Abstract
Organophosphates, a type of neurotoxicant pesticide, are used globally for the treatment of pests on croplands and are therefore found in a large number of conventional foods. These pesticides are harmful and potentially deadly if ingested or inhaled in large quantities by causing a significant reduction in acetylcholinesterase (AChE) activity in the central and peripheral nervous system. However, much less is known about the effects of exposure to small quantities of the pesticides on neural systems and behavior during development. In the current study we used zebrafish larvae in order to determine the effects of two of the most widely used organophosphates, chlorpyrifos and malathion, on zebrafish behavior and AChE activity. Embryos and larvae were exposed to the organophosphates during different time points in development and then tested at 5 days post-fertilization for behavioral, neurodevelopmental and AChE abnormalities. The results of the study indicate that chlorpyrifos and malathion cause opposing behaviors in the larvae such as swim speed (hypoactivity vs. hyperactivity) and rest. Additionally, the pesticides affect only certain behaviors, such as thigmotaxis, during specific time points in development that are unrelated to changes in AChE activity. Larvae treated with malathion but not chlorpyrifos also had significantly smaller forebrain and hindbrain regions compared to controls by 5 days post-fertilization. We conclude that exposure to very low concentrations of organophosphate pesticides during development cause abnormalities in behavior and brain size.
Keywords: Acetylcholinesterase, Organophosphates, Zebrafish larvae, High-throughput screening, Thigmotaxis, Hyperactivity
1. Introduction
Organophosphates are a class of neurotoxicant pesticides that are widely used on croplands worldwide. In the United States, 60 million pounds of organophosphates are used on about 60 million acres of land every year (EPA, 2011). Two of the most widely used organophosphates are chlorpyrifos and malathion (NASS, 2011). At high levels these pesticides work by inhibiting the activity of acetylcholinesterase (AChE) thereby leading to an abundance of acetylcholine in the synapses of neurons. This can lead to paralysis, trouble breathing, and even death (CDC, 2011). These acute effects have been observed in crop workers that have been exposed to these pesticides without proper protection. Lesser information is known about the effects of small quantities of these pesticides over longer durations, especially during development. Some of the developmental effects of organophosphates include delayed motor and digestive tract development, spinal deformities, edema, decreases in body weight and brain volume, reproductive dysfunction and sex dependent abnormalities in responses to social cues (Condette et al., 2015; De Felice et al., 2015; De Felice et al., 2014; Jin et al., 2015; Mullins et al., 2015; Yu et al., 2013).
Exposure to pesticides can occur through several different routes. These include ingestion by mouth, dermal contact, or through inhalation of air and dust particles. Organophosphate pesticides are found on a large number of conventional non-organic fruits, vegetables, and grains (PAN, 2011). They have also been detected in air and dust samples, including in homes and day cares (Morgan et al., 2004). The potential exposure during childhood is concerning because children have more susceptible immune systems and are still physically developing (PAN, 2011). There have already been documented cases of neurodevelopmental and neuropsychological disorders such as attention deficit hyperactivity disorder (ADHD), autism spectrum disorders (ASD), anxiety, and depression in correlation to relatively high levels of pesticide exposure in children (Bouchard et al., 2010; Chen et al., 2011; Rauh et al., 2006).
The zebrafish is an excellent model to study the developmental effects of low levels of pesticides or other toxicants in order to determine behavioral and morphological changes after exposure (Eddins et al., 2010; Levin et al., 2011; Richendrfer and Creton, 2013; Richendrfer et al., 2014; Richendrfer et al., 2012b). Zebrafish embryos develop outside of the mother and can be collected daily in very large numbers and the embryos can be treated directly in a petri dish with various toxicants, drugs, or pesticides. Since zebrafish larvae are transparent, they are frequently used for whole specimen imaging. The large number of transgenic zebrafish available also makes it convenient to visualize gene expression and protein localization using various fluorescent proteins (Park et al., 2000). Zebrafish embryos develop rapidly and exhibit swimming behavior, hunting, avoidance, and escape behaviors within the first week of development (Colwill and Creton, 2011a; Colwill and Creton, 2011b) making them useful for behavioral analysis. A unique behavioral assay has been created in our lab which is used to assess behavioral changes in zebrafish larvae after exposure to toxicants (Pelkowski et al., 2011; Richendrfer and Creton, 2013; Richendrfer et al., 2012a). The assay can detect very subtle differences in behavior such as swim speed, amount of rest, avoidance behavior and thigmotaxis (Pelkowski et al., 2011; Richendrfer and Creton, 2013; Richendrfer et al., 2012a).
In the current study, we exposed zebrafish to low levels of chlorpyrifos and malathion that are relevant to levels that are found in the human diet (Lu et al., 2008; Lu et al., 2006). Larvae were exposed during different days of development and then analyzed for behavior at 5 days post fertilization (dpf), a timepoint chosen because larval activity does not change at this age with external feeding (Clift et al., 2014). Concurrent time points were used in order to analyze AChE activity using the Ellman assay (Ellman et al., 1961). Our results indicate that the pesticides used have opposing effects on behavior and are able to cause changes in behavior without affecting larval AChE activity. In order to determine morphological abnormalities in the brain after treatment with pesticides, measurements were made in larvae at 3, 4, and 5 dpf in the forebrain, midbrain, and hindbrain. Differences in forebrain and hindbrain size were found after treatment of larvae with malathion but not chlorpyrifos. The results presented in the current study suggest that organophosphate pesticides have diverse effects on brain development and behavior, which should be considered when setting health and food guidelines for pregnant women and children.
2. Materials and Methods
2.1 Animal Care and Housing
Adult wild type zebrafish were obtained from Carolina Biological Supply and have been housed at Brown University over several generations in multiple 10 and 20 gallon tanks. The transgenic line Tg(HuC:Kaede) was kindly provided by Dr. Joseph Fetcho in 2011 and has been maintained at Brown University since this time. For breeding purposes, the zebrafish were kept in mixed male and female populations under a 14 hour light/10 hour dark cycle. The fish were fed a combination of Gemma fish food, frozen brine shrimp, and freeze-dried bloodworms. Embryos were collected from the tanks at the beginning of the light cycle (“dawn”) and were immediately transferred to deep Petri dishes (Fisher 08-752-11Z) containing ‘egg water’ (0.06 g of Instant Ocean and 0.25 mg of methylene blue per liter of deionized water). The embryos were then treated with various dilutions of chlorpyrifos (ChemService F2057) and malathion (ChemService F2118), and grown in an incubator at 28.5 °C on a 14 hour light/10 hour dark cycle, and at a density of 60–70 embryos per culture dish.
2.2 Toxicant Exposure
Larvae were exposed to egg water (EW), dimethyl sulfoxide (DMSO) and three concentrations of chlorpyrifos (CPF) (from 0.001– 0.1 μM) or to four concentrations of malathion (from 0.001– 1 μM) during different time points in development for the behavioral assays. These concentrations were chosen based upon previous results indicating that concentrations of chlorpyrifos >0.1μM affect larval morphology (Richendrfer et al., 2012b) and because these concentrations are relevant to the levels found in the human diet (Lu et al., 2008; Lu et al., 2006). On days larvae were not treated, all groups were housed in egg water. Behavioral analyses were performed at 5 dpf. Therefore, some experimental groups were in the pesticide treatment during behavioral testing depending upon the time point of exposure. The same treatments were given to larvae and then AChE activity was evaluated using the Ellman assay (Ellman et al., 1961). Both chlorpyrifos and malathion were dissolved in dimethyl sulfoxide (DMSO) as 1000× stocks that were stored at −20 °C. The control groups were housed in egg water containing 0.1% DMSO. To obtain the desired final concentrations, 50 μL of each 1000× stock was dissolved into 50 ml of egg water in respective culture dishes. Dead embryos were removed daily from culture dishes and all solutions were replaced daily until 5 dpf, when behavioral analyses were conducted.
2.3 Behavioral Analysis
The behavioral assays were carried out in 5 lane plates, which were made by placing a specialized mold into liquid agarose and then letting the mold cool. Five larvae were placed in each lane in 5 lane plates; the solution that the larvae were housed in was used to fill up the agarose lanes. The plates were positioned on top of a laptop screen; four plates fit onto one laptop screen. The assay utilized a PowerPoint presentation shown to the larvae for 30 minutes (Richendrfer and Creton, 2013) (Fig 1). The results of the behavioral assays were used to analyze thigmotaxis, avoidance behavior, rest, and swim speed.
Figure 1. Behavioral assay PowerPoint presentation.
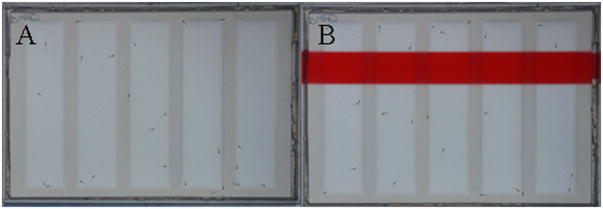
Five lane agarose plates containing five larvae per lane placed on top of a laptop screen displaying a PowerPoint presentation. Image A shows the blank 15 minute portion of the PowerPoint presentation while image B shows 15 minute portion of the PowerPoint presentations with the red aversive bar stimulus.
For the first half of the behavioral assay there were no visual stimuli (Fig 1A), in the second half a red moving bar was shown to the larvae (Fig 1B) which is a visually aversive stimulus and causes the larvae to swim away to the “horizontal” bottom half of the lane. Time lapse imaging was used to capture larval placement within the wells. Images were imported into Image J and a macro developed in our laboratory was used to analyze x, y coordinates of the larvae within the lanes (Richendrfer and Creton, 2013). Results were then imported into Excel to calculate the placement of larvae within the lane, swim speed, and rest. Based on previous reports in our lab, avoidance behavior can be measured by determining “percent down” in the lane and thigmotaxis behavior can be measured by determining “percent on the side, end, and edge” of the lane (Richendrfer et al., 2012a; Richendrfer et al., 2012b). It should be noted that the aversive red bar stimulus always evokes avoidance behavior in the larvae which is determined by comparing it to the blank control background (Pelkowski et al., 2011; Richendrfer et al., 2012a). However, because the treatment groups did not differ from the DMSO controls avoidance behavior, these results were not included in the current study. Behavioral assays for each exposure window were run in triplicates of 10 lanes each time (n = 30) for a total of 150 zebrafish larvae tested per treatment group for each exposure window. For a detailed methodology for the behavioral assay setup see our previous report (Richendrfer and Creton, 2013).
2.4 Ellman Assay
A modified Ellman assay (Ellman et al., 1961) was used to detect the activity of AChE in our larval samples. For the assay, fish were euthanized on ice for 5–10 minutes on the day of analysis. Ten larvae per treatment group were lysed in 500 μL of PBS with 1% Triton X. AChE activity was quantified using DTNB and ASChI as substrates using a multi-plate reader (SpectraMax M5 Microplate Reader) that calculated the changes in absorbance at 405nm over a ten minute period. Triplicates for each treatment group were run on the same day and was repeated a second time on a different day (n=6). The absorbance level at 5 minutes was used to determine AChE activity levels. The 5 minute time point was chosen because it was in the linear phase of the assay. Blanks and buffer solution only were used as negative controls (results not shown). AChE activity levels were compared to larvae treated only with DMSO.
2.5 Brain area measurements
Brain area measurements were made using transgenic larvae Tg(HuC:Kaede) treated with 0.1 μM chlorpyrifos, 1 μM malathion, or corresponding DMSO controls from 1–3, 1–4, and 1–5dpf. Images were taken using a Zeiss fluorescence microscope (Carl Zeiss, LLC, United States) and captured with AxioVision software. Area measurements were made in ImageJ using the freehand drawing tool on days 3, 4, or 5 which corresponded to the last day of exposure. Fifteen to twenty brains for each treatment group were analyzed. The forebrain, midbrain, and hindbrain (not including the spinal cord) were measured for each larva.
2.6 Statistical Analysis
Differences in the effects between duration of treatment, pesticide and concentrations were analyzed using the general linear multivariate analysis in SPSS 22 with Tukey post-hoc analysis. Subsequently, differences in behavior before and during the visual stimuli and between groups were tested for significance for each behavioral variable and pesticide concentration using a one way ANOVA in SPSS 22 to determine the effects of treatment during each specific exposure period. If the treatments showed significance (p<0.05), Tukey’s multiple comparison test was used to determine any significant differences between treatment groups compared to the DMSO control groups (before visual stimulus and during visual stimulus). For brain area measurements, independent sample t-tests were used to determine significance between treatment and DMSO group. ANOVA statistical results are reported in the results section. The asterisks in the graphs indicate a significant difference between the pesticide exposures and the DMSO controls from the Tukey posthoc analysis; *=<0.05 and **=<0.001.
3. Results
Results of the multivariate general linear analysis indicated significant main effects in the duration of treatment (p<0.001), pesticide concentration (p<0.001), and type of pesticide (p<0.001). There were also significant interactions between duration*concentration (p<0.001), duration*type of pesticide (p<0.001), concentration*type of pesticide (p<0.001), and duration*type of pesticide*concentration (p<0.001).
There were four behaviors found to be significantly different in fish before using the aversive visual stimulus. The types of pesticide, concentration and duration of treatment all significantly affected percent of fish on the sides and edges of the lanes, swim speeds, and percent of fish at rest. Duration of treatment had a significant effect on percent of fish on side of lane (p=0.025), swim speed (p<0.001), and percent rest (p<0.001). Pesticide type (malathion vs. chlorpyrifos), concentration, pesticide*concentration and duration*pesticide all had significant effects on percent side, edge, rest, and swim speed (all p<0.001). There was a significant interaction between duration*concentration for swim speed (p<0.001) and rest (p=0.032). There was also a significant triple interaction between duration*pesticide*concentration for percent side (p=0.014), edge (p=0.013), swim speed (p=0.001), and rest (p=0.014).
Similarly, the same four behaviors were significantly different in fish while they were shown the red bar visual stimulus. Duration of treatment had a significant effect on percent of fish on the side of the lane (p=0.008), swim speed and rest (p<0.001). Type of pesticide created significant differences in percent of fish on side and edge, swim speed, and percent rest (all p<0.001). Concentration of pesticide promoted significant differences in percent of fish on the side (p=0.001), edge (p=0.022), swim speed and rest (p<0.001). The interaction of duration of treatment*pesticide had significant effects on percent of fish on the edge (p=0.019), swim speed and rest (p<0.001). The interaction of duration of treatment*concentration had a significant effect on percent rest (p=0.017). The interaction of type of pesticide*concentration had a significant effect on swim speed and rest (p<0.001).
For the main effects of chlorpyrifos, the duration of treatment, concentration, and interaction of duration*concentration all showed significance (p<0.001). Percent of fish on the side, edge, and swim speed and rest were all significantly different (p<0.001) depending on duration of treatment and concentration of chlorpyrifos before fish were presented with the visual stimulus. Similarly, when fish were presented with the visual stimulus, percent of fish on the side (p=0.006), edge (p=0.024), and swim speed and rest (p<0.001) were all significantly affected by the duration of chlorpyrifos treatment. Fish were also significantly affected by the concentration of chlorpyrifos during visual stimulus presentation for percent of the side (p<0.001), edge (p=0.011), and swim speed and rest (p<0.001). More specifically, the percentage of fish on the side of the lane with no visual stimulus was significantly different between days 1–3→3–5 (p=0.038) and 1–5→3–5 (p<0.001) and between days 1–3→3–5 (p=0.016) and 1–5→3–5 (p=0.026) during the visual stimulus. The percentage of fish on the edge of the lane with no visual stimulus was significantly different between days 1–3→3–5 (p=0.023) and 1–5→3–5 (p<0.001) and between days 1–5→3–5 (p=0.022) with a visual stimulus. Swim speeds were significantly different between all treatment periods without and with the visual stimulus (all p<0.001). Percentage of fish at rest was significantly different between days 1–3→3–5 and 1–5→3–5 (p<0.001) without a visual stimulus and between days 1–3→1–5 (p=0.044), 1–3→3–5 and 1–5→3–5 (p<0.001) with the visual stimulus.
The main effects of malathion indicate that there are significant effects of duration of treatment and concentration of malathion (p<0.001). With or without the visual stimulus there were significant effects on swim speed and percentage of fish at rest by the duration of treatment and the concentration of malathion (p<0.001). For fish swim speeds and percentage of fish at rest with or without the visual stimulus, there were significant differences between days of treatment 1–3→3–5 and 1–5→3–5 (all p<0.001).
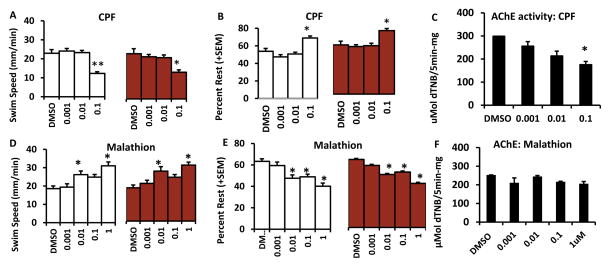
3.1 Chlorpyrifos and Malathion treated larvae show opposite behaviors
Larvae that were treated from 1–3dpf with chlorpyrifos and malathion showed opposite trends in swim speed and rest behaviors (Fig 2). Larvae treated with 0.1 μM chlorpyrifos with or without a visual stimulus showed decreased swim speed and increased rest compared to their respective DMSO control (Fig 2A, B). There was a significant main effect of treatment on swim speed and rest without visual stimulus F(4,140) = 11.84, p<0.001 F(4,140) = 10.05, p<0.001 and with visual stimulus F(4, 140) = 4.73, p=0.001 F(4,140) = 6.44, p<0.001. Similar results were also found in a previous study from our lab using a 6-well plate setup instead of lanes (Richendrfer et al., 2012b). There was also a significant main effect of concentration of chlorpyrifos on acetylcholinesterase activity. Larvae treated with 0.1 μM chlorpyrifos had significantly decreased levels of acetylcholinesterase compared to DMSO controls F(5,30) = 34.7, p<0.001 (Fig 2C). In contrast, larvae treated with 0.01 and 1μM malathion showed increased swim speeds (without visual stimulus F(4, 145) = 8.33, p<0.001 and with visual stimulus F(4, 145) = 7.86, p<0.001). Additionally larvae treated with 0.01–1 μM displayed decreased rest compared to DMSO controls (without visual stimulus F(4, 145) = 10.84, p<0.001 and with visual stimulus F(4, 145) = 10.53, p<0.001) (Fig 2D, E). There were no significant changes in acetylcholinesterase activity with any concentration of malathion (Fig 2F).
Figure 2. Embryonic exposures to chlorpyrifos and malathion from 1–3dpf lead to opposite behavioral defects.
Swim speed of larvae treated with chlorpyrifos (CPF)(A) and malathion (D). Percent rest of larvae treated with chlorpyrifos (B) and malathion (E). The white bars indicate the time that larvae had no visual stimulus during the assay and the red bars indicate the time that larvae were shown the visual aversive stimulus. There were no significant differences in edge or side measurements for either pesticide. Acetylcholinesterase (AChE) activity levels are reported for chlorpyrifos and malathion in C and F. Error bars in the graphs represent +SEM, *=p<0.05, **=p<0.001 in posthoc Tukey analysis. Concentrations are in μM.
3.2 Longer developmental exposures to chlorpyrifos but not malathion from 1–5dpf promote behavioral abnormalities linked to anxiety-related behaviors
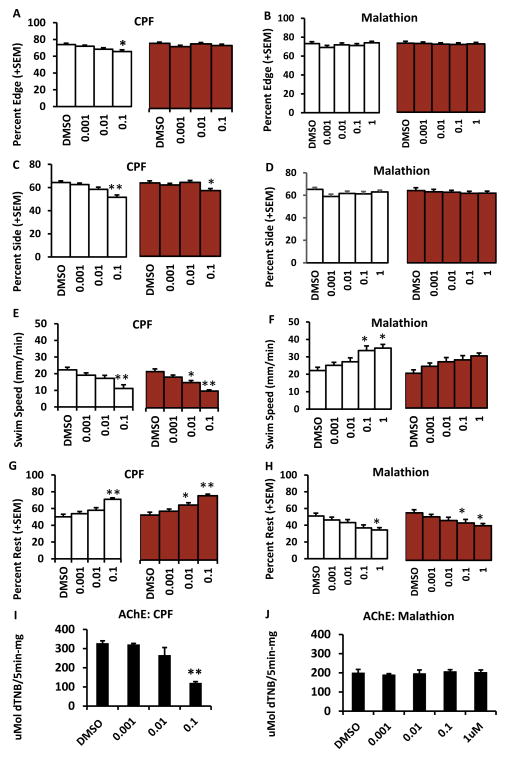
Zebrafish larvae treated with 0.1 μM chlorpyrifos from 1–5dpf showed a larger array of abnormal behaviors such as decreased preferences for the edge and side of the lane (Fig 3A, C) whereas there were no significant differences in these parameters in larvae treated with malathion (Fig 3B, D). There was a significant main effect of chlorpyrifos on edge (without a visual stimulus F(4, 145) = 3.67, p = 0.007) and side preference (without a visual stimulus F(4,145) = 9.27, p<0.001 and with visual stimulus F(4, 145) = 2.92, p = 0.023). Larvae treated with chlorpyrifos from 1–5dpf had decreased swim speed (without a visual stimulus (F(4, 145) = 17.06, p<0.001 and with visual stimulus F(4, 145) = 16.57, p<0.001) and increased rest (without a visual stimulus F(4, 145) = 13.01, p<0.001 and with a visual stimulus F(4, 145) = 14.15, p<0.001) (Fig 3E, G), but with both 0.01 and 0.1 μM concentrations. Malathion treated larvae again showed the opposite trend which was increased swim speed (without visual stimulus F(5, 109) = 4.01, p = 0.002) and decreased rest with 0.1 and 1 μM (without visual stimulus F(5, 109) = 4.04, p = 0.002 and with a visual stimulus F(5, 109) = 2.8, p = 0.02) (Fig 3F, H). Acetylcholinesterase activity in larvae treated with 0.1 μM chlorpyrifos was significantly decreased compared to controls F(5, 30) = 35.11, p<0.001 (Fig 3I). Acetylcholinesterase activity was similar in all groups treated with malathion (Fig 3J).
Figure 3. Developmental exposure to chlorpyrifos from 1–5dpf affects a broad range of behaviors that are opposite in larvae treated with malathion.
A and B show percent edge for larvae treated with chlorpyrifos and malathion. C and D show percentage of larvae treated with chlorpyrifos and malathion on the side of the lane. E and F show the swim speed of the larvae treated with chlorpyrifos and malathion. G and H show percent of larvae treated with chlorpyrifos and malathion that were at rest. The white bars indicate the time that larvae had no visual stimulus and the red bars indicate the time when larvae were shown visual aversive stimulus. Acetylcholinesterase (AChE) activity levels are reported for chlorpyrifos and malathion in I and J. Error bars in the graphs represent +SEM, *=p<0.05, **=p<0.001 in posthoc Tukey analysis. Concentrations are in μM.
3.3 Late developmental exposures (3–5dpf) to chlorpyrifos and malathion affect different behaviors compared to earlier exposures
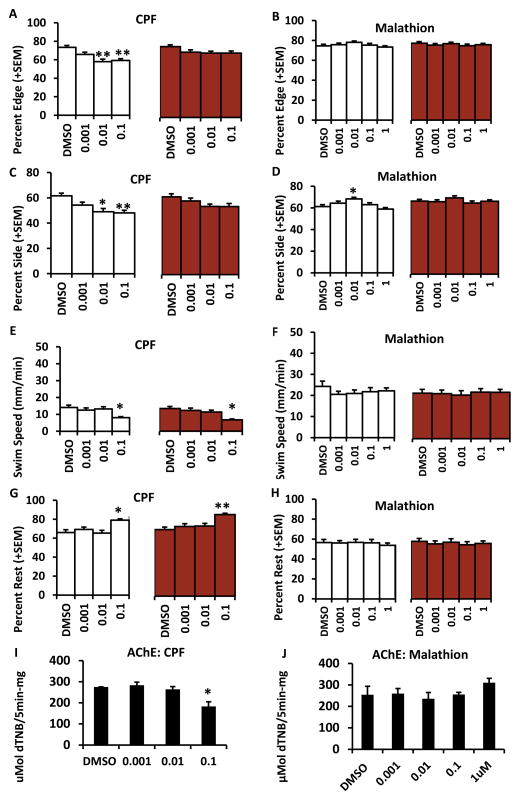
Zebrafish larvae treated with 0.01 and 0.1 μM chlorpyrifos from 3–5 dpf show decreased preferences for the edge and side of the lane without any visual stimuli (F(4, 144) = 5.58, p<0.001; F(4, 144) = 8.64, p<0.001; F(4, 144) = 6.4, p<0.001) (Fig 4A, C). There were no significant differences in edge preference in larvae treated with malathion, however, there was an increased preference for the side of the lane in larvae treated with 0.01 μM malathion without a visual stimulus (F(4, 145) = 4.85, p = 0.001) (Fig 4B, D). Swim speed was decreased (without F(4, 144) = 3.69, p = 0.007 and with visual stimuli F(4, 144) = 5.16, p = 0.001) and rest was increased (without F(4, 144) = 4.72, p = 0.001 and with visual stimuli F(4, 144) = 5.34, p <0.001) in larvae treated with 0.1 μM chlorpyrifos (Fig 4E, G). On the contrary, neither swim speed nor rest was affected with any concentration of malathion (Fig 4, F, H). Acetylcholinesterase activity in larvae treated with 0.1 μM chlorpyrifos was significantly decreased compared to DMSO controls (F(5, 30) = 48.22, p<0.001) (Fig 4I). There were no differences in acetylcholinesterase activity in any groups treated with malathion (Fig 4J).
Figure 4. Late exposures to chlorpyrifos and malathion from 3–5dpf.
Exposures of zebrafish larvae to chlorpyrifos from 3–5dpf invoke similar behaviors to those larvae treated from 1–5 dpf whereas malathion treated larvae show few changes. A and B show percent edge for larvae treated with chlorpyrifos and malathion. C and D show percentage of larvae treated with chlorpyrifos and malathion on the side of the lane. E and F show the swim speed of the larvae treated with chlorpyrifos and malathion. G and H show percent of larvae treated with chlorpyrifos and malathion that were at rest. The white bars indicate the time that larvae had no visual stimulus and the red bars indicate the time when larvae were shown visual aversive stimulus. Acetylcholinesterase (AChE) activity levels are reported for chlorpyrifos and malathion in F and H. Error bars in the graphs represent +SEM, *=p<0.05, **=p<0.001 in posthoc Tukey analysis. Concentrations are in μM.
3.4 Brain sizes affected in larvae only with malathion treatment
Treatment of larvae with malathion but not chlorpyrifos had a significant effect on brain size in comparison to DMSO controls. Figure 5A shows the areas of the zebrafish larval brain that were measured for analysis. These differences emerged by 4dpf in which the forebrain of malathion but not chlorpyrifos treated larvae were significantly smaller than those of DMSO treated larvae (Fig 5B, C) t(59)= 3.56, p=0.001. By 5dpf both the forebrain and hindbrain of malathion but not chlorpyrifos treated larvae were significantly smaller than controls (Fig 5D, E) t(45)= 3.38, p=0.001 and t(45)= 2.92, p=0.006. To verify that the smaller brain regions in malathion treated larvae were not an indication of a developmental delay, the head/trunk angle was measured at days 4 and 5 but no differences were found (data not shown). Head/trunk angle is a commonly measured parameter in zebrafish that is used to determine developmental stage (Kimmel et al., 1995).
Figure 5. Treatment of larvae with malathion but not chlorpyrifos affect forebrain and hindbrain sizes starting at 4dpf.
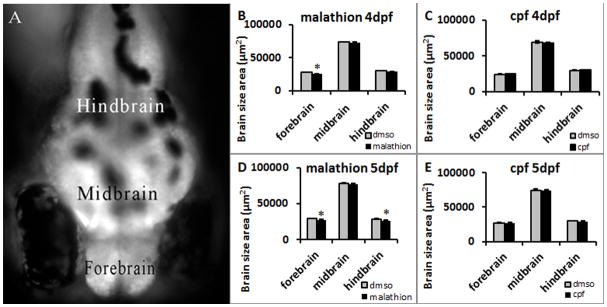
The regions of the brain that were measured are shown in A. By 4dpf the forebrain of malathion but not chlorpyrifos treated larvae was significantly smaller than DMSO controls (B and C). By 5dpf both the forebrain and the hindbrain of larvae treated with malathion but not chlorpyrifos were significantly smaller than DMSO controls (D and E). Error bars in the graphs represent +SEM, *=p<0.05 in t-test analysis.
4. Discussion
4.1 Opposite effects of two different organophosphates
The results from the current experiment indicate that chlorpyrifos and malathion have opposite effects on swim speed and rest behavior. Additionally, brain size was only affected with malathion but not chlorpyrifos treatment. While higher concentrations of chlorpyrifos decreased swim speed and increased rest, malathion treated larvae showed increased swim speed and decreased rest with multiple concentrations. Swim speed and rest behaviors may be correlated to AChE activity in larvae treated with chlorpyrifos but not in larvae treated with malathion. Whereas larval behavior such as edge, end, and side were affected at all three concentrations of chlorpyrifos depending upon the length of exposure, swim speed and rest were typically only affected at the highest concentration (0.1 μM) which was the same concentration in which AChE activity was decreased. On the contrary, larvae treated with malathion had AChE activity levels that were unchanged at any concentration. In order to determine the effects of organophosphates on brain size during development, larvae were exposed to the highest concentrations of malathion and chlorpyrifos. Brains in larvae exposed to malathion but not chlorpyrifos showed decreased forebrain and hindbrain areas. It is possible that the opposite trends seen in behavior with the two organophosphates may also be associated with the brain size differences found in the current study.
Previous studies using zebrafish larvae found that 300nM chlorpyrifos caused an 80% decrease in AChE activity at 5dpf (Yen et al., 2011) indicating a linear downward trend in activity for exposures greater than 0.01–0.1 μM at 5dpf. However, AChE activity in larvae exposed to levels as high as 1 μM chlorpyrifos at 3dpf were unchanged in comparison to controls (Yang et al., 2011) suggesting a potential age dependent physiological response to chlorpyrifos. It is probable that the concentrations of malathion used in the present study were not high enough to cause a change in AChE activity by 5dpf. In a study using carp, it was found that malathion affected AChE activity in much higher concentrations than chlorpyrifos (Chen et al., 2014). It is however intriguing that two types of pesticides with the same mechanism of action cause contrasting behavioral repertoires and effects on brain size.
4.2 Duration of exposures and critical periods
It was apparent that chlorpyrifos had an effect on zebrafish larval behavior that was dependent on the concentration and the window of exposure. The trend appeared to be that longer durations during development and later exposures had more of an impact on behaviors such as percent of larvae on the edge, end, and side of the lane. Swim speed and rest were affected regardless of the exposure time or the window of exposure whereas percentage of larvae on the edge, side, and end of the lane were dependent on exposure window. The trend with malathion exposure seemed to be that fish treated with earlier exposures (from 1dpf) showed a wider range of behavioral changes when compared to fish treated later in development. These results indicate that there may be a ‘critical period’ for susceptibility of embryos to lower concentrations of malathion during development. Moreover, there was a time-dependent effect of malathion on brain size in which longer treatment periods extending to day 5 had the most impact. There are numerous reports of critical period windows of time during development in which an embryo, fetus, or neonate is more susceptible to the effects of organophosphates (Aldridge et al., 2003; Seidler and Slotkin, 2011).
4.3 Visual aversive stimulus
The high-throughput assay used in our lab can measure behavior with and without a visual aversive stimulus, allowing us to compare behaviors before and during a stimulus that the larvae tend to avoid (which is measured by the percentage of larvae “down” in the lane during the visual stimulus). The results of the current study indicate that behaviors are affected, irrespective of the visual stimuli. For example, both swim speed and rest are shown to be affected with or without a visual stimulus with malathion and chlorpyrifos treated larvae indicating that swim speed and rest with or without a stressor. On the other hand, behaviors such as larvae preference for the end, edge, and side are not so clear cut. In most cases, these behaviors were elicited without a visual stimulus but not with a visual stimulus indicating a general decrease in thigmotaxis regardless of aversive stimuli. A decrease in thigmotaxis after treatment with chlorpyrifos was previously seen with the 6-well plate behavioral assay (Richendrfer et al., 2012b).
4.4 Behavioral changes are not necessarily linked to AChE activity levels
The results presented in the current study demonstrate that very low concentrations of organophosphate pesticide can alter the behavior of zebrafish without affecting AChE activity. These results point to the idea that there are other neural parameters affected that influence larval behavioral changes. Organophosphates have been shown to impact synaptic activity of dopamine and serotonin in rats and mice (Aldridge et al., 2005; Slotkin and Seidler, 2007; Venerosi et al., 2010). Chlorpyrifos is known to reduce the expression of serotonin (5HT-1A) receptors in the chick embryo (Slotkin et al., 2008). Malathion and its effects on other neurotransmitters are less documented. One study indicates that malathion affects reproduction via serotonergic systems in female rats (Uluitu et al., 1981). It is also likely that GABAergic and glutamatergic functionality is altered by organophosphates. The glutamatergic system in rats was affected by exposure to malathion (Al-Attar, 2010). Moreover, rats treated with chlorpyrifos had decreased levels of GABA and glutamate in the brain (Montes et al., 2013). Reports of organophosphates affecting neurotrophic factors are also a potential for changes in behavior. Postnatal exposure of rats to organophosphates altered the expression of nerve growth factor (NGF) and brain-derived neurotrophic factor in the hippocampus and cortex (Betancourt et al., 2007). It is likely that these same systems are affected in zebrafish larvae treated with malathion or chlorpyrifos which may subsequently affect behavioral and brain size differences. However, more research will be needed to determine which non-cholinergic systems are altered in zebrafish larvae to promote opposing behaviors and which neuronal subsets are most affected in the alteration of brain size.
4.5 Zebrafish as a model to study neurodevelopmental disorders
The results of the current study indicate that organophosphate pesticides induce a wide range of neurobehavioral phenotypes. We report that malathion treatment had a significant impact of larval swim speed and rest. In this case, larvae were hyperactive and also rested less than control larvae. Hyperactivity in zebrafish can be a measure of attention deficit hyperactivity disorder (ADHD), a disorder associated with alterations in dopamine and norepinephrine (Lange et al., 2012; Levin et al., 2011). There are similar reports of a correlation with ADHD symptoms in human children after developmental exposures to organophosphates (Bouchard et al., 2010; Furlong et al., 2014; Rauh et al., 2006; Yolton et al., 2014). While the human studies were correlational, the results from our study point to a direct link between malathion and hyperactivity as an indication of ADHD. Chlorpyrifos on the other hand, decreased swim speed, increased rest, and decreased thigmotaxis behaviors (preference for end, edge, side). The larvae’s change in swim speed and rest are likely a result of ACh toxicity and AChE inhibition, similar to what was seen in the axolotl and European seabass (Almeida et al., 2010; Robles-Mendoza et al., 2011). Larval decreased preference to be on the side, edge, and end of lane occurred even without changes in AChE activity. These thigmotactic behaviors can be used as indicators of stress and anxiety and suggest an anxiolytic effect of chlorpyrifos. Similar decreased edge preferences were seen in a previous study in our lab when larvae were treated with diazepam (Richendrfer et al., 2012a), a pharmacological agent that enhances the effect of GABA. These results again reinforce the idea that alterations in both non-cholinergic and cholinergic systems are impacted by developmental exposure to organophosphates and suggest the use of zebrafish as an excellent model to study neurodevelopmental disorders.
As a caveat, we cannot exclude the idea that there is a potential for DMSO to be enhancing the actions of malathion and chlorpyrifos. This potentiating effect was seen with teratogens dissolved in DMSO in xenopus embryos(Rayburn et al., 1991). Additionally, DMSO was shown to alter the permeability of fluorescein in zebrafish embryos at a concentration greater than 0.1% (Kais et al., 2013). DMSO concentrations in the current study were 0.1% which leads us to believe that while it is possible that DMSO had a potentiating effect on zebrafish behavior and brain size, more information is needed to determine if there are any adverse reactions with DMSO and organophosphates mixtures in zebrafish larvae.
5. Conclusions
Even very low concentrations of organophosphate pesticides that do not affect AChE activity can significantly impact behavior and brain size during development of the zebrafish. Moreover, the organophosphates used in this study have contrasting effects on behavior and brain size. These results should be taken into consideration when setting up food guidelines especially for young children and pregnant women.
Highlights.
Chlorpyrifos and malathion affect brain size development differently
Chlorpyrifos and malathion inversely affect swim speed and rest
Zebrafish anxiety is affected by chlorpyrifos and malathion without AChE inhibition
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development, (R01 HD060647) and the National Institute of Environmental Health Sciences (F32 ES021342).
Footnotes
We declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Attar A. Physiological and histopathological investigations on the effects of alpha-lipoic acid in rats exposed to malathion. J Biomed Biotechnol. 2010 doi: 10.1155/2010/203503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge J, Seidler F, Meyer A, Thillai I, Slotkin T. Serotonergic systems targeted by developmental exposure to chlorpyrifos: effects during different critical periods. Environ Health Perspect. 2003;111:1736–1743. doi: 10.1289/ehp.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environ Health Perspect. 2005;113:1027–1031. doi: 10.1289/ehp.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida J, Oliveira C, Gravato C, Guilhermino L. Linking behavioural alterations with biomarkers responses in the European seabass Dicentrarchus labrax L. exposed to the organophosphate pesticide fenitrothion. Ecotoxicology. 2010;19:1369–1381. doi: 10.1007/s10646-010-0523-y. [DOI] [PubMed] [Google Scholar]
- Betancourt A, Filipov N, Carr R. Alteration of neurotrophins in the hippocampus and cerebral cortex of young rats exposed to chlorpyrifos and methyl parathion. Toxicol Sci. 2007;100:445–455. doi: 10.1093/toxsci/kfm248. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Bellinger D, Wright R, Weisskopf M. Attention-Deficit/Hyperactivity Disorder and Urinary Metabolites of Organophosphate Pesticides. Pediatrics. 2010;125:e1270–1277. doi: 10.1542/peds.2009-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Nerve Agent and Organophosphate Pesticide Poisoning. 2011 ( http://emergency.cdc.gov/agent/nerve/tsd.asp)
- Chen C, Wang Y, Zhao X, Wang Q, Qian Y. The combined toxicity assessment of carp (Cyprinus carpio) acetylcholinesterase activity by binary mixtures of chlorpyrifos and four other insecticides. Ecotoxicology. 2014;23:221–228. doi: 10.1007/s10646-013-1165-7. [DOI] [PubMed] [Google Scholar]
- Chen W, Yuan L, Xue R, Li Y, Su R, Zhang Y, et al. Repeated exposure to chlorpyrifos alters the performance of adolescent male rats in animal models of depression and anxiety. Neurotoxicology. 2011;32:355–361. doi: 10.1016/j.neuro.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Clift D, Richendrfer H, Thorn R, Colwill RM, Creton R. High-throughput analysis of behavior in zebrafish larvae: effects of feeding. Zebrafish. 2014 doi: 10.1089/zeb.2014.0989. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwill RM, Creton R. Imaging escape and avoidance behavior in zebrafish larvae. Rev Neurosci. 2011a;22:63–73. doi: 10.1515/RNS.2011.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwill RM, Creton R. Locomotor behaviors in zebrafish (Danio rerio) larvae. Behav Processes. 2011b;86:222–229. doi: 10.1016/j.beproc.2010.12.003. (NIHMS264983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condette C, Bach V, Mayeur C, Gay-Queheillard J, Khorsi-Cauet H. Chlorpyrifos exposure during perinatal period impacts intestinal microbiota associate with delay of maturation of digestive tract in rats. J Pediatr Gastroenterol Nutr. 2015 doi: 10.1097/MPG.0000000000000734. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- De Felice A, Scattoni M, Ricceri L, Calamandrei G. Prenatal exposure to a common organophosphate insecticide delays motor development in a mouse model of idiopathic autism. PLoS One. 2015;10:e0121663. doi: 10.1371/journal.pone.0121663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice A, Venerosi A, Ricceri L, Sabbioni M, Scattoni M, Chiarotti F, et al. Sex-dimorphic effects of gestational exposure to the organophosphate insecticide chlorpyrifos on social investigation in mice. Neurotoxicol Teratol. 2014;46:32–39. doi: 10.1016/j.ntt.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Eddins D, Cerutti D, Williams P, Linney E, Levin ED. Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: comparison with nicotine and pilocarpine effects and relationship to dopamine deficits. Neurotoxicol Teratol. 2010;32:99–108. doi: 10.1016/j.ntt.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman G, Courtney K, Andres VJ, Feather-Stone R. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- EPA. Organophosphate Pesticides in Food - A Primer on Reassessment of Residue Limits. 2011 ( http://www.epa.gov/pesticides/)
- Furlong M, Engel S, Barr D, Woff M. Prenatal exposure to organophosphate pesticides and reciprocal social behavior in childhood. Environ Int. 2014;70:125–131. doi: 10.1016/j.envint.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Liu Z, Peng T, Fu Z. The toxicity of chlorpyrifos on the early life stage of zebrafish: A survey on the endpoints at development, locomotor behavior, oxidative stress, and immunotoxicity. Fish Shellfish Immunol. 2015;43:405–414. doi: 10.1016/j.fsi.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Kais B, Schneider KE, Keiter S, Henn K, Ackermann C, Braunbeck T. DMSO modifies the permeability of the zebrafish (Danio rerio) chorion-implications for the fish embryo test (FET) Aquat Toxicol. 2013;140–141:229–238. doi: 10.1016/j.aquatox.2013.05.022. [DOI] [PubMed] [Google Scholar]
- Kimmel C, Ballard W, Kimmel S, Ullmann B, Schilling T. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Lange M, Norton W, Chaminade M, Vernier P, Lesch K, Bally-Cuif L. Zebrafish assays to measure ADHD endophenotypes. In: Spink A, Greieco F, Krips O, Loijens L, Noldus L, Zimmerman P, editors. Measuring Behavior. Utrecht Netherlands: 2012. pp. 259–261. [Google Scholar]
- Levin E, Sledge D, Roach S, Petro A, Donerly S, Linney E. Persistent behavioral impairment cause by embryonic methylphenidate exposure in zebrafish. Neurotoxicol Teratol. 2011;33:668–673. doi: 10.1016/j.ntt.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Barr DB, Pearson MA, Waller LA. Dietary intake and its contribution to longitudinal organophosphorus pesticide exposure in urban/suburban children. Environ Health Perspect. 2008;116:537–542. doi: 10.1289/ehp.10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Toepel K, Irish R, Fenske RA, Barr DB, Bravo R. Organic Diets Significantly Lower Children’s Dietary Exposure to Organophosphorus Pesticides. Environ Health Perspect. 2006:114. doi: 10.1289/ehp.8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes dOL, Moreno M, Cardona D, Campa L, Sunol C, Galofre M, et al. Long term compulsivity on the 5-choice serial reacting time task after acute chlorpyrifos exposure. Toxicol Lett. 2013;216:73–85. doi: 10.1016/j.toxlet.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Morgan MK, Sheldon LS, Croghan CW, Jones PA, Robertson GL, Chuang JC, et al. Exposures of preschool children to chlorpyrifos and its degradation product 3,5,6-trichloro-2-pyridinol in their everyday environments. J Expo Anal Environ Epidemiol. 2004;15:297–309. doi: 10.1038/sj.jea.7500406. [DOI] [PubMed] [Google Scholar]
- Mullins R, Xu S, Pereira E, Pescrille J, Todd S, Mamczarz J, et al. Prenatal exposure of guinea pigs to the organophosphorus pesticide chlorpyrifos disrupts the structural and functional integrity of the brain. Neurotoxicology. 2015;19:9–20. doi: 10.1016/j.neuro.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASS. Agricultural Chemical Use Database. United States Department of Agriculture; 2011. http://www.nass.usda.gov/ [Google Scholar]
- PAN. Pesticide Action Network. 2011. [Google Scholar]
- Pelkowski S, Kapoor M, Richendrfer H, Wang X, Colwill RM, Creton R. A novel high-throughput imaging system for automated analyses of avoidance behavior in zebrafish larvae. Behav Brain Res. 2011;223:135–144. doi: 10.1016/j.bbr.2011.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, et al. Impact of Prenatal Chlorpyrifos Exposure on Neurodevelopment in the First 3 Years of Life Among Inner-City Children. Pediatrics. 2006;118:e1845–1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayburn JR, DeYoung DJ, Bantle JA, Fort DJ, McNew R. Altered developmental toxicity caused by three carrier solvents. J Appl Toxicol. 1991;11:253–260. doi: 10.1002/jat.2550110405. [DOI] [PubMed] [Google Scholar]
- Richendrfer H, Creton R. Automated High-Throughput Behavioral Analyses in Zebrafish Larvae. J Vis Exp. 2013:e50622. doi: 10.3791/50622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richendrfer H, Creton R, Colwill R. The embryonic zebrafish as a model system to study the effects of environmental toxicants. In: Lessman C, Carver E, editors. Zebrafish: Topics in reproduction, toxicology and development. Nova science publishers; 2014. [Google Scholar]
- Richendrfer H, Pelkowski S, Colwill RM, Creton R. On the edge: pharmacological evidence for anxiety-related behavior in zebrafish larvae. Behav Brain Res. 2012a;228:99–106. doi: 10.1016/j.bbr.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richendrfer HA, Pelkowski S, Colwill R, Creton R. Developmental sub-chronic exposure to chlorpyrifos reduces anxiety-related behavior in zebrafish larvae. Neurotoxicol Teratol. 2012b;34:458–465. doi: 10.1016/j.ntt.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles-Mendoza C, Zuniga-Lagunes S, Ponce de Leon-Hill C, Hernandez-Soto J, Vanegas-Perez C. Esterases activity in the axolotl Ambystoma mexicanum exposed to chlorpyrifos and its implication to motor activity. Aquat Toxicol. 2011;105:728–734. doi: 10.1016/j.aquatox.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Seidler F, Slotkin T. Developmental neurotoxicity targeting hepatic and cardiac sympathetic innervation: effects of organophosphates are distinct from those of glucocorticoids. Brain Res Bull. 2011;85:225–230. doi: 10.1016/j.brainresbull.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T, Seidler F, Ryde I, Yanai J. Developmental neurotoxic effects of chlorpyrifos on acetylcholine and serontonin pathways in an avian model. Neurotoxicol Teratol. 2008;30:433–439. doi: 10.1016/j.ntt.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Prenatal chlorpyrifos exposure elicits presynaptic serotonergic and dopaminergic hyperactivity at adolescence: Critical periods for regional and sex-selective effects. Reprod Toxicol. 2007;23:421–427. doi: 10.1016/j.reprotox.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Uluitu M, Boca A, Petec G, Chis R, Catrinescu G. The influence of malathion on the brain serotonin and reproductive function in rats. Physiologie. 1981;18:167–174. [PubMed] [Google Scholar]
- Venerosi A, Ricceri L, Rungi A, Sanghez V, Calamandrei G. Gestational exposure to the organophosphate chlorpyrifos alters social–emotional behaviour and impairs responsiveness to the serotonin transporter inhibitor fluvoxamine in mice. Psychopharmacology. 2010;208:99–107. doi: 10.1007/s00213-009-1713-2. [DOI] [PubMed] [Google Scholar]
- Yang D, Lauridsen H, Buels K, Chi L-H, La Du J, Bruun D, et al. Chlorpyrifos-oxon disrupts zebrafish axonal growth and motor behavior. Toxicol Sci. 2011 doi: 10.1093/toxsci/kfr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen J, Donerly S, Levin ED, Linney E. Differential acetylcholinesterase inhibtion of chlorpyrifos, diazinon, and parathion in larval zebrafish. Neurotoxicol Teratol. 2011;33:735–741. doi: 10.1016/j.ntt.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolton K, Cornelius M, Ornoy A, McGough J, Makris S, Schantz S. Exposure to neurotoxicants and the development of attention deficit hyperactivity disorder and its related behaviors in childhood. Neurotoxicol Teratol. 2014;44C:30–45. doi: 10.1016/j.ntt.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Yu Y, Yang A, Zhang J, Hu S. Maternal exposure to the mixture of organophosphorus pesticides induces reproductive dysfuntion in the offspring. Environ Toxicol. 2013;28:507–515. doi: 10.1002/tox.20741. [DOI] [PubMed] [Google Scholar]