Abstract
Background
Currently, there are no well-established suture protocols to attach fully load-bearing scaffolds which span tendon defects between bone and muscle for repair of critical sized tendon tears. Methods to attach load-bearing tissue repair scaffolds could enable functional repair of tendon injuries.
Methods
Sixteen rabbit shoulders were dissected (New Zealand white rabbits, 1 yr. old, female) to isolate the humeral-infraspinatus muscle complex. A unique suture technique was developed to allow for a 5 mm segmental defect in infraspinatus tendon to be replaced with a mechanically strong bioscaffold woven from pure collagen threads. The suturing pattern resulted in a fully load-bearing scaffold. The tensile stiffness and strength of scaffold repair was compared with intact infraspinatus and regular direct repair.
Findings
The failure load and displacement at failure of the scaffold repair group were 59.9 N (Standard Deviation, SD = 10.7) and 10.3 mm (SD = 2.9), respectively and matched those obtained by direct repair group which were 57.5 N (SD = 15.3) and 8.6 mm (SD = 1.5), (p > 0.05). Failure load, displacement at failure and stiffness of both of the repair groups were half of the intact infraspinatus shoulder group.
Interpretation
With the developed suture technique, scaffolds repair showed similar failure load, displacement at failure and stiffness to the direct repair. This novel suturing pattern and the mechanical robustness of the scaffold at time zero indicates that the proposed model is mechanically viable for future in vivo studies which has a higher potential to translate into clinical uses.
Keywords: segmental rotator cuff defect, scaffold, collagen suture technique, mechanical properties, strength, infraspinatus tendon
1. Introduction
Tendon is a collagen-rich tissue that transfers load from muscle to bone. An estimated 30,000 to 75,000 rotator cuff tendon repairs are performed each year in the United States, and 40% or more of patients older than 60 years old are affected by rotator cuff tendon related disorders3, 40. Initial research for tendon tears has focused on improvement of surgical technique and augmentation of surgical technique by mechanically reinforcing the repair with various graft materials3, 7, 25, 51. Despite the improvements in surgical techniques, revision of tendon tear surgery is high, ranging from 11% to 94% depending on the size of the tear, patient age, tendon quality and the level of tendon degeneration 13, 22.
Existing synthetic or extracellular matrix (ECM) based rotator cuff repair scaffolds are mainly onlay patches which are not fully load-bearing by themselves 2, 15. There are very few studies where scaffolds act as a load-bearing unit and the strength of those scaffold repairs are inadequate14, 27, 43. Therefore, these aforementioned scaffolds need sutures which bridge the gap so as to bear the load. Absence of loads on the scaffold is likely to deprive the cells from the mechanostimulation as it is known that lack of loading translated to inferior tendon formation 10, 28. To our knowledge, there are no reports of a complete load-bearing scaffold model for rotator cuff repair. In association, there are no surgical protocols for suturing bulk scaffolds to bridge the remnant tendon to the bone. The aims of this study were: 1) Develop a suturing method to attach a load-bearing scaffold to span a gap defect between the infraspinatus muscle and humerus in rabbit. 2) Compare the biomechanics of tendon repair using a fully load-bearing scaffold to the biomechanics of direct repair. A recently developed high strength woven collagen biotextile was used as the scaffold to attain these aims55. These woven collagen scaffolds were made out of mechanically strong collagen based threads where the collagen molecules are densely packed and aligned along the length of the thread. These threads are known as electrochemically aligned collagen (ELAC) 12, 24, 48 . The strength of ELAC threads were improved to the level of tendon by genipin crosslinking31, 49, 50, 55 and previous study showed that aligned, dense topography of ELAC threads facilitated tenogenic differentiation of marrow derived mesenchymal stem cells (MSCs) 30. In vivo study of genipin cross linked ELAC threads showed biocompatibility and biodegradability after 8 months32. Therefore, ELAC is a promising biomaterial for tendon repair. Moreover, as tendon’s major constituent is collagen; thus, reconstituted pure collagen, in the form of ELAC is suitable for the woven scaffold. The Current study shows the feasibility of this collagen bioscaffold in a repair model which has a high potential to translate into clinical uses.
2. Materials and Method
2.1 Fabrication of Electrochemically Aligned Collagen Bioscaffolds
Acid soluble monomeric collagen solution (bovine dermis, Advanced Biomatrix, CA; 6 mg/ml) was diluted two-fold, pH was adjusted between 8-10 using 1N NaOH and dialyzed against ultrapure water for 18 hours. Dialyzed collagen was loaded between two stainless steel wire electrodes across which 30 VDC was applied for 2 min. ELAC is formed under the mechanisms previously published12, 24, 48. Briefly, electrical current electrophoretically mobilizes collagen molecules which become packed and aligned in a direction along the longer axes of the electrodes. ELAC threads were fabricated by using a custom-made rotating electrode machine that can generate continuous threads. The resulting ELAC thread is treated in phosphate buffered saline (PBS) for six hours at 37 °C to induce fibril formation and then treated with 2-propanol solution for 12 hours. Threads are crosslinked with 0.625% genipin (Wako Chemical, Japan) in 90% v/v ethanol solution at 37 °C for 3 days.
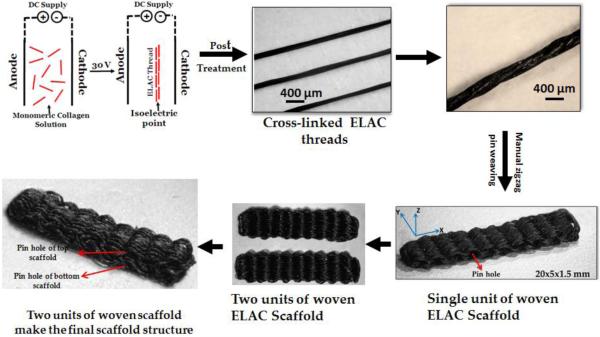
Three individual ELAC threads were twisted together to make a yarn (Fig 1). ELAC yarn was used to fabricate bioscaffolds using a manual pin-weaving method. Briefly, an array of 1 mm diameter pins was secured equidistantly (0.5 mm) from each other onto a solid substrate and an ELAC yarn was woven in a zig-zag pattern around the pins up to the desired width of the bioscaffold. The number of pins and size of pins determine the length and thickness of the bioscaffold, respectively. Once the weaving is complete, 10% PLGA solution was applied onto the bioscaffold to adhere the threads together and the bioscaffold was slid out of the pins, with the former location of the pins left out as holes. An ELAC yarn was then sutured through these holes to hold the woven threads together, resulting in a bioscaffold (Fig 1). Two such bioscaffolds were stacked on top of each other and connected by a long ELAC yarn passing through the holes of both of the scaffolds to hold them together. The resultant bioscaffold (Fig.1) was submerged briefly in chloroform to remove the PLGA coating. The scaffold dimensions were 15 × 5 × 2 mm in accordance with the dimensions of rabbit infraspinatus tendon.
Figure 1.
3-D woven scaffold fabrication process beginning from the monomeric collagen solution to the final scaffold. The black color of ELAC thread is due to the genipin crosslinking.
2.2 Animal tissue collection and surgical procedure
Sixteen rabbit (New Zealand White, 1 yr. old, female) shoulders were dissected from fresh rabbit carcasses obtained from the Animal Resource Center (ARC) of Case Western Reserve University. Since the tissue were collected post-mortem from another study which did not involve the shoulder region, IACUC approval was not applicable. The shoulders were stored at –20 °C wrapped in PBS wetted gauze pad. The right and left shoulders were assigned randomly between different groups. The shoulders were dissected such that all the soft tissues were removed except the humerus–infraspinatus–muscle unit.
2.3 Suture type selection
Before the main study, mechanical tests was done to compare two brands (Ethibond and Polydec) and 4 different sizes (2-0, 3-0, 4-0 and 6-0) of sutures determine the appropriate one for the repair model (N=3/group). A simple loop was made with each suture where each loop contains two strands parallel (i.e. two strands take the load). Tensile tests were performed in the loops until failure at a loading rate of 10 mm/min (Test Resource 800LE3-2, Test Resources Inc., MN, USA). 2-0 Ethibond suture was chosen to repair the tendons because it was the strongest (Table 1) and is widely used for repair5, 6, 44.
Table 1.
Failure loads of sutures. Standard deviations are indicated within parenthesis.
| Size | Brand | Failure Load (N) |
|---|---|---|
| 2-0 | Ethibond | 61 (0.14) |
| 3-0 | Ethibond | 31 (0.32) |
| 3-0 | Polydek | 28.5 (0.21) |
| 4-0 | Polydek | 24 (1.2) |
| 6-0 | Polydek | 8 (0.91) |
2.4 Experimental groups
There were four groups: 1) Intact shoulders (N=6), 2) Direct repair group (N=5) where the infraspinatus was cut at the enthesis and sutured directly, 3) Scaffold repair group (N=5) where infraspinatus was resected and replaced with a scaffold, and 4) Scaffold only group (N=3) where the scaffolds were tested.
2.5 Suture technique
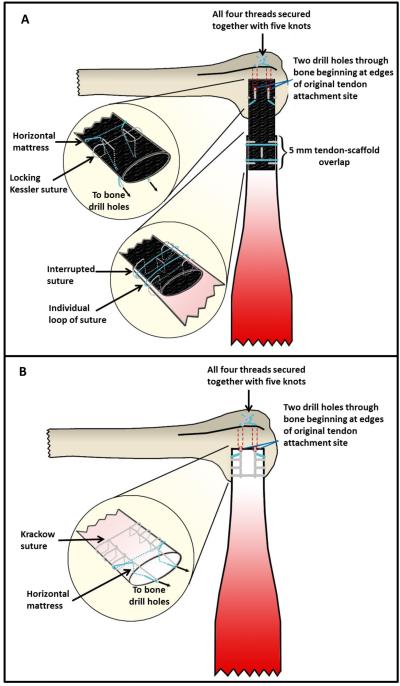
The native infraspinatus tendon was sharply transected at the osteotendinous junction. The humerus was prepared for tendon reconstruction by placement of two drill holes beginning at the original tendon insertion and travelling parallel out the bicipital groove, leaving a 4 mm bone-bridge. In the direct repair group (Fig 2B, 3B), the tendon was positioned such that the lateral end sat flush to the original tendon insertion. A 2-0 non-absorbable braided suture (Ethibond) was placed in the lateral edge of the tendon in a horizontal mattress fashion. The suture ends were passed through the bone tunnels in the humerus, and the ends were secured to each other with five knots. Another suture was used to secure the remnant tendon to the bone by Krackow suture technique. Two Krackow locked throws of suture were placed on each side of the tendon. The suture ends were passed through the bone tunnels in the humerus, and five knots were used to tie the ends. In case of scaffold repair (Fig 2A, 3C), the lateral 5 mm section of tendon was cut and discarded. The collagen scaffold was positioned such that the medial portion of the scaffold overlapped the remnant tendon by 5 mm and the lateral end sat flush to the original tendon insertion. A 2-0 non-absorbable braided suture (Ethibond) was used to secure the medial portion of the scaffold to the remnant tendon by five interrupted sutures, one in each quadrant of the overlapping segments and one placed longitudinally through the center of the overlap. Each suture was secured with four knots. Two individual loops of suture were then tightened around the overlapping segments, one medially and one laterally, and secured with four knots. The middle portion of the scaffold was left untouched to function as the sole load-bearing entity of any force applied across the bone-tendon gap. Another 2-0 non-absorbable braided suture was used to secure the lateral end of the scaffold to the humerus. First, a locking Kessler suture with side loops was placed through the scaffold based on a study by Yotsumoto et al 16, 54. Second, a horizontal mattress suture was placed medial to the locking Kessler suture. All suture ends exited the scaffold posteriorly near its lateral edge. The suture ends were then passed through the bone tunnels in the humerus and all four threads were secured together with five knots [Fig. 2].
Figure 2.
Suture technique. A) Full sized tendon repair with full load-bearing woven collagen scaffold. B) Direct tendon repair with Krackow suture technique
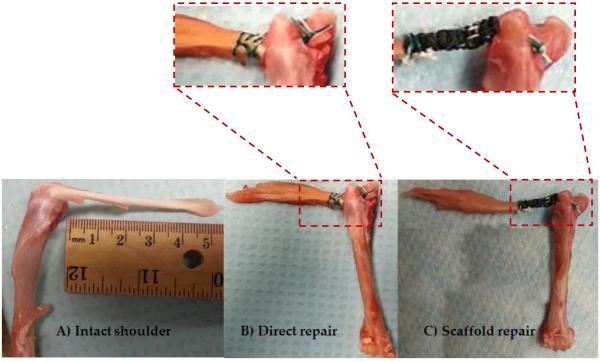
Figure 3.
A) Intact shoulder, B) direct repair suture technique, and, C) woven scaffold repair technique.
2.6 Biomechanical testing
The humeri were potted in (poly) methylmethacrylate cement (Millennium Pour Denture Acrylic, Cherry Hill, NJ) up to 20 mm distant from the humeral head inside a hollow rectangular aluminum pipe which was fixed to the loading frame (Fig.4). In case of intact tendon group and direct repair group, the tendon muscle complex was gripped at the muscle by fixtures at 15 mm distance from the tendon-bone insertion. The muscle was frozen locally at the grip site by a piece of dry ice. The tensile fixture was also cooled by dry ice, all facilitating a solid grip at the fixture. In the case of the scaffold repair group the grip was also 15 mm from the tendon bone insertion and 5 mm above the region of tendon-scaffold overlap. The intact and repaired infraspinatus tendons were loaded in a physiologically relevant direction of the infraspinatus tendon perpendicular to the longitudinal axis of the humerus (Fig 4). The samples were loaded monotonically at a rate of 10 mm/min until failure (Test Resource 800LE3-2, Test Resources Inc., MN, USA). In the scaffold only group, scaffolds were gripped by a tensile fixture at the two ends and loaded at the same displacement rate. A 220 N load cell was used to measure the load. All the samples were kept hydrated at all stages of suturing and testing. Displacement values were normalized by gauge length to obtain strain. Stiffness was calculated at the steepest region of the load-displacement curves. Failure energy was calculated as the area under the load-displacement curves.
Figure 4.
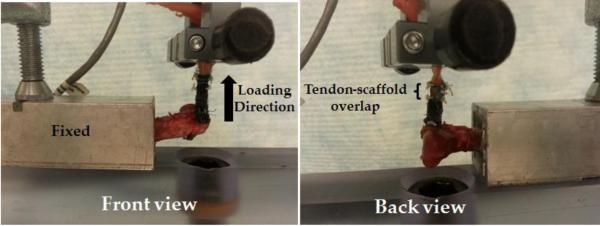
Mechanical testing of the scaffold repair group.
2.7 Statistical Analysis
A one-way analysis of variance (ANOVA) test was performed to evaluate for significant differences between groups (significance level set at P < 0.05). A post-hoc analysis using the Tukey’s test was conducted to compare pairwise differences between groups.
3. Results
3.1 Failure Locations
Direct repair group mostly failed at the repair interface (Table 2). Scaffold repair group failed within the scaffold continuum, indicating that suture technique developed in this study results in a full-load-bearing scaffold. One repair failed at the suture knot on the bone side.
Table 2.
Failure locations at different repaired groups
| Group | Tendon | Suture | Scaffold |
|---|---|---|---|
| Direct Repair | 1 | 4 - Humeral head and tendon suture junction |
N/A |
| Scaffold Repair | 0 | 1- Suture knot on the humeral head side |
4 |
3.2 Mechanical test results
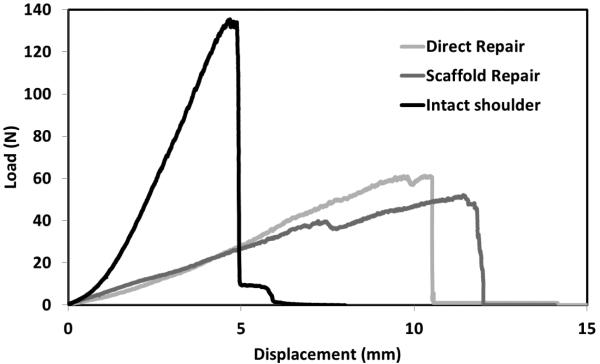
The typical load displacement curves for the intact shoulders and the repaired groups showed that scaffold repair and direct repair groups were comparable during the initial stages of deformation (Fig. 5). The load-displacement curves of the two repair groups were comparable. Both of the repaired groups showed larger displacements and lower loads than the intact group.
Figure 5.
Typical load-displacement curves of intact shoulder and repaired groups.
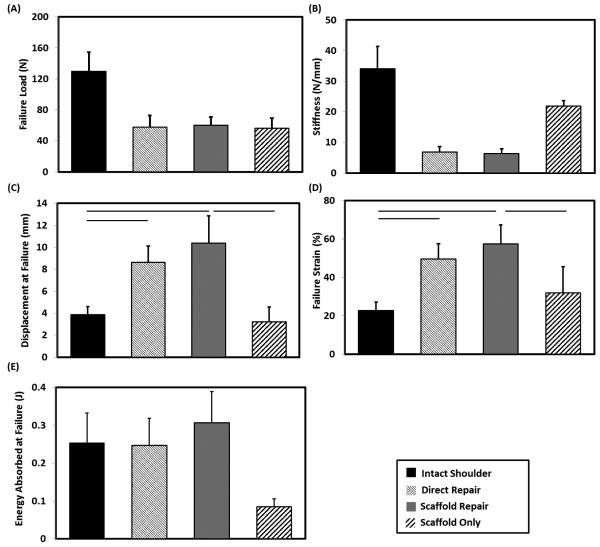
Failure load of direct repair and scaffold repair groups reached almost half of the intact shoulder (Fig. 6A). Failure load of the scaffold repair was not significantly different from that of the scaffold only, indicating that the scaffold suture technique was able to fully transfer loads to the scaffold. The failure strain and displacement at failure of the direct repair group was twice that of the intact shoulder (Fig. 6D & C). The failure strain and displacement of failure (Fig. 6D & C) of scaffold repair matched those of the direct repair (p = 0.21, p=0.20 respectively). Scaffold repair also matched the stiffness (Fig. 6B) of the direct repair (p = 0.47). Individual scaffolds demonstrated similar failure strain and displacement at failure as the intact shoulder (p = 0.16, p=0.35 respectively). The stiffness of the individual scaffold was similar to that of the intact tendon and exceeded those of the repaired shoulders. The failure energy of both scaffold and direct repair group matched that of the intact shoulder whereas scaffold only group showed 3-fold less failure energy than the intact shoulder (Fig. 6E).
Figure 6.
Mechanical evaluation of the intact shoulder, repaired groups and scaffold only. A) Failure load of scaffold repair approached that of the direct repair and scaffold only. (B) Stiffness (C) Displacement at failure and (D) Failure strain of scaffold repair did not significantly differ from those of the direct repair and (E) Failure energy of scaffold repair approached that of intact infraspinatus and direct repair group and significantly higher than scaffold only group. The horizontal line indicates significant difference (p < 0.05).
4. Discussion
To the best of our knowledge, there is a lack of methods for suturing load bearing scaffolds for repair of segmental tendon defects which require attachments on muscle and bone ends separately. Usual suture techniques such as Krackow and mattress may work in direct repair; however, they may be limited in securing porous scaffolds. Premature suture pull out was encountered when we attempted Krackow and mattress suture patterns to affix woven scaffolds during early stages of the project. Therefore, we developed the suture technique presented in Figure 2 for engrafting the load bearing collagen scaffold between muscle and bone. As we demonstrate mechanically, this novel suture technique was able to match the strength of direct repair. Simultaneous use of the locking Kessler suture on the bone side and simple loop at the top and bottom of the scaffold-tendon overlap increased suture pullout load at the bone side and tendon-suture pull out strength on the tendon-scaffold overlap side. This technique would not only be suitable for this woven collagen scaffold but also for other load bearing scaffolds.
Rabbit is a commonly employed animal model to investigate the rotator cuff injury and repair17. The rabbit rotator cuff model, especially the infraspinatus and supraspinatus, has been extensively used for surgical repair technique evaluation38, augmentation repair with autograft9, 42 or scaffolds11, 20, 21, 27, 53, 57, to determine native properties at the tendon insertion56, to check alterations of the mechanical properties19,18, 35or pathology23, 35, 37, 41, 52 of the rotator cuff after chronic detachment, and evaluation of tendon to bone healing26, 33, 34, 46, 47. As evidenced by such extensive literature, rabbit is a useful model for testing emerging technologies for rotator cuff repair before attempting costly large animal models. Different from the prior literature where a load-bearing suture bridged the gap, this study employed a fully load bearing scaffold. We are reporting for the first time a biomechanically sound suturing scheme to intercalate a load bearing scaffold between the muscle and the bone without using sutures for intermediary load bearing.
A previous in vivo study inlaid ELAC threads in patellar tendon and demonstrated their biodegradability and biocompatibility32. However, it is unknown whether the woven scaffold can withstand surgical handling and be employed as a fully-load-bearing tendon replacement material. Most pure-collagen scaffolds lack strength to the extent that they cannot be sutured. Long term cell-culture periods are needed for them to gain sufficient strength for implantation. At the baseline, the lack of mechanical robustness requires load-bearing sutures which bridge the gap. The purpose of this study was to evaluate the biomechanical performance of a mechanically robust, biodegradable32 and bioinductive30 pure collagen 3-D woven scaffold (ELAC) used for repairing segmental defects in the rotator cuff. In the defect model, scaffold acted as a complete load-bearing unit. The performance was evaluated against a conventional tendon repair (direct repair) method. The results indicate that the strength and stiffness of the scaffold repair model were consistent with those of the direct repair model. Strain and displacement at failure of both repair models were less than the intact shoulder and the individual scaffold. Such a reduction in stiffness of the repaired tendon was also reported by a previous study27. The increased compliance of repair models was likely due to the compliance added by suture junctures. The stiffness of the scaffold repair model can be increased by modifying the suture technique.
Although the volume of the scaffold is comparable with the intact infraspinatus, the scaffold contains 81% porosity. The porosity will not only help the population of cell through the continuum of the scaffold but also will help to vascularize which is an advantage over the direct repair.
The majority of the commercially available extracellular matrix based rotator cuff repair patches are too compliant such that they undergo a linear strain of more than 50%2, 15. This is two-fold greater than the strain of the woven scaffold reported in this study. Therefore, the commercially available rotator cuff repair patches may deform excessively under load. In this context, the failure strain and displacement at failure in the ELAC spanning repair models is an improvement over existing alternatives.
Inui et al. showed that a segmental repair of critical sized rabbit infraspinatus had one-third the strength of direct repair group and was 13 times weaker than the intact shoulder at time zero 27. Dejardin et al. showed in the canine model that infraspinatus repaired by porcine small intestinal submucosal scaffold was half the strength of the direct repair group and it was 6 times weaker than the intact group at time zero 14. In the present study, the biomechanics of the scaffold repair matched that of direct repair and was half the strength of the intact infraspinatus at the baseline. The described suture technique was able to translate the strength of the scaffold to the repair in this model. As a result, this may give the scaffold repair an advantage in the early stages of tendon healing.
The direct repair group was the weakest at the tendon-suture junction as most of the repairs failed at that location (Table 2). In contrast, most of the scaffold repair constructs failed in the continuum of the scaffolds. The suture technique developed and used in this study was robust at the location of scaffold-bone and scaffold-tendon junctions allowing for the translation of scaffold strength to the scaffold repair context.
Suture technique features which favor better mechanical strength were chosen to design the proposed scaffold repair model. In knotting the sutures at the bone tunnel ends, all the knots were knotted as double knots in place of two single knots at the end of two bone tunnel end because the double knot gives better strength 4. The locking Kessler suture on the bone side was adopted from Yotsumoto et al. as it does not result in suture pull-out 16, 54. The simple loop at the top and bottom of the scaffold-tendon overlap was adopted from Piskin et al. to increase the tendon-suture pull out strength 39.
A fully load-bearing scaffold may present biological advantages. Mechanical stimulation has an auspicious effect on cells. MSCs exhibited increase in tendon related markers such as type I collagen, scleraxis and also other mechanoresponsive molecules due to mechanostimulation 10, 28. Mechanically stimulated stem cell–collagen sponge constructs for tendon tissue engineering showed 2.5 times higher stiffness than the unstimulated constructs 8, 29, 45. Altman et al. showed that mechanically stimulated MSC seeded collagen gel construct had higher cross sectional density and 2.5 fold increase in cell alignment 1, 36. Therefore, the load-bearing feature of the scaffold repair may help improve the recovery of mechanical strength faster by stimulating resident cells. Future in vivo execution of the proposed surgical model will assess the validity of this assumption.
There have been previous biomechanical studies of synthetic and biomaterial based scaffolds2, 14, 15. However, very few studies have evaluated the performance of the mechanical strength of a scaffold in the context of repairing a spanning defect.14, 27, 43. Tendon repair with scaffolds involves suturing, which constitutionally creates defects in the scaffold and makes the scaffold weaker and vulnerable to failure after repair. The 3-D woven nature of the ELAC scaffold inherently contains pores facilitating the location for sutures and the repair model showed similar mechanical strength as the individual scaffold and the direct repair.
5. Conclusions
Our study was able to establish a repair technique for segmental defects in the rotator cuff using a load-bearing woven collagen bioscaffold. The repair was able to withstand similar load as the direct repair, validating the biomechanics of ELAC as a fully load-bearing scaffold repair construct. A mechanically robust scaffold may decrease early postoperative clinical failures and the load-bearing feature of the scaffold repair may help to improve the recovery of mechanical strength and creating the potential for the resident cells to receive mechanical stimulation. Future work will focus on developing an in vivo model to test the biomechanics and biocompatibility of this construct in tendon repair and to check the feasibility of the scaffold and the suture model for clinical use.
Highlights.
We developed a suture technique to attach load-bearing tendon scaffolds to the remnant muscle and the bone.
A fully load-bearing pure-collagen woven scaffold was implanted to replace the proper of rabbit infraspinatus tendon.
The suture technique was able to fully transfer the robustness of the scaffold to the scaffold repair scheme.
The biomechanical evaluation indicated that with the same suture technique, scaffold repair showed similar failure load as direct repair and significantly higher elastic modulus and toughness than direct repair.
Acknowledgments
This study was funded in part by grants from the National Institute of Health (Grant Number R01 AR063701) and National Science Foundation (Grant Number DMR-1306665).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Altman GH, Horan RL, Martin I, et al. Cell differentiation by mechanical stress. FASEB J. 2002;16(2):270–272. doi: 10.1096/fj.01-0656fje. [DOI] [PubMed] [Google Scholar]
- 2.Aurora A, McCarron J, Iannotti JP, Derwin K. Commercially available extracellular matrix materials for rotator cuff repairs: state of the art and future trends. J Shoulder Elbow Surg. 2007;16(5 Suppl):S171–178. doi: 10.1016/j.jse.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Baker AR, McCarron JA, Tan CD, Iannotti JP, Derwin KA. Does augmentation with a reinforced fascia patch improve rotator cuff repair outcomes? Clin Orthop Relat Res. 2012;470(9):2513–2521. doi: 10.1007/s11999-012-2348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baleani M, Schrader S, Veronesi CA, Rotini R, Giardino R, Toni A. Surgical repair of the rotator cuff: a biomechanical evaluation of different tendon grasping and bone suture fixation techniques. Clin Biomech (Bristol, Avon) 2003;18(8):721–729. doi: 10.1016/s0268-0033(03)00122-0. [DOI] [PubMed] [Google Scholar]
- 5.Boehm TD, Werner A, Radtke S, Mueller T, Kirschner S, Gohlke F. The effect of suture materials and techniques on the outcome of repair of the rotator cuff: a prospective, randomised study. J Bone Joint Surg Br. 2005;87(6):819–823. doi: 10.1302/0301-620X.87B6.15638. [DOI] [PubMed] [Google Scholar]
- 6.Burkhart SS, Wirth MA, Simonich M, Salem D, Lanctot D, Athanasiou K. Knot security in simple sliding knots and its relationship to rotator cuff repair: how secure must the knot be? Arthroscopy. 2000;16(2):202–207. doi: 10.1016/s0749-8063(00)90037-9. [DOI] [PubMed] [Google Scholar]
- 7.Buschmann J, Muller A, Feldman K, et al. Small hook thread (Quill) and soft felt internal splint to increase the primary repair strength of lacerated rabbit Achilles tendons: biomechanical analysis and considerations for hand surgery. Clin Biomech (Bristol, Avon) 2011;26(6):626–631. doi: 10.1016/j.clinbiomech.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Butler DL, Juncosa-Melvin N, Boivin GP, et al. Functional tissue engineering for tendon repair: A multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J Orthop Res. 2008;26(1):1–9. doi: 10.1002/jor.20456. [DOI] [PubMed] [Google Scholar]
- 9.Chang CH, Chen CH, Su CY, Liu HT, Yu CM. Rotator cuff repair with periosteum for enhancing tendon-bone healing: a biomechanical and histological study in rabbits. Knee Surg Sports Traumatol Arthrosc. 2009;17(12):1447–1453. doi: 10.1007/s00167-009-0809-x. [DOI] [PubMed] [Google Scholar]
- 10.Chen JL, Yin Z, Shen WL, et al. Efficacy of hESC-MSCs in knitted silk-collagen scaffold for tendon tissue engineering and their roles. Biomaterials. 2010;31(36):9438–9451. doi: 10.1016/j.biomaterials.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Chen JM, Willers C, Xu J, Wang A, Zheng MH. Autologous tenocyte therapy using porcine-derived bioscaffolds for massive rotator cuff defect in rabbits. Tissue Eng. 2007;13(7):1479–1491. doi: 10.1089/ten.2006.0266. [DOI] [PubMed] [Google Scholar]
- 12.Cheng X, Gurkan UA, Dehen CJ, et al. An electrochemical fabrication process for the assembly of anisotropically oriented collagen bundles. Biomaterials. 2008;29(22):3278–3288. doi: 10.1016/j.biomaterials.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 13.Cheung EV, Silverio L, Sperling JW. Strategies in biologic augmentation of rotator cuff repair: a review. Clin Orthop Relat Res. 2010;468(6):1476–1484. doi: 10.1007/s11999-010-1323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dejardin LM, Arnoczky SP, Ewers BJ, Haut RC, Clarke RB. Tissue-engineered rotator cuff tendon using porcine small intestine submucosa. Histologic and mechanical evaluation in dogs. Am J Sports Med. 2001;29(2):175–184. doi: 10.1177/03635465010290021001. [DOI] [PubMed] [Google Scholar]
- 15.Derwin KA, Baker AR, Spragg RK, Leigh DR, Iannotti JP. Commercial extracellular matrix scaffolds for rotator cuff tendon repair. Biomechanical, biochemical, and cellular properties. J Bone Joint Surg Am. 2006;88(12):2665–2672. doi: 10.2106/JBJS.E.01307. [DOI] [PubMed] [Google Scholar]
- 16.Dogramaci Y, Kalaci A, Sevinc TT, Suner G, Emir A, Yanat AN. Single side locking on the opposite of the modified Kessler tendon repair prevents gap formation and suture pull-out: a biomechanical evaluation in sheep tendons. Eklem Hastalik Cerrahisi. 2009;20(2):102–106. [PubMed] [Google Scholar]
- 17.Edelstein L, Thomas SJ, Soslowsky LJ. Rotator cuff tears: what have we learned from animal models? J Musculoskelet Neuronal Interact. 2011;11(2):150–162. [PubMed] [Google Scholar]
- 18.Fabis J, Kordek P, Bogucki A, Mazanowska-Gajdowicz J. Function of the rabbit supraspinatus muscle after large detachment of its tendon: 6-week, 3-month, and 6-month observation. J Shoulder Elbow Surg. 2000;9(3):211–216. [PubMed] [Google Scholar]
- 19.Fabis J, Kordek P, Bogucki A, Synder M, Kolczynska H. Function of the rabbit supraspinatus muscle after detachment of its tendon from the greater tubercle. Observations up to 6 months. Acta Orthop Scand. 1998;69(6):570–574. doi: 10.3109/17453679808999257. [DOI] [PubMed] [Google Scholar]
- 20.Funakoshi T, Majima T, Iwasaki N, et al. Application of tissue engineering techniques for rotator cuff regeneration using a chitosan-based hyaluronan hybrid fiber scaffold. Am J Sports Med. 2005;33(8):1193–1201. doi: 10.1177/0363546504272689. [DOI] [PubMed] [Google Scholar]
- 21.Funakoshi T, Majima T, Suenaga N, Iwasaki N, Yamane S, Minami A. Rotator cuff regeneration using chitin fabric as an acellular matrix. J Shoulder Elbow Surg. 2006;15(1):112–118. doi: 10.1016/j.jse.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 22.George MS, Khazzam M. Current concepts review: revision rotator cuff repair. J Shoulder Elbow Surg. 2012;21(4):431–440. doi: 10.1016/j.jse.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 23.Grumet RC, Hadley S, Diltz MV, Lee TQ, Gupta R. Development of a new model for rotator cuff pathology: the rabbit subscapularis muscle. Acta Orthop. 2009;80(1):97–103. doi: 10.1080/17453670902807425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurkan UA, Cheng X, Kishore V, Uquillas JA, Akkus O. Comparison of morphology, orientation, and migration of tendon derived fibroblasts and bone marrow stromal cells on electrochemically aligned collagen constructs. J Biomed Mater Res A. 2010;94(4):1070–1079. doi: 10.1002/jbm.a.32783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Habermeyer P, Schmieding R. Method and apparatus for arthroscopic rotator cuff repair: Google Patents. 1996 [Google Scholar]
- 26.Hirose K, Kondo S, Choi HR, Mishima S, Iwata H, Ishiguro N. Spontaneous healing process of a supraspinatus tendon tear in rabbits. Arch Orthop Trauma Surg. 2004;124(6):374–377. doi: 10.1007/s00402-004-0663-8. [DOI] [PubMed] [Google Scholar]
- 27.Inui A, Kokubu T, Mifune Y, et al. Regeneration of rotator cuff tear using electrospun poly(d,l-Lactide-Co-Glycolide) scaffolds in a rabbit model. Arthroscopy. 2012;28(12):1790–1799. doi: 10.1016/j.arthro.2012.05.887. [DOI] [PubMed] [Google Scholar]
- 28.Juncosa-Melvin N, Matlin KS, Holdcraft RW, Nirmalanandhan VS, Butler DL. Mechanical stimulation increases collagen type I and collagen type III gene expression of stem cell-collagen sponge constructs for patellar tendon repair. Tissue Eng. 2007;13(6):1219–1226. doi: 10.1089/ten.2006.0339. [DOI] [PubMed] [Google Scholar]
- 29.Juncosa-Melvin N, Shearn JT, Boivin GP, et al. Effects of mechanical stimulation on the biomechanics and histology of stem cell-collagen sponge constructs for rabbit patellar tendon repair. Tissue Eng. 2006;12(8):2291–2300. doi: 10.1089/ten.2006.12.2291. [DOI] [PubMed] [Google Scholar]
- 30.Kishore V, Bullock W, Sun X, Van Dyke WS, Akkus O. Tenogenic differentiation of human MSCs induced by the topography of electrochemically aligned collagen threads. Biomaterials. 2012;33(7):2137–2144. doi: 10.1016/j.biomaterials.2011.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kishore V, Paderi JE, Akkus A, et al. Incorporation of a decorin biomimetic enhances the mechanical properties of electrochemically aligned collagen threads. Acta Biomaterialia. 2011;7(6):2428–2436. doi: 10.1016/j.actbio.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kishore V, Uquillas JA, Dubikovsky A, et al. In vivo response to electrochemically aligned collagen bioscaffolds. J Biomed Mater Res B Appl Biomater. 2011 doi: 10.1002/jbm.b.31962. [DOI] [PubMed] [Google Scholar]
- 33.Koike Y, Trudel G, Curran D, Uhthoff HK. Delay of supraspinatus repair by up to 12 weeks does not impair enthesis formation: a quantitative histologic study in rabbits. J Orthop Res. 2006;24(2):202–210. doi: 10.1002/jor.20031. [DOI] [PubMed] [Google Scholar]
- 34.Koike Y, Trudel G, Uhthoff HK. Formation of a new enthesis after attachment of the supraspinatus tendon: A quantitative histologic study in rabbits. J Orthop Res. 2005;23(6):1433–1440. doi: 10.1016/j.orthres.2005.02.015.1100230628. [DOI] [PubMed] [Google Scholar]
- 35.Koshima H, Kondo S, Mishima S, et al. Expression of interleukin-1beta, cyclooxygenase-2, and prostaglandin E2 in a rotator cuff tear in rabbits. J Orthop Res. 2007;25(1):92–97. doi: 10.1002/jor.20241. [DOI] [PubMed] [Google Scholar]
- 36.Kuo CK, Marturano JE, Tuan RS. Novel strategies in tendon and ligament tissue engineering: Advanced biomaterials and regeneration motifs. Sports Med Arthrosc Rehabil Ther Technol. 2010;2:20. doi: 10.1186/1758-2555-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minaki Y, Yamashita T, Takebayashi T, Ishii S. Mechanosensitive afferent units in the shoulder and adjacent tissues. Clin Orthop Relat Res. 1999;369:349–356. doi: 10.1097/00003086-199912000-00037. [DOI] [PubMed] [Google Scholar]
- 38.Ozbaydar M, Elhassan B, Esenyel C, et al. A comparison of single-versus double-row suture anchor techniques in a simulated repair of the rotator cuff: an experimental study in rabbits. J Bone Joint Surg Br. 2008;90(10):1386–1391. doi: 10.1302/0301-620X.90B10.20862. [DOI] [PubMed] [Google Scholar]
- 39.Piskin A, Yuceturk A, Tomak Y, et al. [Tendon repair with the strengthened modified Kessler, modified Kessler, and Savage suture techniques: a biomechanical comparison] Acta Orthop Traumatol Turc. 2007;41(3):238–243. [PubMed] [Google Scholar]
- 40.Ricchetti ET, Aurora A, Iannotti JP, Derwin KA. Scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2012;21(2):251–265. doi: 10.1016/j.jse.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Rowshan K, Hadley S, Pham K, Caiozzo V, Lee TQ, Gupta R. Development of Fatty Atrophy After Neurologic and Rotator Cuff Injuries in an Animal Model of Rotator Cuff Pathology. J Bone Joint Surg Am. 2010;92(13):2270–2278. doi: 10.2106/JBJS.I.00812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sano H, Kumagai J, Sawai T. Experimental fascial autografting for the supraspinatus tendon defect: remodeling process of the grafted fascia and the insertion into bone. J Shoulder Elbow Surg. 2002;11(2):166–173. doi: 10.1067/mse.2002.120808. [DOI] [PubMed] [Google Scholar]
- 43.Sawadkar P, Alexander S, Tolk M, et al. Development of a surgically optimized graft insertion suture technique to accommodate a tissue-engineered tendon in vivo. Biores Open Access. 2013;2(5):327–335. doi: 10.1089/biores.2013.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneeberger AG, von Roll A, Kalberer F, Jacob HA, Gerber C. Mechanical strength of arthroscopic rotator cuff repair techniques: an in vitro study. J Bone Joint Surg Am. 2002;84(A(12)):2152–2160. doi: 10.2106/00004623-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Shearn JT, Juncosa-Melvin N, Boivin GP, et al. Mechanical stimulation of tendon tissue engineered constructs: effects on construct stiffness, repair biomechanics, and their correlation. J Biomech Eng. 2007;129(6):848–854. doi: 10.1115/1.2800769. [DOI] [PubMed] [Google Scholar]
- 46.Trudel G, Ramachandran N, Ryan SE, Rakhra K, Uhthoff HK. Supraspinatus tendon repair into a bony trough in the rabbit: mechanical restoration and correlative imaging. J Orthop Res. 2010;28(6):710–715. doi: 10.1002/jor.21045. [DOI] [PubMed] [Google Scholar]
- 47.Uhthoff HK, Sano H, Trudel G, Ishii H. Early reactions after reimplantation of the tendon of supraspinatus into bone. A study in rabbits. J Bone Joint Surg Br. 2000;82(7):1072–1076. doi: 10.1302/0301-620x.82b7.9986. [DOI] [PubMed] [Google Scholar]
- 48.Uquillas JA, Akkus O. Modeling the electromobility of type-I collagen molecules in the electrochemical fabrication of dense and aligned tissue constructs. Ann Biomed Eng. 2012;40(8):1641–1653. doi: 10.1007/s10439-012-0528-1. [DOI] [PubMed] [Google Scholar]
- 49.Uquillas JA, Kishore V, Akkus O. Effects of phosphate-buffered saline concentration and incubation time on the mechanical and structural properties of electrochemically aligned collagen threads. Biomed Mater. 2011;6(3):035008. doi: 10.1088/1748-6041/6/3/035008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uquillas JA, Kishore V, Akkus O. Genipin crosslinking elevates the strength of electrochemically aligned collagen to the level of tendons. J Mech Behav Biomed Mater. 2012;15:176–189. doi: 10.1016/j.jmbbm.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 51.Wall LB, Keener JD, Brophy RH. Double-row vs single-row rotator cuff repair: a review of the biomechanical evidence. J Shoulder Elbow Surg. 2009;18(6):933–941. doi: 10.1016/j.jse.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Yamashita T, Minaki Y, Takebayashi T, Sakamoto N, Ishii S. Neural response of mechanoreceptors to acute inflammation in the rotator cuff of the shoulder joint in rabbits. Acta Orthop Scand. 1999;70(2):137–140. doi: 10.3109/17453679909011251. [DOI] [PubMed] [Google Scholar]
- 53.Yokoya S, Mochizuki Y, Nagata Y, Deie M, Ochi M. Tendon-bone insertion repair and regeneration using polyglycolic acid sheet in the rabbit rotator cuff injury model. Am J Sports Med. 2008;36(7):1298–1309. doi: 10.1177/0363546508314416. [DOI] [PubMed] [Google Scholar]
- 54.Yotsumoto T, Mori R, Uchio Y. Optimum locations of the locking loop and knot in tendon sutures based on the locking Kessler method. J Orthop Sci. 2005;10(5):515–520. doi: 10.1007/s00776-005-0929-1. [DOI] [PubMed] [Google Scholar]
- 55.Younesi M, Islam A, Kishore V, Anderson JM, Akkus O. Tenogenic Induction of Human MSCs by Anisotropically Aligned Collagen Biotextiles. Advanced Functional Materials. 2014 doi: 10.1002/adfm.201400828. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yukata K, Matsui Y, Shukunami C, et al. Differential expression of Tenomodulin and Chondromodulin-1 at the insertion site of the tendon reflects a phenotypic transition of the resident cells. Tissue Cell. 2010;42(2):116–120. doi: 10.1016/j.tice.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 57.Zheng MH, Chen J, Kirilak Y, Willers C, Xu J, Wood D. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J Biomed Mater Res B Appl Biomater. 2005;73(1):61–67. doi: 10.1002/jbm.b.30170. [DOI] [PubMed] [Google Scholar]