Abstract
Luteinizing hormone receptor (LHR) mRNA expression in the ovary is regulated post-transcriptionally by an LH receptor mRNA binding protein (LRBP). Eukaryotic initiation factor 5A (EIF5A), identified as an LRBP-interacting protein plays a crucial role in LHR mRNA expression. In this study, we have demonstrated that during hCG-induced LHR downregulation, a significant upregulation of eIF5A mRNA expression and hypusination of eIF5A protein occurs in a time dependent manner. Pretreatment with H89, a specific inhibitor of PKA, and U0126, a specific inhibitor of ERK1/2 significantly inhibited both hCG-induced eIF5A mRNA expression and hypusination of eIF5A protein. Pretreatment with GC7, a specific inhibitor of eIF5A hypusination significantly abolished hCG-induced LRBP mRNA and protein expression. Furthermore, GC7 pretreatment significantly inhibited hCG-induced interaction of LRBP with LHR mRNA as assessed by RNA electrophoretic mobility gel shift assay (REMSA). GC7 treatment also reversed LHR mRNA downregulation. Taken together, these results suggest that hCG-induced LHR mRNA downregulation is mediated by cAMP-PKA-ERK1/2 signaling leading to activation of eIF5A hypusination.
Keywords: LH receptor, eIF5A, hypusination, cAMP/PKA/ERK1/2, LRBP
1. Introduction
Luteinizing hormone-human choriogonadotropin (LH/hCG) receptor (LHR), a member of the glycoprotein hormone receptor sub family of the large G-protein coupled receptor family is crucial for mammalian reproduction. LHR is expressed primarily in the testis and ovary [1,2]. During normal ovarian cycle, LHR expression undergoes remarkable changes, as revealed by the acquisition of LHR by the growing follicles in response to the combined actions of FSH and estradiol [3] followed by a transient downregulation of LHR in response to the preovulatory LH surge. The latter phenomenon can be mimicked by administration of pharmacological dose of hCG to superovulated rats [4,5]. Studies from our laboratory have elucidated that the transient ligand-induced down-regulation of LHR mRNA occurs through post-transcriptional mechanism involving a specific mRNA binding protein designated as LHR mRNA binding protein (LRBP) leading to accelerated LHR mRNA degradation [6,7]. Since LRBP is devoid of mRNAse activity, we performed yeast two hybrid screening assay and identified eukaryotic initiation factor 5A (eIF5A) as one of the proteins that interact with LRBP [8]. EIF5A is a highly conserved, 17 kDa protein that is expressed ubiquitously in all cells. It undergoes hypusination, a unique post-translational modification by the addition of hypusine residue, an unusual amino acid from spermidine, to the ε-amino group of lysine and this modification of eIF5A is essential for its function [9]. Other known actions of eIF5A include its role as an elongation factor in protein translation [10–12] as well as shuttling proteins regulating mRNA transport [13], and regulation of cell proliferation [14], inflammation and apoptosis [15,16]. Recent studies from our laboratory have shown that hypusinated eIF5A interacts with LHR mRNA-LRBP complex and this interaction is critical for ligand-induced downregulation of LHR mRNA [17]. In the present study, we investigated the upstream signaling pathways of eIF5A hypusination and the role of hypusinated eIF5A in LH/hCG-induced downregulation of LHR mRNA in rat ovaries. Our results show that hCG-induced LHR downregulation is downstream of hCG-induced cAMP/PKA/ERK1/2 signaling pathway leading to eIF5A hypusination culminating in the downregulation of LHR mRNA.
2. Materials and Methods
2.1. Materials
Highly purified human chorionic gonadotropin (hCG; CR127) was purchased from Dr. A.F. Parlow (National Hormone and peptide program, Torrance, California). Pregnant mare serum gonadotropin was obtained from Calbiochem. EDTA-free protease inhibitor cocktail tablets and RNAse inhibitor were products of Roche Applied Science and Promega Corp., respectively. Real-time PCR Primers specific for LH receptor, LRBP, and 18S rRNA (TaqMan Assay-on-Demand Gene Expression Product) and Multiscribe reverse transcriptase were supplied by Applied Biosystems (Foster City, CA). Real-time PCR Primers specific for rat eIF5A and GAPDH were obtained from Qiagen. Anti-N-terminal mevalonate kinase IgG was raised against the first 15 N-terminal amino acids of mevalonate kinase (MLSEVLLVSAPGKVI) and this antibody is referred to as the LRBP antibody in the text. Recombinant eIF5A was purchased from Prospec Bio (Rehovot, Israel) and [3H]-spermidine from Perkin Elmer (Waltham, MA). MEK inhibitor (U0126) was purchased from Promega and PKA inhibitor (H89) was obtained from Calbiochem (La Jolla, CA). EIF5A hypusination inhibitor, N1-Guanyl- 1,7 diaminoheptane (GC7) was purchased from BIOSEARCH Technologies, Petaluma, CA. Monoclonal mouse Anti-eIF5A antibody and anti-β-tubulin antibody were obtained from Sigma Aldrich (St. Louis, MO). The Super Signal West Femto chemiluminescence kit and anti-rabbit/anti-mouse IgG conjugated to horseradish peroxidase were obtained from Pierce (Rockford, IL). Quick spin columns (G-50-Sephadex) for radiolabeled RNA purification were purchased from Roche Molecular Biochemicals. [α-32P]-UTP was obtained from PerkinElmer (Santa Clara, CA), and MAXIscript T7 Kit was the product of Ambion (Austin, TX). All other chemicals and reagents used were conventional commercial products.
2.2. Animals and treatments
Sprague-Dawley female rats (23 day old) were purchased from Charles River Laboratories (Wilmington, MA). All the experimental protocols used in this study were approved by the University Committee on the Use and Care of Animals. Animals were housed in a temperature-controlled room with proper dark-light cycles as per the guidelines provided by the University Committee on the Use and Care of Animals. Superovulation was induced in 23-day-old female rats by subcutaneous injection of 50 IU of pregnant mare serum gonadotropin followed by 25 IU of human chorionic gonadotropin 56 h later. Five days after inducing superovulation, the animals were injected (s.c.) with single dose of hCG (50 IU) to downregulate LHR mRNA expression. The animals were euthanized by CO2 asphyxiation and the ovaries were collected at 0, 1, 2, 4 and 6 hours after hCG injection and were immediately frozen in liquid nitrogen until further use. To block PKA and ERK1/2, the superovulated rats were treated (s.c.) with H-89 (100 mg/kg body weight) or UO126 (10 mg/kg body weight) 1 hour before the second hCG treatment. To block eIF5A hypusination, the superovulated rats were treated with GC7 (16 mg/kg body weight, i, p.) 2 hour before the second dose of hCG.
2.3. Preparation of tissue extracts and Western blot analysis
Ovaries were homogenized in Radioimmunoprecipitation assay (RIPA) buffer containing EDTA-free protease inhibitor cocktail followed by brief periods of sonication. The samples were centrifuged at 14,000 × g for 15 min at 4° C and total protein content of the supernatants was measured by Bicinchoninic acid assay (BCA) reagent method. Proteins (60 µg/lane) were separated by electrophoresis using 12% SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). The membranes were blocked in 5% fat-free skimmed milk in TBS-tween-20 buffer (TBST), pH-7.4 for 1h at room temperature and then incubated overnight at 4° C with primary antibody in 5% fat free milk/TBST. The membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies for 1h at room temperature. The membrane bound antibodies were detected with the west Femto-Supersignal substrate system Western blotting detection kit (Pierce). Protein loading was monitored by stripping and re-probing the same blots with appropriate antibodies (internal control) as indicated in the figure legends.
2.4. In vitro hypusination assay
In vitro hypusination assay was performed using our previously published procedure [17]. Briefly, ovarian S10 fractions (40 g protein) were added to assay mixture containing 50 mM glycine, pH 8.3, 20% glycerol, 2 mM DTT, 150 mM KCl, 10 mM MgCl2, 0.1 mg/ml BSA, 0.1 mM NAD+, 20 ng of recombinant eIF5A protein and 2.0 µCi of [3H]-spermidine in a final volume of 25 µl. The reaction mixture was incubated at 37°C for 2h and terminated by the addition of 5µL of SDS-PAGE sample buffer. The proteins were separated on a 12% SDS-PAGE gel, transferred to nitro-cellulose membrane and subjected to flurography using Kodak BioMax TranScreen LE and BioMax MS film at −70°C for 72 hours. The images were then scanned and quantitated.
2.5. RNA isolation and Real-time PCR
Total RNA was extracted from the ovaries using TRIzol reagent following the manufacturer’s instructions (Life Technologies, Grand Island, NY). Aliquots of total RNA (100 ng) extracted from each sample were reverse-transcribed in a reaction volume of 20 µl using 2.5µM random hexamer, 500µM deoxynucleotide triphosphates, 5.5 mM MgCl2, 8U ribonuclease inhibitor, and 25U multiscribe reverse transcriptase (Applied Biosystems). The resulting cDNA’s were diluted with nuclease free water. The real-time PCR quantitation was then performed using 5 µl of the diluted cDNAs in triplicate using predesigned primers and probes. Reactions were carried out in a final volume of 25 µl using Applied Biosystems 7300 Real-Time PCR system. The fold change in gene expression was calculated using the standard curve method with 18S rRNA or GAPDH as the internal control using the ΔΔCt method [18].
2.6. RNA electrophoretic mobility shift assay (REMSA)
Ovaries were collected from superovulated rats treated with GC7 or saline, followed by s.c. injection with 50 IU hCG and ovaries collected 8h later as described in the section “Animals and treatments”. Ovaries were homogenized in assay buffer (10 mM HEPES pH 7.9, 0.5 mM MgCl2, 50 mM EDTA, 5 mM DTT and 10% glycerol) containing 50 mM KCl and protease inhibitor cocktail at 4° C. The homogenates were centrifuged at 105,000 × g for 90 min at 4° C. The supernatants containing the cytoplasmic proteins (S100) were collected. REMSA was performed by incubating S100 cytosolic fractions with [α-32P]-UTP-labeled LBS, as described previously [6,19,20]. The [α-32P]-labeled RNA for the binding assay was prepared using the Maxiscript kit. The RNA-protein complexes were resolved by 5% native polyacrylamide (70:1) gel electrophoresis and analyzed by autoradiography, as described previously [19].
2.7. Statistical analysis
Statistical analysis was carried out using GraphPad Prism software. The data were analyzed using one-way ANOVA followed by the Tukey multiple comparison test. Values were considered statistically significant for p<0.05. Each experiment was repeated at least 3 times with similar results. Western blots and autoradiograms shown are representative of a minimum of 3 experiments.
3. Results
3.1. hCG induces eIF5A mRNA and protein expression
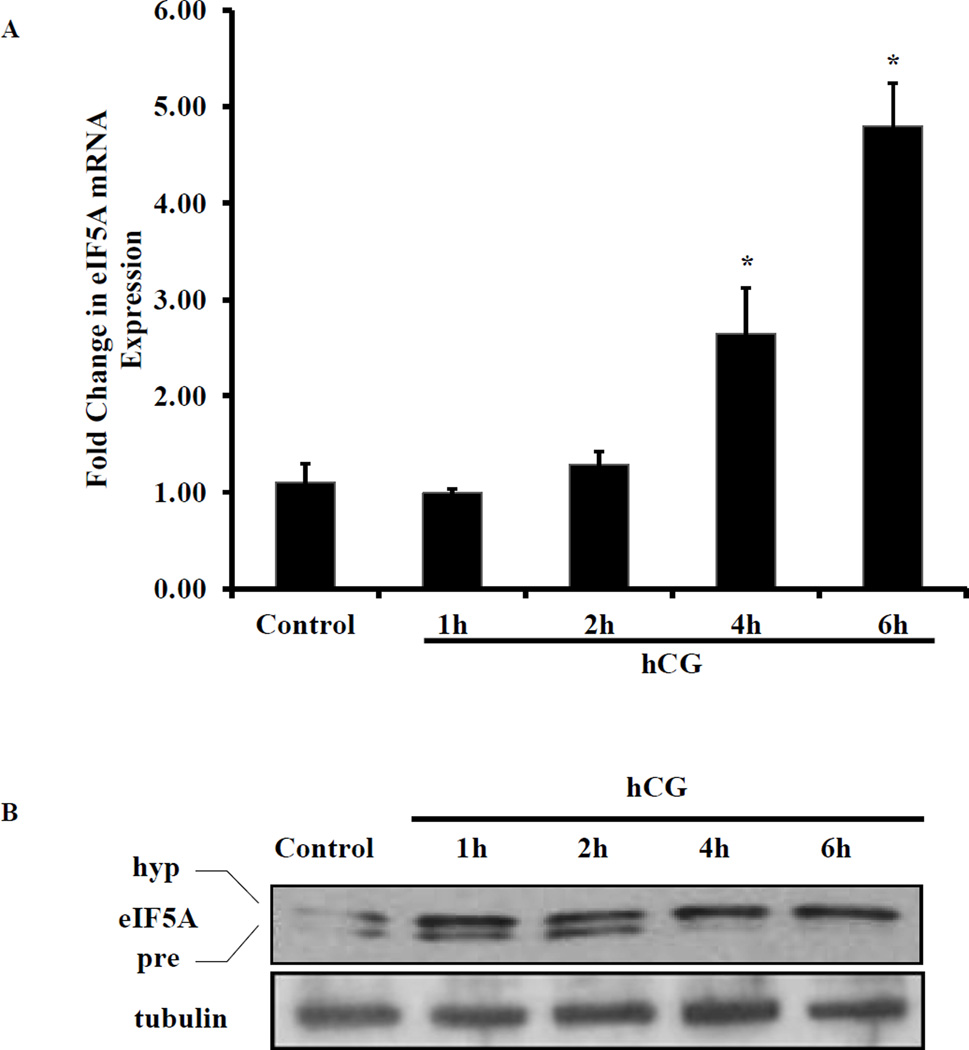
Since our previous study identified that eIF5A interacts with LHR mRNA-LRBP complex during hCG-induced LHR downregulation [17], we examined whether hCG has any effect on eIF5A expression. To examine this, RNA and proteins were extracted from ovaries of superovulated rats treated with hCG for 0, 1, 2, 4 and 6h representing the time periods prior to inducing complete downregulation. The extracts were analyzed for eIF5A mRNA and protein expression using real time PCR and Western blot analysis, respectively. The results showed that hCG treatment causes significant upregulation of eIF5A mRNA expression in a time dependent manner starting with a 1.3 fold increase at 2h, 2.6 fold at 4h and 5 fold at 6h compared to control (Fig. 1A). Furthermore, Western blot results showed that hCG treatment significantly induced eIF5A protein expression in a time dependent manner (Fig. 1B). The unhypusinated (precursor) form of eIF5A (17 kDa) migrates faster in the gel and appears as a faint band directly below the hypusinated form in the Western blot in hCG treated samples (Fig. 1B, upper panel, lanes 1, 2 and 3). At 4h and 6h time points, the unhypusinated precursor form was not visible, however, the intensity of the hypusinated eIF5A band showed significant increase upon hCG treatment (Fig. 1B, upper panel, lanes 4 and 5). These results suggest that hCG causes upregulation of eIF5A expression which subsequently undergoes hypusination.
Fig. 1. hCG treatment for LHR downregulation induces expression and hypusination of eIF5A.
(A) Superovulated rats were injected on day 5 with saline or hCG (50 IU) to induce LHR downregulation. The ovaries were collected at 0, 1, 2, 4 and 6 hours later. RNA was isolated and reverse transcribed and the resulting cDNA were subjected to real time PCR quantitation using specific primers and probes for eIF5A and GAPDH. The graphs represent changes in eIF5A levels normalized to GAPDH and shown as fold change vs control. Error bars represent mean ± SE; *p<0.05 vs control. (B) Ovary lysates were prepared in RIPA buffer by homogenization followed by brief sonication. The samples were centrifuged and supernatants were collected. Equal amounts of protein from each sample were separated on 12% SDS-PAGE. The proteins were transferred to nitrocellulose membrane and probed with eIF5A antibody. The membranes were stripped and reprobed for β-tubulin antibodies. The blots shown are representative of three independent experiments.
3.2. Pretreatment with PKA and ERK1/2 inhibitors abrogates hCG-induced eIF5A expression and hypusination
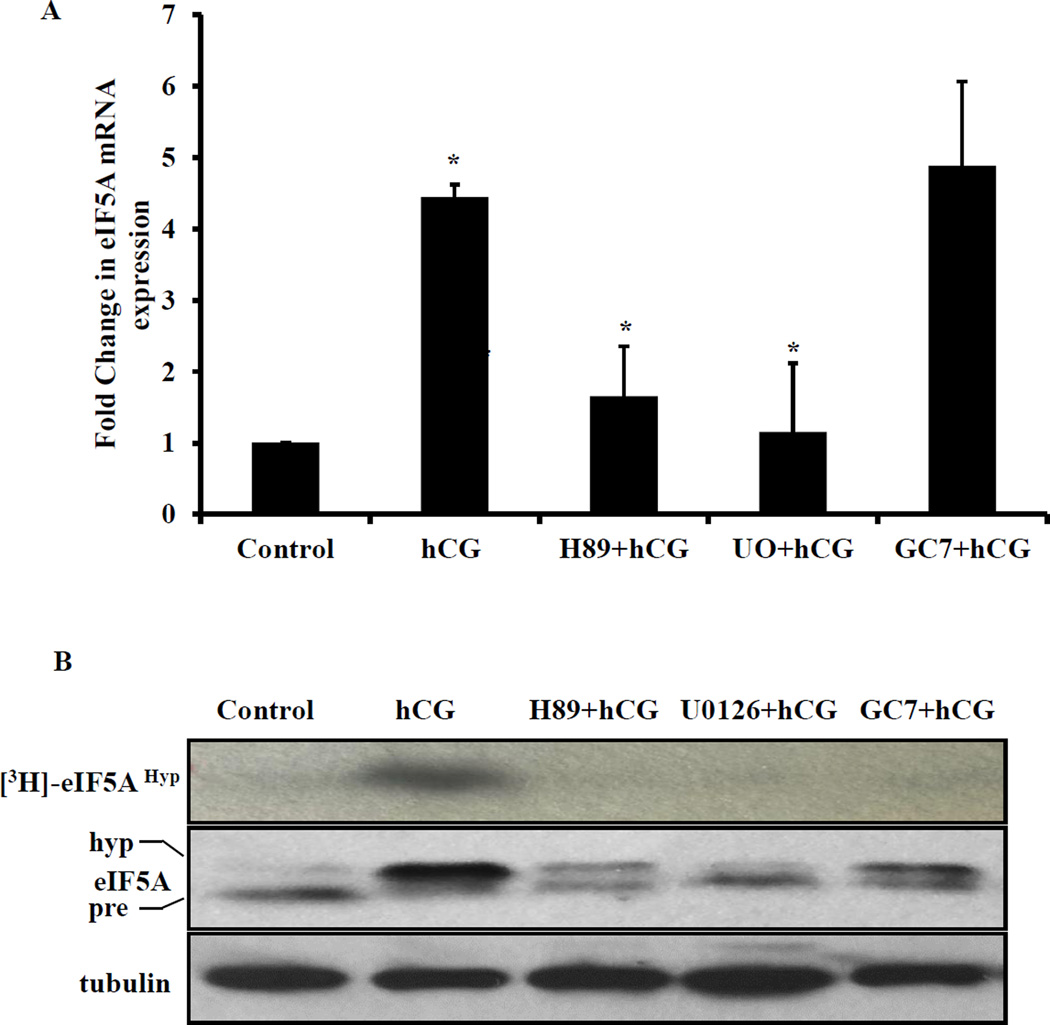
We then tested the signaling pathway that leads to increase in eIF5A mRNA expression and hypusination of eIF5A protein in response to hCG treatment. Specifically, we examined the role of cAMP/PKA/ERK1/2 signaling pathway, since our previous studies have shown that hCG-induced downregulation of LHR mRNA expression is mediated by this pathway [21]. To examine this, superovulated rats were treated with PKA inhibitor (H-89; 100 mg/kg body weight), ERK1/2 inhibitor (U0126; 10 mg/kg body weight) 1 hour before hCG treatment or eIF5A hypusination inhibitor (GC7; 16 mg/kg body weight) 2 hour before hCG treatment. Total RNA was extracted from the ovaries and eIF5A mRNA expression was analyzed by real time PCR. The results showed that, as expected, hCG significantly increased eIF5A mRNA expression. Conversely, pretreatment with PKA and ERK1/2 inhibitors significantly inhibited the hCG-induced upregulation of eIF5A mRNA (Fig. 2A). Western blot analysis of the cytosolic extracts indicated that pretreatment with PKA inhibitor or ERK1/2 inhibitor significantly reduced hCG-mediated eIF5A protein expression and its hypusination. As well as expected, treatment with the hypusination inhibitor, GC7 had no effect on eIF5A mRNA expression (Fig. 2B, middle panel). We also performed hypusination activity assay in vitro by incubating the ovarian cytosolic extracts with [3H]-spermidine and recombinant eIF5A protein. Western blotting followed by fluorography analysis results showed that while hCG treatment increased the incorporation of [3H]-spermidine into eIF5A, pretreatment with PKA inhibitor, ERK1/2 inhibitor and hypusination inhibitor separately produced significant inhibition of hypusination (Fig. 2B. top panel). A summary of the effect of various inhibitors on eIF5A mRNA, protein expression and hypusination is presented in Table -1. Collectively, these results suggest that eIF5A hypusination occurs through hCG-induced activation of cAMP/PKA/ERK1/2 pathway.
Fig. 2. Pretreatment with PKA inhibitor and ERK1/2 inhibitor inhibits hCG-induced eIF5A expression and hypusination.
(A) Superovulated rats were injected with vehicle or PKA inhibitor H-89 (100 mg/kg body weight, s.c) or ERK1/2 inhibitor U0126 (10 mg/kg body weight, s.c) 1 hour before the second hCG treatment. The other set of rats were treated with GC7 (16 mg/kg body weight, i, p.) 2 hour before the second dose of hCG treatment. The ovaries were collected 6 hours later. The RNA was isolated from the ovaries and reverse-transcribed and the resulting cDNA were subjected to real time PCR quantitation using specific primers and probes for eIF5A and GAPDH. The graphs represent changes in eIF5A levels normalized to GAPDH and shown as fold change vs control. Error bars represent mean ± SE; *p<0.05 vs control. (B) Ovaries were homogenized and S10 fractions were prepared and equal amounts of protein from each sample were subjected to in vitro hypusination assay as described in methods section. The fluorography image is shown in the top panel. Western blot analyses of the same samples for eIF5A and tubulin are shown in the bottom panels. The blots shown are representative of three independent experiments.
TABLE-1.
Effect of various inhibitors on hCG-induced eIF5A mRNA, protein expression and eIF5A hypusination.
| Inhibitors | eIF5A mRNA | eIF5A protein | eIF5A hypusination |
|---|---|---|---|
| H-89 | Decrease | Decrease | Decrease |
| U0126 | Decrease | Decrease | Decrease |
| GC7 | No change | No change | Decrease |
3.3. Inhibition of eIF5A hypusination by GC7 abrogates hCG-induced LRBP mRNA and protein expression
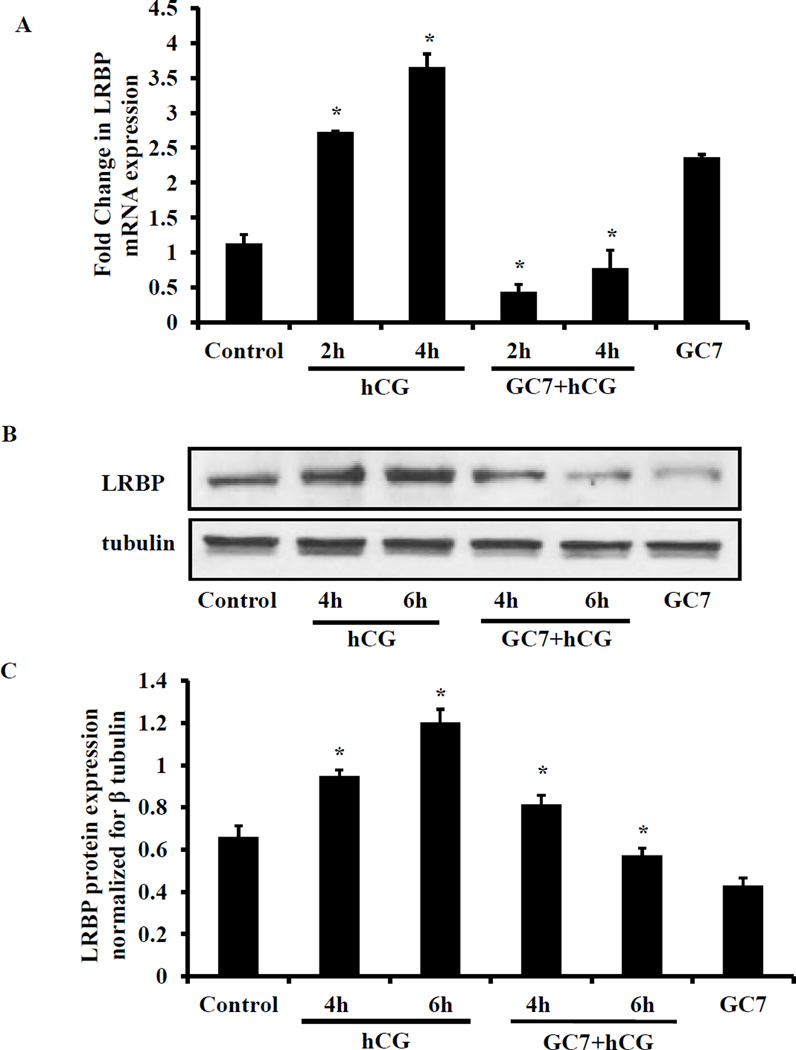
The role of eIF5A hypusination on the induction of LRBP was then examined by eIF5A hypusination inhibitor treatment. LRBP mRNA and protein expression were examined by real time PCR and Western blot analyses, respectively. The results showed that treatment with hCG produced a 2.5 fold increase in LRBP mRNA at 4h and 4 fold increases at 6h. GC7 pretreatment significantly inhibited the hCG-induced LRBP mRNA expression (Fig. 3A). A significant induction of LRBP protein was also seen in response to hCG treatment and this response was significantly inhibited by inhibition of eIF5A hypusination by GC7 (Fig. 3B). These results demonstrate that eIF5A hypusination is required for hCG-induced LRBP expression.
Fig. 3. Inhibition of eIF5A hypusination abrogated hCG-induced increase in LRBP expression.
(A) GC7 (16 mg/kg body weight, i.p) was injected to superovulated rats on day 5 and 2 hours later the rats were injected with hCG (50 IU, s.c.) to induce LHR downregulation. Ovaries were collected and processed for RNA isolation. The RNA was reverse transcribed and the resulting cDNAs were subjected to real-time PCR quantitation using specific primers and probes for LRBP and 18S rRNA. The graphs represent changes in LRBP levels normalized to 18S rRNA and shown as fold change vs control. Error bars represent mean ± SE; *p<0.05 vs control. (B) The ovaries were homogenized in RIPA buffer containing protease inhibitor cocktail and S10 fractions were prepared. Equal amounts of protein from control, hCG-treated or GC7+hCG-treated S10 fractions were subjected to Western blot analysis using LRBP antibody. The membranes were stripped and reprobed for β-tubulin antibody. The bar graph represents the densitometry scanning of LRBP signals normalized with β-tubulin blots and expressed as fold change vs control. The blots shown are representative of three independent experiments and the results of the bar graphs are SEM of three experiments. *P<0.05 vs control.
3.4. Inhibition of eIF5A hypusination by GC7 inhibits hCG-induced LRBP activity and LHR downregulation
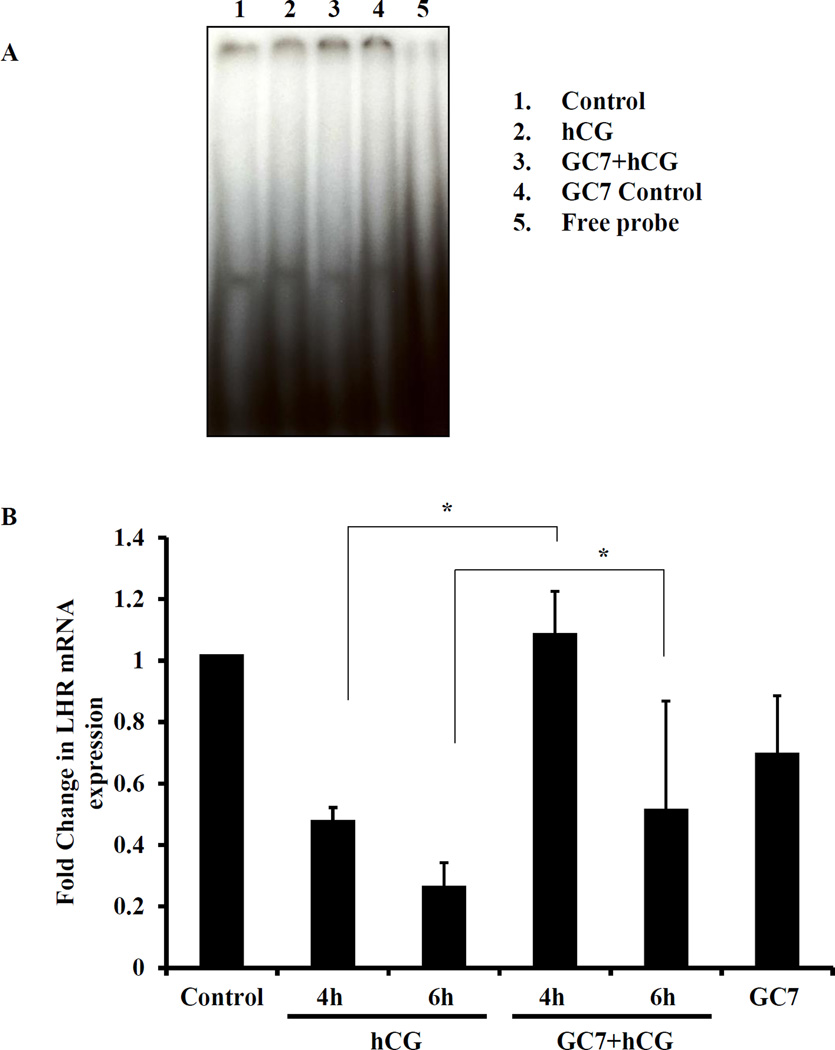
Furthermore, we have previously shown that the hCG-induced inhibition of LHR mRNA expression is mediated by LRBP [7,22]. Since eIF5A hypusination inhibition blocks LRBP mRNA and protein expression as shown in this study, we therefore examined whether this hypusination inhibition would lead to reduced binding of LHR mRNA to LRBP. To test this, RNA electrophoretic mobility gel shift assay (REMSA) was performed with [32P]-labeled rat LHR mRNA fragment that contain LRBP binding sequence (LBS) and S100 fractions from extracts of superovulated ovaries pretreated with vehicle or GC7 followed by hCG treatment. The results showed that hCG treatment showed an increase in the binding activity of LHR mRNA to LRBP compared to control. As expected, there was a significant increase in the intensity of the band in the hCG treated samples when compared to control (Fig. 4A, lane 1 and 2). GC7 pretreatment produced significant decrease in the binding activity as reflected in the reduction in the amounts of the band (Fig. 4A, lane 3) suggesting a role for eIF5A hypusination in hCG-induced increase in the binding of LHR mRNA to LRBP. We also examined the effect of GC7 treatment on LHR mRNA expression by Real time PCR. The results showed that GC7 pretreatment abrogated hCG-induced downregulation of LHR mRNA at 4 and 6h (Fig. 4B) again confirming the role of eIF5A hypusination in the downregulation of LHR mRNA.
Fig. 4. Inhibition of eIF5A hypusination by GC7 inhibits hCG-induced LRBP activity and LHR downregulaton.
Superovulated rats were injected with GC7 (16mg/kg body weight, i.p.), followed 2h later with hCG (50IU, s.c) and the ovaries were collected at different time intervals. (A) S100 fractions were prepared from the ovaries using 1× REMSA buffer. Equal amounts of protein from control, hCG or GC7+hCG-treated S100 fractions were subjected to RNA electrophoretic mobility shift assay as described in methods section. (B) The total RNA was isolated from superovulated rats with hCG or saline (with or without GC7 pretreatment). cDNA was synthesized by reverse transcription PCR and Real-Time PCR was performed using specific primers and probes for rLHR and 18S rRNA. The graphs represent changes in LRBP levels normalized to 18S rRNA and shown as fold change vs control. Error bars represent mean ± SE; *p<0.05 vs control.
4. Discussion
Previous studies from our laboratory have shown that LHR expression in the ovary is downregulated in response to preovulatory LH surge or by the administration of a pharmacological dose of hCG to superovulated rats through a posttranscriptional mechanism [22]. Using a rodent model system, we identified a protein designated as LHR mRNA binding protein (LRBP) in the downregulated rat ovary that binds specifically to the polypyrimidine-rich sequence in the coding region of LHR mRNA and accelerates its degradation [6], [22]. Further studies using yeast two hybrid screening, we identified eukaryotic initiation factor 5A (eIF5A), an 18 kDa protein, that interacts with LRBP during LHR downregulation [8]. Furthermore, we demonstrated that eIF5A functionally associates with LHR mRNA-LRBP complex during downregulation of LHR mRNA. Our recent study showed that eIF5A undergoes hypusination and this is necessary for its function [17]. The current study focused on the upstream signaling pathways of eIF5A hypusination and extends its role in LRBP-mediated downregualtion of LHR mRNA. The results clearly show that hCG also induces eIF5A mRNA and protein expression in addition to stimulating its hypusination. This hCG-induced response was blocked by the PKA inhibitor, H89 and ERK1/2 inhibitor, U0126 suggesting that hypusination of eIF5A occurs through hCG-induced activation of cAMP/PKA/ERK1/2 signaling pathway. Further, eIF5A hypusination inhibitor GC7 blocked LRBP expression and its LHR-mRNA binding activity suggesting that eIF5A hypusination is essential for LRBP-mediated LHR downregulation.
It is known that hypusination of eIF5A protein occurs readily after translation [23]. The unhypusinated eIF5A precursor protein accumulates only when the deoxyhupusine synthesis is blocked by inhibitors of deoxyhupusine synthase, an enzyme involved in the first step of hypusination reaction or by deprivation of spermidine [24,25]. Since eIF5A interacts with LRBP-LHR mRNA complex during the downregulation of LHR mRNA, we hypothesized that hCG treatment might trigger eIF5A synthesis and facilitate its hypusination. The results presented here support this notion by demonstrating that the expression of both eIF5A mRNA and protein increases during downregulation of LHR mRNA. The LH/hCG-mediated upregulation of eIF5A mRNA and protein expression is mediated by cAMP/PKA/ERK1/2 signaling pathway. PKA inhibitor H89 as well as ERK1/2 inhibitor U0126 blocked the expression of eIF5A in response to LH/hCG stimulation. This is consistent with the previous study by Wu et al. who examined the expression of forskolin-induced genes in an ovarian granulosa-like tumor cell line and found a 4.9 fold induction of eIF5A mRNA expression [26]. Likewise, in epithelial cells, eIF5A mRNA and protein expression have been shown to be induced in response to treatment with epidermal growth factor [27]. Thus our results are in agreement with findings in other systems.
We have previously shown that LH/hCG-activated cAMP/PKA/ERK1/2 signaling plays an important role in LHR downregulation by increasing LRBP expression [21]. The present study extends this further showing that this pathway is also instrumental in increasing the hypusination of eIF5A. As shown in Fig 2B, inhibition of PKA and ERK 1/2 abrogated the increases in the expression of hypusinated form of eIF5A seen during hCG treatment. It is possible that hCG might increase hypusination reaction via cAMP/PKA/ERK1/2 pathway by activating deoxy hypusine synthase activity which is an early step in the hypusination reaction. Additionally, the possibility that cAMP/PKA/ERK1/2 pathway increases eIF5A expression, which then results in increased accumulation of the hypusinated form of eIF5A cannot be ruled out. Identification of specific steps that is activated by the cAMP/PKA/ERK1/2 signaling pathways leading to increased hypusination of eIF5A needs additional investigation.
Our previous studies have established that LRBP is a key component of the post-transcriptional mechanism of LH receptor expression by causing accelerated degradation of LHR mRNA during LHR downregulation [21,22]. The present findings extend this by demonstrating that inhibition of eIF5A hypusination by GC7 reduces the binding of LHR mRNA to LRBP as presented in Fig. 4A. The abolishment of LH/hCG-induced increases in LRBP mRNA and protein expression by inhibition of eIF5A hypusination as shown in the present study is consistent with previous reports using other systems showing the effect of hypusination inhibition on transcription and translation. For example, de Almeida et al. have reported that inhibition of eIF5A hypusination by GC7 inhibits mRNA expression of inflammatory mediators [28] and Li et al [11] have shown that eIF5A affects translation elongation in U2OS (human osteosarcoma), COS7, and RDG3 cells.
In conclusion, the present study demonstrates that LH/hCG-induced LHR downregulation in the ovary is proceeded by the activation of cAMP/PKA/ERK1/2 pathway and induction of eIF5A protein and its hypusination. Inhibition of eIF5A hypusination inhibits LRBP expression culminating in LHR downregulation. Thus, hypusination of eIF5A plays an important role in the regulation of LH receptor expression.
Highlights.
-
▪
LH/hCG increases eIF5A expression and hypusination during LHR downregulation.
-
▪
PKA and ERK1/2 inhibitor reduces hCG-induced eIF5A hypusination.
-
▪
EIF5A hypusination inhibitor GC7 blocks LRBP expression and LRBP-LHR mRNA binding activity.
-
▪
LH/hCG-induced LHR mRNA downregulation was preceded by activation of cAMP/PKA-ERK1/2-mediated hypusination of eIF5A.
-
▪
Hypusinated eIF5A regulates LHR expression.
Acknowledgments
This work was supported by National Institutes of Health Grant R01 HD 06656.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev. 2002:141–174. doi: 10.1210/edrv.23.2.0462. [DOI] [PubMed] [Google Scholar]
- 2.Menon KMJ, Munshi UM, Clouser CL, Nair AK. Regulation of luteinizing hormone/human chorionic gonadotropin receptor expression: a perspective. Biol Reprod. 2004;70:861–866. doi: 10.1095/biolreprod.103.024471. [DOI] [PubMed] [Google Scholar]
- 3.Zeleznik AJ. The physiology of follicle selection. Reprod Biol Endocrinol. 2004;2:31. doi: 10.1186/1477-7827-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffman YM, Peegel H, Sprock MJ, Zhang QY, Menon KMJ. Evidence that human chorionic gonadotropin/luteinizing hormone receptor down-regulation involves decreased levels of receptor messenger ribonucleic acid. Endocrinology. 1991;128:388–393. doi: 10.1210/endo-128-1-388. [DOI] [PubMed] [Google Scholar]
- 5.Peegel H, Randolph J, Jr, Midgley AR, Menon KMJ. In situ hybridization of luteinizing hormone/human chorionic gonadotropin receptor messenger ribonucleic acid during hormone-induced down-regulation and the subsequent recovery in rat corpus luteum. Endocrinology. 1994;135:1044–1051. doi: 10.1210/endo.135.3.8070346. [DOI] [PubMed] [Google Scholar]
- 6.Kash JC, Menon KMJ. Identification of a hormonally regulated luteinizing hormone/human chorionic gonadotropin receptor mRNA binding protein. Increased mrna binding during receptor down-regulation. J Biol Chem. 1998;273:10658–10664. doi: 10.1074/jbc.273.17.10658. [DOI] [PubMed] [Google Scholar]
- 7.Nair AK, Menon KMJ. Isolation and characterization of a novel trans-factor for luteinizing hormone receptor mRNA from ovary. J Biol Chem. 2004;279:14937–14944. doi: 10.1074/jbc.M309484200. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Gulappa T, Menon KMJ. Identification and characterization of proteins that selectively interact with the LHR mRNA binding protein (LRBP) in rat ovaries. Biochim Biophys Acta. 2010;1803:591–597. doi: 10.1016/j.bbamcr.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cano VS, Jeon GA, Johansson HE, Henderson CA, Park JH, Valentini SR, Hershey JW, Park MH. Mutational analyses of human eIF5A-1--identification of amino acid residues critical for eIF5A activity and hypusine modification. FEBS J. 2008;275:44–58. doi: 10.1111/j.1742-4658.2007.06172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saini P, Eyler DE, Green R, Dever TE. Hypusine-containing protein eIF5A promotes translation elongation. Nature. 2009;459:118–121. doi: 10.1038/nature08034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li CH, Ohn T, Ivanov P, Tisdale S, Anderson P. eIF5A promotes translation elongation, polysome disassembly and stress granule assembly. PLoS One. 2010;5:e9942. doi: 10.1371/journal.pone.0009942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gregio AP, Cano VP, Avaca JS, Valentini SR, Zanelli CF. eIF5A has a function in the elongation step of translation in yeast. Biochem Biophys Res Commun. 2009;380:785–790. doi: 10.1016/j.bbrc.2009.01.148. [DOI] [PubMed] [Google Scholar]
- 13.Rosorius O, Reichart B, Kratzer F, Heger P, Dabauvalle MC, Hauber J. Nuclear pore localization and nucleocytoplasmic transport of eIF-5A: evidence for direct interaction with the export receptor CRM1. J Cell Sci. 1999;112(Pt 14):2369–2380. doi: 10.1242/jcs.112.14.2369. [DOI] [PubMed] [Google Scholar]
- 14.Park MH, Joe YA, Kang KR, Lee YB, Wolff EC. The polyamine-derived amino acid hypusine: its post-translational formation in eIF-5A and its role in cell proliferation. Amino Acids. 1996;10:109–121. doi: 10.1007/BF00806584. [DOI] [PubMed] [Google Scholar]
- 15.Maier B, Ogihara T, Trace AP, Tersey SA, Robbins RD, Chakrabarti SK, Nunemaker CS, Stull ND, Taylor CA, Thompson JE, Dondero RS, Lewis EC, Dinarello CA, Nadler JL, Mirmira RG. The unique hypusine modification of eIF5A promotes islet beta cell inflammation and dysfunction in mice. J Clin Invest. 2010;120:2156–2170. doi: 10.1172/JCI38924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor CA, Sun Z, Cliche DO, Ming H, Eshaque B, Jin S, Hopkins MT, Thai B, Thompson JE. Eukaryotic translation initiation factor 5A induces apoptosis in colon cancer cells and associates with the nucleus in response to tumour necrosis factor alpha signalling. Exp Cell Res. 2007;313:437–449. doi: 10.1016/j.yexcr.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 17.Menon B, Gulappa T, Menon KMJ. Eukaryotic Initiation Factor 5A Plays an Essential Role in Luteinizing Hormone Receptor Regulation. Mol Endocrinol. 2014;28:1796–1806. doi: 10.1210/me.2014-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Menon B, Peegel H, Menon KMJ. Evidence for the association of luteinizing hormone receptor mRNA-binding protein with the translating ribosomes during receptor downregulation. Biochim Biophys Acta. 2009;1793:1787–1794. doi: 10.1016/j.bbamcr.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kash JC, Menon KMJ. Sequence-specific binding of a hormonally regulated mRNA binding protein to cytidine-rich sequences in the lutropin receptor open reading frame. Biochemistry. 1999;38:16889–16897. doi: 10.1021/bi9915770. [DOI] [PubMed] [Google Scholar]
- 21.Menon B, Franzo-Romain M, Damanpour S, Menon KMJ. Luteinizing hormone receptor mRNA down-regulation is mediated through ERK-dependent induction of RNA binding protein. Mol Endocrinol. 2011;25:282–290. doi: 10.1210/me.2010-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nair AK, Kash JC, Peegel H, Menon KMJ. Post-transcriptional regulation of luteinizing hormone receptor mRNA in the ovary by a novel mRNA-binding protein. J Biol Chem. 2002;277:21468–21473. doi: 10.1074/jbc.M111653200. [DOI] [PubMed] [Google Scholar]
- 23.Park MH, Wolff EC, Folk JE. Hypusine: its post-translational formation in eukaryotic initiation factor 5A and its potential role in cellular regulation. Biofactors. 1993;4:95–104. [PubMed] [Google Scholar]
- 24.Caraglia M, Park MH, Wolff EC, Marra M, Abbruzzese A. eIF5A isoforms and cancer: two brothers for two functions? Amino Acids. 2013;44:103–109. doi: 10.1007/s00726-011-1182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee Y, Kim HK, Park HE, Park MH, Joe YA. Effect of N1-guanyl-1,7-diaminoheptane, an inhibitor of deoxyhypusine synthase, on endothelial cell growth, differentiation and apoptosis. Mol Cell Biochem. 2002;237:69–76. doi: 10.1023/a:1016535217038. [DOI] [PubMed] [Google Scholar]
- 26.Wu Y, Ghosh S, Nishi Y, Yanase T, Nawata H, Hu Y. The orphan nuclear receptors NURR1 and NGFI-B modulate aromatase gene expression in ovarian granulosa cells: a possible mechanism for repression of aromatase expression upon luteinizing hormone surge. Endocrinology. 2005;146:237–246. doi: 10.1210/en.2004-0889. [DOI] [PubMed] [Google Scholar]
- 27.Ding L, Gao LJ, Gu PQ, Guo SY, Cai YQ, Zhou XT. The role of eIF5A in epidermal growth factor-induced proliferation of corneal epithelial cell association with PI3-k/Akt activation. Mol Vis. 2011;17:16–22. [PMC free article] [PubMed] [Google Scholar]
- 28.de Almeida OP, Jr, Toledo TR, Rossi D, Rossetto Dde B, Watanabe TF, Galvao FC, Medeiros AI, Zanelli CF, Valentini SR. Hypusine modification of the ribosome-binding protein eIF5A, a target for new anti-inflammatory drugs: understanding the action of the inhibitor GC7 on a murine macrophage cell line. Curr Pharm Des. 2014;20:284–292. doi: 10.2174/13816128113199990036. [DOI] [PubMed] [Google Scholar]