Abstract
There is increasing recognition that the sex hormones (estrogen, progesterone, and testosterone) have biological and pathophysiological actions in peripheral, non-reproductive organs, including the lung. Clinically, sex differences in the incidence, morbidity and mortality of lung diseases such as asthma, chronic obstructive pulmonary disease (COPD), pulmonary fibrosis, lung cancer and pulmonary hypertension have been noted, although intrinsic sex differences vs. the roles of sex steroids are still not well-understood. Accordingly, it becomes important to ask the following questions: 1) Which sex steroids are involved? 2) How do they affect different components of the lung under normal circumstances? 3) How does sex steroid signaling change in or contribute to lung disease, and in this regard, are sex steroids detrimental or beneficial? As our understanding of sex steroid signaling in the lung improves, it is important to consider whether such information can be used to develop new therapeutic strategies to target lung diseases, perhaps in both sexes or in a sex-specific manner. In this review, we focus on the basics of sex steroid signaling, and the current state of knowledge regarding how they influence structure and function of specific lung components across the life span and in the context of some important lung diseases. We then summarize the potential for sex steroids as useful biomarkers and therapeutic targets in these lung diseases as a basis for future translational research in the area of gender and individualized medicine.
Keywords: Hormone, Estrogen, Progesterone, Testosterone, Airway, Alveoli, Asthma, COPD, Pulmonary Fibrosis
1. Introduction
There are inherent sex differences in the human lung which are apparent from early in life (indeed in utero) and manifest in different forms throughout the lifespan. Here, changes in lung structure and function at key life stages such as puberty, pregnancy, menopause and in aging suggest a modulatory role of sex steroids. This is particularly relevant, given the considerable epidemiological evidence for a role for sex in the incidence, susceptibility and severity of a number of lung diseases that create a healthcare and financial burden of ~35 million individuals and 400,000 deaths in the US (ALA, 2008). For example, asthma is more common in pre-pubescent males, but becomes more common in females after puberty (Becklake & Kauffmann, 1999; Bjornson & Mitchell, 2000; Caracta, 2003; Carey et al., 2007b; Jensen-Jarolim & Untersmayr, 2008; Melgert et al., 2007) such that the incidence, frequency and severity of asthma is greater among adult women, compared to men (Bjornson & Mitchell, 2000; Caracta, 2003; Carey et al., 2007b; Jensen-Jarolim & Untersmayr, 2008; Melgert et al., 2007). Similarly, differences between men and women in the incidence/frequency of and morbidity/mortality associated with smoking-induced lung disease have also been noted, not all of which can be explained by differences in smoking rates between the sexes (Hughes & Jacobson, 2003; Hughes et al., 2008; Mannino et al., 2002; Troisi et al., 1995a). What is less clear from epidemiological data such as these is the relative contributions of intrinsic sex differences in lung structure and/or function vs. sex steroids: a topic of considerable interest and ongoing research.
The contrasts between men and women in a range of diseases have led the Institute of Medicine to emphasize that sex is or should be a biological variable for research as well as clinical practice norms (“Exploring Biological Contributions to Human Health-Does Sex Matter?”; http://www.nap.edu/openbook.php?isbn=0309072816). Although this report really targets sex differences per se in non-reproductive organ systems, the respiratory system (upper and lower conducting airways, lung parenchyma) and lung diseases were surprisingly not highlighted. More recently, the NIH has announced the requirement that grant applicants report the sex of animals and cells in pre-clinical studies. However, this emphasis is also on intrinsic sex differences rather than any modulating role sex steroids may play in human lung health and disease. Nonetheless, perspectives, editorials and reviews in several journals all highlight the idea that sex steroids “do more” than play a role in reproductive function. Certainly, this has been most established in the cardiovascular (Arain et al., 2009; Konhilas, 2010; Leuzzi et al., 2010; Miller, 2010; Perez-Lopez et al., 2010), metabolism (Beierle et al., 1999; Bigos et al., 2009; Greenhill, 2011; Wang et al., 2011), and cognition (Gillies & McArthur; Hines, 2010; Janicki & Schupf, 2010; Manson, 2008; McEwen, 2010; McEwen & Alves, 1999; Reddy, 2010) arenas, but there are now increasing reports of sex steroid effects in different lung components, and how they may play a role in important diseases such as asthma, Chronic obstructive pulmonary disease (COPD), pulmonary fibrosis, cancer and even pulmonary hypertension. Accordingly, the major goal of this review is to highlight this research relating to sex steroids and their effects on lung components in health and disease. Given that most of the research to date is on the adult lung, which also represents the majority of healthcare burden of lung disease, we will focus on this aspect, with brief mention of how influence of sex steroids early in life may have bearing for adult lung health and disease. Finally, we will introduce the concept of sex steroids and their signaling which could form a platform for diagnosis and therapy in lung diseases in the context of individualized medicine. Regarding intrinsic sex differences and the lung, the reader is referred to some recent reviews on this topic (Gonzalez-Arenas & Agramonte-Hevia, 2012; Martin & Pabelick, 2014; Prakash et al., 2014; Tam et al., 2011; Townsend et al., 2012a).
2. Brief review of sex steroid biology
In order to understand sex steroid effects in the lung, it is important to appreciate how estrogens, progesterone, testosterone or even their biologically-active metabolites are produced and exert their action. Here, we emphasize that substantial cellular, tissue, or even species heterogeneity of sex steroid action can occur due to modulation of the basic sex steroid signaling pathways, as well as interactions between sex steroids (particularly in women where estrogens and progesterone are normally present concurrently).
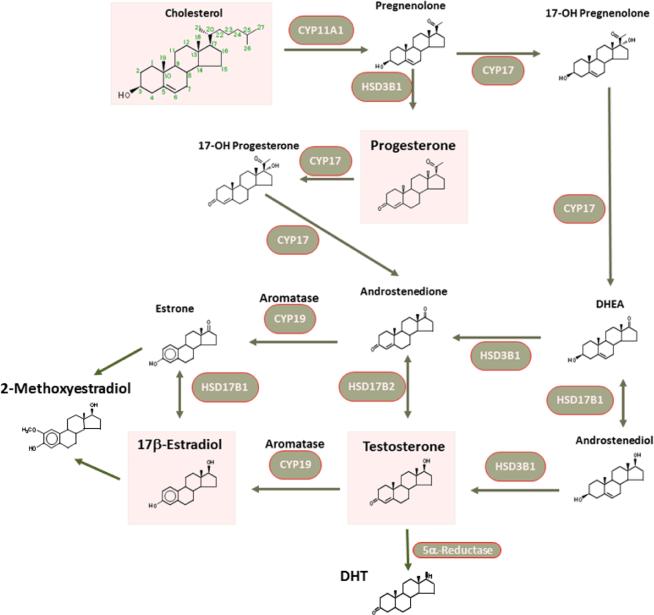
In terms of sex steroid production, estrogen, progesterone and testosterone are all derived from cholesterol via well-established pathways (Ghayee & Auchus, 2007; Payne & Hales, 2004) (Figure 1). Briefly, cholesterol is converted to pregnenolone by the P450 side chain cleavage enzyme located in the mitochondrial membrane. Pregnenolone can be converted either to progesterone (via 3β-hydroxysteroid dehydrogenase, 3β-HSD) or dehydroepiandrosterone (DHEA via cytochrome P450 17). DHEA may be further converted to androstenedione (3β-HSD) and subsequently testosterone (17β-HSD) or estrone (via aromatase). Estrone and testosterone may then be converted to estradiol (via 17β-HSD and aromatase, respectively).
Figure 1.
Synthesis of estrogen, progesterone and testosterone from cholesterol. Pregnenolone is the first product from cholesterol, and can be further processed through 2 pathways, both leading eventually to testosterone (producing progesterone along the way via one pathway), and estradiol as an end product. Here, enzymes such as CYP17 and CYP19 (aromatase) are particularly important and could substantially modulate local (tissue) levels of sex hormones.
Although sex steroids are mainly produced by the gonads, and contribute substantially to circulating levels of these hormones, there is considerable evidence for local production in peripheral tissues of non-reproductive organs such as the heart, multiple vascular beds, breast, brain, and even the lung (Labrie, 2003; Labrie et al., 1991; Luu-The & Labrie, 2010), with the pattern of steroid production being dependent on which enzymes of the cholesterol pathway are present within specific tissues. The relevance of these non-gonadal sources lies in the high possibility that local sex steroid production or its metabolism within specific tissues can lead to substantial differences between local steroid levels vs. that seen in the circulation. Accordingly, it is possible that some of the paradoxical effects of sex steroids between organ systems and diseases involve the effects of local steroid regulation and effects. A prime example of this is the so-called “estrogen paradox” of pulmonary hypertension in young women while estrogens are thought to be protective in the coronary circulation. Multiple experimental animal studies have found estrogen and its metabolites to be protective against pulmonary hypertension, with better outcome in female animals and exacerbation following ovariectomy, but epidemiological data in humans indicate greater incidence of pulmonary hypertension in female patients, with some clinical studies indirectly linking estrogen to increased risk of portopulmonary hypertension, and others showing increased estrogen metabolism and higher levels of estrogen metabolites to be enhancing pulmonary vascular remodeling (see (Martin & Pabelick, 2014; Umar et al., 2012) for recent reviews).
Since both gonadally-derived and local sex steroids (Labrie, 2003; Labrie et al., 1991; Luu-The & Labrie, 2010) contribute to overall concentrations systemically and within tissues, it is important to consider concentrations of specific sex steroids that are typically noted. This is particularly important given life events such as puberty in men and women, pregnancy and menopause in women, and aging in both sexes. Here, it is not uncommon to see different formats (molar concentrations vs. serum levels) being used in studies, which makes comparisons sometimes difficult. We have previously compiled comparative data from different sources (Cummings et al., 1998; Elmlinger et al., 2002; Lippe et al., 1974) in this regard (Townsend et al., 2012a) to provide normal ranges of testosterone, estradiol, and progesterone in men vs. women in different life stages (non-pregnant, pregnant, and post-menopausal). Interestingly, the range of testosterone is usually stable in both males (2-15 ng/ml or 6-50 nM) and females (<1.5 ng/ml or 5 nM) throughout life. While men express pg/ml (pM) estradiol and ng/ml (nM) progesterone levels, the levels of these hormones obviously fluctuate substantially in women, ranging from 20 pg/ml estradiol and 0.3 ng/ml progesterone in follicular phase in non-pregnant and post-menopausal women to 40 ng/ml estradiol and 300 ng/ml progesterone in pregnant women. The importance of these levels fluctuations lies in their relative contribution to the local level of any sex steroid. Thus, for example, tissue production of estrogens (e.g. via aromatase-mediated testosterone conversion) may become more prominent in post-menopausal women as well as in aging men, while separately, the influence of progesterone may wane in these populations. Conversely, the very circulating levels of estrogen and progesterone in women may be more important in pregnancy unless tissue metabolism of these steroids can modulate the local levels that impact cells within an organ. There is currently very little information on these issues relevant to the lung.
2.1. Estrogen
As a primary female sex steroid, the role of estrogens in development of female reproductive organs, the menstrual cycle, and pregnancy are well-known. Estrogen has also been shown to be important in many pathophysiological processes of hormone dependent diseases, the best example being breast cancer (Bai & Gust, 2009; Couse & Korach, 1999; Deroo & Korach, 2006). However, more recent advances in estrogen biology indicate that estrogens have a range of actions in normal physiological process of different organs and tissues, including cell growth, development, differentiation and function (Burns & Korach, 2012; Deroo & Korach, 2006; Gustafsson, 2003; Straub, 2007). Importantly, these actions are not limited to women.
Estrogen effects are commonly mediated by two estrogen receptors ERα and ERβ acting via both classical, genomic mechanisms of altered protein expression/function, as well as acute, non-genomic mechanisms that are being increasingly recognized. In addition to these classical receptors, recent studies have shown that GPR30, a member of the G protein–coupled receptor superfamily, mediates estrogen-dependent kinase functional activation and transcriptional responses (Filardo et al., 2002; Prossnitz et al., 2008). Accordingly, cellular effects of estrogen are likely dependent on the relative expression and actions of ERα vs. ERβ, vs. GPR30, which makes for complex signaling within any tissue.
The classical ERβ and ERβ both belong to the nuclear receptor family of transcription factors (Heldring et al., 2007; Kim et al., 2008; Miller & Duckles, 2008) and are encoded on different genes, with conserved homology in the ligand-binding domain and DNA binding regions, but significant variability at the N-terminus (Heldring et al., 2007). Although ERα and ERβ have similar affinities towards 17-β-estradiol (E2; the major active estrogen in humans), several differences in the transcriptional activation levels have been observed. Some studies suggest that, when both ERs are expressed in a cell, ERβ inhibits ERα mediated transcription (Lindberg et al., 2003; Liu et al., 2002). For example, activation of ERα can lead to aberrant proliferation (Maggiolini et al., 2001; Teng et al., 2008), whereas ERβ activation has anti-proliferative effects via counteraction of ERα effects (Lahm et al., 2008). Such differential effects may underlie protective roles of estrogens in systemic vasculature but the higher incidence of pulmonary hypertension in young women: a topic that is still being investigated. However, it is also important to stress this relationship between ER isoforms has not been shown to be consistent across cell types, and may also be context-specific.
In addition to full-length receptors (hERα-66 in humans), truncated or shorter ERα isoforms (hERα-46 and hERα-36) which lack activation factor AF-1, are known to exist, and have complex effects on full-length ERα activity. This creates another level of modulation of estrogen action, at least in vitro. However, the role of truncated ER isoforms in vivo is not clear (Heldring et al., 2007). Regarding ERα, some splice variants may differentially modulate genomic estrogen signaling (Heldring et al., 2007; Matthews & Gustafsson, 2003). Accordingly, cell-type and context-dependent changes in the relative distribution of the various ER isoforms (truncated or full length) may lead to different overall effects of estrogen. There is currently very little data relevant to these issues in the lung.
Estrogens and ER can activate several distinct pathways to regulate biological processes (Heldring et al., 2007). Estrogen binding to ERs typically leads to nuclear translocation of these ERs, particularly ERα, and can binding to estrogen response elements (ERE) in the promoters of target genes. ERs can also interact with other transcription factor complexes such as c-fos/c-jun (relevant to AP-1-responsive elements) and thus indirectly influence gene transcription. Here, ligand-dependent activation can involve signaling intermediates such as ERK1/2, p38 MAP kinase, and JNK, as well as trigger recruitment of various nuclear co-regulators that are likely cell and context dependent, but not entirely characterized. In addition to the well-recognized transcriptional effects of estradiol, rapid non-genic effects within seconds to minutes have also been observed. Such rapid effects involve activation of cyclic nucleotides, kinases and phosphatases and usually modulate ion fluxes across the cell membrane. What is less clear is which (if any) of the ERs are involved, and what role GPR30 plays in this regard (or alternatively there is a separate, membrane bound receptor).
2.2.Progesterone
Progesterone, the second major endogenous female steroid hormone, is involved in variety of physiological and metabolic changes throughout life including embryogenesis, puberty, the menstrual cycle, and pregnancy. Progesterone is mainly produced in the ovaries although small amounts are produced in the brain and adipose tissue as well.
Progesterone exerts its effect via progesterone receptors (PRs) with target gene transcription. Mammals have two types of PRs: PR-A and PR-B and both these isoforms are transcribed from same gene, although PR-A differs in the truncated N-terminal domain (Edwards, 2005; Giangrande & McDonnell, 1999; Mulac-Jericevic & Conneely, 2004). Classically, progesterone binds to both PRs which enter the nucleus to activate DNA downstream (Giangrande & McDonnell, 1999). Both PRs, PR-A and PR-B has similar ligand binding affinities, but their transcriptional activation differs. For example, PR-B is a strong promoter of transcriptional activities in multiple cell types whereas PR-A isoform is not (Giangrande & McDonnell, 1999). When activated, PRs can recruit regulatory proteins such as SRC-1, SRC-2, SRC-3, CBP/p300 (McKenna et al., 1999) and may also modulate histone acetylation/deacetylation and chromatin remodeling. In addition to this nuclear receptor function, PRs are able to promote and regulate multiple cellular signaling mechanisms independent of nuclear activation (Gellersen et al., 2009).
2.3. Testosterone
Testosterone is the principal circulating androgen secreted by testicular Leydig cells in males. Although traditionally viewed as a “male” hormone, testosterone is also secreted by ovaries and in small amounts by the adrenal gland (Mooradian et al., 1987). Similar to the female sex hormones, testosterone is present and can regulate the structure and function of non-reproductive organs, including bone, skeletal muscle, brain liver, kidney and adipocytes.
Aromatization of testosterone leads to irreversible formation of estrogen as well as dihydrotestosterone. Both testosterone and its highly active metabolite, 5α-dihydrotestosterone (DHT) exert their biological actions via nuclear receptor subfamily NR3C4, also known as androgen receptor (AR). Ligand binding to AR can activate or inhibit androgen responsive elements of target genes (Chang et al., 1995).
2.4. Interaction between sex steroids
It is commonly assumed that an interaction between sex steroids occurs only in women where estrogen and progesterone are usually present concurrently, albeit to different levels depending on the life stage. However, since both men and women have non-zero levels of all three sex steroids, and there is always the potential for local tissue metabolism, interactions between sex steroids may occur in both sexes. There is currently only limited knowledge regarding such interactions, and indeed minimal data in the lung.
All of the classical sex steroid receptors (ERα, ERβPR-A, PR-B and AR) fall under the superfamily of nuclear receptors, and cloning studies reveal that they share common structural domains: C- terminus LBD, variable N-terminus with transcription activation function (AF-1) domain, mid-region DBD and hinge region. A second AF-2 domain is localized to the C-terminus of LBD region, which serves as a second transcriptional activation region with varied function based on ligand activation (Beato & Klug, 2000; McKenna & O'Malley, 2002). Based on these similarities and differences, interactions between sex steroid receptors can occur at multiple levels. For example, both ERα and ERβ have similar affinities towards, E2, but ERβ can antagonize and act as a dominant (negative) regulator of ERα function (Hall & McDonnell, 1999; Zhang & Teng, 2000), as noted for the pro-proliferative cyclin D1 promoter (Liu et al., 2002). ERα and ERβ can act as homodimers or heterodimers in the nucleus. Studies suggest that ERα can also heterodimerize with AR leading to modified transcriptional activity (Bennett et al., 2010).
Interaction between sex steroids can also occur downstream to the receptors. For example, estrogen increases proliferation in endothelial cells by activating MAPK phosphorylation, while in vascular smooth muscle cells estrogen inhibits proliferation via MAPK inhibition (Barchiesi et al., 2002; Dubey et al., 1999). Notably, progesterone also appears to reduce vascular smooth muscle proliferation and seems to facilitate the inhibitory effects of estrogen (Orshal & Khalil, 2004). Furthermore, PR-B is promoter for gene transcription, PR-A is a functional repressor on PR-B as well as for ER transcription (Edwards, 2005; Giangrande & McDonnell, 1999). This ability of PR-A to inhibit transcriptional responses induced by both PRB and ERs may underlie the overall anti-estrogenic activities of progesterone observed in the uterus (Lydon et al., 1995). Furthermore, PR-B can cross-talk with ERs and activate the Src-Ras-ERK pathways (Migliaccio et al., 1998). Separately, AR activation involves c-Src, Raf-1 and ERK-2 pathways followed by MAPK activation (Kousteni et al., 2001; Migliaccio et al., 2000), which can also serve to modulate either estrogen or progesterone signaling.
3. Sex steroids and their receptors in the lung
3.1. Conducting airways
Nasal Passages
Given the incidence of allergic rhinitis, and the well-known changes in nasal epithelial function in pregnancy, there has been some work (albeit limited) on sex steroid signaling in nasal epithelium, particularly estrogens. Both ERs and PRs are present in the nasal mucosa, and are upregulated in the presence of high levels of estrogen and progesterone, may thus contribute to altered nasal epithelial function in pregnancy (Tulchinsky et al., 1972) and the increased edema and nasal mucus secretion seen during the menstrual cycle (Ellegard & Karlsson, 1994; Haeggstrom et al., 2000; Paulsson et al., 1997). Interestingly, AR mRNA has been reported in human turbinate samples (Shirasaki et al., 2004), but not protein, making it likely that female sex steroids are more relevant here.
Bronchial Epithelium
The importance of sex steroid effects on bronchial epithelium lies in modulation of its barrier function, its responses to external stimuli including pathogens and allergens, and as a modulator of airway tone. However, few studies have examined the effect of sex steroids on bronchial epithelium. In immortalized airway epithelial lines, both ERα (Ivanova et al., 2010; Kirsch et al., 1999) and ERβ (Ivanova et al., 2010) have been reported, with ERβ being the predominant isoform. However, we recently reported in freshly isolated human bronchial epithelial tissue, as well as single cells, that the abundant ERα and ERβ expression is comparable (Townsend et al., 2011). Whether PRs or ARs are also expressed in bronchial epithelium is actually not known. However, given the similarities between nasal vs. bronchial epithelium, their expression is likely.
Airway Smooth Muscle
One of the major structural elements of the airway is the smooth muscle layer that is key to regulation of airway caliber and tone. Given the epidemiological and pre-clinical data suggesting sex differences in asthma, and even the potential effects of estrogens on airway function, there has been increasing interest in sex steroid receptor expression and function in airway smooth muscle (ASM). Studies, including our own, have demonstrated the expression of both ERα and ERβ in human airway smooth muscle (Dahlman-Wright et al., 2006; Hughes et al., 2002; Jia et al., 2011; Townsend et al., 2010). Other limited studies have also demonstrated existence of PRs (Abraham et al., 2011), and ARs (Wilson & McPhaul, 1996) in airway smooth muscle of different species.
3.2. Lung Parenchyma
The lung parenchyma comprises ~75% of cells, particularly of the alveoli. In spite of data suggesting sex differences in alveolar diseases, data on sex steroid receptor expression in lung parenchymal cells is limited. All the major sex steroid receptors have been found in the alveolar epithelial layer from biopsy samples (Ishibashi et al., 2005; Marquez-Garban et al., 2009; Mikkonen et al., 2010). Additionally, the enzymes aromatase and 17β-hydroxysteroid dehydrogenase are localized to alveolar epithelium (Marquez-Garban et al., 2009; Plante et al., 2009), indicating that local effects and metabolism of sex steroids may be important.
4. Contribution of sex steroids to airway structure and function
4.1. Lung development
The current review is largely focused on the adult lung. However, it is important to acknowledge the contribution of sex steroids in the developing lung, and the potential of these early influences to modulate postnatal lung growth and function.
During pregnancy, fetuses are exposed to both maternal and fetal sex steroids which in turn are involved in lung development (reviewed in-depth elsewhere (Becklake & Kauffmann, 1999; Carey et al., 2007b; Carey et al., 2007c; Seaborn et al., 2010)). The fetal testes produce testosterone following sex differentiation (Abramovich, 1974) which delays surfactant production during mid-late gestation (Seaborn et al., 2010). On the other hand, androgens and AR in the developing lung aid branching morphogenesis (Kimura et al., 2003; Nielsen, 1985; Provost et al., 2004; Seaborn et al., 2010; Torday, 1990). ERs are also expressed in the fetal lung (Brandenberger et al., 1997; Takeyama et al., 2001) and unlike androgens, promote and accelerate lung maturation (Beyer et al., 2003). Studies have also shown the importance of both ERs in alveolar formation (Massaro & Massaro, 2006; Massaro et al., 1996). Thus, in general, female fetuses have less bronchi, but produce lung surfactant earlier (Fleisher et al., 1985; Torday & Nielsen, 1987), and the lungs mature faster compared to male fetuses (Thurlbeck, 1975, 1982a), thus helping to maintain airway patency. Accordingly, the female lung is smaller and has fewer respiratory bronchioles at birth (Thurlbeck, 1975, 1982b). Nevertheless, female airways and lung parenchyma grow proportionally during childhood and adolescence (Carey et al., 2007b; Hoffstein, 1986; Pagtakhan et al., 1984). Furthermore, alveolar formation, which continues in the postnatal lung may be influenced by ERβ that is abundantly present (Patrone et al., 2003).
4.2. Adult lung
4.2.1. Smooth Muscle
Given clinical data on sex differences in asthma and airway-predominant COPD (Bjornson & Mitchell, 2000; Caracta, 2003; Carey et al., 2007b; Jensen-Jarolim & Untersmayr, 2008; Melgert et al., 2007), there has been much recent interest in how sex steroids may influence ASM, which is a key to contributor to airway hyperresponsiveness and remodeling that occurs in asthma (Carey et al., 2007b; Ebina et al., 1993). Of course, also given that diseases such as asthma or COPD likely involve multiple cell types of the lung, any sex differences that are not intrinsic to the lung, also likely involve sex steroid effects on different cell types. Nonetheless, recent evidence makes it is difficult to argue that steroid effects on ASM are not contributory in this regard.
Sex steroid effects on ASM may target both airway reactivity as well as ASM proliferation, potentially using different signaling pathways: genomic and non-genomic. Earlier studies using animal models (typically ERα or ERβ knockout mice, or alternatively ovariectomy of normal mice with vs. without estrogen replacement) suggest that sex steroids help or promote bronchodilation (Carey et al., 2007c; Degano et al., 2001; Dimitropoulou et al., 2009; Matsubara et al., 2008; Riffo-Vasquez et al., 2007). Although consistent with the idea that estrogens reduce [Ca2+]i in multiple cell types, including vascular smooth muscle, a potential problem with these previous studies is the use of non-physiological concentrations of sex steroids. Furthermore, studies done in vitro did not differentiate between epithelium-independent vs. –dependent effects, thus making it difficult to identify direct effects on ASM. For example, Foster et al. showed the potentiating effect of sex steroids on β-adrenoreceptor-induced relaxation of pig bronchus in vitro (Foster et al., 1983). Degano et al. found that 21 days low dose estradiol exposure (10ug/kg) significantly reduces acetylcholine-induced pulmonary resistance in ovariectomized rats (Degano et al., 2001). In this particular study, they showed that estrogen induced epithelium-dependent increase in cholinesterase activity, (Degano et al., 2001) while in another study the same group reported that acute high-dose estradiol (100ug/kg) directly increases acetylcholine induced airway responsiveness (Degano et al., 2003). Pang et al. showed high dose estradiol relaxes ACh pre-constricted rabbit tracheal strips, and importantly this effect was not reduced by NOS inhibition (Pang et al., 2002), highlighting direct effects of estrogen on ASM. Furthermore, estrogens enhance release of dilatory prostaglandins from tracheal smooth muscle cells and promote cGMP-mediated relaxation. In human ASM, we have shown rapid, likely non-genomic effects of physiologically-relevant concentrations of E2 involving increased cAMP, and potentiation of isoproterenol effects on reducing [Ca2+]i (Townsend et al., 2012b) and force (Townsend et al., 2012b). Additionally, we showed co-localization of β2-AR and ER in human ASM which may play a role in activation and potentiation of relaxation (Townsend et al., 2012b).
The mechanisms by which estrogens affect ASM are still under investigation. For example, in human ASM cells, we found that acute exposure to physiological concentrations of E2 (as low as 100 pM) decreases [Ca2+]i response to agonist: an effect reversed by the ER antagonist ICI-182,780 (Townsend et al., 2010). We further showed full-length ERα and ERβ expression in ASM (Townsend et al., 2010), and that effects on [Ca2+]i largely involve ERα. A previous study showed that estrogens promotes Ca2+ activated K+ channels via NO-cGMP-PKG pathway leading to membrane hyperpolarization and indirect reduction of [Ca2+]i and bronchoconstriction in mice (Dimitropoulou et al., 2005). However, in human ASM, the role of this mechanism appears to be less important, but rather involves inhibition of L-type Ca2+ channels and store operated Ca2+ entry (SOCE) (Townsend et al., 2010). Inhibition of influx may involve activation of the protein kinase A/cAMP pathway. In regards to SOCE mechanism, Orai1 and STIM1 (calcium channel and calcium sensor respectively) are two key regulatory proteins (Ay et al., 2004; Parekh & Putney, 2005; Putney, 2007; Sathish et al., 2012). A recent study showed that E2 non-genomically inhibits basal phosphorylation of STIM1 and reduces SOCE in epithelial cells (Sheridan et al., 2013). In a recently completed study, we have found that E2 inhibits serine phosphorylation of STIM1 in human ASM cells (V. Sathish, M.R. Freeman, M.A Thompson, C.M. Pabelick, Y.S. Prakash, unpublished data).
In contrast to non-genomic effects, there is currently little data on how estrogens could genomically affect ASM. Certainly, given that estrogens can have pro-proliferative effects (e.g. as in cancer), any enhancement of ASM proliferation may negate non-genomic bronchodilatory effects and worsen airway structure/function. Hughes et al. showed that estrogen metabolites, 2-methoxyestradiol (2-ME) and their analogs have anti-proliferative effect on the human airway smooth muscle cells without inducing cytotoxicity (Hughes et al., 2002). In this regard, estrogen effects on proliferative or fibrotic pathways such as MAP kinases, c-fos/c-jun need to be examined.
Compared to in vitro data largely showing estrogen-induced bronchodilation, in vivo studies in mice are less clear. Certainly, in the in vivo situation, it is difficult to isolate effects on ASM per se, especially in models of inflammation and asthma. For example, male C57BL/6 mice show more AHR than females, indicating a protective effect of estrogen (Card et al., 2006), but does not necessarily show an effect of sex steroids. Female mice lacking ERα display AHR, although this has been attributed to neural dysfunction (Carey et al., 2007a). Interestingly, in these animal studies, a direct effect of estrogens on ASM was not observed. A study by Catley et al. in rat models of allergic asthma suggested that selective activation of ERβ is not anti-inflammatory (Catley et al., 2008), but ERβ activation has been established as being anti-inflammatory in other models (Paterni et al., 2014). Overall, these limited data only underline the likely complex role of ER signaling and the airway in vivo.
High concentrations of progesterone potentiate isoprenaline-induced relaxation in preconstricted pig bronchus (Foster et al., 1983). This effect is more potent than testosterone, but not as comparable to E2 (Foster et al., 1983). Other studies show that via inhibition of calcium entry, progesterone and 5β-pregnenalone abolish agonist-induced contraction in guinea pig trachea (Perusquia et al., 1997). On the other hand, Hellings et al. showed that progesterone exacerbates AHR in ovalbumin-sensitized male mice (Hellings et al., 2003), although there are no reports on PR expression per se. Progesterone is known to inhibit oxytocin receptor binding in uterine smooth muscle to maintain quiescence during pregnancy (Grazzini et al., 1998). Oxytocin increases cytosolic Ca2+ in human ASM cells and promotes airway narrowing in murine tracheal rings (Amrani et al., 2010). Accordingly, progesterone may indirectly influence ASM by interference with oxytocin binding: an effect to be demonstrated.
Testosterone non-genomically relaxes pre-constricted rabbit tracheal smooth muscle and this effect seems to be epithelium dependent (Kouloumenta et al., 2006). However, other studies have found that androgens elicit epithelium-independent bronchial relaxation though inhibition of voltage-dependent calcium channels (Bordallo et al., 2008). However, it is interesting to note here that the AR inhibitor flutamide is not effective in preventing such effects. In a recent study, Montanõ et al. showed androgens dose-dependently prevent smooth muscle contraction in response to carbachol and KCl in isolated trachea rings from guinea pigs (Montaño et al., 2014).
In asthma, inflammation effects involve enhanced ASM contractility and cell proliferation as well as altered ECM production. Sex steroid effects on ASM proliferation and remodeling in the context of inflammation is not well-understood. Physiologic concentrations of estrogens, progesterone or testosterone do not appear to affect thrombin-induced human ASM cell proliferation (Stewart et al., 1995). Dehyroepiandrosterone inhibits tracheal smooth muscle proliferation in the rat (Dashtaki et al., 1998). In contrast, in rabbit tracheal smooth muscle cells, ASM proliferation is increased by both testosterone and E2 (Stamatiou et al., 2011). Recent ongoing studies from our group show receptor specific inhibition of human ASM proliferation during inflammation (Sathish et al., 2014) and ERs signaling diverge in inflamed ASM in this regard. With differential effects of different sex steroid receptors on various signaling mechanisms, more detailed studies are warranted to further explore these novel aspects of sex steroid effects in the airway.
4.2.2. Epithelium
Airway epithelium is the initial site of effect for environmental and inflammatory stimuli in the lung. Nasal, bronchial and alveolar epithelia play critical barrier and homeostatic functions (Knight & Holgate, 2003). Furthermore, epithelial cells are involved in the pathophysiology of multiple lung diseases, including asthma, bronchitis, and COPD.
The roles of specific sex steroid receptors in nasal epithelium are less clear. Here, it is possible that ERβ actually serves as a dominant negative regulator for other steroid receptors (Heldring et al., 2007; Levin, 2001), and therefore, for estrogens to induce greater mucus production for example, upregulated ERα expression needs to occur (which has been noted during pregnancy). Interestingly, in spite of PR being present in the nasal mucosa, progesterone has little functional effect (Taylor, 1961). In this regard, the importance of estrogen signaling in the nasal mucosa in the context of rhinitis is also suggested by clinical data showing increased rhinitis symptoms in a significant percentage of women on oral contraceptives (Pelikan, 1978), as well as animal studies showing thickened nasal mucosa following exogenous estrogen administration (Mortimer et al., 1936; Taylor, 1961). In vitro, in cultured human nasal epithelial cells and mucosal microvascular endothelial cells, histamine receptor expression is increased by estrogen and progesterone (Hamano et al., 1998a). However, it is important to note that all of the rhinitis symptoms in women are not explained by estrogens. For example, nasal biopsies from pregnant women with rhinitis show upregulated peri-vascular cholinergic nerve activity (Toppozada et al., 1982). The relevance of these findings lies in identifying novel approaches that target nasal epithelium vs. vasculature (or even innervation) to alleviate nasal symptoms of rhinitis. Furthermore, it is not clear whether rhinitis exacerbation in males involve ARs, or even ERs due to aromatase-induced local conversion of testosterone to estradiol.
The role of estrogens in nitric oxide regulation in the vasculature is well known (Hisamoto & Bender, 2005; Kim & Bender, 2005; Kim et al., 2008). Estrogens facilitate dissociation of endothelial nitric oxide synthase (eNOS) from plasma membrane caveolae resulting in activation of the NO pathway and vasodilation (Sud et al., 2010). NO is produced by bronchial epithelium (Folkerts & Nijkamp, 2006; Hamad et al., 2003), and is increased with inflammation (Jatakanon et al., 1998; Mandhane et al., 2009). In immortalized epithelial cells lines, estradiol, working via ER, can acutely activate eNOS (Kirsch et al., 1999). Using acutely-dissociated human bronchial epithelium, we recently reported that both ERα and ERβ are involved in increasing NO production via eNOS (Townsend et al., 2011). What is not known is whether estradiol can modulate NO production through iNOS, the likely more important enzyme in the context of inflammation.
Studies including our own show constitutive expression of ERα and ERβ in airway epithelial cells (Ivanova et al.; Kirsch et al., 1999; Townsend et al., 2011). In our studies, in epithelium-intact bronchial rings, following stable acetylcholine-induced force levels, 10nM E2 results in rapid relaxation while epithelial denudation blunts this bronchodilatory effect. A similar effect was observed using ERα and ERβ specific agonist, indicating the role of both ERs in human bronchial epithelium (Townsend et al., 2011). A recent report by Sheridan et al showed non-genomic estrogen inhibition of basal STIM1 phosphorylation followed by reduction in SOCE in airway epithelia (Sheridan et al., 2013). Estrogen also increases MUC5AC and is involved in post-translational modification (glycosylation) of mucins in human airways, this effect largely mediated by ERβ (Tam et al., 2014). Overall, these limited data suggest substantial effect of estrogens in the bronchial epithelium.
Co-localization studies using the cilia marker protein acetylated α-tubulin reveal that PRs are expressed in proximal regions of cilia in human tracheal epithelial cells (Jain et al., 2012), with higher PR-B expression in both sexes. Progesterone decreases ciliary motility in epithelial cells: an effect prevented by addition of E2, showing the potentially opposing effects of these sex steroids (Jain et al., 2012).
There is limited and conflicting data regarding sex steroid effects on bronchial epithelial cells growth and proliferation. Estrogen increases proliferation of some immortalized epithelial cell types (Ivanova et al., 2010). There is currently no data on the effects of androgens or progesterone in this regard. Separately, in the context of cystic fibrosis, limited data suggest that estradiol can modulate UTP-induced chloride secretion (Coakley et al., 2008) and thus alter susceptibility to infection and mucus clearance.
4.2.3. Fibroblasts
In bleomycin-induced lung fibrosis model, male mice show decreased lung function and increased fibrosis, while castration restores lung function and conversely DHT replacement therapy worsens it (Voltz et al., 2008). Similarly, studies show increased lung collagen deposition in female rats following bleomycin is greatly reduced by ovariectomy (Gharaee-Kermani et al., 2005). In addition, Langenbach et al suggested that bleomycin-induced pulmonary fibrosis is resistant to treatment with 2ME (Langenbach et al., 2007). However, other studies report a protective role for estrogens in pulmonary fibrosis. For example, in rats, ovariectomy leads to increased pulmonary fibrosis with ASM thickening, while 2ME replacement reverses fibrosis (Tofovic et al., 2009). In vitro, high concentrations of all three sex steroids reduce fetal lung fibroblast density (Kondo et al., 1983). Estrogen inhibits lung myofibroblast proliferation via Raf1-ERK-MAPK (Flores-Delgado et al., 2001), consistent with 2ME data in lung fibroblasts (Tofovic et al., 2009). Studies have also shown the importance of ERβ in maintenance of ECM composition using an ERβ knockout mice model (Morani et al., 2006). However, there is much not known regarding sex steroid regulation of the ECM, particularly effects on specific components such as collagen, fibronectin and laminin as well as matrix metalloproteinases that degrade collagen and ECM components.
4.2.4. Nerves
Both sympathetic and parasympathetic nerves are involved in the regulation of airway caliber. Major functions include defensive reflexes (sneeze and cough) in the upper airways, cholinergic-parasympathetic contraction and non-cholinergic parasympathetic smooth muscle relaxation (Canning & Fischer, 2001). Multiple studies have shown sex differences in ventilatory measures with age, as well as variations with menstrual cycle, and in pregnancy (da Silva et al., 2006; Driver et al., 2005; Saaresranta & Polo, 2002; White et al., 1983). While the role of innervation per se is less clear, neurons and glia have region-specific steroidogenic enzymes. Neurosteroids are synthesized in the central and peripheral nervous system using cholesterol or steroid hormone precursors or from circulating sex steroid hormones (Behan & Wenninger, 2008; Mellon & Griffin, 2002).
A role for progesterone in pregnancy-associated respiratory stimulant activity has been reported (Slatkovska et al., 2006) and furthermore, progesterone is used as an effective respiratory stimulant in patients with breathing disorders (Dempsey & Skatrud, 1986; Kimura et al., 1988; Tatsumi, 1999). Studies have also shown a neuroprotective role for progesterone and estrogen in sleep-disordered breathing in post-menopausal women. (Bixler et al., 2001; Shahar et al., 2003; Young et al., 2003) Some studies suggest that testosterone supplementation in hypogonadal men leads to worsening of obstructive sleep apnea (Saaresranta & Polo, 2002) and increased ventilation, whereas other studies show the opposite (Kapsimalis & Kryger, 2002; Saaresranta & Polo, 2002). Human airway has non-adrenergic, non-cholinergic (NANC) innervation which mediates (excitatory) bronchoconstriction via tachykinins, substance P and neurokinin A. The inhibitory component of NANC mediates bronchodilation via vasoactive intestinal peptide and NO (Belvisi et al., 1995; Joos et al., 2000; Lammers et al., 1992; Linden, 1996; Mackay et al., 1998; van der Velden & Hulsmann, 1999; Ward et al., 1995). Neuronal NOS in NANC nerve fibers generates NO from arginine which then induces ASM relaxation. Previous studies suggest that an imbalance between excitatory vs. inhibitory NANC contributes significantly to asthma (Joos, 2001; Joos et al., 2000). A few studies in urogenitary (Andronowska & Chrusciel, 2008; Scott et al., 2007) and gastrointestinal (Shah et al., 2001) smooth muscles have demonstrated that neurally-derived NO is modulated by estrogens. However, no data is available on estrogen influence on NANC in ASM per se. Nonetheless, in pregnant women, increased cholinergic nerve activity has been found near nasal blood vessels (Toppozada et al., 1982). Carey et al. found that ERα knockout mice showed increased Ach release from isolated tracheas subjected to electrical field stimulation when compared to wild-type control mice, indicating the role of estrogen signaling in neutrally derived signals (Carey et al., 2007a).
4.2.5. Immune cells
Studies show that sex steroids markedly regulate activation, lifespan, and functions of a variety of immune cells including dendritic cells, lymphocytes, regulatory T-cells and B lymphocytes. Although much of the work on sex steroid effects on immune function is relating to rheumatologic disease, immune modulatory role of sex steroids has both physiological and pathological implications in the lung. Differences in male and female immune responses and regulation (immune dimorphism) have been observed in multiple studies (Da Silva, 1999; Huber & Pfaeffle, 1994; Whitacre et al., 1999). Reports show that women generally have enhanced humoral response while men suppress both humoral and cell-mediated immune responses (Gilliver, 2010). Here, we summarize some key features of sex steroid effects on immune cells relevant to airway function. An important consideration gleaned from work in rheumatology (Straub, 2007) is that sex steroid effects on immune cells is dependent on the steroid, its concentration, timing and duration, and furthermore the context of exposure. Obviously, these are not easy to control in experimental settings or to evaluate in clinical settings, and may thus explain the complexity and range of immune cell effects that have been noted. Detailed reviews regarding the immunomodulatory role of sex steroids can be found elsewhere (Gilliver; Straub, 2007).
Dendritic Cells
As initiators of the immune response to antigens and other stimuli, modulation of dendritic cell activity plays an important role in the subsequent response in the context of allergy and asthma. Here, dendritic cells express PR-A, ERα, and ERβ (Gilliver, 2010), but the functional effects of female sex steroids on these cell types within the lung is not known. Estrogen does promote dendritic cell formation in bone marrow (Paharkova-Vatchkova et al., 2004) and to downstream dendritic cell stimulation of T cells (Melgert et al., 2010). Similarly, DHEA promotes maturity of DCs. These limited data suggest that sex steroids modulate very early steps in allergic airway disease and may thus play a role in induction of diseases such as asthma throughout life.
Eosinophils
In ovalbumin-sensitized mice, females show greater eosinophilia compared to males, an effect modulated by female sex hormone levels (Riffo-Vasquez et al., 2007). Eosinophils bind estradiol (Katayama et al., 1998; Klebanoff, 1977) and show increased degranulation and adhesion (Gilliver, 2010; Hamano et al., 1998b; Katayama et al., 1998). Although the expression patterns of PRs or ARs is not known, progesterone can increase eosinophilia-related AHR in sensitized mice (Hellings et al., 2003).
T Cells and B Cells
: Immune cells are well-known to bind estrogens and androgens. All types of T lymphocytes express both ERα and ERβ (Pierdominici et al., 2010), with estrogen being effective in activating intracellular signaling cascades. What is not clear is whether such modulation of T-cell function is suppressive or stimulating to immune function relevant to lung disease. The relevance of such estrogen effects lies in the well-known exacerbation of asthma during the luteal phase of the menstrual cycle and during pregnancy, when high estrogen levels shift skew the immune system towards a Th2-type response (Soldan et al., 2003) with elevated IL-4 and IL-10 (Straub, 2007), but decreased TNFα (Cenci et al., 2000): effects mediated via ERα (Lambert et al., 2005; Straub, 2007). In general, progesterone effects on immune cells parallel those of estrogen although PR expression patterns have not been systematically examined (Hardy et al., 2006; Huck et al., 2005; Miyaura & Iwata, 2002; Piccinni et al., 1995). Accordingly, in both pregnant and non-pregnant women, the two female sex steroids may have additive or synergistic effects in exacerbating asthma. In contrast to estrogen or progesterone, androgens skew the immune system towards a Th1-type response by increasing IFNγ (Huber et al., 1999; Martin, 2000; Namazi, 2009) and IL-2 (Giron-Gonzalez et al., 2000). Overall, these data, while not entirely consistent across studies (and perhaps not valid across species), may be important for understanding sex differences in allergic airway diseases, and especially their modulation at lifestages such as pregnancy and menopause.
Women have higher serum antibody concentrations compared to men. Furthermore, IgE, which causes mast cell degranulation, has been shown to vary with hormonal status in women (Vellutini et al., 1997), and may thus play a role in premenstrual asthma exacerbations. In addition to ERα mast cells also express PR A and PRB B (but not AR). In accordance, estrogen enhances mast cell degranulation and histamine secretion. However, progesterone inhibits mast cell migration and proliferation (Belot et al., 2007; Gilliver, 2010; Vasiadi et al., 2006), overall making the effects of estrogen vs. progesterone different in B-cells compared to T-cells.
5. Sex steroid signaling in disease states
5.1. Asthma
Asthma is a chronic inflammatory disease of the airway and according to WHO reports affects >300 million people worldwide. Asthma involves both intrinsic and environmental factors suggesting that is multifactorial in origin. Asthma is characterized by three major features: airway structural remodeling (involves both airway and epithelial cells), functional changes (airway hyperresponsiveness, edema, mucus production) and inflammatory changes (activation of inflammatory cells) (Frieri, 2005; Hirota et al., 2005; Holgate, 2010; James et al., 2012; Joubert & Hamid, 2005; Prakash, 2013; Siddiqui et al., 2013; West et al., 2013; Wright et al., 2013).
What makes the potential for sex steroid effects in asthma particularly interesting is the changing pattern with life stages that are not simply explained by a one-one relationship between sex hormone levels and asthma symptomatology. Considerable evidence suggests that asthma is more common in pre-pubescent males compared to females, but following puberty, is more common in females(Becklake & Kauffmann, 1999; Bjornson & Mitchell, 2000; Caracta, 2003; Carey et al., 2007b; Jensen-Jarolim & Untersmayr, 2008; Melgert et al., 2007). In the prepubescent period, sex differences in airway growth patterns may help explain greater asthma in boys (Boezen et al., 2004; Carey et al., 2007b; Hoffstein, 1986). Following puberty greater incidence of asthma among females (Melgert et al., 2007; Redline & Gold, 1994; Schatz & Camargo, 2003), may also be due to the fact that female airways and lung sizes are smaller compared to males, and thus any airway remodeling or hyperreactivity is more obvious. Interestingly, with further aging, the differences between men and women decrease. For example, recent clinical study from Swedish asthma patients showed lower total MiniAQLQ score (mini asthma quality of life questionnaire) in younger women than age matched men, while there is no observable difference at older age (Lisspers et al., 2013).
The epidemiological data showing increased post-pubertal asthma in women suggest detrimental effects of female sex hormones. However, during childbearing years, although a higher proportion of women suffer from premenstrual and menstrual worsening and severity of asthma, such exacerbation appears to occur at a time when estrogen is actually at its lowest circulating concentration and progesterone is at its highest circulating concentration (Chhabra, 2005; Farha et al., 2009; Vrieze et al., 2003). However, it is also important to note that some women with moderate asthma observe relief of their premenstrual asthma exacerbations if they use oral contraceptives that suppress large fluctuations in circulating hormones (Dratva et al., 2010; Forbes et al., 1999). These effects of exogenous estrogens negate a simple relationship between sex steroids and asthma in women.
Asthma complicates 8-13% of pregnancies (Kwon et al., 2003). Interestingly, fetal sex may influence asthma, where mothers of boys report improved asthma symptoms (Dodds et al., 1999). However, even in pregnancy, a simple relationship between sex steroid levels (which are high) and asthma is not clear. Among pregnant women with a pre-pregnancy diagnosis of asthma, only ~10% experience acute asthmatic exacerbations during pregnancy, which is less the ~40% of asthmatic women experiencing premenstrual worsening (Caracta, 2003; Pereira & Krieger, 2004). Furthermore, ~30% of asthmatic women actually show an improvement in symptoms in their third trimester, and another 30% show no changes at all (Juniper et al., 1989; Schatz et al., 2003).
Asthma prevalence balances out around menopause designating no sex difference in asthma beyond this age range (Bonner, 1984; Skobeloff et al., 1992). Studies also show that decreased risk of developing de novo asthma in post-menopausal compared to pre-menopausal women (Troisi et al., 1995b). Hormone replacement therapy is associated with increased risk and severity of asthma in post-menopausal women, although some other studies show HRT therapy in women as being protective in asthma (Haggerty et al., 2003; Myers & Sherman, 1994).
In contrast to human data, animal work on the relationship between estrogens and asthma is conflicting. In mice, estrogen appears to protect against airway hyperresponsiveness (Dimitropoulou et al., 2005; Matsubara et al., 2008), while progesterone aggravates allergic airway disease (Hellings et al., 2003). Spontaneous airway hyperresponsiveness has been reported in ERα knockout mice (Carey et al., 2007a). What is not clear is whether the species discrepancies let differences in the immune responses to hormones, or intrinsic differences in the way the airway responds to inflammation.
Compared to women and female sex steroids, the relationship between men and androgens in this context is a bit clearer, albeit supported only by limited data. Androgens appear to be anti-inflammatory by decreasing Th2 cell response, and conversely castration in males exacerbates disease (Hayashi et al., 2003). Severity of asthma in men remains relatively stable from puberty until serum testosterone levels start to decrease with aging, when an increase in asthma is observed (Canguven & Albayrak, 2011; Zannolli & Morgese, 1997), suggesting a beneficial role for testosterone in asthma. Interestingly, even in women, testosterone administration can improve asthma symptoms (Wulfsohn et al., 1964).
Overall, the above data, albeit limited to a certain extent, suggest complex, sometime synergistic, sometimes opposing effects of female sex steroids, and largely opposing effects of male sex steroids in asthma (Figure 2). Considerably more work is needed to better understand the role of specific hormones, particularly in the context of their effects on specific cell types, the life stage of the person when such exposures occur, and the concentration and duration of exposure.
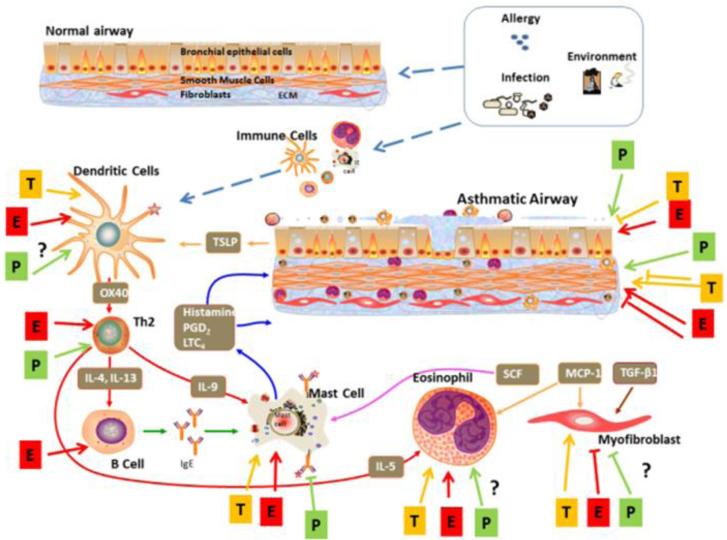
Figure 2.
Sex steroid effects in asthma. It is recognized that asthma is a multifactorial disease involving the effects of allergic, infectious and environmental triggers on both the immune system, and structural cells of the bronchial airway. Overall, inflammation drives structural and functional airway leading to epithelial thickening, increased mucus production, proliferation of epithelial, smooth muscle and fibroblast cells, remodeling of the extracellular matrix and overall airway hyperreactivity and fibrosis. Here, studies to date suggest complex effects of estrogen vs. progesterone vs. testosterone on relevant cell types, involving both cooperative vs. opposing effects of the different sex steroids within a cell type, but not necessarily across cell types. For example, dendritic cells, mast cells, CD4+ T lymphocytes (Th2), and eosinophils are particularly important. The effects of estrogen (E), progesterone (P), or testosterone (T) on these immune cells can vary substantially, particularly in the context of concentration, timing and duration.
5.2. COPD
Chronic obstructive pulmonary disease (COPD) is a progressive disease that includes emphysema and chronic bronchitis. Emphysema is characterized by damaged alveoli while chronic bronchitis is characterized by inflamed airways with excess mucus formation. A recent review by Kamil et al. suggests sex-specific and race-related differences in the manifestation of COPD, although the mechanistic basis for such differences are not completely understood (Kamil et al., 2013). Tobacco smoke is the biggest risk factor for COPD. In the developing world, women are more likely to be exposed to biomass smoke and environmental smokes which are also major causes for COPD (Varkey, 2004). Cigarette smoke exposure in active smokers, as well as secondhand smoke (SHS) exposure exhibit lower airway mucosal inflammation and bronchitis (Jaakkola & Jaakkola, 1997; Sopori, 2002). Updated centers for disease control and prevention (CDC) data in US suggest that death rates for COPD have declined among US men (1999 vs. 2010) whereas no significant change has occurred among women. Coupled with exposure to SHS from spouses, more women now suffer from COPD (Hughes & Jacobson, 2003; Hughes et al., 2008; Mannino et al., 2002; Troisi et al., 1995a). Interestingly, women develop COPD after smoking less number of cigarettes in their lifetime compared to men (Gillum, 2005; Gold et al., 1996). Recent soluble proteome analysis of bronchoalveolar lavage cell from COPD patients reveals distinct, abundant changes in female patients compared to male patients, which form a first step towards understanding these sex differences in COPD (Kohler et al., 2013). What is not clear in this context is whether sex hormones are protective or detrimental in COPD. For example, estrogens can be pro-proliferative and thus potentiate the effects of cigarette smoke and other insults on airway and alveolar epithelial cells leading to bronchitis that is more common in women. Conversely, testosterone may potentiate alveolar destruction and thus contribute to the greater emphysematous changes of the male lung. On the other hand, sex steroids can modulate immune function in complex ways, and thus color the overall response of the lung to insults such as cigarette smoke (Figure 3).
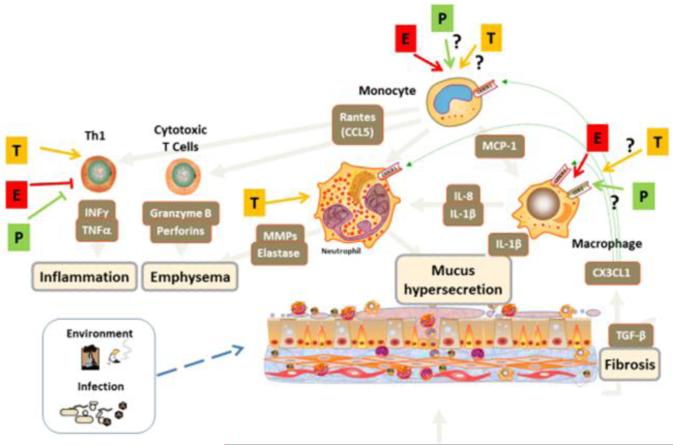
Figure 3.
Sex steroid effects in COPD. Environmental exposures, particularly that from tobacco products, as well certain infects can contribute to COPD, which is also characterized by airway inflammation, mucus hypersecretion, airway fibrosis, alveolar emphysema. The effects of estrogen (E), progesterone (P), or testosterone (T) on resident cells of the airway (as in Figure 2) continue to play a part. In terms of immune cells, Th1 polarized T-lymphocytes as well as CD8+ cytotoxic T cells are also involved. Sex steroid effects on neutrophils and monocyte/macrophages may be particularly important, but there is currently little mechanistic data. In general, female sex steroids appear to be “protective” in terms of lymphocytes.
5.3. Pulmonary fibrosis
Pulmonary fibrosis (PF) is a progressive interstitial lung disease leading to scarring. PF has a higher prevalence in males compared to females (Meltzer & Noble, 2008) with faster progression, and less survival (Han et al., 2008; Olson et al., 2007). Although our understanding of PF biology is improving, sex differences have been studied only in animal models. In contrast to the human clinical data, in bleomycin models of PF, higher mortality and fibrosis has been observed in female rats compared to males, and ovariectomy is protective while estrogen worsens disease (Gharaee-Kermani et al., 2005). Interestingly, this sex difference is reversed in mouse models of bleomycin injury. These conflicting data make it difficult to determine the role of sex steroids per se. Androgens appear to play an exacerbatory role in mediating the decline in lung function in experimental fibrosis (Voltz et al., 2008). A recent study showed that male mice develop more severe PF when compared to age-matched female mice (Redente et al., 2011). Conversely, estrogens show protective effects on airway fibrosis (Lekgabe et al., 2006), as does the estradiol metabolite 2-methoxyesradiol (Tofovic et al., 2009).
6. Targeting sex steroids and their signaling in lung disease
This review highlights the potential importance of sex steroids and their signaling in both structure and function of both normal and diseased lung at different life stages. Better understanding of mechanisms underlying sex steroid effects in regulation of lung structure and function may help in the design of drug-based and other interventions targeting lung diseases in women vs. men, as well as different age groups. Here, an important and appealing advantage is that drug delivery to the lung is comparatively easier than other organs due to the option of inhalation without the need for a systemic route of administration with reduced bioavailability. Therefore, the possibility for targeted delivery of drugs or other interventions to modify sex steroid signaling in specific lung cellular components is appealing.
As discussed above, sex steroid signaling involves both genomic and non-genomic pathways. Accordingly, it would be important to consider what aspect of sex steroid signaling should be manipulated. For example, non-genomic estrogen effects on calcium signaling and bronchodilation has potential application as a β-agonist sparing agent which may help women with β2-AR insensitivity. This may be facilitated by the fact that ERs are frequently found within caveolae, where β2-AR are also localized, thus allowing synergistic signaling between these pathways to promote bronchodilation. Another possibility is targeting epithelium where estrogen promotion of eNOS- mediated NO production could induce bronchodilation. In terms of genomic effects, estrogen appears to play a beneficial modulatory role in ASM remodeling and in pulmonary fibrosis. Accordingly, further exploration of the idea that estrogens could blunt remodeling and fibrosis could lead to novel approaches to addressing a major aspect of multiple lung diseases that are not responsive to corticosteroid therapies. In addition to the standard glucocorticoids and β-AR agonist therapy, the use of oral contraceptives in reducing premenstrual asthma exacerbations highlights the potential alternative therapeutic pathway. Progesterone effect on control of breathing is well known and could be used to treat sleep apnea syndrome. Separately, control of immune responses such as balancing Th1/Th2 signaling is possible via androgens which would be appealing for COPD vs. asthma. Ongoing preliminary studies from our lab showing differential ER expression pattern in asthmatic airway and activation of receptor specific signaling using specific agonist modestly reduce asthmatic airway contractility and remodeling.
7. Future directions
Compelling evidence now suggests that sex steroids and their differential signaling occur in the lungs of both males and females. However, in this regard, it is important to consider multiple factors in delineating the complex signaling of sex steroids that are affected by age, gender, the type of steroids, receptor expression patterns, cell types, and disease type and status, and finally in the case of experimental studies, the species and models being used. Added to these are the concepts of circulating steroids vs. local production and metabolism within tissues. This is exemplified by the complex effects of sex steroids in immune cells, discrepancies between human vs. animal data in the case of asthma, the discrepancies between sex steroid level and asthma exacerbation etc. This makes it difficult to simply determine whether sex steroids are beneficial or detrimental to lung structure and function. Accordingly, it is essential that future research carefully select the most appropriate human or animal models to examine the role of sex steroids within specific tissues, and furthermore correlate data across species and models in order to resolve how complex signaling leads to a disease phenotype.
One major issue in studying sex steroid signaling is crosstalk and interaction between hormones. This can be further confounded by tissue metabolism and conversion, e.g. testosterone to estradiol via aromatase in aged males. Here, differential receptor expression with opposing actions, such as ERβ antagonizing ERα function, can further complicate things. In some cases, ERs can control gene expression of PRs and their function. Overall understanding the cross talk between sex steroids and their receptors is essential for understanding the multifactorial effects they may have in airway diseases, and their contribution to issues such as alterations in menstrual cycle, pregnancy and other life conditions.
Another important area in need of attention is sex steroids effects on various immune cells specifically in the lung. Estrogen shows biphasic dose-dependent responses to Th1 and Th2 signaling, and thus may have different effects in women depending on steroid concentration. In this regard, again interactions with progesterone may be important to consider. Conversely, the stronger immunosuppressive role of androgen needs to be examined further, i.e. are we focusing on female sex steroids being detrimental, when it could be that it is the male sex steroids that are protective. Immune cells can also modulate functions of structural cells of the lung. Accordingly, co-culture studies and integration of sex steroid effect in multiple cell types may provide better understanding in this area.
8. Conclusions
Emerging evidence clearly suggests the influence of sex steroids from molecular level to whole lung function in health and disease. Sex steroids exert their effect from developing lung through aging. Differences in sex steroid signaling are observed in normal physiological and pathophysiological conditions of the lung. Sex steroid effects in the lung can be beneficial or detrimental, but it remains to be established whether and how such effects are overall manifested. The answer may depend on the relative local vs. circulatory concentrations, specific receptor expression/function, interactions between other sex steroids, and cellular component exposure. Overall, complex sex steroid signaling in the multicellular environment of lung with health and diseases remains an important issue to consider from both research and clinical perspectives, with the broader intent of exploring novel therapeutic avenues in the context of individualized medicine on the basis of sex and gender.
Acknowledgement
Funding: Supported by the Flight Attendant Medical Research Institute (Sathish), the Foundation for Anesthesia Education and Research (FAER; Martin), the Department of Anesthesiology (Martin, Prakash), and an NIH Diversity Supplement to Dr. Martin via R01 HL088029.
Abbreviations
- 3β-HSD
β-hydroxysteroid dehydrogenase
- AR
Androgen receptor
- ERE
Estrogen response elements
- ASM
Airway smooth muscle
- cAMP
Cyclic adenosine monophosphate
- COPD
Chronic obstructive pulmonary disease
- CPA
Cyclopiazoinc acid
- CSE
Cigarette smoke extract
- CYP
Cytochrome P450s
- DBD
DNA binding regions
- DCs
Dendritic cells
- DHEA
Dehydroepiandrosterone
- DHT
5α-dihydrotestosterone
- E2
17-β-estradiol
- ECM
Extracellular matrix
- ER
Estrogen receptor
- HRT
Hormone replacement therapy
- IPF
Idiopathic pulmonary fibrosis
- LBD
Ligand-binding domain
- NANC
Non-adrenergic non-cholinergic
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- PF
Pulmonary fibrosis
- PR
Progesterone receptor
- SHS
second hand smoke
- SOCE
Store operated Ca2+ entry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial and Competing Interests Disclosure: The authors have nothing to declare in terms of financial or other conflicts of interest relevant to the subject matter of materials discussed in this review. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- Abraham Z, Lourdes A, Christian G, Martha O, Maria C. Expression And Regulation Of Estrogen, Progesterone And Androgen Receptors In Airway Smooth Muscle Cells In An Allergic Asthma Model. Am J Respir Crit Care Med. 2011;183:A3651. [Google Scholar]
- Abramovich DR. Human sexual differentiation--in utero influences. J Obstet Gynaecol Br Commonw. 1974;81:448–453. doi: 10.1111/j.1471-0528.1974.tb00494.x. [DOI] [PubMed] [Google Scholar]
- ALA, Data American Lung Association: Lung Disease Data. 2008:1–188. http://www.lung.org/assets/documents/publications/lung-disease-data/LDD_2008.pdf.
- Amrani Y, Syed F, Huang C, Li K, Liu V, Jain D, Keslacy S, Sims MW, Baidouri H, Cooper PR, Zhao H, Siddiqui S, Brightling CE, Griswold D, Li L, Panettieri RA., Jr. Expression and activation of the oxytocin receptor in airway smooth muscle cells: Regulation by TNFalpha and IL-13. Respir Res. 2010;11:104. doi: 10.1186/1465-9921-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andronowska A, Chrusciel M. Influence of estradiol-17beta and progesterone on nitric oxide (NO) production in the porcine endometrium during first half of pregnancy. Reprod Biol. 2008;8:43–55. doi: 10.1016/s1642-431x(12)60003-5. [DOI] [PubMed] [Google Scholar]
- Arain FA, Kuniyoshi FH, Abdalrhim AD, Miller VM. Sex/gender medicine. The biological basis for personalized care in cardiovascular medicine. Circ J. 2009;73:1774–1782. doi: 10.1253/circj.cj-09-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ay B, Prakash YS, Pabelick CM, Sieck GC. Store-operated Ca2+ entry in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2004;286:L909–917. doi: 10.1152/ajplung.00317.2003. [DOI] [PubMed] [Google Scholar]
- Bai Z, Gust R. Breast Cancer, Estrogen Receptor and Ligands. Archiv der Pharmazie. 2009;342:133–149. doi: 10.1002/ardp.200800174. [DOI] [PubMed] [Google Scholar]
- Barchiesi F, Jackson EK, Gillespie DG, Zacharia LC, Fingerle J, Dubey RK. Methoxyestradiols mediate estradiol-induced antimitogenesis in human aortic SMCs. Hypertension. 2002;39:874–879. doi: 10.1161/01.hyp.0000013863.25970.ba. [DOI] [PubMed] [Google Scholar]
- Beato M, Klug J. Steroid hormone receptors: an update. Hum Reprod Update. 2000;6:225–236. doi: 10.1093/humupd/6.3.225. [DOI] [PubMed] [Google Scholar]
- Becklake MR, Kauffmann F. Gender differences in airway behaviour over the human life span. Thorax. 1999;54:1119–1138. doi: 10.1136/thx.54.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan M, Wenninger JM. Sex steroidal hormones and respiratory control. Respiratory physiology & neurobiology. 2008;164:213–221. doi: 10.1016/j.resp.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierle I, Meibohm B, Derendorf H. Gender differences in pharmacokinetics and pharmacodynamics. Int J Clin Pharmacol Ther. 1999;37:529–547. [PubMed] [Google Scholar]
- Belot MP, Abdennebi-Najar L, Gaudin F, Lieberherr M, Godot V, Taieb J, Emilie D, Machelon V. Progesterone reduces the migration of mast cells toward the chemokine stromal cell-derived factor-1/CXCL12 with an accompanying decrease in CXCR4 receptors. Am J Physiol Endocrinol Metab. 2007;292:E1410–1417. doi: 10.1152/ajpendo.00286.2006. [DOI] [PubMed] [Google Scholar]
- Belvisi MG, Ward JK, Mitchell JA, Barnes PJ. Nitric oxide as a neurotransmitter in human airways. Arch Int Pharmacodyn Ther. 1995;329:97–110. [PubMed] [Google Scholar]
- Bennett NC, Gardiner RA, Hooper JD, Johnson DW, Gobe GC. Molecular cell biology of androgen receptor signalling. Int J Biochem Cell Biol. 2010;42:813–827. doi: 10.1016/j.biocel.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Beyer C, Kuppers E, Karolczak M, Trotter A. Ontogenetic expression of estrogen and progesterone receptors in the mouse lung. Biol Neonate. 2003;84:59–63. doi: 10.1159/000071445. [DOI] [PubMed] [Google Scholar]
- Bigos KL, Pollock BG, Stankevich BA, Bies RR. Sex differences in the pharmacokinetics and pharmacodynamics of antidepressants: an updated review. Gend Med. 2009;6:522–543. doi: 10.1016/j.genm.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women: effects of gender. American journal of respiratory and critical care medicine. 2001;163:608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- Bjornson CL, Mitchell I. Gender differences in asthma in childhood and adolescence. J Gend Specif Med. 2000;3:57–61. [PubMed] [Google Scholar]
- Boezen HM, Jansen DF, Postma DS. Sex and gender differences in lung development and their clinical significance. Clin Chest Med. 2004;25:237–245. doi: 10.1016/j.ccm.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Bonner JR. The epidemiology and natural history of asthma. Clin Chest Med. 1984;5:557–565. [PubMed] [Google Scholar]
- Bordallo J, de Boto MJ, Meana C, Velasco L, Bordallo C, Suarez L, Cantabrana B, Sanchez M. Modulatory role of endogenous androgens on airway smooth muscle tone in isolated guinea-pig and bovine trachea; involvement of beta2-adrenoceptors, the polyamine system and external calcium. Eur J Pharmacol. 2008;601:154–162. doi: 10.1016/j.ejphar.2008.10.039. [DOI] [PubMed] [Google Scholar]
- Brandenberger AW, Tee MK, Lee JY, Chao V, Jaffe RB. Tissue distribution of estrogen receptors alpha (ER-alpha) and beta (ER-beta) mRNA in the midgestational human fetus. J Clin Endocrinol Metab. 1997;82:3509–3512. doi: 10.1210/jcem.82.10.4400. [DOI] [PubMed] [Google Scholar]
- Burns K, Korach K. Estrogen receptors and human disease: an update. Archives of Toxicology. 2012;86:1491–1504. doi: 10.1007/s00204-012-0868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canguven O, Albayrak S. Do low testosterone levels contribute to the pathogenesis of asthma? Med Hypotheses. 2011;76:585–588. doi: 10.1016/j.mehy.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Fischer A. Neural regulation of airway smooth muscle tone. Respir Physiol. 2001;125:113–127. doi: 10.1016/s0034-5687(00)00208-5. [DOI] [PubMed] [Google Scholar]
- Caracta CF. Gender differences in pulmonary disease. Mt Sinai J Med. 2003;70:215–224. [PubMed] [Google Scholar]
- Card JW, Carey MA, Bradbury JA, DeGraff LM, Morgan DL, Moorman MP, Flake GP, Zeldin DC. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J Immunol. 2006;177:621–630. doi: 10.4049/jimmunol.177.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MA, Card JW, Bradbury JA, Moorman MP, Haykal-Coates N, Gavett SH, Graves JP, Walker VR, Flake GP, Voltz JW, Zhu D, Jacobs ER, Dakhama A, Larsen GL, Loader JE, Gelfand EW, Germolec DR, Korach KS, Zeldin DC. Spontaneous airway hyperresponsiveness in estrogen receptor-alpha-deficient mice. Am J Respir Crit Care Med. 2007a;175:126–135. doi: 10.1164/rccm.200509-1493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MA, Card JW, Voltz JW, Arbes SJ, Jr., Germolec DR, Korach KS, Zeldin DC. It's all about sex: gender, lung development and lung disease. Trends Endocrinol Metab. 2007b;18:308–313. doi: 10.1016/j.tem.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MA, Card JW, Voltz JW, Germolec DR, Korach KS, Zeldin DC. The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am J Physiol Lung Cell Mol Physiol. 2007c;293:L272–278. doi: 10.1152/ajplung.00174.2007. [DOI] [PubMed] [Google Scholar]
- Catley MC, Birrell MA, Hardaker EL, de Alba J, Farrow S, Haj-Yahia S, Belvisi MG. Estrogen receptor beta: expression profile and possible anti-inflammatory role in disease. J Pharmacol Exp Therap. 2008;326:83–88. doi: 10.1124/jpet.108.136275. [DOI] [PubMed] [Google Scholar]
- Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J, Pacifici R. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest. 2000;106:1229–1237. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Saltzman A, Yeh S, Young W, Keller E, Lee HJ, Wang C, Mizokami A. Androgen receptor: an overview. Critical reviews in eukaryotic gene expression. 1995;5:97–125. doi: 10.1615/critreveukargeneexpr.v5.i2.10. [DOI] [PubMed] [Google Scholar]
- Chhabra SK. Premenstrual asthma. Indian J Chest Dis Allied Sci. 2005;47:109–116. [PubMed] [Google Scholar]
- Coakley RD, Sun H, Clunes LA, Rasmussen JE, Stackhouse JR, Okada SF, Fricks I, Young SL, Tarran R. 17beta-Estradiol inhibits Ca2+-dependent homeostasis of airway surface liquid volume in human cystic fibrosis airway epithelia. J Clin Invest. 2008;118:4025–4035. doi: 10.1172/JCI33893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Estrogen Receptor Null Mice: What Have We Learned and Where Will They Lead Us? Endocrine reviews. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- Cummings SR, Browner WS, Bauer D, Stone K, Ensrud K, Jamal S, Ettinger B. Endogenous hormones and the risk of hip and vertebral fractures among older women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1998;339:733–738. doi: 10.1056/NEJM199809103391104. [DOI] [PubMed] [Google Scholar]
- Da Silva JA. Sex hormones and glucocorticoids: interactions with the immune system. Ann N Y Acad Sci. 1999;876:102–117. doi: 10.1111/j.1749-6632.1999.tb07628.x. discussion 117-108. [DOI] [PubMed] [Google Scholar]
- da Silva SB, de Sousa Ramalho Viana E, de Sousa MB. Changes in peak expiratory flow and respiratory strength during the menstrual cycle. Respiratory physiology & neurobiology. 2006;150:211–219. doi: 10.1016/j.resp.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Dahlman-Wright K, Cavailles V, Fuqua SA, Jordan VC, Katzenellenbogen JA, Korach KS, Maggi A, Muramatsu M, Parker MG, Gustafsson J. International union of pharmacology. LXIV. Estrogen receptors. Pharmacol Rev. 2006;58:773–781. doi: 10.1124/pr.58.4.8. [DOI] [PubMed] [Google Scholar]
- Dashtaki R, Whorton AR, Murphy TM, Chitano P, Reed W, Kennedy TP. Dehydroepiandrosterone and analogs inhibit DNA binding of AP-1 and airway smooth muscle proliferation. J Pharmacol Exp Ther. 1998;285:876–883. [PubMed] [Google Scholar]
- Degano B, Mourlanette P, Valmary S, Pontier S, Prevost MC, Escamilla R. Differential effects of low and high-dose estradiol on airway reactivity in ovariectomized rats. Respir Physiol Neurobiol. 2003;138:265–274. doi: 10.1016/j.resp.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Degano B, Prevost MC, Berger P, Molimard M, Pontier S, Rami J, Escamilla R. Estradiol decreases the acetylcholine-elicited airway reactivity in ovariectomized rats through an increase in epithelial acetylcholinesterase activity. Am J Respir Crit Care Med. 2001;164:1849–1854. doi: 10.1164/ajrccm.164.10.2102009. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Skatrud JB. A sleep-induced apneic threshold and its consequences. The American review of respiratory disease. 1986;133:1163–1170. doi: 10.1164/arrd.1986.133.6.1163. [DOI] [PubMed] [Google Scholar]
- Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116:561–570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitropoulou C, Drakopanagiotakis F, Chatterjee A, Snead C, Catravas JD. Estrogen replacement therapy prevents airway dysfunction in a murine model of allergen-induced asthma. Lung. 2009;187:116–127. doi: 10.1007/s00408-008-9129-z. [DOI] [PubMed] [Google Scholar]
- Dimitropoulou C, White RE, Ownby DR, Catravas JD. Estrogen reduces carbachol-induced constriction of asthmatic airways by stimulating large-conductance voltage and calcium-dependent potassium channels. Am J Respir Cell Mol Biol. 2005;32:239–247. doi: 10.1165/rcmb.2004-0331OC. [DOI] [PubMed] [Google Scholar]
- Dodds L, Armson BA, Alexander S. Use of asthma drugs is less among women pregnant with boys rather than girls. BMJ. 1999;318:1011. doi: 10.1136/bmj.318.7189.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dratva J, Schindler C, Curjuric I, Stolz D, Macsali F, Gomez FR, Zemp E. Perimenstrual increase in bronchial hyperreactivity in premenopausal women: results from the population-based SAPALDIA 2 cohort. J Allergy Clin Immunol. 2010;125:823–829. doi: 10.1016/j.jaci.2009.12.938. [DOI] [PubMed] [Google Scholar]
- Driver HS, McLean H, Kumar DV, Farr N, Day AG, Fitzpatrick MF. The influence of the menstrual cycle on upper airway resistance and breathing during sleep. Sleep. 2005;28:449–456. doi: 10.1093/sleep/28.4.449. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Imthurn B, Rosselli M, Jackson EK, Keller PJ. Phytoestrogens inhibit growth and MAP kinase activity in human aortic smooth muscle cells. Hypertension. 1999;33:177–182. doi: 10.1161/01.hyp.33.1.177. [DOI] [PubMed] [Google Scholar]
- Ebina M, Takahashi T, Chiba T, Motomiya M. Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial asthma. A 3-D morphometric study. Am Rev Respir Dis. 1993;148:720–726. doi: 10.1164/ajrccm/148.3.720. [DOI] [PubMed] [Google Scholar]
- Edwards DP. Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol. 2005;67:335–376. doi: 10.1146/annurev.physiol.67.040403.120151. [DOI] [PubMed] [Google Scholar]
- Ellegard E, Karlsson G. Nasal congestion during the menstrual cycle. Clin Otolaryngol Allied Sci. 1994;19:400–403. doi: 10.1111/j.1365-2273.1994.tb01256.x. [DOI] [PubMed] [Google Scholar]
- Elmlinger MW, Kuhnel W, Ranke MB. Reference ranges for serum concentrations of lutropin (LH), follitropin (FSH), estradiol (E2), prolactin, progesterone, sex hormone-binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEAS), cortisol and ferritin in neonates, children and young adults. Clin Chem Lab Med. 2002;40:1151–1160. doi: 10.1515/CCLM.2002.202. [DOI] [PubMed] [Google Scholar]
- Farha S, Asosingh K, Laskowski D, Hammel J, Dweik RA, Wiedemann HP, Erzurum SC. Effects of the menstrual cycle on lung function variables in women with asthma. Am J Respir Crit Care Med. 2009;180:304–310. doi: 10.1164/rccm.200904-0497OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frackelton AR, Jr., Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- Fleisher B, Kulovich MV, Hallman M, Gluck L. Lung profile: sex differences in normal pregnancy. Obstet Gynecol. 1985;66:327–330. [PubMed] [Google Scholar]
- Flores-Delgado G, Bringas P, Buckley S, Anderson KD, Warburton D. Nongenomic estrogen action in human lung myofibroblasts. Biochem Biophys Res Commun. 2001;283:661–667. doi: 10.1006/bbrc.2001.4827. [DOI] [PubMed] [Google Scholar]
- Folkerts G, Nijkamp FP. Nitric oxide in asthma therapy. Curr Pharm Des. 2006;12:3221–3232. doi: 10.2174/138161206778194141. [DOI] [PubMed] [Google Scholar]
- Forbes L, Jarvis D, Burney P. Do hormonal contraceptives influence asthma severity? Eur Respir J. 1999;14:1028–1033. doi: 10.1183/09031936.99.14510289. [DOI] [PubMed] [Google Scholar]
- Foster PS, Goldie RG, Paterson JW. Effect of steroids on beta-adrenoceptor-mediated relaxation of pig bronchus. Br J Pharmacol. 1983;78:441–445. doi: 10.1111/j.1476-5381.1983.tb09409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieri M. Inflammatory issues in allergic rhinitis and asthma. Allergy Asthma Proc. 2005;26:163–169. [PubMed] [Google Scholar]
- Gellersen B, Fernandes MS, Brosens JJ. Non-genomic progesterone actions in female reproduction. Hum Reprod Update. 2009;15:119–138. doi: 10.1093/humupd/dmn044. [DOI] [PubMed] [Google Scholar]
- Gharaee-Kermani M, Hatano K, Nozaki Y, Phan SH. Gender-based differences in bleomycin-induced pulmonary fibrosis. Am J Pathol. 2005;166:1593–1606. doi: 10.1016/S0002-9440(10)62470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghayee HK, Auchus RJ. Basic concepts and recent developments in human steroid hormone biosynthesis. Reviews in endocrine & metabolic disorders. 2007;8:289–300. doi: 10.1007/s11154-007-9052-2. [DOI] [PubMed] [Google Scholar]
- Giangrande PH, McDonnell DP. The A and B isoforms of the human progesterone receptor: two functionally different transcription factors encoded by a single gene. Recent Prog Horm Res. 1999;54:291–313. discussion 313-294. [PubMed] [Google Scholar]
- Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev. 2010;62:155–198. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliver SC. Sex steroids as inflammatory regulators. J Steroid Biochem Mol Biol. 2010;120:105–115. doi: 10.1016/j.jsbmb.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Gillum R. Frequency of attendance at religious services and cigarette smoking in American women and men: the Third National Health and Nutrition Examination Survey. Prev Med. 2005;41:607–613. doi: 10.1016/j.ypmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Giron-Gonzalez JA, Moral FJ, Elvira J, Garcia-Gil D, Guerrero F, Gavilan I, Escobar L. Consistent production of a higher TH1:TH2 cytokine ratio by stimulated T cells in men compared with women. Eur J Endocrinol. 2000;143:31–36. doi: 10.1530/eje.0.1430031. [DOI] [PubMed] [Google Scholar]
- Gold DR, Wang X, Wypij D, Speizer FE, Ware JH, Dockery DW. Effects of cigarette smoking on lung function in adolescent boys and girls. N Engl J Med. 1996;335:931–937. doi: 10.1056/NEJM199609263351304. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Arenas A, Agramonte-Hevia J. Sex steroid hormone effects in normal and pathologic conditions in lung physiology. Mini Rev Med Chem. 2012;12:1055–1062. doi: 10.2174/138955712802762194. [DOI] [PubMed] [Google Scholar]
- Grazzini E, Guillon G, Mouillac B, Zingg HH. Inhibition of oxytocin receptor function by direct binding of progesterone. Nature. 1998;392:509–512. doi: 10.1038/33176. [DOI] [PubMed] [Google Scholar]
- Greenhill C. Metabolism: Sex differences in fatty liver disease. Nat Rev Endocrinol. 2011 doi: 10.1038/nrendo.2011.65. [DOI] [PubMed] [Google Scholar]
- Gustafsson J-Å. What pharmacologists can learn from recent advances in estrogen signalling. Trends Pharmacol Sci. 2003;24:479–485. doi: 10.1016/S0165-6147(03)00229-3. [DOI] [PubMed] [Google Scholar]
- Haeggstrom A, Ostberg B, Stjerna P, Graf P, Hallen H. Nasal mucosal swelling and reactivity during a menstrual cycle. ORL J Otorhinolaryngol Relat Spec. 2000;62:39–42. doi: 10.1159/000027713. [DOI] [PubMed] [Google Scholar]
- Haggerty C, Ness R, Kelsey S, Waterer G. The impact of estrogen and progesterone on asthma. Ann Allergy Asthma Immunol. 2003;90:284–291. doi: 10.1016/S1081-1206(10)61794-2. [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinol. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- Hamad AM, Clayton A, Islam B, Knox AJ. Guanylyl cyclases, nitric oxide, natriuretic peptides, and airway smooth muscle function. Am J Physiol Lung Cell Mol Physiol. 2003;285:L973–983. doi: 10.1152/ajplung.00033.2003. [DOI] [PubMed] [Google Scholar]
- Hamano N, Terada N, Maesako K, Ikeda T, Fukuda S, Wakita J, Yamashita T, Konno A. Expression of histamine receptors in nasal epithelial cells and endothelial cells--the effects of sex hormones. Int Arch Allergy Immunol. 1998a;115:220–227. doi: 10.1159/000023904. [DOI] [PubMed] [Google Scholar]
- Hamano N, Terada N, Maesako K, Numata T, Konno A. Effect of sex hormones on eosinophilic inflammation in nasal mucosa. Allergy Asthma Proc. 1998b;19:263–269. doi: 10.2500/108854198778557773. [DOI] [PubMed] [Google Scholar]
- Han MK, Murray S, Fell CD, Flaherty KR, Toews GB, Myers J, Colby TV, Travis WD, Kazerooni EA, Gross BH, Martinez FJ. Sex differences in physiological progression of idiopathic pulmonary fibrosis. Eur Respir J. 2008;31:1183–1188. doi: 10.1183/09031936.00165207. [DOI] [PubMed] [Google Scholar]
- Hardy DB, Janowski BA, Corey DR, Mendelson CR. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression. Mol Endocrinol. 2006;20:2724–2733. doi: 10.1210/me.2006-0112. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Adachi Y, Hasegawa K, Morimoto M. Less sensitivity for late airway inflammation in males than females in BALB/c mice. Scand J Immunol. 2003;57:562–567. doi: 10.1046/j.1365-3083.2003.01269.x. [DOI] [PubMed] [Google Scholar]
- Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- Hellings PW, Vandekerckhove P, Claeys R, Billen J, Kasran A, Ceuppens JL. Progesterone increases airway eosinophilia and hyper-responsiveness in a murine model of allergic asthma. Clin Exp Allergy. 2003;33:1457–1463. doi: 10.1046/j.1365-2222.2003.01743.x. [DOI] [PubMed] [Google Scholar]
- Hines M. Sex-related variation in human behavior and the brain. Trends Cogn Sci. 2010;14:448–456. doi: 10.1016/j.tics.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota S, Helli PB, Catalli A, Chew A, Janssen LJ. Airway smooth muscle excitation-contraction coupling and airway hyperresponsiveness. Can J Physiol Pharmacol. 2005;83:725–732. doi: 10.1139/y05-070. [DOI] [PubMed] [Google Scholar]
- Hisamoto K, Bender JR. Vascular cell signaling by membrane estrogen receptors. Steroids. 2005;70:382–387. doi: 10.1016/j.steroids.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Hoffstein V. Relationship between lung volume, maximal expiratory flow, forced expiratory volume in one second, and tracheal area in normal men and women. Am Rev Respir Dis. 1986;134:956–961. doi: 10.1164/arrd.1986.134.5.956. [DOI] [PubMed] [Google Scholar]
- Holgate ST. A brief history of asthma and its mechanisms to modern concepts of disease pathogenesis. Allergy, asthma & immunology research. 2010;2:165–171. doi: 10.4168/aair.2010.2.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SA, Kupperman J, Newell MK. Hormonal regulation of CD4(+) T-cell responses in coxsackievirus B3-induced myocarditis in mice. J Virol. 1999;73:4689–4695. doi: 10.1128/jvi.73.6.4689-4695.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SA, Pfaeffle B. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J virol. 1994;68:5126–5132. doi: 10.1128/jvi.68.8.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck B, Steck T, Habersack M, Dietl J, Kammerer U. Pregnancy associated hormones modulate the cytokine production but not the phenotype of PBMC-derived human dendritic cells. Eur J Obstet Gynecol Reprod Biol. 2005;122:85–94. doi: 10.1016/j.ejogrb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Hughes RA, Harris T, Altmann E, McAllister D, Vlahos R, Robertson A, Cushman M, Wang Z, Stewart AG. 2-Methoxyestradiol and analogs as novel antiproliferative agents: analysis of three-dimensional quantitative structure-activity relationships for DNA synthesis inhibition and estrogen receptor binding. Mol Pharmacol. 2002;61:1053–1069. doi: 10.1124/mol.61.5.1053. [DOI] [PubMed] [Google Scholar]
- Hughes TL, Jacobson KM. Sexual orientation and women's smoking. Curr Wom Health Rep. 2003;3:254–261. [PubMed] [Google Scholar]
- Hughes TL, Johnson TP, Matthews AK. Sexual orientation and smoking: results from a multisite women's health study. Substance use & misuse. 2008;43:1218–1239. doi: 10.1080/10826080801914170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi H, Suzuki T, Suzuki S, Niikawa H, Lu L, Miki Y, Moriya T, Hayashi S, Handa M, Kondo T, Sasano H. Progesterone receptor in non-small cell lung cancer--a potent prognostic factor and possible target for endocrine therapy. Cancer Res. 2005;65:6450–6458. doi: 10.1158/0008-5472.CAN-04-3087. [DOI] [PubMed] [Google Scholar]
- Ivanova MM, Mazhawidza W, Dougherty SM, Klinge CM. Sex Differences in Estrogen Receptor Subcellular Location and Activity in Lung Adenocarcinoma Cells. Am J Respir Cell Mol Biol. 2010;42:320–330. doi: 10.1165/rcmb.2009-0059OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakkola MS, Jaakkola JJK. Assessment of exposure to environmental tobacco smoke. Eur Respir J. 1997;10:2384–2397. doi: 10.1183/09031936.97.10102384. [DOI] [PubMed] [Google Scholar]
- Jain R, Ray JM, Pan J.-h., Brody SL. Sex Hormone–Dependent Regulation of Cilia Beat Frequency in Airway Epithelium. Am J Respir Cell Mol Biol. 2012;46:446–453. doi: 10.1165/rcmb.2011-0107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AL, Elliot JG, Jones RL, Carroll ML, Mauad T, Bai TR, Abramson MJ, McKay KO, Green FH. Airway smooth muscle hypertrophy and hyperplasia in asthma. American journal of respiratory and critical care medicine. 2012;185:1058–1064. doi: 10.1164/rccm.201110-1849OC. [DOI] [PubMed] [Google Scholar]
- Janicki SC, Schupf N. Hormonal influences on cognition and risk for Alzheimer's disease. Curr Neurol Neurosci Rep. 2010;10:359–366. doi: 10.1007/s11910-010-0122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatakanon A, Lim S, Kharitonov SA, Chung KF, Barnes PJ. Correlation between exhaled nitric oxide, sputum eosinophils, and methacholine responsiveness in patients with mild asthma. Thorax. 1998;53:91–95. doi: 10.1136/thx.53.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen-Jarolim E, Untersmayr E. Gender-medicine aspects in allergology. Allergy. 2008;63:610–615. doi: 10.1111/j.1398-9995.2008.01645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S, Zhang X, He DZZ, Segal M, Berro A, Gerson T, Wang Z, Casale TB. Expression and Function of a Novel Variant of Estrogen Receptor–α36 in Murine Airways. Am J Respir Cell Mol Biol. 2011;45:1084–1089. doi: 10.1165/rcmb.2010-0268OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joos GF. The role of neuroeffector mechanisms in the pathogenesis of asthma. Curr Allergy Asthma Rep. 2001;1:134–143. doi: 10.1007/s11882-001-0081-8. [DOI] [PubMed] [Google Scholar]
- Joos GF, Germonpre PR, Pauwels RA. Neural mechanisms in asthma. Clin Exp Allergy. 2000;30(Suppl 1):60–65. doi: 10.1046/j.1365-2222.2000.00100.x. [DOI] [PubMed] [Google Scholar]
- Joubert P, Hamid Q. Role of airway smooth muscle in airway remodeling. J Allergy Clin Immunol. 2005;116:713–716. doi: 10.1016/j.jaci.2005.05.042. [DOI] [PubMed] [Google Scholar]
- Juniper EF, Daniel EE, Roberts RS, Kline PA, Hargreave FE, Newhouse MT. Improvement in airway responsiveness and asthma severity during pregnancy. A prospective study. Am Rev Respir Dis. 1989;140:924–931. doi: 10.1164/ajrccm/140.4.924. [DOI] [PubMed] [Google Scholar]
- Kamil F, Pinzon I, Foreman MG. Sex and race factors in early-onset COPD. Curr Opin Pulm Med. 2013;19:140–144. doi: 10.1097/MCP.0b013e32835d903b. 110.1097/MCP.1090b1013e32835d32903b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapsimalis F, Kryger MH. Gender and obstructive sleep apnea syndrome, part 2: mechanisms. Sleep. 2002;25:499–506. [PubMed] [Google Scholar]
- Katayama ML, Federico MH, Brentani RR, Brentani MM. Eosinophil accumulation in rat uterus following estradiol administration is modulated by laminin and its integrin receptors. Cell Adhes Commun. 1998;5:409–424. doi: 10.3109/15419069809010785. [DOI] [PubMed] [Google Scholar]
- Kim KH, Bender JR. Rapid, estrogen receptor-mediated signaling: why is the endothelium so special? Sci STKE. 2005;2005:pe28. doi: 10.1126/stke.2882005pe28. [DOI] [PubMed] [Google Scholar]
- Kim KH, Moriarty K, Bender JR. Vascular cell signaling by membrane estrogen receptors. Steroids. 2008;73:864–869. doi: 10.1016/j.steroids.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H, Tatsumi K, Kunitomo F, Okita S, Tojima H, Kouchiyama S, Masuyama S, Shinozaki T, Mikami M, Watanabe S, et al. Obese patients with sleep apnea syndrome treated by progesterone. The Tohoku journal of experimental medicine. 1988;156(Suppl):151–157. doi: 10.1620/tjem.156.suppl_151. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Suzuki T, Kaneko C, Darnel AD, Akahira J, Ebina M, Nukiwa T, Sasano H. Expression of androgen receptor and 5alpha-reductase types 1 and 2 in early gestation fetal lung: a possible correlation with branching morphogenesis. Clin Sci (Lond) 2003;105:709–713. doi: 10.1042/CS20030236. [DOI] [PubMed] [Google Scholar]
- Kirsch EA, Yuhanna IS, Chen Z, German Z, Sherman TS, Shaul PW. Estrogen acutely stimulates endothelial nitric oxide synthase in H441 human airway epithelial cells. Am J Respir Cell Mol Biol. 1999;20:658–666. doi: 10.1165/ajrcmb.20.4.3241. [DOI] [PubMed] [Google Scholar]
- Klebanoff SJ. Estrogen binding by leukocytes during phagocytosis. J Exp Med. 1977;145:983–998. doi: 10.1084/jem.145.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DA, Holgate ST. The airway epithelium: Structural and functional properties in health and disease. Respirology. 2003;8:432–446. doi: 10.1046/j.1440-1843.2003.00493.x. [DOI] [PubMed] [Google Scholar]
- Kohler M, Sandberg A, Kjellqvist S, Thomas A, Karimi R, Nyrén S, Eklund A, Thevis M, Sköld CM, Wheelock ÅM. Gender differences in the bronchoalveolar lavage cell proteome of patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2013;131:743–751. e749. doi: 10.1016/j.jaci.2012.09.024. [DOI] [PubMed] [Google Scholar]
- Kondo H, Kasuga H, Noumura T. Effects of various steroids on in vitro lifespan and cell growth of human fetal lung fibroblasts (WI-38). Mech Ageing Dev. 1983;21:335–344. doi: 10.1016/0047-6374(83)90050-7. [DOI] [PubMed] [Google Scholar]
- Konhilas JP. What we know and do not know about sex and cardiac disease. J Biomed Biotechnol. 2010;2010:562051. doi: 10.1155/2010/562051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouloumenta V, Hatziefthimiou A, Paraskeva E, Gourgoulianis K, Molyvdas PA. Non-genomic effect of testosterone on airway smooth muscle. Br J Pharmacol. 2006;149:1083–1091. doi: 10.1038/sj.bjp.0706936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- Kwon HL, Belanger K, Bracken MB. Asthma Prevalence among Pregnant and Childbearing-aged Women in the United States: Estimates from National Health Surveys. Ann Epidemiol. 2003;13:317–324. doi: 10.1016/s1047-2797(03)00008-5. [DOI] [PubMed] [Google Scholar]
- Labrie F. Extragonadal synthesis of sex steroids: intracrinology. Ann Endocrinol (Paris) 2003;64:95–107. [PubMed] [Google Scholar]
- Labrie F, Simard J, Luu-The V, Trudel C, Martel C, Labrie C, Zhao HF, Rheaume E, Couet J, Breton N. Expression of 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase (3 beta-HSD) and 17 beta-hydroxysteroid dehydrogenase (17 beta-HSD) in adipose tissue. Int J Obes. 1991;15(Suppl 2):91–99. [PubMed] [Google Scholar]
- Lahm T, Crisostomo PR, Markel TA, Wang M, Wang Y, Tan J, Meldrum DR. Selective estrogen receptor-alpha and estrogen receptor-beta agonists rapidly decrease pulmonary artery vasoconstriction by a nitric oxide-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1486–1493. doi: 10.1152/ajpregu.90667.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert KC, Curran EM, Judy BM, Milligan GN, Lubahn DB, Estes DM. Estrogen receptor alpha (ERalpha) deficiency in macrophages results in increased stimulation of CD4+ T cells while 17beta-estradiol acts through ERalpha to increase IL-4 and GATA-3 expression in CD4+ T cells independent of antigen presentation. J Immunol. 2005;175:5716–5723. doi: 10.4049/jimmunol.175.9.5716. [DOI] [PubMed] [Google Scholar]
- Lammers JW, Barnes PJ, Chung KF. Nonadrenergic, noncholinergic airway inhibitory nerves. Eur Respir J. 1992;5:239–246. [PubMed] [Google Scholar]
- Langenbach SY, Wheaton BJ, Fernandes DJ, Jones C, Sutherland TE, Wraith BC, Harris T, Schuliga MJ, McLean C, Stewart AG. Resistance of fibrogenic responses to glucocorticoid and 2-methoxyestradiol in bleomycin-induced lung fibrosis in mice. Can J Physiol Pharmacol. 2007;85:727–738. doi: 10.1139/Y07-065. [DOI] [PubMed] [Google Scholar]
- Lekgabe ED, Royce SG, Hewitson TD, Tang ML, Zhao C, Moore XL, Tregear GW, Bathgate RA, Du XJ, Samuel CS. The effects of relaxin and estrogen deficiency on collagen deposition and hypertrophy of nonreproductive organs. Endocrinol. 2006;147:5575–5583. doi: 10.1210/en.2006-0533. [DOI] [PubMed] [Google Scholar]
- Leuzzi C, Sangiorgi GM, Modena MG. Gender-specific aspects in the clinical presentation of cardiovascular disease. Fundam Clin Pharmacol. 2010;24:711–717. doi: 10.1111/j.1472-8206.2010.00873.x. [DOI] [PubMed] [Google Scholar]
- Levin ER. Cell localization, physiology, and nongenomic actions of estrogen receptors. J Appl Physiol. 2001;91:1860–1867. doi: 10.1152/jappl.2001.91.4.1860. [DOI] [PubMed] [Google Scholar]
- Lindberg MK, Moverare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, Ohlsson C. Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a “ying yang” relationship between ERalpha and ERbeta in mice. Mol Endocrinol. 2003;17:203–208. doi: 10.1210/me.2002-0206. [DOI] [PubMed] [Google Scholar]
- Linden A. NANC neural control of airway smooth muscle tone. Gen Pharmacol. 1996;27:1109–1121. doi: 10.1016/0306-3623(95)02142-6. [DOI] [PubMed] [Google Scholar]
- Lippe BM, LaFranchi SH, Lavin N, Parlow A, Coyotupa J, Kaplan SA. Serum 17-alpha-hydroxyprogesterone, progesterone, estradiol, and testosterone in the diagnosis and management of congenital adrenal hyperplasia. J Pediatr. 1974;85:782–787. doi: 10.1016/s0022-3476(74)80340-9. [DOI] [PubMed] [Google Scholar]
- Lisspers K, Ställberg B, Janson C, Johansson G, Svärdsudd K. Sex-differences in quality of life and asthma control in Swedish asthma patients. J Asthma. 2013;50:1090–1095. doi: 10.3109/02770903.2013.834502. [DOI] [PubMed] [Google Scholar]
- Liu M-M, Albanese C, Anderson CM, Hilty K, Webb P, Uht RM, Price RH, Pestell RG, Kushner PJ. Opposing Action of Estrogen Receptors α and β on Cyclin D1 Gene Expression. J Biol Chem. 2002;277:24353–24360. doi: 10.1074/jbc.M201829200. [DOI] [PubMed] [Google Scholar]
- Luu-The V, Labrie F. The intracrine sex steroid biosynthesis pathways. Prog Brain Res. 2010;181:177–192. doi: 10.1016/S0079-6123(08)81010-2. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr., Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes & development. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Mackay TW, Hulks G, Douglas NJ. Non-adrenergic, non-cholinergic function in the human airway. Respir Med. 1998;92:461–466. doi: 10.1016/s0954-6111(98)90292-x. [DOI] [PubMed] [Google Scholar]
- Maggiolini M, Bonofiglio D, Marsico S, Panno ML, Cenni B, Picard D, Ando S. Estrogen receptor alpha mediates the proliferative but not the cytotoxic dose-dependent effects of two major phytoestrogens on human breast cancer cells. Mol Pharmacol. 2001;60:595–602. [PubMed] [Google Scholar]
- Mandhane PJ, Hanna SE, Inman MD, Duncan JM, Greene JM, Wang HY, Sears MR. Changes in exhaled nitric oxide related to estrogen and progesterone during the menstrual cycle. Chest. 2009;136:1301–1307. doi: 10.1378/chest.09-0604. [DOI] [PubMed] [Google Scholar]
- Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance--United States, 1971-2000. MMWR Surveill Summ. 2002;51:1–16. [PubMed] [Google Scholar]
- Manson JE. Prenatal exposure to sex steroid hormones and behavioral/cognitive outcomes. Metabol. 2008;57(Suppl 2):S16–21. doi: 10.1016/j.metabol.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Marquez-Garban DC, Chen HW, Goodglick L, Fishbein MC, Pietras RJ. Targeting aromatase and estrogen signaling in human non-small cell lung cancer. Ann N Y Acad Sci. 2009;1155:194–205. doi: 10.1111/j.1749-6632.2009.04116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JT. Sexual dimorphism in immune function: the role of prenatal exposure to androgens and estrogens. Eur J Pharmacol. 2000;405:251–261. doi: 10.1016/s0014-2999(00)00557-4. [DOI] [PubMed] [Google Scholar]
- Martin YN, Pabelick CM. Sex differences in the pulmonary circulation: implications for pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2014;306:H1253–1264. doi: 10.1152/ajpheart.00857.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaro D, Massaro GD. Estrogen receptor regulation of pulmonary alveolar dimensions: alveolar sexual dimorphism in mice. Am J Physiol Lung Cell Mol Physiol. 2006;290:L866–870. doi: 10.1152/ajplung.00396.2005. [DOI] [PubMed] [Google Scholar]
- Massaro GD, Mortola JP, Massaro D. Estrogen modulates the dimensions of the lung's gas-exchange surface area and alveoli in female rats. Am J Physiol. 1996;270:L110–114. doi: 10.1152/ajplung.1996.270.1.L110. [DOI] [PubMed] [Google Scholar]
- Matsubara S, Swasey CH, Loader JE, Dakhama A, Joetham A, Ohnishi H, Balhorn A, Miyahara N, Takeda K, Gelfand EW. Estrogen determines sex differences in airway responsiveness after allergen exposure. Am J Respir Cell Mol Biol. 2008;38:501–508. doi: 10.1165/rcmb.2007-0298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;1204(Suppl):E38–59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocrine reviews. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- McKenna NJ, Lanz RB, O'Malley BW. Nuclear Receptor Coregulators: Cellular and Molecular Biology. Endocrine reviews. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- McKenna NJ, O'Malley BW. Minireview: nuclear receptor coactivators--an update. Endocrinol. 2002;143:2461–2465. doi: 10.1210/endo.143.7.8892. [DOI] [PubMed] [Google Scholar]
- Melgert BN, Oriss TB, Qi Z, Dixon-McCarthy B, Geerlings M, Hylkema MN, Ray A. Macrophages: regulators of sex differences in asthma? Am J Respir Cell Mol Biol. 2010;42:595–603. doi: 10.1165/rcmb.2009-0016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melgert BN, Ray A, Hylkema MN, Timens W, Postma DS. Are there reasons why adult asthma is more common in females? Curr Allergy Asthma Rep. 2007;7:143–150. doi: 10.1007/s11882-007-0012-4. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD. Neurosteroids: biochemistry and clinical significance. Trends in endocrinology and metabolism: TEM. 2002;13:35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- Meltzer EB, Noble PW. Idiopathic pulmonary fibrosis. Orphanet J Rare Dis. 2008;3:8. doi: 10.1186/1750-1172-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini MS, Abbondanza C, Auricchio F. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. Embo J. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. Embo J. 1998;17:2008–2018. doi: 10.1093/emboj/17.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkonen L, Pihlajamaa P, Sahu B, Zhang FP, Janne OA. Androgen receptor and androgen-dependent gene expression in lung. Mol Cell Endocrinol. 2010;317:14–24. doi: 10.1016/j.mce.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Miller VM. Sex-based differences in vascular function. Womens Health (Lond Engl) 2010;6:737–752. doi: 10.2217/whe.10.53. [DOI] [PubMed] [Google Scholar]
- Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60:210–241. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaura H, Iwata M. Direct and indirect inhibition of Th1 development by progesterone and glucocorticoids. J Immunol. 2002;168:1087–1094. doi: 10.4049/jimmunol.168.3.1087. [DOI] [PubMed] [Google Scholar]
- Montaño LM, Espinoza J, Flores-Soto E, Chávez J, Perusquía M. Androgens are bronchoactive drugs that act by relaxing airway smooth muscle and preventing bronchospasm. J Endocrinol. 2014;222:1–13. doi: 10.1530/JOE-14-0074. [DOI] [PubMed] [Google Scholar]
- Mooradian AD, Morley JE, Korenman SG. Biological Actions of Androgens. Endocrine reviews. 1987;8:1–28. doi: 10.1210/edrv-8-1-1. [DOI] [PubMed] [Google Scholar]
- Morani A, Barros RP, Imamov O, Hultenby K, Arner A, Warner M, Gustafsson JA. Lung dysfunction causes systemic hypoxia in estrogen receptor beta knockout (ERbeta−/−) mice. Proc Natl Acad Sci U S A. 2006;103:7165–7169. doi: 10.1073/pnas.0602194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer H, Wright RP, Collip JB. The Effect of the Administration of OEstrogenic Hormones on the Nasal Mucosa of the Monkey (Macaca Mulatta). Can Med Assoc J. 1936;35:503–513. [PMC free article] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Conneely OM. Reproductive tissue selective actions of progesterone receptors. Reproduction. 2004;128:139–146. doi: 10.1530/rep.1.00189. [DOI] [PubMed] [Google Scholar]
- Myers JR, Sherman CB. Should supplemental estrogens be used as steroid-sparing agents in asthmatic women? Chest. 1994;106:318–319. doi: 10.1378/chest.106.1.318. [DOI] [PubMed] [Google Scholar]
- Namazi MR. The Th1-promoting effects of dehydroepiandrosterone can provide an explanation for the stronger Th1-immune response of women. Iran J Allergy Asthma Immunol. 2009;8:65–69. [PubMed] [Google Scholar]
- Nielsen HC. Androgen receptors influence the production of pulmonary surfactant in the testicular feminization mouse fetus. J Clin Invest. 1985;76:177–181. doi: 10.1172/JCI111943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med. 2007;176:277–284. doi: 10.1164/rccm.200701-044OC. [DOI] [PubMed] [Google Scholar]
- Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233–R249. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- Pagtakhan RD, Bjelland JC, Landau LI, Loughlin G, Kaltenborn W, Seeley G, Taussig LM. Sex differences in growth patterns of the airways and lung parenchyma in children. J Appl Physiol. 1984;56:1204–1210. doi: 10.1152/jappl.1984.56.5.1204. [DOI] [PubMed] [Google Scholar]
- Paharkova-Vatchkova V, Maldonado R, Kovats S. Estrogen preferentially promotes the differentiation of CD11c+ CD11b(intermediate) dendritic cells from bone marrow precursors. J Immunol. 2004;172:1426–1436. doi: 10.4049/jimmunol.172.3.1426. [DOI] [PubMed] [Google Scholar]
- Pang JJ, Xu XB, Li HF, Zhang XY, Zheng TZ, Qu SY. Inhibition of beta-estradiol on trachea smooth muscle contraction in vitro and in vivo. Acta Pharmacol Sin. 2002;23:273–277. [PubMed] [Google Scholar]
- Parekh AB, Putney JW., Jr. Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- Paterni I, Granchi C, Katzenellenbogen JA, Minutolo F. Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids. 2014;90:13–29. doi: 10.1016/j.steroids.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrone C, Cassel TN, Pettersson K, Piao YS, Cheng G, Ciana P, Maggi A, Warner M, Gustafsson JA, Nord M. Regulation of postnatal lung development and homeostasis by estrogen receptor beta. Mol Cell Biol. 2003;23:8542–8552. doi: 10.1128/MCB.23.23.8542-8552.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson B, Gredmark T, Burian P, Bende M. Nasal mucosal congestion during the menstrual cycle. J Laryngol Otol. 1997;111:337–339. doi: 10.1017/s0022215100137259. [DOI] [PubMed] [Google Scholar]
- Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Pelikan Z. Possible immediate hypersensitivity reaction of the nasal mucosa to oral contraceptives. Ann Allergy. 1978;40:211–219. [PubMed] [Google Scholar]
- Pereira A, Krieger BP. Pulmonary complications of pregnancy. Clin Chest Med. 2004;25:299–310. doi: 10.1016/j.ccm.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Perez-Lopez FR, Larrad-Mur L, Kallen A, Chedraui P, Taylor HS. Gender differences in cardiovascular disease: hormonal and biochemical influences. Reprod Sci. 2010;17:511–531. doi: 10.1177/1933719110367829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perusquia M, Hernandez R, Montano LM, Villalon CM, Campos MG. Inhibitory effect of sex steroids on guinea-pig airway smooth muscle contractions. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1997;118:5–10. doi: 10.1016/s0742-8413(97)00029-7. [DOI] [PubMed] [Google Scholar]
- Piccinni MP, Giudizi MG, Biagiotti R, Beloni L, Giannarini L, Sampognaro S, Parronchi P, Manetti R, Annunziato F, Livi C, et al. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol. 1995;155:128–133. [PubMed] [Google Scholar]
- Pierdominici M, Maselli A, Colasanti T, Giammarioli AM, Delunardo F, Vacirca D, Sanchez M, Giovannetti A, Malorni W, Ortona E. Estrogen receptor profiles in human peripheral blood lymphocytes. Immunol Lett. 2010;132:79–85. doi: 10.1016/j.imlet.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Plante J, Simard M, Rantakari P, Cote M, Provost PR, Poutanen M, Tremblay Y. Epithelial cells are the major site of hydroxysteroid (17beta) dehydrogenase 2 and androgen receptor expression in fetal mouse lungs during the period overlapping the surge of surfactant. J Steroid Biochem Mol Biol. 2009;117:139–145. doi: 10.1016/j.jsbmb.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Prakash YS. Airway smooth muscle in airway reactivity and remodeling: what have we learned? Am J Physiol Lung Cell Mol Physiol. 2013;305:L912–933. doi: 10.1152/ajplung.00259.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash YS, Sathish V, Townsend E. Sex Steroid Signaling in the Airway. In: Wang Y-X, editor. Calcium Signaling In Airway Smooth Muscle Cells. Springer International Publishing; 2014. pp. 321–332. [Google Scholar]
- Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen Signaling through the Transmembrane G Protein–Coupled Receptor GPR30. Annu Rev Physiol. 2008;70:165–190. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- Provost PR, Simard M, Tremblay Y. A link between lung androgen metabolism and the emergence of mature epithelial type II cells. Am J Respir Crit Care Med. 2004;170:296–305. doi: 10.1164/rccm.200312-1680OC. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr. Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here). Cell Calcium. 2007;42:103–110. doi: 10.1016/j.ceca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Neurosteroids: Endogenous role in the human brain and therapeutic potentials. In: Ivanka S, editor. Progress in Brain Research. Vol. 186. Elsevier; 2010. pp. 113–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redente EF, Jacobsen KM, Solomon JJ, Lara AR, Faubel S, Keith RC, Henson PM, Downey GP, Riches DWH. Age and sex dimorphisms contribute to the severity of bleomycin-induced lung injury and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2011;301:L510–L518. doi: 10.1152/ajplung.00122.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline S, Gold D. Challenges in interpreting gender differences in asthma. Am J Respir Crit Care Med. 1994;150:1219–1221. doi: 10.1164/ajrccm.150.5.7952543. [DOI] [PubMed] [Google Scholar]
- Riffo-Vasquez Y, Ligeiro de Oliveira AP, Page CP, Spina D, Tavares-de-Lima W. Role of sex hormones in allergic inflammation in mice. Clin Exp Allergy. 2007;37:459–470. doi: 10.1111/j.1365-2222.2007.02670.x. [DOI] [PubMed] [Google Scholar]
- Saaresranta T, Polo O. Hormones and breathing. Chest. 2002;122:2165–2182. doi: 10.1378/chest.122.6.2165. [DOI] [PubMed] [Google Scholar]
- Sathish V, Abcejo AJ, Thompson MA, Sieck GC, Prakash YS, Pabelick CM. Caveolin-1 regulation of store-operated Ca2+ influx in human airway smooth muscle. Eur Respir J. 2012;40:470–478. doi: 10.1183/09031936.00090511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish V, Freeman M,R, Manlove L,J, Thompson M,A, Pabelick C,M, Prakash Y,S. Estrogen Receptor Beta (ERβ) Blunts Inflammation-Induced Human Airway Smooth Muscle Proliferation And Remodeling. Am J Respir Crit Care Med. 2014;189:A5318. [Google Scholar]
- Schatz M, Camargo CA., Jr. The relationship of sex to asthma prevalence, health care utilization, and medications in a large managed care organization. Ann Allergy Asthma Immunol. 2003;91:553–558. doi: 10.1016/S1081-1206(10)61533-5. [DOI] [PubMed] [Google Scholar]
- Schatz M, Dombrowski MP, Wise R, Thom EA, Landon M, Mabie W, Newman RB, Hauth JC, Lindheimer M, Caritis SN, Leveno KJ, Meis P, Miodovnik M, Wapner RJ, Paul RH, Varner MW, O'Sullivan M J, Thurnau GR, Conway D, McNellis D. Asthma morbidity during pregnancy can be predicted by severity classification. J Allergy Clin Immunol. 2003;112:283–288. doi: 10.1067/mai.2003.1516. [DOI] [PubMed] [Google Scholar]
- Scott PA, Tremblay A, Brochu M, St-Louis J. Vasorelaxant action of 17 - estradiol in rat uterine arteries: role of nitric oxide synthases and estrogen receptors. Am J Physiol Heart Circ Physiol. 2007;293:H3713–3719. doi: 10.1152/ajpheart.00736.2007. [DOI] [PubMed] [Google Scholar]
- Seaborn T, Simard M, Provost PR, Piedboeuf B, Tremblay Y. Sex hormone metabolism in lung development and maturation. Trends Endocrinol Metab. 2010;21:729–738. doi: 10.1016/j.tem.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Shah S, Nathan L, Singh R, Fu YS, Chaudhuri G. E2 and not P4 increases NO release from NANC nerves of the gastrointestinal tract: implications in pregnancy. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1546–1554. doi: 10.1152/ajpregu.2001.280.5.R1546. [DOI] [PubMed] [Google Scholar]
- Shahar E, Redline S, Young T, Boland LL, Baldwin CM, Nieto FJ, O'Connor GT, Rapoport DM, Robbins JA. Hormone replacement therapy and sleep-disordered breathing. American journal of respiratory and critical care medicine. 2003;167:1186–1192. doi: 10.1164/rccm.200210-1238OC. [DOI] [PubMed] [Google Scholar]
- Sheridan JT, Gilmore RC, Watson MJ, Archer CB, Tarran R. 17beta-Estradiol inhibits phosphorylation of stromal interaction molecule 1 (STIM1) protein: implication for store-operated calcium entry and chronic lung diseases. J Biol Chem. 2013;288:33509–33518. doi: 10.1074/jbc.M113.486662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki H, Watanabe K, Kanaizumi E, Konno N, Sato J, Narita S, Himi T. Expression and localization of steroid receptors in human nasal mucosa. Acta Otolaryngol. 2004;124:958–963. doi: 10.1080/00016480310017063. [DOI] [PubMed] [Google Scholar]
- Siddiqui S, Redhu NS, Ojo OO, Liu B, Irechukwu N, Billington C, Janssen L, Moir LM. Emerging airway smooth muscle targets to treat asthma. Pulm Pharmacol Ther. 2013;26:132–144. doi: 10.1016/j.pupt.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Skobeloff EM, Spivey WH, St Clair SS, Schoffstall JM. The influence of age and sex on asthma admissions. Jama. 1992;268:3437–3440. [PubMed] [Google Scholar]
- Slatkovska L, Jensen D, Davies GA, Wolfe LA. Phasic menstrual cycle effects on the control of breathing in healthy women. Respir Physiol Neurobiol. 2006;154:379–388. doi: 10.1016/j.resp.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Soldan SS, Alvarez Retuerto AI, Sicotte NL, Voskuhl RR. Immune modulation in multiple sclerosis patients treated with the pregnancy hormone estriol. J Immunol. 2003;171:6267–6274. doi: 10.4049/jimmunol.171.11.6267. [DOI] [PubMed] [Google Scholar]
- Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2:372–377. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- Stamatiou R, Paraskeva E, Papagianni M, Molyvdas PA, Hatziefthimiou A. The mitogenic effect of testosterone and 17beta-estradiol on airway smooth muscle cells. Steroids. 2011;76:400–408. doi: 10.1016/j.steroids.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Stewart AG, Fernandes D, Tomlinson PR. The effect of glucocorticoids on proliferation of human cultured airway smooth muscle. Br J Pharmacol. 1995;116:3219–3226. doi: 10.1111/j.1476-5381.1995.tb15127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- Sud N, Wiseman DA, Black SM. Caveolin 1 is required for the activation of endothelial nitric oxide synthase in response to 17beta-estradiol. Mol Endocrinol. 2010;24:1637–1649. doi: 10.1210/me.2010-0043. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Takeyama J, Suzuki T, Inoue S, Kaneko C, Nagura H, Harada N, Sasano H. Expression and cellular localization of estrogen receptors alpha and beta in the human fetus. J Clin Endocrinol Metab. 2001;86:2258–2262. doi: 10.1210/jcem.86.5.7447. [DOI] [PubMed] [Google Scholar]
- Tam A, Morrish D, Wadsworth S, Dorscheid D, Man S, Sin D. The role of female hormones on lung function in chronic lung diseases. BMC Women's Health. 2011;11:24. doi: 10.1186/1472-6874-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam A, Wadsworth S, Dorscheid D, Man SF, Sin DD. Estradiol increases mucus synthesis in bronchial epithelial cells. PloS one. 2014;9:e100633. doi: 10.1371/journal.pone.0100633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi K. Effects of female hormones on respiratory and cardiovascular regulation]. Nihon Kokyuki Gakkai zasshi = the journal of the Japanese Respiratory Society. 1999;37:359–367. [PubMed] [Google Scholar]
- Taylor M. An experimental study of the influence of the endocrine system on the nasal respiratory mucosa. J Laryngol Otol. 1961;75:972–977. doi: 10.1017/s0022215100058746. [DOI] [PubMed] [Google Scholar]
- Teng J, Wang ZY, Jarrard DF, Bjorling DE. Roles of estrogen receptor alpha and beta in modulating urothelial cell proliferation. Endocr Relat Cancer. 2008;15:351–364. doi: 10.1677/erc.1.01255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlbeck WM. Postnatal growth and development of the lung. Am Rev Respir Dis. 1975;111:803–844. doi: 10.1164/arrd.1975.111.6.803. [DOI] [PubMed] [Google Scholar]
- Thurlbeck WM. The pathology of small airways in chronic airflow limitation. Eur J Respir Dis Suppl. 1982a;121:9–18. [PubMed] [Google Scholar]
- Thurlbeck WM. Postnatal human lung growth. Thorax. 1982b;37:564–571. doi: 10.1136/thx.37.8.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofovic SP, Zhang X, Jackson EK, Zhu H, Petrusevska G. 2-methoxyestradiol attenuates bleomycin-induced pulmonary hypertension and fibrosis in estrogen-deficient rats. Vascul Pharmacol. 2009;51:190–197. doi: 10.1016/j.vph.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppozada H, Michaels L, Toppozada M, El-Ghazzawi I, Talaat M, Elwany S. The human respiratory nasal mucosa in pregnancy. An electron microscopic and histochemical study. J Laryngol Otol. 1982;96:613–626. doi: 10.1017/s0022215100092902. [DOI] [PubMed] [Google Scholar]
- Torday JS. Androgens delay human fetal lung maturation in vitro. Endocrinol. 1990;126:3240–3244. doi: 10.1210/endo-126-6-3240. [DOI] [PubMed] [Google Scholar]
- Torday JS, Nielsen HC. The sex difference in fetal lung surfactant production. Exp Lung Res. 1987;12:1–19. doi: 10.3109/01902148709068811. [DOI] [PubMed] [Google Scholar]
- Townsend EA, Meuchel LW, Thompson MA, Pabelick CM, Prakash YS. Estrogen increases nitric-oxide production in human bronchial epithelium. J Pharmacol Exp Therap. 2011;339:815–824. doi: 10.1124/jpet.111.184416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocrine reviews. 2012a;33:1–47. doi: 10.1210/er.2010-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Sathish V, Thompson MA, Pabelick CM, Prakash YS. Estrogen effects on human airway smooth muscle involve cAMP and protein kinase A. Am J Physiol Lung Cell Mol Physiol. 2012b;303:L923–928. doi: 10.1152/ajplung.00023.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Thompson MA, Pabelick CM, Prakash YS. Rapid effects of estrogen on intracellular Ca2+ regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2010;298:L521–530. doi: 10.1152/ajplung.00287.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi R, Speizer F, Willett W, Trichopoulos D, Rosner B. Menopause, postmenopausal estrogen preparations, and the risk of adult-onset asthma. A prospective cohort study. American journal of respiratory and critical care medicine. 1995a;152:1183–1188. doi: 10.1164/ajrccm.152.4.7551368. [DOI] [PubMed] [Google Scholar]
- Troisi RJ, Speizer FE, Willett WC, Trichopoulos D, Rosner B. Menopause, postmenopausal estrogen preparations, and the risk of adult-onset asthma. A prospective cohort study. Am J Respir Crit Care Med. 1995b;152:1183–1188. doi: 10.1164/ajrccm.152.4.7551368. [DOI] [PubMed] [Google Scholar]
- Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. I. Normal pregnancy. Am J Obstet Gynecol. 1972;112:1095–1100. doi: 10.1016/0002-9378(72)90185-8. [DOI] [PubMed] [Google Scholar]
- Umar S, Rabinovitch M, Eghbali M. Estrogen paradox in pulmonary hypertension: current controversies and future perspectives. Am J Respir Crit Care Med. 2012;186:125–131. doi: 10.1164/rccm.201201-0058PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velden VH, Hulsmann AR. Autonomic innervation of human airways: structure, function, and pathophysiology in asthma. Neuroimmunomodulation. 1999;6:145–159. doi: 10.1159/000026376. [DOI] [PubMed] [Google Scholar]
- Varkey AB. Chronic obstructive pulmonary disease in women: exploring gender differences. Curr Opin Pulm Med. 2004;10:98–103. doi: 10.1097/00063198-200403000-00003. [DOI] [PubMed] [Google Scholar]
- Vasiadi M, Kempuraj D, Boucher W, Kalogeromitros D, Theoharides TC. Progesterone inhibits mast cell secretion. Int J Immunopathol Pharmacol. 2006;19:787–794. doi: 10.1177/039463200601900408. [DOI] [PubMed] [Google Scholar]
- Vellutini M, Viegi G, Parrini D, Pedreschi M, Baldacci S, Modena P, Biavati P, Simoni M, Carrozzi L, Giuntini C. Serum immunoglobulins E are related to menstrual cycle. Eur J Epidemiol. 1997;13:931–935. doi: 10.1023/a:1007472407010. [DOI] [PubMed] [Google Scholar]
- Voltz JW, Card JW, Carey MA, Degraff LM, Ferguson CD, Flake GP, Bonner JC, Korach KS, Zeldin DC. Male sex hormones exacerbate lung function impairment after bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2008;39:45–52. doi: 10.1165/rcmb.2007-0340OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze A, Postma DS, Kerstjens HA. Perimenstrual asthma: a syndrome without known cause or cure. J Allergy Clin Immunol. 2003;112:271–282. doi: 10.1067/mai.2003.1676. [DOI] [PubMed] [Google Scholar]
- Wang X, Magkos F, Mittendorfer B. Sex Differences in Lipid and Lipoprotein Metabolism: It's Not Just about Sex Hormones. J Clin Endocrinol Metab. 2011;96:885–893. doi: 10.1210/jc.2010-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JK, Barnes PJ, Springall DR, Abelli L, Tadjkarimi S, Yacoub MH, Polak JM, Belvisi MG. Distribution of human i-NANC bronchodilator and nitric oxide-immunoreactive nerves. Am J Respir Cell Mol Biol. 1995;13:175–184. doi: 10.1165/ajrcmb.13.2.7542897. [DOI] [PubMed] [Google Scholar]
- West AR, Syyong HT, Siddiqui S, Pascoe CD, Murphy TM, Maarsingh H, Deng L, Maksym GN, Bosse Y. Airway contractility and remodeling: links to asthma symptoms. Pulm Pharmacol Ther. 2013;26:3–12. doi: 10.1016/j.pupt.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Whitacre CC, Reingold SC, O'Looney PA. A gender gap in autoimmunity. Science. 1999;283:1277–1278. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- White DP, Douglas NJ, Pickett CK, Weil JV, Zwillich CW. Sexual influence on the control of breathing. Journal of applied physiology: respiratory, environmental and exercise physiology. 1983;54:874–879. doi: 10.1152/jappl.1983.54.4.874. [DOI] [PubMed] [Google Scholar]
- Wilson CM, McPhaul MJ. A and B forms of the androgen receptor are expressed in a variety of human tissues. Mol Cell Endocrinol. 1996;120:51–57. doi: 10.1016/0303-7207(96)03819-1. [DOI] [PubMed] [Google Scholar]
- Wright D, Sharma P, Ryu M-H, Rissé P-A, Ngo M, Maarsingh H, Koziol-White C, Jha A, Halayko AJ, West AR. Models to study airway smooth muscle contraction in vivo, ex vivo and in vitro: Implications in understanding asthma. Pulm Pharmacol Ther. 2013;26:24–36. doi: 10.1016/j.pupt.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Wulfsohn NL, Politzer WM, Henrico JS. Testosterone Therapy in Bronchial Asthma. S Afr Med J. 1964;38:170–172. [PubMed] [Google Scholar]
- Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. American journal of respiratory and critical care medicine. 2003;167:1181–1185. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- Zannolli R, Morgese G. Does puberty interfere with asthma? Med Hypotheses. 1997;48:27–32. doi: 10.1016/s0306-9877(97)90020-7. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Teng CT. Estrogen receptor-related receptor alpha 1 interacts with coactivator and constitutively activates the estrogen response elements of the human lactoferrin gene. J Biol Chem. 2000;275:20837–20846. doi: 10.1074/jbc.M001880200. [DOI] [PubMed] [Google Scholar]