Abstract
Strigolactone (SL), auxin and cytokinin (CK) interact to regulate shoot branching. CK has long been considered to be the only key phytohormone to promote lateral bud outgrowth. Here we report that gibberellin also acts as a positive regulator in the control of shoot branching in the woody plant Jatropha curcas. We show that gibberellin and CK synergistically promote lateral bud outgrowth, and that both hormones influence the expression of putative branching regulators, J. curcas BRANCHED1 and BRANCHED2, which are key transcription factors maintaining bud dormancy. Moreover, treatment with paclobutrazol, an inhibitor of de novo gibberellin biosynthesis, significantly reduced the promotion of bud outgrowth by CK, suggesting that gibberellin is required for CK-mediated axillary bud outgrowth. In addition, SL, a plant hormone involved in the repression of shoot branching, acted antagonistically to both gibberellin and CK in the control of lateral bud outgrowth. Consistent with this, the expression of JcMAX2, a J. curcas homolog of Arabidopsis MORE AXILLARY GROWTH 2 encoding an F-box protein in the SL signaling pathway, was repressed by gibberellin and CK treatment. We also provide physiological evidence that gibberellin also induces shoot branching in many other trees, such as papaya, indicating that a more complicated regulatory network occurs in the control of shoot branching in some perennial woody plants.
Keywords: Axillary bud, Bud outgrowth, Cytokinin, Gibberellin, Shoot branching, Strigolactone
Introduction
Plant shoot architecture is determined by the primary apical meristem and the derived lateral branches. In many crops, the number of lateral branches has a profound effect on yield (Kebrom et al. 2013). For decades, intensive research has focused on identifying and characterizing the phytohormones that control lateral bud outgrowth. How plants flexibly optimize their architecture to adapt to the changing environment through manipulation of their endogenous phytohormone balance remains largely unknown.
Depending on environmental conditions, axillary buds either develop into lateral branches or remain dormant. Earlier studies showed that basipetally transported auxin inhibits lateral bud outgrowth (Thimann and Skoog 1934, Morris 1977), and that interactions between auxin and cytokinin (CK) play a central role in the establishment of apical dominance (Wickson and Thimann 1958, Scott et al. 1967). However, auxin does not directly inhibit bud outgrowth, since apically supplied auxin does not travel into the lateral bud after decapitation (Hall and Hillman 1975). This result supports the hypothesis that auxin controls lateral bud outgrowth in conjunction with secondary messengers (Sachs and Thimann 1967, Bangerth 1994, Li et al. 1995). CK was postulated to function as a second messenger that relays the auxin signal into the lateral buds (Snow 1937, Bangerth 1994, Leyser 2003). Auxin can directly inhibit CK biosynthesis through the AXR1-dependent auxin signaling pathway (Nordström et al. 2004), and CK is the only identified phytohormone to date known to control lateral bud outgrowth positively in pea (Pisum sativum) and Arabidopsis thaliana. Indeed, a few hours after decapitation, the CK level increased several fold in the lateral buds of pea and chickpea (Turnbull et al. 1997, Tanaka et al. 2006). Tanaka et al. (2006) found that the expression level of the pea gene adenosine phosphate isopentenyltransferase (PsIPT), encoding a key enzyme in CK biosynthesis, was increased at the node after decapitation, and proposed that lateral bud outgrowth after decapitation was due to locally increased accumulation of CK. Recently, an excellent study by Mason et al. (2014) showed that sucrose acts as the second messenger that transduces the decapitation signal, and highlighted the specific role of sugar in the control of lateral bud outgrowth.
It has long been postulated that a carotenoid-derived root signal inhibits shoot branching and acts as a graft-transmissible factor that moves up the shoot and is required for auxin-mediated repression of lateral bud outgrowth (Beveridge et al. 1994, Beveridge et al. 1997, Morris et al. 2001). This signal was identified as strigolactones (SLs), a group of phytohormones discovered relatively recently (Gomez-Roldan et al. 2008, Umehara et al. 2008). SLs act downstream of auxin to inhibit lateral bud outgrowth (Brewer et al. 2009, Agusti et al. 2011). Direct application of GR24 (an analog of SL) efficiently suppresses the lateral bud outgrowth induced by decapitation or CK treatment (Brewer et al. 2009, Dun et al. 2012).
Gibberellins are a group of key hormones regulating many aspects of plant growth and development (Olszewski et al. 2002, Yamaguchi 2008). In pea, gibberellin seems to play an inhibitory role in lateral bud outgrowth (Scott et al. 1967). In Arabidopsis, a gibberellin-insensitive (gai) mutant shows a reduction in apical dominance and an increased number of axillary shoots (Koornneef et al. 1985). In turfgrass and Populus trees, overexpression of the gibberellin-catabolizing gene GA2ox led to an increased number of tillers or branches, suggesting that gibberellin may also play a negative role in the control of shoot branching in these species (Agharkar et al. 2007, Mauriat et al. 2011, Zawaski and Busov 2014). However, stimulation by gibberellin of axillary bud development was reported in citrus and snapdragon (Marth et al. 1956) and in sweet cherry (Elfving et al. 2011). The involvement of gibberellin biosynthesis in the light effect on bud burst was also demonstrated in rose (Choubane et al. 2012). Here we present evidence that gibberellin is a positive regulator in controlling shoot branching in the perennial woody plant Jatropha curcas, a promising biofuel feedstock (Sato et al. 2011, Chen et al. 2014, Wu et al. 2015). We also investigated the interactions between gibberellin and CK in the control of lateral bud outgrowth.
Results
Gibberellin treatment promotes lateral bud outgrowth
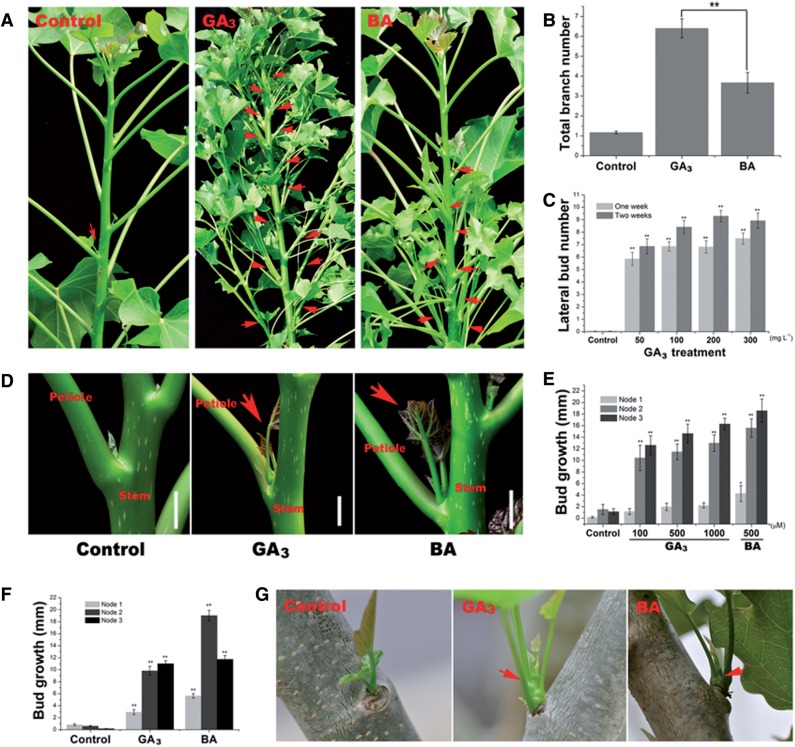
We found that lateral branch outgrowth could be efficiently stimulated by treatment with GA3 or the synthetic CK, 6-benzyladenine (BA), in 2-year-old J. curcas trees (Fig. 1A). Interestingly, GA3 was more effective at promoting shoot branching (Fig. 1A, B). We then investigated how the axillary buds respond to different concentrations of GA3. Treatment with higher concentrations of GA3 led to an increased number of stimulated lateral buds (Fig. 1C), although it caused severe side effects, such as shoot apex necrosis. Typically, lateral branches induced by GA3 or BA treatment had an actively growing stem with several small leaves (Fig. 1D).
Fig. 1.
GA3 and BA promote shoot branching in J. curcas. (A) Shoot branching stimulated by GA3 or BA treatment in the young stems of 2-year-old J. curcas trees. (B) The number of induced branches was counted 4 weeks after GA3 (500 mg l–1) or BA (500 mg l–1) treatment (n = 30). (C) The number of stimulated axillary buds was counted 1 and 2 weeks after the application of various concentrations of GA3 to the top region of the branch (∼20 cm; n = 28–45). (D) Representative lateral bud stimulated by GA3 treatment in 2-year-old J. curcas trees. (E) Axillary bud length at nodes 1–3 of 4-week-old J. curcas seedlings measured 2 weeks after GA3 or BA treatment (n = 12–14). (F) Buds at nodes 1–3 of 5-week-old J. curcas seedlings showed different responsiveness to GA3 or BA (500 μM) treatment (n > 20). (G) Lateral bud on an old stem of a 3-year-old J. curcas tree, stimulated by GA3 or BA (500 μM) treatment. Values are means ± SE for (B), (C), (E) and (F). Student’s t-test was used to determine significant differences between the indicated groups in (B) or between the treated and control groups in (C), (E) and (F). Significance levels: *P < 0.05; **P < 0.01. Red arrows indicate the stimulated axillary buds.
GA3 and BA were also applied to the shoots of 4-week-old J. curcas seedlings. Approximately 2–3 d after treatment, the axillary buds had undergone marked outgrowth (Fig. 1E), and treatment with BA promoted bud outgrowth to a greater extent than did GA3 (Fig. 1E).
In pea, axillary buds at different locations vary in responsiveness to CK treatment and decapitation (King and Vanstaden 1988, Morris et al. 2005). We investigated the responsiveness to GA3 and BA treatment of axillary buds located at different positions in 5-week-old J. curcas seedlings. Whereas all buds were successfully stimulated by the treatments, those located at higher positions (nodes 2 and 3) were the most responsive (Fig. 1F).
Axillary buds located on old stems are dormant and seldom develop into branches in J. curcas (Ghosh et al. 2011). We investigated whether axillary buds on old stems (approximately 1.5 m above the ground) of 3-year-old J. curcas trees were also sensitive to GA3 or BA treatment. We detected marked outgrowth of the axillary buds 1 week after GA3 or BA treatment (Fig. 1G), suggesting that the dormant axillary buds located on old stems can still be activated by these phytohormones.
We further generated transgenic J. curcas overexpressing a gibberellin biosynthesis gene, JcGA20ox1 (GenBank accession No. KM454465), which encodes a gibberellin 20-oxidase in J. curcas (Supplementary Fig. S1). Since the transgenic shoots failed to produce roots, which probably resulted from the increased gibberellin level produced by overexpression of the transgene JcGA20ox1, they were grafted onto rootstocks of wild-type J. curcas seedlings. The grafted JcGA20ox1 transgenic plants showed accelerated growth and stem elongation (Supplementary Fig. S1C), and enhanced lateral bud outgrowth (Supplementary Fig. S1D). An enhanced branching phenotype was observed in the young transgenic trees a few months after transplantation in the field (Supplementary Fig. S1E). These results support our finding that gibberellin promotes lateral bud outgrowth.
Interactions of gibberellin and CK in the control of lateral bud outgrowth
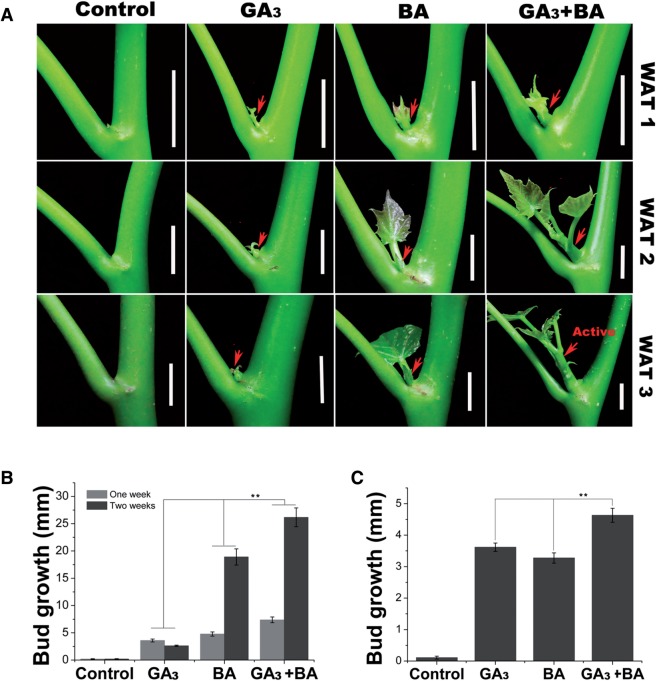
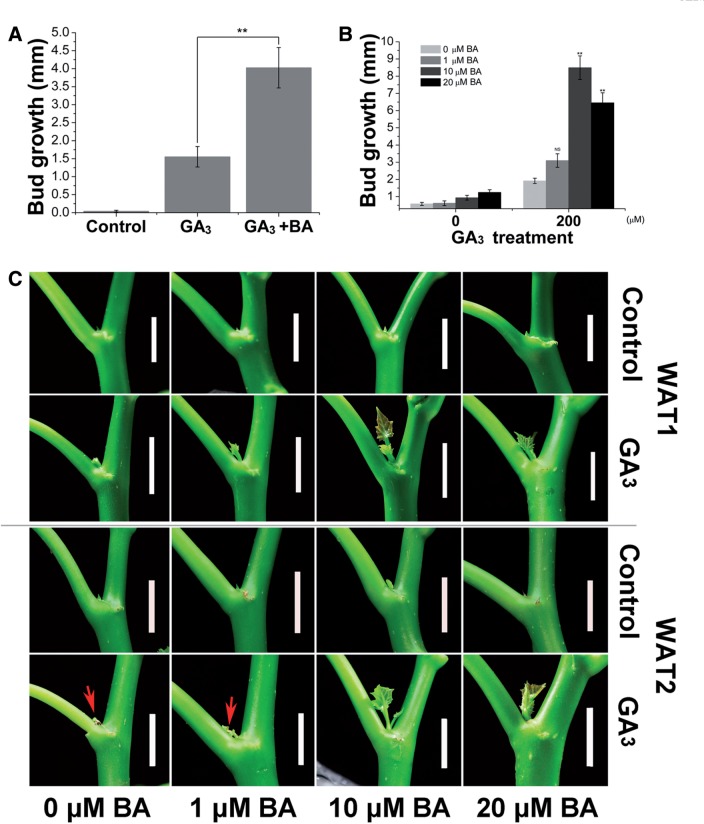
As shown above, the lateral bud outgrowth in J. curcas seedlings can be stimulated by either GA3 or CK treatment; we thus evaluated the synergistic effect of these two hormones on the outgrowth of axillary buds in seedlings. Unlike on the adult J. curcas trees (Fig. 1A, B), bud treatment with only GA3 or BA had a very limited effect in promoting axillary buds in the seedlings to grow into complete branchlets, and the growth of the stimulated buds lasted only for 1 or 2 weeks (Fig. 2A). In contrast, co-application of the two phytohormones to buds revealed a significant synergistic effect on bud outgrowth in the seedlings (Fig. 2A, B). Axillary buds located at node 2 were also stimulated by the treatments (Fig. 2C), which may result from the hormone being transported up the stem after bud treatment at node 1. In addition, we found that the co-application of lower concentrations of GA3 and BA (e.g. 10 μM of each hormone) also successfully stimulated the outgrowth of axillary buds (Fig. 3). The promotive effect of the combined treatment of GA3 and BA at a low concentration (e.g. 10 μM of each, Fig. 3B) was greater than that of the separate treatment with GA3 or BA alone at a much higher concentration (500 μM of each, Fig. 2B). These results suggest that gibberellin and CK synergistically regulate lateral bud outgrowth in J. curcas.
Fig. 2.
GA3 and BA synergistically stimulate bud outgrowth in 3-week-old seedlings of J. curcas. (A) Photographs showing representative outgrowth of the buds at node 1 at 1, 2 and 3 weeks after treatment (WAT 1, 2 and 3), respectively. Red arrows indicate the stimulated axillary buds. Scale bars = 1 cm. (B) Outgrowth of the buds at node 1 (n = 24). (C) Outgrowth of the buds at node 2 at 2 weeks after treatment (n > 20). Treatments were conducted at node 1 with GA3 (500 μM), BA (500 μM) or GA3 + BA. Values are means SE. Student’s t-test was used to determine significant differences between the treated and control groups. Significance levels: **P < 0.01.
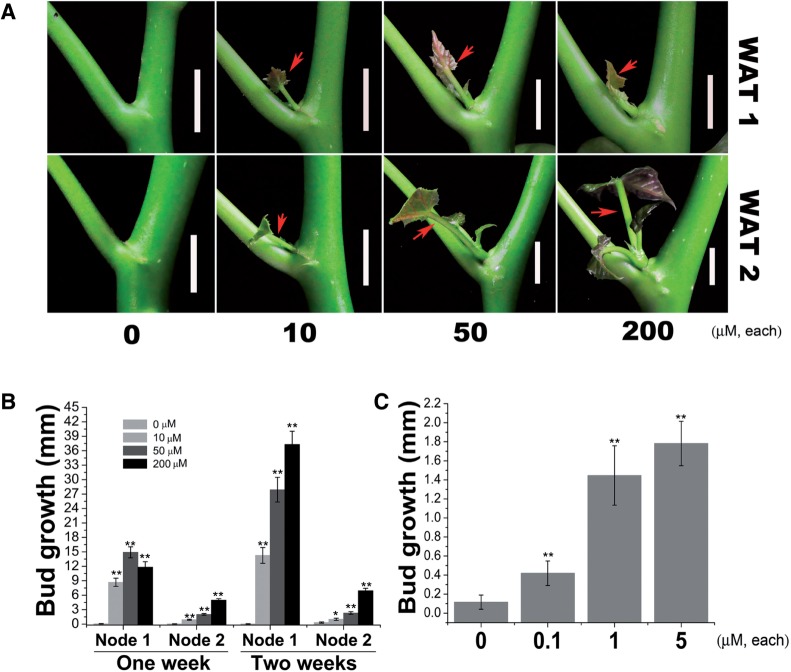
Fig. 3.
Co-application of GA3 and BA at low concentrations effectively promoted lateral bud outgrowth in 3-week-old seedlings of J. curcas. (A) Outgrowth of the buds at node 1 stimulated by GA3 + BA treatment. Photos were taken at 1 and 2 weeks after treatment (WAT 1 and 2). Red arrows indicate the stimulated axillary buds. Scale bars = 1 cm. (B) The length of the buds at nodes 1 and 2 was measured at 1 or 2 weeks after treatment with 10–200 μM GA3 + BA. (C) The length of the buds at nodes 1 was measured at 1 week after treatment with 0.1–5 μM GA3 + BA. Data are means ±SE (n = 20). Student’s t-test was used to determine significant differences between the treated and control groups. Significance levels: *P < 0.05; **P < 0.01.
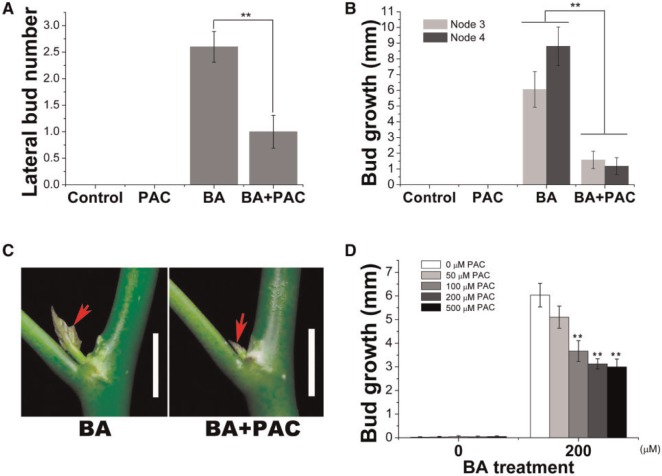
We then investigated whether the inhibition of endogenous gibberellin biosynthesis could affect the promotion of bud outgrowth by BA in J. curcas. We found that the number of stimulated axillary buds was significantly reduced when paclobutrazol (PAC), an inhibitor of gibberellin biosynthesis (Ghosh et al. 2010, Ghosh et al. 2011), was co-applied with BA (Fig. 4A). The bud outgrowth promoted by BA was also severely inhibited upon PAC treatment (Fig. 4B, C), in a concentration-dependent manner (Fig. 4D). Since CK is the only phytohormone known to regulate shoot branching positively in the model plant pea (Domagalska and Leyser 2011, Janssen et al. 2014), we tested whether gibberellin also promoted shoot branching in pea, and whether the inhibition of gibberellin biosynthesis also affected the BA-mediated promotion of lateral bud outgrowth in pea. In contrast to the marked increase in lateral bud formation observed in J. curcas plants treated with GA3 (Fig. 1), GA3 had no obvious effect on bud outgrowth in pea (Supplementary Fig. S2A, B). The addition of PAC did not significantly affect the BA-mediated promotion of lateral bud outgrowth at node 2 or 3 (Supplementary Fig. S2), suggesting that gibberellin is not required during the early stages of bud outgrowth promoted by CK in pea.
Fig. 4.
Paclobutrazol (PAC) inhibits promotion of bud outgrowth by BA. (A, B) PAC decreased the number of stimulated axillary buds and the outgrowth of buds in 4-week-old seedlings subjected to shoot treatment with BA (n > 15). The concentrations of PAC and BA used in single or combined treatments in (A) and (B) are 200 and 500 μM respectively. (C) Promotion of outgrowth of buds at node 1 by bud treatment with BA (200 μM) was inhibited by co-application of PAC (200 μM). Red arrows indicate the stimulated axillary buds. Scale bars = 1 cm. (D) Bud outgrowth of buds at node 1 by bud treatment with BA (200 μM) in conjunction with different concentrations of PAC (n = 20). All data and photos in (A–D) were taken at 1 week after treatment. Student’s t-test was used to determine significant differences between the treated and control groups. Significance levels: **P < 0.01.
Since CKs, especially trans-zeatin (tZ), are produced mostly in the roots (Ko et al. 2014), we excised the roots of 3-week-old J. curcas seedlings to mimic immediate CK depletion, and cultured the shoots in the liquid medium to investigate further whether CK affects the bud promotion in J. curcas caused by GA3 treatment. The outgrowth of lateral buds in the root-excised seedlings of J. curcas was weakly stimulated by GA3 treatment, whereas co-application with BA or addition of BA in the aqueous culture significantly increased GA3-stimulated bud outgrowth (Fig. 5). Thus, a CK supply is required for the GA3-stimulated increase in bud formation in J. curcas.
Fig. 5.
CK is required for promotion of bud outgrowth by gibberellin in the root-excised 3-week-old seedlings of J. curcas. (A) The length of buds at node 1 at 1 week after treatment with GA3 (200 μM) or GA3 (200 μM) + BA (20 μM) (n = 20). (B) Addition of various concentrations of BA in the liquid culture promoted the outgrowth of buds at node 1 at 1 week after GA3 treatment (n = 20). (C) Photographs of outgrowth of lateral buds at node 1 promoted by treatment with GA3 (200 μM) in conjunction with or without various concentrations of BA in the liquid culture. Red arrows indicated the buds that became dormant after 2 weeks growth. Scale bars = 1 cm. Root excision was conducted 2 d before hormone treatment. Values are means ± SE. Student’s t-test was used to determine significant differences between the treated and control groups. Significance levels: **P < 0.01; NS, not significant.
GA3, but not BA, induces lateral bud outgrowth on the newly developed shoots
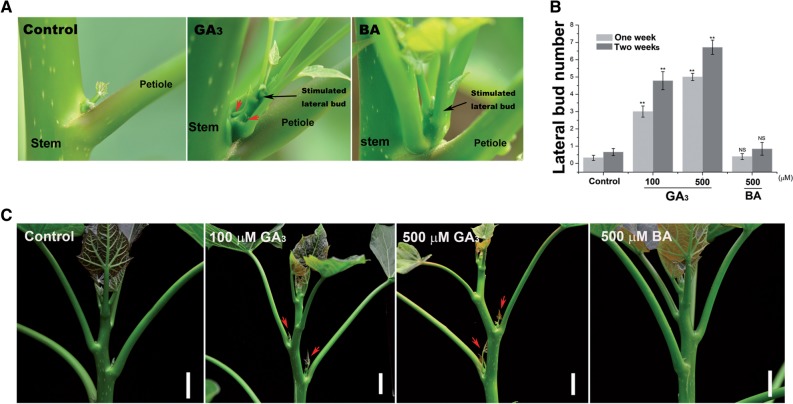
As shown above, treatment of shoots with both GA3 and BA can effectively promote branching in J. curcas; however, GA3 treatment produced more branches than did BA treatment (Fig. 1A, B). We further found that GA3 induced formation of secondary buds in the axils of the newly developed lateral buds, whereas BA treatment did not (Fig. 6A). In addition, treatment with GA3, but not BA, at the shoot apex led to an obvious outgrowth of lateral buds on the newly developed shoots (Fig. 6C), which resulted in a significant increase in the number of lateral buds (Fig. 6B).
Fig. 6.
GA3, but not BA, induces lateral bud outgrowth on the newly developed shoots of 2-year-old J. curcas trees. (A) Secondary lateral buds (indicated by red arrows) were induced by treatment of shoots with GA3 (500 μM), but not BA (500 μM). (B) The number of lateral buds induced by treatment of shoot apex with GA3 or BA (n = 31–33). Values are means ± SE. Student’s t-test was used to determine significant differences between the treated and control groups. Significance levels: **P < 0.01; NS indicates no significant differences. (C) GA3 treatment at the shoot apex led to an obvious outgrowth of lateral buds (indicated by red arrows) on the newly developed shoots, while BA treatment did not. Scale bars = 1 cm.
GA3, BA and decapitation all influence the expression of putative branching regulators JcBRC1 and JcBRC2
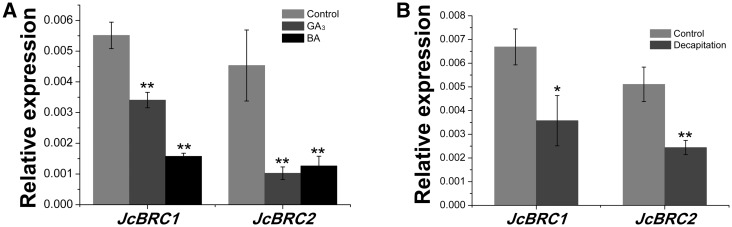
Previous studies showed that BRANCHED1 (BRC1) and BRANCHED2 (BRC2) promoted bud arrest in response to environmental and endogenous signals, and that mutation of these genes caused ectopic shoot branching in Arabidopsis (Aguilar-Martínez et al. 2007). BRC1 acts as a local integrator controlling bud outgrowth that is positively regulated by SLs, and down-regulated by CK treatment in Arabidopsis and pea (Aguilar-Martínez et al. 2007, Braun et al. 2012). We identified the homologs of BRC1 and BRC2 in J. curcas, JcBRC1 and JcBRC2 (GenBank accession Nos. KM454467 and KM454466), and analyzed their expression at 24 h after GA3, BA and decapitation treatment. We found that the expression of JcBRC1 and JcBRC2 declined within 24 h of treatment with GA3 or BA (Fig. 7A). Furthermore, JcBRC1 and JcBRC2 were also down-regulated within 24 h of decapitation (Fig. 7B). These results suggest that the promotion of lateral bud outgrowth by GA3, BA and decapitation treatment may be due to the inhibition of the expression of JcBRC1 and JcBRC2. However, although the expression of BRC1 and BRC2 was significantly up-regulated by the branching suppressor GR24 (a synthetic SL) in pea (Braun et al. 2012), the expression of JcBRC1 and JcBRC2 was slightly but not significantly increased after 12 h of GR24 treatment (Supplementary Fig. S3), which is in agreement with findings in maize and rice (Minakuchi et al. 2010, Guan et al. 2012).
Fig. 7.
Effects of GA3, BA and decapitation on the expression of JcBRC1 and JcBRC2 at node 1 of 3-week-old J. curcas seedlings. (A) GA3 or BA (500 μM) treatment decreased the expression of JcBRC1 and JcBRC2 (n = 3). (B) Decapitation decreased the expression of JcBRC1 and JcBRC2 (n = 3). Decapitation was conducted on the 3-week-old seedlings by removing the shoot tip (approximately 1 cm). Samples for qPCR analysis were collected at 24 h after treatment. Values are means ± SE. Student’s t-test was used to determine significant differences between the treated and control groups. Significance levels: *P < 0.05; **P < 0.01.
Bud outgrowth promoted by gibberellin or CK is inhibited by SL
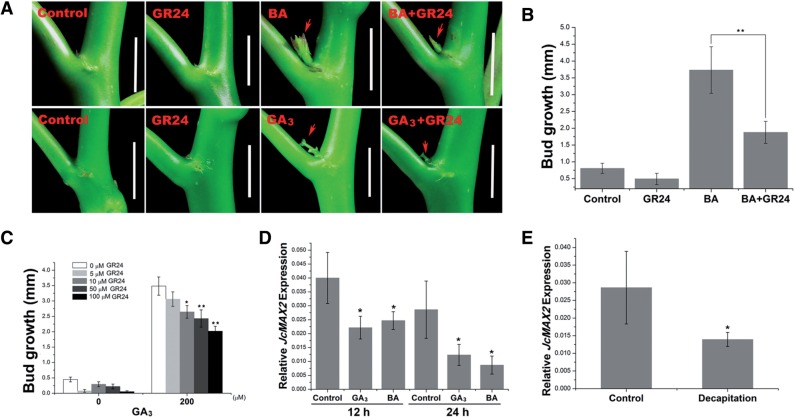
In pea, CK and SL antagonistically regulate bud outgrowth; exogenous application of the synthetic SL GR24 inhibits BA-stimulated bud formation in a dose-dependent manner (Dun et al. 2012). Here, we found that BA- or GA3-mediated bud promotion was significantly inhibited by GR24 in J. curcas (Fig. 8A–C). This result suggests that in J. curcas SL also acts antagonistically with gibberellin and CK in the control of lateral bud outgrowth. Arabidopsis MORE AXILLARY GROWTH 2 (MAX2) plays a pivotal role in SL signaling transduction, and mutation of this gene results in an ectopic shoot branching phenotype (Stirnberg et al. 2002, Stirnberg et al. 2007). We showed that the expression of JcMAX2, a homolog of MAX2 from J. curcas (GenBank accession No. KM454470), was decreased within 12 or 24 h of BA and GA3 treatment (Fig. 8D). In addition, JcMAX2 expression was also down-regulated within 24 h of decapitation (Fig. 8E). These observations suggest that the down-regulation of JcMAX2 expression could contribute to the promotion of bud outgrowth by gibberellin, CK and decapitation in J. curcas.
Fig. 8.
Strigolactones antagonistically regulates lateral bud outgrowth in J. curcas seedlings treated with GA3 and BA. (A–C) Bud outgrowth at node 1 of 3-week-old seedlings 1 week after GA3, BA, GR24, BA + GR24 or GA3 + GR24 treatments. Scale bars = 1 cm (n = 20). (D) GA3 and BA decreased the expression of JcMAX2 at node 1 (n = 3). (E) Decapitation decreased the expression of JcMAX2 at 24 h after treatment (n = 3). The concentration of BA, GA3 and GR24 used in (A), (B) and (D) was 200, 200 and 50 μM, respectively. Values are means ± SE for (B–E). Student’s t-test was used to determine significant differences between the indicated groups in (B) or between the treated and control groups in (C–E). Significance levels: *P < 0.05; **P < 0.01.
Discussion
In this work, we report that gibberellin acts as a positive regulator of shoot branching in the woody plant J. curcas. We provided physiological and molecular evidence that gibberellin interacts with two other well-studied phytohormones, CK and SL, in the control of lateral bud outgrowth in J. curcas.
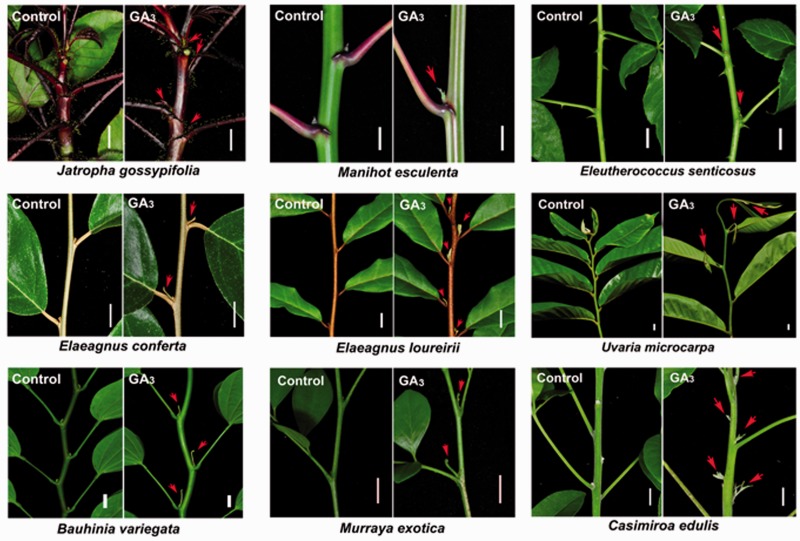
Although a negative correlation between gibberellin levels and branching or tillering has been observed in some species, such as Arabidopsis (Silverstone et al. 1997), pea (Scott et al. 1967, Luisi et al. 2011), rice (Lo et al. 2008, Qi et al. 2011), barley (Jia et al. 2009, Jia et al. 2011), turfgrass (Agharkar et al. 2007) and Populus trees (Mauriat et al. 2011, Zawaski and Busov 2014), we found that gibberellin acts as a positive regulator in the regulation of shoot branching in the perennial woody plant J. curcas, demonstrated by direct GA3 treatment (Fig. 1) and by overexpressing the gibberellin biosynthesis gene JcGA20ox1 (Supplementary Fig. S1). On the basis of the results of this study, we hypothesize that gibberellin may function as a potent promoter of shoot branching in many perennial woody plants. Indeed, significant GA3-mediated promotion of bud outgrowth was also found in another woody plant, papaya (Fig. 9). By shoot treatment with GA3 and BA on the 2-year-old papaya trees, the outgrowth of the lateral buds on the stem was successfully stimulated (Fig. 9A). On the papaya seedlings, the lateral buds also showed strong response to GA3 or BA treatment (Fig. 9B, C). These results indicated that gibberellin was also involved in the positive regulation of bud outgrowth in papaya as it was in J. curcas. To investigate whether the positive role of gibberellin in the regulation of shoot branching is common in the perennial woody or shrub plants, we then conducted GA3 treatment on more tree species. It turned out that most of them showed accelerated outgrowth of lateral buds after GA3 treatment (Fig. 10). However, some species (e.g. Bischofia javanica and Glochidion eriocarpum) showed no obvious responses to GA3 (Supplementary Fig. S4), which is similar to the results we found in pea whereby GA3 treatment cannot promote bud outgrowth (Supplementary Fig. S2). It is possible that in those species, gibberellin may play a role as a negative factor in controlling lateral bud outgrowth as reported in pea (Scott et al. 1967, Luisi et al. 2011), Arabidopsis (Silverstone et al. 1997) and Populus (Mauriat et al. 2011, Zawaski and Busov 2014). Collectively, our results suggest that compared with the model plants Arabidopsis and pea, a more complex network regulates shoot branching in many perennial trees, in which gibberellin plays an important positive role.
Fig. 9.
GA3 promotes lateral bud outgrowth in papaya. (A) One-year-old papaya tree. (B) Six-week-old papaya seedlings. (C) Bud length at node 2 of 6-week-old papaya seedlings was measured 3 weeks after GA3 or BA treatment (n = 32–34). The axillary buds of papaya were directly treated with GA3 (500 μM) or BA (500 μM) solution. Values are means ± SE for (C). Student’s t-test was used to determine significant differences between the treated and control groups. Significance levels: **P < 0.01.
Fig. 10.
GA3 promotes the lateral bud outgrowth in other tree plants. The top approximately 20 cm of the selected branches was sprayed with 500 μM GA3 or the control solutions every other day twice. The red arrows indicate the stimulated lateral buds. Scale bars = 1 cm.
SL, auxin and CK are three key phytohormones that co-ordinately regulate shoot branching. In pea and Arabidopsis, CK is the only phytohormone playing a positive role in regulating bud outgrowth, while gibberellin seems to inhibit this process. The lateral bud outgrowth is believed to be closely correlated with CK levels in the bud (Turnbull et al. 1997, Tanaka et al. 2006). After decapitation, the expression levels of CK biosynthesis genes (IPT genes) were rapidly increased at nodal stems, suggesting the locally biosynthesized CK rather than those derived from the roots controls bud outgrowth (Tanaka et al. 2006). Consistently in J. curcas, we also found that the expression level of IPT genes was rapidly increased at the nodal stems within 6 h after decapitation (Supplementary Fig. S5), which indicates that enhanced local CK biosynthesis is also a prerequisite for successful bud outgrowth in J. curcas. Initially, it was thought that bud outgrowth promoted by gibberellin in J. curcas could also be correlated with the increased CK biosynthesis at the nodal stem after GA3 treatment. However, the gene expression analysis at the nodal stems revealed that GA3 treatment significantly down-regulated the expression of most of the IPT genes (Supplementary Fig. S5), demonstrating that bud outgrowth induced by GA3 treatment is not through regulation of CK biosynthesis at the node, which is different from that by decapitation.
Our physiological results showed that both gibberellin and CK could effectively promote bud outgrowth in J. curcas and papaya. This raises the question of whether these two hormones act redundantly in the regulation of bud outgrowth in these species. In J. curcas, single GA3 or BA treatment can effectively promote bud outgrowth on the 2-year-old J. curcas trees (Fig. 1A), but the promotion is less effective on the 4-week-old seedlings (Fig. 2A). Unlike on the adult trees, growth of lateral buds on the seedlings after single GA3 or BA treatment only lasted for a few days, then the buds became dormant again (Fig. 2A), indicating that bud outgrowth was more tightly regulated on the seedlings than on the adult trees. The co-operation of gibberellin and CK in the regulation of bud outgrowth can be more obviously observed on the seedlings. Co-application of GA3 and BA had profound stimulative effects, which led to an enhanced and continued outgrowth of the lateral buds on seedlings (Fig. 2A, B). Co-application of GA3 and BA at much lower concentrations (e.g. 10 µM each) showed stronger stimulative effects on lateral bud outgrowth (Fig. 3B) compared with that by single GA3 or BA (500 µM) treatment (Fig. 2B). However, the successful outgrowth of the lateral buds severely inhibits the growth of apical buds (Supplementary Fig. S6), which probably resulted from competition for resources between lateral buds and apical buds. Moreover, we found that the bud outgrowth stimulated by BA treatment could be significantly inhibited by additional application of PAC (Fig. 4). However, we found that lateral buds of the root-excised shoots cultured in the liquid medium showed a weak response to GA3 treatment, whereas co-application with BA or addition of BA in the liquid medium rescued the response. These results demonstrate that in J. curcas, especially in the seedlings, gibberellin and CK are both required for the successful bud outgrowth. However, further work will be needed to elucidate the mechanism of how gibberellin and CK interact with each other in the promotion of bud outgrowth.
The findings in this study show that gibberellin also interacts with SL to regulate shoot branching in J. curcas. Nakamura et al. (2013) showed that rice DWARF14 (D14), an essential component of plant SL signaling, interacts with a gibberellin signaling repressor SLR1 in an SL-dependent manner, thus contributing to the negative regulation of gibberellin signaling, suggesting that SL and gibberellin signaling are co-ordinated during the control of shoot development and growth. It will be interesting to identify the mediators of the cross-talk between gibberellin and SL and other regulators of shoot branching in woody plants. Our findings indicate that the mechanism controlling shoot branching in perennial trees is not completely conserved with regard to that in annual species, emphasizing the importance of further studies to identify the components involved in the control of shoot branching in woody plants.
Materials and Methods
Plant materials and growth conditions
Jatropha curcas trees and seedlings were propagated from the cultivar ‘Flowery’. Jatropha curcas and Carica papaya (papaya) were grown in the experimental field and greenhouse at the Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences (21°54'N, 101°46'E) (Pan et al. 2014). The photosynthetic active radiation reached 1,850 μmol m−2 s−1 in summer, and 1,550 μmol m−2 s−1 in winter. The J. curcas seedlings used in the experiments were grown under long-day conditions (14 h light/10 h dark) at 22°C. The seedlings were grown in peat soil. Plants were fertilized with 1/4 Murashige and Skoog (MS) solution. For the root excision experiment, the shoots of 3-week-old seedlings were separated from the roots, leaving two-thirds of the hypocotyl tissues intact, and cultured in MS medium with or without BA in darkness overnight. Phytohormone treatments were administered at node 1 one day after the root excision. The excised stems were kept in an incubator (Sanyo) with 80% humidity, a 12 h light/12 h dark photoperiod, 25°C light/20°C dark and 80 μmol m−2 s−1 radiation.
Phytohormone application
To make stock solutions (10 mM) of phytohormones, GA3 (Sigma) was dissolved in ethanol, BA (Sigma) in 0.5 M NaOH solution, and rac-GR24 (Chiralix) in acetone. These stock solutions were used to prepare working solutions of different concentrations, and the working solutions contained 0.05% (v/v) Tween-20 (BBI). All working solutions for each experiment had the same solvent. For the bud treatment, around 20 μl of working solution was directly dropped onto the leaf axil with a pipettor. For the shoot treatment, the stem was sprayed with working solution using a 100 ml plastic sprayer. Approximately 2 ml of working solution was used for each shoot treatment.
J. curcas transformation
The full-length cDNA for JcGA20ox1 was cloned into the binary vector pOCA30. Transformation of J. curcas was performed according to a previously described protocol (Pan et al. 2010). The transgenic shoots failed to generate roots in the root generation culture, so instead the transgenic shoots were grafted onto the rootstocks of wild-type J. curcas seedlings to generate transgenic plants (Li et al. 2014). The population of the transgenic lines was increased by grafting the transgenic shoots to the wild-type stock.
RNA extraction and quantitative real-time PCR (qPCR)
For analysis of gene expression in the seedlings, the nodal stem at node 1 (approximately 10–20 mg) of 3-week-old J. curcas was sliced and immediately frozen in liquid nitrogen and kept at –80°C. For the expression profile analysis of gibberellin and SL biosynthesis genes, samples were collected from the 2-year-old J. curcas trees. All the samples were prepared in triplicate. Total RNA was isolated using pBIOZOL RNA extraction reagent (Bioer). RNA was quantified using a NanoDrop 2000 spectrophotometer (Thermo). Approximately 1 μg of total RNA was used for cDNA synthesis according to the method described in the TAKARA PrimeScript™ RT Reagent Kit (TAKARA Biotechnology). qPCR was performed on the LightCycler 480II (Roche). Primers are listed in Supplementary Table S1.
Supplementary data
Supplementary data are available at PCP online.
Funding
This work was supported by the National Natural Science Foundation of China [31370595]; the Top Science and Technology Talents Scheme of Yunnan Province [2009CI123]; the Natural Science Foundation of Yunnan Province [2011FA034]; the CAS 135 Program [XTBG-T02].
Supplementary Material
Acknowledgments
We thank Miss Mei-Li Zhao for her assistance with the field work, Dr. Bo Pan for the identification of the plant species in this study, and Dr. Diqiu Yu for generously providing the binary vector pOCA30. The authors gratefully acknowledge the Central Laboratory of the Xishuangbanna Tropical Botanical Garden for providing research facilities.
Disclosures
The authors have no conflicts of interest to declare.
Glossary
Abbreviations
- BA
6-benzyladenine
- CK
cytokinin
- IPT
adenosine phosphate isopentenyl transferase
- MS
Murashige and Skoog
- PAC
paclobutrazol
- qPCR
quantitative real-time PCR
- SL
strigolactone
- tZ
trans-zeatin
References
- Agharkar M., Lomba P., Altpeter F., Zhang H., Kenworthy K., Lange T. (2007) Stable expression of AtGA2ox1 in a low-input turfgrass (Paspalum notatum Flugge) reduces bioactive gibberellin levels and improves turf quality under field conditions. Plant Biotechnol. J. 5: 791–801. [DOI] [PubMed] [Google Scholar]
- Aguilar-Martínez J.A., Poza-Carrión C., Cubas P. (2007) Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 19: 458–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agusti J., Herold S., Schwarz M., Sanchez P., Ljung K., Dun E.A., et al. (2011) Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants. Proc. Natl Acad. Sci. USA 108: 20242–20247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangerth F. (1994) Response of cytokinin concentration in the xylem exudate of bean (Phaseolus vulgaris L.) plants to decapitation and auxin treatment, and relationship to apical dominance. Planta 194: 439–442. [Google Scholar]
- Beveridge C.A., Ross J.J., Murfet I.C. (1994) Branching mutant rms-2 in Pisum sativum-grafting studies and endogenous indole-3-acetic acid levels. Plant Physiol. 104: 953–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge C.A., Symons G.M., Murfet I.C., Ross J.J., Rameau C. (1997) The rms1 mutant of pea has elevated indole-3-acetic acid levels and reduced root-sap zeatin riboside content but increased branching controlled by graft-transmissible signal(s). Plant Physiol. 115: 1251–1258. [Google Scholar]
- Braun N., de Saint Germain A., Pillot J.P., Boutet-Mercey S., Dalmais M., Antoniadi I., et al. (2012) The pea TCP transcription factor PsBRC1 acts downstream of strigolactones to control shoot branching. Plant Physiol. 158: 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer P.B., Dun E.A., Ferguson B.J., Rameau C., Beveridge C.A. (2009) Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol. 150: 482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.-S., Pan B.-Z., Wang G.-J., Ni J., Niu L., Xu Z.-F. (2014) Analysis of the transcriptional responses in inflorescence buds of Jatropha curcas exposed to cytokinin treatment. BMC Plant Biol. 14: 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choubane D., Rabot A., Mortreau E., Legourrierec J., Péron T., Foucher F., et al. (2012) Photocontrol of bud burst involves gibberellin biosynthesis in Rosa sp. J. Plant Physiol. 169: 1271–1280. [DOI] [PubMed] [Google Scholar]
- Domagalska M.A., Leyser O. (2011) Signal integration in the control of shoot branching. Nat. Rev. Mol. Cell Biol. 12: 211–221. [DOI] [PubMed] [Google Scholar]
- Dun E.A., de Saint Germain A., Rameau C., Beveridge C.A. (2012) Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol. 158: 487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfving D.C., Visser D.B., Henry J.L. (2011) Gibberellins stimulate lateral branch development in young sweet cherry trees in the orchard. Int. J. Fruit Sci. 11: 41–54. [Google Scholar]
- Ghosh A., Chikara J., Chaudhary D.R. (2011) Diminution of economic yield as affected by pruning and chemical manipulation of Jatropha curcas L. Biomass Bioenerg. 35: 1021–1029. [Google Scholar]
- Ghosh A., Chikara J., Chaudhary D.R., Prakash A.R., Boricha G., Zala A. (2010) Paclobutrazol arrests vegetative growth and unveils unexpressed yield potential of Jatropha curcas. J. Plant Growth Regul. 29: 307–315. [Google Scholar]
- Gomez-Roldan V., Fermas S., Brewer P.B., Puech-Pagès V., Dun E.A., Pillot J.-P., et al. (2008) Strigolactone inhibition of shoot branching. Nature 455: 189–194. [DOI] [PubMed] [Google Scholar]
- Guan J.C., Koch K.E., Suzuki M., Wu S., Latshaw S., Petruff T., et al. (2012) Diverse roles of strigolactone signaling in maize architecture and the uncoupling of a branching-specific subnetwork. Plant Physiol. 160: 1303–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S.M., Hillman J.R. (1975) Correlative inhibition of lateral bud growth in Phaseolus vulgaris L. Timing of bud growth following decapitation. Planta 123: 137–143. [DOI] [PubMed] [Google Scholar]
- Janssen B.J., Drummond R.S., Snowden K.C. (2014) Regulation of axillary shoot development. Curr. Opin. Plant Biol. 17: 28–35. [DOI] [PubMed] [Google Scholar]
- Jia Q.J., Zhang J.J., Westcott S., Zhang X.Q., Bellgard M., Lance R., et al. (2009) GA-20 oxidase as a candidate for the semidwarf gene sdw1/denso in barley. Funct. Integr. Genomics 9: 255–262. [DOI] [PubMed] [Google Scholar]
- Jia Q.J., Zhang X.Q., Westcott S., Broughton S., Cakir M., Yang J.M., et al. (2011) Expression level of a gibberellin 20-oxidase gene is associated with multiple agronomic and quality traits in barley. Theor. Appl. Genet. 122: 1451–1460. [DOI] [PubMed] [Google Scholar]
- Kebrom T.H., Spielmeyer W., Finnegan E.J. (2013) Grasses provide new insights into regulation of shoot branching. Trends Plant Sci. 18: 41–48. [DOI] [PubMed] [Google Scholar]
- King R.A., Vanstaden J. (1988) Differential responses of buds along the shoot of Pisum sativum to isopentenyladenine and zeatin application. Plant Physiol. Biochem. 26: 253–259. [Google Scholar]
- Ko D., Kang J., Kiba T., Park J., Kojima M., Do J., et al. (2014) Arabidopsis ABCG14 is essential for the root-to-shoot translocation of cytokinin. Proc. Natl Acad. Sci. USA 111: 7150–7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M., Elgersma A., Hanhart C.J., Vanloenenmartinet E.P., Vanrijn L., Zeevaart J.A.D. (1985) A gibberellin insensitive mutant of Arabidopsis thaliana. Physiol. Plant. 65: 33–39. [Google Scholar]
- Leyser O. (2003) Regulation of shoot branching by auxin. Trends Plant Sci. 8: 541–545. [DOI] [PubMed] [Google Scholar]
- Li C., Luo L., Fu Q., Niu L., Xu Z.-F. (2014) Isolation and functional characterization of JcFT, a FLOWERING LOCUS T (FT) homologous gene from the biofuel plant Jatropha curcas. BMC Plant Biol. 14: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.J., Guevara E., Herrera J., Bangerth F. (1995) Effect of apex excision and replacement by 1-naphthylacetic acid on cytokinin concentration and apical dominance in pea plants. Physiol. Plant. 94: 465–469. [Google Scholar]
- Lo S.-F., Yang S.-Y., Chen K.-T., Hsing Y.-I., Zeevaart J.A., Chen L.-J., et al. (2008) A novel class of gibberellin 2-oxidases control semidwarfism, tillering, and root development in rice. Plant Cell 20: 2603–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisi A., Lorenzi R., Sorce C. (2011) Strigolactone may interact with gibberellin to control apical dominance in pea (Pisum sativum). Plant Growth Regul 65: 415–419. [Google Scholar]
- Marth P.C., Audia W.V., Mitchell J.W. (1956) Effects of gibberellic acid on growth and development of plants of various genera and species. Bot. Gaz. 118: 106–111. [Google Scholar]
- Mason M.G., Ross J.J., Babst B.A., Wienclaw B.N., Beveridge C.A. (2014) Sugar demand, not auxin, is the initial regulator of apical dominance. Proc. Natl Acad. Sci. USA 111: 6092–6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauriat M., Sandberg L.G., Moritz T. (2011) Proper gibberellin localization in vascular tissue is required to control auxin-dependent leaf development and bud outgrowth in hybrid aspen. Plant J. 67: 805–816. [DOI] [PubMed] [Google Scholar]
- Minakuchi K., Kameoka H., Yasuno N., Umehara M., Luo L., Kobayashi K., et al. (2010) FINE CULM1 (FC1) works downstream of strigolactones to inhibit the outgrowth of axillary buds in rice. Plant Cell Physiol. 51: 1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. (1977) Transport of exogenous auxin in two-branched dwarf pea seedlings (Pisum sativum L.). Planta 136: 91–96. [DOI] [PubMed] [Google Scholar]
- Morris S.E., Cox M.C., Ross J.J., Krisantini S., Beveridge C.A. (2005) Auxin dynamics after decapitation are not correlated with the initial growth of axillary buds. Plant Physiol. 138: 1665–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S.E., Turnbull C.G., Murfet I.C., Beveridge C.A. (2001) Mutational analysis of branching in pea. Evidence that rms1 and rms5 regulate the same novel signal. Plant Physiol. 126: 1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H., Xue Y.L., Miyakawa T., Hou F., Qin H.M., Fukui K., et al. (2013) Molecular mechanism of strigolactone perception by DWARF14. Nat. Commun. 4: 2613. [DOI] [PubMed] [Google Scholar]
- Nordström A., Tarkowski P., Tarkowska D., Norbaek R., Åstot C., Dolezal K., et al. (2004) Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin–cytokinin-regulated development. Proc. Natl Acad. Sci. USA 101: 8039–8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski N., Sun T.P., Gubler F. (2002) Gibberellin signaling: biosynthesis, catabolism, and response pathways. Plant Cell 14: S61–S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B.-Z., Chen M.-S., Ni J., Xu Z.-F. (2014) Transcriptome of the inflorescence meristems of the biofuel plant Jatropha curcas treated with cytokinin. BMC Genomics 15: 974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J., Fu Q., Xu Z.-F. (2010) Agrobacterium tumefaciens-mediated transformation of biofuel plant Jatropha curcas using kanamycin selection. Afr. J. Biotechnol. 9: 6477–6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W.W., Sun F., Wang Q.J., Chen M.L., Huang Y.Q., Feng Y.Q., et al. (2011) Rice ethylene-response AP2/ERF factor OsEATB restricts internode elongation by down-regulating a gibberellin biosynthetic gene. Plant Physiol. 157: 216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs T., Thimann K.V. (1967) The role of auxins and cytokinins in the release of buds from dominance. Amer. J. Bot. 54: 136–144. [Google Scholar]
- Sato S., Hirakawa H., Isobe S., Fukai E., Watanabe A., Kato M., et al. (2011) Sequence analysis of the genome of an oil-bearing tree, Jatropha curcas L. DNA Res. 18: 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott T.K., Case D.B., Jacobs W.P. (1967) Auxin–gibberellin interaction in apical dominance. Plant Physiol. 42: 1329–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone A.L., Mak P.Y.A., Martinez E.C., Sun T.P. (1997) The new RGA locus encodes a negative regulator of gibberellin response in Arabidopsis thaliana. Genetics 146: 1087–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow R. (1937) On the nature of correlative inhibition. New Phytol. 36: 283–300. [Google Scholar]
- Stirnberg P., Furner I.J., Ottoline Leyser H. (2007) MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J. 50: 80–94. [DOI] [PubMed] [Google Scholar]
- Stirnberg P., van De Sande K., Leyser H.O. (2002) MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 129: 1131–1141. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Takei K., Kojima M., Sakakibara H., Mori H. (2006) Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J. 45: 1028–1036. [DOI] [PubMed] [Google Scholar]
- Thimann K.V., Skoog F. (1934) On the inhibition of bud development and other functions of growth substance in Vicia faba. Proc. R. Soc. B: Biol. Sci . 114: 317–339. [Google Scholar]
- Turnbull C.G.N., Raymond M.A.A., Dodd I.C., Morris S.E. (1997) Rapid increases in cytokinin concentration in lateral buds of chickpea (Cicer arietinum L) during release of apical dominance. Planta 202: 271–276. [Google Scholar]
- Umehara M., Hanada A., Yoshida S., Akiyama K., Arite T., Takeda-Kamiya N., et al. (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455: 195–200. [DOI] [PubMed] [Google Scholar]
- Wickson M., Thimann K.V. (1958) The antagonism of auxin and kinetin in apical dominance. Physiol. Plant. 11: 62–74. [Google Scholar]
- Wu P., Zhou C., Cheng S., Wu Z., Lu W., Han J., et al. (2015) Integrated genome sequence and linkage map of physic nut (Jatropha curcas L.), a biodiesel plant. Plant J. 81: 810–821. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S. (2008) Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 59: 225–251. [DOI] [PubMed] [Google Scholar]
- Zawaski C., Busov V.B. (2014) Roles of gibberellin catabolism and signaling in growth and physiological response to drought and short-day photoperiods in Populus trees. PLoS One 9: e86217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.