Abstract
The hypothalamus-pituitary-adrenal (HPA) axis is particularly sensitive to conditions of maltreatment. In particular, neglected children have shown a flatter slope with lower wake-up values relative to non-neglected children. An intervention, Attachment and Biobehavioral Catch-up (ABC), was developed to enhance biological and behavioral regulation in young children at risk for neglect. The effectiveness of the intervention was assessed in a randomized clinical trial for children with involvement with Child Protective Services. Following the interventions, children receiving the ABC intervention (n = 49) showed more typical cortisol production, with higher wake-up cortisol values and a steeper diurnal slope than children receiving the control intervention (n = 51). These results suggest that the ABC intervention is effective in enhancing biological regulation.
When infants face chronic stress in childhood, such as neglect and poverty, their behavioral and biological regulation is compromised (Gunnar & Vazquez, 2001). The hypothalamus-pituitary-adrenal (HPA) axis is especially vulnerable to the effects of early adversity and non-optimal caregiving (e.g., Bernard, Butzin-Dozier, Rittenhouse, & Dozier, 2010; Gunnar & Vazquez, 2001). Among humans and other primates, cortisol represents an end product of the HPA system. Cortisol typically follows a diurnal pattern, characterized by high wake-up values and low bedtime values. Early adversity has been associated with a blunting of this diurnal pattern, with children living under high-risk conditions showing a blunted pattern of cortisol production across the day (Bernard et al., 2010; Kroupina et al., 2012). These findings, as well as experimental studies with animals (Mirescu, Peters, & Gould, 2004; Sanchez, Ladd, & Plotsky, 2001), suggest that significant stress experienced in early life may disrupt children’s developing regulation of the HPA system. An intervention, Attachment and Biobehavioral Catch-up (ABC), was designed to support children’s development of regulatory capabilities. Through a randomized clinical trial, the current study assessed whether children who received the ABC intervention show more normative diurnal production of cortisol than children who received a control intervention.
HPA axis functioning
There are two relatively orthogonal functions of the HPA axis: mounting a stress response, and maintaining a circadian rhythm.
Stress reactivity
The HPA axis is perhaps best known for its role in mounting a stress response. At of stress, as the result of other limbic system input, the hypothalamus secretes corticotrophin-releasing hormone (CRH), which signals the pituitary to release adrenocorticotropic hormone (ACTH). ACTH is released into the blood stream, which then signals the adrenal cortex to release cortisol. Cortisol is an end product of this system and has a negative feedback function of signaling the shutdown of ACTH and CRH production.
Whereas most older children and adults reliably show a cortisol response under some types of stressful experiences, there is evidence that young children undergo a stress hypo-responsive period (SHRP), during which cortisol is not elevated in response to stressors. Such a period parallels that seen among rodents (Gunnar & Quevado, 2007; Sapolsky & Meaney, 1986), and may function to protect the developing brain from high levels of circulating glucocorticoids (Gunnar & Cheatham, 2003). Among rodents and humans, the availability of a sensitive mother is necessary to maintain this stress hypo-responsive period (Levine, 2001). For example, human infants with organized attachments do not show an increase in cortisol to the Strange Situation, a procedure involving maternal separations, whereas infants with disorganized attachments do show a cortisol response (Bernard & Dozier, 2010; Gunnar, Broderson, Nachmias, Buss, & Rigatuso, 1996). Because differences in reactivity can be difficult to interpret among young children, much of the work on intervention effects has focused instead on diurnal patterns of cortisol production (e.g., Dozier, Peloso, et al., 2006; Fisher, Stoolmiller, Gunnar, & Burraston, 2007).
Diurnal production
Among humans and other diurnal creatures, cortisol has a diurnal pattern, with high morning values and low evening levels. Cortisol begins to rise prior to wake-up and is relatively high at wake-up. It increases to a peak 30 minutes post-wake-up, and then decreases quickly (typically by mid-morning), and is flat to decreasing across the day to near zero levels at the bedtime nadir. This diurnal pattern serves to mobilize energy in the morning and prepare the organism for sleep at night, among other things. The system is important in promoting similar sleep-wake cycles among members of a species.
At birth, human infants do not show an established diurnal rhythm but instead exhibit two cortisol peaks within a 24-hour period (Sippell, Becker, Versmold, Bidlingmaier, & Knorr, 1978). By three months of age, a single diurnal peak and evening nadir is seen (Gunnar & White, 2001; Larson, White, Cochran, Donzella, & Gunnar, 1998; Price, Close, Fielding, 1983), with the pattern gradually approximating the adult pattern over the first two years of life among typically developing children (Gunnar & Donzella, 2002).
Perturbations in diurnal cortisol production have been observed across a variety of conditions of early adversity, including maltreatment, placement into foster care, and extreme institutional deprivation (Bernard et al., 2010; Bruce, Fisher, Pears, & Levine, 2009; Carlson et al., 1995; Gunnar & Vazquez, 2001). The specific experience of neglect, especially early in life, has been associated with a blunting of the HPA axis across a variety of settings and populations. Children reared in extremely depriving institutional settings have shown blunting of diurnal HPA activity relative to children raised with their biological parents (Carlson et al., 1995; Carlson & Earls, 1997; Gunnar & Vazquez, 2001; Kroupina et al., 2012). Lower morning values and atypically flat cortisol production have been observed among foster children removed from their biological caregivers primarily due to the experience of neglect (Bruce et al., 2009; Dozier, Manni, et al., 2006). Bernard et al. (2010) found that children living with their high-risk birth parents who were identified by Child Protective Services due to risk of neglect showed more blunted patterns than even foster children. It has been theorized that the neglecting environments may lead to a downregulation of the HPA axis in response to chronic stress, as reflected in the atypically flat diurnal cortisol rhythms among neglected children (Bruce et al., 2009). Although it is not yet clear whether these blunted cortisol rhythms predict problematic long term outcomes, there is evidence that low, flat diurnal cortisol patterns are associated with increased risk for mood disorders (Gunnar & Vazquez, 2001), aggression (McBurnett, Lahey, Rathouz, & Loeber, 2000), and conduct problems (Pajer, Gardner, Rubin, Perel, & Neal, 2001).
Taken together, recent research suggests that a blunted diurnal cortisol rhythm, as opposed to an overall heightened production of cortisol, reflects the characteristic pattern of HPA axis dysregulation among children who experience early adversity. It is important to note, however, that the literature is mixed, with some studies finding higher cortisol levels among subgroups of children who experience early life stress relative to comparison children. The patterns of cortisol dysregulation (i.e., hyper- versus hypocortisolism) among maltreated children have been found to vary depending on gender, concurrent psychopathology (e.g., internalizing problems), and characteristics of the experienced maltreatment (Bruce et al., 2009; Cicchetti & Rogosch, 2001; Doom, Cicchetti, Rogosh, & Dackis, 2013). In addition to these between-individual factors, the pattern of cortisol dysregulation within maltreated individuals may change over time (e.g., Cicchetti & Rogosch, 2001; Tricket, Noll, Susman, Shenk, & Putnam, 2010). For example, Trickett et al. (2010) found that females who experienced childhood abuse showed a different developmental trajectory in basal cortisol production from childhood through adulthood (assessed 6 times from 6 to 30 years old), compared to non-abused females. In childhood, abused females showed higher cortisol levels relative to control females, whereas the same group of abused females showed lower cortisol levels relative to control females in adulthood. This down-regulation or attenuation of cortisol production over time suggests that both hypo- and hyperactivation of the HPA axis can follow maltreatment. Thus, although we focus our attention here on cortisol dysregulation characterized by blunted diurnal patterns, it is possible that young children experience high cortisol levels at earlier points of development.
Intervening to support children’s regulation of diurnal cortisol production
There is evidence that therapeutic, nurturing environments can normalize cortisol production among previously maltreated children (Cicchetti, Rogosch, Toth, & Sturge-Apple, 2011; Dozier, Peloso, et al., 2006; Fisher et al., 2007). In an exploratory study, Dozier, Peloso, et al. (2006) found that foster infants whose caregivers completed the ABC intervention showed a more normative pattern of cortisol production following the intervention than did foster infants in a control intervention condition. More specifically, foster infants who received the ABC intervention showed lower levels of cortisol that were comparable to a low-risk comparison group, as contrasted with foster infants in the control condition. Fisher et al. (2007) found that preschoolers (the majority with prior experiences of neglect) assigned to an experimental condition (Multidimensional Treatment Foster Care) showed steeper slopes from morning to evening than those receiving regular foster care. Those assigned to the control intervention continued to show a persisting pattern of blunted cortisol reactivity typical of neglected children (Fisher et al., 2007). Improvements among children adopted from orphanage care suggest the robustness of these effects. Once removed from the neglecting environment, previously institutionalized children eventually no longer exhibit the flattened patterns across the day, but develop more normative diurnal rhythms (Gunnar & Donzella, 2002; Kertes, Gunnar, Madsen, & Long, 2008).
Cicchetti et al. (2011) examined mid-morning cortisol of maltreated infants longitudinally across two years. Maltreating parents were randomly assigned to receive an intervention (either child-parent psychotherapy or a psychoeducational parenting intervention) or community services as usual, and morning cortisol was assessed at pre-intervention, mid-intervention, post-intervention, and at a long-term follow-up (1 year post-intervention). Whereas maltreated infants receiving care as usual showed progressively lower levels of morning cortisol across the 2-year period, cortisol levels of maltreated infants who received either of the early parenting interventions remained similar to children in a nonmaltreated comparison group. Thus, the parenting interventions essentially prevented biological dysregulation among maltreated children. Notably, this study only examined cortisol collected at mid-morning prior to laboratory-based assessments; thus, findings do not speak to intervention effects on the diurnal pattern of cortisol production from wake-up to bedtime.
Although results have varied somewhat from one study to another, it seems that blunted cortisol production is the most consistent pattern seen among children exposed to adversity (Bernard et al., 2010; Cicchetti & Rogosch, 2001; Kroupina et al., 2012). Thus, consistent with other findings (Fisher et al., 2007; Kroupina et al., 2012), we were interested in assessing whether an early intervention for the parents of young children referred for risk of neglect resulted in a steeper diurnal cortisol pattern relative to the slope of young children whose parents received a control intervention.
Attachment and Biobehavioral Catch-up was developed to enhance children’s self-regulatory capabilities. The objective of the ABC intervention in this study was to intervene with neglecting biological parents and their young children prior to the point at which a foster care intervention might be necessary. Bernard et al. (2012) have previously shown that the ABC intervention was effective in enhancing attachment security. The current study investigated whether the intervention effectively promoted children’s diurnal cortisol regulation. We expected that children who received the ABC intervention would show enhanced physiological regulation, as indexed by higher wake-up levels of cortisol and a steeper wake-up to bedtime decline in cortisol, compared with children who received the control intervention.
Method
Participants
Primary analyses included 101 children receiving services as part of a diversion from foster care program who were assessed by Child Protective Services as being at risk for neglect. In this sample, there were 3 sets of siblings (with both children within the targeted age range at the time of referral; data were analyzed with full sample, and with reduced sample removing one sibling). Parents were referred to this randomized clinical trial as one of the services provided. Among the conditions most often noted for these parents were: maltreating other children, domestic violence, parental substance abuse, homelessness, and mental disorders. We did not have access to formal records, however, and we were limited to reports of referring agencies and parent report.
At the time of post-intervention assessment of cortisol, children ranged in age 5.0 to 34.2 months (M = 17.6, SD = 7.8). Most (62%) of the children were African American, with 17% bi-racial, 13% Hispanic, and 8% White/non-Hispanic. All of the primary parents were female, with the exception of 2 males. Parents ranged in age from 15.1 to 46.6 years (M = 26.9, SD = 7.6); 8 parents did not provide information about their age. Most (65%) of the parents were African American, 6% were Biracial, 13% were Hispanic, and 16% were White/non-Hispanic. Table 1 presents demographic characteristics of the two groups.
Table 1.
Child Demographic Characteristics
| Variable | ABC Intervention (n = 49) | DEF Control Intervention (n = 52) | ||
|---|---|---|---|---|
|
| ||||
| n | % | n | % | |
| Gender | ||||
| Male | 29 | 59% | 29 | 56% |
| Female | 20 | 41% | 23 | 44% |
| Ethnicity | ||||
| White | 5 | 10% | 3 | 6% |
| African American | 29 | 59% | 34 | 65% |
| Hispanic | 2 | 4% | 11 | 21% |
| Bi-racial | 13 | 27% | 4 | 8% |
| Mean (SD) | Min – Max | Mean (SD) | Min – Max | |
|
|
||||
| Child age (months) | 17.7 (7.6) | 5.0 – 33.8 | 17.2 (7.8) | 5.8 – 34.2 |
| Months post intervention | 2.75 (2.49) | .20 – 10.9 | 2.59 (2.19) | .37 – 10.0 |
Procedures
Figure 1 displays the Consolidated Standards of Reporting Trials (CONSORT) flow diagram, which includes information about participant referral, enrollment/randomization, and follow-up. We report more detailed information about participants included in the analyses for the current sample below.
Figure 1.
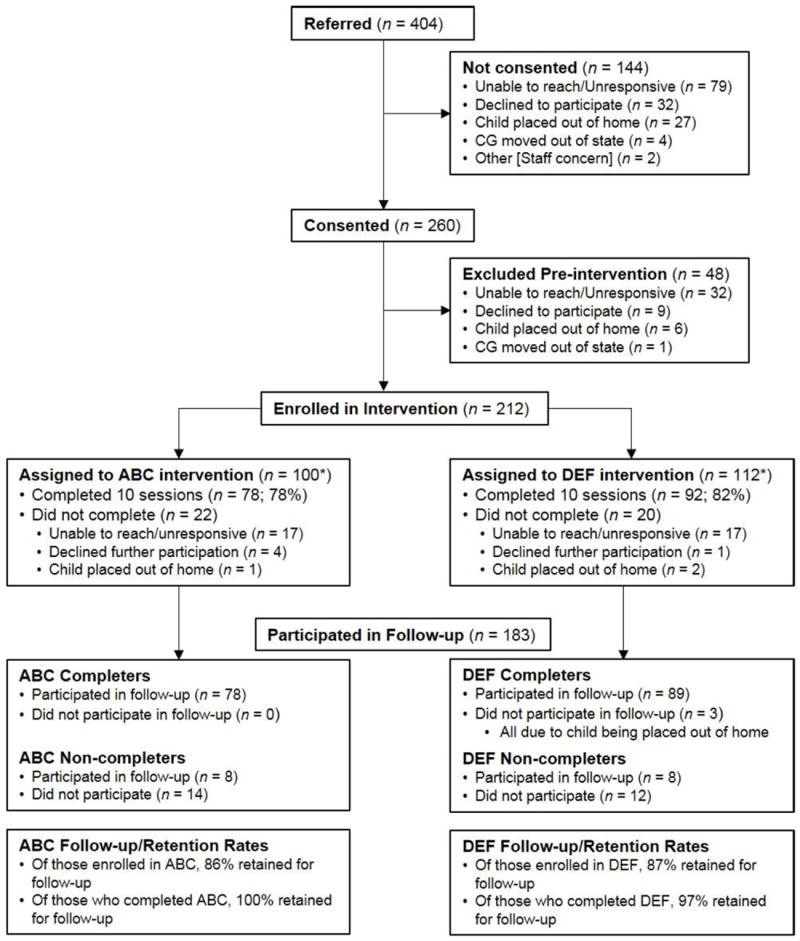
Consolidated Standards of Reporting Trials flow diagram. *We report numbers of children enrolled in ABC (n = 100) and DEF (n = 112) following completion of pre-intervention baseline visits. However, participants were randomly assigned to group upon consenting (N = 260; ABC n = 129, DEF n = 131), at which time the intervention group sample sizes were more similar. Follow-up numbers include participants seen for any post-intervention visits. More specific information is provided in Method section.
Participant recruitment
Parents were recruited through a foster care diversion program in a large mid-Atlantic city. Besides enrollment in the city’s diversion program (following involvement with Child Protective Services), the only criteria for inclusion was the child being under 2 years old at the time of referral. Thus, families demonstrated a broad range of experiences that led to CPS involvement, from homelessness to a history of neglect. Referrals were received directly from agency staff, and parents were contacted individually by phone. When parents expressed initial interest in the program, research staff met with families individually to describe participation fully and to obtain consent. Given the study’s lack of exclusionary criteria, the sample may best be described as a diverse, high-risk, community sample. All components of this research study were approved by an internal review board at the University of [Deleted for Blind Review].
Pre-intervention and post-intervention assessments
After consenting, parents were randomly assigned to receive either the Attachment and Biobehavioral Catch-up (ABC; experimental) intervention or the Developmental Education for Families (DEF; control) intervention. Randomization occurred immediately after a parent provided consent. Of the 260 children that were consented, 129 were randomly assigned to receive the ABC intervention and 131 were randomly assigned to receive the DEF intervention. We did not consider children “enrolled” in the intervention until they completed pre-intervention research visits and had their first intervention session scheduled. Of the 212 children enrolled in the intervention phase, 100 were in the ABC group and 112 were in the DEF group. All pre- and post-intervention assessments were the same for the two groups, and the two interventions were of the same duration (i.e., ten 1-hour sessions) and frequency (i.e., weekly). All intervention sessions and the assessment of cortisol production were conducted in families’ homes.
The intended follow-up schedule included one visit a month after the last intervention session, and then annual follow-up visits around the time of the child’s birthday. For the present study, cortisol was assessed as part of the first follow-up visit. Although efforts were made to collect data approximately one-month after the last session, there was considerable variability in the timing of this assessment, with some of these visits ranging up to a year post-intervention. The mean time between the last session and the cortisol assessment from the first follow-up visit was 2.67 months (SD = 2.33), with the majority (88%) collected within the first 6 months following the last session.
As can be seen from Figure 1, 183 participants were retained during the post-intervention phase of the study (representing 86% of the 212 children enrolled in the intervention). Of these participants, cortisol data were collected from 120 children within the time window of the first follow-up visit. Of these 120 children, 19 provided samples that were not useable (15 children had insufficient volumes of saliva for all samples, 2 had insufficient volumes of saliva for two samples and other samples were excluded as outliers, and 2 had all samples excluded as outliers), resulting in a sample size for the present study of 101 children. For the remaining 63 children, cortisol data were not available because parents did not collect or did not return the samples (n = 48), or because parents could not be reached to schedule a follow-up visit at that time (n = 15).
Interventions
Parent coaches with extensive experience working with children and with strong clinical skills were selected to implement both the ABC and DEF interventions. All sessions were videotaped, which allowed supervision and fidelity monitoring. Sessions were typically conducted in parents’ homes, or in shelters or other facilities as needed.
Experimental intervention
Attachment and Biobehavioral Catch-up Intervention (ABC)
The ABC intervention was designed to help parents become more synchronous and nurturing, and less frightening, in their interactions with their children. The first two sessions provide an assessment of parents’ beliefs and behaviors, and begin to emphasize the importance of nurturing behavior. During these sessions, parent coaches help parents to recognize that children need them even when they fail to signal their need clearly. Sessions 3 and 4 focus on synchrony, helping the parent recognize the importance of following the child’s lead. Sessions 5 and 6 focus on helping parents behave in ways that are not intrusive or frightening, respectively. Sessions 7 and 8 introduce consideration of how parents’ own issues can affect their ability to behave in synchronous, nurturing, and non-frightening ways. In particular, the parent coach helps the parent identify “voices from the past” (i.e., influences from the past that influence parenting), and consider how these “voices” affect parenting. The final two sessions of the ABC intervention help consolidate gains made through the prior sessions and celebrate change.
Throughout all ten sessions, parent coaches observe the parent’s behavior and make comments on behaviors that relate to the intervention targets. This “in the moment” feedback is used to focus attention on intervention targets, promoting behavioral change during the intervention sessions. Along with “in the moment” comments, parent coaches provide video feedback to highlight parents’ strengths, challenge weaknesses, and celebrate changes in behaviors.
Control intervention
Developmental Education for Families (DEF)
The DEF intervention is designed to enhance motor, cognitive, and language skills. It was adapted from a home-visiting program that was previously shown to be effective in enhancing intellectual functioning (Brooks-Gunn, Klebanov, Liaw, & Spiker, 1993; Ramey, McGinness, Cross, Collier, & Barrie-Blackley, 1982; Ramey, Yeates & Short, 1984). Components that targeted maternal sensitivity were removed to keep the interventions distinct. The DEF intervention followed a manual, with sessions tailored to the developmental level of the child. During each session, DEF parent coaches provided general psychoeducation about developmental milestones, presented age-appropriate activities for parents to use to support their children’s learning, and used video-feedback to review session activities. Importantly, during discussions, activities, and video feedback, DEF parent coaches focused on the child’s abilities with respect to the targeted areas of development (i.e., motor, cognitive, language). DEF parent coaches did not provide information, guidance, or feedback to parents about parenting behaviors or how to interact with their children during the activities.
Saliva sampling
The procedures used for collecting and assaying cortisol followed established protocol (e.g., Gunnar & White, 2001). Research staff trained parents to collect and store saliva samples in their homes. Additionally, step-by-step pictorial directions of the sampling procedure were given to parents along with the sampling materials. Parents collected saliva samples from children twice per day over a 2- or 3-day period. Initially, parents were asked to collect saliva samples for 2 days, but this was increased to 3 days later in data collection in order to increase the number of samples for analyses. Each day, parents collected one sample when the child first woke up and one sample at bedtime. Parents were asked to complete data collection on “typical” days, and to collect samples at least 30 minutes prior to or following mealtime or eating. Parents completed daily questionnaires about infant health status variables such as whether children were teething, were sick, or had eaten prior to sampling. If children were sick, parents were asked to delay sampling until the children were healthy again.
Samples were obtained by placing the end of the cotton roll in the child’s mouth. Flavored sugar-free beverage crystals (cherry-flavored drink mix) were provided to facilitate sampling. Parents were instructed to first wet the cotton in the child’s mouth, then dip the cotton in a cup containing 0.8g of the flavored crystals and place it back in the child’s mouth until the cotton was soaking wet. Controlled studies have reported that flavored crystals only minimally affect cortisol levels when radioimmunoassay is used (Gordon, Peloso, Auker, & Dozier, 2005; Talge, Donzella, Kryzer, Gierens, & Gunnar, 2005). The saturated cotton roll was returned to a prelabeled vial and stored in the freezer until it was collected by a research assistant.
The saliva samples were stored in a freezer at −20°C prior to assay procedures. Samples were assayed using a high-sensitivity salivary cortisol enzyme immunoassay kit (Salimetrics, LLC, State College, Pennsylvania). All samples from a child were assayed in duplicate on the same plate to minimize variability. The intra-assay and interassay coefficients of variation fell below 3.7% and 6.4%, respectively.
Primary analyses focused on post-intervention cortisol data collected within the year following the completion of the intervention. There were no intervention group differences in the time between the last session and the time of saliva sample data collection. Wake-up samples were collected between 4:20 a.m. and 11:46 a.m. (M = 8:45 a.m.) and bedtime samples were collected between 5:45 p.m. and 12:45 a.m. (M = 9:11 p.m.). Table 2 shows descriptive statistics of sampling times. Although it is important to also consider the actual time of waking with respect to the sample collection time, this information was not consistently available across participants.
Table 2.
Descriptive Statistics
| N | Time of sample
|
Cortisol value (in ug/dl)
|
Log-transformed cortisol value
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M (SD) | Min | Max | M (SD) | Min | Max | M (SD) | Min | Max | ||
| ABC Intervention (n = 49) | ||||||||||
| Wake- Day 1 | 39 | 8:08 (1:33) | 4:20 | 11:17 | .24 (.20) | .033 | 1.03 | -.75 (.35) | -1.48 | .01 |
| Wake- Day 2 | 42 | 8:21 (1:18) | 5:25 | 11:46 | .23 (.17) | .023 | .61 | -.79 (.39) | -1.64 | -.21 |
| Wake- Day 3 | 17 | 8:28 (1:16) | 6:00 | 11:00 | .27 (.17) | .055 | .79 | -.64 (.27) | -1.26 | -.31 |
| Bed- Day 1 | 44 | 8:58 (1:04) | 6:15 | 11:25 | .16 (.16) | .019 | .63 | -1.01 (.46) | -1.72 | -.20 |
| Bed- Day 2 | 39 | 9:23 (1:12) | 6:18 | 12:00 | .16 (.16) | .019 | .66 | -.98 (.42) | -1.72 | -.18 |
| Bed- Day 3 | 15 | 9:22 (1:09) | 7:19 | 12:30 | .18 (.14) | .020 | .50 | -.93 (.45) | -1.70 | -.31 |
| DEF Control Intervention (n = 52) | ||||||||||
| Wake- Day 1 | 44 | 8:12 (1:28) | 4:20 | 11:45 | .18 (.13) | .006 | .60 | -.92 (.45) | -2.22 | -.22 |
| Wake- Day 2 | 39 | 8:23 (1:16) | 5:25 | 11:46 | .19 (.17) | .010 | .61 | -.90 (.43) | -2.00 | -.19 |
| Wake- Day 3 | 21 | 8:39 (1:11) | 6:00 | 11:25 | .14 (.14) | .004 | .53 | -.1.09 (.57) | -2.40 | -.28 |
| Bed- Day 1 | 45 | 8:58 (1:07) | 6:15 | 11:32 | .15 (.13) | .010 | .65 | -1.04 (.46) | -2.00 | -.19 |
| Bed- Day 2 | 42 | 9:16 (1:08) | 5:45 | 12:00 | .15 (.16) | .004 | .62 | -1.11 (.55) | -2.40 | -.28 |
| Bed- Day 3 | 19 | 9:33 (1:22) | 7:00 | 12:45 | .13 (.11) | .010 | .42 | -1.09 (.46) | -2.00 | -.38 |
Of the participants with post-intervention data included in the present study, 59 (28 ABC and 31 DEF) had pre-intervention cortisol data available for preliminary analyses. Preliminary analyses were conducted to ensure that there were no group differences in cortisol at baseline. There were multiple reasons that data were not available for the full sample of participants, including: children were considered too young at the time of the pre-intervention assessment to provide valid physiological data (excluded children under 4 months), samples were not returned prior to beginning the intervention (thus not representing a true baseline), and not enough saliva was collected by parents.
Cortisol Data Preparation
Following procedures commonly used in previous studies (e.g., Fisher et al., 2007), biologically implausible cortisol values (i.e., defined as values greater than 2.0) were deleted. Additionally, cortisol values greater than 3 SDs above the mean were considered outliers and excluded from analyses. Each child could have up to 4 or 6 cortisol values (i.e., 2 wake-up and 2 bedtime samples for the 59 children with 2 days of data collection, or 3 wake-up and 3 bedtime samples for the 42 children with 3 days of data collection). Of 488 possible samples, 406 were included in analyses, with 3.7% removed as outliers and 13.1% missing due to an inadequate volume of saliva or because no sample was collected. Missing data patterns were comparable for the two groups, with children from the ABC group missing 15.5%, and children in the DEF group missing 18.0%. Of the 49 ABC children, 31 had cortisol collected across 2 days (18 with four samples included in analyses, 6 with three samples, 6 with two samples, and 1 with one sample), and 18 had cortisol collected across 3 days (9 with six samples included in analyses, 5 with five samples, 2 with four samples, and 2 with three samples). Of the 52 DEF children, 28 had cortisol collected across 2 days (16 with four samples included in analyses, 4 with three samples, 4 with two samples, and 4 with one sample), and 24 had cortisol collected across 3 days (14 with six samples included in analyses, 4 with five samples, 1 with four samples, 4 with three samples, and 1 with two samples). Patterns of missingness of cortisol samples were examined in multiple ways to determine whether data could be considered missing at random. First, we conducted t tests for each sampling time point, with missingness for a particular sample (i.e., missing versus not missing) as the independent variable and cortisol value for all other samples as the dependent variables. All of these were non-significant (ps > .05), indicating that there was no association between whether a particular cortisol sample was missing and cortisol level based on available observed measurements. Second, we examined whether missingness was associated with demographic information or group assignment, using Chi-square tests for categorical variables (i.e., minority, gender, intervention group) and t tests for continuous variables (i.e., child age). Missingness was not associated with child demographic variables or intervention group (ps > .05). Based on these analyses, the data meet criteria for missing at random (MAR; Schafer & Graham, 2002). Finally, there were 7 samples that had cortisol levels below the detectable limit of the assay; these samples were replaced with a value of .004 μg/dl. Log10 transformation was used to normalize the distribution of cortisol values due to a positive skew. See Table 2 for descriptive statistics regarding cortisol values (raw and log-transformed) and sampling times.
Results
Preliminary Analyses
Pre-intervention cortisol data for the subset of participants were analyzed using the data analytic approach described in detail below. At pre-intervention, children randomly assigned to receive the ABC intervention or control (DEF) intervention did not differ with regard to wake-up cortisol levels (β01 = 0.14, p > .05), bedtime cortisol levels (β01 = -.01, p > .05), or wake-up to bedtime slope in cortisol production (β11 = -0.15, p > .05). There were no pre-intervention differences between the ABC and DEF groups in child or caregiver age and percentage of minority versus non-minority participants (p values > .05). Demographic variables were examined to determine whether child characteristics were associated with log-transformed post-intervention cortisol values. Child gender and minority status were not associated with cortisol values at any of the time points (p values > .05). Child age was negatively correlated with log-transformed bedtime cortisol levels on day 1 (r = -.36, p = .001) and day 2 (r = -.24, p < .05), and with log-transformed wake-up cortisol levels on day 3 (r = -.38, p < .05). Given these associations and findings from previous studies (e.g., Larson et al., 1998; Watamura, Donzella, Kertes, & Gunnar, 2004), child age was included as a covariate in primary analyses. Although time of sample collection was not associated with cortisol values at any of the time points (p values > .05), it was included as a covariate in primary analyses based on previous studies.
Data Analytic Strategy
Intervention group differences in cortisol levels at wake-up and bedtime as well as change in cortisol levels across the day were analyzed using hierarchical linear modeling (HLM; Raudenbush & Bryk, 2002). HLM accounts for the non-independence of repeated measures by modeling multiple data points as nested within individuals. This approach allows for separate estimates of within-subject and between-subject variation. Whereas other approaches collapse across samples to create an average wake-up level and average bedtime level, HLM models all samples individually and accounts for measurement error associated with each sample (Raudenbush, Brennan, & Barnett, 1995). Thus, this approach is considered more appropriate than aggregating all wake-up samples or all bedtime samples together, as it accounts for the error associated with each measurement occasion. Given that HLM allows for variability in the timing and number of repeated data points, participants can be included even if they are missing 1 or more points of data.
The dependent variable was the log-transformed cortisol value, measured in micrograms per deciliter (μg/dl). Cortisol sample collection time (in hours since the average wake-up sample collection time) was included as a time-varying covariate at level 1. The following level 1 within-individual model was specified:
where Log cortti represents the log-transformed cortisol value for child i at time t; π0i represents child i’s estimated log-transformed cortisol value at wake-up when controlling sampling time; π1i is the estimated slope of cortisol change from wake-up to bedtime; π2i is the regression coefficient representing the effect of the time-varying covariate (i.e., sample collection time); SAMPLE represents whether the sample was collected at wake-up or bedtime (with 0 representing wake-up and 1 representing bedtime); TIME represents the collection time of the sample in hours from the mean time for wake-up sample collection (i.e., 8:45 a.m.); and eti is the within-individual error in child i’s log-transformed cortisol value.
Level 2 (i.e., between-subject) variables were included to examine whether there were intervention group effects on cortisol levels at wake-up or bedtime and in change across the day. Child age was included as a covariate given that it was associated with cortisol levels in preliminary analyses. The following level 2 model was specified:
where π0i represents the wake-up log-transformed cortisol value (intercept) for an individual and π1i represents the linear change (slope) in log-transformed cortisol across the day for an individual; the term β00 represents the average estimated log-transformed cortisol level at wake-up for children in the DEF group, controlling for child’s age; β01 represents the difference between the wake-up log-transformed cortisol value between the children in the DEF group and the ABC; β02 is the regression coefficient representing the effect of the child’s age (grand centered at the mean); ABC represents the dummy-coded intervention group (with 0 representing DEF and 1 representing ABC); ChAGE represents the child’s age in months; and r0i is the between-subject differences left unexplained by the level 2 predictors The equations for linear change (i.e., β1i) contain the same predictors allowing for examination of intervention group effects on cortisol change across the day.
Primary Analyses
In order to test intervention effects on the diurnal pattern of cortisol production, we examined whether intervention group predicted the wake-up level of cortisol (intercept) and the change in cortisol level from waking to bedtime (slope). The log-transformed cortisol value at wake-up differed significantly between children in the ABC group and children the DEF group, controlling for time of sample collection and age (β01 = 0.21, p < .01). Specifically, children in the ABC group showed a higher wake-up level of cortisol, relative to children in the DEF group (Table 3). Intervention effects on bedtime cortisol levels were examined by re-running the model with the bedtime sample as the intercept (by re-coding the dummy-coded SAMPLE variable with 0 representing bedtime and 1 representing wake-up). Log-transformed cortisol values at bedtime did not differ significantly between the intervention groups (β01 = 0.06, p > .05). There was a significant effect of intervention group on the change in cortisol across the day, with children in the ABC group showing a steeper wake-up to bedtime pattern (i.e., more negative slope) than children in the DEF intervention group (β11 = -0.15, p = 0.051). Thus, children in the DEF intervention group showed a more blunted diurnal cortisol pattern, relative to children in the ABC intervention group. Figure 2 presents the model estimates of the wake-up and bedtime values for each intervention group.
Table 3.
Multilevel Modeling Coefficients of Intervention Effects on Diurnal Cortisol Production
| Effect | Log-transformed Cortisol
|
||||
|---|---|---|---|---|---|
| Coefficient | SE | t | df | p | |
| Intercept, β00 | -.95 | .05 | -17.37 | 98 | .000 |
| ABC, β01 | .21 | .08 | 2.67 | 98 | .009 |
| ChAGE, β02 | -.01 | .00 | -1.89 | 98 | .061 |
| SAMPLE Slope, β10 | -.09 | .05 | -1.75 | 98 | .083 |
| ABC, β11 | -.15 | .08 | -1.98 | 98 | .051 |
| ChAGE, β12 | -.00 | .00 | -1.60 | 98 | .114 |
| TIME Slope, β20 | .02 | .03 | .73 | 98 | .469 |
| ABC, β21 | -.04 | .04 | -1.03 | 98 | .304 |
| ChAGE, β22 | -.00 | .00 | -.16 | 98 | .875 |
Note. β00 and β10 represent the wake-up level of cortisol and the slope of cortisol production across the day, respectively, for DEF (control) children. β01 and β11 represent the difference in the wake-up level of cortisol and slope of cortisol production across the day, respectively, between DEF children and ABC children.
Figure 2.
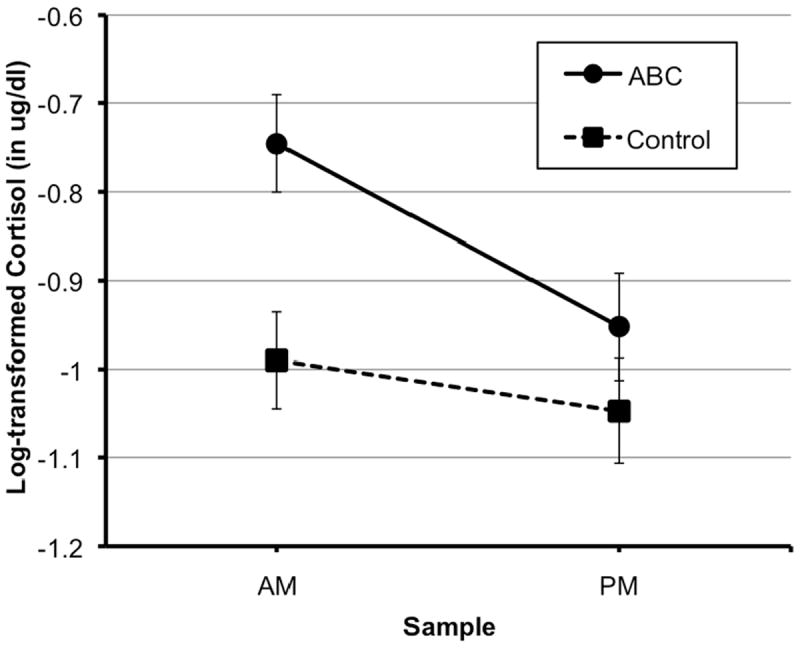
Cortisol patterns for neglected children who received the ABC intervention versus neglected children who received the control (DEF) intervention. Error bars represent SE.
Effect sizes were computed to determine the magnitude of the intervention effect on wake-up cortisol level and the diurnal slope following procedures recommended for clinical trials (Feingold, 2009; Karna et al., 2011). Specifically, Cohen’s d was computed by dividing the unstandardized estimate of the intervention effect (taking into account level 1 and level 2 covariates) by the pooled within-group standard deviation. Estimates of standard deviation for each group were computed using the raw data for wake-up cortisol (to examine the intervention effect on the intercept) and raw data for wake-up to bedtime change in cortisol (to examine the intervention effect on the slope). Based on conventions for small (0.2), medium (0.5), and large (0.8) effect sizes, the effect size for group difference in wake-up cortisol was approximately medium (d = 0.48), and the effect size for the group difference in the diurnal slope of cortisol was small to medium (d = -0.38).
Additional analyses were conducted to determine whether findings held if (1) children who did not complete the interventions as intended were excluded, and (2) children who were part of a sibling pair were excluded. Whereas all ABC children included in the present study completed the full 10 sessions, there were 4 DEF children who provided follow-up cortisol data that did not complete the full 10 sessions (considered “non-completers” but retained for follow-up visits). When these 4 non-completers were excluded from analyses, findings held for the effect of the ABC intervention on wake-up cortisol (β01 = 0.19, p < .05), and the diurnal slope (β11 = -0.16, p < 0.05). Second, there were 3 sets of siblings in the sample (2 in ABC, 1 in DEF). When one sibling from each pair was excluded from analyses, the intervention effect held for wake-up cortisol (β01 = 0.20, p < .05), and was marginally significant for the diurnal slope (β11 = -0.14, p = 0.07).
Discussion
This study assessed the effectiveness of a 10-session attachment-based parenting program in supporting the regulation of diurnal production of cortisol among neglected children who were living with their birth parents. Results showed differences in the post-intervention diurnal cortisol patterns of children assigned to the Attachment and Biobehavioral Catch-up intervention when compared to those assigned to the control intervention. Children randomly assigned to the ABC intervention showed higher wake-up values of cortisol and a steeper wake-up to bedtime decline in cortisol than children randomly assigned to the control intervention. This pattern is an important index of the intervention’s effectiveness, as it suggests that the intervention serves to support children’s cortisol regulation.
Among humans and animals, cortisol (or corticosterone in rodents) is an integral component of the HPA axis, necessary for adaptation to chronic and immediate stress, and maintenance of circadian rhythms and homeostasis (Sapolsky, Romero, & Munck, 2000). Children exposed to chronic neglect are especially prone to low morning cortisol values, (Bruce, Kroupina, Parker, & Gunnar, 2001; Dozier, Manni, et al., 2006; Fisher et al., 2007), perhaps due to a downregulation of the HPA axis in response to elevated glucocorticoid production in a chronically stressful environment. Such flat or blunted diurnal patterns, characterized primarily by low wake-up cortisol values, represent dysfunction in this important regulatory system (Gunnar & Vazquez, 2001). Indeed, low wake-up cortisol represents a biomarker for clinical, social, and physical maladjustment (Finsterward, Selig, Schieche, Wurmser, & Papousek, 2000; Pruessner, Hellmammer, & Kirschbaum, 1999; White, Gunnar, Larson, Donzella, & Barr, 2000). Given these risks and consequences of low morning cortisol values and associated blunted diurnal patterns, we find our results especially promising.
An important remaining question concerns the mechanism by which the Attachment and Biobehavioral Catch-up intervention leads to more typical cortisol regulation. The ABC intervention was designed to target parenting behaviors that were expected to promote biological regulation among young children at risk for neglect. Specifically, parents were supported to behave in synchronous ways to children’s signals, respond with nurturance when children were distressed, and not engage in frightening behaviors. Synchronous interactions, in particular, may help children develop a sense of control over their environment, and thus support biological and behavioral regulation (Feldman, Greenbaum, & Yirmiya, 1999; Raver, 1996). Thus, a critical next step is to identify mechanisms of intervention effectiveness, by examining the specific ways in which parenting behaviors change, and how these changes in parenting behaviors contribute to changes in child outcome. Though important, demonstrating that specific changes to parent behavior mediate the effect of the intervention on cortisol regulation (or other outcomes) may be challenging. Meta-analyses indicate that maternal sensitivity often underperforms in predicting child attachment, relative to its expected role based on attachment theory (De Wolff & van IJzendoorn, 1997; van IJzendoorn, 1995). In part, these modest effect sizes may be due to variable or even inadequate procedures for measuring maternal sensitivity (Cassidy et al., 2005; Lindhiem, Bernard, & Dozier, 2011). Given that the primary purpose of the present study was to examine the effect of the intervention on children’s cortisol regulation, we did not aim to test the mediating role of changes in parenting in this paper. However, testing mediation in future studies will help us clarify mechanisms of intervention effectiveness, as well as inform our understanding of basic processes within developmental psychopathology.
The ABC intervention is similar to other short-term interventions that emphasize the importance of synchrony and nurturance for at-risk parent-infant populations in general (Bakermans-Kranenburg, van IJzendoorn, & Juffer, 2003; Hoffman, Marvin, Cooper, & Powell, 2006; Lieberman & van Horn, 2009; van den Boom, 1994). Enhancing these behaviors among parents of young children has strong support in the literature, with effects primarily tested and seen with regard to children’s quality of attachment relationships. Thus, we are excited to extend this work by showing that the intervention, designed to increase synchrony and nurturance among high-risk birth parents, can also have effects on children’s diurnal cortisol regulation. Given that the effectiveness of the ABC intervention has now been demonstrated on both behavioral outcomes (i.e., attachment organization, Bernard et al., 2012; cognitive flexibility, Lewis-Morrarty, Dozier, Bernard, Terracciano, & Moore, 2012) and biological outcomes, it is important to consider potential models for how such changes influence each other and other outcomes. As we suggest above, changes to parenting behavior, such as enhanced synchrony, may serve a direct role in helping children develop typical biological regulation. Similarly, changes to parenting behavior, such as enhanced nurturance, may serve a direct role in supporting the development of organized, secure attachment relationships. Whether changes to specific parenting behaviors (e.g., synchrony versus nurturance) uniquely predict changes to specific child outcomes (e.g., cortisol regulation versus attachment quality) remains to be tested. It may also be the case that enhancing attachment organization leads to more normative cortisol regulation or vice versa. Indeed, multiple studies report associations between attachment quality and cortisol responses (e.g., Bernard & Dozier, 2010; Hertsgaard, Gunnar, Erickson, Farrell, & Nachmias, 1995). Further, these more immediate targets (i.e., attachment organization and cortisol regulation) may serve as mediators to later outcomes, such as emotion regulation, attention/behavioral regulation, and physical health.
In a previous study of infants in foster care (Dozier, Peloso, et al., 2006), the ABC intervention was shown to be effective in leading to more typical cortisol regulation. However, the pattern of findings differs between that previous study and the current study. In the Dozier, Peloso, et al. (2006) study, infants whose foster parents received the ABC intervention showed lower wake-up and bedtime levels than infants whose foster parents received the control intervention. Foster infants in the ABC group showed cortisol levels that were similar to a low-risk comparison group of similar age. Whereas lower cortisol values were seen as reflective of a more normative diurnal pattern among infants in foster care, we suggest here that a steeper slope and higher wake-up cortisol values may reflect a more normative diurnal pattern among young children living with their high-risk birth parents. The reason for the discrepancy across studies in unclear, but may reflect differences between young children who remain in care with their high-risk birth parents and young children who are placed in foster care. Fisher et al. (2007) showed that preschoolers in foster care who received a psychosocial parenting intervention had steeper wake-up to bedtime slopes of cortisol production relative to children in a control condition. Given that the Fisher et al. (2007) findings parallel those of the current study, we cannot attribute discrepancies between findings of Dozier, Peloso, et al. (2006) and our current findings solely to differences between children in foster care and children living with high-risk birth parents. However, it is possible that infants and toddlers in foster care represent a unique group in terms of cortisol regulation, compared to preschool-aged children in foster care or to infants and toddlers living with high-risk birth parents. In addition to infants in foster care (Dozier, Peloso, et al., 2006), high levels of cortisol have also been observed among other subsets of maltreated children, including foster children that experience severe emotional maltreatment (Bruce et al., 2009), as well as in post-institutionalized children with significant growth delays (Kertes et al., 2008). Both atypically high and atypically low values of cortisol are considered problematic, and future research is needed to better understand what early experiences differentially predict these profiles. It will be critical to replicate and extend findings from these studies by examining diurnal cortisol regulation longitudinally among neglected children living in foster care and children living with their neglecting birth parents.
Unlike the Fisher et al. (2007) and Dozier, Peloso, et al. (2006) studies that examined interventions that target children after they have been removed from their neglecting parents and placed into foster care, the current study focused on improving children’s neuroendocrine regulation in an environment of ongoing, chronic adversity. Cicchetti et al. (2011) intervened with a similar population of infants that were living with their maltreating parents, but examined a laboratory assessment of cortisol, rather than the diurnal pattern. Here, we show that an intervention designed to enhance synchronous and nurturing parenting, even under chronically challenging conditions, may support children’s cortisol regulation. Presumably the ABC intervention helps parents serve a buffering role, in protecting their children from the negative effects of a chronically stressful environment. Thus, findings of the effectiveness of this short-term parenting intervention for highly vulnerable children have significant implications for prevention, which can be explored in future research. In addition to preventing short-term risk indicators (e.g., cortisol dysregulation, attachment disorganization) that are linked to later mental and physical health problems, the ABC intervention may have effects on child welfare system involvement.
Certain limitations to the current design must be recognized. We had limited information to children’s experiences of neglect. This sample of parents and children was being monitored for neglect by child welfare services. In general, the experiences of neglect were likely not as severe as those that warrant removal of a child from a parent’s care. However, families in the current study varied with regard to histories of neglect. Given this variability, it will be important for future research to consider whether different experiences of maltreatment are associated with treatment outcomes, ideally characterizing the type, severity, and frequency of maltreatment experiences and other stressors. Additionally, we were unable to characterize change in cortisol patterns from pre- to post-intervention. Thus, it is unclear whether cortisol patterns became more typical over time for children in the ABC group, or whether cortisol patterns became more atypical (i.e., blunted) over time for children in the DEF group, or both. Future longitudinal analyses can further examine whether the ABC intervention serves to normalize cortisol patterns following early dyregulation, or prevent later dysregulation. Further, although we established between-group comparability in cortisol profiles for a subset of children at pre-intervention, we did not have cortisol data from a low-risk comparison group. A low-risk age-matched comparison group would also help clarify the degree of dysregulation that was observed pre- and post-intervention. Finally, we did not test whether changes to parenting behavior mediate the association between the intervention and children’s cortisol regulation. This leaves the question about the mechanism by which the intervention is effective unanswered. Although we have previously demonstrated that the ABC intervention leads to changes in parent sensitivity among foster parents (Bick & Dozier, 2013), it will be important to examine whether such improvements to parenting then explain changes in child functioning, such as cortisol regulation.
The results from the current study offer several directions for future research. The sustainability of ABC intervention effects on diurnal cortisol regulation and long-term advantages of children’s improved cortisol regulation are currently unknown. Thus, it will be important to examine whether changes in cortisol regulation are maintained over time and whether changes contribute to improvements in other physical or mental health outcomes. As mentioned above, future research should test the mediating role of specific changes in parenting behavior that may drive effects of the intervention on child outcomes. Furthermore, it will be important to examine factors (i.e., moderators) that enhance or interfere with intervention effects. For example, variability in children’s experiences of neglect may be associated with the effectiveness of the ABC intervention on influencing cortisol outcomes, as both the severity and chronicity of neglect have been associated with the degree to which diurnal HPA patterns are blunted (Gunnar, Fisher, & The Early Experience, Stress, and Prevention Network, 2006; Gunnar & Vazquez, 2001). In line with a differential susceptibility model, Bakermans-Kranenburg, van IJzendoorn, Mesman, Alink, & Juffer (2008) demonstrated that the dopamine receptor D4 (DRD4) gene moderated the effectiveness of an attachment-based parenting program on basal cortisol levels among children with elevated externalizing behavior problems. More specifically, children with the DRD4 7-repeat allele, which has been associated with increased externalizing problems, were responsive to the intervention, whereas children without the DRD4 7-repeat allele were not. Future research examining genetic and environmental factors that moderate the effectiveness of interventions can enhance our ability to match particular treatment approaches to the individuals in need.
In conclusion, the Attachment and Biobehavioral Catch-up intervention, designed to enhance synchronous and nurturing responses among parents at-risk for neglecting their children, supports young children’s diurnal cortisol regulation. In addition to supporting the efficacy of the ABC intervention, this study highlights the need to better understand of associations between responsive caregiving and biological regulation. Among young children living in chronically challenging environments, responsive caregiving characterized by synchronous and nurturing interactions, may be especially important for the development of basic biological regulation.
Acknowledgments
This research was supported by funding from Edna Bennett Pierce and from NIH grants R01 MH074374, R01 MH084135, and R01 MH052135 to M. Dozier.
References
- Bakermans-Kranenburg MJ, van IJzendoorn MH, Juffer F. Less is more: Meta-analysis of sensitivity and attachment interventions in early childhood. Psychological Bulletin. 2003;129:195–215. doi: 10.1037/0033-2909.129.2.195. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH, Mesman J, Alink LRA, Juffer F. Effects of an attachment-based intervention on daily cortisol moderated by dopamine receptor D4: A randomized control trial on 1- to 3-year-olds screened for externalizing behavior. Development and Psychopathology. 2008;20:805–820. doi: 10.1017/S0954579408000382. [DOI] [PubMed] [Google Scholar]
- Bernard K, Butzin-Dozier Z, Rittenhouse J, Dozier M. Cortisol production patterns in young children living with birth parents vs children placed in foster care following involvement of Child Protective Services. Archives of Pediatrics and Adolescent Medicine. 2010;164:438–443. doi: 10.1001/archpediatrics.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Dozier M. Examining infants’ cortisol responses to laboratory tasks among children varying in attachment disorganization: Stress reactivity or return to baseline? Developmental Psychology. 2010;46:1771–1778. doi: 10.1037/a0020660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Dozier M, Bick J, Lewis-Morrarty E, Lindhiem O, Carlson E. Enhancing attachment organization among maltreated children: Results of a randomized clinical trial. Child Development. 2012;83:623–636. doi: 10.1111/j.1467-8624.2011.01712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J, Dozier M. The effectiveness of an attachment-based intervention in promoting foster mothers’ sensitivity toward foster infants. Infant Mental Health Journal. 2013;34:95–103. doi: 10.1002/imhj.21373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Gunn J, Klebanov PK, Liaw F, Spiker D. Enhancing the development of low-birthweight, premature infants: Changes in cognition and behavior over the first three years. Child Development. 1993;64:736–753. doi: 10.2307/1131215. [DOI] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol levels in preschool-aged foster children: Differential effects of maltreatment type. Developmental Psychobiology. 2009;51:14–23. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce J, Kroupina M, Parker S, Gunnar MR. The relationships between cortisol patterns, growth retardation, and developmental delay in post-institutionalized children; 2000, July; Paper presented at the International Conference on Infant Studies; Brighton, England. [Google Scholar]
- Carlson M, Dragomir C, Earls F, Farrell M, Macovei O, Nystrom P, Sparling J. Cortisol regulation in home-reared and institutionalized Romanian children. Society of Neuroscience Abstracts. 1995;218:12. [Google Scholar]
- Carlson M, Earls F. Psychological and neuro-endocrinological sequelae of early social deprivation in institutionalized children in Romania. Annals of the New york Academy of Sciences. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch F. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Development and Psychopathology. 2001;13:783–804. [PubMed] [Google Scholar]
- Cicchetti D, Rogosch F, Toth SL, Sturge-Apple ML. Normalizing the development of cortisol regulation in maltreated infants through preventive interventions. Development and Psychopathology. 2011;23:789–800. doi: 10.1017/S0954579411000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Doom JR, Cicchetti D, Rogosch FA, Dackis MN. Child maltreatment and gender interactions as predictors of differential neuroendocrine profiles. Psychoneuroendocrinology. 2013;38:1442–1454. doi: 10.1016/j.psyneuen.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozier M, Manni M, Gordon MK, Peloso E, Gunnar MR, Stovall-McClough K, Levine S, et al. Foster children’s diurnal production of cortisol: An exploratory study. Child Maltreatment. 2006;11:189–197. doi: 10.1177/1077559505285779. [DOI] [PubMed] [Google Scholar]
- Dozier M, Peloso E, Lindhiem O, Gordon MK, Manni M, Sepulveda S, Levine S, et al. Developing evidence-based intervention for foster children: An example of a randomized clinical trial with infant and toddlers. Journal of Social Issues. 2006;62:767–785. doi: 10.1111/j.1540-4560.2006.00486.x. [DOI] [Google Scholar]
- Feingold A. Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychological Methods. 2009;14:43–53. doi: 10.1037/a0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Greenbaum CW, Yirmiya N. Mother-infant affect synchrony as an antecedent of the emergence of self-control. Developmental Psychology. 1999;35:223–231. doi: 10.1037/0012-1649.35.1.223. [DOI] [PubMed] [Google Scholar]
- Finsterward J, Selig MA, Schieche M, Wurmser H, Papousek M. Individual differences in self-regulation in excessively crying infants: A microanalytic approach; Paper presented at the International Conference of Infant Studies; Brighton, England. 2000. [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007;32:892–905. doi: 10.1016/j.psyneuen.2007.06.008. 892-905.2007-18151-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MK, Peloso E, Auker A, Dozier M. Effect of flavored beverage crystals on salivary cortisol enzyme immunoreactive assay measurements. Developmental Psychobiology. 2005;4:189–195. doi: 10.1002/dev.2008. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Brodersen L, Nachmias M, Buss K, Rigatuso J. Stress reactivity and attachment security. Developmental Psychobiology. 1996;29:191–204. doi: 10.1002/(SICI)1098-2302(199604)29:3<191∷AID-DEV1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Cheatham CL. Brain and behavior interfaces: Stress and the developing brain. Infant Mental Health Journal. 2003;24:195–211. doi: 10.1002/imhj.10052. [DOI] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/S0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Fisher PA The Early Experience, Stress, and Prevention Network. Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Developmental Psychopathology. 2006;18:651–677. doi: 10.1017/0S0954579406060330. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Quevedo KM. Early care experiences and HPA axis regulation in children: A mechanisms for later trauma and vulnerability. Progress in Brain Research. 2007;167:137–149. doi: 10.1016/S0079-6123(07)67010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13:515–737. doi: 10.1017/S0954579401003066. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, White BP. Salivary cortisol measures in infant and child assessment. In: Singer LT, Zeskind PS, editors. Biobehavioral Assessment of the Infant. New York: Guilford Press; 2001. pp. 167–189. [Google Scholar]
- Hoffman KT, Marvin RS, Cooper G, Powell B. Changing toddlers’ and preschoolers’ attachment classifications: The circle of security intervention. Journal of Consulting and Clinical Psychology. 2006;74:1017–1026. doi: 10.1037/0022-006X.74.6.1017. [DOI] [PubMed] [Google Scholar]
- Karna A, Voeten M, Little TD, Poskiparta E, Kalijonen A, Salmivalli C. A large-scale evaluation of the KiVa Antibullying Program: Grades 4 – 6. Child Development. 2011;82:311–330. doi: 10.1111/j.1467-8624.2010.01557.x. [DOI] [PubMed] [Google Scholar]
- Kertes DA, Gunnar MR, Madsen NJ, Long JD. Early deprivation and home basal cortisol levels: A study of internationally adopted children. Development And Psychopathology. 2008;20:473–491. doi: 10.1017/S0954579408000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroupina MG, Fuglestad AJ, Iverson SL, Himes JH, Mason PW, Gunnar MR, Johnson DE, et al. Adoption as an intervention for institutionally reared children: HPA functioning and developmental status. Infant Behavior and Development. 2012;35:829–837. doi: 10.1016/j.infbeh.2012.07.011. doi.org.proxy.nss.udel.edu/10.1016/j.infbeh.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Larson MC, White BP, Cochran A, Donzella B, Gunnar M. Dampening of the cortisol response to handling at 3 months in human infants and its relations to sleep, circadian cortisol activity, and behavioral distress. Developmental Psychobiology. 1998;33:327–337. doi: 10.1002/(SICI)1098-2302(199812)33:4<327∷AID-DEV4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Lewis-Morrarty E, Dozier M, Bernard K, Terracciano SM, Moore SV. Cognitive flexibility and theory of mind outcomes among foster children: Preschool follow-up results of a randomized clinical trial. Journal of Adolescent Health. 2012;51:17–22. doi: 10.1016/j.jadohealth.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic–pituitary–adrenal axis in the rat. Physiology & Behavior. 2001;73:255–260. doi: 10.1016/S0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Lieberman AF, van Horn P. Child-parent psychotherapy: A developmental approach to mental health treatment in infancy and early childhood. In: Lieberman AF, van Horn P, editors. Handbook of Infant Mental Health. 3. New York: Guilford; 2009. [Google Scholar]
- McBurnett K, Lahey BB, Rathouz PJ, Loeber R. Low salivary cortisol and persistent aggression in boys referred for disruptive behavior. Archives of General Psychiatry. 2000;57:38–43. doi: 10.1001/archpsyc.57.1.38. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nature Neuroscience. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- Pajer K, Gardner W, Rubin RT, Perel J, Neal S. Decreased cortisol levels in adolescent girls with conduct disorder. Archives of General Psychiatry. 2001;58:297–302. doi: 10.1001/archpsyc.58.3.297. [DOI] [PubMed] [Google Scholar]
- Price DA, Close GC, Fielding BA. Age of appearance of circadian rhythm in salivary cortisol values in infancy. Archives of Disease in Childhood. 1983;58:454–456. doi: 10.1136/adc.58.6.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Low self-esteem, induced failure and the adrenocortical stress response. Personality And Individual Differences. 1999;27:477–489. doi: 10.1016/S0191-8869(98)00256-6. [DOI] [Google Scholar]
- Ramey CT, McGinness GD, Cross L, Collier AM, Barrie-Blackley S. The Abecedarian approach to social competence: Cognitive and linguistic intervention for disadvantaged preschoolers. In: Borman K, editor. The Social Life of Children in a Changing Society. Hillsdale, NJ: Erlbaum; 1982. pp. 145–174. [Google Scholar]
- Ramey CT, Yeates KO, Short EJ. The plasticity of intellectual development: Insights from preventive intervention. Child Development. 1984;55:1913–1925. [PubMed] [Google Scholar]
- Raudenbush SW, Brennan RT, Barnett RC. A multivariate hierarchical model for studying psychological change within married couples. Journal of Family Psychology. 1995;9:161–174. doi: 10.1037/0893-3200.9.2.16. [DOI] [Google Scholar]
- Raudenbush S, Bryk A. Hierarchical linear models: Applications and data analysis methods (Advanced Quantitative Techniques in the Social Sciences) 2. Newbury Park, CA: Sage; 2002. [Google Scholar]
- Raver CC. Relations between social contingency in mother-child interactions and 2-year-olds’ social competence. Developmental Psychology. 1996;32:850–859. doi: 10.1037/0012-1649.32.5.850. [DOI] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Development and Psychopathology. 2001;13:419–449. doi: 10.1017/S0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: Neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Research Reviews. 1986;11:65–76. doi: 10.1016/0165-0173(86)90010-X. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress-responses? Integrating permissive, suppressive, stimulatory, and adaptive actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. doi: 10.1037//1082-989X.7.2.147. [DOI] [PubMed] [Google Scholar]
- Sippell WG, Becker H, Versmold HT, Bidlingmaier F, Knorr D. Longitudinal studies of plasma aldosterone, corticosterone, deoxycosterone, progesterone 17-hydroxyprogesterone, cortisol, and cortisone determined simultaneously in mother and child at birth and during the early neonatal period. I. Spontaneous delivery. Journal of Clinical Endocrinology and Metabolism. 1978;46:971–985. doi: 10.1210/jcem-46-6-971. [DOI] [PubMed] [Google Scholar]
- Talge NM, Donzella B, Kryzer EM, Gierens A, Gunnar MR. It’s not that bad: Error introduced by oral stimulants in salivary cortisol. Developmental Psychobiology. 2005;47:369–376. doi: 10.1002/dev.20097. doi.org.proxy.nss.udel.edu/10.1002/de v.20097. [DOI] [PubMed] [Google Scholar]
- Trickett PK, Noll JG, Susman E, Shenk CE, Putnam FW. Attenuation of cortisol across development for victims of sexual abuse. Development and Psychopathology. 2010;22:165–175. doi: 10.1017/S0954579409990332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Boom DC. The influence of temperament and mothering on attachment and exploration: An experimental manipulation of sensitive responsiveness among lower-class mothers with irritable infants. Child Development. 1994;65:1457–1477. doi: 10.2307/1131511. [DOI] [PubMed] [Google Scholar]
- Watamura SE, Donzella B, Kertes DA, Gunnar MR. Developmental changes in baseline cortisol activity in early childhood: Relations with napping and effortful control. Developmental Psychobiology. 2004;45:125–133. doi: 10.1002/dev.20026. [DOI] [PubMed] [Google Scholar]
- White BP, Gunnar MR, Larson MC, Donzella B, Barr RG. Behavioral and physiological responsivity, and patterns of sleep and daily salivary cortisol in infants with and with out colic. Child Development. 2000;71:862–877. doi: 10.1111/1467-8624.00196. [DOI] [PubMed] [Google Scholar]


