general hypothesis of an importance of the SLV/glutamate system in differing mechanosensory endings.
PLD-mGluR pharmacology in spindle and lanceolate endings
As part of the author’s PhD study, this observation of spontaneous FM1-43 uptake was extended, by finding FM1-43 uptake into lanceolate endings was also enhanced by exogenous glutamate. This labelling reflected changes in basal spontaneous uptake, as the hair follicles were not stimulated mechanically during these experiments. Subsequent extensive pharmacological characterisation of the glutamate receptor in both these mechanosensory systems then revealed that both physiological responses (increases in spindle afferent firing and lanceolate SLV turnover) relied exclusively on the PLD-mGluR, with little evidence for the presence of other receptors. The glutamate pharmacological characterisation of the labelling of lanceolate endings with FM1-43 has been published (Banks et al. 2013), while the pharmacology of the stretch-induced afferent firing in muscle spindles is currently being submitted elsewhere. Here, additional aspects arising from this pharmacology are presented: one from each experimental system. The first uses the electrophysiology of spindle firing to identify a PLD-mGluR ligand that could be functionalised to use for receptor isolation; and the second describes how the pharmacology of the lanceolate FM1-43 uptake assay suggests ‘the’ PLD-mGluR may actually be a receptor family. The methods used here are as detailed in Bewick et al. (2005) for the muscle spindle work, and Banks et al. (2013) for the lanceolate work. In brief, the spindle firing was assessed by quantifying the total firing frequency in the whole muscle nerve leading from the rat 4th lumbrical muscle when stretched by 10% in a ramp-hold-release trapezoid. As under control conditions, the response to stretch varies between preparations, a pre-drug control set of responses was taken for each preparation. The response following drug incubation was then expressed as a percentage change from pre-drug control. For lanceolate ending uptake of FM1-43, mouse ear skin was pinned dermal side up and exposed to FM1-43 in the absence or presence of various glutamate receptor ligands. The resulting net fluorescence intensity was then quantified in an annulus demarcating the ring of terminals surrounding each follicle. In both preparations, data from all terminals under a given paradigm were pooled and analysed as a homogenous population, reflecting the overall response from all terminals.
Developing a functionalised ligand for PLD-mGluR isolation
The author’s research revealed mechanosensory endings are clearly an enriched and essentially pure source of PLD-mGluRs from which they might perhaps be isolated for further study at the molecular and cellular level. The aim here, therefore, was to identify a suitable agonist from the range of compounds tested as a basis for the synthesis of biotinylated and fluorescent versions to extract this unsequenced receptor from spindle homogenates. The ideal agonist would produce strong receptor activation and have a chemical structure that either already facilitated side-group addition or could be easily modified to do so without destroying the activity of the core structure. Unfortunately, PCCG-13 failed the second criterion. It is difficult to manufacture and its selectivity is exquisitely dependent on a specific three-dimensional configuration (Pellicciari et al. 1999).
From the range of compounds that enhanced stretch-induced afferent firing in muscle spindles, kainate was chosen as the parent compound for further development. Medicinal chemist colleagues in the Kosterlitz Centre for Therapeutics in the Institute of Medical Sciences suggested the structure of kainate could easily be modified to make it suitable for ‘click-chemistry’ (Kolb et al. 2001). This technique requires a core molecule with certain specific properties to be synthesised to allow the direct addition and removal of side-chains (e.g. biotin, fluorophores) in a relatively direct manner using the established procedures of ‘click-chemistry’. This avoids the laborious task of de novo synthesis of each variant from its simpler constituents, which is required by other synthetic approaches. Our goal was to use this technique to facilitate the relatively simple synthesis of biotinylated and fluorescent analogues of the core molecule.
Using spindle firing to assay kainate derivatives as tools for PLD-mGluR isolation
Kainate was identified as a suitable ligand structure for the following reasons. It is a potent enhancer of stretch-induced afferent firing in muscle spindles (Fig.2). Importantly, this enhancement is not attenuated by NBQX, the kainate receptor antagonist, but it is by the selective PLD-mGluR antagonist, PCCG-13. Because spindles are also not inhibited by kynurenic acid (Bewick et al. 2005), kainate is clearly not acting through a classical iGluR kainate receptor either, but rather via the PLD-mGluR. Finally, its structure was much less constrained in three dimensions than PCCG-13.
Fig 2.
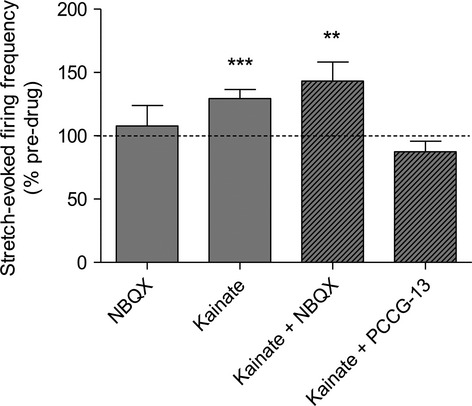
Kainate’s enhancement of stretch-induced afferent firing is blocked by PCCG-13 but not the kainate receptor antagonist NBQX. Mean percentage increase in stretch-induced afferent firing measured in the whole muscle nerve as a percentage of its pre-drug control. Each muscle contains 8–12 muscle spindles. All drugs were applied at 10 μm, determined previously to produce a maximal effect. NBQX: n = 4; kainate: n = 7; kainate + NBQX: n = 7; kainate + PCCG-13: n = 7. **P < 0.001, ***P < 0.0001.
Three functionalising side-groups were assessed for kainate. It was essential that any modification must still bind the receptor, i.e. it retained agonist properties. The details of their synthesis can be found elsewhere (Zanato et al. 2014), but their structures are given in Fig.3. Two of the functionalising modifications (ZCZ49 and ZCZ50) destroyed all agonist activity in muscle spindles. However, adding a triazole group (ZCZ90) retained the potent agonist activity of kainate (Fig.4). Agonism of ZCZ90 was found to be blocked by PCCG-13, indicating the activity was through the PLD-mGluR (Fig.4d). A biotinylated (ZCZ180) and a fluorescent (ZCZ172) version of ZCZ90 were then synthesised by ‘click-chemistry’, and these also both retained the ability to enhance stretch-induced afferent firing in muscle spindles (Fig.5). The biotinylated compound (ZCZ180) is now being used in another project seeking to isolate the receptor from spindle homogenates in two ways: labelling protein bands in ligand blots of spindle homogenates following sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE); and immobilised on Sephadex beads in affinity columns to pull out binding proteins.
Fig 3.
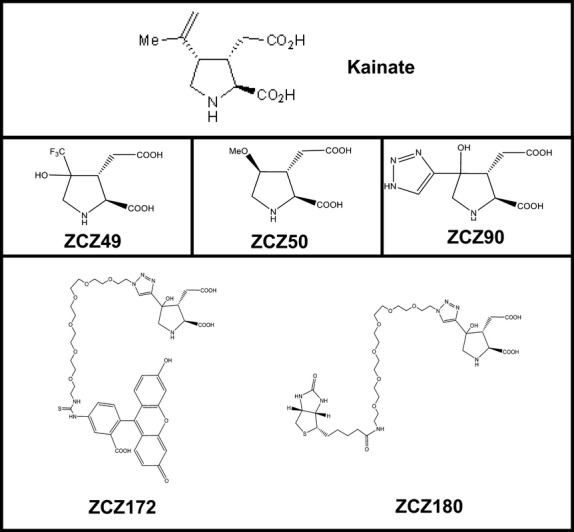
Chemical structures of novel kainate derivatives – the ZCZ compounds. The upper panel shows the original structure of kainate. The middle row shows kainate derivative compounds, each functionalised by the addition of a side-group at carbon 3 to make it amenable to ‘click-chemistry’. To achieve this, each required to be synthesised from different simpler constituents to arrive at the final structure illustrated, each synthesis requiring different low-yield and complex intermediates and chemical reactions. Only ZCZ90 retained its activity (Fig.4). The lower row shows further development of the triazole derivative ZCZ90 to add either a fluorescein (ZCZ172) or biotin (ZCZ180) side-chain directly to the compound by click-chemistry. The fact that both compounds started directly from ZCZ90 as the initial component, and not its constituent parts, indicates the great utility of this technique.
Fig 4.
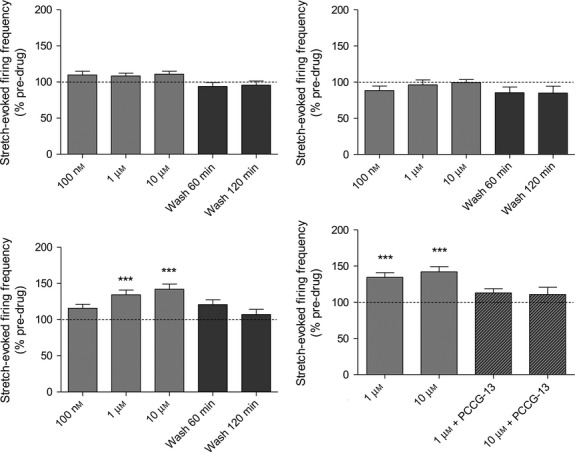
Only ZCZ90 retained its agonism of stretch-induced afferent firing in muscle spindles, and this action is attenuated by PCCG-13. Kainate derivatives. (a) ZCZ49 and (b) ZCZ50 lost the potent agonist action of kainate on stretch-induced afferent firing in muscle spindles, even at 10 μm, while derivative (c) ZCZ90 significantly increased firing at only 1 μm, (d) Agonism of ZCZ90 is blocked by PCCG-13 (10 μm), showing it retains affinity for the PLD-mGluR. ***P = 0.0001 vs. control.
Fig 5.
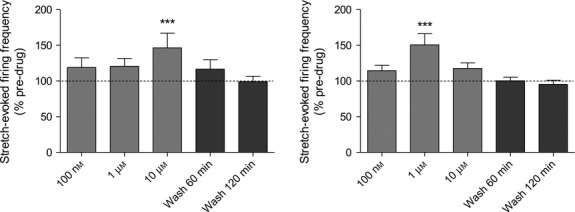
ZCZ172 and ZCZ180 retain agonist activity on stretch-evoked muscle spindle firing. (a) ZCZ172 and (b) ZCZ180, the fluorescent and biotinylated versions of ZCZ90, respectively, both retain their ability to enhance evoked firing in muscle spindles. ***P = 0.0001
Evidence from comparative pharmacology that ‘the’ PLD-mGluR may constitute a receptor family
As mentioned above, dye uptake (SLV endocytosis) is enhanced by exogenous glutamate (Simon et al. 2010). Because SLVs are found in all mechanosensory endings, a further aim of the author’s PhD work was to compare the glutamate pharmacology of SLV endocytosis with that of afferent firing (in spindles) and PLD activity (in the hippocampus), which are both regulated by the PLD-mGluR. As lanceolate endings, the mechanosensory endings associated with hair follicles, took up and released FM1-43 much more quickly than spindles, this is the preparation used in these investigations.
The lanceolate ending is one of a group of afferent terminals forming a palisade-like structure around individual hair shafts. This entire system is all enclosed by a sebaceous gland (Fig.6A). After being stained with FM1-43, this palisade structure produces a characteristic ring of labelling when viewed en face from the dermal surface (Fig.6). Electron micrographs of transverse sections of follicles show each terminal runs parallel to the long axis of the hair shaft. Each is sandwiched between processes from a glial support cell, with the blade-like terminals forming a ring of endings round the hair shaft (Fig.6C). Figure6C also particularly shows a sensory terminal full of SLVs in the terminal axoplasm. As FM1-43 uptake is indicative of SLV endocytosis (Bewick et al. 2005), measuring how the staining intensity of FM1-43 is affected by various glutamate ligands allows the pharmacology of SLV endocytosis to be monitored. Some key aspects of this pharmacology are presented here.
Fig 6.
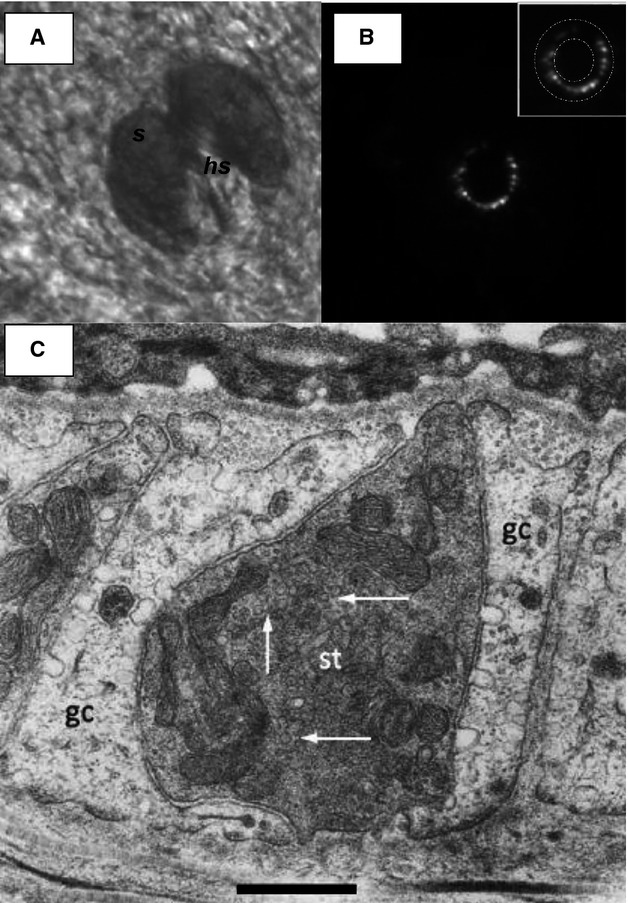
Lanceolate endings stain with FM1-43. (A) Electron micrograph through a lanceolate ending. (A) Brightfield image of a hair follicle in mouse ear skin in situ, from the dermal aspect showing the sebaceous gland (s) surrounding a central hair shaft (hs). Scale bar (also for B) indicates 50 μm. (B) FM1-43 fluorescence of lanceolate ending in the same follicle as (A), showing the appearance of the endings viewed along their length, orthogonal to their long axis. Note the almost complete circle of lanceolate terminals, forming a palisade to effectively detect hair movement in almost any direction. The inset shows the area of analysis used to measure follicle-ending fluorescence intensity. The intensity of a nearby background area was then subtracted to determine the final net intensity. (C) The more darkly stained sensory terminal (st) can be seen to be almost completely surrounded by glial cell processes (gc). SLVs present in the terminal axoplasm are indicated by white arrows. Scale bar: 0.5 μm. Micrograph from Banks et al. (2013).
As with stretch-evoked firing in muscle spindles, both glutamate and kainate increased, while PCCG-13 decreased, FM1-43 uptake (Fig.7). This, along with responses to most other ligands, including the lack of antagonism with MCPG, CPPG and kynurenate, which block all the cloned receptors (Banks et al. 2013), indicates the modulation of SLV endocytosis has a very similar pharmacology to that of the PLD-mGluR of spindles and the hippocampus. However, there were subtle differences in detail. While it had no effect on stretch-induced afferent firing in muscle spindles, at 100 μm the broad spectrum antagonist of cloned mGluRs LY341495 did inhibit FM1-43 uptake. Differences were also found between the two mechanosensory assay systems in response to certain other ligands. For example, L-CSA, which potently enhances stretch-induced afferent firing in muscle spindles, has no effect on FM1-43 uptake in lanceolates. Thus, while the overall pharmacology is very similar in lanceolates and spindles, details differ for a small number of specific ligands. Moreover, despite large instances of commonality, there are also small differences (e.g. sensitivity to ibotenate) from the pharmacology of the PLD-mGluR in the hippocampus. Thus, these data suggest that although a receptor inhibited by the selective PLD-mGluR inhibitor PCCG-13 appears pivotal in all three tissues, there are key differences between them. This subtle variability may indicate that there is either a family of such receptors, or a range of downstream signalling partners to which it can be coupled. Untangling these possibilities must await the isolation and sequencing on the receptor involved, which is being undertaken in another project in the laboratory. It is clearly important that these pharmacological differences are taken into consideration when interpreting outcomes of further studies. However, they may also indicate the intriguing opportunity that the receptors may be selectively targeted with drugs. This could be useful if different tissues need to be targeted by drug treatments for mechanosensory diseases. For example, there is increasing evidence that spindle hyper-excitability contributes to muscle spasm, spasticity and pain following spinal cord injury (Rosales & Dressler, 2010), which may be alleviated by selectively inhibiting the pharmacological variant of the PLD-mGluR receptor in muscle receptors. Another intriguing example is its potential as a target in antihypertensive treatments. Funding has just received from the British Heart Foundation to explore a receptor with similar pharmacology in baroreceptors, the stretch-sensitive endings monitoring blood pressure in major blood vessels. There is increasing evidence that increasing activity of baroreceptors successfully reduces blood pressure by ∼30 mmHg in resistant hypertensives. This is usually achieved by implanting electrical electrodes to stimulate the carotid sinus (Zhang, Zhou et al. 2014). However, the expense, surgical risks and discomfort may be avoided by a simple agonist drug targeting the same endings.
Fig 7.
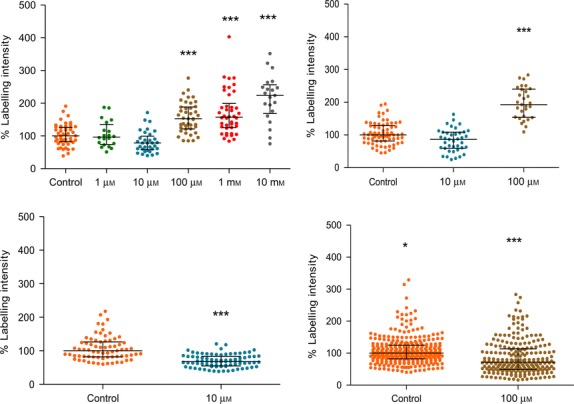
FM1-43 uptake of lanceolate endings is modulated by glutamate receptor ligands, indicating it involves the PLD-mGluR. Scatter dot plots of change in FM1-43 intensity as a percentage of drug-free controls. It can be seen that glutamate and kainate both increase, while PCCG-13 decreases intensity, i.e. SLV endocytosis. This is in line with findings in muscle spindles, where they have analogous effects on afferent discharge rates. The effect of LY341495, however, shows that there are differences between these systems, as here it decreases SLV endocytosis whereas it has no effect in muscle spindle firing. Each dot represents a single lanceolate’s FM1-43 intensity as a percentage of the control median. Lines show median intensity of all lanceolates measured, and whiskers indicate the interquartile range of data values (25% and 75%). The width of the distribution is arbitrary, but predominantly reflects the frequency of follicles of that intensity, as the first point is plotted in centrally, and subsequent points increasingly laterally, to minimise overlap and display as many points as possible. For the LY341495 data, many points are seen to overlap, to constrain the lateral spread of the column. Each data set is from 20 to 316 follicles (1 pallisade of lanceolate endings per follicle) per preparation, from 4 to 12 preparations. *P = 0.01, ***P = 0.0001.
References
- Aguado F, Majó G, Ruiz-Montasell B. Syntaxin 1A and 1B display distinct distribution patterns in the rat peripheral nervous system. Neuroscience. 1999;88:437–446. doi: 10.1016/s0306-4522(98)00247-4. [DOI] [PubMed] [Google Scholar]
- Akoev G, Alekseev N, Krylov B. Mechanoreceptors: Their Functional Organization. Berlin: Springer; 1988. [Google Scholar]
- Albani-Torregrossa S, Attucci S, Marinozzi M. Antagonist pharmacology of metabotropic glutamate receptors coupled to phospholipase D activation in adult rat hippocampus: focus on (2R,1’S,2’R,3’S)-2-(2’-carboxy-3’-phenylcyclopropyl)glycine versus 3, 5-dihydroxyphenylglycine. Mol Pharmacol. 1999;55:699–707. [PubMed] [Google Scholar]
- Banks RW, Cahusac PMB, Graca A. Glutamatergic modulation of synaptic-like vesicle recycling in mechanosensory lanceolate nerve terminals of mammalian hair follicles. J Physiol. 2013;591:2523–2540. doi: 10.1113/jphysiol.2012.243659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W, Bewick G. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science. 1992;255:200–203. doi: 10.1126/science.1553547. [DOI] [PubMed] [Google Scholar]
- Bewick GS, Reid B, Richardson C, et al. Autogenic modulation of mechanoreceptor excitability by glutamate release from synaptic-like vesicles: evidence from the rat muscle spindle primary sensory ending. J Physiol. 2005;562:381–394. doi: 10.1113/jphysiol.2004.074799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss V, Conn PJ. Metabotropic excitatory amino acid receptor activation stimulates phospholipase D in hippocampal slices. J Neurochem. 1992;59:2340–2343. doi: 10.1111/j.1471-4159.1992.tb10131.x. [DOI] [PubMed] [Google Scholar]
- Cauna N. Fine structure of the receptor organs and its probable functional significance. In: De Reuck A, Knight J, editors. Touch, Heat and Pain. Churchill, London: John Wiley; 1966. pp. 117–136. [Google Scholar]
- De Camilli P, Vitadello M, Canevini MP. The synaptic vesicle proteins synapsin I and synaptophysin (protein P38) are concentrated both in efferent and afferent nerve endings of the skeletal muscle. J Neurosci. 1988;8:1625–1631. doi: 10.1523/JNEUROSCI.08-05-01625.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halata Z. Sensory innervation of the hairy skin (light- and electronmicroscopic study. J Invest Dermatol. 1993;101(Suppl 1):75S–81S. doi: 10.1111/1523-1747.ep12362877. [DOI] [PubMed] [Google Scholar]
- Kolb HC, Finn MG, Sharpless KB. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew Chem Int Ed Engl. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Landon DN. The fine structure of the equatorial regions of developing muscle spindles in the rat. J Neurocytol. 1972;1:189–210. doi: 10.1007/BF01099184. [DOI] [PubMed] [Google Scholar]
- Merrillees N. The fine structure of muscle spindles in the lumbrical muscles of the rat. J Biophys Biochem Cytol. 1960;7:725–742. doi: 10.1083/jcb.7.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini-Giampietro DE, Torregrossa SA, Moroni F. Pharmacological characterization of metabotropic glutamate receptors coupled to phospholipase D in the rat hippocampus. Br J Pharmacol. 1996;118:1035–1043. doi: 10.1111/j.1476-5381.1996.tb15503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicciari R, Marinozzi M, Costantino G. (2R,1’S,2’R,3’S)-2-(2’-Carboxy-3’-phenylcyclopropyl)glycine (PCCG-13), the first potent and selective competitive antagonist of phospholipase D-coupled metabotropic glutamate receptors: asymmetric synthesis and preliminary biological properties. J Med Chem. 1999;42:2716–2720. doi: 10.1021/jm990128v. [DOI] [PubMed] [Google Scholar]
- Queiroz LS, Duchen LW. Effects of Latrodectus spider venoms on sensory and motor nerve terminals of muscle spindles. Proc R Soc Lond B Biol Sci. 1982;216:103–110. doi: 10.1098/rspb.1982.0063. [DOI] [PubMed] [Google Scholar]
- Rosales RL, Dressler D. On muscle spindles, dystonia and botulinum toxin. Eur J Neurol. 2010;17(Suppl 1):71–80. doi: 10.1111/j.1468-1331.2010.03056.x. [DOI] [PubMed] [Google Scholar]
- Simon A, Shenton F, Hunter I, Banks RW, Bewick GS. Amiloride-sensitive channels are a major contributor to mechanotransduction in mammalian muscle spindles. J Physiol. 2010;588:171–185. doi: 10.1113/jphysiol.2009.182683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanato C, Watson S, Bewick GS, et al. Synthesis and biological evaluation of (-)-kainic acid analogues as phospholipase D-coupled metabotropic glutamate receptor ligands. Org Biomol Chem. 2014;12:9638–9643. doi: 10.1039/c4ob02002b. [DOI] [PubMed] [Google Scholar]
- Zelena J. Nerves and Mechanoreceptors. London: Chapman & Hall; 1994. [Google Scholar]
- Zhang X, Zhou Z, Steiner TJ, et al. Validation of ICHD-3 beta diagnostic criteria for 13.7 Tolosa-Hunt syndrome: Analysis of 77 cases of painful ophthalmoplegia. Cephalalgia. 2014;34:624–632. doi: 10.1177/0333102413520082. [DOI] [PubMed] [Google Scholar]


