Abstract
Background and Purpose
Caffeine (a non-selective adenosine receptor antagonist) prevents memory deficits in aging and Alzheimer’s disease, an effect mimicked by adenosine A2A receptor, but not A1 receptor, antagonists. Hence, we investigated the effects of adenosine receptor agonists and antagonists on memory performance and scopolamine-induced memory impairment in mice.
Experimental Approach
We determined whether A2A receptors are necessary for the emergence of memory impairments induced by scopolamine and whether A2A receptor activation triggers memory deficits in naïve mice, using three tests to assess short-term memory, namely the object recognition task, inhibitory avoidance and modified Y-maze.
Key Results
Scopolamine (1.0 mg·kg−1, i.p.) impaired short-term memory performance in all three tests and this scopolamine-induced amnesia was prevented by the A2A receptor antagonist (SCH 58261, 0.1–1.0 mg·kg−1, i.p.) and by the A1 receptor antagonist (DPCPX, 0.2–5.0 mg·kg−1, i.p.), except in the modified Y-maze where only SCH58261 was effective. Both antagonists were devoid of effects on memory or locomotion in naïve rats. Notably, the activation of A2A receptors with CGS 21680 (0.1–0.5 mg·kg−1, i.p.) before the training session was sufficient to trigger memory impairment in the three tests in naïve mice, and this effect was prevented by SCH 58261 (1.0 mg·kg−1, i.p.). Furthermore, i.c.v. administration of CGS 21680 (50 nmol) also impaired recognition memory in the object recognition task.
Conclusions and Implications
These results show that A2A receptors are necessary and sufficient to trigger memory impairment and further suggest that A1 receptors might also be selectively engaged to control the cholinergic-driven memory impairment.
Tables of Links
| TARGETS | |
|---|---|
| A1 receptor | M1 receptor |
| A2A receptor |
| LIGANDS | ||
|---|---|---|
| ACh | CGS 21680 | Scopolamine |
| Adenosine | DPCPX | SCH 58261 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
Adenosine plays an essential role in the maintenance of brain homeostasis mainly acting through inhibitory adenosine A1 receptors and facilitatory adenosine A2A receptors (Fredholm et al., 2005). The parallel and combined effects mediated by A1 and A2A receptors control basal synaptic transmission and plasticity, respectively, and contribute to the encoding of salient information in neuronal circuits (Cunha, 2008), thus affecting different behaviours ranging from locomotion to mood (Chen et al., 2013). In particular, the participation of adenosine receptors in cognitive processes has been recognized over the years, as heralded by the ability of caffeine, a non-selective adenosine receptor antagonist, to control memory performance (reviewed in Cunha and Agostinho, 2010). Thus, the acute administration of caffeine improves the performance of rodents in the object recognition task (Costa et al., 2008; Botton et al., 2010) and inhibitory avoidance (Angelucci et al., 1999) as well as the performance of discrimination tasks in humans (Borota et al., 2014). Furthermore, the chronic consumption of caffeine attenuates cognitive dysfunction observed during aging or Alzheimer’s disease (AD) in humans (Eskelinen et al., 2009; Cao et al., 2012) and animal models (Arendash et al., 2006; Dall’Igna et al., 2007; Espinosa et al., 2013; Laurent et al., 2014). From results obtained in animal models, it was further concluded that the anti-amnesic effect of caffeine in AD was mimicked by the selective blockade of A2A, but not of A1 receptors (Dall’Igna et al., 2007; Canas et al., 2009).
The hypofunction of the cholinergic system is considered a trigger for memory deterioration in aging and AD (reviewed in Bartus, 2000), in accordance with the well-documented amnesic effects of the muscarinic receptor antagonist, scopolamine (reviewed in Klinkenberg and Blokland, 2010). Notably, caffeine can prevent scopolamine-induced memory impairment in rodents (Nikodijević et al., 1993; Botton et al., 2010) and humans (Riedel et al., 1995). However, since A2A receptor activation enhances, while A1 receptors inhibit, the evoked release of ACh in the limbic cortex (Cunha et al., 1994; Rodrigues et al., 2008), it would be expected that blockade of A1 rather than of A2A receptors might be able to prevent cholinergic hypofunction. Thus, we compared the effects of selectively blocking A2A and A1 receptors on the impairment of memory caused by scopolamine.
Methods
Animals and housing
Adult male CF1 mice (3–4 months old), obtained from Fundação Estadual de Produção e Pesquisa em Saúde (Porto Alegre/RS, Brazil), were housed in standard polypropylene cages (4–5 animals per cage), under a 12/12 h light/dark cycle (lights on at 07:00 h) with food and water ad libitum. Independent groups of mice were used for each behavioural test, which were performed between 08:00 h and 14:00 h. All experimental procedures were approved by the ethical committee of the Federal University of Rio Grande do Sul (Proc. n° 22534) and followed the recommendations of the NIH Guide for Care and Use of Laboratory Animals and of the Sociedade Brasileira de Neurociências e Comportamento (SBNeC). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Drugs
1,3-Dipropyl-8-cyclopentylxanthine (DPCPX, a selective A1R antagonist), 7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine (SCH 58261, a selective A2AR antagonist) and 3-[4-[2-[6-amino-9-[(2R,3R,4S,5S)-5-(ethylcarbamoyl)-3,4-dihydroxy-oxolan-2-yl]purin-2-yl]amino]ethyl]phenyl]propanoic acid (CGS 21680, an A2AR agonist) were from Tocris (São Paulo/SP, Brazil) and were prepared as 5 mM stock solutions in saline with 20% DMSO and later dissolved to the desired concentration in saline. CGS 21680 was also infused into the lateral ventricles (coordinates: AP −1, LM 1.5 and DV +1.5) in a volume of 1 μL per side. Scopolamine hydrobromide trihydrate, a non-selective muscarinic receptor antagonist, was from Sigma-Aldrich (São Paulo/SP, Brazil), and was dissolved in saline. Drugs were administered i.p. in a volume of 10 mL·kg−1 of body weight at a minimal dose of each drug, with no effects on locomotion (El Yacoubi et al., 2000a,b; Botton et al., 2010).
Behavioural procedures
Different groups of mice were used for each behavioural task. Mice were handled for 2 min for 4 days before beginning the behavioural tasks and transferred to the testing room one hour before the habituation session. The testing room was sound-attenuated room with a low-intensity light (15 lux) uniformly distributed throughout the arena and a temperature of 21 ± 2°C. The experiments were monitored by two observers blind to experimental protocols and recorded with a video camera positioned above the arena. The data were collected and analysed using the ANY-Maze video-tracking system (Stoelting CO, Woods Dale, IL, USA).
Open field
Mice were exposed to an open-field arena to evaluate locomotor activity and further the object recognition task. The first day corresponded to habituation to the apparatus and the second day to training and test sessions of 90 min. The apparatus was made of black-painted Plexiglas measuring 50 × 50 cm and was surrounded by 50-cm-high walls. Each mouse was placed in the centre of the arena and the distance travelled, the time and average speed of locomotor activity in metres was recorded for 10 min. The experiments were conducted in a sound-attenuated room under low-intensity light (12 lux); activity was recorded with a video camera positioned above the arena and monitored in an adjacent room by an observer blinded to the treatment of the animals. The open-field apparatus was cleaned after the end of each session.
Novel object recognition task
The object recognition task was carried out in a grey-painted wood open field apparatus (50 × 25 × 25 cm). For the habituation session, the mice were placed in the open field 24 h before the training session and allowed to explore the arena for 10 min. The training session consisted of allowing the mouse to explore an apparatus containing two similar objects (A and A′) for 10 min. The objects were identical sized (13 cm height) glass bottles, but of different shapes and colours, and were positioned in two adjacent corners, 9 cm from the walls. Each mouse was always placed in the apparatus facing the wall. The tests sessions, after the training, were performed either for 90 min for short-term memory or for 24 h for long-term memory. In the test session, one of the objects was replaced by a novel object and the mice were allowed to explore the objects for 10 min. The apparatus and objects were cleaned with a 70% ethanol solution between trials to eliminate odor cues. The locomotor activity was measured as the total distance travelled, as the percentage of total time engaged in locomotion and as the average speed. To calculate the object discrimination ratio, we first measured the time spent exploring each object, defined as the time a mouse spent directing its nose to the object at a distance ≤2 cm and/or touching the object with the nose or forepaws; the discrimination ratio was then expressed as TN/(TN + TF) where TF is the time spent exploring familiar object and TN is the time spent exploring the novel object.
Inhibitory avoidance task
The inhibitory avoidance task was assessed in an apparatus consisting of an acrylic box (30 cm × 25 cm × 20 cm) with a floor containing parallel stainless-steel bars (1 mm diameter) spaced 1 cm apart and 25 cm platform length on the left. In the training session, animals were gently placed on the platform facing the left rear corner of the training box. When they stepped down and placed their four paws on the grid, they received a 2 s, 0.5 mA scrambled footshock and were immediately withdrawn from the training box. The test session was carried out 90 min after the training session (short-term memory) or 24 h after the training session (long-term memory). No footshock was given in the test session, and step-down latencies (180 s ceiling) were taken as a measure of retention.
Modified Y-maze
The Y-maze apparatus consisted of three arms (18 cm long, 6 cm wide and 6 cm high) made of wood covered with impermeable Formica elevated to a height of 50 cm above the floor. We used this task to assess short-term spatial memory, which is based on the innate preference of animals to explore areas that have not been previously explored. This task consisted of two trials (training and test) of 8 min each separated by an intertrial interval of 120 min. During the training trial, one arm (novel) was blocked by a removable door and mice were placed at the end of the one arm (start) facing the centre and they could chose between the start and the ‘other’ arm. At the end of the training trial, the mouse was removed from the maze and back housing box during the inter-trial interval (120 min). During the test trial, the novel arm was opened and the animals were once again placed at the start arm and allowed to explore freely the three arms for 8 min. The number of entries and the time spent in each arm were recorded. Entry into an arm was defined as placement of all four paws into the arm.
Experimental design
The schedules of administration of drugs and of experimental manipulations are depicted in the timelines displayed in Figure 1.
Figure 1.
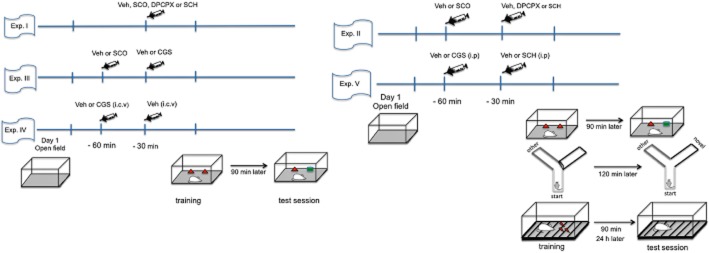
Schematic overview of the experimental design. In all experiments, all groups of mice were submitted to open-field analysis 24 h before the training session. In experiment 1 (Exp. 1), DPCPX (A1 receptor antagonist, 0.2–5 mg·kg−1, i.p.) or SCH 58261 (A2A receptor antagonist, 0.1–1 mg·kg−1, i.p.) or scopolamine (SCO; 0.1–1 mg·kg−1, i.p.) was administered to different mice 30 min before training of the object recognition task. The test session was performed either 90 min or 24 h after training. In Experiment 2 (Exp. 2), scopolamine (1.0 mg·kg−1, i.p.) was administered 30 min before injecting DPCPX (1.0 mg·kg−1, i.p.) or SCH 58261 (0.5 mg·kg−1, i.p.). The training session was carried out 30 min after and the test session was performed 90 min for object recognition and inhibitory avoidance tasks, and also 24 h for inhibitory avoidance or 120 min for the modified Y-maze task. In experiment 3 (Exp. 3), scopolamine (1.0 mg·kg−1, i.p.) was administered 30 min before injecting the A2A receptor agonist CGS 21680 (0.1 mg·kg−1, i.p.). In experiment 4 (Exp. 4), CGS 21680 (50 nmol) was infused into the lateral ventricles (1 μL per side) 30 min before training. Test session was performed 90 min after training in the object recognition task. In experiment 5 (Exp. 5), CGS 21680 (0.1 mg·kg−1, i.p.) was administered 30 min before SCH 58261 (0.5 mg·kg−1, i.p.). The training session was carried out for 30 min and the test session was performed after 90 min for object recognition task, 90 min and 24 h for inhibitory avoidance task and 120 min for the modified Y-maze task.
Experiment 1
Mice received a single i.p. injection of either vehicle (veh – 0.9% saline) or scopolamine (0.1, 0.3 or 1.0 mg·kg−1) 30 min before the training session. The same procedure was performed in another group of animals for DPCPX (0.2, 1.0 or 5.0 mg·kg−1, i.p.) or SCH 58261 (0.1, 0.5 or 1.0 mg·kg−1, i.p.) and for their respective controls (0.9% saline with 20% DMSO). The pharmacokinetic profile of both antagonists shows that they rapidly reach the brain parenchyma where their concentration remains at effective levels for over 120 min (Yang et al., 2007; Elmenhorst et al., 2013). In this experiment, each animal received only one drug in a single dose before the training session.
Experiment 2
Mice first received scopolamine (1.0 mg·kg−1, i.p.) or vehicle 60 min before the training session. After 30 min, the mice received a second injection of vehicle, DPCPX (1.0 mg·kg−1, i.p.) or SCH 58261 (0.5 mg·kg−1, i.p.). The experimental groups were as follows: vehicle (veh + veh), scopolamine (SCO + veh), scopolamine + DPCPX (SCO + DPCPX) and scopolamine + SCH 58261 (SCO + SCH). In order to minimize the number of animals and based on the data from experiment 1, we did not administer DPCPX or SCH 58261 to mice previously injected with vehicle for the novel object recognition task. In the inhibitory avoidance task and modified Y-maze, DPCPX (1.0 mg·kg−1, i.p.) or SCH 58261 (0.5 mg·kg−1, i.p.) were administered with vehicle.
Experiment 3
Mice received scopolamine (1.0 mg·kg−1, i.p.) or vehicle 60 min before the training session. After 30 min, the mice received a second injection of either vehicle or CGS 21680 (0.1 mg·kg−1, i.p.). We also tested the effects of CGS 21680 (0.05 mg·kg−1, i.p.), and the groups in this experiment were vehicle (veh + veh), scopolamine (SCO + veh), CGS 21680 (veh + CGS) and scopolamine + CGS 21680 (SCO + CGS). The test session was performed only 90 min after training.
Experiment 4
Intracerebroventricular infusion: all surgical procedures were carried out under anaesthesia (ketamine: xylazine – 80:10 mg·kg−1 i.p.) and aseptic conditions. Cannulae (27-gauge) were bilaterally implanted into the lateral ventricles following coordinates: A −1.0, L ± 1.5, V −1.5, from the atlas of Paxinos and Watson. Animals were allowed to recover from surgery for 7 days. Mice received vehicle or CGS (50 nmol, 1 μL per side), 30 min before training. Infusions were carried out over 60 s with an infusion pump and cannulae were left for an additional 60 s to minimize backflow. The placement of the cannulae was verified postmortem 2–4 h after the end of behavioural test by staining with 1 μL of 4% methylene blue solution. Only data from animals with correct implants were analysed (95 %). The test session was performed only 90 min after the training session.
Systemic administration: mice received CGS 21680 (0.1 mg·kg−1, i.p.) or vehicle 60 min before the training session. After 30 min, the mice received either vehicle or SCH 58261 (0.5 mg·kg−1, i.p.). The groups in this experiment were vehicle (veh + veh), CGS 21680 (veh + CGS) and CGS 21680 + SCH 58261 (CGS + SCH). In order to minimize the number of animals and based on the results from experiment 2, we did not administer SCH 58261 to mice previously injected with vehicle. The test session was performed only 90 min after the training session.
Statistical analysis
Data are presented as mean ± SEM. Two-way anova was used to analyse the discrimination ratio with repeated measures (within-subject factor: sessions of behavioural test; between-subject factor: treatments) followed by a Tukey’s multiple comparisons test. In some cases, Student’s paired t-test was also used to analyse differences between training and test sessions within the same group. The locomotor activity and modified Y-maze task was analysed by one-way anova followed by either a Tukey’s multiple comparisons test or a Kruskal–Wallis test and a Dunn’s multiple comparisons procedure. Data from inhibitory avoidance task are presented as median values (interquartile range) and analysed by Wilcoxon paired test for differences within groups (training vs. test sessions) and Mann–Whitney test for differences between groups. GraphPad Prism 6 software (São Paulo/SP, Brazil) was used for statistical analysis and significance was considered as P < 0.05.
Results
Experiment 1
No differences were found in either the total distance travelled or the time engaged in locomotion or the average speed for each dose of DPCPX (Table 2008), SCH 58261 (Table 2013) or scopolamine tested (Table 1999), when administered 30 min before the training session, in the object recognition task. The administration of DPCPX [F(3,45) = 2.150; P = 0.1071] or SCH 58261 [F(3,72) = 1.429; P = 0.2413] did not alter the discrimination ratio. In fact, all groups of mice were equally able to discriminate the novel from the familiar object with all doses of either DPCPX [F(2,90) = 42.33; P < 0.0001] or SCH 58261 [F(2,144) = 26.39; P < 0.0001] (Figure 2A and 2B respectively).
Figure 2.
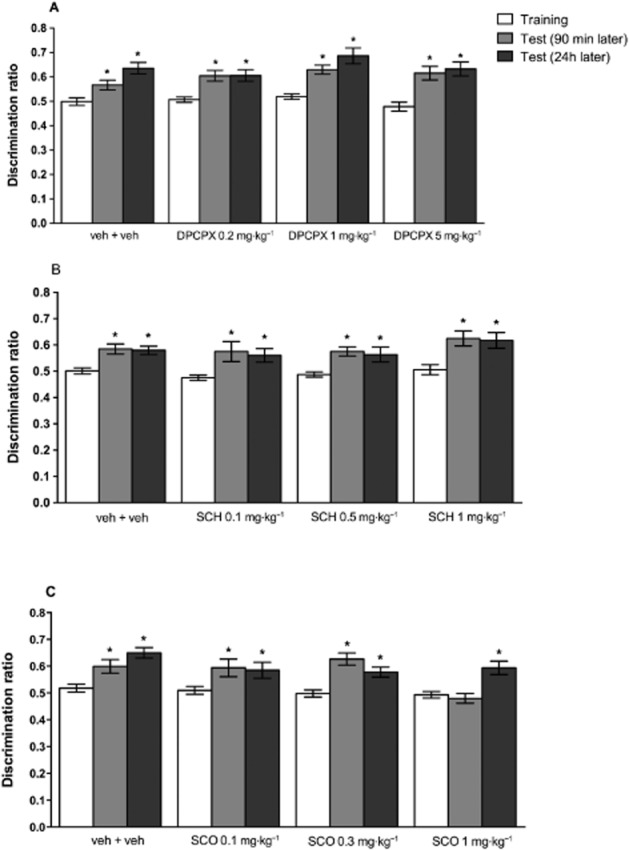
Dose-dependent effects of the adenosine receptor antagonists and of scopolamine (SCO) in the object recognition task (experiment 1). (A) The selective blockade of A1 receptors by i.p. administration of DPCPX 30 min before the training session did not modify the discrimination ratio. Results are means ± SEM of n = 12–13 mice per group; *P < 0.05 versus training session. (B) The selective blockade of A2A receptors by administration of SCH 58261 (SCH, i.p.) 30 min before the training session did not modify the discrimination ratio. Results are mean ± SEM of n = 16–20 mice per group; *P < 0.05 versus training session. (C) The blockade of muscarinic receptors by SCO (i.p.) 30 min before the training session only decreased the discrimination ratio at the highest tested dose and when testing was carried out 90 min after training. Results are means ± SEM of n = 10–12 mice per group; *P < 0.05 versus training session.
Table 1.
Locomotor activity during the training session of object recognition task for different doses of DPCPX
| 0.0 mg·kg−1 | 0.2 mg·kg−1 | 1.0 mg·kg−1 | 5.0 mg·kg−1 | F value | P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SEM | M | SEM | M | SEM | M | SEM | |||
| Distance travelled (m) | 34.77 | 1.95 | 36.34 | 2.15 | 34.49 | 1.60 | 31.92 | 2.58 | 0.764 | 0.520 |
| Time of locomotor activity (s) | 489.3 | 7.18 | 486.6 | 10.87 | 500.7 | 8.59 | 443.2 | 25.23 | 3.103a | 0.376 |
| Average speed of locomotion (m·min−1) | 4.25 | 0.21 | 4.48 | 0.25 | 4.14 | 0.20 | 4.31 | 0.21 | 0.435 | 0.29 |
Results from 12 to 13 animals per group.
Statistical analysis performed by one-way anova or
Kruskal–Wallis test.
No significant differences.
M, mean.
Table 2.
Locomotor activity during the training session of object recognition task for different doses of SCH58261
| 0.0 mg·kg−1 | 0.1 mg·kg−1 | 0.5 mg·kg−1 | 1.0 mg·kg−1 | KW value | P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SEM | M | SEM | M | SEM | M | SEM | |||
| Distance travelled (m) | 27.76 | 0.85 | 30.56 | 2.74 | 29.03 | 0.77 | 29.02 | 1.09 | 1.427 | 0.699 |
| Time of locomotor activity (s) | 485.6 | 6.52 | 488.2 | 14.50 | 501.8 | 6.56 | 508.5 | 7.61 | 4.024 | 0.259 |
| Average speed of locomotion (m·min−1) | 3.43 | 0.09 | 3.64 | 0.24 | 3.47 | 0.07 | 3.41 | 0.08 | 0.642 | 0.887 |
Results from 16 to 24 animals per group.
Statistical analysis performed by Kruskal–Wallis test.
M, mean.
Table 3.
Locomotor activity during the training session of object recognition task for different doses of scopolamine
| 0.0 mg·kg−1 | 0.1 mg·kg−1 | 0.3 mg·kg−1 | 1.0 mg·kg−1 | KW value | P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SEM | M | SEM | M | SEM | M | SEM | |||
| Distance travelled (m) | 34.98 | 1.77 | 33.45 | 2.94 | 36.08 | 1.13 | 38.71 | 1.41 | 5.212 | 0.157 |
| Time of locomotor activity (s) | 451.3 | 25.80 | 455.6 | 24.41 | 481.7 | 12.36 | 471.2 | 8.78 | 2.352 | 0.503 |
| Average speed of locomotion (m·min−1) | 4.82 | 0.47 | 4.36 | 0.26 | 4.39 | 0.18 | 4.61 | 0.16 | 2.150 | 0.542 |
Results from 10 to 12 animals per group.
Statistical analysis performed by Kruskal–Wallis test.
M, mean.
The highest dose of scopolamine tested, administered 30 min before the training session, impaired recognition memory. Thus, two-way anova with repeated measures revealed differences between sessions [F(2,84) = 25.05; P < 0.0001], doses [F(3,42) = 3.736; P = 0.0182] and interaction between sessions and doses [F(6,84) = 4.006; P = 0.0014]. Mice that received vehicle and scopolamine at 0.1 and 0.3 mg·kg−1 were still able to discriminate the novel from the familiar object in both test sessions, whereas the administration of scopolamine (1.0 mg·kg−1) impaired recognition memory when assessed 90 min after training, but not 24 h later (Figure 2C). Thus, all subsequent experiments focused only on short-term memory, with the test session performed 90 min after the training session, since this was the condition most sensitive to scopolamine.
Experiment 2
The role of A1 or A2A receptors in the scopolamine-induced memory impairment was evaluated by testing the effects of either the A1 receptor antagonist DPCPX (1.0 mg·kg−1) or of the A2A receptor antagonist SCH 58261 (0.5 mg·kg−1), administered 30 min after scopolamine (1.0 mg·kg−1), in the different memory tests. Neither DPCPX nor SCH 58261 without or with scopolamine affected locomotor activity (Table 2006). By contrast, both adenosine receptor antagonists altered the effect of scopolamine on recognition memory. Two-way anova with repeated measures revealed differences between sessions [F(1,40) = 51.69; P < 0.0001], treatments [F(3,40) = 3.842; P = 0.0165] and an interaction between sessions and treatments [F(3,40) = 3.139; P = 0.0357]. Mice that received scopolamine (1.0 mg·kg−1) 60 min before training (scopolamine + veh) were not able to discriminate the novel from the familiar object. However, mice that received the A1 receptor antagonist DPCPX (1.0 mg·kg−1) or the A2A receptor antagonist SCH 58261 (0.5 mg·kg−1) 30 min after the scopolamine injection (SCO + DPCPX or SCO + SCH groups, respectively) were able to discriminate the objects (Figure 3A). In the inhibitory avoidance task, all groups presented differences between training and test sessions (P < 0.05), except mice treated with scopolamine (P > 0.05). However, the A1 receptor antagonist DPCPX seemed more effective than the A2A receptor antagonist at preventing the memory impairment caused by scopolamine (Figure 3B). The adenosine receptor antagonists had a different effect on the scopolamine-impaired memory performance in the modified Y-maze. Analysis of the percentage of exploration in three arms revealed that mice treated with vehicle spent more time exploring the novel arm compared with the other arms [F(2,18) = 18.61; P < 0.0001] (Figure 3C). By contrast, mice treated with scopolamine did not show any differences (P > 0.05) in the time spent searching the different arms (Figure 3C). Notably, scopolamine-treated mice that received the A2A receptor antagonist (SCO + SCH group) displayed again differences in the exploration of new arm when compared with the others [F(2,21) = 3.788; P = 0.0394], whereas the SCO + DPCPX group still displayed no differences in the percentage of exploration of the different arms (P > 0.05) (Figure 3C). Finally, the antagonists were devoid of effects by themselves since both the SCH + veh [F(2,15) = 24.65; P < 0.0001] and the DPCPX + veh [F(2,15) = 10.10; P = 0.0017] groups spent more time searching the novel arm compared with the others.
Figure 3.
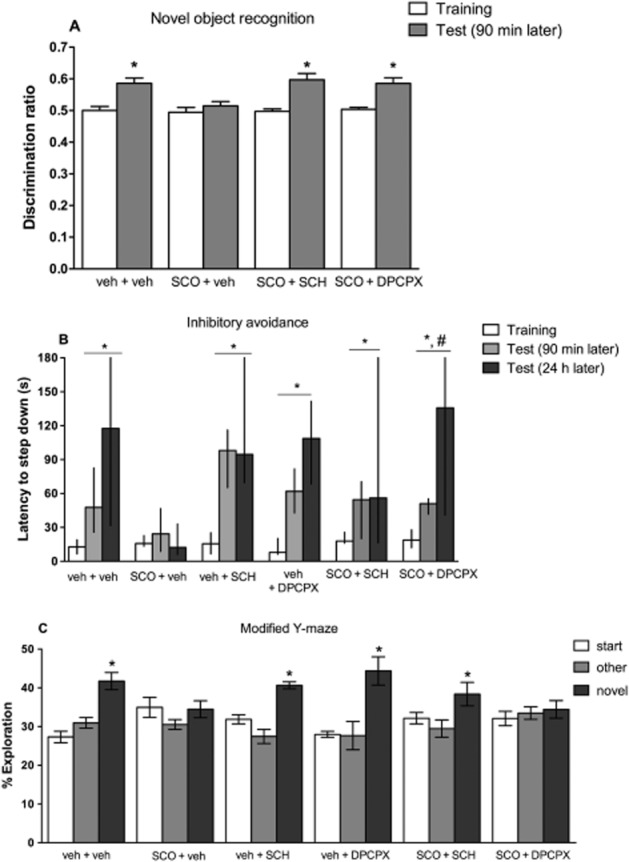
The selective blockade of either A1 or A2A receptors prevented the scopolamine (SCO)-induced impairment in short-term memory (experiment 2). DPCPX (A1 receptor antagonist, 1.0 mg·kg−1, i.p.) or SCH 58261 (SCH, A2A receptor antagonist, 0.5 mg·kg−1, i.p.) were administered 30 min after SCO (1.0 mg·kg−1, i.p.), which was injected 60 min before training in three different tests, namely (A) discrimination ratio for object recognition task; data are mean ± SEM of n = 10–11 mice per group; *P < 0.05 versus training session. (B) Latencies in seconds (s) to step down from the platform in the inhibitory avoidance task; data are median and interquartile range of n = 6–10 mice per group; *P < 0.05, Wilcoxon paired t-test comparing training and test session within group; #P < 0.05, Mann–Whitney test comparing the groups SCO + veh and SCO + DPCPX in the test sessions; (C) percentage of the time of exploration in the three arms (start, other and novel) in the test trial of the modified Y-maze task; data are means ± SEM of n = 6–9 mice per group; *P < 0.05, one-way anova and Tukey’s multiple comparison test comparing the novel and other arms (start and other) within group. The tested groups were: veh + veh (vehicle); SCO + veh (SCO); DPCPX + veh (DPCPX) SCH + veh (SCH 58261); SCO + DPCPX (SCO + DPCPX) and SCO + SCH (SCO + SCH 58261).
Table 4.
Locomotor activity during the training session of object recognition task for experiment 2
| veh + veh | Scop + veh | Scop + DPCPX | Scop + SCH58261 | F value | P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SEM | M | SEM | M | SEM | M | SEM | |||
| Distance travelled (m) | 35.83 | 2.84 | 36.76 | 3.22 | 33.44 | 2.42 | 32.89 | 2.45 | 2.823a | 0.4198 |
| Time of locomotor activity (s) | 482.1 | 12.20 | 487.5 | 37.13 | 484.9 | 18.57 | 476.9 | 16.98 | 2.724a | 0.4361 |
| Average speed of locomotion (m·min−1) | 4.44 | 0.31 | 4.48 | 0.21 | 4.15 | 0.29 | 4.11 | 0.22 | 0.535 | 0.6610 |
Results from 11 animals per group.
One-way anova or
Kruskal–Wallis test.
No significant differences.
M, mean; Scop, scopolamine; veh, vehicle.
Experiment 3
We then investigated if the activation of A2A receptors further accentuates scopolamine-induced impairment of recognition memory. Thus, we administered the A2A receptor agonist CGS 21680 (0.1 mg·kg−1) or vehicle 30 min after scopolamine (1.0 mg·kg−1), which was injected 60 min before training. CGS 21680 did not affect locomotor activity alone or when administered with scopolamine (Table 2013). This contrasted with the decreased locomotion observed with CPA (0.3–1.0 mg·kg−1; El Yacoubi et al., 2000b), which precluded testing the effect of A1 receptor activation on the scopolamine-induced depression of recognition memory. The analysis of the effect of CGS 26180 showed that it did not significantly exacerbate the scopolamine-induced memory impairment, probably because it already caused memory impairment by itself: in fact, control mice (veh + veh group) were able to discriminate the novel from the familiar object and this was the only group displaying a significant difference between the training and test session (paired t-test, t = 5.729; P = 0.0004). In all other groups (SCO + veh, veh + CGS and SCO + CGS), no differences were found between sessions, suggesting that these mice were not able to discriminate the novel from the familiar object (Figure 4).
Figure 4.
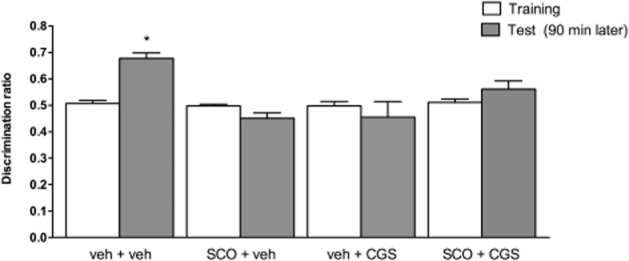
The activation of adenosine A2A receptors did not exacerbate the scopolamine (SCO)-induced impairment of the discrimination ratio in the recognition memory test (experiment 3). The adenosine A2A receptor agonist CGS 21680 (0.1 mg·kg−1) was administered i.p. 30 min before SCO (1.0 mg·kg−1, i.p.), which was injected 60 min before training. Data are means ± SEM of n = 9–16 mice per group; *P < 0.05 versus training session.
Table 5.
Locomotor activity during the training session of object recognition task for experiment 3
| veh + veh | SCO + veh | veh + CGS21680 | SCO + CGS21680 | F value | P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SEM | M | SEM | M | SEM | M | SEM | |||
| Distance travelled (m) | 38.00 | 2.594 | 36.97 | 2.771 | 39.34 | 1.315 | 35.20 | 1.398 | 2.405a | 0.4927 |
| Time of locomotor activity (s) | 407.1 | 28.99 | 380.9 | 22.13 | 428.4 | 23.00 | 375.8 | 30.52 | 0.7127 | 0.5499 |
| Average speed of locomotion (m·min−1) | 5.922 | 0.7482 | 6.128 | 0.6264 | 5.702 | 0.5255 | 6.059 | 0.5923 | 0.0104a | 0.9997 |
Results from 8 to 16 animals per group.
One-way anova or
Kruskal–Wallis.
No significant differences.
M, mean; Scop, scopolamine; veh, vehicle.
Experiment 4
In order to rule out peripheral effects of CGS 21680 as a cause for the memory impairment observed in the object recognition task, and in attempt to ensure that the effect found is predominantly central, CGS 21680 was directly infused into the lateral ventricles. As shown in Figure 5, the infusion of CGS 21680 (50 nmol) directly into the ventricles 30 min before training abolished recognition memory (Student’s paired t-test, t = 0.01216; P = 0.9906; Figure 5).
Figure 5.
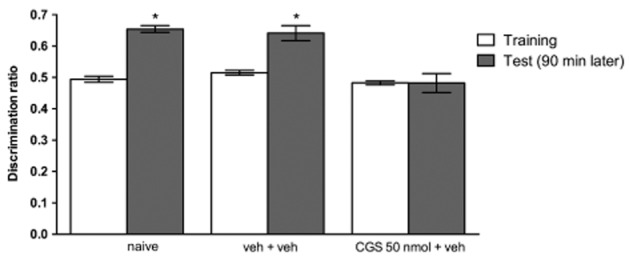
The i.c.v. infusion of CGS 21680 impaired the discrimination ratio in the recognition memory test (experiment 4). The adenosine A2A receptor agonist CGS 21680 (50 nmol, 1 μL per side) was infused into lateral ventricles 30 min before training. Test session was carried out 90 min later. Naïve (n = 5); veh + veh (vehicle, n = 8) and CGS + veh (CGS 21680, n = 8). Data are means ± SEM *P < 0.05 versus training session.
Experiment 5
We next tested if the activation of A2A receptors was sufficient to impair other types of short-term memory, beyond recognition memory, in control mice. Thus, we investigated if CGS 21680 (0.1 mg·kg−1) would impair aversive and spatial memory and if the blockade of A2A receptors with SCH 58261 (0.5 mg·kg−1) would prevent the CGS 21680-induced memory impairment in the three tasks. Neither CGS 21680 nor SCH 58261 alone or in combination modified locomotor activity (Table 2000). In contrast to control mice (veh + veh group), which recognized the novel from the familiar object as concluded from the differences between the training and test sessions (paired t-test, t = 7.859, P < 0.0001), the administration of CGS 21680 (0.1 mg·kg−1) 60 min before the training session (CGS + veh group) impaired recognition memory, as evidenced by the lack of difference between training and test session (P > 0.05). Mice that received SCH 58261 (0.5 mg·kg−1) 30 min after CGS 21680 (0.1 mg·kg−1; SCH + CGS group) were again able to discriminate the novel from the familiar object, as judged by the difference between the training and test sessions (paired t-test, t = 2.715, P = 0.0264; Figure 6A).
Figure 6.
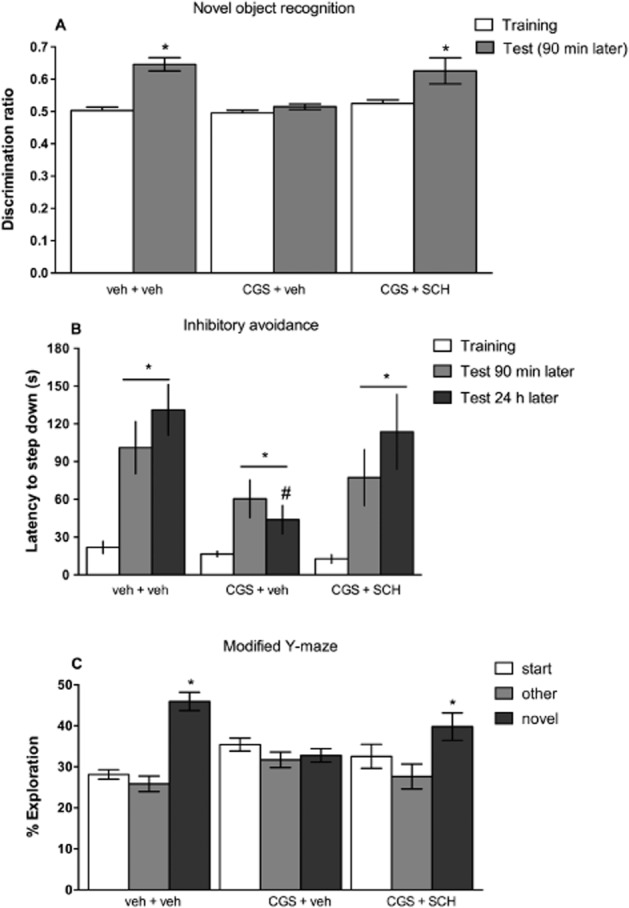
The blockade of adenosine A2A receptors prevents the deterioration in short-term memory triggered by exposure to CGS 21680 (experiment 5). CGS 21680 (0.1 mg·kg−1, i.p.) was administered 30 min before SCH 58261 (0.5 mg·kg−1, i.p.), which was administered 30 min before training. CGS 21680 impaired: (A) the discrimination ratio in the recognition memory test (data are means ± SEM of n = 9–10 mice per group; *P < 0.05 vs. training session); (B) the latency in seconds (s) to step down in the inhibitory avoidance task (data are median and interquartile range of n = 6–8 mice per group; *P < 0.05, Wilcoxon paired t-test comparing training and test session within group; #P < 0.05, Mann–Whitney test comparing veh + veh and CGS + SCH group in the test sessions); (C) the percentage of the time of exploration in the three arms (start, other and novel) in the test trial of the modified Y-maze task. Data are means ± SEM of n = 7–8 mice per group; *P < 0.05 one-way anova and Tukey’s multiple comparison test comparing the novel and other arms within group.
Table 6.
Locomotor activity during the training session of object recognition task for experiment 4
| veh + veh | CGS21680 + veh | CGS21680 + SCH58261 | F value | P value | ||||
|---|---|---|---|---|---|---|---|---|
| M | SEM | M | SEM | M | SEM | |||
| Distance travelled (m) | 33.06 | 3.182 | 35.12 | 3.640 | 31.88 | 2.891 | 0.2302 | 0.7961 |
| Time of locomotor activity (s) | 398.9 | 24.37 | 394.0 | 25.43 | 393.4 | 10.11 | 0.0524a | 0.9741 |
| Average speed of locomotion (m·min−1) | 5.097 | 0.5375 | 5.628 | 0.7866 | 4.937 | 0.3934 | 0.3672 | 0.6962 |
One-way anova (or Kruskal–Wallis). Results from 8 to 11 animals per group.
Kruskal–Wallis test was used.
M, mean; veh, vehicle.
In the inhibitory avoidance test, all groups of mice presented differences between training and both test sessions (90 min or 24 h after training; P < 0.05). However, CGS 21680 (0.1 mg·kg−1) worsened the long-term memory performance, as testified by the different latencies measured in the test session between CGS + veh and veh + veh groups (P < 0.05). The selective blockade of A2A receptors effectively prevented the CGS 21680-induced mnemonic deficit, as gauged by the different latencies measured in the test session between the CGS + veh and CGS + SCH groups (P < 0.05; Figure 6B).
In the modified Y-maze, it was observed that mice treated only with 0.1 mg·kg−1 CGS 21680 (CGS + veh) were the only group that did not show differences in the exploration of the novel arm when compared with the others arms [F(2,18) = 1.240; P = 0.3129], whereas both control mice (veh + veh) [F(2,21) = 36.75; P < 0.0001] and mice treated with the A2A receptor antagonist, SCH 58261 before CGS 21680 (CGS + SCH) [F(2,21) = 3.890; P = 0.0385] spent more time exploring the novel arm than the others (Figure 6C).
Discussion
The present study shows that the selective blockade of A2A receptors reproducibly prevented the scopolamine-induced impairment in short-term memory in three different behavioural tasks, which was also prevented by the A1 receptor antagonist in most tasks. This implies that the previously observed beneficial effect of caffeine to prevent scopolamine-induced amnesia (Botton et al., 2010) probably results from its dual ability to antagonize A1 and A2A receptors (Fredholm et al., 1999). Furthermore, we now showed that the activation of A2A receptors in naïve animals is sufficient to disrupt short-term memory in the three different tests, an effect mimicked by the direct brain activation of A2A receptors. Therefore, we now provide the first integrated evidence that activation of A2A receptors is necessary and sufficient for the impairment of short-term memory.
By using selective antagonists of A1 and A2A receptors that are devoid of effects in anxiety-related tests (El Yacoubi et al., 2000a,b) and by controlling for their lack of effect on spontaneous locomotion, we can now conclude that the blockade of A2A receptors prevents scopolamine-induced deficits in short-term recognition memory in three different behavioural paradigms. Notably, the selective blockade of A1 receptors also attenuated the scopolamine-induced deficits in short-term recognition memory in the object recognition test and in the inhibitory avoidance test, but not in the modified Y-maze, which is the test with the lowest sensory or aversive influence. Since we have previously shown that such deficits in recognition memory are also attenuated by caffeine (Botton et al., 2010), which is a mixed A1 and A2A receptor antagonist at non-toxic doses (Fredholm et al., 1999), we can tentatively conclude that this prevention by caffeine of scopolamine-induced amnesia is likely to depend on both A1 and A2A receptors. This provides a conclusion different from that previously derived from the comparison of the effects of caffeine and of A1 and A2A receptors on memory impairment in different animal models of brain disease (see Cunha and Agostinho, 2010). In fact, in animal models of aging (Prediger et al., 2005), AD (Dall’Igna et al., 2007), attention deficit and hyperactivity disorder (Pandolfo et al., 2013), early life convulsions (Cognato et al., 2010) or early life stress (Batalha et al., 2013), the ability of caffeine to prevent memory dysfunction is mimicked by the selective blockade of A2A, but not of A1 receptors.
Our results imply that A1 receptors are selectively involved in this model of scopolamine-induced amnesia, in particular in tasks relying on sensory or nociceptive components (see also Suzuki et al., 1993). This is in agreement with the particular importance of A1 receptors to control the cholinergic system, which is expected to be at the core of the scopolamine-induced amnesia: in fact, A1 receptors control the actions of muscarinic receptors (Pedata et al., 1986; Oliveira et al., 2002) and efficiently inhibit the release of ACh in the limbic cortex (e.g. Jackisch et al., 1984; Cunha et al., 1994). Accordingly, A1 receptor antagonists can bolster the release of ACh (Pedata et al., 1986; Cunha et al., 1994), potentially counteracting the scopolamine-induced cholinergic hypofunction that underlies memory deficits (reviewed in Fisher, 2012).
In contrast, A2A receptor antagonists inhibit the release of ACh from limbic cortical preparations (Rodrigues et al., 2008), an effect that is not compatible with the beneficial effect of A2A receptor antagonists to alleviate memory impairment; furthermore, other central responses triggered by the activation of muscarinic receptors, such as rapid eye movement sleep (Marks et al., 2003) or tremor (Salamone et al., 2013), are prevented by A2A receptor antagonists. Thus, it is most likely that the beneficial effect of A2A receptor antagonists found in the present study may not result from a direct effect on the cholinergic system but might instead be related to their ability to control synaptic plasticity in cortical circuits (Rebola et al., 2008; Costenla et al., 2011), which is argued to be the neurophysiological basis of learning and memory (Martin et al., 2000). This would explain the general ability of A2A receptor antagonists to prevent memory impairment under different conditions, including upon exposure to scopolamine as now reported, especially since scopolamine might decrease LTP in slices from the hippocampus or perirhinal cortex (e.g. Hirotsu et al., 1989; Warburton et al., 2003; but see Tanaka et al., 1989). Thus, the effect of A2A receptor antagonists would be a balance between its beneficial effect normalizing synaptic plasticity and its detrimental effects on the cholinergic system. Furthermore, this hypothesis might explain a previous report using different doses of scopolamine and A2A receptor antagonists that concluded that the blockade of A2A receptors was not completely effective at preventing the impairment of spatial memory induced by scopolamine (Cunha et al., 2008).
However, it is important to stress that the mechanism(s) underlying this beneficial effect of A2A and A1 receptor antagonists on scopolamine-induced amnesia are currently difficult to unravel. One of the reasons is essentially because the neurophysiological bases of scoplamine-induced amnesia are still unclear given that the effects of scopolamine on synaptic plasticity are not consistently observed (e.g. Hirotsu et al., 1989; Warburton et al., 2003; but see Tanaka et al., 1989). Besides, scopolamine actually bolsters ACh release in a manner mimicked by muscarinic M1 receptor antagonists (Vannucchi et al., 1997), in contrast to the beneficial effect of muscarinic M1 receptor agonists and acetylcholinesterase inhibitors to alleviate memory impairment (reviewed in Fisher, 2012). Furthermore, it has been observed that an injection of scopolamine into the perirhinal cortex can actually improve object recognition memory (Winters et al., 2007), which argues for the involvement of circuit-mediated effects (i.e. affecting different brain regions and their connectivity) in the amnesia induced by systemic administration of scopolamine rather than single neurochemical events (i.e. restricted to a single molecular alteration in a defined brain region).
In contrast to the effectiveness of A2A receptors at controlling memory dysfunction under different brain conditions, we observed that neither A2A nor A1 receptor antagonists modified recognition memory in control mice that were not challenged with scopolamine. The effect of A1 receptor blockade on memory performance is rather controversial, with reports of beneficial (Pereira et al., 2002; Mioranzza et al., 2011; Harvey et al., 2012) and detrimental or lack of effects (Kopf et al., 1999; Giménez-Llort et al., 2002; Vollert et al., 2013), probably depending on the schedule of administration of the A1 receptor antagonists and on the different dependence of the memory task on anxiety and locomotion, since the activation of A1 receptors can cause a profound sedative effect (Snyder et al., 1981; Bruns et al., 1983).
As for the effect of A2A receptor blockade on control rodents, a similar lack of effect of A2A receptor antagonists on memory function of naïve adult animals was also reported in previous studies (Prediger et al., 2005; Dall’Igna et al., 2007; Cunha et al., 2008; Canas et al., 2009; Cognato et al., 2010; Batalha et al., 2013). This is confirmed in transgenic mice with A2A receptor deletion, which display an unaltered reference memory (Canas et al., 2009; Augusto et al., 2013; but see Wang et al., 2006) and an enhanced working memory performance (Zhou et al., 2009; Augusto et al., 2013). Thus, it seems that A2A receptors are selectively engaged upon perturbation of brain function to control recognition memory and become the preferential target for the benefits of caffeine in promoting neuroprotection against the mnemonic deficits observed in experimental models of AD and aging (Prediger et al., 2005; Arendash et al., 2006; Dall’Igna et al., 2007; Canas et al., 2009; Espinosa et al., 2013).
Notably, in the present study we observed that the activation of A2A receptors in control mice was sufficient to trigger a memory deficit, that is, CGS 21680 attenuated short-term memory in all three behavioural tests, an effect prevented by SCH 58261. Furthermore, infusion of CGS 21680 i.c.v. mimicked the impairment in recognition memory caused by its systemic administration. CGS 21680 infused directly into the posterior cingulate cortex has previously been demonstrated to worsen memory retrieval in the inhibitory avoidance task (Pereira et al., 2005). Similar deficits in memory performance in the object recognition task were observed in transgenic rats overexpressing A2A receptors in the forebrain (Giménez-Llort et al., 2007) and an up-regulation of limbic cortical A2A receptors is observed upon memory impairment in patients (Albasanz et al., 2008) and in animal models of brain disease (Cognato et al., 2010; Espinosa et al., 2013). Finally, we also observed that CGS 21680 did not further exacerbate the scopolamine-induced impairment in recognition memory, as this effect probably results from the full engagement of A2A receptors. This body of evidence not only indicates that the activation of A2A receptors is detrimental for memory performance but also prompts the hypothesis that the over-activation of A2A receptors may actually be a cause of memory impairment. This makes the elucidation of the signalling mechanism operated by A2A receptors particularly important (Fredholm et al., 2007; Zezula and Freissmuth, 2008), as this may shed light on transducing systems critically associated with the implementation and recall of memory traits.
In conclusion, the present study establishes the involvement of both A1 and A2A receptor antagonism as likely mechanisms underlying the beneficial effect of caffeine on scopolamine-induced deficits in recognition memory. Furthermore, the present observation that A2A receptor activation is sufficient to trigger memory deficits prompts the hypothesis that an over-activation of A2A receptors might actually be a causative factor for the emergence of memory deficits. Overall, these results reinforce the therapeutic interest in targeting A2A receptors to manage memory dysfunction.
Acknowledgments
We are grateful to CNPq and CAPES for fellowships. Giovanna C. Chenet received a fellowship from PROBIC/FAPERGS. A. S. A. and D. M. M. are fellows from CNPq and FAPERGS. This study was supported by PRONEX, PRONEM (FAPERGS/CNPq), FCT/CAPES and Ciência sem Fronteiras.
Glossary
- AD
Alzheimer’s disease
- CGS 21680
3-[4-[2-[6-amino-9-[(2R,3R,4S,5S)-5-(ethylcarbamoyl)-3,4-dihydroxy-oxolan-2-yl]purin-2-yl]amino]ethyl]phenyl]propanoic acid
- DPCPX
1,3-dipropyl-8-cyclopentylxanthine
- M1
muscarinic receptors type 1
- SCH 58261
7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine
- SCO
scopolamine
- veh
vehicle
Author contributions
N. P., A. S. A., D. M. M., P. H. S. B., S. M., F. N., G. C. C. and C. M. L. performed the experiments and help to analyse the data. N. P., R. A. C. and L. O. P. designed the research study. N. P., R. A. C. and L. O. P. analysed the data and wrote the paper.
Conflicts of interest
The authors declare no conflicts of interest.
References
- Albasanz JL, Perez S, Barrachina M, Ferrer I, Martín M. Up-regulation of adenosine receptors in the frontal cortex in Alzheimer’s disease. Brain Pathol. 2008;18:211–219. doi: 10.1111/j.1750-3639.2007.00112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelucci ME, Vital MA, Cesário C, Zadusky CR, Rosalen PL, Da Cunha C. The effect of caffeine in animal models of learning and memory. Eur J Pharmacol. 1999;373:135–140. doi: 10.1016/s0014-2999(99)00225-3. [DOI] [PubMed] [Google Scholar]
- Arendash GW, Schleif W, Rezai-Zadeh K, Jackson EK, Zacharia LC, Cracchiolo JR, et al. Caffeine protects Alzheimer’s mice against cognitive impairment and reduces brain beta-amyloid production. Neuroscience. 2006;142:941–952. doi: 10.1016/j.neuroscience.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Augusto E, Matos M, Sévigny J, El-Tayeb A, Bynoe MS, Müller CE, et al. Ecto-5′-nucleotidase (CD73)-mediated formation of adenosine is critical for the striatal adenosine A2A receptor functions. J Neurosci. 2013;33:11390–11399. doi: 10.1523/JNEUROSCI.5817-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol. 2000;163:495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- Batalha VL, Pego JM, Fontinha BM, Costenla AR, Valadas JS, Baqi Y, et al. Adenosine A2A receptor blockade reverts hippocampal stress-induced deficits and restores corticosterone circadian oscillation. Mol Psychiatry. 2013;18:320–331. doi: 10.1038/mp.2012.8. [DOI] [PubMed] [Google Scholar]
- Borota D, Murray E, Keceli G, Chang A, Watabe JM, Ly M, et al. Post-study caffeine administration enhances memory consolidation in humans. Nat Neurosci. 2014;17:201–203. doi: 10.1038/nn.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botton PH, Costa MS, Ardais AP, Mioranzza S, Souza DO, da Rocha JB, et al. Caffeine prevents disruption of memory consolidation in the inhibitory avoidance and novel object recognition tasks by scopolamine in adult mice. Behav Brain Res. 2010;214:254–259. doi: 10.1016/j.bbr.2010.05.034. [DOI] [PubMed] [Google Scholar]
- Bruns RF, Katims JJ, Annau Z, Snyder SH, Daly JW. Adenosine receptor interactions and anxiolytics. Neuropharmacology. 1983;22:1523–1529. doi: 10.1016/0028-3908(83)90121-1. [DOI] [PubMed] [Google Scholar]
- Canas PM, Porciúncula LO, Cunha GM, Silva CG, Machado NJ, Oliveira JM, et al. Adenosine A2A receptor blockade prevents synaptotoxicity and memory dysfunction caused by beta-amyloid peptides via p38 mitogen-activated protein kinase pathway. J Neurosci. 2009;29:14741–14751. doi: 10.1523/JNEUROSCI.3728-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Loewenstein DA, Lin X, Zhang C, Wang L, Duara R, et al. High Blood caffeine levels in MCI linked to lack of progression to dementia. J Alzheimers Dis. 2012;30:559–572. doi: 10.3233/JAD-2012-111781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets – what are the challenges? Nat Rev Drug Discov. 2013;12:265–286. doi: 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognato GP, Agostinho PM, Hockemeyer J, Müller CE, Souza DO, Cunha RA. Caffeine and an adenosine A(2A) receptor antagonist prevent memory impairment and synaptotoxicity in adult rats triggered by a convulsive episode in early life. J Neurochem. 2010;112:453–462. doi: 10.1111/j.1471-4159.2009.06465.x. [DOI] [PubMed] [Google Scholar]
- Costa MS, Botton PH, Mioranzza S, Ardais AP, Moreira JD, Souza DO, et al. Caffeine improves adult mice performance in the object recognition task and increases BDNF and TrkB independent on phospho-CREB immunocontent in the hippocampus. Neurochem Int. 2008;53:89–94. doi: 10.1016/j.neuint.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Costenla AR, Diógenes MJ, Canas PM, Rodrigues RJ, Nogueira C, Maroco J, et al. Enhanced role of adenosine A2A receptors in the modulation of LTP in the rat hippocampus upon ageing. Eur J Neurosci. 2011;34:12–21. doi: 10.1111/j.1460-9568.2011.07719.x. [DOI] [PubMed] [Google Scholar]
- Cunha GM, Canas PM, Melo CS, Hockemeyer J, Müller CE, Oliveira CR, et al. Adenosine A2A receptor blockade prevents memory dysfunction caused by beta-amyloid peptides but not by scopolamine or MK-801. Exp Neurol. 2008;210:776–781. doi: 10.1016/j.expneurol.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Cunha RA. Different cellular sources and different roles of adenosine: A1 receptor-mediated inhibition through astrocytic-driven volume transmission and synapse-restricted A2A receptor-mediated facilitation of plasticity. Neurochem Int. 2008;52:65–72. doi: 10.1016/j.neuint.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Agostinho PM. Chronic caffeine consumption prevents memory disturbance in different animal models of memory decline. J Alzheimers Dis. 2010;20(Suppl. 1):S95–S116. doi: 10.3233/JAD-2010-1408. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Milusheva E, Vizi ES, Ribeiro JA, Sebastião AM. Excitatory and inhibitory effects of A1 and A2A adenosine receptor activation on the electrically evoked [3H]acetylcholine release from different areas of the rat hippocampus. J Neurochem. 1994;63:207–214. doi: 10.1046/j.1471-4159.1994.63010207.x. [DOI] [PubMed] [Google Scholar]
- Dall’Igna OP, Fett P, Gomes MW, Souza DO, Cunha RA, Lara DR. Caffeine and adenosine A2A receptor antagonists prevent beta-amyloid (25–35)-induced cognitive deficits in mice. Exp Neurol. 2007;203:241–245. doi: 10.1016/j.expneurol.2006.08.008. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois J. SCH 58261 and ZM 241385 differentially prevent the motor effects of CGS 21680 in mice: evidence for a functional ‘atypical’ adenosine A2A receptor. Eur J Pharmacol. 2000a;401:63–77. doi: 10.1016/s0014-2999(00)00399-x. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Ménard JF, Parmentier M, Costentin J, Vaugeois JM. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A2A receptors. Br J Pharmacol. 2000b;129:1465–1473. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmenhorst D, Kroll T, Wedekind F, Weisshaupt A, Beer S, Bauer A. In vivo kinetic and steady-state quantification of 18F-CPFPX binding to rat cerebral A1 adenosine receptors: validation by displacement and autoradiographic experiments. J Nucl Med. 2013;54:1411–1419. doi: 10.2967/jnumed.112.115576. [DOI] [PubMed] [Google Scholar]
- Eskelinen MH, Ngandu T, Tuomilehto J, Soininen H, Kivipelto M. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis. 2009;16:85–91. doi: 10.3233/JAD-2009-0920. [DOI] [PubMed] [Google Scholar]
- Espinosa J, Rocha A, Nunes F, Costa MS, Schein V, Kazlauckas V, et al. Caffeine consumption prevents memory impairment, neuronal damage, and adenosine A2A receptors upregulation in the hippocampus of a rat model of sporadic dementia. J Alzheimers Dis. 2013;34:509–518. doi: 10.3233/JAD-111982. [DOI] [PubMed] [Google Scholar]
- Fisher A. Cholinergic modulation of amyloid precursor protein processing with emphasis on M1 muscarinic receptor: perspectives and challenges in treatment of Alzheimer’s disease. J Neurochem. 2012;120(Suppl. 1):22–33. doi: 10.1111/j.1471-4159.2011.07507.x. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Bättig K, Holmén J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol. 2005;63:191–270. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Chern Y, Franco R, Sitkovsky M. Aspects of the general biology of adenosine A2A signaling. Prog Neurobiol. 2007;83:263–276. doi: 10.1016/j.pneurobio.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Giménez-Llort L, Fernández-Teruel A, Escorihuela RM, Fredholm BB, Tobeña A, Pekny M, et al. Mice lacking the adenosine A1 receptor are anxious and aggressive, but are normal learners with reduced muscle strength and survival rate. Eur J Neurosci. 2002;16:547–550. doi: 10.1046/j.1460-9568.2002.02122.x. [DOI] [PubMed] [Google Scholar]
- Giménez-Llort L, Schiffmann SN, Shmidt T, Canela L, Camón L, Wassholm M, et al. Working memory deficits in transgenic rats overexpressing human adenosine A2A receptors in the brain. Neurobiol Learn Mem. 2007;87:42–56. doi: 10.1016/j.nlm.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Harvey AL, Young LC, Kornisiuk E, Snitcofsky M, Colettis N, Blanco C, et al. A novel dihydro-pyrazolo(3,4d)(1,2,4)triazolo(1,5a)pyrimidin-4-one (AJ23) is an antagonist at adenosine A1 receptors and enhances consolidation of step-down avoidance. Behav Brain Res. 2012;234:184–191. doi: 10.1016/j.bbr.2012.06.023. [DOI] [PubMed] [Google Scholar]
- Hirotsu I, Hori N, Katsuda N, Ishihara T. Effect of anticholinergic drug on long-term potentiation in rat hippocampal slices. Brain Res. 1989;482:194–197. doi: 10.1016/0006-8993(89)90561-1. [DOI] [PubMed] [Google Scholar]
- Jackisch R, Strittmatter H, Kasakov L, Hertting G. Endogenous adenosine as a modulator of hippocampal acetylcholine release. Naunyn Schmiedebergs Arch Pharmacol. 1984;327:319–325. doi: 10.1007/BF00506243. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkenberg I, Blokland A. The validity of scopolamine as a pharmacological model for cognitive impairment: a review of animal behavioral studies. Neurosci Biobehav Rev. 2010;34:1307–1350. doi: 10.1016/j.neubiorev.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Kopf SR, Melani A, Pedata F, Pepeu G. Adenosine and memory storage: effect of A1 and A2 receptor antagonists. Psychopharmacology (Berl) 1999;146:214–219. doi: 10.1007/s002130051109. [DOI] [PubMed] [Google Scholar]
- Laurent C, Eddarkaoui S, Derisbourg M, Leboucher A, Demeyer D, Carrier S, et al. Beneficial effects of caffeine in a transgenic model of Alzheimer’s disease-like tau pathology. Neurobiol Aging. 2014;35:2079–2090. doi: 10.1016/j.neurobiolaging.2014.03.027. [DOI] [PubMed] [Google Scholar]
- Marks GA, Shaffery JP, Speciale SG, Birabil CG. Enhancement of rapid eye movement sleep in the rat by actions at A1 and A2A adenosine receptor subtypes with a differential sensitivity to atropine. Neuroscience. 2003;116:913–920. doi: 10.1016/s0306-4522(02)00561-4. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mioranzza S, Costa MS, Botton PH, Ardais AP, Matte VL, Espinosa J, et al. Blockade of adenosine A1 receptors prevents methylphenidate-induced impairment of object recognition task in adult mice. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:169–176. doi: 10.1016/j.pnpbp.2010.10.022. [DOI] [PubMed] [Google Scholar]
- Nikodijević O, Jacobson KA, Daly JW. Locomotor activity in mice during chronic treatment with caffeine and withdrawal. Pharmacol Biochem Behav. 1993;44:199–216. doi: 10.1016/0091-3057(93)90299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira L, Timóteo MA, Correia-de-Sá P. Modulation by adenosine of both muscarinic M1-facilitation and M2-inhibition of [3H]-acetylcholine release from the rat motor nerve terminals. Eur J Neurosci. 2002;15:1728–1736. doi: 10.1046/j.1460-9568.2002.02020.x. [DOI] [PubMed] [Google Scholar]
- Pandolfo P, Machado NJ, Köfalvi A, Takahashi RN, Cunha RA. Caffeine regulates frontocorticostriatal dopamine transporter density and improves attention and cognitive deficits in an animal model of attention deficit hyperactivity disorder. Eur Neuropsychopharmacol. 2013;23:317–328. doi: 10.1016/j.euroneuro.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedata F, Giovannelli L, De Sarno P, Pepeu G. Effect of adenosine, adenosine derivatives, and caffeine on acetylcholine release from brain synaptosomes: interaction with muscarinic autoregulatory mechanisms. J Neurochem. 1986;46:1593–1598. doi: 10.1111/j.1471-4159.1986.tb01781.x. [DOI] [PubMed] [Google Scholar]
- Pereira GS, Mello e Souza T, Vinadé ER, Choi H, Rodrigues C, Battastini AM, et al. Blockade of adenosine A1 receptors in the posterior cingulate cortex facilitates memory in rats. Eur J Pharmacol. 2002;437:151–154. doi: 10.1016/s0014-2999(02)01307-9. [DOI] [PubMed] [Google Scholar]
- Pereira GS, Rossato JI, Sarkis JJ, Cammarota M, Bonan CD, Izquierdo I. Activation of adenosine receptors in the posterior cingulate cortex impairs memory retrieval in the rat. Neurobiol Learn Mem. 2005;83:217–223. doi: 10.1016/j.nlm.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Prediger RD, Fernandes D, Takahashi RN. Blockade of adenosine A2A receptors reverses short-term social memory impairments in spontaneously hypertensive rats. Behav Brain Res. 2005;159:197–205. doi: 10.1016/j.bbr.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Rebola N, Lujan R, Cunha RA, Mulle C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron. 2008;57:121–134. doi: 10.1016/j.neuron.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Riedel W, Hogervorst E, Leboux R, Verhey F, van Praag H, Jolles J. Caffeine attenuates scopolamine-induced memory impairment in humans. Psychopharmacology (Berl) 1995;122:158–168. doi: 10.1007/BF02246090. [DOI] [PubMed] [Google Scholar]
- Rodrigues RJ, Canas PM, Lopes LV, Oliveira CR, Cunha RA. Modification of adenosine modulation of acetylcholine release in the hippocampus of aged rats. Neurobiol Aging. 2008;29:1597–1601. doi: 10.1016/j.neurobiolaging.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Collins-Praino LE, Pardo M, Podurgiel SJ, Baqi Y, Müller CE, et al. Conditional neural knockout of the adenosine A2A receptor and pharmacological A2A antagonism reduce pilocarpine-induced tremulous jaw movements: studies with a mouse model of parkinsonian tremor. Eur Neuropsychopharmacol. 2013;23:972–977. doi: 10.1016/j.euroneuro.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Snyder SH, Katims JJ, Annau Z, Bruns RF, Daly JW. Adenosine receptors and behavioral actions of methylxanthines. Proc Natl Acad Sci U S A. 1981;78:3260–3264. doi: 10.1073/pnas.78.5.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki F, Shimada J, Shiozaki S, Ichikawa S, Ishii A, Nakamura J, et al. Adenosine A1 antagonists. 3. Structure–activity relationships on amelioration against scopolamine- or N6-((R)-phenylisopropyl) adenosine-induced cognitive disturbance. J Med Chem. 1993;36:2508–2518. doi: 10.1021/jm00069a009. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Sakurai M, Hayashi S. Effect of scopolamine and HP 029, a cholinesterase inhibitor, on long-term potentiation in hippocampal slices of the guinea pig. Neurosci Lett. 1989;98:179–183. doi: 10.1016/0304-3940(89)90506-5. [DOI] [PubMed] [Google Scholar]
- Vannucchi MG, Scali C, Kopf SR, Pepeu G, Casamenti F. Selective muscarinic antagonists differentially affect in vivo acetylcholine release and memory performances of young and aged rats. Neuroscience. 1997;79:837–846. doi: 10.1016/s0306-4522(97)00091-2. [DOI] [PubMed] [Google Scholar]
- Vollert C, Forkuo GS, Bond RA, Eriksen JL. Chronic treatment with DCPCX, an adenosine A1 antagonist, worsens long-term memory. Neurosci Lett. 2013;548:296–300. doi: 10.1016/j.neulet.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Ma YY, van den Buuse M. Improved spatial recognition memory in mice lacking adenosine A2A receptors. Exp Neurol. 2006;199:438–445. doi: 10.1016/j.expneurol.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Koder T, Cho K, Massey PV, Duguid G, Barker GR, et al. Cholinergic neurotransmission is essential for perirhinal cortical plasticity and recognition memory. Neuron. 2003;38:987–996. doi: 10.1016/s0896-6273(03)00358-1. [DOI] [PubMed] [Google Scholar]
- Winters BD, Bartko SJ, Saksida LM, Bussey TJ. Scopolamine infused into perirhinal cortex improves object recognition memory by blocking the acquisition of interfering object information. Learn Mem. 2007;14:590–596. doi: 10.1101/lm.634607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Soohoo D, Soelaiman S, Kalla R, Zablocki J, Chu N, et al. Characterization of the potency, selectivity, and pharmacokinetic profile for sixadenosine A2A receptor antagonists. Naunyn Schmiedebergs Arch Pharmacol. 2007;375:133–144. doi: 10.1007/s00210-007-0135-0. [DOI] [PubMed] [Google Scholar]
- Zezula J, Freissmuth M. The A2A-adenosine receptor: a GPCR with unique features? Br J Pharmacol. 2008;153(Suppl. 1):S184–S190. doi: 10.1038/sj.bjp.0707674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SJ, Zhu ME, Shu D, Du XP, Song XH, Wang XT, et al. Preferential enhancement of working memory in mice lacking adenosine A2A receptors. Brain Res. 2009;1303:74–83. doi: 10.1016/j.brainres.2009.09.082. [DOI] [PubMed] [Google Scholar]


