Abstract
Purpose
Dysplastic lesions of the oral epithelium are known precursors of oral cancer. A significant proportion of oral dysplastic lesions have functional defects in p53 response pathways. The ONYX-015 adenovirus is selectively cytotoxic to cells carrying defects in p53-dependent signaling pathways. The current study sought to establish the feasibility and activity of ONYX-015 administered topically as a mouthwash to patients with clinically apparent and histologically dysplastic lesions of the oral mucosa.
Patients and Methods
A total of 22 patients (19 assessable patients) were enrolled onto the study. ONYX-015 was administered on three different schedules to consecutive cohorts. Biopsies of the involved mucosa were performed to evaluate histologic response and changes in expression of putative markers of malignant potential, including p53, cyclin D1, and Ki-67. Serology was performed to measure antiadenoviral titers.
Results
Histologic resolution of dysplasia was seen in seven (37%) of 19 patients, and the grade of dysplasia improved in one additional patient. The majority of responses were transient. No toxicity greater than grade 2 (febrile episode in one patient) was observed. Only one of seven patients demonstrated an increase in circulating antiadenoviral antibody titer while on therapy. Although responding and resistant lesions had similar mean p53 staining at baseline, histologic response correlated with a decrease in p53 positivity over time. Significant changes in cyclin D1 or Ki-67 were not observed. Viral replication was confirmed in two of three lesions examined.
Conclusion
This novel approach to cancer prevention is tolerable, feasible, and has demonstrable activity.
Approximately 40,000 cases of head and neck squamous cell carcinoma (HNSCC) are diagnosed annually in the United States, of which approximately 27,000 derive from the oral cavity and pharynx.1 The long-term survival for patients with oral cancer has remained approximately 50% over the past 40 years. Among the factors contributing to this poor outcome is the frequency of multiple synchronous or metachronous primary tumors.2 Among patients with early-stage disease, second primary tumors are the most common cause of treatment failure and death.3 The rate of second primary tumors in these patients has been reported to be 3% to 5% per year, a higher rate than for any other malignancy.4 Therefore, oral cancer is the classic example of field carcinogenesis: multiple individual primary tumors develop independently as a result of chronic carcinogenic exposure.5 The model of field carcinogenesis is supported by both epidemiologic and molecular studies and accounts for the failure of surgical excision to substantially reduce the risk of cancer in patients with oral dysplasia.6–11 Successful therapeutic intervention for premalignant lesions could have a major impact on the long-term survival of these patients.
The clinical presentation of premalignant disease in the oral cavity is highly heterogeneous and includes both erythroplakia and leukoplakia. Within such lesions, the finding of cytologic dysplasia is an important predictor of malignant potential.12–14 The direct clonal relationship between oral dysplasia and invasive HNSCC has been well documented.15,16
Despite recognition of the premalignant nature of oral dysplastic lesions, there are few, if any, effective therapies to prevent progression of oral carcinogenesis. Surgical excision can remove grossly evident disease but cannot address the spread of clonally related cells into normal-appearing mucosa or the existence of multiple independent premalignant lesions.16–19 Systemic treatment with differentiating agents, such as isotretinoin, has been shown to delay but ultimately not prevent development of oral cancer in patients with premalignant disease, and the use of isotretinoin at maximally effective doses has been limited by adverse systemic effects.20–24 New approaches based on directed cytotoxic therapies with minimal systemic toxicity are needed.
Approximately 40% to 50% of HNSCC carry an inactivating mutation of the p53 tumor suppressor gene.25 Altered p53 expression is found in up to 45% of dysplastic mucosal lesions of the head and neck.26 Dysregulation of p53 in mucosal epithelium correlates with increased proliferation and dedifferentiation.27 Metachronous primary tumors of the head and neck may carry discordant p53 mutations, confirming their independent etiology.8 Together, these findings suggest that mutational inactivation of p53 is a critical and relatively early event in oral carcinogenesis, often preceding clinically evident malignant transformation.
ONYX-015 is an attenuated adenovirus designed to preferentially replicate in and destroy p53-mutant cells.28–30 ONYX-015 carries an inactivating deletion in the gene encoding the E1B 55-kd protein. The E1B 55-kd protein binds to and inactivates cellular p53 and is required for efficient viral replication in most human cells.31 In cells lacking p53 function, adenoviral replication can proceed in the absence of E1B 55-kd protein. Thus, ONYX-015 may be selectively lytic in cells in which p53-dependent signaling pathways are nonfunctional.30
Recent studies demonstrate that ONYX-015 can replicate in several tumor lines containing intact p53, in contrast to initial expectations.32 Cell lines that support ONYX-015 replication despite normal p53 genotype seem to carry other defects in p53-dependent response pathways, including abnormal expression of MDM2 and p14ARF.33,34 These observations extend the potential antitumor activity of ONYX-015 to include not only cells with inactivating mutations of p53 but also cells harboring other functional defects in p53-dependent response pathways.
We hypothesized that topical application of ONYX-015 might have significant efficacy against oral dysplasia. Because dysplastic lesions are confined to the epithelial layer, cells within such lesions might be accessible by direct topical viral administration. The frequent association of oral dysplasia with disruption of p53 function would be expected to support productive replication of ONYX-015. Mouthwash delivery allows concurrent treatment of the entire oral mucosal field; cells harboring premalignant genetic alterations in areas not demonstrating grossly evident dysplasia might also be susceptible to viral infection and lysis. Finally, topical administration was predicted to limit potential adverse effects by minimizing systemic viral exposure, an important consideration in preventive therapy.
Based on these considerations, we initiated a clinical trial for patients with oral dysplasia using ONYX-015 in suspension as an oral rinse. The goals of this study included evaluation of toxicity and feasibility of viral mouthwash administration, quantitative analysis of histologic response in oral dysplasia, and investigation of potential associations between response and molecular determinants of hyperproliferation or transformation associated with oral cancer, including p53, Ki-67, and cyclin D1.35
PATIENTS AND METHODS
Patients
Enrollment was limited to adults (age ≥ 18 years) with a Zubrod performance status of 2 or less. Eligible patients had grossly evident oral leukoplakia or erythroplakia with histologic evidence of dysplasia on biopsy. All patients provided written informed consent before study enrollment or performance of study-related procedures, in accordance with institutional and federal guidelines.
Virus Administration
ONYX-015 was administered in a total volume of 15 mL of 5% dextrose and 0.45 N saline. Patients were asked to hold the viral suspension in their mouths for a duration of 30 minutes and then to expectorate any remaining solution.
Schedules of Administration
Cohorts of patients were treated on three different sequentially tested regimens (Fig 1). Cohort 1 (n = 7) consisted of patients who were administered ONYX-015 1010 plaque forming units (pfu) daily for 5 days, with cycles repeated every 4 weeks for a maximum of 12 cycles. The rationale behind this schedule was to maximize viral infection over a week and to provide a treatment-free interval of 3 weeks to monitor for toxicity. Patients underwent lesion biopsy before initiation of therapy and during cycles 6 and 12. Cohort 2 (n = 12) were administered a similar dose-intensity, receiving once-weekly administration of ONYX-015 1010 pfu for 24 weeks. Patients underwent biopsy before initiation of therapy and in week 12. Patients demonstrating a histologic response continued on therapy to week 24. Cohort 3 (n = 3) was designed to evaluate a more dose-intensive regimen and to combine the potentially beneficial aspects of both prior regimens. Patients in cohort 3 received ONYX-015 1011 pfu daily for 5 days, followed by weekly administration over the next 5 weeks. This 6-week block was repeated in weeks 7 to 12.
Fig 1.
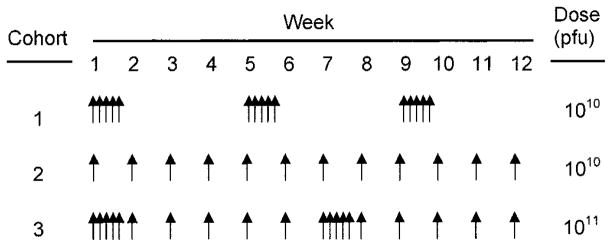
Schematic comparison of schedules of administration. Cohort 1 received ONYX-015 1010 plaque forming units (pfu) for 5 consecutive days. Cohort 2 received ONYX-015 1010 pfu weekly. Cohort 3 received ONYX-015 1011 pfu daily for 5 days, followed by weekly administration for 5 weeks.
Toxicity and Response Evaluation
Toxicity was assessed using National Cancer Institute common toxicity criteria version 2.0. Any toxicity attributable to therapy greater than or equal to grade 2 was considered dose limiting. Histologic evaluation of all biopsies was performed independently by two pathologists at different institutions who were blinded to clinical data. Each biopsy sample was assigned one of the following scores reflecting the extent of dysplasia: 0, none; 1, mild (dysplastic features restricted to the lower one third of the epithelium); 2, moderate (dysplasia involving the lower two thirds of the epithelium); 3, severe (full thickness dysplasia); and 4, invasive carcinoma. Scores from both pathologists were averaged to assign a final histologic score. Histologic response was defined as follows: complete response, no evident dysplasia; partial response, decrease of at least one point in histologic score; progressive disease, any increase in histologic score; and stable disease, reevaluation meeting none of the above criteria.
Immunohistochemical Analyses
Biopsy samples were formalin-fixed and paraffin-embedded before sectioning (sections, 8 to 10 μm). Sections were deparaffinized and taken through a graded alcohol series. After heat-mediated antigen retrieval, slides were incubated in Tris 3% H2O2 and incubated with primary antibodies at room temperature, including anti-p53 (Ab6; Oncogene Research Products, San Diego, CA), anti–Ki-67 (Ki-S; DAKO, Carpenteria, CA), and anticyclin D1 (antisera; Cell Marque, Austin, TX). After washing with buffer, slides were incubated with appropriate secondary antibodies, and antigen-antibody binding was detected using the 3,3′ diaminobenzidine substrate chromogen system. Slides were counterstained with hematoxylin. The immunohistochemical grading scale, which was performed by observers blinded to patient data and time of biopsy, was as follows: 0, 0% to 5% positive staining; 1, more than 5% to 20% positive; 2, more than 20% to 50% positive; or 3, more than 50% positive. Positivity of p53 was based on nuclear staining with the anti-p53 antibody.
In Situ Hybridization
Slides were deparaffinized in xylene and washed in ethanol. Slides were immersed in phosphate-buffered saline and Triton X-100, followed by proteinase K digestion and fixation in 4% paraformaldehyde. After dehydration through a series of ethanol washes (70% to 100%), slides were incubated overnight with Adenovirus BioProbe or negative control probe according to the manufacturer’s instructions (Enzo Life Sciences, Farmingdale, NY). Slides were washed and treated with antibiotin and alkaline phosphatase conjugate (Vector Laboratories, Burlingame, CA). The alkaline phosphatase substrate, 5-bromo-4-chloro-3-indolyl phosphate nitroblue tetrazolium salt, was applied, followed by counterstaining with nuclear fast red.
Statistical Methods
The changes over time in molecular correlates including p53, Ki-67, and cyclin D1 in responding and nonresponding patients were compared using the Wilcoxon rank-sum test and Spearman correlation.
RESULTS
Patient Characteristics
A total of 22 patients were enrolled onto this study. Patient characteristics are listed in Table 1. All patients treated had grossly evident abnormalities of the oral mucosa consisting of areas of leukoplakia, erythroplakia, or both. All patients were required to have mucosal dysplasia. Three patients in the first cohort were started on therapy based on pretreatment biopsies initially read by outside pathologists as demonstrating oral dysplasia that could not be confirmed by the study pathologists; two patients demonstrated mucosal hyperplasia without definitive dysplasia, and, in one patient, a focus of microinvasive carcinoma was noted. Because these three patients did not fulfill eligibility criteria, therapy was stopped after pathologic review, and these patients were excluded from analysis. In cohorts 2 and 3, pathologic confirmation of pretreatment dysplasia was performed by study pathologists before initiation of therapy (Table 2).
Table 1.
Patient Characteristics
| Patient Characteristics | No. of Patients | % |
|---|---|---|
| Total enrollment | 22 | |
| Age, years | ||
| Range | 29–80 | |
| Median | 53 | |
| Sex | ||
| Male | 8 | 37 |
| Female | 14 | 64 |
| Location | ||
| Oral tongue | 16 | |
| Oral mucosa* | 5 | |
| Floor of mouth | 1 | |
| Cohort | ||
| 1 | 7 | 32 |
| 2 | 12 | 55 |
| 3 | 3 | 14 |
Buccal, gingival, and palatal surfaces.
Table 2.
Initial Pathology
| Histology | No. of Patients |
|---|---|
| Cohort 1 | |
| SCC in situ* | 1 |
| Dysplasia | 4 |
| Nondysplastic* | 2 |
| Cohorts 2 and 3† | |
| Mild | 5 |
| Moderate | 6 |
| Severe | 4 |
Abbreviation: SCC, squamous cell carcinoma.
These patients were registered but not included in analysis because they did not fulfill eligibility criteria.
All patients on cohort 3 were required to have moderate or severe dysplasia.
Patient Cohort 1
Four patients with initial biopsies demonstrating mucosal dysplasia were treated on this schedule. Two of four patients had histologic resolution of dysplasia after six cycles of viral therapy. One of these patients, who had a history of resected squamous cell carcinoma of the tongue and recurrent severe oral dysplasia and leukoplakia, experienced complete resolution of clinically evident disease after one cycle of therapy, associated with resolution of histologic dysplasia demonstrated on biopsy after cycle 6 (24 weeks, Fig 2). Despite no recurrence of leukoplakia or erythroplakia, a biopsy of normal-appearing mucosa after 12 cycles (48 weeks) demonstrated recurrent severe dysplasia. The other responding patient had a similar course, with resolution of histologic dysplasia after cycle 6 but recurrence and progression of disease after cycle 12.
Fig 2.
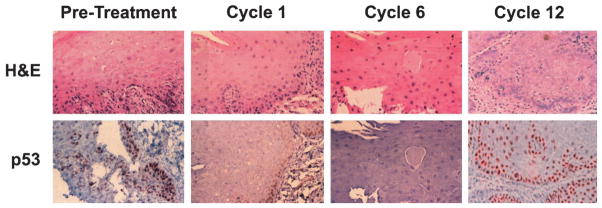
Histologic response. Serial biopsies of a patient in cohort 1 are shown. Top, stained with hematoxylin and eosin (H&E); bottom, anti-p53 staining. The transient complete histologic response and transient disappearance of p53 positivity was associated with complete resolution of clinically evident leukoplakia.
Immunohistochemical staining for p53 paralleled response in the first of these two patients. The initial biopsy demonstrated strong p53 positivity that resolved after cycle 6 but recurred in conjunction with histologic dysplasia after cycle 12 (Fig 2). The dysplastic lesion of the second responding patient was negative for p53.
Cohorts 2 and 3
Because of the observed recurrence of disease in the responding patients in cohort 1, alternative schedules of administration were investigated. The rationale for adopting a weekly schedule for cohort 2 was to maintain similar dose-intensity to that given in cohort 1, while reducing the intervals between viral administrations from 3 weeks to 1 week. Cohort 3 was designed to evaluate the toxicity of a higher intensity regimen, combining aspects of both prior schedules.
Histologic responses were observed in both cohorts 2 and 3 (Table 3). Complete resolution of dysplasia was observed in four of 12 patients in cohort 2 and in one of three patients in cohort 3 (Fig 3). Partial response, from an initial histologic score of 3 to 1.5, was noted in one patient in cohort 2. Recurrent dysplasia after complete histologic response was noted in two patients, both in cohort 2; one of these responses was documented while on therapy (week 24), and the other was documented on first follow-up (week 48). Of the remaining two patients in cohort 2 with complete response, one was lost to follow-up after completing therapy, and one has had no recurrent dysplasia on biopsy after 27 months. The patient with complete response in cohort 3 has also had no evidence of recurrence, with the most recent follow-up being 30 months after completion of therapy.
Table 3.
Histologic Response
| Cohort | Total | No. of Patients
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Best Response
|
Final Response*
|
||||||||
| CR | PR | SD | PD | CR | PR | SD | PD | ||
| 1 | 4 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 4 |
| 2 | 12 | 4 | 1 | 4 | 3 | 2 | 1 | 4 | 5 |
| 3 | 3 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 2 |
Abbreviations: CR, complete response; PR, progressive disease; SD, stable disease; PD, progressive disease.
Defined as response after completion of therapy.
Fig 3.
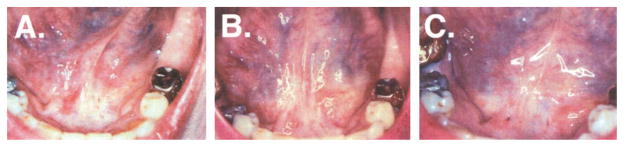
Resolution of clinically evident leukoplakia. Serial photographs of a leukoplakic lesion in a patient in cohort 2 are shown: (A) day 1; (B) week 12; and (C) week 16. The clinical resolution of leukoplakia was associated with a complete histologic response.
Toxicity
Administration of ONYX-015 as a mouthwash was well tolerated in all three cohorts (Table 4). Grade 1 diarrhea, possibly associated with therapy, was noted in four patients. A single episode of grade 2 toxicity was observed (cohort 2); an organ transplantation recipient with a history of recurrent oral herpes simplex virus lesions presented with fever, stomatitis, flu-like symptoms, and oral pain, all typical of previous herpes simplex virus recrudescence in this patient. These symptoms resolved over the course of 1 week, and the patient continued on therapy without further incident.
Table 4.
Toxicities Observed
| Toxicity | Toxicity Grade (No.)
|
||
|---|---|---|---|
| 1 | 2* | 3/4 | |
| Stomatitis | 1 | 1 | 0 |
| Diarrhea | 4 | 0 | 0 |
| Fatigue | 2 | 1 | 0 |
| Pain | 5 | 0 | 0 |
| Fever | 1 | 1 | 0 |
| Flu symptoms | 3 | 1 | 0 |
| Dysphagia | 2 | 0 | 0 |
| Vertigo | 1 | 0 | 0 |
| Infection | 0 | 1 | 0 |
All grade 2 toxicities were related to a single episode of oral herpes simplex virus infection.
Systemic Exposure
One theoretical advantage of topical therapy for oral premalignant disease is avoidance of systemic exposure to cytotoxic therapy, thus potentially minimizing adverse effects. The lack of significant adverse effects attributable to the ONYX-015 therapy in this trial suggested that, in fact, such exposure was minimal. Systemic exposure of adenoviral vectors, including ONYX-015, is typically associated with an increase in circulating antiadenoviral antibody titers. Circulating antiadenoviral antibody titers were measured before therapy and after 12 weeks of therapy in seven patients in cohort 2. Only one of seven patients demonstrated any increase (two-fold) in antiadenoviral titer over the course of treatment.
Molecular Correlates
All patients in cohorts 2 and 3 underwent mucosal biopsies before initiation and after 12 weeks of therapy for immunohistochemical and histologic evaluation. Mutation of the p53 gene can result in production of a stabilized dominant negative form, leading to marked increase in p53 protein levels detectable by immunohistochemistry. Constitutive overexpression of cyclin D1 has been strongly implicated in oral carcinogenesis. Ki-67, a marker of cellular proliferation, is frequently upregulated in oral cancer. Pretreatment staining indices for p53, cyclin D1, and Ki-67, performed on biopsies from all patients in cohorts 2 and 3, were found to be indistinguishable in responding and nonresponding patients (Fig 4) and, thus, do not seem to be predictive of response to ONYX-015.
Fig 4.
Response to ONYX-015 correlates with decline in p53 staining. Biopsies were performed in cohorts 2 and 3 before initiation of ONYX-015 therapy (PreRx) and after 12 weeks of therapy (Wk 12). Black bars indicate responding patients, gray bars indicate nonresponding patients, and error bars indicate SEM. Asterisk indicates significant change from baseline.
Although the pretreatment level of p53 expression did not differ between responding and nonresponding lesions, after 12 weeks of therapy, there was marked difference in p53 expression in responding versus nonresponding lesions; p53 level was significantly suppressed only in responding lesions (Fig 4). The change in p53 staining between responding and nonresponding patients was significant using the Wilcoxon rank-sum test (P = .027). Because both histologic change and p53 staining were measured on a graded scale, this association was further analyzed through derivation of a Spearman correlation (r = .74), confirming the association between histologic response and change in p53 (P = .0017). In contrast, no significant change in either cyclin D1 or Ki-67 expression was observed in responding or nonresponding patients on this study (Wilcoxon rank-sum test: for change in cyclin D1, P = .74; for change in Ki-67, P = .82).
Viral Replication In Vivo
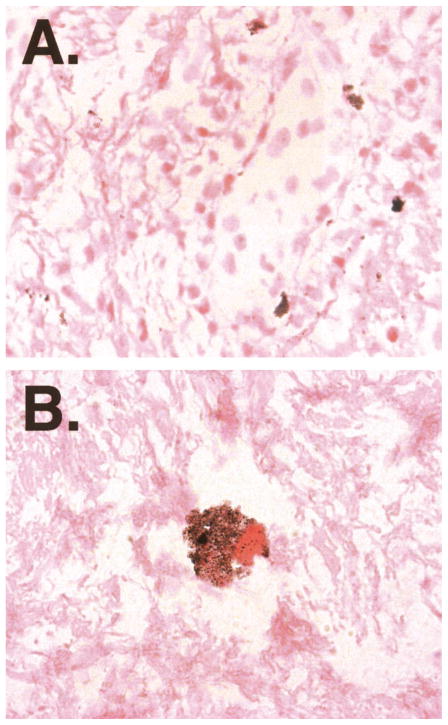
Despite the clinical and histologic responses observed with this therapy, one concern was whether this attenuated adenovirus could actually gain access to and replicate within dysplastic mucosa when applied by topical oral administration. The three patients enrolled onto cohort 3, therefore, underwent an additional mucosal biopsy on day 8 of therapy. The goal of this early biopsy was to attempt to document productive ONYX-015 infection through detection of intracellular genome amplification by in situ hybridization; day 8 was expected to be early in the first round of productive viral synthesis. Scattered cells demonstrating strongly positive in situ hybridization throughout the cytoplasm were noted in biopsies of two of three patients, confirming the potential for productive ONYX-015 infection in oral dysplasia (Fig 5).
Fig 5.
ONYX-015 replication in mucosal tissue of two patients with oral dysplasia. In situ hybridization for adenovirus type 5 was performed. (A) Magnification × 40 demonstrates scattered cells with staining characteristic of intracellular viral proliferation. (B) Magnification × 100 demonstrates punctate staining confined to the cytoplasm.
DISCUSSION
This trial explored three schedules of administration of a replication-competent adenovirus engineered for preferential replication in cells lacking functional p53-dependent response pathways. The rationale for this therapeutic approach was to selectively target a known signaling pathway implicated in oral carcinogenesis. The trial also explored the feasibility of topical oral administration of a chemopreventive agent as a mouthwash, treating the entire oral mucosa as a field and avoiding systemic toxicities in a preventive strategy. ONYX-015, administered as an oral rinse, was found to be extremely well tolerated at doses of up to 1011 pfu/d and was associated with complete histologic response in a subset of patients. Single daily doses of greater than 1011 pfu become impractical because of volume considerations. Given the lack of evident toxicity at even the highest dose evaluated, we recommend further exploration of activity based on the dose and schedule of cohort 3.
A significant negative correlation was observed between histologic response to ONYX-015 therapy and the fraction of cells with immunohistochemically detectable p53 protein. These data are consistent with the hypothesis that biologic response to ONYX-015 is dependent on the capacity to suppress or eliminate growth of p53 mutant cells. An alternative hypothesis could be that p53 staining correlates with histologic severity, regardless of response to therapy. However there was no apparent correlation between p53 staining and histologic severity in pretreatment biopsies. Prior studies of chemopreventive therapy for oral dysplasia have found no correlation between response and change in p53 expression.36 Furthermore, the two other biomarkers used in this study, cyclin D1 and Ki-67, have both been implicated in carcinogenic progression in oral cancer. In contrast to p53, no interval change in expression of either of these two markers was observed in responding or nonresponding patients. Thus, the correlation between change in biomarker expression levels and response to ONYX-015 was found exclusively for the abnormal p53 expression targeted by this agent. In addition to the immunohistochemical assessment used in this trial, incorporation of p53 gene sequencing in future trials involving ONYX-015 might provide further prognostic and mechanistic information.
Despite the observed histologic and clinical responses seen in this cohort of patients, this pilot trial cannot be taken as evidence of ONYX-015 efficacy for oral premalignancy. Although at least two patients remain without evidence of recurrence more than 2 years after therapy, the majority of responses were transient. The clinical course of oral dysplasia is variable and does include spontaneous regression of disease.14 The observed lack of improvement in cyclin D1 and Ki67 is of concern in this respect and suggests that not all genetic changes associated with premalignancy were reversed by ONYX-015 administration. Determination of response rate attributable to ONYX-015 will require a larger, randomized, phase II assessment.
Most chemoprevention trials for premalignant oral cavity lesions have been based on either naturally occurring compounds (vitamin A, vitamin E, and beta-carotene) or synthetically derived retinoids structurally related to vitamin A.37 Initial promising response rates reported with vitamin E and beta-carotene have not been consistently reproduced.38–40 The most extensively evaluated chemopreventive agent for oral cancer is isotretinoin, or 13-cis-retinoic acid.22–24,41,42 In a randomized, placebo-controlled study of high-dose isotretinoin in 44 patients with leukoplakia, both clinical and histologic response rates were significantly higher in the actively treated patients.20 Isotretinoin administration can be limited by toxicity, including both xero-derma and conjunctivitis, and responses are typically of short duration, with the majority of responding patients relapsing within 3 months. Progression of disease can be delayed but not prevented by continued administration of low-dose (0.5 mg/kg/d) isotretinoin.22,24
Retinoid therapy has been evaluated in placebo-controlled trials as a chemopreventive agent in the context of localized head and neck cancer after therapy with curative intent. In the first of these trials, high-dose isotretinoin did not affect either locoregional or distant relapse rates relative to placebo, but it was associated with a reduction in the incidence of second primary tumors.41 A subsequent Eastern Cooperative Oncology Group trial evaluating low-dose isotretinoin versus placebo as secondary chemoprevention in 189 patients with a history of early-stage head and neck cancer failed to demonstrate an effect on the rates of relapse or second primary tumors.43 In both of these trials, toxicity was significantly higher in patients receiving isotretinoin therapy.
Topical administration of ONYX-015 for chemoprevention in the oral cavity has some theoretical advantages over systemic drug therapy. First, topical ONYX-015 administration limits exposure to the involved oral mucosa, minimizing the potential for adverse systemic effects. Second, this approach targets a signaling pathway implicated in malignant transformation. Unlike isotretinoin, which functions primarily as a differentiating agent, ONYX-015 has been shown to be selectively cytotoxic for cells with defects in p53-dependent response pathways. The lack of adverse effects with ONYX-015 combined with evidence of potential efficacy supports the rationale for phase II studies of this agent both in patients with oral dysplasia and as preventive therapy in patients at high risk of local relapse after resection of oral cancer.
Finally, ONYX-015 may have potential synergy with retinoid therapy in oral cancer chemoprevention. High-dose isotretinoin is primarily active against dysplastic lesions without upregulated p53 and does not suppress p53 levels in dysplasia.36 Through its differentiating activity, retinoid therapy may promote transient resolution of the hyperplastic and hyperkeratotic thickening of mucosa associated with early dysplastic lesions. Retinoid therapy might be predicted to increase the accessibility of the basal mucosal layers of dysplastic lesions to viral infection with ONYX-015. In addition to phase II studies of single-agent ONYX-015 in chemoprevention for oral cancer, analysis of the combined efficacy of the differentiating activity of retinoid therapies with the cytotoxic effect of ONYX-015 is warranted.
Acknowledgments
Supported by the Francis Lederer Foundation, grant no. NIH 5 P50 DECA11921-04 from the National Institutes of Health, and ONYX Pharmaceuticals.
We thank Rosalyn Williams, Allison Dekker, and Anthea Atwell for data management and coordination of patient care, David Kirn for contributions to initial trial design, Scott Freeman and Qing Wang for assistance with in situ hybridization, and Dezheng Huo for statistical analysis.
Footnotes
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The authors indicated no potential conflicts of interest.
References
- 1.Jemal A, Murray T, Samuels A, et al. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Anderson WF, Hawk E, Berg CD. Secondary chemoprevention of upper aerodigestive tract tumors. Semin Oncol. 2001;28:106–120. doi: 10.1016/s0093-7754(01)90048-x. [DOI] [PubMed] [Google Scholar]
- 3.Lippman SM, Hong WK. Second malignant tumors in head and neck squamous cell carcinoma: The overshadowing threat for patients with early-stage disease. Int J Radiat Oncol Biol Phys. 1989;17:691–694. doi: 10.1016/0360-3016(89)90126-0. [DOI] [PubMed] [Google Scholar]
- 4.Day GL, Blot WJ. Second primary tumors in patients with oral cancer. Cancer. 1992;70:14–19. doi: 10.1002/1097-0142(19920701)70:1<14::aid-cncr2820700103>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 5.Slaughter DP, Southwick HW, Smejkal W. “Field cancerization” in oral stratified squamous epithelium. Cancer. 1953;6:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 6.Vokes EE, Weichselbaum RR, Lippman SM, et al. Head and neck cancer. N Engl J Med. 1993;328:184–194. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 7.Jones AS, Morar P, Phillips DE, et al. Second primary tumors in patients with head and neck squamous cell carcinoma. Cancer. 1995;75:1343–1353. doi: 10.1002/1097-0142(19950315)75:6<1343::aid-cncr2820750617>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 8.Chung KY, Mukhopadhyay T, Kim J, et al. Discordant p53 gene mutations in primary head and neck cancers and corresponding second primary cancers of the upper aerodigestive tract. Cancer Res. 1993;53:1676–1683. [PubMed] [Google Scholar]
- 9.Scholes AG, Woolgar JA, Boyle MA, et al. Synchronous oral carcinomas: Independent or common clonal origin? Cancer Res. 1998;58:2003–2006. [PubMed] [Google Scholar]
- 10.Bedi GC, Westra WH, Gabrielson E, et al. Multiple head and neck tumors: Evidence for a common clonal origin. Cancer Res. 1996;56:2484–2487. [PubMed] [Google Scholar]
- 11.Worsham MJ, Wolman SR, Carey TE, et al. Common clonal origin of synchronous primary head and neck squamous cell carcinomas: Analysis by tumor karyotypes and fluorescence in situ hybridization. Hum Pathol. 1995;26:251–261. doi: 10.1016/0046-8177(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 12.Waldron CA, Shafer WG. Leukoplakia revisited: A clinicopathologic study 3256 oral leukoplakias. Cancer. 1975;36:1386–1392. doi: 10.1002/1097-0142(197510)36:4<1386::aid-cncr2820360430>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 13.Axell T. A prevalence study of oral mucosal lesions in an adult Swedish population. Odontol Revy. 1976;27:1–103. [PubMed] [Google Scholar]
- 14.Sciubba JJ. Oral leukoplakia. Crit Rev Oral Biol Med. 1995;6:147–160. doi: 10.1177/10454411950060020401. [DOI] [PubMed] [Google Scholar]
- 15.Califano J, Westra WH, Koch W, et al. Unknown primary head and neck squamous cell carcinoma: Molecular identification of the site of origin. J Natl Cancer Inst. 1999;91:599–604. doi: 10.1093/jnci/91.7.599. [DOI] [PubMed] [Google Scholar]
- 16.Califano J, Westra WH, Meininger G, et al. Genetic progression and clonal relationship of recurrent premalignant head and neck lesions. Clin Cancer Res. 2000;6:347–352. [PubMed] [Google Scholar]
- 17.Califano J, van der Riet P, Westra W, et al. Genetic progression model for head and neck cancer: Implications for field cancerization. Cancer Res. 1996;56:2488–2492. [PubMed] [Google Scholar]
- 18.Mao L, Lee JS, Fan YH, et al. Frequent microsatellite alterations at chromosomes 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. Nat Med. 1996;2:682–685. doi: 10.1038/nm0696-682. [DOI] [PubMed] [Google Scholar]
- 19.Brennan JA, Mao L, Hruban RH, et al. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:429–435. doi: 10.1056/NEJM199502163320704. [DOI] [PubMed] [Google Scholar]
- 20.Hong WK, Endicott J, Itri LM, et al. 13-cis-retinoic acid in the treatment of oral leukoplakia. N Engl J Med. 1986;315:1501–1505. doi: 10.1056/NEJM198612113152401. [DOI] [PubMed] [Google Scholar]
- 21.Mao L, El-Naggar AK, Papadimitrakopoulou V, et al. Phenotype and genotype of advanced premalignant head and neck lesions after chemopreventive therapy. J Natl Cancer Inst. 1998;90:1545–1551. doi: 10.1093/jnci/90.20.1545. [DOI] [PubMed] [Google Scholar]
- 22.Lippman SM, Batsakis JG, Toth BB, et al. Comparison of low-dose isotretinoin with beta carotene to prevent oral carcinogenesis. N Engl J Med. 1993;328:15–20. doi: 10.1056/NEJM199301073280103. [DOI] [PubMed] [Google Scholar]
- 23.Lotan R, Xu XC, Lippman SM, et al. Suppression of retinoic acid receptor-beta in premalignant oral lesions and its up-regulation by isotretinoin. N Engl J Med. 1995;332:1405–1410. doi: 10.1056/NEJM199505253322103. [DOI] [PubMed] [Google Scholar]
- 24.Papadimitrakopoulou VA, Hong WK, Lee JS, et al. Low-dose isotretinoin versus beta-carotene to prevent oral carcinogenesis: Long-term follow-up. J Natl Cancer Inst. 1997;89:257–258. doi: 10.1093/jnci/89.3.257. [DOI] [PubMed] [Google Scholar]
- 25.Koch WM, Brennan JA, Zahurak M, et al. p53 mutation and locoregional treatment failure in head and neck squamous cell carcinoma. J Natl Cancer Inst. 1996;88:1580–1586. doi: 10.1093/jnci/88.21.1580. [DOI] [PubMed] [Google Scholar]
- 26.Shin DM, Kim J, Ro JY, et al. Activation of p53 gene expression in premalignant lesions during head and neck tumorigenesis. Cancer Res. 1994;54:321–326. [PubMed] [Google Scholar]
- 27.Nees M, Homann N, Discher H, et al. Expression of mutated p53 occurs in tumor-distant epithelia of head and neck cancer patients: A possible molecular basis for the development of multiple tumors. Cancer Res. 1993;53:4189–4196. [PubMed] [Google Scholar]
- 28.Cohen EE, Rudin CM. ONYX-015: Onyx Pharmaceuticals. Curr Opin Investig Drugs. 2001;2:1770–1775. [PubMed] [Google Scholar]
- 29.Ries S, Korn WM. ONYX-015: Mechanisms of action and clinical potential of a replication-selective adenovirus. Br J Cancer. 2002;86:5–11. doi: 10.1038/sj.bjc.6600006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heise C, Sampson-Johannes A, Williams A, et al. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 31.Burgert HG, Ruzsics Z, Obermeier S, et al. Subversion of host defense mechanisms by adenoviruses. Curr Top Microbiol Immunol. 2002;269:273–318. doi: 10.1007/978-3-642-59421-2_16. [DOI] [PubMed] [Google Scholar]
- 32.Rothmann T, Hengstermann A, Whitaker NJ, et al. Replication of ONYX-015, a potential anticancer adenovirus, is independent of p53 status in tumor cells. J Virol. 1998;72:9470–9478. doi: 10.1128/jvi.72.12.9470-9478.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCormick F. ONYX-015 selectivity and the p14ARF pathway. Oncogene. 2000;19:6670–6672. doi: 10.1038/sj.onc.1204096. [DOI] [PubMed] [Google Scholar]
- 34.Ries SJ, Brandts CH, Chung AS, et al. Loss of p14ARF in tumor cells facilitates replication of the adenovirus mutant dl1520 (ONYX-015) Nat Med. 2000;6:1128–1133. doi: 10.1038/80466. [DOI] [PubMed] [Google Scholar]
- 35.Papadimitrakopoulou VA, Shin DM, Hong WK. Molecular and cellular biomarkers for field cancerization and multistep process in head and neck tumorigenesis. Cancer Metastasis Rev. 1996;15:53–76. doi: 10.1007/BF00049487. [DOI] [PubMed] [Google Scholar]
- 36.Lippman SM, Shin DM, Lee JJ, et al. p53 and retinoid chemoprevention of oral carcinogenesis. Cancer Res. 1995;55:16–19. [PubMed] [Google Scholar]
- 37.Rudin CM. Chemoprevention in oral dysplasia. In: Rose BD, editor. UpToDate: Oncology. Wellesley, MA: UpToDate; 2001. [Google Scholar]
- 38.Liede K, Hietanen J, Saxen L, et al. Long-term supplementation with alpha-tocopherol and beta-carotene and prevalence of oral mucosal lesions in smokers. Oral Dis. 1998;4:78–83. doi: 10.1111/j.1601-0825.1998.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 39.Jyothirmayi R, Ramadas K, Varghese C, et al. Efficacy of vitamin A in the prevention of loco-regional recurrence and second primaries in head and neck cancer. Eur J Cancer B Oral Oncol. 1996;32B:373–376. doi: 10.1016/s0964-1955(96)00010-3. [DOI] [PubMed] [Google Scholar]
- 40.van Zandwijk N, Dalesio O, Pastorino U, et al. EUROSCAN, a randomized trial of vitamin A and N-acetylcysteine in patients with head and neck cancer or lung cancer: For the European Organization for Research and Treatment of Cancer Head and Neck and Lung Cancer Cooperative Groups. J Natl Cancer Inst. 2000;92:977–986. doi: 10.1093/jnci/92.12.977. [DOI] [PubMed] [Google Scholar]
- 41.Hong WK, Lippman SM, Itri LM, et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med. 1990;323:795–801. doi: 10.1056/NEJM199009203231205. [DOI] [PubMed] [Google Scholar]
- 42.Khuri FR, Lippman SM, Spitz MR, et al. Molecular epidemiology and retinoid chemoprevention of head and neck cancer. J Natl Cancer Inst. 1997;89:199–211. doi: 10.1093/jnci/89.3.199. [DOI] [PubMed] [Google Scholar]
- 43.Pinto H, Li Y, Loprinzi C, et al. Phase III trial of low-dose 13-cis-retinoic acid for prevention of second primary cancers in stage I–II head and neck cancer: An Eastern Cooperative Oncology Group study. Proc Am Soc Clin Oncol. 2001;20:222a. (abstr 866) [Google Scholar]