Abstract
Background
The number of separable cognitive dimensions in schizophrenia has been debated. Guided by the extant factor analytic literature, the NIMH Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative selected seven cognitive domains relevant to treatment studies in schizophrenia: speed of processing, attention/vigilance, working memory, verbal learning, visual learning, reasoning and problem solving, and social cognition (Nuechterlein et al., 2004). These domains are assessed in the MATRICS Consensus Cognitive Battery (MCCB). The aim of the present study was to conduct a confirmatory factor analysis (CFA) of the beta battery of the MCCB to compare the fit of the MATRICS consensus seven-domain model to other models in the current literature on cognition in schizophrenia.
Methods
Using data from 281 schizophrenia outpatients, we compared the seven correlated factors model with alternative models. Specifically, we compared the seven-factor model to a) a single-factor model, b) a three correlated factors model including speed of processing, working memory, and general cognition, and c) a hierarchical model in which seven first-order factors loaded onto a second-order general cognitive factor.
Results
Multiple fit indices indicated the seven correlated factors model was the best fit for the data and provided significant improvement in model fit beyond the comparison models.
Conclusions
These results support the assessment of these seven cognitive dimensions in clinical trials of interventions to improve cognition in schizophrenia. Because these cognitive factors are separable to some degree, it is plausible that specific interventions may have differential effects on the domains.
Keywords: neurocognition, schizophrenia, confirmatory factor analysis, MCCB
Introduction
Schizophrenia is associated with marked cognitive impairment, with effects in the medium to large range across a variety of cognitive tests (Heinrichs and Zakzanis, 1998, Mesholam-Gately et al., 2009). Various conceptualizations of the underlying or latent structure of cognition in schizophrenia have been proposed in the literature. The models vary with respect to their complexity, and can be broadly categorized as: 1) single-factor models, in which all cognitive tasks load onto a single cognitive factor, 2) correlated factors models, in which a certain number of cognitive domains are modeled as separable but associated factors, and 3) hierarchical models, in which cognitive domains are modeled as loading on a higher-order general cognitive factor.
Identifying the latent structure of cognition in schizophrenia has implications for clinical research. The various models make different assumptions about the associations among cognitive domains and they offer differing conceptualizations about the separability of causal factors in cognitive impairment. In turn, the various models have implications for development of potential treatments to improve cognitive functioning.
The NIMH Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative proposed that seven cognitive domains were relevant to treatment studies in schizophrenia, including: speed of processing, attention/vigilance, working memory, verbal learning, visual learning, reasoning and problem solving, and social cognition (Nuechterlein, et al., 2004). These domains were selected based on a review of existing factor analyses of cognitive performance in schizophrenia patients. The seven domains were conceptualized as separable, but inter-correlated with each other. These cognitive factors are not unique to schizophrenia, as they can be found in other multi-factorial models of cognition in healthy samples (Genderson et al., 2007; Schretlen et al., 2013; Tulsky & Price, 2003). Likewise, although fewer factor analytic studies have been conducted in bipolar disorder, a similar multi-factorial structure of cognition has also been reported in that disorder (Czobor et al., 2007; Schretlen et al., 2013).
The seven cognitive domains are represented by ten tests in the MATRICS Consensus Cognitive Battery (MCCB) (Nuechterlein et al., 2008), but this factor structure was not specifically evaluated with the MCCB. In fact, because five of the seven domains are represented by only one measure each, it is not possible to fully confirm the factor structure using only the final 10-test MCCB. The psychometric study that was used to validate the MCCB included a beta battery with at least two tests per domain. Hence, the beta version can be used to determine whether the underlying structure of cognition can be validly represented by seven factors, or if alternative factor structures better capture the variance in performance.
The number of separable cognitive dimensions in schizophrenia has been debated. Starting with the simplest model, some authors have hypothesized that cognition in schizophrenia is sufficiently represented by a single cognitive factor. A single factor may reflect a generalized deficit in performance across neuropsychological tests that may be attributable to diffuse dysfunction of the central nervous system in schizophrenia (Dickinson & Harvey, 2008). When single factor models have been tested empirically, the conclusions are partly dependent on which statistical method is used. Studies that use principal components analysis indicate that a single component captures a large proportion of the total variance (i.e., both the shared variance and the variance unique to individual cognitive measures) (Keefe et al., 2006, Keefe et al., 2004). However, studies that have tested the single factor model using confirmatory factor analysis (CFA), a technique in which the latent structure of the shared variance is modeled, generally do not support this conceptualization (e.g., (Gladsjo et al., 2004, Ojeda et al., 2012, Schretlen et al., 2013), but see (Keefe et al., 2006)).
In contrast to the single factor model, others have proposed models with multiple correlated factors, and these models vary with respect to the number of factors specified. In healthy samples, speed of processing and working memory factors reliably emerge in factor analytic studies of the WAIS (Arnau & Thompson, 2000). Similarly, these factors emerged in a recent CFA conducted on 9 of the 10 core MCCB tests (Burton et al., 2013). Thus, a potential model of intermediate complexity between the single factor model and the seven-factor model is a three-factor model consisting of speed of processing and working memory factors, as well as a general factor (i.e., the remaining measures).
Hierarchical models include features of the single factor model and multi-factorial models. Specifically, hierarchical models consist of multiple cognitive factors that arise from a single, second-order general cognitive factor. Some have argued that these models can explain cognitive impairment in schizophrenia (Dickinson, Ragland, Calkins, Gold, & Gur, 2006), perhaps reflecting dysfunction of an underlying central mechanism, such as cognitive control, that serves a rate-limiting function for performance across various cognitive domains (Barch and Ceaser, 2012, Braver et al., 1999). Hierarchical models are also consistent with models of intelligence in healthy samples (e.g., combinations of a psychometric “g” as well as separable intellectual factors (Arnau and Thompson, 2000; Carroll, 1993).
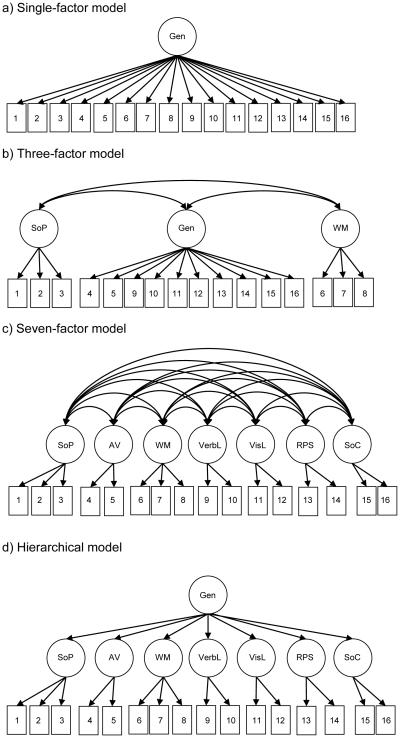
The aim of this paper was to directly compare these competing models of the latent structure of cognition in schizophrenia by conducting CFA of a modified beta version of the MCCB. The modified beta version of MCCB is comprised of 16 tests and has at least two measures for each cognitive domain. Thus, it is suitable for CFA. We compared four possible models (see Figure 1). These include the seven correlated factors model (which was the basis for the MCCB) with alternative models including: 1) a single-factor solution, 2) a three correlated factors solution with processing speed, working memory, and general cognition, and 3) a hierarchical model where the seven MCCB domains load onto a higher-order general cognitive factor.
Figure 1.
Simplified graphical representations of the various confirmatory factor analysis (CFA) models
Note: An error term associated with each observed variable has been omitted for clarity. Gen: General cognitive factor, SoP: Speed of Processing, AV: Attention & Vigilance, WM: Working Memory, VerbL: Verbal Learning, VisL: Visual Learning, RPS: Reasoning and Problem Solving, SoC: Social Cognition, 1: BACS Symbol Coding, 2: Category Fluency, 3: Trail Making Test A, 4: 3-7 Continuous Performance Test, 5: Continuous Performance Test, Identical Pairs, 6: BACS Digit Sequencing, 7: Maryland Letter-Number Span, 8: WAIS-III Spatial Span, 9: Hopkins Verbal Learning Test – Revised, 10: NAB Daily Living Memory, 11: Benton Verbal Memory Test – Revised, 12: NAB Shape Learning, 13: NAB Mazes, 14: WAIS-III Block Design, 15: MSCEIT Managing Emotions, 16: MSCEIT Perceiving Emotions
Method
Participants
A total of 281 patients from two separate samples provided data for these analyses. One hundred five were from the UCLA Aftercare Research Program, an outpatient clinical research program for recent-onset schizophrenia (n=56 with schizophrenia, n=13 with schizoaffective disorder, depressed type, n=36 with schizophreniform disorder). In addition, 176 patients (n=151 with schizophrenia and n=25 with schizoaffective disorder, depressed type) participated in the MATRICS Psychometric and Standardization Study (MATRICS PASS) (Kern et al., 2011, Nuechterlein et al., 2008). The PASS included five academic sites: Duke University, Harvard University, University of Kansas, Maryland Psychiatric Research Center, and UCLA. The mean age of the combined sample was 35.8 (s.d.=14.0) years of age, and the mean level of education was 12.5 (s.d.=2.2) years. The combined sample was 75.8% male, 19.2% were of Hispanic ethnicity, and 29.9% were African American. Additional demographic information can be found in Supplementary Table 1.
Recent-onset patients from the Aftercare Program were all taking oral risperidone at the time of this baseline MCCB assessment (Subotnik et al., in press). The majority of the MATRICS PASS participants were prescribed atypical antipsychotic medications (83%). Informed consent was obtained from all patients using consent forms that were approved by the local universities. Inclusion criteria for patients from both studies were: 1) a DSM-IV (American Psychiatric Association, 1994) diagnosis of schizophreniform, schizophrenia, or schizoaffective disorder, depressed subtype based on SCID interview (First, Spitzer, Gibbon, & Williams, 2001), 2) age 18–65 years (MATRICS PASS) or 18-45 years (Aftercare Program), and 3) clinical stability, indicated by stable outpatient or rehabilitation center status and no medication changes in the month prior to testing. Exclusion criteria were: 1) evidence of a neurological disorder or head injury, and 2) current substance or alcohol abuse or dependence.
Procedure
All participants completed a modified beta version of the MCCB. Aftercare Program participants completed testing as part of a baseline assessment, which typically occurred within three months of program entry. Recruitment and testing procedures for the MATRICS PASS sample are described elsewhere (Nuechterlein et al., 2008). For patients prescribed anti-parkinsonian medication, it was discontinued (if judged clinically feasible) 48 hours prior to testing to avoid anticholinergic effects on cognitive measures. Assessments were conducted by testers trained to certification criteria. Ongoing quality assurance included periodic checks on test administration and scoring practices.
The modified beta version of the MCCB consists of 16 tests assessing seven cognitive domains. A brief description of the domains and associated tests is presented in Table 1. The original beta version of the MCCB included 20 tests that were reduced to 10 tests in the final battery (Nuechterlein et al., 2008). The Aftercare Program assessments included 16 of the 20 tests in the beta version of the MCCB; therefore, they are the tests used in this data analysis. The remaining four beta battery tests were excluded from the Aftercare protocol due to psychometric or practicality concerns, or redundancy with included measures.
Table 1.
Description of MCCB beta version tests and descriptive data (n=281)
| Domain | Test | Dependent Variable | Mean (s.d.) |
|---|---|---|---|
| Speed of Processing |
BACS, Symbol Coding (SC)*
(Keefe, 1999) |
Total number correct | 43.48 (12.28) |
| Category Fluency Test, Animal Naming (Fluency)* (Spreen & Strauss, 1998) |
Total number of animals named in 60 seconds |
18.45 (5.22) | |
| Trail Making, Part A (TMT)*
(Army Individual Test Battery: Manual of Directions and Scoring, 1944) |
Time to completion | 38.66 (15.22) | |
|
| |||
| Attention/ Vigilance |
3-7 CPT (Nuechterlein et al., 1986) |
Overall d’ | 3.94 (1.01) |
| CPT, Identical Pairs (CPT-IP)* (Cornblatt et al., 1988) |
Mean d’ across 2-, 3-, and 4-digit conditions |
2.21 (0.80) | |
|
| |||
| Working Memory |
BACS, Digit Sequencing Test (DS) (Keefe, 1999) |
Total number correct | 17.78 (4.99) |
| Letter-Number Span Test (LNS)*
(Gold et al., 1997) |
Number of correct trials | 12.39 (4.19) | |
| WMS, 3rd ed. Spatial Span (SS)*
(Wechsler, 1987) |
Sum of raw scores, forward and backward |
14.54 (3.95) | |
|
| |||
| Verbal Learning |
Hopkins Verbal Learning Test – Revised (HVLT-R)* (Brandt, 2001) |
Total recall over three learning trials |
21.67 (5.44) |
| NAB, Daily Living Memory (DLM) (White & Stern, 2003) |
Total recall across three trials |
44.41 (11.41) | |
|
| |||
| Visual Learning |
Brief Visual Memory Test – Revised (BVMT-R)* (Benedict, 1997) |
Total recall score over three learning trials |
19.15 (7.93) |
| NAB Shape Learning (SL) (White & Stern, 2003) |
Total learning score over three trials |
14.73 (4.57) | |
|
| |||
| Reasoning & Problem Solving |
NAB Mazes (Mazes)*
(White & Stern, 2003) |
Total raw score | 14.26 (7.14) |
| WAIS – 3rd ed. Block Design (BD) (Wechsler, 1995) |
Total raw score | 32.75 (13.88) | |
|
| |||
| Social Cognition |
MSCEIT, Managing Emotions Branch (ME)* (Mayer et al., 2002) |
Branch score using general consensus scoring |
97.48 (17.30) |
| MSCEIT, Perceiving Emotions Branch (PE) (Mayer et al., 2002) |
Branch score using general consensus scoring |
85.76 (11.08) | |
Note: BACS: Brief Assessment of Cognition in Schizophrenia, CPT: Continuous Performance Test, MSCEIT: Mayer-Salovey-Caruso Emotional Intelligence Test, NAB: Neuropsychological Assessment Battery, WAIS: Wechsler Adult Intelligence Scale, WMS: Wechsler Memory Scale,
included in the final 10-test MCCB.
Data Analysis
Prior to analysis, the data were screened for univariate and multivariate normality. Missing data were minimal (1.27% of data points) and were imputed using the expectation-maximization (EM) algorithm. The MCCB scoring program does not provide age- and gender-corrected T-scores for the additional beta version tasks. Thus, raw scores for all of the tests were standardized (z-score) so that convergence of the statistical model would not be complicated by differences in test variance. These preliminary analyses were conducted using IBM SPSS version 21 (IBM, 2012). CFA was conducted using EQS version 6.2 (Bentler & Wu, 2005). We were particularly interested in a model based on the seven factors (allowed to correlate with each other) that was the basis of the MCCB (Nuechterlein, et al., 2004). We then compared this seven-factor model to three alternative models of varying complexity: a) a single factor model, b) a model of three correlated factors that included speed of processing, working memory, and all remaining tests (i.e., general cognition), and c) a hierarchical model with seven first-order factors that loaded onto a single second-order general factor.
The single-factor and 3-factor models were nested within the seven-factor model: constraining the correlations between all seven factors to 1 is equivalent to the single-factor model, while constraining the correlations between all factors except speed of processing and working memory to 1 is equivalent to the 3-factor model. The hierarchical model was non-nested as it contained an additional second-order general cognitive factor that was not present in any of the other models.
Nested models were evaluated using the chi-square difference test (Δχ2), which determines whether the model fit is significantly changed as a result of adding or removing free parameters from the model (Bollen, 1989). Specifically, the Δχ2 explicitly tests whether the change in χ2 value is meaningful considering the change in df, and a significant Δχ2 statistic favors the model with the lower chi-square value. Non-nested models were compared using parsimony-adjusted information criterion-based fit indices: the Akaike Information Criterion (AIC) and corrected AIC (AICc), and the Bayesian Information Criterion (BIC) and sample-size adjusted BIC (SABIC), in which the model with lower values is favored. All models were evaluated using model χ2 divided by degrees of freedom (χ2/df), in which lower values are preferred, as well as parsimony-adjusted fit indices root mean square error of approximation (RMSEA) and comparative fit index (CFI). For the RMSEA, varying recommendations for cut-points have been suggested, typically ranging from <0.05 to <0.10 (Hooper et al., 2008). For the CFI, values <0.90 suggest that the model could be substantially improved (Bentler & Bonett, 1980). Hu and Bentler (1999) recommend cut-off values close to 0.06 for RMSEA and 0.95 for CFI to reduce the number of misspecified models in the literature.
Results
Descriptive data and bivariate correlations for all modified MCCB beta version tests are presented in Table 1 and Supplementary Table 2.
Results of the CFA, including fit indices for the competing models, are presented in Table 2. The results of the Δχ2 tests for nested models are presented in Table 3. The single-factor solution, where all modified MCCB beta version indicators loaded onto a single general cognitive latent factor, provided a poor fit for the data (see Table 2, “Single-factor”). The single-factor model was then compared to the three correlated factors solution (working memory, speed of processing, and all remaining tests). The three-factor solution yielded a significant increase in model fit over the single-factor solution [Δχ2(3)=61.65, p<0.001], but the fit was still relatively poor (see Table 2, “3-factor”). Next, we compared the three-factor model to the seven correlated factors solution. For this model, latent factors with only two indicators were constrained so that the loadings were equivalent. The seven-factor solution further improved the model fit beyond the three-factor solution [Δχ2(13)=191.48, p<0.001], and was a reasonable fit for the data, as the RMSEA is less than 0.10 and the CFI is greater than 0.90 (see Table 2, “7-factor”; Figure 2). Finally, a hierarchical model, with seven first-order factors loading onto a second-order general cognitive factor was fit to the data. The larger values for the information criterion-based fit indices (i.e., AIC, AICc, BIC, SABIC) for the hierarchical model, along with the poorer fit indices (see Table 2, “Hierarchical”), demonstrate that addition of a general cognitive factor did not yield improved model fit over the seven correlated factors solution.
Table 2.
Confirmatory factor analysis results
| Model |
df (# est. parameters) |
χ 2 | χ2/df | AIC | AICc | BIC | SABIC | CFI | RMSEA [90%CI] |
|---|---|---|---|---|---|---|---|---|---|
| Nested | |||||||||
| Single-factor | 104 (32) | 508.69* | 4.89 | 572.69 | 546.98 | 689.12 | 516.22 | 0.82 | 0.12 [0.11,0.13] |
| 3-factor | 101 (35) | 447.04* | 4.43 | 517.04 | 489.76 | 644.38 | 517.04 | 0.84 | 0.11 [0.10,0.12] |
| Alternative model (5-factor) | 97 (39) | 333.89* | 3.44 | 411.89 | 382.72 | 553.79 | 411.89 | 0.89 | 0.09 [0.08,0.10] |
| 7-factor | 88 (48) | 255.56* | 2.90 | 351.56 | 318.96 | 526.20 | 351.56 | 0.93 | 0.08 [0.07,0.09] |
| Non-Nested | |||||||||
| Hierarchical | 95 (41) | 358.30* | 3.77 | 440.30 | 410.27 | 589.47 | 440.30 | 0.88 | 0.10 [0.09,0.11] |
Note:
p<0.05,
AIC=Akaike Information Criterion, AICc=Corrected Akaike Information Criterion, BIC=Bayesian Information Criterion, SABIC=Sample-size Adjusted Bayesian Information Criterion, CFI=Comparative Fit Index, RMSEA=Root Mean Square Error of Approximation.
Table 3.
χ2 difference tests for nested models
| Model | χ 2 | df | Contrast | Δ χ 2 | Δ df | p |
|---|---|---|---|---|---|---|
| Single-factor | 508.69 | 104 | -- | -- | -- | -- |
| 3-factor | 447.04 | 101 | Single- vs. 3-factor | 61.65 | 3 | <0.001 |
| Alternative model (5-factor) | 333.89 | 97 | 3- vs. 5-factor | 113.15 | 4 | <0.001 |
| 7-factor | 255.56 | 88 | 5- vs. 7-factor | 78.33 | 9 | <0.001 |
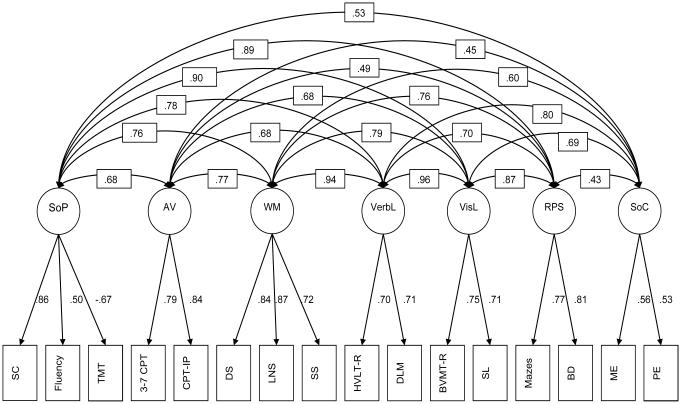
Figure 2.
Seven-factor model, standardized solution
Note: SoP: Speed of Processing, AV: Attention & Vigilance, WM: Working Memory, VerbL: Verbal Learning, VisL: Visual Learning, RPS: Reasoning and Problem Solving, SoC: Social Cognition, SC: BACS Symbol Coding, Fluency: Category Fluency, TMT: Trail Making Test A, 3-7 CPT: 3-7 Continuous Performance Test, CPT-IP: Continuous Performance Test, Identical Pairs, DS: BACS Digit Sequencing, LNS: Maryland Letter-Number Span, SS: WAIS-III Spatial Span, HVLT-R: Hopkins Verbal Learning Test – Revised, DLM: NAB Daily Living Memory, BVMT-R: Benton Verbal Memory Test – Revised, SL: NAB Shape Learning, Mazes: NAB Mazes, BD: WAIS-III Block Design, ME: MSCEIT Managing Emotions, PE: MSCEIT Perceiving Emotions.
For the seven-factor model, high correlations were observed between working memory and verbal learning (r=0.94), and between visual learning and verbal learning (r=0.96), suggesting that these factors may be tapping into facets of a common memory construct. The seven-factor solution was re-specified to a nested model that contained five correlated factors: speed of processing, attention/vigilance, reasoning and problem solving, social cognition, and a memory factor encompassing indicators for working memory (i.e., digit span, letter-number span, spatial span), verbal learning (i.e., HVLT-R, daily living memory), and visual learning (BVMT-R, shape learning). The alternative model provided a reasonable fit (see Table 2, “Alternative model”), however, the seven-factor solution remained a superior fit for the data [Δχ2(9)=78.33, p<0.001].
Discussion
In this paper, we used CFA in a reasonably large sample of patients with schizophrenia to examine the factor structure of a modified beta version of the MCCB in which there were at least two measures per domain. Specifically, we compared four models that have been proposed in the schizophrenia literature and that vary in their complexity. These include: 1) a single-factor model, in which all cognitive tasks represent a single cognitive factor, 2) a correlated factors model in which three cognitive factors were modeled as separable but intercorrelated, 3) a correlated factors model in which seven cognitive domains were modeled as separable but intercorrelated, and 4) a hierarchical model in which seven cognitive domains were modeled as loading on a higher-order general cognitive factor. Our results best support a seven-factor model, which provided a better fit for the data than the other models. Further, collapsing the highly intercorrelated memory factors into a single factor resulted in a significant loss of model fit compared to the seven-factor solution.
These findings support the seven-factor model that guided development of the MCCB. A review of the factor analytic literature led to the inclusion of seven cognitive domains that were relevant to treatment studies in schizophrenia (Nuechterlein, et al., 2004). However, the existing factor analytic literature at that time was limited in that no single study had assessed all of the proposed factors. The factor analytic literature is larger now; however, conclusions about predominant factor structure in schizophrenia still vary.
Similar to previous CFA studies (Gladsjo et al., 2004, Ojeda et al., 2012, Schretlen et al., 2013), the single factor solution provided a poor fit for our data. Although we found clear evidence for a seven-factor solution, other studies have supported models with a fewer number of factors or a hierarchical model. There are several possible reasons for these discrepant findings, including the range of domains assessed and the number of measures included for each domain in a given study. For example, a recent CFA of nine of the ten core MCCB tests supported a three-factor solution that included processing speed, working memory/attention, and learning (Burton et al., 2013). In that study, six of the seven MCCB domains were represented and four of the six domains were assessed with a single test. CFA requires at least two indicators per factor for model identification (Bollen, 1989). In fact, the core MCCB with ten tests and seven factors is not well-suited for CFA without supplementary tests, as five of the seven domains are assessed by a single test.
In some ways, the hierarchical models are most intuitive and fit the common claim that impairment in schizophrenia exists at both the general and specific level. Previous studies supported the hierarchical model of cognition in schizophrenia (Dickinson et al., 2011, Dickinson et al., 2006). However, in the current analyses, the hierarchical model was comparable but not a superior fit for the data relative to the seven-factor model. In the papers that have compared hierarchical models to correlated factors models in schizophrenia, the advantage of hierarchical models was typically slight and inconsistent across reported fit indices (Dickinson et al., 2011, Dickinson et al., 2006). In addition, the discrepant findings may be partly attributable to inclusion of social cognition indicators in the current analyses but not in the previous studies that supported a hierarchical model. For example, although associations among the non-social neurocognitive factors were moderate to large in magnitude, associations between social cognition and the remaining factors were somewhat weaker. This is congruent with the pattern of MCCB intercorrelations reported previously (August, Kiwanuka, McMahon, & Gold, 2012). There is growing evidence surrounding the separability of social and non-social cognition in schizophrenia (Allen et al., 2007, Bell et al., 2009, Hoe et al., 2012, Sergi et al., 2007, Van Hooren et al., 2008, Williams et al., 2008). Thus, the inclusion of tasks assessing social cognition in the current study may have contributed to the reduced fit of the hierarchical model in comparison to the seven-factor model.
One limitation of this study is the number of indicators. Although a minimum of two indicators per factor are required for model identification in CFA, it would be ideal to use three or more indicators per factor (Bollen, 1989). However, in studies of a severely impaired group such as schizophrenia patients, lengthy test batteries are associated with fatigue. In addition, although the current sample size is reasonably large, there were many estimated parameters, and a larger sample might have yielded a more stable solution with improved fit indices. In addition, a larger sample size would permit examination of more complex models (e.g., bifactor models), and consistency of model fit across different sub-groups of patients (e.g., recent-onset versus chronic patients; entire sample versus schizophrenia only). Finally, since our list of alternative models was not exhaustive, it is possible that an untested model may provide a better fit for the data that the seven-factor model.
In conclusion, these analyses provide support for the seven-factor model of cognition in schizophrenia, and support the assessment of these cognitive dimensions in clinical trials of interventions to improve cognition in schizophrenia. Moreover, because these factors are separable to some degree, it is at least plausible that specific neuropharmacological or training-based interventions may impact some domains more than others. Although the 7-factor structure of cognition was evaluated in a large sample with schizophrenia in the current study, there is increasing evidence that similar models of cognitive structure apply to healthy samples and to bipolar disorder. Thus, schizophrenia patients may differ from the healthy population in having substantially poorer level of cognitive performance, while retaining a very similar cognitive structure.
Supplementary Material
Acknowledgements
We thank the patient participants for their help with this study. We gratefully acknowledge the assistance of MATRICS PASS staff and UCLA Aftercare Research Program research assistants and clinical staff.
Financial support
A. McCleery is supported by the Canadian Institutes of Health Research fellowship award (MFE-120919). This work was supported by NIMH research grant MH037705 (K.H. Nuechterlein, PI), Center grant MH066286 (K.H. Nuechterlein, PI), and Contract N01MH22006 (S. Marder, PI; M.F. Green, Co-PI; W. Fenton, Project Officer).
Footnotes
Conflicts of interest
M.F. Green and K.H. Nuechterlein are officers within MATRICS Assessment, Inc., the non-profit publisher of the MCCB, but do not receive any financial remuneration for their respective roles. M.F. Green has been a consultant to AbbVie, DSP, EnVivo, FORUM Pharmaceuticals, and Roche, and he is on the scientific advisory board of Mnemosyne. He has received research funds from Amgen (20130132). J.M. Gold receives royalty payments from The Brief Assessment of Cognition in Schizophrenia (BACS) and has been a consultant to Amgen, Hoffman-LaRoche, Lundback, and Pfizer. R.S. Kern has been an officer for MATRICS Assessment, Inc. and received financial compensation for his role within the non-profit organization. K.L. Subotnik has received research funding from Janssen Scientific Affairs, LLC, and Genentech, Inc, through grants to K.H. Nuechterlein and J. Ventura. K.L. Subotnik is a consultant to Otsuka America Pharmaceutical, Inc. J. Ventura has received funding from Genentech, Inc. (C4-150335). He has served as a consultant to Brain Plasticity, Inc., and Boehringer-Ingelheim GmbH. K.H. Nuechterlein has received research grants from Janssen Scientific Affairs (R092670SCH4005; RIS-NAP-4009), Genentech (ML28264), and Posit Science (BPI-1000-11) and has been a consultant to Otsuka, Janssen Scientific Affairs, and Genentech. All other authors declare they have no conflicts of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- Allen DN, Strauss GP, Donohue B, van Kammen DP. Factor analytic support for social cognition as a separable cognitive domain in schizophrenia. Schizophrenia Research. 2007;93:325–33. doi: 10.1016/j.schres.2007.02.008. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) American Psychiatric Press; Washington, DC: 1994. [Google Scholar]
- Army US. Army Individual Test Battery: Manual of Directions and Scoring. Adjutant General's Office, War Department; Washington, D C: 1944. [Google Scholar]
- Arnau RC, Thompson B. Second-order confirmatory factor analysis of the WAIS-III. Wechsler Adult Intelligence Scale. Assessment. 2000;7:237–46. doi: 10.1177/107319110000700304. [DOI] [PubMed] [Google Scholar]
- August SM, Kiwanuka JN, McMahon RP, Gold JM. The MATRICS Consensus Cognitive Battery (MCCB): clinical and cognitive correlates. Schizophrenia Research. 2012;134:76–82. doi: 10.1016/j.schres.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends in Cognitive Sciences. 2012;16:27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell M, Tsang HWH, Greig TC, Bryson GJ. Neurocognition, social cognition, perceived social discomfort, and vocational outcomes in schizophrenia. Schizophrenia Bulletin. 2009;35:738–747. doi: 10.1093/schbul/sbm169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RHB. Brief Visuospatial Memory Test - Revised: Professional Manual. Psychological Assessment Resources, Inc.; Odessa: 1997. [Google Scholar]
- Bentler PM, Bonett DG. Significance tests and goodness of fit in the analysis of covariance structures. Psychological Bulletin. 1980;88:588–606. [Google Scholar]
- Bentler PM, Wu EJ. EQS 6.1 for Windows. Multivariate Software; Encino, CA: 2005. [Google Scholar]
- Bollen KA. Structural Equations with Latent Variables. John Wiley & Sons; New York: 1989. [Google Scholar]
- Brandt J BR. The Hopkins Verbal Learning Test—Revised: Professional Manual. Psychological Assessment Resources, Inc.; Odessa, FL: 2001. [Google Scholar]
- Braver TS, Barch DM, Cohen JD. Cognition and control in schizophrenia: a computational model of dopamine and prefrontal function. Biological Psychiatry. 1999;46:312–328. doi: 10.1016/s0006-3223(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Burton CZ, Vella L, Harvey PD, Patterson TL, Heaton RK, Twamley EW. Factor structure of the MATRICS Consensus Cognitive Battery (MCCB) in schizophrenia. Schizophrenia Research. 2013;146:244–8. doi: 10.1016/j.schres.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JB. Human cognitive abilities: A survey of factor-analytic studies. Cambridge University Press; 1993. [Google Scholar]
- Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L. The Continuous Performance Test, Identical Pairs Version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatric Research. 1988;26:223–238. doi: 10.1016/0165-1781(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Czobor P, Jaeger J, Berns SM, Gonzalez C, Loftus S. Neuropsychological symptom dimensions in bipolar disorder and schizophrenia. Bipolar Disorders. 2007;9:71–92. doi: 10.1111/j.1399-5618.2007.00428.x. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Goldberg TE, Gold JM, Elvevag B, Weinberger DR. Cognitive factor structure and invariance in people with schizophrenia, their unaffected siblings, and controls. Schizophrenia Bulletin. 2011;37:1157–67. doi: 10.1093/schbul/sbq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Harvey PD. Systemic Hypotheses for Generalized Cognitive Deficits in Schizophrenia: A New Take on An Old Problem. Schizophrenia Bulletin. 2008;35:403–414. doi: 10.1093/schbul/sbn097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Ragland JD, Calkins ME, Gold JM, Gur RC. A comparison of cognitive structure in schizophrenia patients and healthy controls using confirmatory factor analysis. Schizophrenia Research. 2006;85:20–9. doi: 10.1016/j.schres.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P) Biometrics Research Department, New York State Psychiatric Institute; New York: 2001. [Google Scholar]
- Genderson MR, Dickinson D, Diaz-Asper CM, Egan MF, Weinberger DR, Goldberg TE. Factor analysis of neurocognitive tests in a large sample of schizophrenic probands, their siblings, and healthy controls. Schizophrenia Research. 2007;84:231–239. doi: 10.1016/j.schres.2006.12.031. [DOI] [PubMed] [Google Scholar]
- Gladsjo JA, McAdams LA, Palmer BW, Moore DJ, Jeste DV, Heaton RK. A six-factor model of cognition in schizophrenia and related psychotic disorders: Relationships with clinical symptoms and functional capacity. Schizophrenia Bulletin. 2004;30:739–754. doi: 10.1093/oxfordjournals.schbul.a007127. [DOI] [PubMed] [Google Scholar]
- Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Archives of General Psychiatry. 1997;54:159–65. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficits in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12:426–45. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Hoe M, Nakagami E, Green MF, Brekke JS. The causal relationships between neurocognition, social cognition and functional outcome over time in schizophrenia: a latent difference score approach. Psychological Medicine. 2012;42:2287–99. doi: 10.1017/S0033291712000578. [DOI] [PubMed] [Google Scholar]
- Hooper D, Coughlan J, Mullen MR. Structural equation modelling: Guidelines for determining model fit. Electronic Journal of Business Research Methods. 2008;6:53–60. [Google Scholar]
- Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6:1–55. [Google Scholar]
- IBM . IBM SPSS Statistics for Windows, Version 21.0. IBM Corp.; Armonk, NY: 2012. [Google Scholar]
- Keefe RS, Bilder RM, Harvey PD, Davis SM, Palmer BW, Gold JM, Meltzer HY, Green MF, Miller del D, Canive JM, Adler LW, Manschreck TC, Swartz M, Rosenheck R, Perkins DO, Walker TM, Stroup TS, McEvoy JP, Lieberman JA. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology. 2006;31:2033–2046. doi: 10.1038/sj.npp.1301072. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Seidman LJ, Christensen BK, Hamer RM, Sharma T, Sitskoorn MM, Lewine RR, Yurgelun-Todd DA, Gur RC, Tohen M, Tollefson GD, Sanger TM, Lieberman JA. Comparative effect of atypical and conventional antipsychotic drugs on neurocognition in first-episode psychosis: a randomized, double-blind trial of olanzapine versus low doses of haloperidol. Americal Journal of Psychiatry. 2004;161:985–95. doi: 10.1176/appi.ajp.161.6.985. [DOI] [PubMed] [Google Scholar]
- Keefe RSE. Brief Assessment of Cognition in Schizophrenia (BACS) Manual - A: Version 2.1. Duke University Medical Center; Durham, NC: 1999. [Google Scholar]
- Kern RS, Gold JM, Dickinson D, Green MF, Nuechterlein KH, Baade LE, Keefe RS, Mesholam-Gately RI, Seidman LJ, Lee C, Sugar CA, Marder SR. The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophrenia Research. 2011;126:124–31. doi: 10.1016/j.schres.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer JD, Salovey P, Caruso DR. Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT) User's Manual. Multi-Health System Publishers; Toronto: 2002. [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–36. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophrenia Research. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Edell WS, Norris M, Dawson ME. Attentional vulnerability indicators, thought disorder, and negative symptoms. Schizophrenia Bulletin. 1986;12:408–26. doi: 10.1093/schbul/12.3.408. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, 3rd, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR. The MATRICS Consensus Cognitive Battery, part 1: Test selection, reliability, and validity. American Journal of Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Ojeda N, Pena J, Schretlen DJ, Sanchez P, Aretouli E, Elizagarate E, Ezcurra J, Gutierrez M. Hierarchical structure of the cognitive processes in schizophrenia: the fundamental role of processing speed. Schizophrenia Research. 2012;135:72–78. doi: 10.1016/j.schres.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Schretlen DJ, Peña J, Aretouli E, Orue I, Cascella NG, Pearlson GD, Ojeda N. Confirmatory factor analysis reveals a latent cognitive structure common to bipolar disorder, schizophrenia, and normal controls. Bipolar Disorders. 2013;15:422–433. doi: 10.1111/bdi.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergi MJ, Rassovsky Y, Widmark C, Reist C, Erhart S, Braff DL, Marder SR, Green MF. Social cognition in schizophrenia: relationships with neurocognition and negative symptoms. Schizophrenia Research. 2007;90:316–324. doi: 10.1016/j.schres.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests: Administration, norms, and commentary. Oxford University Press; New York: 1998. [Google Scholar]
- Subotnik KL, Casaus LR, Ventura J, Luo JS, Hellemann GS, Gretchen-Doorly D, Marder S, Nuechterlein KH. Efficacy of risperidone long-acting injection after a recent first episode of schizophrenia: Relapse prevention and control of breakthrough symptoms. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2015.0270. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsky DS, Price LR. The joint WAIS-III and WMS-III factor structure: Development and cross-validation of a six-factor model of cognitive functioning. Psychological Assessment. 2003;15:149–162. doi: 10.1037/1040-3590.15.2.149. [DOI] [PubMed] [Google Scholar]
- Van Hooren S, Versmissen D, Janssen I, Myin-Germeys I. Social cognition and neurocognition as independent domains in psychosis. Schizophrenia Research. 2008;103:257–265. doi: 10.1016/j.schres.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale - Revised, manual. Psychological Corporation; New York: 1987. [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale III. The Psychological Corporation; New York: 1995. [Google Scholar]
- White T, Stern RA. Neuropsychological Assessment Battery: Psychometric and technical manual. Psychological Assessment Resources, Inc.; Lutz, FL: 2003. [Google Scholar]
- Williams LM, Whitford TJ, Flynn G, Wong W, Liddell BJ, Silverstein S, Galletly C, Harris AW, Gordon E. General and social cognition in first episode schizophrenia: identification of separable factors and prediction of functional outcome using the IntegNeuro test battery. Schizophrenia Research. 2008;99:182–191. doi: 10.1016/j.schres.2007.10.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.