Abstract
A vital property of the brain is its plasticity, which manifests as changes in behavioral performance. Invasive studies at the cellular level in animal models reveal time-restricted windows during which existing memories that are reactivated become susceptible to modification through reconsolidation, and evidence suggests similar effects in humans. In this review, we summarize recent work utilizing noninvasive brain stimulation in humans to uncover the systems-level mechanisms underlying memory reconsolidation. This novel understanding of memory dynamics may have far reaching clinical implications, including the potential to modulate reconsolidation in patients with memory disorders.
Keywords: Noninvasive brain stimulation, TMS, tDCS, memory, episodic, motor skill
Memory consolidation and modification through reconsolidation
Memory plays a crucial role in everyday life. It encompasses one's ability to recall an event that occurred in the past, retrieve knowledge that is stored in the brain, and execute motor skills that one has learned. From a cognitive perspective, memories are acquired (encoding), stored, maintained, and later retrieved (retrieval). The process that transforms the acquired information into long-term memory (LTM) is known as memory consolidation. The consolidation model assumes that memories are labile and unstable (i.e., susceptible to interference) for a limited time after encoding, but that as time passes, memories stabilize and become resistant to interference [1].
Two levels of description and analysis are used to describe the consolidation process. Synaptic consolidation involves the activation of intracellular signaling cascades, modulation of gene expression, and synthesis of gene products that alter synaptic efficacy. This form of consolidation is completed within hours from its initiation [2]. System-level consolidation refers to the reorganization of LTM over distributed brain networks. This process may last from days to years, depending on the memory system [2]. It is now known that sleep optimizes the consolidation of some types of newly encoded information in memory [3].
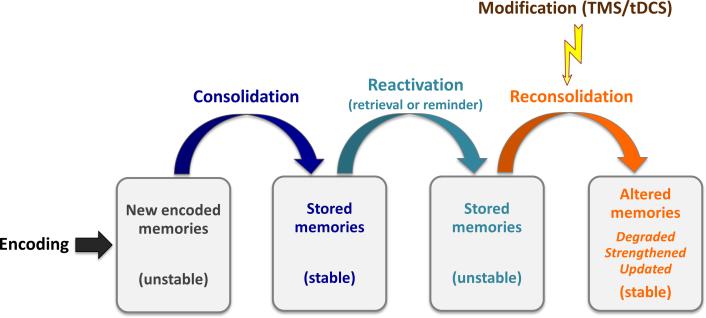
It is now widely accepted that memories are dynamic, even after their initial stabilization through consolidation. Accumulating evidence has shown that consolidated memories can re-enter unstable states when they are reactivated during retrieval (i.e. the process of recalling or recognizing previous stored information) or by a reminder cue (i.e. external information that is associated with the stored information). These memories must then be consolidated again, or reconsolidated, in order to persist over longer periods of time [4,5]. Thus, reconsolidation refers to the processes that re-stabilize the consolidated memories after reactivation [6-9]. During the time-limited reconsolidation window, existing memories are vulnerable to modifications. There is evidence that memories can be strengthened, weakened or updated by the inclusion of new information through reconsolidation (Figure 1) [6-9]. However, reconsolidation does not occur every time the existing memory is reactivated. Different boundary conditions have been identified so far, such as the age of the memory [10,11], the strength of training [10,12,13], the reactivation length [10,13-15], and the requirement of novel information at the time of the reactivation session (prediction error) [16-19].
Figure 1. Schematic illustration of memory formation and modification through reconsolidation.
Shortly after encoding, new memories are in an unstable/labile state until they are consolidated. Memory reactivation returns the consolidated memories from a stable state to an unstable state again, from which they need to be reconsolidated. During reconsolidation, noninvasive brain stimulation techniques (i.e. TMS, tDCS) can modify the unstable memories, revealing the systems-level mechanisms underlying memory reconsolidation. Thus, existing memories can be degraded, strengthened or updated by the inclusion of new information through reconsolidation. Modified from [9] with permission from Elsevier.
In this review, we refer to reconsolidation as the process that allows modification of memory strength or mediates updating of memory content by allowing the integration of new information into the original memory.
Most work on reconsolidation has been done in animal models because this permits the use of invasive methods such as the infusion of protein synthesis inhibitors to designated brain areas to interfere with neural processes underlying memory (e.g., [4]). However, noninvasive brain stimulation (NIBS) [20] has recently provided a powerful approach for studying brain function in humans. Indeed, transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) [20] have been used in the past few years to reveal the mechanisms underlying consolidation and reconsolidation of human memories. Combining NIBS with neuroimaging [21] through interdisciplinary efforts has also yielded novel insights into human reconsolidation's neural mechanisms at the brain network systems-level [22,23]. This work, for example, demonstrates that modification of previously consolidated human motor skill memories is possible, and shows that processing in primary motor cortex (M1) during memory reactivation is essential for efficient reconsolidation of the memory [24]. In addition, correlated co-activations of M1 and sensorimotor striatum are altered following interference with a consolidated motor memory, revealing a network in which both regional activity and inter-regional functional connectivity are involved in the reactivation-reconsolidation process [22,23].
In addition to discussing reconsolidation of human motor skill memories, we will describe studies that used NIBS to modulate prefrontal cortex (PFC) function [25-27] and demonstrate its causal role in the reconsolidation of episodic memories [27]. We will also discuss clinical implications, outstanding questions and future research directions of such work. Overall, this article will illustrate how NIBS studies complement crucial findings on reconsolidation at the regional cellular level.
Insights from animal models
Extensive studies have been performed in animal models (mainly rodents) in order to study memory reconsolidation. The classical interventions used to modify memory strength through reconsolidation were electroconvulsive shocks [5] and administration of amnesic agents, such as protein synthesis inhibitors [4]. For instance, in a study using the auditory fear-conditioning paradigm [4], a neutral stimulus (conditioned stimulus, i.e. a tone) was paired with an aversive outcome (unconditioned stimulus, i.e. a footshock). After the memory was consolidated, the protein synthesis inhibitor anisomycin was infused into the lateral and basal amygdala shortly after the memory for fear was reactivated by the presentation of the tone alone. In the control group, a vehicle (i.e. inactive variant of the drug) infusion was administered after the reactivation of the fear memory. Memory for fear was tested 24 hours later by presenting only the tone. Animal's immobility (freezing) was used as a measure of the retention of the fear response. The results showed that memory for fear was disrupted in the experimental group whereas in the control group fear conditioning was still present as well as in another control group in which the drug was infused without memory reactivation (no tone), suggesting that indeed the reactivation rendered the fear memory unstable again. Moreover, if the drug was infused 6 hours after memory reactivation, there was no effect on memory tested the following day, suggesting a limited time-window during which the memory is unstable following its reactivation [4].
The hippocampus has also been shown to be involved in reconsolidation mechanisms [11,17,28,29]. For example, intra-hippocampal infusions of the protein synthesis inhibitor anisomycin blocked hippocampal-dependent contextual fear memory, but only if the memory was reactivated prior to infusion [29].
Existing memories can be not only weakened but also strenghtened through reconsolidation. Some studies have shown memory enhancement during reconsolidation by using different pharmacological agents or modulators that affect the re-stabilization phase [30,31]. Other studies have addressed the strengthening function of reconsolidation by simply triggering this process without any treatment [28,32,33].
Overall, these studies indicate that memory modification through reconsolidation may include changes in memory strength. However, there is also evidence of changes in content. Existing memories can also be updated by the inclusion of new information [16-18]. This approach capitalizes on reconsolidation as an update mechanism. For instance, during the reconsolidation window, fear memories can be updated and attenuated by extinction training [34], a manipulation in which the memory for fear is diminished by repeated presentations of the conditioned stimulus without the aversive outcome.
Insights from modifying human memories
Until recently, human reconsolidation could only be studied using safe pharmacological or behavioral interventions. One influential example of this approach involved administering propranolol (a beta-adrenergic antagonist) to understand how fear memory strength can be modified [35]. Subjects were fear-conditioned and the memory was reactivated by the presentation of a conditioned stimulus 24 hours later. The authors found that the pharmacological intervention before memory reactivation disrupted the behavioral expression of the fear memory 24 hours later and prevented the return of fear. Importantly, propranolol without memory reactivation, as well as the combination of a placebo pill and memory reactivation, did not alter the memory. In addition, the results were replicated with propranolol administered after the reactivation [36], thereby ruling out the possibility of an effect of the drug on fear reactivation rather than reconsolidation (but see also [37]). Importantly, this behavioral effect was long-lasting [38] and generalized to semantically related stimuli [36, 39] as well as to other contexts [40]. Moreover, a recent functional magnetic resonance imaging (fMRI) study found that impairments in emotional episodic memories induced by propranolol during reactivation were associated with altered amygdala and hippocampus activation [41]. Most interestingly, the same structures were active during memory reactivation, suggesting that the brain areas that are recruited during reactivation undergo changes in activity that are associated with subsequent changes in memory strength [41].
Behavioral methods have also been used to understand how the strength of existing memories is degraded through reconsolidation. For example, in one study of procedural motor memory [42], subjects learned a new motor sequence following reactivation of a previously consolidated original motor sequence memory. A control group learned the new sequence without reactivation of the original motor sequence memory. Memory for the original motor sequence was tested 24 hours later. The results showed that the initial improvement achieved when learning the original sequence was reduced in subjects that learned a new motor sequence after reactivation [42].
Results from a recent behavioral study in motor learning suggest that reactivation length determines the extent of memory degradation [15]. As this study acknowledges, these findings are consistent with work in rodents showing that the length of memory reactivation and extinction training sessions is a factor that determines the extent to which reconsolidation can be blocked [10,13-14].
New learning after reactivation of existing memories can also impair episodic memories for paired associate verbal memory [43], and for more real-life related materials, such as a movie of a fictional terrorist attack [44], emotional pictures [45] or autobiographical memories [46]. In addition, emotional (fearful) faces may also affect the reconsolidation of episodic memories [47].
Beyond these behavioral modifications of reconsolidation and in line with early animal work [5,48], recent evidence showed that a single application of electroconvulsive therapy (ECT) following memory reactivation in patients with unipolar depression disrupted reactivated, but not non-reactivated, emotional episodic memories [49]. Using a technique that has been applied to treat major depression for decades, this study shows that it is possible to alter episodic memory for emotional experiences during reconsolidation.
Memory strengthening as a result of reconsolidation [33] has also been reported in humans with exposure to stress [50-52, but see also 53], administration of glucose [52] or clonazepam (i.e. a gamma-amynobutyric acidergic agonist) [54], and repeated presentations of the reminder [55], a finding in line with animal work using repeated reactivation of existing memories [28].
In addition to memory weakening and strengthening, reconsolidation can also mediate updating of the memory content [56-58]. In a series of elegant experiments of episodic memories [56], subjects learned a new list of objects, either following a reminder of the previously consolidated list of objects or without a reminder. Memory recall for the original list was tested 24h later. The results showed the inclusion of objects of the second list in the recall of the first list, but only if the original memory was reactivated by a reminder. Thus, the original memory was still expressed, but it was merged with new information presented during reconsolidation [56]. Additional work demonstrated that the exposure to the spatial context where the first list of objects was learned was critical for memory reactivation [57]. Thus, the role of context in reactivating the memories points to the involvement of the hippocampus, as shown in contextual reconsolidation work in rodents [17,29].
On the basis of animal work [34], a study showed that human fear memories can be updated by allowing the introduction of new information during the reconsolidation window [59]. Subjects who received extinction training during the reconsolidation window showed no return of fear 24 hours and up to 1 year later, as measured by skin conductance responses. Importantly, memory for fear was observed in a control group that received extinction training outside of the reconsolidation window and, hence, after completed reconsolidation [59]. These results are consistent with animal work using a similar protocol [34] and were replicated in later studies [60,61]. Recent findings suggest that, similar to young memories [59], older fear memories can also be updated and attenuated using extinction training after memory reactivation [62]. In addition, it has been shown that for labilization of fear memory to occur, new information has to be presented during reactivation (prediction error) [19] as demonstrated in animal work [16-18], supporting the notion that a function of reconsolidation is memory updating.
Recent human fMRI studies have revealed the brain regions that mediate extinction when it occurs during reconsolidation of fear memories [60,61]. After a fear memory was formed, subjects who underwent an extinction session during the reconsolidation window showed no return of fear and reduced activation of the amygdala compared to subjects who received the extinction training outside of the reconsolidation window [60]. As was previously shown in rodents [34], fear memory suppression resulting from behavioral disruption of reconsolidation is amygdala-dependent in humans as well [60].
There is evidence that controlling fear memory through extinction does not alter the memory itself, but rather regulates its expression via inhibitory influence of the PFC over the amygdala [63,64]. A recent study tested the hypothesis that targeting reconsolidation should eliminate the necessity of PFC inhibition by contrasting standard extinction with extinction during reconsolidation [61]. The results indicate that extinction training shortly after the reactivation of fear memories reduced ventromedial PFC (vmPFC) involvement and also altered the functional connectivity between vmPFC and amygdala relative to extinction training without memory reactivation (i.e. standard extinction). This altered connectivity might play a role in enabling extinction learning to more persistently modify the original fear memory trace within the amygdala [61].
In conclusion, animal and human evidence reveal that reconsolidation allows changes of the memory strength or mediates updating of the memory content. The idea that memories can be updated by the inclusion of new information through reconsolidation could explain the malleable nature of memory after retrieval extensively documented by cognitive psychology [65]. Memory distorsions, such as the misinformation effect (i.e. recall of episodic memories becomes less accurate because of post-event information) [65], illustrate that memories can be modified. Thus, reconsolidation seems to link cognitive findings in memory research with novel mechanistic insights emerging from systems and basic neuroscience [66].
Modulating reconsolidation by noninvasive brain stimulation
In recent years, the development of TMS and tDCS techniques has created a promising new way to modify human memories and study reconsolidation [20,67-71] (Box 1). Whereas neuroimaging techniques (e.g. fMRI) provide correlational data, NIBS techniques allow scientists to establish a causal link between neural processing in a cortical region and a human memory function [20]. Based on stimulation parameters, such as frequency or polarity, and the initial neural activation state of the stimulated area, NIBS applied to a cortical area can impair or enhance behavioral performance [69]. Frameless stereotactic brain navigation systems using each subject's structural MRI data allow mapping of the target for stimulation with accuracy [70]. Since tDCS and rTMS can induce long-lasting effects, these techniques can be also used as adjuvant strategies for rehabilitation of neurological deficits and treatment of psychiatric disorders [72,73].
There has been a significant gap in knowledge regarding the systems-levels mechanisms underlying human memory reconsolidation. Recent studies combining NIBS and neuroimaging have enabled researchers to identify the functional networks underlying memory reconsolidation. Using a motor sequence learning task, a series of experiments showed that processing in M1 is critical for successful modification of motor memory strength. This was demonstrated by applying inhibitory repetitive TMS (rTMS) over M1 in humans after reactivating the motor sequence memory, which subsequently blocked modification of memory strength [24]. The transient virtual lesion of M1 by inhibitory NIBS [20] resembles a reversible pharmacological lesion used in animal models. This technique allows researchers to temporarily down-regulate cortical processing during memory reactivation to neuromodulate the resultant memory.
Although it is important to understand the role of specific brain regions in human memory reconsolidation as described above, the next valuable step is to identify the underlying network-level (inter-regional) brain function. The effects of rTMS not only on human memory but also on fMRI activity and inter-regional functional connectivity have been measured in motor sequence learning. The results revealed a cortico-subcortical neuronal circuitry associated with modification of human procedural memories, suggesting that both regional activity and inter-regional interactions are critical for motor memory reconsolidation [22]. Previous studies have commonly segregated motor skill learning into two main distinct stages: early learning, in which cerebellar components are more dominant, and late learning, in which striatal components are commonly engaged [74]. Both cerebellar and striatal components are engaged during memory modification. These results suggest that reconsolidation may serve as a mechanism connecting the early and late stages of procedural learning, making skill acquisition possible.
An open question has been whether there are intrinsic task-free signatures of modified memories. It was recently shown using resting-state fMRI that inhibitory rTMS [23] applied in association with a reactivated motor memory altered M1-striatal inter-regional coherence measured at rest the following day (Figure 2). The reduction in coherence predicted the magnitude of memory modification. Thus, systems-level brain activity can be modulated by noninvasive interaction with existing memories, and strongly correlates with behavioral measurements of changes in memory strength.
Figure 2. Neuromodulation of motor memory.

(A) Measuring the effects of rTMS interference applied to M1 and synchronous with motor memory reactivation, on subsequent behavioral memory strength and resting-state fMRI. (B) M1 and sensorimotor striatum regions of interest were identified from a baseline fMRI measurement. Single subjects’ examples of time courses for M1 and sensorimotor striatum before (Post-Test) and after (Pre-Retest) interference (upper quadrants) and control stimulation (lower quadrants). Correlations depicted for each region-of-interest pair. Adapted from [23] with permission from Elsevier.
There is also evidence of memory enhancement after neuromodulation of lateral PFC [25-27,75]. A recent study used facilitatory anodal tDCS to modulate fear memories [25]. Shortly after memory reactivation, facilitatory anodal tDCS over the right lateral PFC, a region involved in negative affect [76], enhanced fear memories compared to sham stimulation [25]. In a study involving episodic memory, tDCS was applied over the left lateral PFC [26], a region critically involved in encoding of verbal episodic memories [77,78]. Subjects memorized words in the first session. Three hours later, in the second session, subjects underwent tDCS while the existing memories were reactivated. The results showed that facilitatory anodal tDCS enhanced episodic memory recognition (tested 5 hours after the tDCS session) compared to control stimulation conditions, such as sham or cathodal (commonly inhibitory) tDCS (Box 1). Importantly, anodal tDCS did not enhance memory performance when applied to the left PFC in the absence of reactivation [26]. Because these tDCS studies [25, 26] did not include the stimulation of a control site it remains to be determined if this effect is topographically specific. A TMS study of episodic memory used an experimental design that did include stimulation of a control site [27]. rTMS was applied to the right lateral PFC, a region critically involved in episodic memory retrieval [78] and reactivation [79], after a contextual reminder [57]. This resulted in enhanced verbal episodic memory recall 24 hours later compared to recall following rTMS of the right PFC without a contextual reminder or to rTMS of a control site (i.e. vertex) after a contextual reminder. This demonstrates the causal role of lateral PFC in strengthening of verbal episodic memories through reconsolidation [27]. Considering the role of hippocampus in contextual reconsolidation [17,29], and evidence showing that rTMS affects not only the targeted local region but also induces remote effects in regions (including subcortical) interconnected to the stimulated site [21-23], rTMS during reconsolidation in this study might have strengthened the functional connectivity between PFC and hippocampus [80] and therefore enhanced recollection. However, it is also possible that rTMS enhanced the functional efficacy of the PFC or promoted a feedback process that enhanced hippocampal memory in the absence of lasting change in PFC function or hippocampus-PFC connectivity. Regardless, the combination of TMS with resting-state fMRI is starting to shed light on how functional interactions between remote but interconnected brain regions may support strengthening of episodic memories through reconsolidation.
Although there is evidence of reconsolidation of episodic memories, it remains unknown whether reconsolidation might change with an individual's age. To address this question, facilitatory anodal tDCS was used to strengthen verbal episodic memories through reconsolidation in the elderly [75]. Using the paradigm of a previous study [27], tDCS was applied over the left lateral PFC, a brain region critically involved in retrieval of verbal episodic memories in older adults [81]. The results showed that anodal tDCS, either preceded by a contextual reminder or not (i.e. same or different spatial context of the learning session [27,57]) strengthened existing episodic memories and reduced forgetting for up to 1 month compared to sham tDCS after a contextual reminder. These memory enhancement effects among older adults, observed in both anodal tDCS groups, differ from effects seen in younger individuals [27,57]. The pattern shown by older adults suggests that their existing memories might have been reactivated by other factors (e.g. the context may have been encoded at a general level but without distinctive detail [82]), or the contextual reminder might have not been sufficient to trigger memory reconsolidation, as shown in a recent study using the same contextual reminder in older adults [83]. Thus, the facilitation effects might simply be attributable to tDCS over the left lateral PFC without affecting the reconsolidation process. Future studies are needed to determine the mechanisms underlying this age difference, the specific reminder cues that trigger reconsolidation in older subjects, and the effects of aging on reconsolidation in different memory domains.
Concluding Remarks
Previous experimental approaches in animals and humans have provided insights into reconsolidation mechanisms. More recently, new strategies and tools have uncovered systems-level dynamics of human memories by modulating memory reconsolidation. This exciting new framework of understanding is leading to the development of neuromodulatory strategies for reshaping human memories in health and disease. Such strategies are geared to facilitate or inhibit target brain regions or networks, thus enabling enhancement of memory functions or down-regulation of maladaptive memories (Box 2). For this to occur, several outstanding questions remain to be addressed. There is a need to better understand the systems-level correlates of memory reconsolidation across memory modalities (Box 3). In turn, this would enable the development of mechanism-based modulation of reconsolidation using noninvasive brain stimulation, including emerging techniques geared to entrain in a frequency-specific fashion the underlying oscillatory activity in cortical networks (Box 1).
Using these techniques, a window of opportunity is opening to modulate memory reconsolidation, with the potential to facilitate neuroplasticity and learning in healthy individuals and in patients with brain and memory disorders, as well as to mediate the forgetting of negative memories such as in post-traumatic stress disorders.
Box 1: Noninvasive brain stimulation techniques.
Transcranial Magnetic Stimulation (TMS)
TMS uses electromagnetic induction to induce weak electric current through the skull, affecting neuronal activity in the stimulated region. Thus, TMS can depolarize or hyperpolarize neurons in the brain [67]. Standard focal TMS coils can stimulate cortical regions and affect indirectly deep regions. Deep TMS coils enable stimulation (though less focal) of deeper brain structures [20,88]. TMS can be applied not only as single pulses but also as a train of pulses, and in this case is named repetitive TMS (rTMS). rTMS is generally applied for 15-20 minutes at low frequency (≤ 1 Hz) and then task performance is measured. This is based on studies showing changes in cortex excitability beyond the duration of the stimulation [20,70]. To increase the duration of these TMS after-effects, researchers can use a type of stimulation named Theta Burst Stimulation (TBS) [90], in which trains at 50 Hz are applied every 200 milliseconds continuously for 40 seconds (cTBS) or intermittently for 190 seconds (iTBS). Low frequency rTMS and cTBS applied to the primary motor cortex decrease motor cortical excitability as assessed by Motor Evoked Potentials (MEPs), whereas high-frequency rTMS and iTBS increase motor cortical excitability [20]. However, these effects can vary, especially for cognitive functions involving non-motor brain regions [70].
Transcranial Direct Current Stimulation (tDCS)
tDCS is a portable device, which uses constant, low intensity current (usually between 1 and 2 milliampere) delivered directly to the cortical area via two surface electrodes (i.e. 5×5 or 5×7 cm2), anode and cathode [20,68]. One electrode is placed over the target region and the other more distant. tDCS differs from TMS in that it does not induce neuronal action potentials [68]. tDCS modifies spontaneous neuronal excitability and activity by a tonic de- or hyperpolarization of resting membrane potential [68]. Anodal tDCS applied to primary motor cortex generally increases cortical excitability as assessed by MEPs induced by TMS, whereas cathodal tDCS decreases cortical excitability [20,68]. Anodal tDCS applied to non-motor areas often enhances behavioral performance, whereas it is rare that cathodal tDCS impairs performance [91].
Transcranial alternating current stimulation (tACS)
tACS is a promising technique by which an alternating current may entrain in a frequency-specific fashion the underlying oscillatory activity in cortical networks [92]. In addition to rhythmic rTMS [93], tACS offers the opportunity to causally link brain oscillations in a specific frequency range to cognitive processes [92].
Box 2: Clinical implications.
The observation that existing memories can be modified by interventions (i.e. drug treatment, behavioral means or noninvasive brain stimulation) during reconsolidation opens up the possibility of using these strategies to treat memory disorders. These interventions can be administered to disrupt maladaptive memories (i.e. aversive, appetitive) or strengthen motor skills or episodic memories through reconsolidation. In one of these studies, patients with posttraumatic stress disorder (PTSD) reactivated the traumatic experience by describing it, after which they received a single dose of propranolol or a placebo. Patients given propranolol showed reduced physiological signs of fear (i.e. skin conductance and heart rate) when they were asked to describe once again the traumatic experience a week later [94]. Replications of these findings are under way [95,96].
Preclinical studies indicate that reconsolidation of appetitive (drug) memories may also be disrupted [97]. Post-reactivation extinction training [34,59] was used to disrupt reconsolidation of drug memories in abstinent heroin addicts [98], resulting in reduced heroin craving up to 6 months later.
Another potential therapeutic intervention is offered by noninvasive brain stimulation. After initial animal work showing impairment of aversive memory reconsolidation by intracranial electrical stimulation to the insular cortex [99], a pilot study found that the combination of brief exposure to a traumatic event with repeated medial PFC deep rTMS (see Box 1) induced beneficial effects in PTSD patients [100]. In another study using the same technique, nicotine addicts received multiple daily sessions of deep rTMS to the lateral PFC and insula following, or without, presentation of smoking cues. The results showed that high frequency rTMS reduced cigarette consumption. However, rTMS following smoking cues enhanced reduction in cigarette consumption, leading to an abstinence rate of 44% at the end of the treatment and an estimated 33% after 6 months [88].
Although these interventions suggest potential clinical applications, further research is needed to determine their efficacy. Finally, noninvasive brain stimulation during reconsolidation might also be a novel strategy to delay dementia or enhance memory function after brain lesions.
Box 3: Outstanding questions.
How are reconsolidation mechanisms at the cellular level related to systems-level reconsolidation? To what extent are reconsolidation mechanisms identified in non-human animals similar to reconsolidation processes evident in human behavior [2,7,9] in health and disease?
What are the key systems-level mechanistic differences between consolidation and reconsolidation? Although dissociable mechanisms underlying consolidation and reconsolidation have been suggested at the cellular level [6,8], further evidence is required in order to support such distinction at the brain systems-level.
What are the systems-level correlates of reconsolidation that are common across different memory domains [101], and how can the identified commonalities be utilized in order to neuromodulate reconsolidation?
How can emerging techniques geared to target specific frequencies underlying oscillatory activity in cortical networks (Box 1) be utilized and further developed in order to neuromodulate reconsolidation?
Can NIBS protocols applied in association with memory reactivation be effective as clinical treatment interventions (Box 2)?
Acknowledgements
This work was supported by funding from US Department of Defense in the Center for Neuroscience and Regenerative Medicine (CNRM) to M.S., by the Intramural Research Program of the NINDS-NIH (US) to L.G.C. and M.S., and by the I-CORE program of the Planning and Budgeting Committee and The Israel Science Foundation (grant No. 51/11) to N.C.
Glossary
- Consolidation
The processes that stabilize memories after encoding, transforming them into long-term memories.
- Electroconvulsive therapy (ECT) or electroconvulsive shock
Refers to the application of a short-acting anesthesia followed by electrical stimulation to the cranium, evoking generalized seizure activity. The mechanism of action by which ECT affects memory and may disrupt reconsolidation remains largely unknown [5,49].
- Episodic memory
A type of declarative memory that refers to the conscious recollection of events [84]. It is mediated primarily by the prefrontal cortex (PFC) [27,78,85] and medial temporal lobe [85], particularly the hippocampus. Variations in the strength of links between hippocampus and neocortex are at the heart of different studies in the field of memory consolidation [2]. The standard model of system-level consolidation posits that encoding, storage, and retrieval of information is initially dependent on the hippocampus as well as neocortical areas relevant to the encoded stimuli. Over time, this information reorganizes and becomes integrated in the neocortex and independent of the hippocampus [2]. An alternative model, the multiple trace theory, posits that the links between hippocampal and cortical representation remain critical and so the hippocampus is continuously involved in the storage and retrieval of memories [2].
- Extinction training
Manipulation in which the fear memory is diminished by repeated presentations of the conditioned stimulus without the aversive outcome.
- Functional connectivity
Correlation between remote neurophysiological events in the temporal domain [86].
- Nondeclarative memory
Refers to processes in which learning has occurred, which is reflected in performance rather than through overt remembering (e.g. fear memories, procedural motor skills) [84]. Although memories created by aversive or rewarding reinforcement rely primarily on the PFC [25,61,88] and the amygdala [60,61,87], memories of motor skills rely on activity in a distributed network that includes the motor cortex, striatum and cerebellum [74].
- Prediction error
A discrepancy between actual and expected events. It can be influenced by previous learning, reinforcement or sensory information, and their relation to the actual subsequent events [16-19].
- Protein synthesis inhibitors
Infused following memory encoding or reactivation in order to causally inhibit protein synthesis required for consolidation and reconsolidation, thereby identifying the regional processes involved and linking them with the behavioral expressions of memory functions [89].
- Reconsolidation
The processes that re-stabilize the consolidated memories after reactivation.
- Vertex
The scalp midline location referring to the highest point of the skull. Commonly considered as a neutral stimulation site used to control for topographic specificity of the TMS-induced effects [70].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 2.Dudai Y. The restless engram: consolidations never end. Annu. Rev. Neurosci. 2012;35:227–247. doi: 10.1146/annurev-neuro-062111-150500. [DOI] [PubMed] [Google Scholar]
- 3.Diekelmann S, Born J. The memory function of sleep. Nat. Rev. Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 4.Nader K, et al. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 5.Misanin JR, et al. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160:554–555. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- 6.Alberini CM, Ledoux JE. Memory reconsolidation. Curr. Biol. 2013;23:R746–50. doi: 10.1016/j.cub.2013.06.046. [DOI] [PubMed] [Google Scholar]
- 7.Agren T. Human reconsolidation: a reactivation and update. Brain Res. Bull. 2014;105:70–82. doi: 10.1016/j.brainresbull.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Nader K, Hardt O. A single standard for memory: the case for reconsolidation. Nat. Rev. Neurosci. 2009;10:224–234. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- 9.Schwabe L, et al. Reconsolidation of human memory: brain mechanisms and clinical relevance. Biol. Psychiatry. 2014;76:274–280. doi: 10.1016/j.biopsych.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki A, et al. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J. Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milekic MH, Alberini CM. Temporally graded requirement for protein synthesis following memory reactivation. Neuron. 2002;36:521–525. doi: 10.1016/s0896-6273(02)00976-5. [DOI] [PubMed] [Google Scholar]
- 12.Wang SH, et al. Cellular and systems mechanisms of memory strength as a constraint on auditory fear reconsolidation. Nat. Neurosci. 2009;12:905–912. doi: 10.1038/nn.2350. [DOI] [PubMed] [Google Scholar]
- 13.Eisenberg M, et al. Stability of retrieved memory: inverse correlation with trace dominance. Science. 2003;301:1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- 14.Pedreira ME, Maldonado H. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron. 2003;38:863–869. doi: 10.1016/s0896-6273(03)00352-0. [DOI] [PubMed] [Google Scholar]
- 15.de Beukelaar TT, et al. Gone for 60 seconds: reactivation length determines motor memory degradation during reconsolidation. Cortex. 2014;59:138–145. doi: 10.1016/j.cortex.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Pedreira ME, et al. Mismatch between what is expected and what actually occurs triggers memory reconsolidation or extinction. Learn. Mem. 2004;11:579–585. doi: 10.1101/lm.76904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris RG, et al. Memory reconsolidation: sensitivity of spatial memory to inhibition of protein synthesis in dorsal hippocampus during encoding and retrieval. Neuron. 2006;50:479–489. doi: 10.1016/j.neuron.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Díaz-Mataix L, et al. Detection of a temporal error triggers reconsolidation of amygdala-dependent memories. Curr. Biol. 2013;23:467–472. doi: 10.1016/j.cub.2013.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sevenster D, et al. Prediction error governs pharmacologically induced amnesia for learned fear. Science. 2013;339:830–833. doi: 10.1126/science.1231357. [DOI] [PubMed] [Google Scholar]
- 20.Dayan E, et al. Noninvasive brain stimulation: from physiology to network dynamics and back. Nat. Neurosci. 2013;16:838–844. doi: 10.1038/nn.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shafi MM, et al. Exploration and modulation of brain network interactions with noninvasive brain stimulation in combination with neuroimaging. Eur. J. Neurosci. 2012;35:805–825. doi: 10.1111/j.1460-9568.2012.08035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Censor N, et al. Cortico-subcortical neuronal circuitry associated with reconsolidation of human procedural memories. Cortex. 2014;58:281–288. doi: 10.1016/j.cortex.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Censor N, et al. Interference with existing memories alters offline intrinsic functional brain connectivity. Neuron. 2014;81:69–76. doi: 10.1016/j.neuron.2013.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Censor N, et al. Modification of existing human motor memories is enabled by primary cortical processing during memory reactivation. Curr. Biol. 2010;20:1545–1549. doi: 10.1016/j.cub.2010.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mungee A, et al. Transcranial direct current stimulation of the prefrontal cortex: a means to modulate fear memories. Neuroreport. 2014;25:480–484. doi: 10.1097/WNR.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 26.Javadi AH, Cheng P. Transcranial direct current stimulation (tDCS) enhances reconsolidation of long-term memory. Brain Stimul. 2013;6:668–674. doi: 10.1016/j.brs.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Sandrini M, et al. Causal role of prefrontal cortex in strengthening of episodic memories through reconsolidation. Curr. Biol. 2013;23:2181–2184. doi: 10.1016/j.cub.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inda MC, et al. Memory retrieval and the passage of time: from reconsolidation and strengthening to extinction. J. Neurosci. 2011;31:1635–1643. doi: 10.1523/JNEUROSCI.4736-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Debiec J, et al. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:527–538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 30.Frenkel L, et al. Memory strengthening by a real-life episode during reconsolidation: an outcome of water deprivation via brain angiotensin II. Eur. J. Neurosci. 2005;22:1757–1766. doi: 10.1111/j.1460-9568.2005.04373.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee JL, et al. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J. Neurosci. 2006;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JL. Memory reconsolidation mediates the strengthening of memories by additional learning. Nat. Neurosci. 2008;11:1264–1266. doi: 10.1038/nn.2205. [DOI] [PubMed] [Google Scholar]
- 33.Forcato C, et al. Strengthening a consolidated memory: the key role of the reconsolidation process. J. Physiol. Paris. 2014;108:323–333. doi: 10.1016/j.jphysparis.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Monfils MH, et al. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kindt M, et al. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat. Neurosci. 2009;12:256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- 36.Soeter M, Kindt M. Stimulation of the noradrenergic system during memory formation impairs extinction learning but not the disruption of reconsolidation. Neuropsychopharmacology. 2012;37:1204–1215. doi: 10.1038/npp.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bos MG, et al. Noradrenergic blockade of memory reconsolidation: a failure to reduce conditioned fear responding. Front. Behav. Neurosci. 2014;28:8, 412. doi: 10.3389/fnbeh.2014.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soeter M, Kindt M. Dissociating response systems: erasing fear from memory. Neurobiol. Learn. Mem. 2010;94:30–41. doi: 10.1016/j.nlm.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Soeter M, Kindt M. Noradrenergic enhancement of associative fear memory in humans. Neurobiol. Learn. Mem. 2011;96:263–271. doi: 10.1016/j.nlm.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Soeter M, Kindt M. Erasing fear for an imagined threat event. Psychoneuroendocrinology. 2012;37:1769–1779. doi: 10.1016/j.psyneuen.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 41.Schwabe L, et al. Neural signature of reconsolidation impairments by propranolol in humans. Biol. Psychiatry. 2012;71:380–386. doi: 10.1016/j.biopsych.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 42.Walker MP, et al. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- 43.Forcato C, et al. Reconsolidation of declarative memory in humans. Learn. Mem. 2007;14:295–303. doi: 10.1101/lm.486107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan JC, LaPaglia JA. Impairing existing declarative memory in humans by disrupting reconsolidation. Proc. Natl. Acad. Sci. U S A. 2013;110:9309–9313. doi: 10.1073/pnas.1218472110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wirkner J, et al. New learning following reactivation in the human brain: Targeting emotional memories through rapid serial visual presentation. Neurobiol. Learn. Mem. 2015;119C:63–68. doi: 10.1016/j.nlm.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Schwabe L, Wolf OT. New episodic learning interferes with the reconsolidation of autobiographical memories. PLoS One. 2009;4:e7519. doi: 10.1371/journal.pone.0007519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strange BA, et al. Emotion causes targeted forgetting of established memories. Front. Behav. Neurosci. 2010;4:175. doi: 10.3389/fnbeh.2010.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneider AM, Sherman W. Amnesia: a function of the temporal relation of footshock to electroconvulsive shock. Science. 1968;159:219–221. doi: 10.1126/science.159.3811.219. [DOI] [PubMed] [Google Scholar]
- 49.Kroes MC, et al. An electroconvulsive therapy procedure impairs reconsolidation of episodic memories in humans. Nat. Neurosci. 2014;17:204–206. doi: 10.1038/nn.3609. [DOI] [PubMed] [Google Scholar]
- 50.Bos MG, et al. Stress enhances reconsolidation of declarative memory. Psychoneuroendocrinology. 2014;46:102–113. doi: 10.1016/j.psyneuen.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 51.Coccoz V, et al. The enhancement of reconsolidation with a naturalistic mild stressor improves the expression of a declarative memory in humans. Neuroscience. 2011;185:61–72. doi: 10.1016/j.neuroscience.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 52.Coccoz V, et al. The temporal dynamics of enhancing a human declarative memory during reconsolidation. Neuroscience. 2013;246:397–408. doi: 10.1016/j.neuroscience.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 53.Schwabe L, Wolf OT. Stress impairs the reconsolidation of autobiographical memories. Neurobiol. Learn. Mem. 2010;94:153–157. doi: 10.1016/j.nlm.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Rodríguez ML, et al. Enhancing a declarative memory in humans: the effect of clonazepam on reconsolidation. Neuropharmacology. 2013;64:432–442. doi: 10.1016/j.neuropharm.2012.06.059. [DOI] [PubMed] [Google Scholar]
- 55.Forcato C, et al. Repeated labilization-reconsolidation processes strengthen declarative memory in humans. PLoS One. 2011;6:e23305. doi: 10.1371/journal.pone.0023305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hupbach A, et al. Reconsolidation of episodic memories: a subtle reminder triggers integration of new information. Learn. Mem. 2007;14:47–53. doi: 10.1101/lm.365707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hupbach A, et al. The dynamics of memory: context-dependent updating. Learn. Mem. 2008;15:574–579. doi: 10.1101/lm.1022308. [DOI] [PubMed] [Google Scholar]
- 58.Forcato C, et al. Reconsolidation in humans opens up declarative memory to the entrance of new information. Neurobiol. Learn. Mem. 2010;93:77–84. doi: 10.1016/j.nlm.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 59.Schiller D, et al. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Agren T, et al. M. Disruption of reconsolidation erases a fear memory trace in the human amygdala. Science. 2012;337:1550–1552. doi: 10.1126/science.1223006. [DOI] [PubMed] [Google Scholar]
- 61.Schiller D, et al. Extinction during reconsolidation of threat memory diminishes prefrontal cortex involvement. Proc. Natl. Acad. Sci. U S A. 2013;110:20040–20045. doi: 10.1073/pnas.1320322110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steinfurth EC, et al. Young and old Pavlovian fear memories can be modified with extinction training during reconsolidation in humans. Learn. Mem. 2014;21:338–341. doi: 10.1101/lm.033589.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pare D, Duvarci S. Amygdala microcircuits mediating fear expression and extinction. Curr. Opin. Neurobiol. 2012;22:717–723. doi: 10.1016/j.conb.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr. Opin. Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Loftus EF. Planting misinformation in the human mind: a 30-year investigation of the malleability of memory. Learn. Mem. 2005;12:361–366. doi: 10.1101/lm.94705. [DOI] [PubMed] [Google Scholar]
- 66.Hardt O, et al. A bridge over troubled water: reconsolidation as a link between cognitive and neuroscientific memory research traditions. Annu. Rev. Psychol. 2010;61:141–167. doi: 10.1146/annurev.psych.093008.100455. [DOI] [PubMed] [Google Scholar]
- 67.Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 68.Nitsche MA, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 69.Miniussi C, et al. Modelling non-invasive brain stimulation in cognitive neuroscience. Neurosci. Biobehav. Rev. 2013;37:1702–1712. doi: 10.1016/j.neubiorev.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 70.Sandrini M, et al. The use of transcranial magnetic stimulation in cognitive neuroscience: a new synthesis of methodological issues. Neurosci. Biobehav. Rev. 2011;35:516–536. doi: 10.1016/j.neubiorev.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 71.Censor N, Cohen LG. Using repetitive transcranial magnetic stimulation to study the underlying neural mechanisms of human motor learning and memory. J. Physiol. 2011;589:21–28. doi: 10.1113/jphysiol.2010.198077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sandrini M, Cohen LG. Noninvasive brain stimulation in neurorehabilitation. Handb. Clin. Neurol. 2013;116:499–524. doi: 10.1016/B978-0-444-53497-2.00040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.George MS, et al. Treating the depressions with superficial brain stimulation methods. Handb. Clin. Neurol. 2013;116:399–413. doi: 10.1016/B978-0-444-53497-2.00033-4. [DOI] [PubMed] [Google Scholar]
- 74.Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron. 2011;72:443–454. doi: 10.1016/j.neuron.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sandrini M, et al. Noninvasive stimulation of prefrontal cortex strengthens existing episodic memories and reduces forgetting in the elderly. Front. Aging. Neurosci. 2014;6:289. doi: 10.3389/fnagi.2014.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn. Sci. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- 77.Javadi AH, Walsh V. Transcranial direct current stimulation (tDCS) of the left dorsolateral prefrontal cortex modulates declarative memory. Brain Stimul. 2012;5:231–241. doi: 10.1016/j.brs.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 78.Sandrini M, et al. The role of prefrontal cortex in verbal episodic memory: rTMS evidence. J. Cogn Neurosci. 2003;15:855–861. doi: 10.1162/089892903322370771. [DOI] [PubMed] [Google Scholar]
- 79.Diekelmann S, et al. Labile or stable: opposing consequences for memory when reactivated during waking and sleep. Nat. Neurosci. 2011;14:381–386. doi: 10.1038/nn.2744. [DOI] [PubMed] [Google Scholar]
- 80.Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr. Biol. 2013;23:R764–73. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Manenti R, et al. Enhancing verbal episodic memory in older and young subjects after non-invasive brain stimulation. Front. Aging. Neurosci. 2013;5:49. doi: 10.3389/fnagi.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Glisky EL, et al. Source memory in older adults: An encoding or retrieval problem? J. Exp. Psychol. Learn Mem Cogn. 2001;27:1131–1146. doi: 10.1037//0278-7393.27.5.1131. [DOI] [PubMed] [Google Scholar]
- 83.Jones BJ, et al. Contextual reminders fail to trigger memory reconsolidation in aged rats and aged humans. Neurobiol. Learn. Mem. 2015;120:7–15. doi: 10.1016/j.nlm.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 84.Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiol. Learn. Mem. 2004;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 85.Dickerson BC, Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology. 2010;35:86–104. doi: 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Friston KJ, et al. Functional connectivity: the principal-component analysis of large (PET) data sets. J. Cereb. Blood Flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- 87.Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dinur-Klein L, et al. A. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol. Psychiatry. 2014;76:742–749. doi: 10.1016/j.biopsych.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 89.Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J. Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang YZ, et al. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 91.Jacobson L, et al. tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Exp. Brain Res. 2012;216:1–10. doi: 10.1007/s00221-011-2891-9. [DOI] [PubMed] [Google Scholar]
- 92.Battleday RM, et al. Mapping the mechanisms of transcranial alternating current stimulation: a pathway from network effects to cognition. Front. Psychiatry. 2014;5:162. doi: 10.3389/fpsyt.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thut G, Miniussi C. New insights into rhythmic brain activity from TMS-EEG studies. Trends Cogn. Sci. 2009;13:182–189. doi: 10.1016/j.tics.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 94.Brunet A, et al. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J. Psychiatr. Res. 2008;42:503–506. doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 95.Brunet A, et al. Trauma reactivation under the influence of propranolol decreases posttraumatic stress symptoms and disorder: 3 open-label trials. J. Clin. Psychopharmacol. 2011;31:547–550. doi: 10.1097/JCP.0b013e318222f360. [DOI] [PubMed] [Google Scholar]
- 96.Wood NE, et al. Pharmacological blockade of memory reconsolidation in posttraumatic stress disorder: three negative psychophysiological studies. Psychiatry Res. 2015;225:31–39. doi: 10.1016/j.psychres.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 97.Taylor JR, et al. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology. 2009;1:186–195. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xue YX, et al. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science. 2012;336:241–245. doi: 10.1126/science.1215070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stehberg J, et al. Impairment of aversive memory reconsolidation by localized intracranial electrical stimulation. Eur. J. Neurosci. 2009;29:964–969. doi: 10.1111/j.1460-9568.2009.06634.x. [DOI] [PubMed] [Google Scholar]
- 100.Isserles M, et al. Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder: a pilot study. Brain. Stimul. 2013;6:377–383. doi: 10.1016/j.brs.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 101.Censor N, et al. Common mechanisms of human perceptual and motor learning. Nat. Rev. Neurosci. 2012;13:658–664. doi: 10.1038/nrn3315. [DOI] [PMC free article] [PubMed] [Google Scholar]