Abstract
Background and Purpose
Classically, ligands of GPCRs have been classified primarily upon their affinity and efficacy to activate a signal transduction pathway. Recent reports indicate that the efficacy of a particular ligand can vary depending on the receptor-mediated response measured (e.g. activating G proteins, other downstream responses, internalization). Previously, we reported that inverse agonists induce both homo- and heterologous desensitization, similar to agonist stimulation, at the Gs-coupled 5-HT7 receptor. The primary objective of this study was to determine whether different inverse agonists at the 5-HT7 receptor also induce internalization and/or degradation of 5-HT7 receptors.
Experimental Approach
HEK293 cells expressing 5-HT7(a, b or d) receptors were pre-incubated with 5-HT, clozapine, olanzapine, mesulergine or SB269970 and their effects upon receptor density, AC activity, internalization, recruitment of β-arrestins and lysosomal trafficking were measured.
Key Results
The agonist 5-HT and three out of four inverse agonists tested increased internalization independently of β-arrestin recruitment. Among these, only the atypical antipsychotics clozapine and olanzapine promoted lysosomal sorting and reduced 5-HT7 receptor density (∼60% reduction within 24 h). Inhibition of lysosomal degradation with chloroquine blocked the clozapine- and olanzapine-induced down-regulation of 5-HT7 receptors. Incubation with SB269970 decreased both 5-HT7(b) constitutive internalization and receptor density but increased 5-HT7(d) receptor density, indicating differential ligand regulation among the 5-HT7 splice variants.
Conclusions and Implications
Taken together, we found that various ligands differentially activate regulatory processes governing receptor internalization and degradation in addition to signal transduction. Thus, these data extend our understanding of functional selectivity at the 5-HT7 receptor.
Tables of Links
| TARGETS | ||
|---|---|---|
| GPCRsa | ||
| 5-HT2A receptor | CCR5 | Opioid receptors |
| 5-HT7 receptor | D2 receptor | PTH1 receptor |
| β1-adrenoceptor | ETA receptor | V2 receptor |
| β2-adrenoceptor | H2 receptor | Y1 receptor |
| Angiotensin receptors | MC3 receptor | Enzymesb |
| CCK1 receptor | MC4 receptor | Adenylyl cyclase (AC) |
| LIGANDS | |
|---|---|
| 5-HT | Isoprenaline |
| [3H]-5-CT | Mesulergine |
| [3H]-CGP12177 | Metergoline |
| Chloroquine | SB269970 |
| Clozapine | |
| Olanzapine |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,bAlexander et al., 2013a,b).
Introduction
Until recently, ligands towards GPCRs have been classified based on their affinity and their efficacy at conveying a specified response through a specific receptor. Based on the response pattern, ligands have been characterized either as full agonists, partial agonists, neutral antagonists, partial or full inverse agonists. Recent studies indicate the need to redefine ligand efficacy. It has been demonstrated for both agonists and antagonists/inverse agonists that different ligands can stabilize distinct receptor conformations (Kobilka and Deupi, 2007; Rochais et al., 2007). It has also been hypothesized that these different receptor conformations can lead to differential signal transduction patterns (Kenakin, 1995; 2002; 2005; 2013,,,), for example, different agonists towards one receptor can activate different downstream effectors with varying efficacy, as demonstrated for the β1- and β2-adrenoceptors, D2 dopamine, CCR5, 5-HT2A, angiotensin, histamine, vasopressin and opioid receptors (reviewed by Galandrin et al., 2007; Kenakin, 2007; Rajagopal et al., 2011; Reiter et al., 2012; Seifert, 2013; Luttrell, 2014). The term ‘functional selectivity’ has been suggested as a description of how two ligands could stabilize different receptor conformations leading to differential effects (Urban et al., 2007).
The human 5-HT7 receptor is coupled to Gs and expressed as three different splice variants [5-HT7(a, b and d)], differing only in their carboxy termini (Heidmann et al., 1997; Krobert et al., 2001; Gellynck et al., 2013). The 5-HT7 receptor displays several interesting pharmacological properties; it behaves as if it is physically pre-associated with Gs in the absence of ligand (Bruheim et al., 2003) and expression of the 5-HT7 receptor is sufficient to attenuate signalling of other Gs-coupled receptors (Andressen et al., 2006). The 5-HT7 receptor also displays high constitutive activity (Krobert and Levy, 2002; Andressen et al., 2006) and several atypical antipsychotics have high affinity towards the 5-HT7 receptor (Roth et al., 1994). Of particular interest, the atypical antipsychotics clozapine and olanzapine function as inverse agonists at the 5-HT7 receptor (Thomas et al., 1998; Krobert and Levy, 2002). Functional selectivity has also been observed for the 5-HT7 receptor: long-term incubation with the endogenous agonist 5-HT and the inverse agonist SB269970 invoked both homologous and heterologous desensitization without down-regulating receptor densities (Krobert et al., 2006). The desensitizing effects of SB269970 were not reproduced by two other inverse agonists (clozapine and mesulergine), indicating that efficacy towards G-protein activation and desensitization differs between ligands at the 5-HT7 receptor. Although the three 5-HT7 splice variants share nearly identical functional and pharmacological profiles (Krobert et al., 2001; Krobert and Levy, 2002; Andressen et al., 2006), one difference among the splice variants is that 5-HT7(d) receptors display a marked constitutive internalization in the absence of agonist, and that SB269970 stabilized 5-HT7(d) receptors on the cell membrane (Guthrie et al., 2005). The objective of this study was to build upon the above findings by determining whether ligands differentially mediate down-regulation of 5-HT7 receptors and if so whether differences in down-regulation occur among the 5-HT7 splice variants.
Methods
Construction of expression vectors
For the construction of 5-HT7(b) yellow fluorescent protein (YFP), a SalI-flanked primer with a mutated stop codon was used to generate a PpuMI/SalI fragment covering the C-terminal end of the 5-HT7(b) receptor. After subcloning and sequence verification, this PpuMI/SalI fragment was ligated to a PpuMI/XbaI fragment of ph5-HT7(b) (De Martelaere et al., 2007) and a SalI/XbaI fragment of peYFPN1 (Clontech, Mountain View, CA, USA). The resulting vector was sequence verified in both directions. For the construction of 5-HT7(b) receptor lacking a C-terminal PDZ domain (Δ430FVL), a stop codon was introduced after E429 in the human FLAG-5-HT7(b) (De Martelaere et al., 2007) by site-directed mutagenesis and this construct was sequence verified.
Transfection of HEK293 cells
The three human 5-HT7 receptor splice variants were stably expressed in HEK293 cells and cultured in 5-HT-free medium (UltraCULTURE™ general purpose serum-free medium; BioWhittaker, Walkersville, MD, USA), as described previously (Krobert et al., 2001). HEK293 cells (ATCC, Rockville, MD, USA) were grown in DMEM (Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal calf serum (EuroClone, Milano, Italy), L-glutamine (2 mM), penicillin (100 U·mL−1) and streptomycin (100 μg·mL−1) and were transiently transfected with LipofectAMINE2000™ (Life Technologies) according to the manufacturer’s protocol. Cells were transfected with the following plasmids: human 5-HT7(a) (Krobert et al., 2001), human FLAG-5-HT7(b) (De Martelaere et al., 2007), human 5-HT7(b)-YFP, human β2 adrenoceptor (Andressen et al., 2006), CD63-RFP (generated as described for the CD63-YFP; Sherer et al., 2003), LAMP-1-YFP (both generous gifts from Dr Walther Mothes, Section of Microbial Pathogenesis, Yale University School of Medicine, New Haven, CT, USA: Sherer et al., 2003) or β-arrestin1-GFP and β-arrestin2-GFP (both generous gifts from Professor Håvard Attramadal, Institute of Surgical Research, University of Oslo and Oslo University Hospital), where indicated. After transfection, HEK293 cells were cultured in UltraCULTURE, supplemented with L-glutamine (2 mM), penicillin (100 U·mL−1) and streptomycin (100 μg·mL−1) for 48 h.
Membrane preparation, AC assay, radioligand binding and cell surface receptor binding
Crude membranes were prepared exactly as described previously (Krobert et al., 2001). For pre-incubation experiments, the cells were subjected to a more vigorous washing protocol to remove residual drug. Briefly, following 30 min to 24 h pre-incubation with saturating concentrations of 5-HT, mesulergine, clozapine, olanzapine or SB269970, or 200 μM chloroquine (only where indicated), the cells were rapidly washed with 37°C HBSS (Life Technologies) followed by two 1 h incubations at 37°C in DMEM, then membranes were prepared as described, or cells were trypsinized, pelleted and resuspended in UltraCULTURE and subjected to cell surface receptor binding (see below). AC assays and radioligand binding assays on membrane preparations were performed exactly as previously described (Krobert et al., 2001), estimating Kd and Bmax based on saturation binding with [3H]-5-CT or [3H]-mesulergine as radiochemicals and the pKi of olanzapine was determined by displacing [3H]-5-CT with increasing concentrations of olanzapine. Bmax of membrane preparations from cells expressing β2-adrenoceptors pre-incubated with isoprenaline, clozapine or olanzapine, which was subsequently washed (as described earlier), was measured exactly as for 5-HT7 receptors, but with [3H]-CGP12177 as the radioligand and isoprenaline to determine non-specific binding. Cell-surface 5-HT7 receptor density was determined as described previously (Andressen et al., 2006) with the following modification: cells were incubated with the lipophilic compound [3H]-SB269970 (5 nM) with or without the lipophilic mesulergine (100 μM) or the hydrophilic agonist 5-HT (100 μM). The incubation was carried out for 3 h at 13°C before harvesting, which allows ligand binding to reach equilibrium while inhibiting sequestration or the return of sequestered receptors to the cell surface (Andressen et al., 2006). The percentage of receptors on the cell surface was calculated as specific radioligand binding displaced by the hydrophilic ligand, and the percentage of internalized receptors was derived from this.
Confocal laser-scanning microscopy
HEK293 cells were co-transfected with FLAG-5-HT7(b), LAMP-1-YFP and CD63-RFP (where indicated) 48 h before the measurement was taken. Cells were stimulated with the indicated ligand for 6 h at 37°C, washed in PBS and subsequently fixed with 4% paraformaldehyde for 10 min. Cells were then washed with PBS for 10 min, permeabilized with 0.2% Triton-X 100 for 10 min and washed twice in PBS for 10 min before being blocked with 10% BSA in PBS for an additional 30 min at 37°C. Cells were then incubated with anti-FLAG M2 overnight at 4°C. After the cells had been washed twice in PBS for 10 min, immunobinding was detected by incubating the cells with Cy5-conjugated donkey anti-mouse IgG for 1 h. After being washed with PBS, the cells were mounted in DakoCytomation Fluorescent Mounting Medium (DakoCytomation, Carpinteria, CA, USA). As controls for cross-reactivity of the secondary reagent, the primary antibody was omitted from the staining sequence. For the β-arrestin recruitment assay, cells transiently expressing β-arrestin1-GFP or β-arrestin2-GFP were incubated with ligand from 20 min up to 3 h, then washed in PBS before fixation with 4% paraformaldehyde for 10 min. Cells were then washed twice in PBS for 10 min and mounted in DakoCytomation Fluorescent Mounting Medium.
Cells were analysed using a Leica TCS SP confocal microscope (Leica, Heidelberg, Germany) equipped with an Ar (488 nm) and two He/Ne (543 and 633 nm) lasers and 100× oil immersion objective, or an Olympus FV1000/BX61 (Olympus Corporation, Tokyo, Japan) with an Ar (488 nm) laser and an oil immersion objective (60 × 1.35 NA). Images were taken of approximately 10 cells in up to five experiments at the largest diameter of the nucleus. Multi-labelled images were acquired sequentially and single-image TIF files were exported to Adobe Photoshop CS3 (Adobe, Mountain View, CA, USA) or ImageJ (US National Institutes of Health, Bethesda, MD, USA) for creating an overlay image.
Live imaging of β-arrestin recruitment to the plasma membrane
HEK293 cells were transiently transfected with β2-adrenoceptor and either β-arrestin1-GFP or β-arrestin2-GFP, transferred to poly-L-lysine-coated cover slides and imaged in a watertight imaging chamber (Attofluor; Life Technologies) at room temperature with buffer A (mM): MgCl2 (1), KCl (1.97), KH2PO4 (0.43), K2HPO4 (1.5), CaCl2 (1), NaCl (144) and glucose (10) through a motorized digital-inverted fluorescent microscope (iMIC; FEI Munich GmbH, Munich, Germany) with an oil immersion objective (60 × 1.35 NA). Cells were excited at 500 ± 10 nm for 30–50 ms using a monochromator (Polychrome V: FEI Munich), and imaged on an EM-CCD camera chip (EVOLVE 512, Photometrics, Tucson, AZ, USA) and acquired by Live Acquisition browser (FEI Munich). Image stacks were exported to Fiji software and movies were converted to AVI files with JPG compression.
Protein measurements
Protein concentration in membrane preparations was measured with the Micro BC Assay Reagent Kit (Uptima Interchim, Montluçon, France) using BSA as a standard.
Statistics
Statistical significance was determined using GraphPad Prism 6.01 for Windows (GraphPad Software, San Diego, CA, USA) with one-way anova with Bonferroni’s adjustment or Dunnett’s multiple comparison test, two-way anova with Bonferroni’s adjustment or Student’s t-test, as indicated. P < 0.05 was considered statistically significant, but lower α-values are also reported.
Materials
5-HT (5-HT hydrochloride), clozapine (8-chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo[b,e][1,4]diazepine), mesulergine (N′-[(8a)-1,6-dimethylergolin-8-yl]-N,N-dimethylsulfamide hydrochloride), metergoline ([[(8β)-1,6-dimethylergolin-8-yl]-methyl]carbamic acid phenylmethyl ester), isoprenaline hydrochloride, chloroquine (N4-(7-chloro-4-quinolinyl)-N1,N1-dimethyl-1,4-pentanediamine diphosphate) and mouse anti-FLAG M2 were from Sigma-Aldrich (St. Louis, MO, USA). SB269970 ((R)-3-(2-(2-(4-methyl-piperidin-1-yl)ethyl)-pyrrolidine-1-sulphonyl)-phenol) was from Tocris (Bristol, UK). Olanzapine (ad injectabila) was from Eli Lilly & Co. (Indianapolis, IN, USA). G418, penicillin-streptomycin, L-glutamine and LipofectAMINE2000™ were from Life Technologies). Cy5-conjugated donkey anti-mouse IgG was from Jackson ImmunoResearch (West Grove, PA, USA). [3H]-5-CT (5-carboxamidotryptamine) (60–102 Ci·mmol−1), [N6-methyl-3H]-mesulergine (87 Ci·mmol−1), [3H]-SB269970 (36 Ci·mmol−1) and [3H]-CGP12177 (37 Ci·mmol−1) were from GE Healthcare (Buckinghamshire, UK).
Nomenclature
We adopted the nomenclature proposed by Galandrin and Bouvier (2006) to provide clarity and to differentiate between agonism and inverse agonism upon the three major measured responses in the current work. ACago and ACinv are agonists and inverse agonists on AC activation respectively. Intago and Intinv are agonists and inverse agonists at internalization. Dregago and Dreginv are agonists and inverse agonists on down-regulation.
Results
We have previously reported that clozapine is a full inverse agonist at the 5-HT7 receptor (Krobert and Levy, 2002). Here, we wanted to determine the efficacy of four different inverse agonists (ACinv) to reduce constitutive AC activity at the 5-HT7 receptor. As shown in Figure 1, olanzapine behaved as a full ACinv (104 ± 6% of clozapine), whereas mesulergine and SB269970 were partial ACinv (24 ± 6% and 53 ± 1% of clozapine respectively). Olanzapine was almost six times less potent than clozapine (pEC50 values were 7.2 ± 0.1 and 7.9 ± 0.1, respectively), whereas mesulergine and SB269970 were more potent (pEC50 values were 8.7 ± 0.1 and 9.0 ± 0.3 respectively). The constitutive activity of 5-HT7(b) receptors accounted for 3.5 ± 0.2% of maximal 5-HT-stimulated AC activity (n = 3) as shown in the inset of Figure 1.
Figure 1.
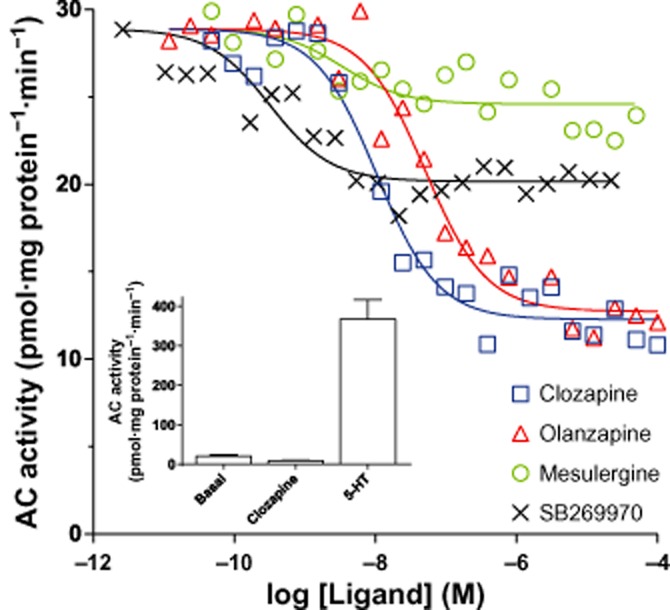
Clozapine and olanzapine are full inverse agonists at AC activation. AC activity in response to increasing concentrations of the indicated ligands in the membranes of HEK293 cells stably expressing the human 5-HT7(b) receptor. AC activity was measured as described in the Methods section and the data shown are representative of those obtained from three independent experiments. pEC50 was 7.9 ± 0.1, 7.2 ± 0.1, 8.7 ± 0.1, 9.0 ± 0.3 and 7.6 ± 0.1 (n = 3) for clozapine, olanzapine, mesulergine, SB269970 and 5-HT respectively. Inset: basal, clozapine- and 5-HT-stimulated AC activity presented as mean ± SEM of the three experiments.
Next, we determined whether incubation with these ACinv or the agonist (ACago), 5-HT, modified receptor density. As shown in Figure 2, 5-HT7 receptor density was differentially modified across both the splice variants and the applied ligand. Incubation with the ACago 5-HT increased only 5-HT7(b) receptor density. Mesulergine increased only 5-HT7(d) receptor density. The full ACinv clozapine reduced 5-HT7 receptor density in all three splice variants, but was significantly less efficacious at the 5-HT7(a) receptor (P < 0.05). Therefore, clozapine can be considered as an agonist with respect to mediating down-regulation (Dregago). Whereas the partial ACinv SB269970 did not modify 5-HT7(a) receptor density, it reduced 5-HT7(b) receptor density compared to the control (Dregago) and increased 5-HT7(d) receptor density (Dreginv). The affinity of [3H]-5-CT or [3H]-mesulergine at the 5-HT7(a, b or d) receptors was not significantly modified after 24 h of incubation with 5-HT, mesulergine, clozapine or SB269970 in any of three 5-HT7 splice variants (Supporting Information Tables S1 and S2), indicating that the change in Bmax did not result from remaining bound ligand. To determine if the clozapine-mediated down-regulation was dependent upon high receptor expression, we evaluated two low-expressing 5-HT7(b) receptor cell lines (Bmax 254 ± 35 fmol·mg protein−1) and found that 24 h of incubation with clozapine reduced receptor density to 51 ± 7% of control (n = 3), which is not significantly different from that obtained with higher receptor expression.
Figure 2.
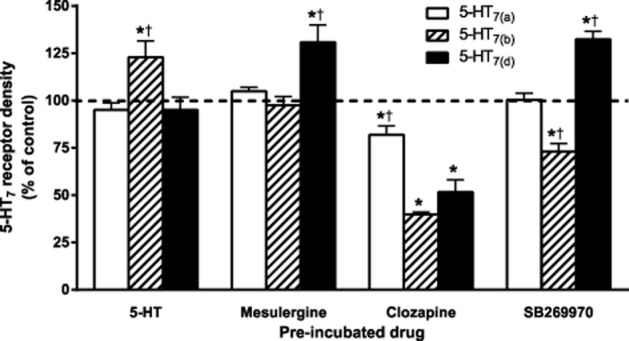
Long-term incubation with agonists or inverse agonists modifies 5-HT7 receptor density. Receptor density (Bmax) in the membranes of HEK293 cells stably expressing the human 5-HT7 receptor splice variants after 24 h of pre-incubation with either 10 μM 5-HT or 1 μM mesulergine, clozapine or SB269970. Radioligand binding was performed with increasing concentrations of [3H]-5-CT in the absence (total binding) and presence (non-specific binding) of 10 μM 5-HT for 1 h at 24°C. Bmax and Kd were determined as described in the Methods section. Data are presented as a % of control (sister plates of cells pre-incubated without drug or vehicle). The data shown are mean ± SEM of 5–14 experiments from two to three different clonal cell lines of each splice variant. *P < 0.01 versus control (one-way anova with Dunnett’s multiple comparison test), †P < 0.05 versus the other two 5-HT7 splice variants pre-incubated with the same ligand (two-way anova with Bonferroni adjustment).
It has been reported that high-affinity agonist binding ([3H]-5-HT) only labels 60% of the 5-HT7(a) receptors labelled with an antagonist ([3H]-mesulergine; Alberts et al., 2001). Therefore, we also determined 5-HT7 receptor densities using [3H]-mesulergine in parallel with [3H]-5-CT binding on the same membrane preparations. No significant differences in 5-HT7 receptor density (Bmax) were observed between estimates calculated from [3H]-mesulergine or [3H]-5-CT radioligand binding (Table 2001 and Supporting Information Fig. S1).
Table 1.
Affinity (Kd) and receptor density (Bmax in pmol·mg protein−1) of [3H]-5-CT and [3H]-mesulergine binding was determined in the membranes of HEK293 stably expressing the indicated 5-HT7 splice variant
| Radioligand | [3H]-5-CT | [3H]-mesulergine | ||
|---|---|---|---|---|
| Bmax | Kd (nM) | Bmax | Kd (nM) | |
| 5-HT7(a) | 13.8 ± 1.5 | 0.39 ± 0.09 | 13.7 ± 1.5 | 9.2 ± 1.6 |
| 5-HT7(b) | 19.2 ± 3.0 | 0.40 ± 0.05 | 19.7 ± 2.9 | 6.2 ± 0.7 |
| 5-HT7(d) | 17.6 ± 1.4 | 0.53 ± 0.07 | 18.8 ± 2.8 | 9.6 ± 1.2 |
Membranes were incubated with increasing concentrations of the indicated radioligand in the absence (total binding) or presence (non-specific binding) of 10 μM 5-HT or 10 μM metergoline, for [3H]-5-CT or [3H]-mesulergine respectively. Bmax and Kd were determined as described in the Methods section. The data shown are mean ± SEM obtained from non-pre-incubated control membranes. n = 5, 5 and 13 for 5-HT7(a), 5-HT7(b) and 5-HT7(d) respectively. No significant differences were observed in Kd across splice variants or Bmax between radioligands (one-way anova).
Down-regulation of 5-HT7 receptors induced by the atypical antipsychotics clozapine and olanzapine is time-dependent
We and others have previously reported that 5-HT7 receptor density was not modified after 30–60 min of incubation with the atypical antipsychotic clozapine (Krobert et al., 2006; Smith et al., 2006). Because 24 h of incubation with clozapine down-regulated 5-HT7 receptor density (Figure 2), we wanted to determine the kinetics of clozapine-mediated down-regulation of 5-HT7 receptors. For comparison, we evaluated the ability of another atypical antipsychotic, olanzapine [pKi = 5.9 ± 0.2 at the 5-HT7(b) receptor, n = 3], to mediate down-regulation of 5-HT7 receptor density. Because the 5-HT7(b) receptor was more sensitive than the other splice variants to down-regulation by clozapine, these studies were only conducted with this splice variant. As shown in Figure 3, both olanzapine and clozapine decreased 5-HT7(b) receptor density with similar kinetics over the 24 h incubation period, reaching a maximal reduction after ∼12 h. The affinity of [3H]-5-CT for the 5-HT7(b) receptor was not modified after incubation with olanzapine or clozapine (Figure 3, inset). Olanzapine and clozapine have high affinity for a wide range of receptors (Roth et al., 1994; Schotte et al., 1996) and mediate receptor-independent effects (Park et al., 2001). Under the same experimental conditions, β2-adrenoceptor density was down-regulated to 70% of control after 24 h of incubation with isoprenaline. Neither olanzapine nor clozapine modified β2-adrenoceptor density, indicating 5-HT7 receptor-specific effects (Supporting Information Table S3).
Figure 3.
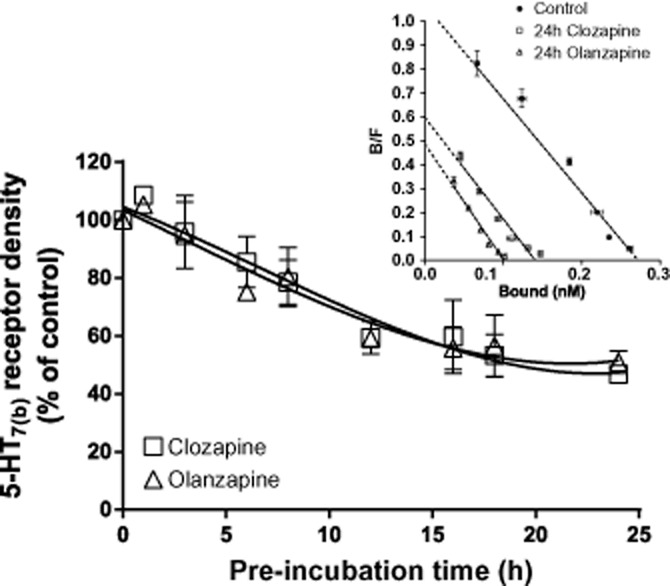
Clozapine- and olanzapine-mediated down-regulation of 5-HT7(b) receptors is time-dependent. Receptor density in the membranes of HEK293 cells stably expressing the 5-HT7(b) receptor after pre-incubation with either 20 μM olanzapine or 1 μM clozapine for 0–24 h. Receptor density was determined by [3H]-5-CT binding, as described in the Methods section, and is presented as a % of control (sister plates of cells pre-incubated without drug). The data are mean ± SEM of four to five experiments. Inset: A representative Scatchard plot of the data for control and 24 h pre-incubation with clozapine and olanzapine with mean ± SD of triplicate measurement.
Clozapine, a lipophilic ligand, could either bind to receptors at the cell surface, resulting in internalization and subsequent degradation, or act as a negative chaperone on newly synthesized receptors. To discriminate between these options, we incubated with either 1 μM clozapine alone or 100 μM 5-HT or a combination of both. As shown in Figure 4, a high concentration of the hydrophilic ligand 5-HT prevented clozapine-mediated down-regulation of 5-HT7(b) receptors, indicating that clozapine binds to receptors on the cell surface and probably promotes internalization and sorting of receptors for degradation.
Figure 4.
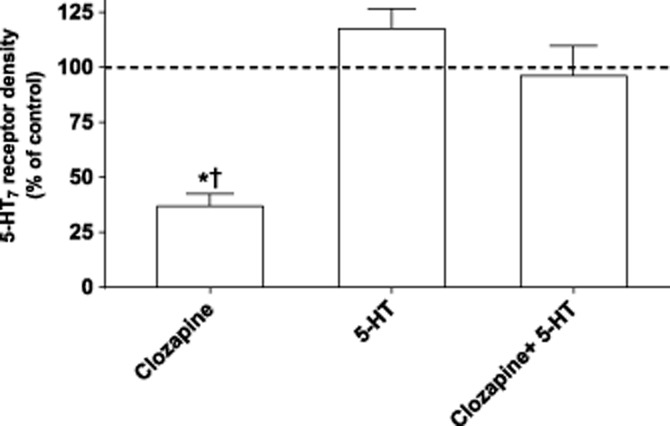
Clozapine down-regulates 5-HT7(b) receptors by binding to receptors at the cell surface. Receptor density in the membranes of HEK293 cells stably expressing the 5-HT7(b) receptor after pre-incubation with either 1 μM clozapine, 100 μM 5-HT or the combination for 24 h. Receptor density was determined by [3H]-5-CT binding, as described in the Methods section, and is presented as a percentage of control (sister plates of cells pre-incubated without drug). The data are mean ± SEM of four experiments. No difference in Kd of [3H]-5-CT was observed (data not shown). *P < 0.01 versus control (one-way anova with Dunnett’s multiple comparison test); †P < 0.01 versus both 5-HT alone and clozapine + 5-HT (one-way anova with Bonferroni adjustment).
Ligands modify internalization of 5-HT7 receptors
To determine whether the ligands modify the internalization of 5-HT7 receptors, we performed a binding assay on intact cells that can distinguish intracellular receptors from the total receptor pool. We found that 30 ± 3% of 5-HT7(b) receptors are constitutively internalized, a value in accordance with a previous report (Guthrie et al., 2005). After an 8.5 h incubation (when clozapine had down-regulated receptor levels to 79 ± 8% of control; Figure 3), a significantly higher fraction of 5-HT7(b) receptors were internalized with either 5-HT, mesulergine, olanzapine or clozapine compared with control (parallel incubations in the absence of ligand; Figure 5A). In contrast, an incubation with SB269970 significantly decreased the fraction of internalized 5-HT7(b) receptors and SB269970 can thus be classified as an inverse agonist regarding internalization (Intinv).
Figure 5.
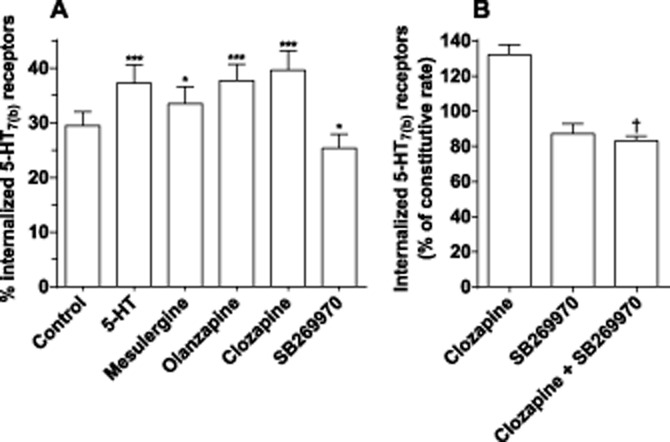
Internalization of 5-HT7(b) receptors is modified after incubation with different ligands. Intact cells stably expressing 5-HT7(b) receptors were pre-incubated for 8.5 h with the indicated ligand. To determine the percentage of internalized receptors, intact cells were subsequently incubated with 5 nM [3H]-SB269970 in the absence or presence of either 5-HT or mesulergine (both at 100 μM) as described in the Methods section. (A) The data shown are mean ± SEM of three experiments. (B) Cells were incubated with clozapine or SB269970 alone or in combination for 8.5 h. Data are presented as a % of control (sister plates with cells pre-incubated without drug to reflect the constitutive internalization rate) and are mean ± SEM of two experiments. *P < 0.05 and ***P < 0.001 versus control (one-way anova with Bonferroni’s multiple comparison test). †P < 0.05 versus clozapine alone (Student’s t-test).
SB269970 displays about 10 times higher potency than clozapine for the 5-HT7 receptor (Figure 1). To determine if clozapine and SB269970 are competing for the same pool of receptors, we co-incubated the cells with 1 μM clozapine and 1 μM SB269970 and found that internalization of 5-HT7(b) receptors was reduced, similar to incubation with SB269970 alone (Figure 5B), suggesting that clozapine-mediated internalization is competitive.
Internalization and trafficking of GPCRs is normally initiated and coordinated by recruitment of cytosolic β-arrestins to the receptor on the plasma membrane (Lefkowitz, 2013). To determine if clozapine or 5-HT recruited β-arrestin 1 or 2, we co-expressed GFP-tagged arrestins in stable-expressing 5-HT7(b) cell lines, incubated with ligands for 30 min to 3 h and determined recruitment of β-arrestin-GFP to the plasma membrane. Incubation with 5-HT, clozapine or SB269970 did not recruit β-arrestin 1 or 2 to the plasma membrane (Figure 6), in contrast to activation of β2-adrenoceptors, which resulted in an observable membrane recruitment of β-arrestin 1 and 2 (Figure 6 and Supporting Information Movies). Therefore, we conclude that β-arrestin recruitment is not involved in the internalization or down-regulation of the 5-HT7(b) receptor.
Figure 6.
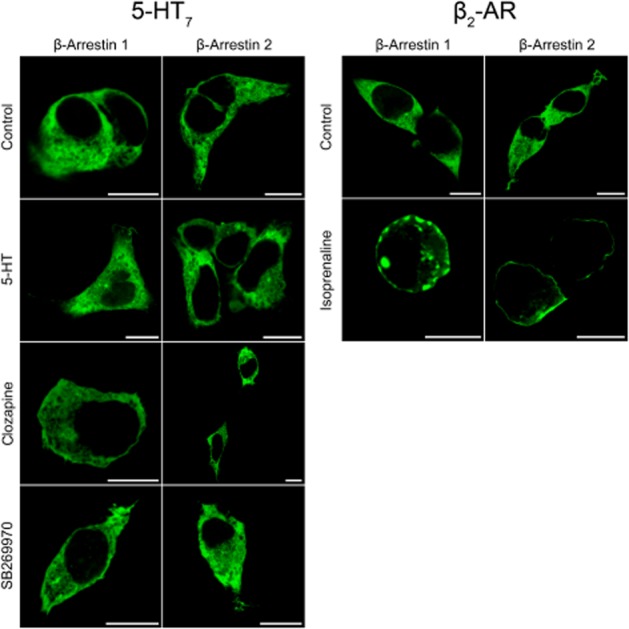
β-Arrestin 1 or 2 are not recruited to the 5-HT7 receptor. 5-HT7: HEK293 cells stably expressing 5-HT7(b) receptors were transfected with either β-arrestin1-GFP or β-arrestin2-GFP, incubated with either vehicle, 10 μM 5-HT, 1 μM clozapine or 1 μM SB269970 for 30 min at 37°C. Similar results were obtained for 3 h incubation with ligands at the 5-HT7 receptor. β2-adrenoceptor (β2-AR): HEK293 cells were transiently transfected with either β-arrestin1-GFP or β-arrestin2-GFP and twice as much β2-adrenoceptor DNA and incubated with either vehicle or 10 μM isoprenaline for 30 min at 37°C. After fixation, cellular distribution of β-arrestin-GFP was visualized by a confocal microscope as described in the Methods section. Only cells expressing β2-adrenoceptors and incubated with isoprenaline recruited β-arrestins to the plasma membrane. Scale bar: 10 μm.
Clozapine- and olanzapine-mediated internalization is followed by lysosomal degradation of 5-HT7 receptors
Internalized receptors are either recycled back to the plasma membrane or transported to lysosomes for degradation. Using an N-terminally FLAG-tagged 5-HT7(b) receptor, we determined whether clozapine- and olanzapine-internalized 5-HT7(b) receptors were targeted to lysosomes for degradation. The FLAG tag did not modify the affinity of [3H]-5-CT, ability to activate AC or clozapine-mediated down-regulation of the 5-HT7(b) receptor, but the FLAG-tagged receptors were expressed at a lower Bmax than wild-type receptors (Table 2013a). However, tagging YFP to the C-terminal end of the 5-HT7(b) receptor inhibited clozapine-mediated down-regulation (Table 2013a), indicating that a freely available carboxy terminus is important for down-regulation. Lysosomes were visualized by co-transfecting fluorescently tagged LAMP-1 and CD63 (LAMP-3), both known to be localized in lysosomes (Sherer et al., 2003). In untreated cells, 5-HT7(b) receptors did not co-localize with the lysosomal marker LAMP-1 (Figure 7). After 6 h of incubation (a time point with significant clozapine- or olanzapine-mediated receptor down-regulation; Figure 3) with either clozapine or olanzapine, internalized 5-HT7(b) receptors were present in lysosomes, whereas less co-localization of internalized 5-HT7(b) receptors was observed in lysosomes after incubation with SB269970, and incubation with 5-HT did not differ from control (Figure 7). To verify that the lysosomal marker LAMP-1-YFP was only present in the lysosomes, we co-expressed another fluorescently tagged lysosomal marker, CD63-RFP. As shown in Supporting Information Fig. S2, the lysosomal proteins LAMP-1 and CD63 co-localized to a high degree (shown as yellow colour in overlay). 5-HT7(b) receptors co-localized with both LAMP-1 and CD63 only in cells incubated with clozapine or olanzapine (shown as white colour in overlay). Less co-localization was observed in cells pre-incubated with SB269970, whereas cells pre-incubated with 5-HT or mesulergine did not differ from control cells with respect to lysosomal co-localization (Figure 7 and data not shown). In some of the cells visualized (e.g. clozapine in Figure 7), there was little staining of 5-HT7(b) receptors on the plasma membrane in the section chosen (where LAMP-1 and CD63 were strongest). However, in other sections (e.g. olanzapine and SB269970 in Figure 7), a staining in both the plasma membrane and the lysosomes was visualized. This variation, complicating the interpretation of these images, represents a possible limitation of our study.
Figure 7.
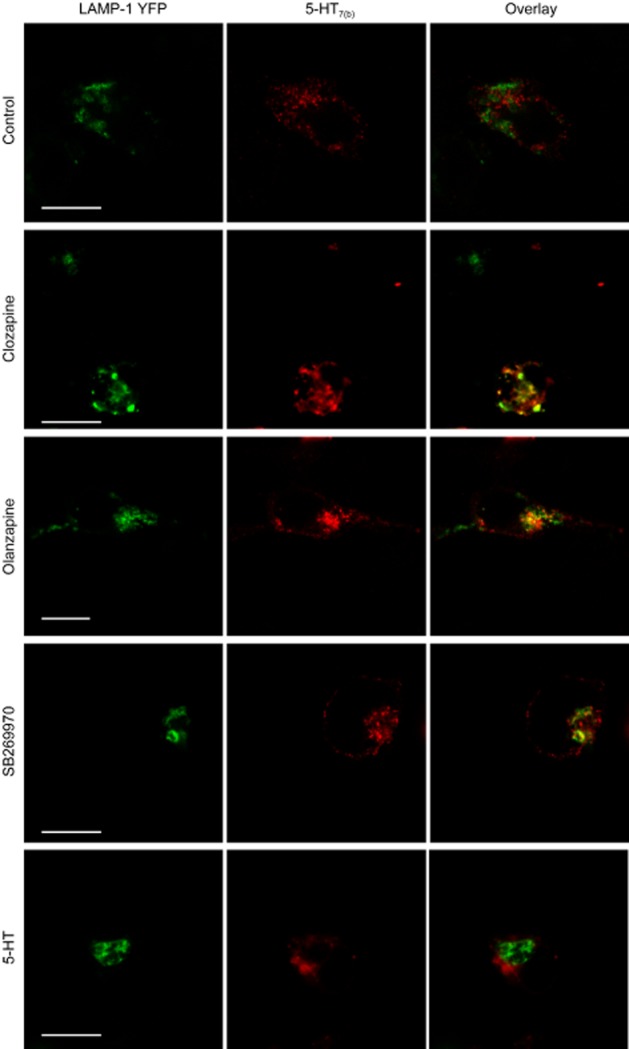
Clozapine and olanzapine target 5-HT7(b) receptors to LAMP-1-positive lysosomes. HEK293 cells were transfected with LAMP-1-YFP and FLAG-tagged 5-HT7(b) receptors and left untreated (control) or stimulated with either 1 μM clozapine, 20 μM olanzapine, 1 μM SB269970 or 10 μM 5-HT for 6 h at 37°C. After fixation, a mouse anti-FLAG antibody followed by Cy5-conjugated donkey anti-mouse antibody was applied for visualization of FLAG-5-HT7(b) receptors as described in the Methods section. Confocal microscopy was used to generate consecutive images of LAMP-1-YFP (green) and FLAG-tagged 5-HT7(b) receptors (red), which were then superimposed (overlay, yellow indicating colocalization). No Cy5 fluorescence was observed in cells not transfected with 5-HT7(b) receptors. Scale bar: 10 μm.
Table 2.
Affinity (Kd), receptor density Bmax and potency to activate AC (EC50) were determined in membranes of HEK293 cells transiently transfected with either wild-type (WT), N-terminally FLAG-tagged or C-terminally YFP-tagged 5-HT7(b) receptors incubated with either vehicle or 1 μM clozapine for 24 h
| Receptor | Kd (nM) | Bmax after clozapine pre-incubation (% of control) | pEC50 | Efficacy (% of WT) |
|---|---|---|---|---|
| 5-HT7(b) WT | 0.17 ± 0.02 | 72 ± 4* | 7.38 ± 0.04 | 100 |
| FLAG-5-HT7(b) | 0.18 ± 0.02 | 76 ± 3* | 7.46 ± 0.20 | 70 ± 12 |
| 5-HT7(b)-YFP | 0.14 ± 0.05 | 105 ± 5 | 7.32 ± 0.04 | 122 ± 7 |
Membranes were incubated with increasing concentration of [3H]-5-CT in the absence (total binding) or presence (non-specific binding) of 10 μM 5-HT. Bmax and Kd were determined as described in the Methods section. Bmax of WT, FLAG- and YFP-labelled 5-HT7(b) receptors were 2.4 ± 0.7, 0.9 ± 0.2 and 2.6 ± 1.2 pmol·mg protein−1. To determine pEC50 and efficacy, vehicle-treated membranes were incubated with increasing concentrations of 5-HT (95 pM–100 μM) and AC activity was measured. The data shown are mean ± SEM from two to five experiments.
P < 0.01 versus 5-HT7(b)-YFP, one-way anova with Bonferroni’s adjustment for multiple comparisons.
Next, we determined whether inhibiting lysosomal degradation with chloroquine (Law et al., 1984) would prevent the clozapine- and olanzapine-mediated down-regulation of 5-HT7(b) receptors. Chloroquine doubled 5-HT7(b) receptor density in the absence of ligand (102% above control), suggesting a high degree of constitutive degradation due to high constitutive internalization (Guthrie et al., 2005). Chloroquine partially inhibited clozapine- and olanzapine-mediated down-regulation and completely inhibited SB269970-mediated down-regulation of 5-HT7(b) receptors (Figure 8). These results indicate that the 5-HT7(b) receptor is transported to lysosomes for degradation upon incubation with clozapine, olanzapine and SB269970.
Figure 8.
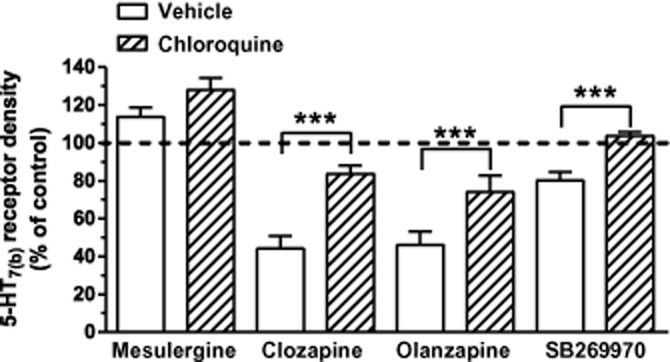
Clozapine-, olanzapine- and SB269970-mediated down-regulation is attenuated by inhibiting lysosomal degradation. HEK293 cells stably expressing the 5-HT7(b) receptor were incubated with the indicated ligand for 24 h without (vehicle) and with chloroquine (200 μM). Data are receptor densities determined by [3H]-5-CT binding, as described in the Methods section, and presented as a % of their respective control (sister plates incubated without or only with chloroquine alone). The data shown are mean ± SEM of four to five experiments. ***P < 0.001 of indicated ligand alone versus ligand + chloroquine (one-way anova with Bonferroni’s multiple comparison test).
Discussion
The primary findings of this study are that (i) different ligands bound to 5-HT7 receptors can elicit differential regulatory mechanisms known to govern receptor responsiveness (e.g. by modifying internalization, intracellular transport and receptor degradation) and (ii) the atypical antipsychotics clozapine and olanzapine mediate internalization and the transport of 5-HT7 receptors to lysosomes for degradation. Of particular interest is the finding that some ACinv (defined by the ability to decrease basal AC activity) not only decrease AC activity but also induce activation of regulatory mechanisms. The pattern of effects elicited by one ACinv varies from another as well as from those induced by ACago. Specifically, the full ACinv clozapine and olanzapine internalized 5-HT7(b) receptors and sorted these for lysosomal degradation in a time-dependent manner. This was not observed for the ACago 5-HT, which only internalized 5-HT7(b) receptors without a subsequent down-regulation. Mesulergine, another ACinv, also promoted internalization, but no down-regulation was observed. With the partial ACinv SB269970, the pattern of responses measured was different, as we observed a reduced internalization of 5-HT7(b) receptors and yet observed down-regulation of these receptors. The SB269970-mediated down-regulation was specific to the 5-HT7(b) receptor, as there was an up-regulation of 5-HT7(d) receptors and no effect on the 5-HT7(a) splice variant, indicating differential regulation among the three splice variants. The ligand-dependent internalization and down-regulation were not related to binding affinities or ACinv efficacies. The current results therefore provide strong support that the 5-HT7 receptor displays functional selectivity, whereby each ligand stabilizes a set of receptor conformations (as defined by Kenakin, 1995; Kenakin, 2002; Urban et al., 2007) governing the ability to activate AC, induce receptor internalization, homo- and heterologous desensitization or receptor degradation, as illustrated in Figure 9.
Figure 9.
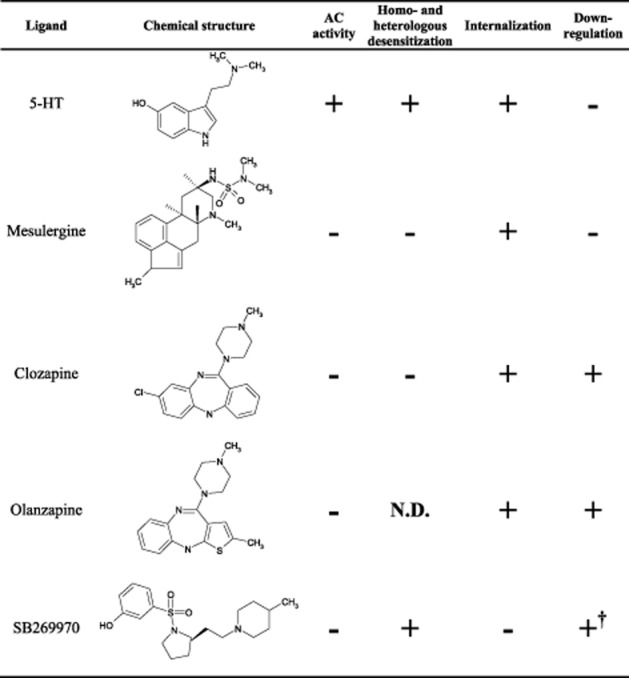
Regulation of AC activity, homo- and heterologous desensitization, internalization and down-regulation of 5-HT7(b) receptors. Summary of the effects of various ligands upon 5-HT7(b) receptor signalling, regulation and trafficking. Data on AC activity are from Krobert and Levy (2002) and Thomas et al. (1998) and the current study. Data on homo- and heterologous desensitization are from Krobert et al. (2006). Data on internalization and down-regulation are from the current study. †5-HT7 receptor density varied among the three splice variants in response to incubation with SB269970 (see Figure 2). N.D., not determined.
From a clinical perspective, it is interesting that the atypical antipsychotics clozapine and olanzapine are full ACinv (Figure 1; Thomas et al., 1998; Krobert and Levy, 2002), and also induce increased internalization of 5-HT7 receptors (Figure 5) with subsequent trafficking to lysosomes for degradation (Figure 7 and Supporting Information Fig. S2). As a consequence of this, clozapine and olanzapine would not only compete with 5-HT for receptors on the cell surface but could also internalize and degrade receptors, severely limiting activation by the endogenous agonist 5-HT. The concentration of clozapine used in the current study is within the range measured in treated patients (Olesen et al., 1995). Clozapine displays about equally high affinity for both 5-HT7 and 5-HT2A receptors and a lower affinity for D2 receptors (Roth et al., 1994), two important receptor targets for antipsychotic therapy. 5-HT7 receptors have also been suggested to be involved in the treatment effect in schizophrenia (Meneses, 2004; Thomas and Hagan, 2004; Ikeda et al., 2006; Matthys et al., 2011). Evidence indicating that 5-HT7 receptor blockade is beneficial for treating schizophrenia is currently debatable, particularly as the receptor-selective inverse agonist SB258741 was not beneficial in animal models of schizophrenia, whereas SB269970 has been shown to be beneficial (recently reviewed by Matthys et al., 2011), indicating that these ligands mediate different G-protein-independent signalling, indicative of functional selectivity. Therefore, it is conceivable that clozapine-mediated internalization and degradation of 5-HT7 receptors might be partially responsible for clozapine’s antipsychotic effect. Indeed, at the 5-HT2A receptor, in vitro and in vivo studies have shown that clozapine and olanzapine induce internalization of 5-HT2A receptors (Willins et al., 1999; Bhatnagar et al., 2001) and clozapine-mediated down-regulation of 5-HT2A receptors in vivo has also been observed (reviewed by Gray and Roth, 2001). Given the numerous receptors affected by clozapine and olanzapine (Roth et al., 1994; Schotte et al., 1996), it is difficult to conclude about the potential clinical relevance of the clozapine- and olanzapine-mediated blockade and down-regulation of 5-HT7 receptors. Although 5-HT7 receptor density was down-regulated equally by clozapine and olanzapine at high ligand concentrations, unlike clozapine, olanzapine is unlikely to mediate down-regulation of 5-HT7 receptors at serum concentrations achieved in patients (serum concentrations are 8–50 times lower than the affinity towards 5-HT7 receptors measured in the current study; Olesen and Linnet, 1999). Perhaps the more efficacious antipsychotic effect of clozapine compared with olanzapine (Serretti et al., 2004) results in part from its ability to antagonize and down-regulate the 5-HT7 receptor.
We report here that the ACago 5-HT, ACinv mesulergine, clozapine and olanzapine increase internalization of 5-HT7 receptors (Figure 5). Antagonist-/inverse agonist-mediated internalization of GPCRs is not exclusive to 5-HT7 and 5-HT2A receptors, as the phenomenon has also been demonstrated for endothelin ETA, neuropeptide Y1, cholecystokinin CCK1, parathyroid hormone PTH1, vasopressin V2 and melanocortin MC3 and 4 receptors (Roettger et al., 1997; Bhowmick et al., 1998; Pfeiffer et al., 1998; Pheng et al., 2003; Sneddon et al., 2003; Breit et al., 2006). SB269970 was the only ligand that stabilized the 5-HT7(b) receptor on the cell surface, indicating that SB269970 stabilizes receptor conformations less prone to internalization. Such ACinv-mediated stabilization on the cell surface has been reported for the H2 histamine receptor and suggested to be a general phenomenon for inverse agonists (Smit et al., 1996; Osawa et al., 2005). Given the opposing results on internalization seen with different ACinv in the current study and similar findings at the V2 receptor (Pfeiffer et al., 1998), stabilization of receptors on the cell surface is not due to the property as an inverse agonist, but rather reflects the receptor conformations that a ligand stabilizes. Although SB269970 reduced internalization of 5-HT7(b) receptors, the remaining intracellular receptors must be accessible for transport to lysosomes for degradation as chloroquine inhibited SB266970-mediated down-regulation (Figure 8). Down-regulation of 5-HT7(b) receptors was more pronounced after clozapine and olanzapine incubation compared with SB269970, and likely results, in part, from increased availability of intracellular receptors for degradation due to enhanced internalization. Alternatively, clozapine- and olanzapine-mediated degradation of 5-HT7(b) receptors may utilize proteasomal degradation in addition, as chloroquine did not completely block down-regulation.
Whereas clozapine and olanzapine increase internalization (Figure 5) and facilitate transportation of 5-HT7 receptors to lysosomes for degradation (Figures 8), 5-HT and mesulergine increased internalization with no accompanying down-regulation (Figures 2 and 5). These data indicate that at least one receptor conformation able to initiate internalization is not adequate for the receptor to be targeted for degradation. Internalized 5-HT7 receptors are possibly sorted to different pathways (recycled or degraded) depending on the ligand remaining bound. It is conceivable that 5-HT increases internalization of 5-HT7(b) receptors and that these receptors recycle back to the cell surface. These recycled receptors are therefore more resistant to constitutive degradation that results in a net increase in receptor density. At the neuropeptide Y1 and 5-HT2A receptors, different ligands have been shown to induce internalization by different mechanisms (Pheng et al., 2003; Raote et al., 2013). Interestingly, clozapine and 5-HT internalize by different mechanisms and display different recycling kinetics at the 5-HT2A receptor (Raote et al., 2013). Therefore, it would be interesting to determine the mechanism of 5-HT-mediated internalization of 5-HT7 receptors and whether clozapine internalizes 5-HT7 receptors through the same β-arrestin-independent mechanism. Regardless of the mechanism, the C-terminal tail of 5-HT7 receptors is involved in binding proteins sorting receptors to lysosomes, as adding YFP to the C-tail of 5-HT7(b) prevented clozapine-mediated down-regulation (Table 2013a). Interestingly, the C-tail of 5-HT7 receptors has been shown to bind G-protein-associated binding protein (GASP) in vitro (Simonin et al., 2004), a protein involved in sorting receptors to lysosomes (Whistler et al., 2002), and the possible consequences of this is discussed in a follow-up paper to the present study (Manfra et al., 2015).
Different regulatory effects were observed across the 5-HT7 splice variants for each ligand evaluated. The fact that the 5-HT7(a) receptor appears relatively resistant to down-regulation by any ligand is particularly interesting, especially as the C-terminus of the 5-HT7(a) receptor has only 13 extra amino acids compared with the 5-HT7(b) receptor. Interestingly, only the 5-HT7(b) receptor has a putative PDZ binding domain (430FVL) in its extreme C-terminus (Vanhoenacker et al., 2000; Gellynck et al., 2013). However, we did not find this PDZ binding domain vital for down-regulation, as clozapine-mediated down-regulation was also observed with a 5-HT7(b) receptor lacking this domain (Δ430FVL; data not shown). It is interesting to note that placing a fluorescent protein after the C-terminus of the 5-HT7(b) receptor prevented clozapine-mediated internalization of a 5-HT7(a)-GFP receptor (Smith et al., 2006) and rendered the 5-HT7(b)-YFP receptor resistant to clozapine-mediated degradation (Table 2013a). Tagging the receptor with such fluorescent proteins might prevent modifications to the receptor (phosphorylation, palmitoylation or ubiquitylation) or docking of regulatory proteins, such as GASP. Guthrie et al. (2005) have reported that the 5-HT7(d) splice variant displays a higher constitutive internalization and that SB269970 decreases internalization. Our data showing an up-regulation of 5-HT7(d) receptor density after incubation with SB269970 are consistent with this. These observations demonstrate how the different C-termini of the 5-HT7 splice variants are involved in regulating the number of receptors expressed at the cell surface and the fate of internalized receptors.
Most GPCRs recruit β-arrestin after agonist activation, which is requisite for internalization, G-protein-independent signalling and trafficking of receptors. However, we did not observe that the 5-HT7(b) receptor recruited β-arrestins constitutively or upon ligand-binding (Figure 6). β-Arrestin and G-protein compete for binding sites on GPCRs. Because the 5-HT7 receptor may be pre-associated with G-protein (Bruheim et al., 2003; Andressen et al., 2006), this may sterically hinder β-arrestin binding to the receptor. Therefore, unlike most receptors, the 5-HT7 receptor may possibly utilize other proteins for coordinating internalization and lysosomal trafficking. Alternatively, ligand stabilization may produce different conformations that may result in differential phosphorylation, similar to that reported at μ opioid and M3 muscarinic receptors (Butcher et al., 2011; Doll et al., 2011), which may determine the pattern of internalization and/or down-regulation.
It has become relatively well established that ligand–receptor interactions are much more complex than simply activating (agonism) or inhibiting (antagonism) a specific signalling pathway. For example, at 5-HT7 receptors, various ligands can induce receptor conformations that mediate numerous effects, from regulating G-protein-activation, inducing homo- and heterologous desensitization, internalization or mediating lysosomal degradation (Figure 9). The chemical structures of the ligands used in the current study differ considerably, providing a molecular basis for the ability to induce different receptor conformations. Although clozapine and olanzapine do not differ substantially in chemical structure, clozapine has over 10-fold higher affinity for the 5-HT7 receptor (pKi of 5.9 vs 7.8 for olanzapine and clozapine, respectively; Krobert et al., 2001) and a sixfold higher potency for ACinv (Figure 1), indicating that ligand affinity is not a determinant for internalization and down-regulation.
In summary, we demonstrate functional selectivity at the 5-HT7 receptor with regard to regulatory events (internalization, desensitization and down-regulation). Most importantly, the atypical antipsychotics clozapine and olanzapine both inhibit receptor activity and initiate 5-HT7 receptor degradation. This unique property could be important for the antipsychotic effects of these drugs, particularly since clozapine also down-regulates the critical 5-HT2A receptor (Gray and Roth, 2001). The kinetics of these regulatory events is quite slow, which would be consistent with the slow onset of action of these drugs. We also demonstrated that ligand-mediated internalization of 5-HT7 receptors was not associated with β-arrestin recruitment, which has been thought of as a universal mechanism of internalization for all GPCRs.
Acknowledgments
We are grateful to Dr Walther Mothes (Yale University School of Medicine, New Haven) for providing the plasmids encoding CD63-RFP and LAMP-1-YFP, Prof Håvard Attramadal (Institute of Surgical Research, University of Oslo and Oslo University Hospital) for providing plasmids encoding β-arrestin1-GFP and β-arrestin2-GFP and Prof Henrik Huitfeldt (Department of Pathology, University of Oslo) for helpful discussions on confocal microscopy. This work was supported by The Norwegian Council on Cardiovascular Diseases, The Research Council of Norway, The Kristian Gerhard Jebsen Foundation, The Norwegian Cancer Society, Anders Jahre’s Foundation for the Promotion of Science, The Novo Nordisk Foundation, The Family Blix Foundation, grants from the University of Oslo and the COST Action CM1207 (GLISTEN, http://www.glisten-gpcr.eu). P. V. was supported by a grant of the IWT (Instituut voor de aanmoediging van innovatie door Wetenschap en Technologie in Vlaanderen) (project 990173) and a grant from GSKE (Geneeskundige Stichting Koningin Elisabeth).
Glossary
- 5-CT
5-carboxamidotryptamine
- YFP
yellow fluorescent protein
Author contributions
K. W. A., O. M., C. H. B., A. H. U., P. V., F. O. L. and K. A. K. participated in research design. K. W. A., O. M., C. H. B. and A. H. U. conducted the experiments. P. V. contributed new reagents or analytic tools. K. W. A., O. M., C. H. B., A. H. U., F. O. L. and K. A. K. performed data analysis. K. W. A., O. M., C. H. B., A. H. U., P. V., F. O. L. and K. A. K. wrote or contributed to the writing of the manuscript and read and approved the final version.
Conflict of interest
None.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1 [3H]-mesulergine- and [3H]-5-CT-binding labels the same receptor pool. Scatchard plot of binding data for [3H]-5-CT and [3H]-mesulergine at various concentrations in the membranes of HEK293 cells stably expressing the 5-HT7(a) receptor. Radioligand binding was performed with increasing concentrations of [3H]-5-CT or [3H]-mesulergine (0.1–5 and 0.5–20 nM, respectively) on the same membrane preparations in parallel. Non-specific binding was measured in the presence of 10 μM 5-HT or 10 μM metergoline for [3H]-5-CT and [3H]-mesulergine respectively. The data were analysed by non-linear regression as described in the Methods section and plotted as a Scatchard plot for illustration. The data presented are from a representative experiment of the data listed in Table 1.
Figure S2 Clozapine and olanzapine traffic 5-HT7(b) receptors to lysosomes. HEK293 cells were transfected with LAMP-1-YFP, CD63-RFP and FLAG-tagged 5-HT7(b) receptors and left untreated (control) or stimulated with either 1 μM clozapine, 20 μM olanzapine or 1 μM SB269970 for 6 h at 37°C. After fixation, FLAG-5-HT7(b) receptors were visualized by a mouse anti-FLAG antibody followed by Cy5-conjugated donkey anti-mouse antibody, as described in the Methods section. Confocal microscopy was used to generate consecutive images of LAMP-1-YFP (green), CD63-RFP (red) and FLAG-tagged 5-HT7(b) receptors (blue), which were then superimposed (overlay, yellow indicates colocalization of LAMP1 and CD63, whereas white indicates colocalization of LAMP-1, CD63 and 5-HT7(b) receptors). No Cy5 fluorescence was observed in cells not transfected with 5-HT7(b) receptors. Scale bar: 10 μm.
Table S1 Total receptor levels (Bmax) and binding affinity (Kd) determined by radioligand binding assays with [3H]-5-CT obtained from membranes of stably expressing 5-HT7(a, b and d) receptors. Data are mean ± SEM for Bmax and Kd determined as described in the Methods section [5-HT7(a), n = 9; 5-HT7(b), n = 5; 5-HT7(d), n = 14]. Bmax of the pre-incubated membranes is presented as a percentage of control (non-pre-incubated). *P < 0.05 versus respective control group; one-way anova with Bonferroni correction of α-value for multiple post hoc comparisons. aP < 0.05 of 5-HT7(a) versus 5-HT7(b); b5-HT7(b) versus 5-HT7(d); c5-HT7(d) versus 5-HT7(a), two-way anova; dP < 0.01 versus all respective controls, one-way anova.
Table S2 Total receptor levels (Bmax) and binding affinity (Kd) determined by radioligand binding assays with [3H]mesulergine obtained from membranes of HEK293 cells stably expressing 5-HT7(a, b and d) receptors. Data are mean ± SEM for Bmax and Kd determined as described in the Methods section [5-HT7(a), n = 7; 5-HT7(b), n = 5; 5-HT7(d), n = 11]. Bmax of the pre-incubated membranes is presented as a percentage of control (non-pre-incubated). *P < 0.05 versus respective control group; one-way anova with Bonferroni correction of α-value for multiple post hoc comparisons. aP < 0.05 of 5-HT7(a) versus 5-HT7(b); b5-HT7(b) versus 5-HT7(d); c5-HT7(d) versus 5-HT7(a), two-way anova.
Table S3 Total receptor levels (Bmax) and binding affinity (Kd) determined by a radioligand binding assay with [3H]CGP12177 in the membranes of HEK293 cells transiently transfected with the β2adrenoceptor and incubated with either vehicle, 1 μM clozapine, 20 μM olanzapine or 10 μM isoprenaline for 24 h and then subsequently washed (as described in the Methods section). Bmax of the pre-incubated membranes is presented as a percentage of control (non-pre-incubated) that was 6.3 pmol·mg protein−1.
Movie S1 β-Arrestin1-GFP recruitment to isoprenaline-stimulated β2-adrenoceptor. HEK293 cells transiently expressing β2-adrenoceptor and β-arrestin1-GFP were visualized as described in the Methods section. Cells were incubated with 10 μM isoprenaline and subsequently continuously visualized for 30 min. After ∼4 min, a punctate membrane staining is observed, reflecting recruitment of β-arrestin1-GFP to the plasma membrane. Scale bar: 10 μm.
Movie S2 β-Arrestin2-GFP recruitment to isoprenaline-stimulated β2-adrenoceptor. HEK293 cells transiently expressing β2-adrenoceptor and β-arrestin2-GFP were visualized as described in the Methods section. Cells were incubated with 10 μM isoprenaline and subsequently continuously visualized for 10 min. After ∼2.5 min, a punctate membrane staining is observed, reflecting recruitment of β-arrestin2-GFP to the plasma membrane. Scale bar: 10 μm.
References
- Alberts GL, Chio CL, Im WB. Allosteric modulation of the human 5-HT7A receptor by lipidic amphipathic compounds. Mol Pharmacol. 2001;60:1349–1355. doi: 10.1124/mol.60.6.1349. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: enzymes. Br J Pharmacol. 2013b;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andressen KW, Norum JH, Levy FO, Krobert KA. Activation of adenylyl cyclase by endogenous Gs-coupled receptors in HEK293 cells is attenuated by 5-HT7 receptor expression. Mol Pharmacol. 2006;69:207–215. doi: 10.1124/mol.105.015396. [DOI] [PubMed] [Google Scholar]
- Bhatnagar A, Willins DL, Gray JA, Woods J, Benovic JL, Roth BL. The dynamin-dependent, arrestin-independent internalization of 5-hydroxytryptamine 2A (5-HT2A) serotonin receptors reveals differential sorting of arrestins and 5-HT2A receptors during endocytosis. J Biol Chem. 2001;276:8269–8277. doi: 10.1074/jbc.M006968200. [DOI] [PubMed] [Google Scholar]
- Bhowmick N, Narayan P, Puett D. The endothelin subtype A receptor undergoes agonist- and antagonist-mediated internalization in the absence of signaling. Endocrinology. 1998;139:3185–3192. doi: 10.1210/endo.139.7.6105. [DOI] [PubMed] [Google Scholar]
- Breit A, Wolff K, Kalwa H, Jarry H, Buch T, Gudermann T. The natural inverse agonist agouti-related protein induces arrestin-mediated endocytosis of melanocortin-3 and -4 receptors. J Biol Chem. 2006;281:37447–37456. doi: 10.1074/jbc.M605982200. [DOI] [PubMed] [Google Scholar]
- Bruheim S, Krobert KA, Andressen KW, Levy FO. Unaltered agonist potency upon inducible 5-HT7(a) but not 5-HT4(b) receptor expression indicates agonist-independent association of 5-HT7(a) receptor and Gs. Receptors Channels. 2003;9:107–116. [PubMed] [Google Scholar]
- Butcher AJ, Prihandoko R, Kong KC, McWilliams P, Edwards JM, Bottrill A, et al. Differential G-protein-coupled receptor phosphorylation provides evidence for a signaling bar code. J Biol Chem. 2011;286:11506–11518. doi: 10.1074/jbc.M110.154526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martelaere K, Lintermans B, Haegeman G, Vanhoenacker P. Novel interaction between the human 5-HT7 receptor isoforms and PLAC-24/eIF3k. Cell Signal. 2007;19:278–288. doi: 10.1016/j.cellsig.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Doll C, Konietzko J, Poll F, Koch T, Hollt V, Schulz S. Agonist-selective patterns of micro-opioid receptor phosphorylation revealed by phosphosite-specific antibodies. Br J Pharmacol. 2011;164:298–307. doi: 10.1111/j.1476-5381.2011.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galandrin S, Bouvier M. Distinct signaling profiles of β1 and β2 adrenergic receptor ligands toward adenylyl cyclase and mitogen-activated protein kinase reveals the pluridimensionality of efficacy. Mol Pharmacol. 2006;70:1575–1584. doi: 10.1124/mol.106.026716. [DOI] [PubMed] [Google Scholar]
- Galandrin S, Oligny-Longpre G, Bouvier M. The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol Sci. 2007;28:423–430. doi: 10.1016/j.tips.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Gellynck E, Heyninck K, Andressen KW, Haegeman G, Levy FO, Vanhoenacker P, et al. The serotonin 5-HT7 receptors: two decades of research. Exp Brain Res. 2013;230:555–568. doi: 10.1007/s00221-013-3694-y. [DOI] [PubMed] [Google Scholar]
- Gray JA, Roth BL. Paradoxical trafficking and regulation of 5-HT(2A) receptors by agonists and antagonists. Brain Res Bull. 2001;56:441–451. doi: 10.1016/s0361-9230(01)00623-2. [DOI] [PubMed] [Google Scholar]
- Guthrie CR, Murray AT, Franklin AA, Hamblin MW. Differential agonist-mediated internalization of the human 5-hydroxytryptamine 7 receptor isoforms. J Pharmacol Exp Ther. 2005;313:1003–1010. doi: 10.1124/jpet.104.081919. [DOI] [PubMed] [Google Scholar]
- Heidmann DE, Metcalf MA, Kohen R, Hamblin MW. Four 5-hydroxytryptamine7 (5-HT7) receptor isoforms in human and rat produced by alternative splicing: species differences due to altered intron-exon organization. J Neurochem. 1997;68:1372–1381. doi: 10.1046/j.1471-4159.1997.68041372.x. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Iwata N, Kitajima T, Suzuki T, Yamanouchi Y, Kinoshita Y, et al. Positive association of the serotonin 5-HT7 receptor gene with schizophrenia in a Japanese population. Neuropsychopharmacology. 2006;31:866–871. doi: 10.1038/sj.npp.1300901. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Agonist-receptor efficacy. II. Agonist trafficking of receptor signals. Trends Pharmacol Sci. 1995;16:232–238. doi: 10.1016/s0165-6147(00)89032-x. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Drug efficacy at G protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2002;42:349–379. doi: 10.1146/annurev.pharmtox.42.091401.113012. [DOI] [PubMed] [Google Scholar]
- Kenakin T. New concepts in drug discovery: collateral efficacy and permissive antagonism. Nat Rev Drug Discov. 2005;4:919–927. doi: 10.1038/nrd1875. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Collateral efficacy in drug discovery: taking advantage of the good (allosteric) nature of 7TM receptors. Trends Pharmacol Sci. 2007;28:407–415. doi: 10.1016/j.tips.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Kenakin T. New concepts in pharmacological efficacy at 7TM receptors: IUPHAR review 2. Br J Pharmacol. 2013;168:554–575. doi: 10.1111/j.1476-5381.2012.02223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilka BK, Deupi X. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:397–406. doi: 10.1016/j.tips.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Krobert KA, Levy FO. The human 5-HT7 serotonin receptor splice variants: constitutive activity and inverse agonist effects. Br J Pharmacol. 2002;135:1563–1571. doi: 10.1038/sj.bjp.0704588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krobert KA, Bach T, Syversveen T, Kvingedal AM, Levy FO. The cloned human 5-HT7 receptor splice variants: a comparative characterization of their pharmacology, function and distribution. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:620–632. doi: 10.1007/s002100000369. [DOI] [PubMed] [Google Scholar]
- Krobert KA, Andressen KW, Levy FO. Heterologous desensitization is evoked by both agonist and antagonist stimulation of the human 5-HT7 serotonin receptor. Eur J Pharmacol. 2006;532:1–10. doi: 10.1016/j.ejphar.2005.11.039. [DOI] [PubMed] [Google Scholar]
- Law PY, Hom DS, Loh HH. Down-regulation of opiate receptor in neuroblastoma x glioma NG108-15 hybrid cells. Chloroquine promotes accumulation of tritiated enkephalin in the lysosomes. J Biol Chem. 1984;259:4096–4104. [PubMed] [Google Scholar]
- Lefkowitz RJ. Arrestins come of age: a personal historical perspective. Prog Mol Biol Transl Sci. 2013;118:3–18. doi: 10.1016/B978-0-12-394440-5.00001-2. [DOI] [PubMed] [Google Scholar]
- Luttrell LM. Minireview: more than just a hammer: ligand ‘bias’ and pharmaceutical discovery. Mol Endocrinol. 2014;28:281–294. doi: 10.1210/me.2013-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfra O, Van Craenenbroeck K, Skieterska K, Frimurer T, Schwartz TW, Levy FO, et al. Downregulation of 5-HT7 Serotonin Receptors by the Atypical Antipsychotics Clozapine and Olanzapine. Role of Motifs in the C-Terminal Domain and Interaction with GASP-1. ACS Chem Neurosci. 2015 doi: 10.1021/cn500339p. DOI: 10.1021/cn500339p. [DOI] [PubMed] [Google Scholar]
- Matthys A, Haegeman G, Van CK, Vanhoenacker P. Role of the 5-HT7 receptor in the central nervous system: from current status to future perspectives. Mol Neurobiol. 2011;43:228–253. doi: 10.1007/s12035-011-8175-3. [DOI] [PubMed] [Google Scholar]
- Meneses A. Effects of the 5-HT7 receptor antagonists SB-269970 and DR 4004 in autoshaping Pavlovian/instrumental learning task. Behav Brain Res. 2004;155:275–282. doi: 10.1016/j.bbr.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Olesen OV, Linnet K. Olanzapine serum concentrations in psychiatric patients given standard doses: the influence of comedication. Ther Drug Monit. 1999;21:87–90. doi: 10.1097/00007691-199902000-00013. [DOI] [PubMed] [Google Scholar]
- Olesen OV, Thomsen K, Jensen PN, Wulff CH, Rasmussen NA, Refshammer C, et al. Clozapine serum levels and side effects during steady state treatment of schizophrenic patients: a cross-sectional study. Psychopharmacology (Berl) 1995;117:371–378. doi: 10.1007/BF02246112. [DOI] [PubMed] [Google Scholar]
- Osawa S, Kajimura M, Yamamoto S, Ikuma M, Mochizuki C, Iwasaki H, et al. Alteration of intracellular histamine H2 receptor cycling precedes antagonist-induced upregulation. Am J Physiol Gastrointest Liver Physiol. 2005;289:G880–G889. doi: 10.1152/ajpgi.00536.2004. [DOI] [PubMed] [Google Scholar]
- Park T, Bae S, Choi S, Kang B, Kim K. Inhibition of nicotinic acetylcholine receptors and calcium channels by clozapine in bovine adrenal chromaffin cells. Biochem Pharmacol. 2001;61:1011–1019. doi: 10.1016/s0006-2952(01)00577-9. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer R, Kirsch J, Fahrenholz F. Agonist and antagonist-dependent internalization of the human vasopressin V2 receptor. Exp Cell Res. 1998;244:327–339. doi: 10.1006/excr.1998.4159. [DOI] [PubMed] [Google Scholar]
- Pheng LH, Dumont Y, Fournier A, Chabot JG, Beaudet A, Quirion R. Agonist- and antagonist-induced sequestration/internalization of neuropeptide Y Y1 receptors in HEK293 cells. Br J Pharmacol. 2003;139:695–704. doi: 10.1038/sj.bjp.0705306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal S, Ahn S, Rominger DH, Gowen-MacDonald W, Lam CM, Dewire SM, et al. Quantifying ligand bias at seven-transmembrane receptors. Mol Pharmacol. 2011;80:367–377. doi: 10.1124/mol.111.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raote I, Bhattacharyya S, Panicker MM. Functional selectivity in serotonin receptor 2A (5-HT2A) endocytosis, recycling, and phosphorylation. Mol Pharmacol. 2013;83:42–50. doi: 10.1124/mol.112.078626. [DOI] [PubMed] [Google Scholar]
- Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol. 2012;52:179–197. doi: 10.1146/annurev.pharmtox.010909.105800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochais F, Vilardaga JP, Nikolaev VO, Bünemann M, Lohse MJ, Engelhardt S. Real-time optical recording of β1-adrenergic receptor activation reveals supersensitivity of the Arg389 variant to carvedilol. J Clin Invest. 2007;117:229–235. doi: 10.1172/JCI30012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roettger BF, Ghanekar D, Rao R, Toledo C, Yingling J, Pinon D, et al. Antagonist-stimulated internalization of the G protein-coupled cholecystokinin receptor. Mol Pharmacol. 1997;51:357–362. [PubMed] [Google Scholar]
- Roth BL, Craigo SC, Choudhary MS, Uluer A, Monsma FJ, Jr, Shen Y, et al. Binding of typical and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J Pharmacol Exp Ther. 1994;268:1403–1410. [PubMed] [Google Scholar]
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, et al. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl) 1996;124:57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- Seifert R. Functional selectivity of G-protein-coupled receptors: from recombinant systems to native human cells. Biochem Pharmacol. 2013;86:853–861. doi: 10.1016/j.bcp.2013.07.029. [DOI] [PubMed] [Google Scholar]
- Serretti A, De RD, Lorenzi C, Berardi D. New antipsychotics and schizophrenia: a review on efficacy and side effects. Curr Med Chem. 2004;11:343–358. doi: 10.2174/0929867043456043. [DOI] [PubMed] [Google Scholar]
- Sherer NM, Lehmann MJ, Jimenez-Soto LF, Ingmundson A, Horner SM, Cicchetti G, et al. Visualization of retroviral replication in living cells reveals budding into multivesicular bodies. Traffic. 2003;4:785–801. doi: 10.1034/j.1600-0854.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- Simonin F, Karcher P, Boeuf JJ, Matifas A, Kieffer BL. Identification of a novel family of G protein-coupled receptor associated sorting proteins. J Neurochem. 2004;89:766–775. doi: 10.1111/j.1471-4159.2004.02411.x. [DOI] [PubMed] [Google Scholar]
- Smit MJ, Leurs R, Alewijnse AE, Blauw J, Nieuw Amerongen GP, Van De Vrede Y, et al. Inverse agonism of histamine H2 antagonist accounts for upregulation of spontaneously active histamine H2 receptors. Proc Natl Acad Sci U S A. 1996;93:6802–6807. doi: 10.1073/pnas.93.13.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Rahman T, Toohey N, Mazurkiewicz J, Herrick-Davis K, Teitler M. Risperidone irreversibly binds to and inactivates the h5-HT7 serotonin receptor. Mol Pharmacol. 2006;70:1264–1270. doi: 10.1124/mol.106.024612. [DOI] [PubMed] [Google Scholar]
- Sneddon WB, Syme CA, Bisello A, Magyar CE, Rochdi MD, Parent JL, et al. Activation-independent parathyroid hormone receptor internalization is regulated by NHERF1 (EBP50) J Biol Chem. 2003;278:43787–43796. doi: 10.1074/jbc.M306019200. [DOI] [PubMed] [Google Scholar]
- Thomas DR, Hagan JJ. 5-HT7 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:81–90. doi: 10.2174/1568007043482633. [DOI] [PubMed] [Google Scholar]
- Thomas DR, Gittins SA, Collin LL, Middlemiss DN, Riley G, Hagan J, et al. Functional characterisation of the human cloned 5-HT7 receptor (long form); antagonist profile of SB-258719. Br J Pharmacol. 1998;124:1300–1306. doi: 10.1038/sj.bjp.0701946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Vanhoenacker P, Haegeman G, Leysen JE. 5-HT7 receptors: current knowledge and future prospects. Trends Pharmacol Sci. 2000;21:70–77. doi: 10.1016/s0165-6147(99)01432-7. [DOI] [PubMed] [Google Scholar]
- Whistler JL, Enquist J, Marley A, Fong J, Gladher F, Tsuruda P, et al. Modulation of postendocytic sorting of G protein-coupled receptors. Science. 2002;297:615–620. doi: 10.1126/science.1073308. [DOI] [PubMed] [Google Scholar]
- Willins DL, Berry SA, Alsayegh L, Backstrom JR, Sanders-Bush E, Friedman L, et al. Clozapine and other 5-hydroxytryptamine-2A receptor antagonists alter the subcellular distribution of 5-hydroxytryptamine-2A receptors in vitro and in vivo. Neuroscience. 1999;91:599–606. doi: 10.1016/s0306-4522(98)00653-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 [3H]-mesulergine- and [3H]-5-CT-binding labels the same receptor pool. Scatchard plot of binding data for [3H]-5-CT and [3H]-mesulergine at various concentrations in the membranes of HEK293 cells stably expressing the 5-HT7(a) receptor. Radioligand binding was performed with increasing concentrations of [3H]-5-CT or [3H]-mesulergine (0.1–5 and 0.5–20 nM, respectively) on the same membrane preparations in parallel. Non-specific binding was measured in the presence of 10 μM 5-HT or 10 μM metergoline for [3H]-5-CT and [3H]-mesulergine respectively. The data were analysed by non-linear regression as described in the Methods section and plotted as a Scatchard plot for illustration. The data presented are from a representative experiment of the data listed in Table 1.
Figure S2 Clozapine and olanzapine traffic 5-HT7(b) receptors to lysosomes. HEK293 cells were transfected with LAMP-1-YFP, CD63-RFP and FLAG-tagged 5-HT7(b) receptors and left untreated (control) or stimulated with either 1 μM clozapine, 20 μM olanzapine or 1 μM SB269970 for 6 h at 37°C. After fixation, FLAG-5-HT7(b) receptors were visualized by a mouse anti-FLAG antibody followed by Cy5-conjugated donkey anti-mouse antibody, as described in the Methods section. Confocal microscopy was used to generate consecutive images of LAMP-1-YFP (green), CD63-RFP (red) and FLAG-tagged 5-HT7(b) receptors (blue), which were then superimposed (overlay, yellow indicates colocalization of LAMP1 and CD63, whereas white indicates colocalization of LAMP-1, CD63 and 5-HT7(b) receptors). No Cy5 fluorescence was observed in cells not transfected with 5-HT7(b) receptors. Scale bar: 10 μm.
Table S1 Total receptor levels (Bmax) and binding affinity (Kd) determined by radioligand binding assays with [3H]-5-CT obtained from membranes of stably expressing 5-HT7(a, b and d) receptors. Data are mean ± SEM for Bmax and Kd determined as described in the Methods section [5-HT7(a), n = 9; 5-HT7(b), n = 5; 5-HT7(d), n = 14]. Bmax of the pre-incubated membranes is presented as a percentage of control (non-pre-incubated). *P < 0.05 versus respective control group; one-way anova with Bonferroni correction of α-value for multiple post hoc comparisons. aP < 0.05 of 5-HT7(a) versus 5-HT7(b); b5-HT7(b) versus 5-HT7(d); c5-HT7(d) versus 5-HT7(a), two-way anova; dP < 0.01 versus all respective controls, one-way anova.
Table S2 Total receptor levels (Bmax) and binding affinity (Kd) determined by radioligand binding assays with [3H]mesulergine obtained from membranes of HEK293 cells stably expressing 5-HT7(a, b and d) receptors. Data are mean ± SEM for Bmax and Kd determined as described in the Methods section [5-HT7(a), n = 7; 5-HT7(b), n = 5; 5-HT7(d), n = 11]. Bmax of the pre-incubated membranes is presented as a percentage of control (non-pre-incubated). *P < 0.05 versus respective control group; one-way anova with Bonferroni correction of α-value for multiple post hoc comparisons. aP < 0.05 of 5-HT7(a) versus 5-HT7(b); b5-HT7(b) versus 5-HT7(d); c5-HT7(d) versus 5-HT7(a), two-way anova.
Table S3 Total receptor levels (Bmax) and binding affinity (Kd) determined by a radioligand binding assay with [3H]CGP12177 in the membranes of HEK293 cells transiently transfected with the β2adrenoceptor and incubated with either vehicle, 1 μM clozapine, 20 μM olanzapine or 10 μM isoprenaline for 24 h and then subsequently washed (as described in the Methods section). Bmax of the pre-incubated membranes is presented as a percentage of control (non-pre-incubated) that was 6.3 pmol·mg protein−1.
Movie S1 β-Arrestin1-GFP recruitment to isoprenaline-stimulated β2-adrenoceptor. HEK293 cells transiently expressing β2-adrenoceptor and β-arrestin1-GFP were visualized as described in the Methods section. Cells were incubated with 10 μM isoprenaline and subsequently continuously visualized for 30 min. After ∼4 min, a punctate membrane staining is observed, reflecting recruitment of β-arrestin1-GFP to the plasma membrane. Scale bar: 10 μm.
Movie S2 β-Arrestin2-GFP recruitment to isoprenaline-stimulated β2-adrenoceptor. HEK293 cells transiently expressing β2-adrenoceptor and β-arrestin2-GFP were visualized as described in the Methods section. Cells were incubated with 10 μM isoprenaline and subsequently continuously visualized for 10 min. After ∼2.5 min, a punctate membrane staining is observed, reflecting recruitment of β-arrestin2-GFP to the plasma membrane. Scale bar: 10 μm.


