Abstract
Objective
Determine if genetic variation in enzymes/transporters influencing extracellular adenosine homeostasis, including adenosine kinase (ADK), ecto-5'-nucleotidase (NT5E, CD73), and equilibrative nucleoside transporter type-1 (ENT-1), is significantly associated with epileptogenesis and post-traumatic epilepsy (PTE) risk, as indicated by time to first seizure analyses.
Methods
Nine ADK, three CD73, and two ENT-1 tagging SNPs were genotyped in 162 white adults with moderate/severe TBI and no history of premorbid seizures. Kaplan Meier models were used to screen for genetic differences in time to first seizure occurring >1 week post-TBI. SNPs remaining significant after correction for multiple comparisons were examined using Cox Proportional Hazards analyses, adjusting for subdural hematoma, injury severity score, and isolated TBI status. SNPs significant in multivariate models were then entered simultaneously into an adjusted Cox model.
Results
Comparing Kaplan Meier curves, rs11001109 (ADK) rare allele homozygosity and rs9444348 (NT5E) heterozygosity were significantly associated with shorter time to first seizure and increased seizure rate 3 years post-TBI. Multivariate Cox Proportional Hazard models showed these genotypes remained significantly associated with increased PTE hazard up to 3yrs post-TBI after controlling for variables of interest [rs11001109: HR=4.47, 95%CI (1.27–15.77), p=0.020; rs9444348: HR=2.95, 95%CI (1.19–7.31), p=0.019].
Significance
Genetic variation in ADK and NT5E may help explain variability in time to first seizure and PTE risk, independent of previously identified risk factors, after TBI. Once validated, identifying genetic variation in adenosine regulatory pathways relating to epileptogenesis and PTE may facilitate exploration of therapeutic targets and pharmacotherapy development.
Keywords: TBI, post-traumatic epilepsy, epileptogenesis, genetics, adenosine, rehabilomics
Introduction
Traumatic brain injury (TBI) is a leading cause of death and disability in the United States, affecting over 1.5 million people annually.1 Those who survive can develop secondary complications such as post-traumatic epilepsy (PTE). Prevalence and likelihood of developing PTE varies widely in the literature and depends on injury type and severity2. Though, PTE accounts for 10–20% of symptomatic epilepsy in the general population and 5% of all epilepsy.3 Importantly, PTE is associated with less favorable recovery and increases the risk of poor functional outcome following TBI4. PTE often requires long-term AED treatment, increasing the risk of adverse side effects, need for regular monitoring, and limiting cognitive recovery. However, not every patient presenting with clinical risk factors will develop PTE. Recent studies suggest differences in the secondary injury response post-TBI and individual genetic variability may contribute to epileptogenesis.5,6 Therefore, identifying genetic variation in pathways involved in epileptogenesis may help explain variation in PTE risk and present future points of intervention.
Adenosine is a potent endogenous anticonvulsant, regulated by a complex adenosine regulatory cycle7. Within the brain, adenosine homeostasis is largely under astrocyte control, providing a metabolic clearance system for intra- and extracellular adenosine.8 Briefly, extracellular ATP is dephosphorylated into adenosine via a chain of processes involving ectonucleotidases (including the 5'ectonucleotidase CD73, encoded by the NT5E gene), equilibrative nucleoside transporters (including the primary type-1 ENT-1 transporter, encoded by the SLC29A1 gene) for maintenance of intra/extra-cellular adenosine levels, and intracellular adenosine re-phosporylation to inactive 5'-AMP (via adenosine kinase ADK, encoded by the ADK gene). Differential regulation of the biochemical processes within this cycle, as well as extracellular adenosine receptors and intracellular epigenetic functions mediated by adenosine, represent a potentially large group of both therapeutic targets and biomarkers for evaluating epileptogenesis.
Reactive astrogliosis is prevalent in both human epilepsy and in TBI9,10, potentially contributing to PTE development. Previous research shows intra- and extracellular adenosine homeostasis actively influences epileptogenesis11 in non-traumatic and post-traumatic seizure models12,13. Additionally, adenosine activation of the inhibitory adenosine A1 receptor (A1R) has a powerful anticonvulsant effect following chemically induced as well as chronic recurrent seizures12 and on post-traumatic seizure development in a TBI mouse model.13 Our previous work shows variation at two single nucleotide polymorphisms (SNPs) within the adenosine A1R gene (ADORA1) is associated with an increased PTE risk in a population with severe TBI.5
Thus, using a Rehabilomics approach14, identifying mechanisms and biomarkers of epileptogenesis may have significant prognostic and personalized medical management value in TBI populations to prevent PTE throughout their rehabilitation and recovery. SNPs are a potentially useful biomarker for PTE, but few studies have assessed the link between genetic variation and epileptogenesis. We hypothesized genetic variation within candidate genes encoding components critical for maintenance of adenosine homeostasis, ADK, NT5E, and SLC29A1, would be associated with epileptogenesis and increased PTE risk, as demonstrated by time to event analysis.
Methods
Study design and subjects
This study was approved by the University of Pittsburgh Institutional Review Board. Candidate SNPs were genotyped and subject information regarding PTE was abstracted from medical charts for 208 subjects with severe, non-penetrating, TBI (admission Glasgow Coma Scale (GCS) score ≤ 8, head region Abbreviated Injury Score (AIS) ≥ 3) from a single academic medical center as part of a larger study investigating genetic relationships with TBI outcome. Subjects included in this longitudinal historical cohort study were 18–70 years old, had a positive computed topography (CT) scan confirming TBI, and no history of premorbid seizures. This study was restricted to subjects that self-reported race as white due to racial differences in allelic frequency obtained from the database of Single Nucleotide Polymorphisms (dbSNP: http://www.ncbi.nlm.nih.gov/snp). Four subjects were subsequently excluded (n=204).
Critical Care Management
All subjects were admitted to the neurotrauma intensive care unit at our level 1 trauma center and received treatment consistent with The Guidelines for the Management of Severe Head Injury.15 Treatment initially consisted of extraventricular drain (EVD) placement, central venous and arterial catheters, and further surgical intervention when clinically necessary. Intermittent EEGs were obtained by treating physicians as part of standard of care when suspicion of clinical or non-convulsive seizures arose. In accordance with guidelines for seizure prophylaxis therapy,16 the majority of subjects received anti-epileptic drugs (AEDs) for the first week post-injury.
Demographic and Injury Data
Subject demographic and injury information was abstracted from electronic medical records. Demographic and premorbid variables, including age and sex, were recorded. Injury information including mechanism of injury, GCS score (best in the first 24 hours post-injury), subdural hematoma (SDH), depressed skull fracture, and acute care length of stay (LOS) was obtained from ambulance, emergency room, surgical, and radiographic reports. Information regarding AED administration during the acute care period was considered only if used for seizure prophylaxis or treatment. Isolated TBI status and Injury Severity Scores (ISS) were calculated using the AIS scoring system, with data for these measures obtained from the University of Pittsburgh Medical Center (UPMC) trauma registry. The AIS scoring system determines the severity of a specific injury based on survivability of that injury.17 ISS is defined as the sum of squares of AIS scores from the three most severely affected body regions.18 Isolated TBI was defined using AIS criteria, where a head region AIS score of ≥ 3 and a score of <3 in all other body regions was considered an isolated TBI. Non-isolated TBI was defined as a head region AIS score of ≥ 3 and an AIS score ≥ 3 in at least one extra-cranial region, while isolated TBI status was defined as an AIS head region of ≥ 3, with AIS scores in other body regions <3.
Seizure Assessment
Time to first seizure was the primary variable of interest, and our cohort was further restricted to comply with the most current definition of PTE.19 PTE was defined as at least one documented seizure occurring after the first week post-injury. Therefore, subjects who seized (n=11) or died (n=31) during the first week post-injury were excluded. A final cohort of 162 subjects met the inclusion criteria based on our PTE definition.
Information regarding PTE occurrence was collected using all available inpatient and outpatient electronic medical records from our health system. Inpatient records were reviewed to determine time to first seizure and included ambulance and emergency room reports, nursing notes, progress reports, EEG reports, patient history and physical reports, and discharge or transfer summaries. PTE follow-up was censored at 3 years post-injury to allow for comparable and complete ascertainment of PTE information for all subjects. Any indications of seizures, convulsions, status epilepticus (SE), or diagnosed seizure disorders were classified as PTE occurrence. Any mention of possible seizure activity that was either ambiguous or non-conclusive was categorized as no-PTE.
DNA Extraction
DNA was extracted from whole blood samples or CSF collected from each subject. Blood was collected into ethylenediaminetetraacetic acid (EDTA) tubes. Samples were centrifuged and processed to retrieve the buffy coat, and DNA was extracted using a simple salting-out procedure.20 If no blood sample was available for DNA extraction, DNA was extracted from white blood cells obtained from passively drained CSF using QIAamp DNA extraction protocol for extraction from body fluids (Qiagen Corporation Venlo, The Netherlands).
SNP Selection and Genotyping
SNPs were selected in order to assess the variability across the entire ADK, NT5E, and SLC29A1 genes. This required a total of 14 tagging SNPs (tSNPs), including nine ADK (rs10824218, rs11001111, rs10824094, rs946185, rs11001109, rs11000980, rs1908335, rs4746209, and rs7899674), three NT5E (rs9444348, rs4431401, and rs9450282), and two SCL29A1 (rs324148 and rs760370) tSNPs. These tSNPs were selected via HapMap database build 36, using a minor allele frequency (MAF) ≥ 20% and CEPH (Utah residents with northern/western European ancestry) data. All 14 tSNPs were genotyped using the i-PLEX Gold SNP Assay (Sequenom Incorporated San Diego, CA, U.S.A). Hardy-Weinberg equilibrium was verified for all SNPs, indicating genotype distributions were within the expected proportions. Information regarding the degree of linkage disequilibrium (LD) between SNPs in this study was obtained using HapMap database build 36 (http://hapmap.ncbi.nlm.nih.gov). LD and MAF were examined within the specific study population using Haploview21 software (Broad Institute Cambridge, MA, U.S.A; Supplemental Table 1). We used the most recent Genome Reference Consortium release, GRCh38, to determine SNP location and to examine reported LD from the CEPH population when assessing SNPs with significant associations with time to first seizure and PTE risk.
Statistical Analysis
Statistical analyses were performed using SAS version 9.3 (Cary, NC, U.S.A) and R version 3.03 (http://www.r-project.org/). Summary statistics were calculated for demographic and injury characteristics of interest. Chi-square, using Fischer's exact test when appropriate, and Mann-Whitney statistics were used to assess differences in demographic and injury variables between PTE/no-PTE groups. All mean values were reported as the mean±SEM.
There were no a priori hypotheses regarding genetic models of disease for the selected SNPs of interest. Therefore, we initially examined autosomal dominant and autosomal recessive models for ADK and ENT-1 to determine if either genetic model was associated with seizure activity using chi-square analyses. Because ecto-5'-nucleotidase is known to form homodimers22, we compared seizure activity between heterozygous and homozygous individuals for the NT5E SNPs, in addition to autosomal dominant and recessive models. For all genes, the most significant genetic model was selected for further examination in primary analysis.
To screen for SNPs potentially associated with time to first seizure, Kaplan-Meier survival curves were generated for each SNP, using the genetic groupings indicated above, and compared using the log-rank statistic. Due to the observed correlation among the SNPs of interest, as evidenced by LD from examination using Haploview (described above), it was likely the number of truly independent tests would be less than the number of SNPs analyzed. Therefore, the minimum number of effective tests (Meff) was calculated individually for each gene23 and summed across genes to generate a total Meff of five. Because NT5E and SLC29A1 both reside on chromosome 6, a Meff was also calculated for both genes simultaneously to determine if there was significant correlation between genes. When examined together, the Meff for NT5E and SLC29A1 did not show correlation between genes, and the total Meff of five was subsequently used as the true number of independent tests. To correct for multiple comparisons during screening procedures, a Sidak correction was applied to the initial screening level of significance (α =0.10), using the Meff as the number of tests. Log-rank statistics were then compared to a corrected level of significance of α =0.021.
SNPs that were significant using the Sidak corrected α, and that met assumptions of Cox Proportional Hazards modeling, were further examined in multivariate Cox models, adjusted for demographic and injury characteristics found to differ by PTE status in univariate analysis (p<0.10). Following examination of individual SNPs in multivariate Cox models, SNPs were entered simultaneously into the adjusted model.
Additional post-hoc descriptive analyses were conducted to assess genetic variant concordance between nominally significant SNPs and SNPs surviving correction for multiple comparisons. Cross tabulations were used to explore concordance among SNPs within genes.
Results
Study Population
One hundred sixty-two subjects met inclusion criteria for PTE analysis. The average age was 33.27±1.10 years; 80.2% were male, and 33 subjects were deceased at the conclusion of the 3 year follow-up. The median GCS score was 6, and the mean ISS score was 35.42±0.72. Common mechanisms of injury included motor vehicle accidents (59.3%). The acute care LOS ranged 7–61 days, with a mean of 23.62±0.84 days. During the first week post-injury, 95.7% of subjects received AEDs for seizure prophylaxis including all subjects with PTE. Consistent with previous PTE studies,3 14.8% (n=24) of subjects had documented evidence of PTE upon medical record review. Two additional subjects developed PTE outside of the three year follow-up but were included in the “No PTE” group for analyses. Of those with PTE, 30.8% had ≥ 1 EEG during acute care, and 50% had an EEG at the time of initial seizure presentation. There were significant differences in demographic variables by PTE status (Table 1). Those with isolated TBI more often developed PTE versus those with non-isolated TBI (p=0.033). Subjects with SDH tended to have higher PTE frequencies (p=0.089). Mortality was inversely associated with PTE (p=0.007), with those who died having lower PTE rates. Age was not a significant factor associated with PTE.
Table 1.
Comparison of Demographic and Injury Variables between Subjects Who Did and Did Not Develop PTE
| Variable | Adenosine Genetics Population (n=162) | ||
|---|---|---|---|
|
| |||
| No PTE | PTE | p-value | |
| Sex | |||
|
| |||
| Women | 25(78.1%) | 7(21.9%) | p=0.210 |
| Men | 113(86.9%) | 17(13.1%) | |
|
| |||
| AED treatment | |||
|
| |||
| Yes | 131 (84.5%) | 24(15.5%) | p=0.259 |
| No | 7(100%) | 0 (0.00%) | |
|
| |||
| GCS Score | |||
|
| |||
| 3–4 | 50 (87.7%) | 7(12.3%) | p=0.339 |
| 5–8 | 73 (83.0%) | 15(17.0%) | |
| 9–12 | 13(92.9%) | 1 (7.1%) | |
| 13–15 | 1 (50.0%) | 1 (50.0%) | |
|
| |||
| Depressed Skull Fracture | |||
|
| |||
| Yes | 18(75.0%) | 6 (25.0%) | p=0.128 |
| No | 120(87.0%) | 18(13.0%) | |
|
| |||
| Injury Mechanism | |||
|
| |||
| Motor Vehicle | 83 (86.5%) | 13(13.5%) | p=0.695 |
| Fall | 15(88.2%) | 2 (11.8%) | |
| Motorcycle | 21 (77.8%) | 6 (22.2%) | |
| Other | 19(86.4%) | 3(13.6%) | |
|
| |||
| Isolated Head Injury | |||
|
| |||
| Yes | 43 (76.8%) | 13(23.2%) | p=0.033 |
| No | 93 (89.4%) | 11 (10.6%) | |
|
| |||
| Mortality | |||
|
| |||
| Alive | 105(81.4%) | 24(18.6%) | p=0.007 |
| Dead | 33(100%) | 0 (0.00%) | |
|
| |||
| Age | 33.6 ± 1.23 | 31.3 ± 2.27 | p=0.736 |
|
| |||
| ISS | 35.9 ± 0.78 | 32.4 ± 1.82 | p=0.047 |
|
| |||
| Acute Care LOS | 23.3 ± 0.91 | 25.6 ± 2.10 | p=0.288 |
|
| |||
| Subdural Hematoma | |||
|
| |||
| Yes | 78(81.3%) | 18(18.7%) | p=0.089 |
| No | 60 (90.9%) | 6(9.1%) | |
Evaluation of Tagging SNPs and PTE Risk
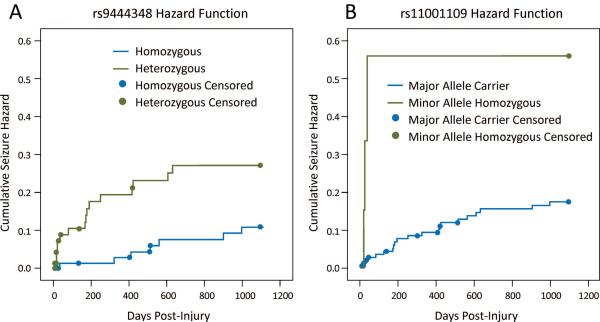
Tagging SNPs for ADK, NT5E, and SLC29A1 were independently evaluated for associations with time to seizure using Kaplan Meier models (Table 2). Within the ADK gene, individuals homozygous for the minor allele at rs11001109 (AA) had significantly shorter times to first seizure and higher seizure rates compared to major allele carriers (p=0.018) (Figure 1). Within NT5E, rs9444348 heterozygous subjects had shorter times to first seizure and higher seizure rates compared to homozygous subjects (p=0.021) (Figure 1). rs946185 (TT; ADK) minor allele homozygous and rs9450282 (NT5E) heterozygous subjects tended to have shorter time to seizure and higher seizure rates, though these findings were not statistically significant. Remaining SNPs evaluated showed no association with time to first seizure.
Table 2.
Comparison of Kaplan-Meier Curves for SNP Associations with PTE using Log-Rank Statistics
| SNP | Allele | P-value | 3 Year Seizure Rate (%) | Mean Days To Seizure (SE) |
|---|---|---|---|---|
| ADK Gene | ||||
| rslO824218 (n=157) | TT | 0.101 | 25.3 | 430.8 (31.6) |
| AA+AT | 14.3 | 903.3 (25.4) | ||
| rs11001111 (n=159) | GG | 0.071 | 29.5 | 265.5 (25.7) |
| AA+AG | 15.3 | 898.4 (23.9) | ||
| rs10824094 (n=156) | TT | 0.436 | 27.3 | 459.1 (50.5) |
| CC+CT | 16.9 | 878.1 (26.0) | ||
| rs946185(n=158) | TT | 0.046 | 31.0 | 406.0 (48.7) |
| CC+CT | 15.3 | 895.5 (24.5) | ||
| rs11001109(n=158) | AA | 0.018 | 42.9 | 30.6 (3.3) |
| GG+AG | 16.0 | 890.5 (23.4) | ||
| rs11000980 (n=151) | AA | 0.355 | 10.3 | 578.8 (36.1) |
| GG+AG | 18.6 | 860.6 (28.9) | ||
| rs1908335 (n=157) | GG | 0.264 | 28.6 | 446.0 (44.5) |
| TT+GT | 16.2 | 883.8 (25.6) | ||
| rs4746209 (n=159) | TT | 0.841 | 16.8 | 805.3 (31.5) |
| GG+GT | 17.8 | 868.1 (35.6) | ||
| rs7899674 (n=157) | CC | 0.228 | 14.6 | 905.5 (28.3) |
| GG+GC | 22.2 | 540.5 (27.4) | ||
| NT5E Gene | ||||
| rs9444348(n=156) | GG+AA | 0.021 | 10.2 | 948.5 (21.8) |
| AG | 23.8 | 529.8 (25.6) | ||
| rs4431401 (n=152) | CC+TT | 0.431 | 16.0 | 903.8 (30.7) |
| CT | 20.4 | 543.3 (24.6) | ||
| rs9450282 (n=151) | GG+AA | 0.034 | 12.9 | 912.3 (26.9) |
| AG | 27.4 | 726.3 (47.4) | ||
| SLC29A1 Gene | ||||
| rs324148 (n=158) | CC | 0.161 | 20.9 | 860.9 (36.9) |
| TT+CT | 11.4 | 584.6 (19.0) | ||
| rs760370 (n=155) | AA | 0.410 | 13.7 | 814.4 (37.3) |
| GG+AG | 20.1 | 859.5 (32.7) | ||
SNPs significant using the adjusted α of 0.021 are shown in bold
Figure 1.
Kaplan Meier curves depicting significantly different time to first seizure and 3 year seizure rate by genetic model for A) NT5E: rs9444348, p=0.021 and B) ADK: rs11001109, p=0.018.
Multivariate SNP Associations with PTE
After adjusting for ISS, isolated TBI status, and SDH in multivariate Cox models, there were significant differences in hazard ratios (HR) for rs11001109 (p=0.020) and rs9444348 (p=0.019) (Table 3). The risk of developing PTE was ~4.5X higher for individuals who were minor allele homozygotes for rs11001109 compared to major allele carriers. rs9444348 heterozygous individuals had ~3X higher risk of developing PTE than homozygous individuals within three years post-TBI. When entered simultaneously into a multivariate Cox model, both SNPs remain marginally significant (rs11001109: p=0.067, rs9444348: p=0.050) (Table 3).
Table 3.
Multivariate Cox Proportional Hazard Models
| Model | Hazard Ratio | Confidence Interval | p-value |
|---|---|---|---|
| Model 1 (n=156) | |||
| rs11001109: AA (compared to AG+GG) | 4.47 | 1.27–15.77 | 0.020 |
| ISS | 0.99 | 0.92–1.05 | 0.674 |
| IHI | 2.50 | 0.80–7.83 | 0.116 |
| Model2(n=154) | |||
| rs9444348: AG (compared to GG+AA) | 2.95 | 1.19–7.31 | 0.019 |
| ISS | 0.98 | 0.91–1.04 | 0.492 |
| IHI | 1.51 | 0.48–4.69 | 0.481 |
| Model3‡(n=151) | |||
| rs11001109: AA (compared to AG+GG) | 3.30 | 0.91–11.90 | 0.069 |
| rs9444348: AG (compared to GG+AA) | 2.52 | 1.00–6.32 | 0.050 |
| ISS | 0.98 | 0.91–1.04 | 0.474 |
| IHI | 0.58 | 0.18–1.87 | 0.36 |
All models adjusted for ISS, IHI and SDH
Due to non-proportionality, models are stratified by SDH status
SNPs entered simultaneously
Post-hoc Descriptive Analyses
Nominally or statistically significant variants in Kaplan Meier models were explored to examine levels of within gene concordance among these SNPs. Interestingly, when SNPs located within the ADK gene were examined, all subjects that were minor allele homozygous at rs11001109 were also minor allele homozygous at rs946185 and rs11001111. These subjects represented a minority of the population (4.5%). In the NT5E gene, 29.3% of subjects were found to be heterozygous for both rs9450282 and rs9444348, while 41.5% were homozygous.
Discussion
To determine whether or not genetic variation within integral components of the adenosine regulatory cycle is associated with epileptogenesis and PTE risk, we investigated a total of 14 SNPs within the ADK, NT5E, and SLC29A1 genes (9, 3, 2 SNPs, respectively). Importantly, SNPs associated with increased HR, rs11001109 and rs9444348, remain significantly associated with PTE risk after adjusting for potential confounders, including SDH, which is consistently cited as a risk factor for post-traumatic seizure2,3. To our knowledge, this is the first clinical investigation evaluating how ADK, NT5E, and SCL29A1 genetic variation influences time to first seizure and PTE risk. Based on International League Against Epilepsy (ILAE) definitions19 and the time to event analytical approach, our data show there is a genetic risk within ADK and NT5E for PTE, over the first 3 years after severe TBI. Both rs11001109 and rs9444348 are tagging SNPs with no known functionality at this time. However, these data do support the hypothesis that genetic variation within adenosine regulatory pathways can accelerate or shorten the latent period between injury and PTE, suggesting a possible genetic role in epileptogenesis following TBI.
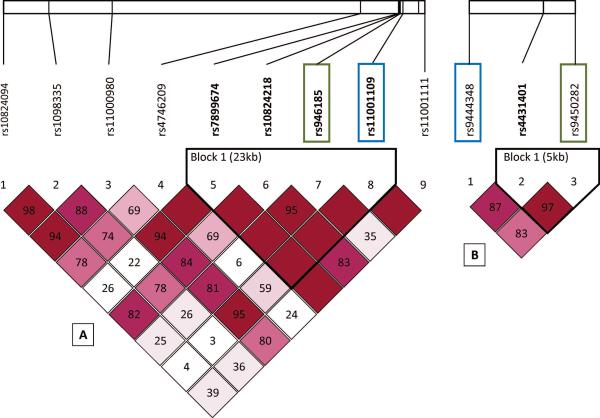
The ADK gene, located on chromosome 10q11-q24, is 546-kb long and is one of the largest in the human genome.7 Despite its size, the coding sequence produced by the ADK gene is only about 1.1kb long and is comprised of 11 short exons, each of which range from 36–765 nucleotides in length.7 rs11001109 is located within intron 10, about 13 kb from the end of exon 10 and 25kb from the 3'-end of the ADK gene, and it is in LD with multiple other tagging SNPs across a large DNA block of ~80kb, ranging from intron 9 into the 3' end of the ADK gene. The region of LD tagged by rs11001109 includes exon 10, which contains the catalytic site of ADK24. Variation within rs11001109 may represent functional variation within this exon of the ADK gene. Also, a missense polymorphism, rs397514452, causing a residue change from alanine to glutamic acid has been identified in this exonic region within a small case series of individuals with hypermethioninemia and epileptic seizures25. These findings further suggest rs11001109 may reflect functional variation in the represented DNA block. However, future studies are needed to determine what, if any, effects in ADK protein or gene expression result from variation represented by tagging SNPs explored in this current PTE study.
NT5E is located on chromosome 6q14.3, is about 1/10th the size of ADK (55kb), and consists of 9 exons. rs9444348, is located within intron 1, and as shown from the 1000 Genomes CEPH population, is in LD with a region extending across exon 1 and approximately 22kb 5' of NT5E (http://www.broadinstitute.org). Using data obtained from RegulomeDB (www.regulomedb.org), variation represented by rs9444384 may affect binding capacity. The lack of association between ENT-1 genetic variation and PTE in our study is consistent with findings by Wiesner et al. (1999) and further specifies the importance of enzymatic regulation of adenosine in epileptogenesis compared to adenosine transport.
The importance of adenosine regulation and its neuroprotective role has been well established through in vitro and rodent models and extensive reviews of the literature26–28. Physiologically, ADK and NT5E are critical components to adenosine regulation as part of a complex cycle. In addition, multiple adenosine receptors (i.e. A1R and A2AR), with regional expression patterns and functionality, contribute to this regulatory cycle. As part of the adenosine cycle, two metabolic processes contribute to extracellular adenosine. Bi-directional nucleoside transporters are driven by intracellular adenosine metabolism via ADK, and extracellular ATP dephosphorylation to adenosine occurs via ectonucleotidases, including 5'-ectonucleotidase. Numerous studies consistently document significantly higher levels of extracellular adenosine during pathophysiological processes, such as those initiated by TBI26,27. Importantly, increased adenosine can contribute to the development of reactive astrogliosis through increased activation of A2ARs29. Astrogliosis is a common pathology associated with both TBI and epilepsy9,10 and associated with ADK upregulation30. Genetic variation in NT5E, whose activity parallels A2AR's, and/or ADK may increase gliosis, possibly impacting epileptogenesis.
Experimental manipulation of adenosine cycle components and adenosine receptors has shed light on mechanisms of epileptogenesis and the pathophysiology of epilepsy. The extracellular tone of adenosine has a direct inhibitory effect on ictogenesis via A1R activation that couples to inhibitory G-proteins, mediates presynaptic inhibition, and stabilizes the postsynaptic membrane potential7,31. The failure of endogenous seizure prevention mechanisms could promote epileptogenesis in the sense that `seizures beget seizures'. A1R agonists have been shown to reduce seizure activity. However, application of various ADK inhibitors achieve similar results with an improved side effect profile32,33. Combined A1R and A2AR pharmacological modulation may provide more fine-tuned seizure control.
Rodent studies investigating kainic acid (KA) induced status epilepticus (SE) report ADK over-expression and increases in ADK enzymatic activity contribute to epileptogenesis.11,33,34 Experiments in a similar model demonstrate seizures originate from focal areas of ADK over-expression and generalize to the cortex following disruption of adenosine signaling through A1R blockade34. ADK over-expression, and the resulting adenosine deficiency, is associated with non-traumatic epilepsy development in humans and rodents.35 Genetically modified mice having reduced ADK expression results in reduced incidence of KA-induced SE, protection against brain injury associated with acute seizures, and resistance to epileptogenesis.11 Together, this work suggests ADK's role in epileptogenesis may be based on the ability of ADK to regulate extra-/intracellular adenosine homeostasis.
Interestingly, recent data suggest intracellular adenosine may facilitate epileptogenesis by regulating the DNA methylome36. Adenosine is an obligatory end-product of DNA methylation and needs to be metabolically cleared by ADK, presumably via the nucleus-based enzyme isoform28,36 in order to maintain transmethylation reactions. Importantly, increased ADK activity leads to DNA hypermethylation, thought relevant for epileptogenesis28,36. Indeed the methylation hypothesis of epileptogenesis suggests seizures by themselves can induce epigenetic modifications, thereby aggravating the epileptogenic condition37. Also, the NT5E gene has a CpG island regulated through methylation38. Importantly, experimental TBI studies suggest injury itself reduces epigenetic marks compared to controls39, lending greater importance to epigenetics as a mechanism for PTE development. ADK polymorphism associations with epigenetic marking profiles could shed light on the contribution of epigenetic mechanisms and possibly lead to the identification of additional biomarkers to inform PTE risk and pathology.
While the literature regarding manipulation of 5'-ectonucleotidase is not as expansive as the literature involving ADK, synthesis of current evidence suggests disruption in 5'-ectonulceotidase function affects adenosine regulation and epileptogenesis. Data suggest 5'-ectonulceotidase is the enzyme most responsible for the last step of ATP degradation, dephosphorylation of AMP to adenosine and a free phosphate40. Significant reductions in extracellular adenosine have been shown when using 5'-ectonucleotidase inhibitors and NT5E knock-out mice41. In vitro studies also demonstrate reduced adenosine production following 5'-ectonulceotidase inhibitor administration42. It is possible genetic variation, as observed in our results, may alter 5'-ectonucleotidase function, decrease extracellular adenosine, and increase seizure activity.
There are two functional domains for NT5E, with the N-terminal domain responsible for catalytic activity, and the C-terminal domain for substrate binding43. Two binding conformations result in different orientation of these two functional domains43, and variation within this gene may impact any of these components required for normal enzymatic function. Outside of its purinergic enzymatic activity, NT5E influences T-cell immunoactivation and inflammatory cell adhesion43, potentially implicating this enzyme with inflammatory pathways affecting epileptogenesis after TBI. Our previous work implicates inflammation, specifically genetic variation in the IL-1β gene and IL-1 β levels, to PTE risk and accelerated epileptogenesis.6
rs11001109 and rs9444348 were marginally significant when presented in the same multivariate model, suggesting these variants capture some shared variance with regard to PTE risk. This finding is not surprising, given the interrelatedness of these two genes in managing the adenosine regulatory cycle pathway. Multivariate models also linked isolated TBI status and increased PTE occurrence. Several studies have reported differences in inflammatory responses in serum following isolated TBI compared to non-isolated TBI44, including IL-1β 45, suggesting individuals with isolated TBI may have an inflammatory response more conducive to epileptogenesis compared to those with non-isolated TBI. Additionally, SDH was associated with PTE in univariate analysis, supporting previous work reporting an association between SDH and PTE2. Some literature suggests age may be a risk factor for PTE2,3. However, there were no significant differences between individuals that did/did not seize when assessing age as a continuous variable (see results) or when dichotomizing age at previously published cut-points (data not shown).
It is important to acknowledge limitations when interpreting the study findings. Because the primary hypothesis centered on genetic variation in epileptogenesis following TBI, time to first seizure was the primary variable of interest and was collected via medical record abstraction. While the medical system whose comprehensive electronic record system was reviewed is the largest provider in the geographic area, subjects may have presented to physicians outside the system for subsequent seizure development, resulting in misclassification. Non-convulsive seizures frequently occur early after TBI46. Thus, seizure incidence could also have been misclassified based on the first convulsive seizure. To control for population stratification, we limited analyses to white individuals. Without ancestral data regarding race, it is possible that residual population stratification exists. Conversely, limiting analyses to self-reported white subjects prevents generalization to other racial populations. Although this cohort is one of the largest single center TBI cohorts with genetic data, analyses are limited by small sample size. Future studies are needed to validate the current findings.
Additionally, one SNP within the ADK and one SNP within the NT5E genes were marginally associated with time to first seizure using Kaplan Meier models, but were not statistically significant using the α level appropriately corrected for multiple comparisons (Table 2). With a larger sample size, there could be significant associations with time to first seizure and accelerated epileptogenesis that survives correction for multiple comparisons with these SNPs that are only nominally/marginally significant with our sample size. Future studies can investigate how variation at functional SNPs (e.g. rs201127930, ADK) and the 5' region of NT5E, located within the regions of LD identified, are important to epileptogenesis to validate our findings. Despite these limitations, this study presents biologically plausible preliminary evidence that genetic variation in the adenosine regulatory cycle is a risk factor for epileptogenesis and PTE in a clinical population with severe TBI. If validated, appropriate therapeutics targeting this pathway can be explored.
Supplementary Material
Key Points.
Genetic variation in the adenosine regulatory cycle may accelerate epileptogenesis & increase post-traumatic epilepsy (PTE) risk after TBI.
rs11001109 rare allele homozygosity & rs9444348 heterozygosity are linked with shorter time to first seizure & higher 3 year seizure rates.
Confirmatory studies are needed to establish adenosine cycle genetic variation associations with accelerated epileptogenesis and PTE risk.
If confirmed, adenosine regulatory pathways may be a viable therapeutic target & point of patient stratification for clinical intervention.
Figure 2.
Haploview LD maps of D' values (within diamonds) using a LOD heatmap (LOD≥2 in shades of pink/red) for A) ADK and B) NT5E. SNPs significant in Kaplan Meier after correction for multiple comparisons shown in blue boxes; SNPs marginally significant in Kaplan Meier shown in green. LD blocks outlined in black as calculated by 95% confidence bounds on D'.
Acknowledgements
This work was supported by NIH-R01HD048162, DODW81XWH-071-0701, NIH R01NR013342, DOD W81XWH-12-1-0283, and NIH-R01NS087978. Thanks to Sandra Deslouche for support with genotyping and Dr. Candice Kammerer for statistical genetic guidance, and the subjects and their families for their generous participation. Thanks to the UPMC Trauma Registry for assisting with some elements of data collection.
Footnotes
Declarations and Disclosures We confirm that we have read the Journal's position on issues involved in ethical publication and affirm this report is consistent with those guidelines. No author has any conflict of interest to disclose.
References
- 1.Rutland-Brown W, Langlois JA, Thomas KE, et al. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil. 2006;21:544–8. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Temkin NR. Risk factors for posttraumatic seizures in adults. Epilepsia. 2003;44(Suppl 10):18–20. doi: 10.1046/j.1528-1157.44.s10.6.x. [DOI] [PubMed] [Google Scholar]
- 3.Englander J, Bushnik T, Duong TT, et al. Analyzing risk factors for late posttraumatic seizures: a prospective, multicenter investigation. Arch Phys Med Rehabil. 2003;84:365–73. doi: 10.1053/apmr.2003.50022. [DOI] [PubMed] [Google Scholar]
- 4.Kolakowsky-Hayner SA, Wright J, Englander J, et al. Impact of late post-traumatic seizures on physical health and functioning for individuals with brain injury within the community. Brain Inj BI. 2013;27:578–86. doi: 10.3109/02699052.2013.765595. [DOI] [PubMed] [Google Scholar]
- 5.Wagner AK, Miller MA, Scanlon J, et al. Adenosine A1 receptor gene variants associated with post-traumatic seizures after severe TBI. Epilepsy Res. 2010;90:259–72. doi: 10.1016/j.eplepsyres.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamond ML, Ritter AC, Failla MD, et al. IL-1β associations with posttraumatic epilepsy development: A genetics and biomarker cohort study. Epilepsia. 2014;55:1109–19. doi: 10.1111/epi.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boison D. Adenosine dysfunction in epilepsy. Glia. 2012;60:1234–43. doi: 10.1002/glia.22285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pascual O, Casper KB, Kubera C, et al. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–6. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 9.Robel S, Buckingham SC, Boni JL, et al. Reactive astrogliosis causes the development of spontaneous seizures. J Neurosci Off J Soc Neurosci. 2015;35:3330–45. doi: 10.1523/JNEUROSCI.1574-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGraw J, Hiebert GW, Steeves JD. Modulating astrogliosis after neurotrauma. J Neurosci Res. 2001 Jan 15;63:109–15. doi: 10.1002/1097-4547(20010115)63:2<109::AID-JNR1002>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 11.Li T, Ren G, Lusardi T, et al. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Invest. 2008;118:571–82. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fedele DE, Li T, Lan JQ, et al. Adenosine A1 receptors are crucial in keeping an epileptic focus localized. Exp Neurol. 2006;200:184–90. doi: 10.1016/j.expneurol.2006.02.133. [DOI] [PubMed] [Google Scholar]
- 13.Kochanek PM, Vagni VA, Janesko KL, et al. Adenosine A1 receptor knockout mice develop lethal status epilepticus after experimental traumatic brain injury. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2006;26:565–75. doi: 10.1038/sj.jcbfm.9600218. [DOI] [PubMed] [Google Scholar]
- 14.Wagner AK, Zitelli KT. A Rehabilomics focused perspective on molecular mechanisms underlying neurological injury, complications, and recovery after severe TBI. Pathophysiol Off J Int Soc Pathophysiol ISP. 2013;20:39–48. doi: 10.1016/j.pathophys.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care, AANS/CNS, Bratton SL, Chestnut RM, et al. Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J Neurotrauma. 2007;24(Suppl 1):S37–44. doi: 10.1089/neu.2007.9990. [DOI] [PubMed] [Google Scholar]
- 16.Temkin NR, Dikmen SS, Wilensky AJ, et al. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323:497–502. doi: 10.1056/NEJM199008233230801. [DOI] [PubMed] [Google Scholar]
- 17.Gennarelli TA, Wodzin E. AIS 2005: a contemporary injury scale. Injury. 2006;37:1083–91. doi: 10.1016/j.injury.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Baker SP, O'Neill B, Haddon W, Jr, et al. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–96. [PubMed] [Google Scholar]
- 19.Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–82. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 20.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 22.Knapp K, Zebisch M, Pippel J, et al. Crystal Structure of the Human Ecto-5'-Nucleotidase (CD73): Insights into the Regulation of Purinergic Signaling. Structure. 2012;20:2161–73. doi: 10.1016/j.str.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity. 2005;95:221–7. doi: 10.1038/sj.hdy.6800717. [DOI] [PubMed] [Google Scholar]
- 24.Schumacher MA, Scott DM, Mathews II, et al. Crystal structures of Toxoplasma gondii adenosine kinase reveal a novel catalytic mechanism and prodrug binding†1. J Mol Biol. 2000;298:875–93. doi: 10.1006/jmbi.2000.3753. [DOI] [PubMed] [Google Scholar]
- 25.Bjursell MK, Blom HJ, Cayuela JA, et al. Adenosine kinase deficiency disrupts the methionine cycle and causes hypermethioninemia, encephalopathy, and abnormal liver function. Am J Hum Genet. 2011;89:507–15. doi: 10.1016/j.ajhg.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunha RA. Neuroprotection by adenosine in the brain: From A1 receptor activation to A2A receptor blockade. Purinergic Signal. 2005;1:111–34. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomes CV, Kaster MP, Tomé AR, et al. Adenosine receptors and brain diseases: Neuroprotection and neurodegeneration. Biochim Biophys Acta BBA - Biomembr. 2011;1808:1380–99. doi: 10.1016/j.bbamem.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Boison D. Adenosine kinase: exploitation for therapeutic gain. Pharmacol Rev. 2013;65:906–43. doi: 10.1124/pr.112.006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ke R-H, Xiong J, Liu Y, et al. Adenosine A2a receptor induced gliosis via Akt/NF-β B pathway in vitro. Neurosci Res. 2009;65:280–5. doi: 10.1016/j.neures.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Fedele DE, Gouder N, Güttinger M, et al. Astrogliosis in epilepsy leads to overexpression of adenosine kinase, resulting in seizure aggravation. Brain. 2005;128:2383–95. doi: 10.1093/brain/awh555. [DOI] [PubMed] [Google Scholar]
- 31.Diógenes MJ, Neves-Tomé R, Fucile S, et al. Homeostatic control of synaptic activity by endogenous adenosine is mediated by adenosine kinase. Cereb Cortex N Y N 1991. 2014;24:67–80. doi: 10.1093/cercor/bhs284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiesner JB, Ugarkar BG, Castellino AJ, et al. Adenosine kinase inhibitors as a novel approach to anticonvulsant therapy. J Pharmacol Exp Ther. 1999;289:1669–77. [PubMed] [Google Scholar]
- 33.Gouder N, Scheurer L, Fritschy J-M, et al. Overexpression of adenosine kinase in epileptic hippocampus contributes to epileptogenesis. J Neurosci Off J Soc Neurosci. 2004;24:692–701. doi: 10.1523/JNEUROSCI.4781-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li T, Lytle N, Lan J-Q, et al. Local disruption of glial adenosine homeostasis in mice associates with focal electrographic seizures: a first step in epileptogenesis? Glia. 2012;60:83–95. doi: 10.1002/glia.21250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aronica E, Zurolo E, Iyer A, et al. Upregulation of adenosine kinase in astrocytes in experimental and human temporal lobe epilepsy. Epilepsia. 2011;52:1645–55. doi: 10.1111/j.1528-1167.2011.03115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams-Karnesky RL, Sandau US, Lusardi TA, et al. Epigenetic changes induced by adenosine augmentation therapy prevent epileptogenesis. J Clin Invest. 2013;123:3552–63. doi: 10.1172/JCI65636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobow K, Blümcke I. The methylation hypothesis: do epigenetic chromatin modifications play a role in epileptogenesis? Epilepsia. 2011;52(Suppl 4):15–9. doi: 10.1111/j.1528-1167.2011.03145.x. [DOI] [PubMed] [Google Scholar]
- 38.Lo Nigro C, Monteverde M, Lee S, et al. NT5E CpG island methylation is a favourable breast cancer biomarker. Br J Cancer. 2012;107:75–83. doi: 10.1038/bjc.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao W-M, Chadha MS, Kline AE, et al. Immunohistochemical analysis of histone H3 acetylation and methylation--evidence for altered epigenetic signaling following traumatic brain injury in immature rats. Brain Res. 2006;1070:31–4. doi: 10.1016/j.brainres.2005.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lovatt D, Xu Q, Liu W, et al. Neuronal adenosine release, and not astrocytic ATP release, mediates feedback inhibition of excitatory activity. Proc Natl Acad Sci U S A. 2012;109:6265–70. doi: 10.1073/pnas.1120997109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chu S, Xiong W, Parkinson FE. Effect of ecto-5'-nucleotidase (eN) in astrocytes on adenosine and inosine formation. Purinergic Signal. 2014;10:603–9. doi: 10.1007/s11302-014-9421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parkinson FE, Xiong W. Stimulus- and cell-type-specific release of purines in cultured rat forebrain astrocytes and neurons. J Neurochem. 2004;88:1305–12. doi: 10.1046/j.1471-4159.2003.02266.x. [DOI] [PubMed] [Google Scholar]
- 43.Sträter N. Ecto-5'-nucleotidase: Structure function relationships. Purinergic Signal. 2006;2:343–50. doi: 10.1007/s11302-006-9000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar RG, Diamond ML, Boles JA, et al. Acute CSF interleukin-6 trajectories after TBI: Associations with neuroinflammation, polytrauma, and outcome. Brain Behav Immun. 2015;45:253–62. doi: 10.1016/j.bbi.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 45.Maegele M, Sauerland S, Bouillon B, et al. Differential immunoresponses following experimental traumatic brain injury, bone fracture and “two-hit”-combined neurotrauma. Inflamm Res Off J Eur Histamine Res Soc Al. 2007;56:318–23. doi: 10.1007/s00011-007-6141-3. [DOI] [PubMed] [Google Scholar]
- 46.Vespa PM, Nuwer MR, Nenov V, et al. Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J Neurosurg. 1999;91:750–60. doi: 10.3171/jns.1999.91.5.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.