Abstract
Background and Purpose
Increased level of very low-density lipoprotein (VLDL) is a key feature of the metabolic syndrome and is associated with cardiovascular diseases. PPAR-δ agonists play a protective role in lipid metabolism and vascular function. In this study, we aimed to investigate the role of PPAR-δ in the uptake of VLDL in endothelial cells and its underlying mechanism(s).
Experimental Approach
Uptake of VLDL in HUVECs was assessed by Dil-fluorescent labelling of VLDL. Levels of VLDL receptor mRNA and microRNA (miR-100) were detected by quantitative PCR. The target genes of miR-100 were predicted using bioinformatics analysis. 3′-Untranslated region (3′-UTR) luciferase reporter and Argonaute 1 pull-down assays were used to validate the target of miR-100.
Key Results
PPAR-δ agonist GW501516 decreased uptake of VLDL and expression of VLDL receptor at mRNA and protein levels. GW501516 inhibited the luciferase reporter activity of the 3′-UTR of VLDL receptor. VLDL receptor was a direct target of miR-100. miR-100 was significantly increased by GW501516 in HUVECs. Transfection of a miR-100 mimic decreased the mRNA and protein levels of VLDL receptor and uptake of VLDL. Furthermore, a miR-100 inhibitor abolished the inhibitory effect of PPAR-δ on VLDL receptor expression and VLDL uptake.
Conclusions and Implications
In endothelial cells, activation of PPAR-δ decreased VLDL receptor expression and VLDL uptake via the induction of miR-100. These results provided a novel mechanism for the vascular protective effect of PPAR-δ agonists.
Tables of Links
| TARGETS |
|---|
| Nuclear hormone receptorsa |
| PPAR-δ (NR1C2) |
| Transportersb |
| ABCA1, cholesterol efflux regulatory protein |
| LIGANDS |
|---|
| GSK0660 |
| GW501516 |
| GW0742 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,bAlexander et al., 2013a,b).
Introduction
Hypertriglyceridaemia is widely recognized as a risk factor for cardiovascular diseases, especially coronary artery diseases (Carroll et al., 2005; Miller et al., 2011). Very low-density lipoprotein (VLDL) is a major carrier of plasma triglyceride. Epidemiological studies showed that a high level of plasma VLDL predicts coronary events and contributes to atherogenesis (Hodis, 1999; Kugiyama et al., 1999; Nordestgaard et al., 2007). Also, VLDL increased endothelial permeability and apoptosis and enhanced inflammatory response, suggesting a direct impact of VLDL on atherogenesis through endothelial cell (EC) uptake (Eiselein et al., 2007; Libby, 2007). The VLDL receptor, a member of the LDL receptor superfamily, binds and internalizes VLDL and other apoE-containing lipoproteins (Takahashi et al., 1992). VLDL receptor is expressed ubiquitously in peripheral tissues including vascular tissues and particularly increased in atherosclerotic lesions (Karls et al., 1992; Wyne et al., 1996; Hiltunen et al., 1998), which suggests the involvement of VLDL receptor in the development of atherosclerosis.
PPARs are ligand-activated transcription factors belonging to the nuclear receptor superfamily. Three isoforms of PPAR (α, β/δ and γ) play important roles in controlling lipid metabolism and energy homeostasis. PPAR-δ has many endogenous ligands, including saturated and polyunsaturated fatty acid. Synthetic ligands include GW501516, GW0742 and L0165041 (Wang, 2008). These synthetic ligands have been used in mouse models of obesity and diabetes. GW501516, which is 1000-fold selective for PPAR-δ over the other PPAR subtypes, reduces weight gain and circulating triglyceride level, while increasing HDL in high-fat diet-induced obese mice, db/db and ob/ob mice, through stimulating peripheral fatty acid catabolism (Oliver et al., 2001; Tanaka et al., 2003). GW501516 and GW0742 both improve insulin sensitivity and rescue hepatic steatosis in diabetic models (Lee et al., 2006; Qin et al., 2008). Moreover, PPAR-δ agonists significantly reduce atherosclerotic lesions in LDL receptor-/- mice and restore endothelial function in diabetic mice. The potential mechanism for the vascular protective effects is that PPAR-δ regulates endothelial survival, activation and inflammation (Li et al., 2004; Graham et al., 2005; Barish et al., 2008; Tian et al., 2012). In the context of lipoprotein transport, GW501516 up-regulates expression of the cholesterol efflux regulatory protein ABCA1 and increases apolipoprotein A1-specific cholesterol efflux in human THP-1 monocytes. PPAR-δ is a VLDL sensor in macrophages (Chawla, 2003) and VLDL directly regulates gene expression via the activation of PPAR-δ. However, the role of PPAR-δ in the regulation of VLDL uptake has not been assessed.
MicroRNAs (miRNAs) are a family of highly conserved, small non-coding RNA molecules, that regulate gene expression at the post-transcriptional level via the degradation or translational repression of their target mRNAs (Filipowicz et al., 2008). As a transcription factor, PPAR-δ regulates both protein-coding and non-coding gene expressions (Yin et al., 2010). In this study, we have identified miR-100 as a potential target of PPAR-δ and that this miRNA consequently regulates the expression of VLDL receptor and VLDL uptake.
Methods
Cells
HUVECs were cultured as described previously (Qin et al., 2007). The investigation conformed to the principles outlined in the Declaration of Helsinki. Informed consent was documented and the protocol was approved by Institutional Ethic Committee. HEK293 cells (ATCC CRL-1573) were cultured in DMEM with 10% FBS.
Quantitative RT-PCR
Total RNA was isolated with TRIzol reagent. Quantitative RT-PCR (qRT-PCR) for microRNA was performed using the Taqman miR assay kits and TaqMan real-time PCR Master Mixes (Applied Biosystems, Foster City, CA, USA). Levels of miRs were normalized to the internal control U6 snRNA. Levels of mRNA for the VLDL receptor were measured using specific primers (forward: 5′-ACC TGA ATG ATG CCC AAG AC; reverse: 5′- CTC GGC CAT TTT CCT CTA CA) and normalized to the housekeeping gene GAPDH using SYBR Green real-time PCR Master Mixes (Applied Biosystems). The relative levels of gene expression were expressed as fold changes in relation to the control treatment.
Immunoblotting
Cellular proteins were extracted with lysis buffer [50 mmol·L−1 Tris-HCl, pH 7.5, 15 mmol·L−1 EGTA, 100 mmol·L−1 NaCl, 0.1% (wt/vol) Triton X-100] supplemented with the protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA). The samples were resolved on a 10% SDS-PAGE and blotted to nitrocellulose membranes. Immunoblots were reacted with primary antibodies at 1:1000 dilution, detected with HRP-conjugated secondary antibodies and visualized by enhanced chemoluminescence system.
Transfection, plasmid and reporter assay
The double-stranded miRNA mimic was designed to supplement endogenous miRNA activity, while the single-stranded inhibitor was designed to specifically bind to and inhibit endogenous miRNA activity. The miRNA mimic and inhibitor were transfected into HUVECs using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) at a final concentration of 5 nM or 40 nM respectively. Cells were harvested after 24 h of transfection.
The VLDL receptor 3′-untranslated region (3′-UTR) was cloned by PCR from the genomic DNA with specific primers (forward: 5′- AGC TTT GGA TGT GGT TAC CG; reverse: 5′- CTC AGT CTT TGC AAA CCT CCA). The amplified product was cloned into Hind III and Spe I of the pMir-Report luciferase vector (Ambion, Austin, TX, USA) to generate VLDL receptor 3′-UTR reporter construct (wt-VLDL receptor 3′-UTR-luc). The VLDL receptor 3′-UTR mutation vector (mu-VLDLR 3′-UTR) was generated using the method of site-directed mutagenesis as previous described (Laible and Boonrod, 2009). The parental plasmid (wt-VLDL receptor 3′-UTR-luc) was amplified using the primer-contained mutated sequence (GGT TGG GAC AAT GGC AAT AGG ACA AAG AAT TCT ACT AAG ATG AAA TTG CCA AAA AAA) and KOD DNA polymerase (Millipore, Billerica, MA, USA). After DpnI digestion, the plasmid was subjected to the transformation. The mutant construct was confirmed by sequencing. pcDNA3.1-PPAR-δ and miR-100 mimic were co-transfected into HEK293 cells using Lipofectamine 2000. For luciferase assay, the pRSV-β-galactosidase plasmid was co-transfected with the luciferase reporter vector to normalize the transfection efficiency. Luciferase and β-galactosidase activities were measured as described previously (Wang et al., 2013).
Immunoprecipitation of argonaute (AGO)-miRNA-mediated silencing complex (miRISC)
HUVECs were harvested and lysed with a buffer containing 50 mM Tris-HCl at pH 7.4, 150 mM NaCl, 0.5% NP-40, 0.5% sodium deoxycholate, 100 U·μL−1 RNase inhibitor and 1 mM PMSF. The lysates were incubated with anti-Ago1 antibody. After incubation at 4°C overnight, the immune complexes were pulled down with protein A/G sepharose beads for 4 h and washed with the lysis buffer. Following the last wash, the beads were then centrifuged and the immunoprecipitated RNAs were extracted with TRIzol and analysed by qRT-PCR (Chen et al., 2013). The level of VLDL mRNA indicated the association of VLDL receptor mRNA with AGO-containing miRISC.
VLDL uptake assay
The uptake of VLDL was measured by Dil-VLDL. HUVECs were cultured on glass-bottom culture dishes (MatTek Corp., Ashland, MA, USA) and treated with GW501516 for 36 h. The assay was performed in a serum-free medium containing 1% BSA. Cells were incubated with Dil-VLDL (10 mg·mL−1 in pre-incubation medium) for 2 h at 37°C, then washed three times with probe-free medium (Takahashi et al., 1992). Fluorescence confocal microscopy was performed using 514 nm excitation and 550 nm emission filters to visualize Dil-VLDL. Hoechst dye was used to counterstain nuclei. The fluorescence intensity was determined from 200 cells normalized to the areas of the cells.
Data analysis
Data were expressed as means ± SEM from at least three independent experiments. Student’s t-test or two-way anova were used for statistical analyses. P < 0.05 was considered significant.
Materials
GW501516 (({4-[({4-methyl-2-[4-(trifluoromethyl)phenyl]-1,3-thiazol-5-yl}methyl)sulfanyl]-2-methylphenoxy}acetic acid)), a selective agonist of PPAR-δ, was synthetized by WuXi Pharmatech Inc. (Shanghai, China), as described previously (Oliver et al., 2001; Fan et al., 2007). The specific PPAR-δ antagonist GSK0660 was obtained from Tocris Bioscience (Bristol, UK). Dil-labelled VLDL (Dil-VLDL) (BT-922) was from Biomedical Technologies Inc. (Stoughton, MA, USA). The VLDL receptor (6A6) mouse monoclonal antibody (sc-18824) was obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA) and the rabbit polyclonal argonaute 1 (Ago1) antibody (#9338) was from Cell Signaling Inc. (Danvers, MA, USA). The miRNA mimic and inhibitors were from Genepharma (Shanghai, China).
Results
Activation of PPAR-δ decreases VLDL receptor expression and VLDL uptake in vascular ECs
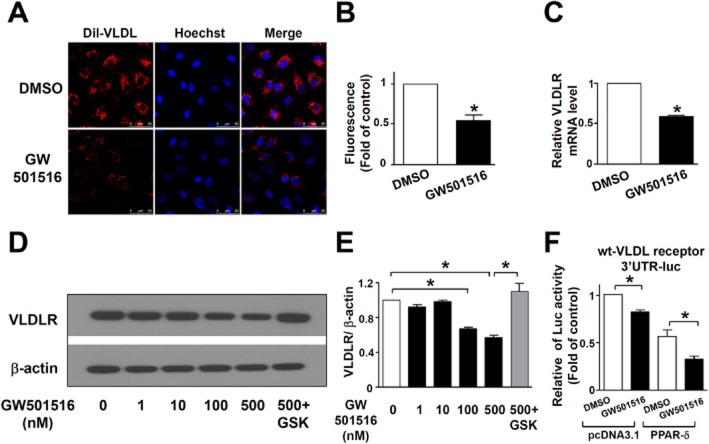
To examine the effect of PPAR-δ on endothelial VLDL uptake, HUVECs were pretreated with the PPAR-δ agonist GW501516 for 24 h before incubation with Dil-VLDL. By tracing Dil fluorescence, we found that GW501516 decreased Dil-VLDL uptake into HUVECs compared with DMSO control (Figure 1A and B).
Figure 1.
GW501516 reduces VLDL uptake through decreased expression of VLDL receptor (VLDLR). (A) Representative fluorescence micrographs showing the fluorescently labelled VLDL (Dil-VLDL) uptake into HUVECs after 36 h treatment with GW501516 (500 nM) or DMSO control. Dil-VLDL is shown in red and nuclei in blue; n = 4. (B) Quantification of Dil-VLDL uptake into HUVECs. (C) VLDL receptor mRNA level after 36 h GW501516 (500 nM) or DMSO control treatment in HUVECs; n = 4. (D) Representative immunoblots for VLDL receptor in HUVECs after 36 h GW501516 in different concentrations or in the present of GSK0660. DMSO treatment was used as a control; n = 6. (E) Quantification of VLDL receptor immunoblotting results. (F) The effect of GW501516 (500 nM) or DMSO control treatment on the luciferase activity of the wt-VLDL receptor 3′-UTR-luc in HEK293 cells co-transfected with pcDNA3.1-PPAR-δ or pcDNA3.1 control vector; n = 3. Data are presented as mean ± SEM. *P < 0.05, significantly different from DMSO control.
To determine if the effect of GW501516 was mediated by modulation of VLDL receptor we measured the levels of VLDL receptor protein and mRNA by Western blotting and qRT-PCR. Both the mRNA and protein levels of the VLDL receptor were decreased by GW501516 (Figure 1C–E).
To assess the involvement of miRNAs in the regulation of VLDL receptor expression by PPAR-δ, we co-transfected wild-type VLDL receptor 3′-UTR (wt-VLDL receptor 3′-UTR-luc) with either pcDNA 3.1-PPAR-δ or pcDNA 3.1 control vector in HEK293 cells. As shown in Figure 1F, the luciferase activity was decreased with GW501516 treatment in control cells and was further decreased in cells with PPAR-δ overexpression, indicating that the 3′-UTR of the VLDL receptor gene was a target of the PPAR-δ action.
VLDL receptor is a target of miR-100
By using Pictar (http://pictar.mdc-berlin.de/) to predict microRNA target sites in the 3′-UTR of VLDL receptor, we found that miR-100 might conservatively target this 3′-UTR mRNA in several species (human, chimpanzee, mouse, rat, dog and chicken; Supporting Information Fig. S1). This analysis led us to focus on the role of miR-100 in our subsequent experiments. We first transfected a miR-100 mimic in HUVECs and, as shown in Figure 2A, found this transfection increased the miR-100 level ∼20-fold, compared with the control RNA. Immunoblotting and qRT-PCR analyses revealed that this transfection also decreased both VLDL receptor protein and mRNA levels (Figure 2B–D). The functional consequence of the transfection of miR-100 mimic was a significantly decreased Dil-VLDL uptake (Figure 2E and F),.
Figure 2.
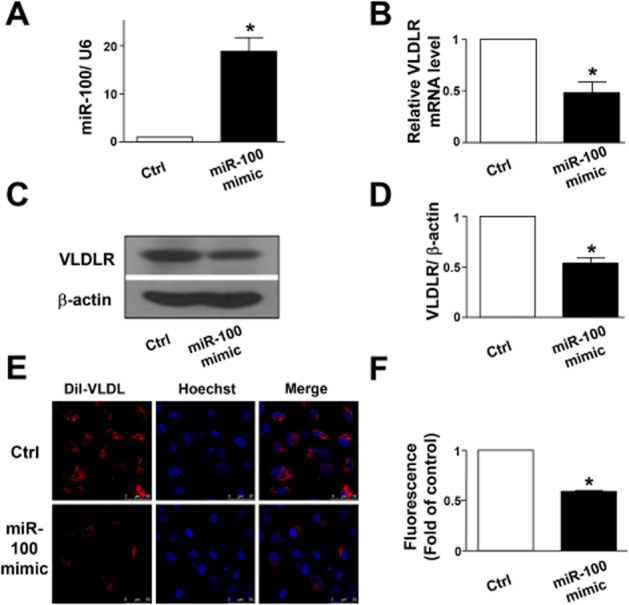
miR-100 suppresses VLDL receptor (VLDLR) expression and VLDL uptake. (A) Quantitative PCR for validation of the efficiency of HUVECs transfected with miR-100 mimic (5 nM). (B) Quantitative PCR analysing VLDL receptor mRNA level after 24 h transfection with miR-100 mimic (5 nM) or control RNA (Ctrl). (C) Representative immunoblot for VLDL receptor in HUVECs after 24 h miR-100 mimic (5 nM) or control RNA (Ctrl) transfection. (D) Quantification of VLDL receptor immunoblotting results. (E) Representative fluorescence micrographs showing the Dil-VLDL uptake into HUVECs following 24 h transfection with miR-100 mimic (5 nM) or control RNA (Ctrl). Dil-VLDL is shown in red and nuclei in blue. (F) Quantification of Dil-VLDL uptake into HUVECs. Data are presented as mean ± SEM of three independent experiments. *P < 0.05, significantly different from control.
To confirm that miR-100 directly targeted VLDL receptor, we co-transfected HEK293 cells with wt-VLDL receptor 3′UTR-luc reporter plasmid and the miR-100 mimic. As shown in Figure 3A, the miR-100 mimic significantly decreased the luciferase activity of wt-VLDL receptor 3′-UTR-luc. However, after mutating the seed sequence of miR-100 in the 3′UTR of VLDL receptor, we found that the luciferase activity was not decreased by miR-100 mimic (Figure 3B).
Figure 3.
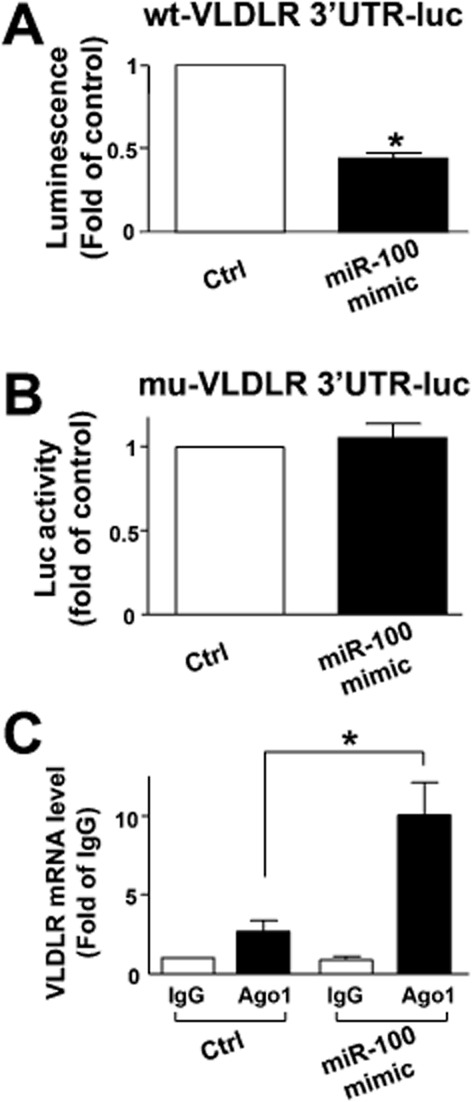
MicroRNA-100 targets VLDL receptor 3′UTR. (A and B) Luciferase activity of the reporter of wt-VLDL receptor 3′-UTR-luc plasmid (A), but not mutated VLDL receptor 3′-UTR-luc plasmid (B), was decreased in miR-100 mimic transfected cells. (C) Quantitative PCR analysing the Ago1 immunocomplexes after 48 h of miR-100 mimic or control RNA transfection. Data are presented as the mean ± SEM from three independent experiments. *P < 0.05, significantly different from control.
As a post-transcriptional regulation, miRNAs control gene expression by guiding AGO-containing miRISC complexes to target mRNA. To investigate the association of VLDL receptor mRNA with Ago1, we transfected HUVECs with the miR-100 mimic or control RNA, then quantitated mRNA level after Ago1 immunoprecipitation. The equal input of cell lysate as well as the equal amount of Ago1 pulled down were shown in Supporting Information Fig. S2. As expected, the miR-100 mimic transfection increased the level of VLDL receptor mRNA complexed to Ago1 compared with the IgG control (Figure 3C), indicating a direct targeting action of miR-100.
miR-100 mediates the effect of PPAR-δ on VLDL uptake
To investigate whether PPAR-δ activation induced miR-100 expression, HUVECs were treated with the PPAR-δ agonist GW501516 and the level of miR-100 was quantified. As shown in Figure 4, GW501516 increased the expression of miR-100, both time- and dose-dependently. This effect was blocked by GSK0660, a selective PPAR-δ antagonist. Furthermore, a miR-100 inhibitor blocked the inhibitory effects of GW501516 on Dil-VLDL uptake (Figure 5A and B) and expression of the VLDL receptors (Figure 5C and D).
Figure 4.
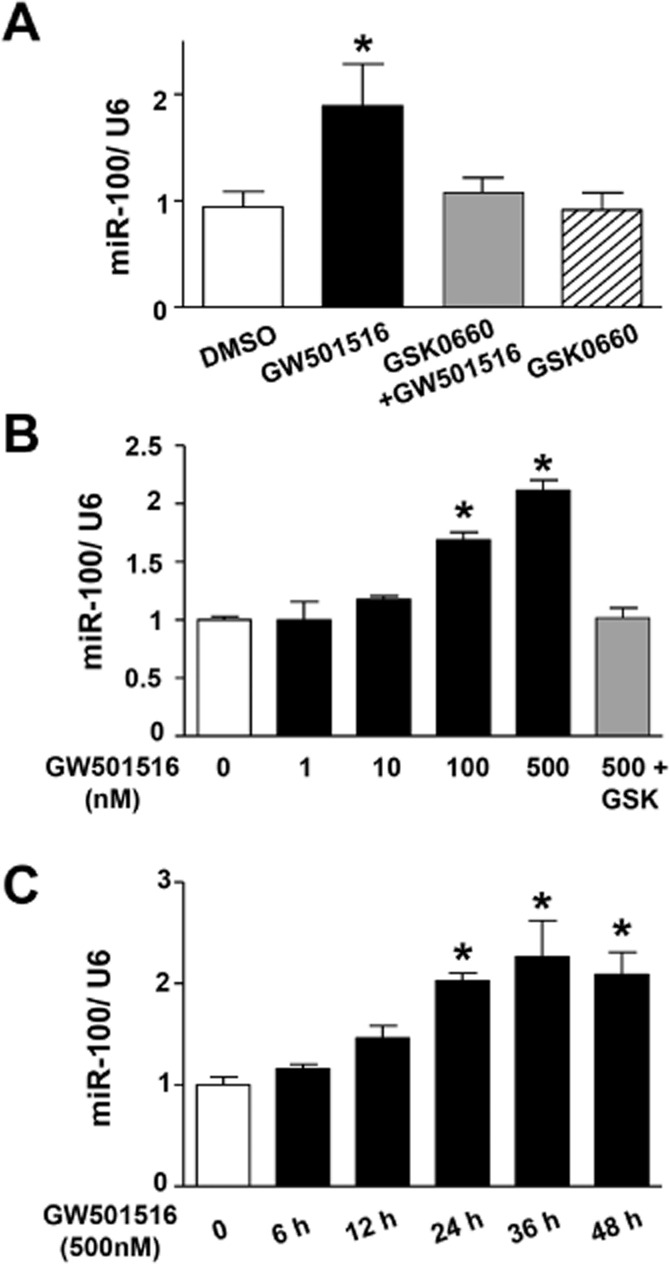
Specific ligand for PPAR-δ induces miR-100 expression. (A) Quantitative PCR analysing miR-100 level in HUVECs after treatment with DMSO control, GSK0660 (2.5 μM) or GW501516 (500 nM) in the presence or absence of GSK0660 (2.5 μM); n = 7. (B) Quantitative PCR analysing miR-100 level in HUVECs after 24 h treatment with GW501516 at different concentrations or in the present of GSK0660. DMSO treatment was used as a control; n = 4. (C) Quantitative PCR analysing miR-100 level in HUVECs after treatment with GW501516 (500 nM) for different time; n = 5. Data are presented as the mean ± SEM. *P < 0.05, significantly different from control.
Figure 5.
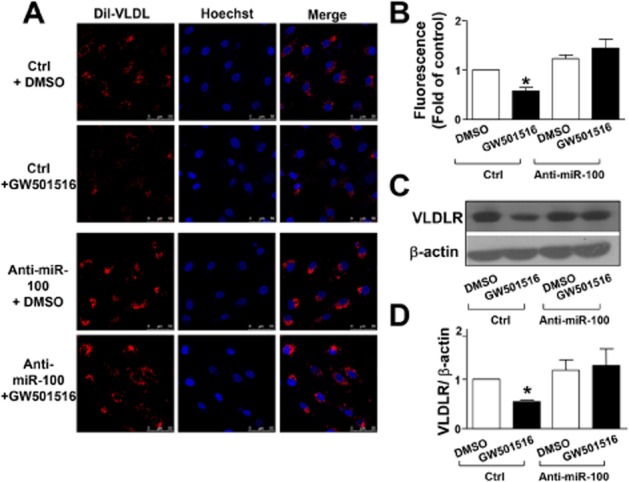
miR-100 mediates the suppressive effect of GW501516 on VLDL receptor (VLDLR) in HUVECs. (A) Representative fluorescence micrographs showing the Dil-VLDL uptake into HUVECs following 36 h treatment with GW501516 (500 nM) or DMSO control after 24 h of 40 nM miR-100 antagomir (anti-miR100) or control RNA (Ctrl) transfection. Dil-VLDL is shown in red and nuclei in blue. (B) Quantification of Dil- VLDL uptake into HUVECs. (C) Representative immunoblot for VLDL receptor in HUVECs following 36 h treatment with GW501516 (500 nM) or DMSO control after 24 h 40 nM miR-100 inhibitor (anti-miR100) or control RNA (Ctrl) transfection. (D) Quantification of VLDL receptor immunoblotting results. Data are presented as the mean ± SEM from three independent experiments. *P < 0.05, significantly different from control.
Discussion
In this study, we have demonstrated that activation of PPAR-δ induced miR-100 expression and subsequently decreased the expression of VLDL receptor, resulting in the reduction of VLDL uptake in ECs. PPAR-δ, a ligand-dependent transcription factor, plays an important role on lipid metabolism and vascular protection. For instance, PPAR-δ activation inhibited inflammation, oxidative stress and apoptosis (Liou et al., 2006; Fan et al., 2007) in ECs. In vivo evidence showed that a PPAR-δ agonist restored endothelial function in diabetic and atherosclerosis models (Graham et al., 2005; Tian et al., 2012). Importantly, PPAR-δ acts as a sensor for VLDL, regulating triglyceride homeostasis locally in peripheral tissues such as macrophages and vessel walls (Chawla, 2003). In this study, we found that the PPAR-δ agonist triggered translational repression of VLDL receptor and the decrease of VLDL internalization, effects that would provide endothelial protection. Chawla had shown that the expression of VLDL receptors was dramatically increased in PPAR-δ null macrophages, suggesting that VLDL receptor was either directly or indirectly decreased by activation of PPAR-δ (Chawla, 2003). Our finding provides a novel mechanism for the inhibiting effect of PPAR-δ on the expression of VLDL receptors, via miRNAs.
The major finding of this study was that miR-100, which was induced by PPAR-δ, directly targeted VLDL receptor expression. This was validated by the reporter assay and Western blotting analysis. AGO pull-down assay demonstrated the direct binding of miR-100 and miRISC to VLDL receptor mRNA. Functionally, we showed that miR-100 decreased VLDL uptake in ECs, via this target gene. As shown in Figure 4, the PPAR-δ agonist GW501516 increased miR-100 expression, while the specific antagonist GSK0660 attenuated this induction. A genome-wide analysis by chromatin immunoprecipitation sequencing revealed a PPARβ/δ-enrichment peak in the promoter of miR-100 (−4016 bp from the putative transcription start site) (Tora et al., 2011), which indicates that PPAR-δ may transcriptionally regulate the expression of miR-100. The specific role of miR-100 in the inhibition of VLDL expression by the PPAR-δ agonist GW501516 was addressed by using a miR-100 inhibitor. GW501516 was unable to decrease the protein level of VLDL receptor in the presence of anti-miR-100 (Figure 5C and D), which confirmed that GW501516 suppressed the expression of VLDL receptor mainly through the action of miR-100. Treatment with GW501516 and transfection of miR-100 had a similar efficacy in terms of the inhibition of VLDL receptors, although very different levels of miR-100 were achieved. The reasons for the disproportionate dose–effect relationship are unclear although it has been a common observation that an exogenously transfected gene and an endogenously induced gene are similarly effective. One interpretation may be that overexpressed miRNAs potentially saturated RISC complexes and consequently reached a plateau state for the silencing effect (Khan et al., 2009). This miRNA, miR-100, is highly expressed in ECs. Recently, Grundmann et al. showed that miRNA-100 was significantly down-regulated after induction of hind-limb ischaemia in mice and modulated ECs proliferation, tube formation and sprouting activity, which established an anti-angiogenic role of miR-100 (Grundmann et al., 2011). In this study, we have identified miR-100 as a mediator regulating VLDL internalization through the direct inhibition of the expression of VLDL receptor in ECs.
Being a member of the LDL receptor superfamily, the VLDL receptor plays a major role in VLDL internalization and metabolism. Although VLDL receptor knockout mice had no lipoprotein abnormalities, reconstitution of VLDL receptor expression in VLDL receptor knockout mice greatly increased atherosclerotic lesion development, indicating a pro-atherogenic role of this receptor (Eck et al., 2005). Moreover, the increased expression of VLDL receptor in atherosclerotic lesions suggests an important contribution to the endothelial dysfunction and the development of atherosclerosis (Hiltunen et al., 1998). Our results in this study showed that PPAR-δ decreased VLDL receptor expression and VLDL uptake in ECs, via miR-100. This evidence has provided a new mechanism for the endothelial protective effect of PPAR-δ. This regulation could be a tissue-/organ-specific effect. The decreased uptake by ECs would restore the endothelial function in hyperlipidaemia. However, these results are limited to in vitro experiments. Understanding the physiological significance of PPAR-δ-induced miR-100 needs further investigation in animal models, using EC-specific miR-100 overexpression or knockout.
In summary, we have demonstrated that PPAR-δ activation induced the expression of miR-100 and decreased the expression of VLDL receptor and, in turn, attenuated VLDL internalization in ECs. Our finding provides evidence that PPAR-δ regulates VLDL in ECs via a miRNA mechanism.
Acknowledgments
This work was supported by the grants from the National Natural Science Foundation of China (grant numbers 31430045, 81470373 and 30881220108005).
Glossary
- 3′-UTR
3′-untranslated region
- Ago1
argonaute 1
- ECs
endothelial cells; miRISC, miRNA-mediated silencing complex
- miRNAs
microRNAs
- VLDL
very low-density lipoprotein
Author contributions
X. F. and N. W. designed the research. X. F., L. F., A. L., X. W. and B. Z. performed the research. X. F. analysed the data. X. F. and N. W. wrote the paper.
Conflict of interest
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1 Comparison of the seed region of miR-100 with putative target sequences of the 3′UTR conserved regions at human, chimpanzee, mouse, rat, dog and chicken VLDL receptor mRNA.
Figure S2 Western blot analysing the Ago1 immunocomplexes after 48 h of miR-100 mimic or control RNA transfection in input of cell lysate as well as IgG and Ago1 pulled-down sample. Data are representative of three independent experiments.
References
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: nuclear hormone receptors. Br J Pharmacol. 2013a;170:1652–1675. doi: 10.1111/bph.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Transporters. Br J Pharmacol. 2013b;170:1706–1796. doi: 10.1111/bph.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish GD, Atkins AR, Downes M, Olson P, Chong LW, Nelson M, et al. PPARdelta regulates multiple proinflammatory pathways to suppress atherosclerosis. Proc Natl Acad Sci U S A. 2008;105:4271–4276. doi: 10.1073/pnas.0711875105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll MD, Lacher DA, Sorlie PD, Cleeman JI, Gordon DJ, Wolz M, et al. Trends in serum lipids and lipoproteins of adults, 1960–2002. JAMA. 2005;294:1773–1781. doi: 10.1001/jama.294.14.1773. [DOI] [PubMed] [Google Scholar]
- Chawla A. PPARdelta is a very low-density lipoprotein sensor in macrophages. Proc Natl Acad Sci U S A. 2003;100:1268–1273. doi: 10.1073/pnas.0337331100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Lai TC, Jan YH, Lin FM, Wang WC, Xiao H, et al. Hypoxia-responsive miRNAs target argonaute 1 to promote angiogenesis. J Clin Invest. 2013;123:1057–1067. doi: 10.1172/JCI65344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eck MV, Oost J, Goudriaan JR, Hoekstra M, Hildebrand RB, Bos IS, et al. Role of the macrophage very-low-density lipoprotein receptor in atherosclerotic lesion development. Atherosclerosis. 2005;183:230–237. doi: 10.1016/j.atherosclerosis.2005.03.045. [DOI] [PubMed] [Google Scholar]
- Eiselein L, Wilson DW, Lame MW, Rutledge JC. Lipolysis products from triglyceride-rich lipoproteins increase endothelial permeability, perturb zonula occludens-1 and F-actin, and induce apoptosis. Am J Physiol Heart Circ Physiol. 2007;292:H2745–H2753. doi: 10.1152/ajpheart.00686.2006. [DOI] [PubMed] [Google Scholar]
- Fan Y, Wang Y, Tang Z, Zhang H, Qin X, Zhu Y, et al. Suppression of pro-inflammatory adhesion molecules by PPAR- in human vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2007;28:315–321. doi: 10.1161/ATVBAHA.107.149815. [DOI] [PubMed] [Google Scholar]
- Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- Graham TL, Mookherjee C, Suckling KE, Palmer CN, Patel L. The PPARdelta agonist GW0742X reduces atherosclerosis in LDLR(-/-) mice. Atherosclerosis. 2005;181:29–37. doi: 10.1016/j.atherosclerosis.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Grundmann S, Hans FP, Kinniry S, Heinke J, Helbing T, Bluhm F, et al. MicroRNA-100 regulates neovascularization by suppression of mammalian target of rapamycin in endothelial and vascular smooth muscle cells. Circulation. 2011;123:999–1009. doi: 10.1161/CIRCULATIONAHA.110.000323. [DOI] [PubMed] [Google Scholar]
- Hiltunen TP, Luoma JS, Nikkari T, Yla-Herttuala S. Expression of LDL receptor, VLDL receptor, LDL receptor-related protein, and scavenger receptor in rabbit atherosclerotic lesions: marked induction of scavenger receptor and VLDL receptor expression during lesion development. Circulation. 1998;97:1079–1086. doi: 10.1161/01.cir.97.11.1079. [DOI] [PubMed] [Google Scholar]
- Hodis HN. Triglyceride-rich lipoprotein remnant particles and risk of atherosclerosis. Circulation. 1999;99:2852–2854. doi: 10.1161/01.cir.99.22.2852. [DOI] [PubMed] [Google Scholar]
- Karls U, Muller U, Gilbert DJ, Copeland NG, Jenkins NA, Harbers K. Structure, expression, and chromosome location of the gene for the beta subunit of brain-specific Ca2+/calmodulin-dependent protein kinase II identified by transgene integration in an embryonic lethal mouse mutant. Mol Cell Biol. 1992;12:3644–3652. doi: 10.1128/mcb.12.8.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AA, Betel D, Miller ML, Sander C, Leslie CS, Marks DS. Transfection of small RNAs globally perturbs gene regulation by endogenous microRNAs. Nat Biotechnol. 2009;27:549–555. doi: 10.1038/nbt.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugiyama K, Doi H, Takazoe K, Kawano H, Soejima H, Mizuno Y, et al. Remnant lipoprotein levels in fasting serum predict coronary events in patients with coronary artery disease. Circulation. 1999;99:2858–2860. doi: 10.1161/01.cir.99.22.2858. [DOI] [PubMed] [Google Scholar]
- Laible M, Boonrod K. Homemade site directed mutagenesis of whole plasmids. J Vis Exp. 2009;27:1135. doi: 10.3791/1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Olson P, Hevener A, Mehl I, Chong LW, Olefsky JM, et al. PPAR regulates glucose metabolism and insulin sensitivity. Proc Natl Acad Sci U S A. 2006;103:3444–3449. doi: 10.1073/pnas.0511253103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AC, Binder CJ, Gutierrez A, Brown KK, Plotkin CR, Pattison JW, et al. Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARalpha, beta/delta, and gamma. J Clin Invest. 2004;114:1564–1576. doi: 10.1172/JCI18730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. Fat fuels the flame: triglyceride-rich lipoproteins and arterial inflammation. Circ Res. 2007;100:299–301. doi: 10.1161/01.RES.0000259393.89870.58. [DOI] [PubMed] [Google Scholar]
- Liou JY, Lee S, Ghelani D, Matijevic-Aleksic N, Wu KK. Protection of endothelial survival by peroxisome proliferator-activated receptor-delta mediated 14-3-3 upregulation. Arterioscler Thromb Vasc Biol. 2006;26:1481–1487. doi: 10.1161/01.ATV.0000223875.14120.93. [DOI] [PubMed] [Google Scholar]
- Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- Oliver WR, Jr, Shenk JL, Snaith MR, Russell CS, Plunket KD, Bodkin NL, et al. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci U S A. 2001;98:5306–5311. doi: 10.1073/pnas.091021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl Acids Res. 2014;42:D1098–D1106. doi: 10.1093/nar/gkt1143. (Database Issue): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Tian J, Zhang P, Fan Y, Chen L, Guan Y, et al. Laminar shear stress up-regulates the expression of stearoyl-CoA desaturase-1 in vascular endothelial cells. Cardiovasc Res. 2007;74:506–514. doi: 10.1016/j.cardiores.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Xie X, Fan Y, Tian J, Guan Y, Wang X, et al. Peroxisome proliferator-activated receptor-δ induces insulin-induced gene-1 and suppresses hepatic lipogenesis in obese diabetic mice. Hepatology. 2008;48:432–441. doi: 10.1002/hep.22334. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Kawarabayasi Y, Nakai T, Sakai J, Yamamoto T. Rabbit very low density lipoprotein receptor: a low density lipoprotein receptor-like protein with distinct ligand specificity. Proc Natl Acad Sci U S A. 1992;89:9252–9256. doi: 10.1073/pnas.89.19.9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Yamamoto J, Iwasaki S, Asaba H, Hamura H, Ikeda Y, et al. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci U S A. 2003;100:15924–15929. doi: 10.1073/pnas.0306981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian XY, Wong WT, Wang N, Lu Y, Cheang WS, Liu J, et al. PPARδ activation protects endothelial function in diabetic mice. Diabetes. 2012;61:3285–3293. doi: 10.2337/db12-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tora L, Adhikary T, Kaddatz K, Finkernagel F, Schönbauer A, Meissner W, et al. Genomewide analyses define different modes of transcriptional regulation by peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) PLoS ONE. 2011;6:e16344. doi: 10.1371/journal.pone.0016344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N. PPAR-delta in vascular pathophysiology. PPAR Res. 2008;2008:164163. doi: 10.1155/2008/164163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Fang X, Zhou J, Chen Z, Zhao B, Xiao L, et al. Shear stress activation of nuclear receptor PXR in endothelial detoxification. Proc Natl Acad Sci U S A. 2013;110:13174–13179. doi: 10.1073/pnas.1312065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyne KL, Pathak K, Seabra MC, Hobbs HH. Expression of the VLDL receptor in endothelial cells. Arterioscler Thromb Vasc Biol. 1996;16:407–415. doi: 10.1161/01.atv.16.3.407. [DOI] [PubMed] [Google Scholar]
- Yin KJ, Deng Z, Hamblin M, Xiang Y, Huang H, Zhang J, et al. Peroxisome proliferator-activated receptor regulation of miR-15a in ischemia-induced cerebral vascular endothelial injury. J Neurosci. 2010;30:6398–6408. doi: 10.1523/JNEUROSCI.0780-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Comparison of the seed region of miR-100 with putative target sequences of the 3′UTR conserved regions at human, chimpanzee, mouse, rat, dog and chicken VLDL receptor mRNA.
Figure S2 Western blot analysing the Ago1 immunocomplexes after 48 h of miR-100 mimic or control RNA transfection in input of cell lysate as well as IgG and Ago1 pulled-down sample. Data are representative of three independent experiments.