Abstract
Many behaviors necessary for organism survival are learned anew and become organized as complex sequences of actions. Recent studies suggest that cortico-basal ganglia circuits are important for chunking isolated movements into precise and robust action sequences that permit the achievement of particular goals. During sequence learning many neurons in the basal ganglia develop sequence-related activity - related to the initiation, execution, and termination of sequences - suggesting that action sequences are processed as action units. Corticostriatal plasticity is critical for the crystallization of action sequences, and for the development of sequence-related neural activity. Furthermore, this sequence-related activity is differentially expressed in direct and indirect basal ganglia pathways. These findings have implications for understanding the symptoms associated with movement and psychiatric disorders.
“Action may not always bring happiness; but there is no happiness without action.”
- Benjamin Disraeli (1804–1881)
Animals survive and reproduce by behaving, i.e. by interacting with, adapting to and changing the environment around them. Many of the behaviors animals generate are innate, genetically determined, and pre-wired during development [1]. Although these behaviors may be modulated by an animal’s experience or internal state, they are essentially fixed action patterns produced by either reflex-like stimulus-response circuits [2], or by central pattern generators (CPGs) - intrinsic neuronal networks capable of generating organized motor activity [3,4]. However, we and other animals have the extraordinary capacity of developing novel, sophisticated action skills to communicate with each other, seek resources and build new environments. This amazing ability raises several interesting challenges in neuroscience. One is to understand the mechanisms by which an organism can generate novel actions. Another is to understand how these new actions are organized through experience into precise sequences of movements to produce complex skills that are accurately performed. Still another challenge is to understand how the brain then executes different motor sequences and switches between them, e.g. how each sequence is initiated and terminated in a given situation. Dissecting the molecular and circuit mechanisms underlying these processes will greatly advance our understanding of how neural circuits generate novel complex behavior [5], and hopefully give important insights into a wide range of neurological and psychiatric diseases [6–8].
There is increasing evidence that cortico-basal ganglia circuits, including the mesencephalic dopamine system, play a critical role in generating, shaping, and executing action sequences [9–15]. “Chunking” in psychology was first proposed as a phenomenon to associate a collection of elements together because of the limited ‘channel capacity’ in memory systems [16]. The sequential organization of behavior may result from a sensorimotor form of “chunking”, and has been proposed to include a hierarchical representation of actions [17,18]. Numerous studies in humans, non-human primates, rodents, pigeons, etc, show that something akin to “chunking” takes place during action learning, as revealed by the systematic decrease in response times following the training of motor sequences, and by the increase in precision and accuracy [14,19–22]. The basal ganglia, an ancient set of circuits streaming through a series of interconnected nuclei functionally conserved in virtually all vertebrates [5,23], has been proposed to be critically involved in “chunking” of sequences of actions [9,13,14,24]. In this review, we discuss how basal ganglia circuits contribute to the shaping of action sequences, focusing heavily on recent genetic and physiological studies in mice.
Taming Variability and Shaping Action Sequences
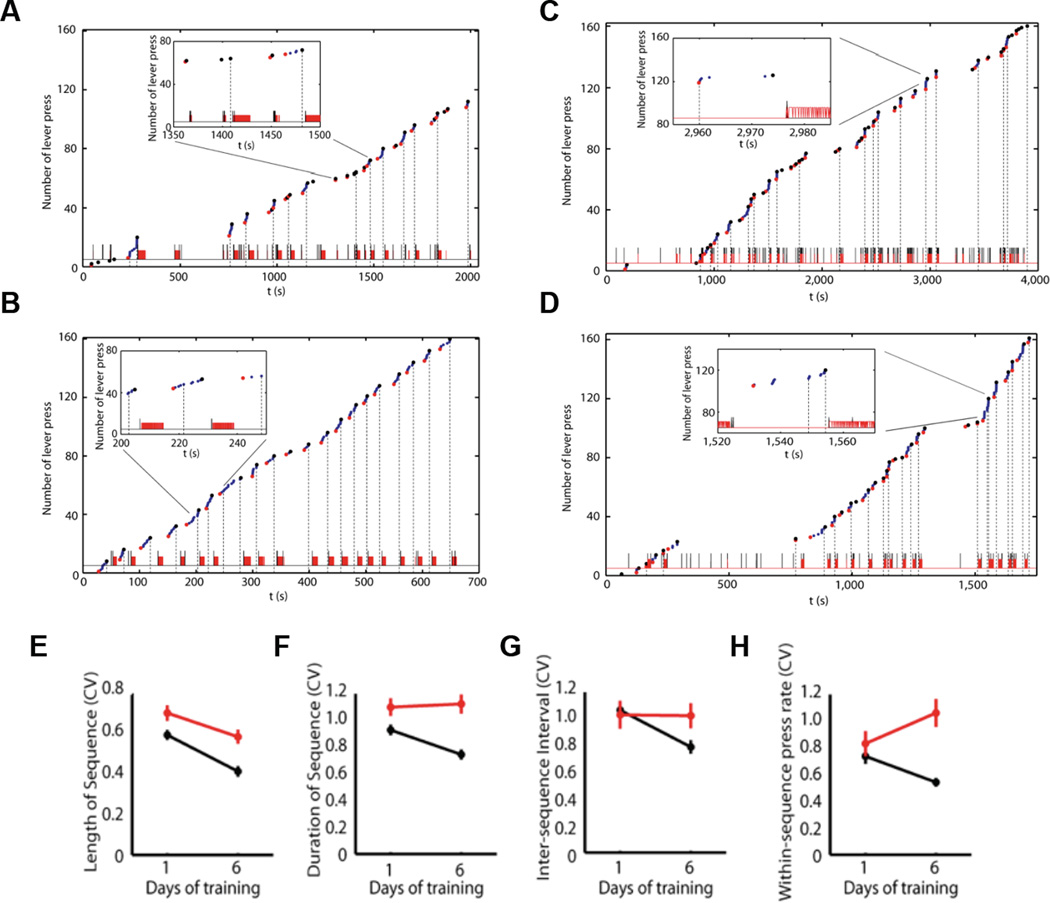
Learning new actions often starts from trying. Selection from variability has been proposed as a general feature contributing to a wide range of biological phenomena, from evolution to gene expression, to development, and behavior and learning [25–28]. Although generation of action variability is essential for new learning, the selection of movements and improvement of motor accuracy, speed, and efficiency as actions are repeated is critical for survival. There are plenty examples of decreased action variability during sequence learning - handwriting and computer keyboard typing are among those experienced by most in everyday life. Songbirds, like humans, crystallize their songs into a precise, stable template after learning [11,29]. This is also evident in monkeys trained to perform a visuomotor sequence task [9,24]. Furthermore, it appears that this decrease in motor variability and organization of behavior as action sequences is commonly observed during skill learning, even if there are no explicit rules dictating that behavior should be organized into sequences[14,19]. Rather it appears that the statistics of the interaction with the environment are essential for the selection of the appropriate elements in a sequence, the order in which they should be executed, the speed, the pauses, eventual division into subsequences, etc [28]. For example, if animals are trained in self-paced operant tasks where they obtain a sucrose reinforcer after pressing a certain number of times (e.g. eight times, with no explicit signal indicating when reward is available. And no specific need of doing theses presses in sequences or bouts) they eventually start pressing in bouts or clusters of presses of about the number of presses needed for reward delivery [14] (Figure 1). With training animals execute the sequences better and faster by decreasing sequence duration and inter-sequence interval (ISI), while increasing within-sequence press rate, indicating that performance speed increased with training. Most importantly though, the within-animal variability (measured for example by coefficient of variation) for all the behavioral features decreases with training (Figure 1), indicating that an individual but robust structure of lever–pressing behavior emerges with training [14]. If the training rule is different, for example with animals being reinforced for faster and faster sequences of presses the variability of the press rate decreases [15].
Figure 1.
Learning-related decrease of behavior variability and crystallization of action sequences depends on striatal NMDA receptors. (A–B) Behavioral microstructure during the first day (A) and last day (B) of self-paced fixed-ratio eight (FR8) sequence training. (C–D) Behavioral microstructure of a striatal NMDA-KO mouse during the first day (C) and last day (D) of self-paced FR8 sequence training. (E–H) Variability of sequence length (E), sequence duration (F), inter-sequence interval (G) and within-sequence press rate (H), measured as coefficient of variation (CV), in control (black) and striatal NMDA-KO mice (red) during training. Adapted from Jin and Costa (2010).
What are the neural mechanism underlying the decrease in variability and increased speed during sequence learning? Again, theoretical and experimental studies have suggested that changes in basal ganglia circuits are critical for the decrease in variability and improvement in performance observed in sequence learning [9,13,14,24,30]. Clinical observations in human patients with basal ganglia disorders, like Parkinson’s and Huntington’s diseases, have also revealed a critical role of basal ganglia in sequence learning [31–33].
A natural prediction from the above findings is that variability in neuronal activity activity patterns in cortico-basal ganglia circuits is critical for the generation of action diversity, and that the decrease in behavioral variability observed during sequence learning may result from a reduction in the variability of neuronal activity patterns in these circuits. Measurements of the variation in neural activity of motor cortex and dorsal striatum ensembles throughout learning of a novel skill strongly supported these predictions [34,35]. It was found that early training there was a large variability in neuronal ensemble activity between trials in both striatum and motor cortex. This variability decreased substantially with training as the skill was consolidated [34,35]. It is interesting to note that this training-related decrease in neuronal ensemble variability occurred in the absence of any detectable changes in average firing rate modulation of the same neuronal ensembles [34], suggesting that coordinated, network-wide plasticity in corticostrital circuits may have taken place. These data suggest that as skills are crystallized there is a selection of particular activity patterns in particular ensembles of cortico-basal ganglia circuits, which form circuits akin to reflexive stimulus-response type circuits, which may contribute to faster and more accurate motor performance after learning.
Sequence Learning and Corticostriatal Plasticity
What mechanisms could mediate the selection of particular activity patterns in specific neuronal ensembles? A somewhat obvious answer would be that synaptic plasticity in cortico-basal ganglia circuits could select some subcircuits/patterns and dismiss others. There is increasing evidence of synaptic plasticity in cortico-basal ganglia circuits during skill or sequence learning [36–39].
In the striatum, learning an operant task where rodents pressed a lever for intracranial self-stimulation induced long-term potentiation of glutamatergic inputs onto striatal medium spiny projection neurons, and the changes in synaptic efficacy were correlated with behavioral learning and performance [36]. It has been also been shown that skill learning on an accelerating rotarod leads to long-term potentiation of glutamatergic inputs onto striatal projection neurons [37]. Additionally, genetic deletion of NMDA receptors in dopaminergic neurons, which selectively disrupts the burst firing of dopamine neurons, resulted in impairments in action learning and formation of habit [40–42].
Is this plasticity of glutamatergic inputs onto striatal projection neurons (SPN) necessary for the reduction in behavioral variability observed during the “chunking” of sequences? Long-term potentiation of glutamatergic inputs onto striatal projection neurons is NMDA receptor-dependent [43,44]. Selective deletion of NMDA receptors in striatal projection neurons [45] severely impairs the ability of mutant animals to consolidate precise behavioral sequences and reduce behavioral variability with training (Figure 1, comparison between mutant and littermate controls) [14]. Importantly, the ability to learn to perform the sequences faster with training was not affected in these mutant mice, indicating that learning to precisely organize behavioral sequences and to perform them faster are dissociable processes [14]. These data suggest that corticostriatal plasticity is necessary to select and consolidate particular motor patterns. This consolidation of particular motor patterns could be subserved by the selection and consolidation of particular neural patterns [28,39]. Consistently, NMDA-dependent corticostriatal plasticity seems to be necessary to select and consolidate particular neural activity patterns in motor cortex of mice performing a closed-loop brain-machine interface paradigm [46].
Still, the selection of particular neuronal patterns underlying the action crystallization process should involve bidirectional modification of synaptic connections within a network, which poses the question of the role of corticostriatal long-term depression (LTD) in sequence learning. Corticostriatal synapses onto SPNs exhibit LTD after high-frequency stimulation of excitatory afferents in vitro [47–49], which depends on the activation of presynaptic CB1 receptors by endocannabinoids [50,51]. Consistently, CB1 knockout mutants do not have LTD at corticostriatal synapses [51]. Interestingly, these mutants are impaired in habit stimulus-response learning [52]. Mice carrying a point mutation in Foxp2 similar to that found in humans of the KE family, which have speech deficits [53], also have impaired LTD in the dorsolateral striatum [54]. These mice are impaired in skill learning [54,55] and display dramatic higher basal striatal firing rate and abnormal plasticity in vivo during skill learning [55]. Interestingly, it was also found that knockdown of Foxp2 in songbird Area X, a region homologous to mammalian basal ganglia, impairs song learning [56]. Consistently, introduction of a humanized version of Foxp2 into the mice also affects skill learning and striatal neuroplasticity [57,58].
These studies underscore the importance of plasticity in cortico-basal ganglia circuits in sequence learning, and suggest that this plasticity is important for the selection of the neuronal activity patterns underlying the shaping of action sequences.
Sequence-Related Neural Activity in Basal Ganglia Circuits
How are learned sequences encoded and executed? Identifying the first and the last elements within a sequence is critical not only in the sensory domain for perceptual recognition [59], but also in the motor domain for behavioral execution. Consolidated or crystallized sequences of movements can be reliably reproduced once activated, much like reflexes or stimulus-response type circuits producing innate actions, but how are these learned action sequences initiated and terminated? Previous studies have reported changes in neuronal activity of basal ganglia neurons, including neurons in the dorsal striatum and the substantia nigra pars reticulata (SNr), during the initiation of natural grooming sequences [60,61]. In agreement, lesions of the dorsal striatum have been shown to disrupt syntactic grooming chains without disrupting constituent movements [62], and lack of SAPAP3 in striatum results in compulsive grooming [63]. Human patients with Parkinson’s disease, which results from the degeneration of dopamine-containing cells in the substantia nigra pars compacta (SNc), or Huntington’s disease, which shows degeneration of projection neurons in the striatum, have profound difficulty in the initiation and termination of voluntary actions, especially for sequential movements [64,65]. These data suggest a crucial role for basal ganglia circuits in the initiation and termination of learned action sequences.
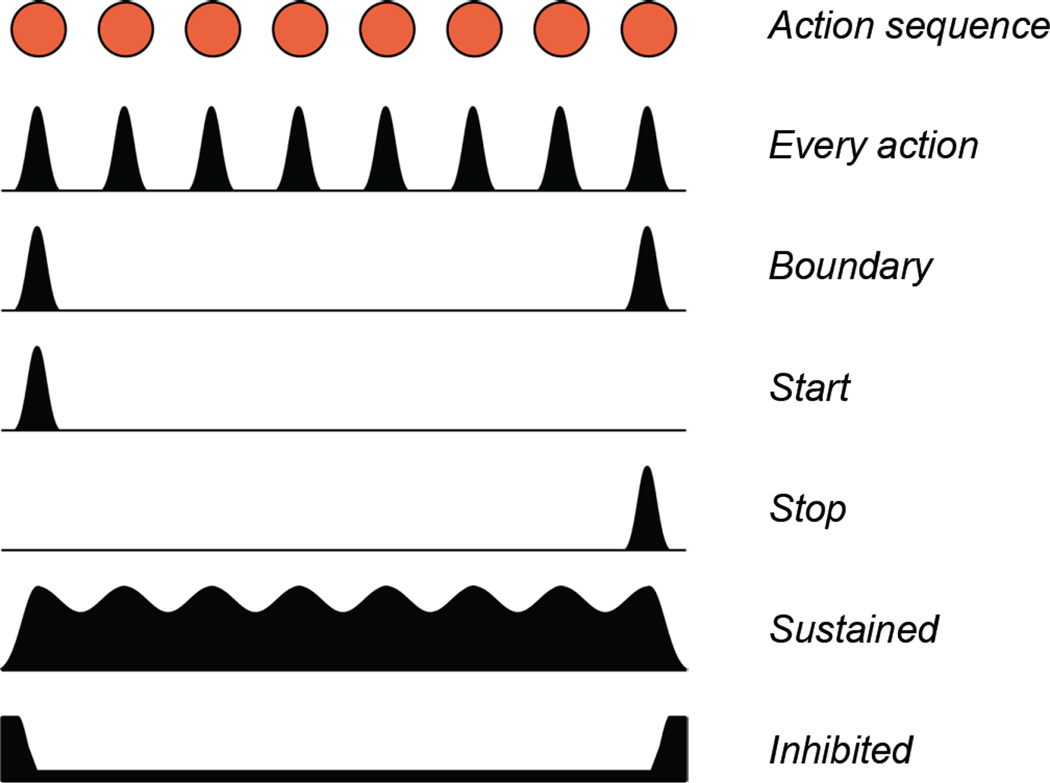
In the striatum, neural activity selectively encoding the initiation or termination of visually-guided repetitive or heterogeneous action sequences has been repeatedly observed [66–68]. Similarly, using a T-maze procedure-learning task in rats, it has been reported striatal neuronal activity exhibits phasic increase during cue-signaled running initiation and goal arrival, marking the boundary of the running procedure by the same striatal cells [69]. Recordings of neuronal activity in nigrostriatal circuits (SNc dopaminergic neurons, striatal projection neurons, and SNr basal ganglia output neurons) while mice developed robust action sequences in a self-paced lever-pressing task revealed that many striatal SPNs, SNr GABAergic and SNc dopaminergic neurons show activity related to every action (lever press) in a sequence (Figure 2) [14]. However, a large proportion of neurons in these three brain areas display phasic changes in activity specifically before the first (start) or around the final (stop) lever press within a lever press sequence. This start/stop activity increases with learning and seems to be dependent on corticostraiatal plasticity, as striatal NR1-KOs develop less start/stop activity compared to control animals [14]. Few neurons in striatum, SNr and SNc displayed activity signaling both the initiation and the termination of the sequence (i.e. sequence boundary) (Figure 2) [14]. Furthermore, start/stop activity in nigrostriatal circuits seems to be specific for particular action sequences, e.g. if a neuron signals initiation of a particular sequence of lever presses, it would likely not signal the initiation of a sequence of presses on a different lever, suggesting they are signaling the specific action of initiating and the specific action of terminating a particular sequence, rather than marking the overall boundary of the sequence [14,70]. This finding contrasts with the fact many striatal neurons were found signaling the boundary (both the beginning and end) of procedural running in a T-maze task [69], but this could be because in a locomotor sequence initiating and terminating locomotion may be more similar actions. This would be consistent with the finding of putative VTA DA neurons exhibited both onset and offset related burst activity during a running wheel locomotion task [71]. Consistent with what was observed in primate cortex [10,72,73], boundary activity was prominent in the primary motor cortex of mice performing a self-paced action sequence task [15]. These data suggest that basal ganglia circuits are more engaged in the specific actions of initiating or terminating a particular sequence (which are different actions), while frontal cortices may have a more abstract representation of sequences with many neurons signaling the boundary of sequences (Figure 2). In addition to start/stop activity, many neurons in basal ganglia circuits display sustained or inhibited activity throughout the whole period execution of the sequence (Figure 2) [15], again indicating that basal ganglia circuits can encode actions sequences as modules or chunks.
Figure 2.
Different types of neural signals during action sequences. There are neurons showing phasic firing modulation during the execution of every element in a sequence (‘Every action’), neurons signaling selectively the initiation (‘Start’), termination (‘Stop’) or both initiation and termination (‘Boundary’) of the action sequence, neurons exhibiting sustained oscillation firing (‘Sustained’) or inhibition (Inhibited) throughout the whole action sequence. See text for detailed references.
Distinct sequence-related activity in direct/indirect pathways
Basal ganglia circuitry comprises two major pathways linking input (striatum) and output (SNr and GPi): a monosynaptic GABAergic projection from dopamine D1 receptor-expressing striatal projection neurons (dSPNs) to the output nuclei including substantia nigra pars reticulata (SNr), called ‘direct pathway’; and a polysynaptic projection from dopamine D2 receptor-expressing striatal projection neurons (iSPNs) to the output nuclei through external globus pallidus (GP) via subthalamic nucleus (STN), called ‘indirect pathway’ [74]. Traditional functional models of basal ganglia suggested that these two pathways work in an antagonistic manner to facilitate and inhibit movements respectively [75,76]. According to this view, movement initiation would relate to activation of direct pathway, while movement inhibition or arrest would relate to activation of indirect pathway [75,76]. Imbalance of activity in the two pathways would lead to many basal ganglia related disorders. For instance, Parkinson’s disease would results from over-excitement of the indirect vs. direct pathway after loss of dopamine, leading to greater inhibition of thalamocortical circuits and development of akinesia [75,76].
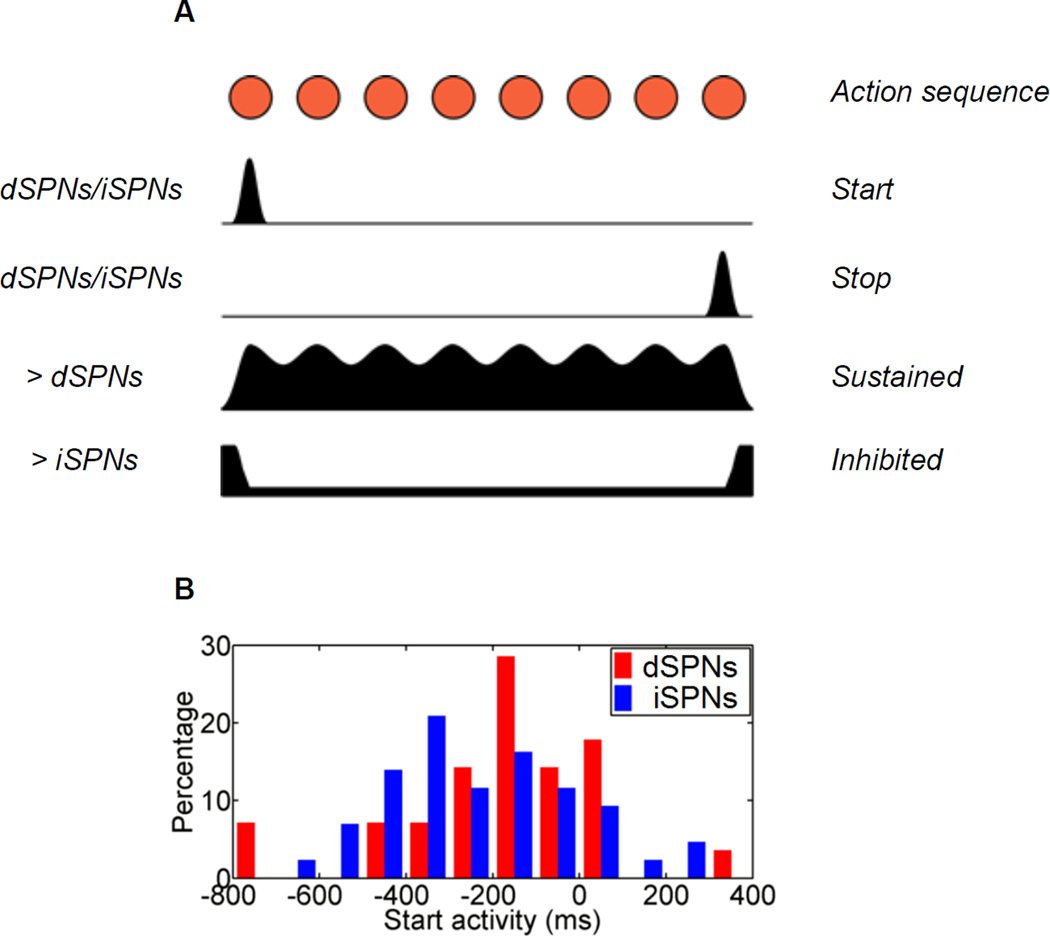
Surprisingly, neural activity recordings using optogenetics-aided cell identification [77] revealed that a similar proportion of striatonigral (dSPNs) and striatopallidal neurons (iSPNs) were phasically active during action initiation. The same was observed for action termination (Figure 3). However, more dSPNs show sequence-related sustained than inhibited activity (Figure 3). Conversely, more iSPNs show sequence-related inhibited activity than sustained. The downstream nuclei of SNr and GPe, which receive GABAergic innervation from striatonigral and striatopallidal neurons, behave in a symmetric way, indicating that the direct and indirect pathways indeed have different activity during the execution of action sequences [15]. These data highlight the diverse and heterogeneous neuronal activity in different striatal cell types during sequence performance, and supports a model advocating complementary but distinct roles of direct and indirect pathway neurons in action selection/execution [15].
Figure 3.
Heterogeneous sequence-related neuronal activity in the direct vs. indirect pathways. (A) dSPNs and iSPNs are co-activated during both sequence initiation (‘Start’) and sequence termination (‘Stop’). There are more D1 neurons exhibited ‘sustained’activity, and more D2 neurons showed ‘inhibited’ activity during sequence execution. (B) Distribution of activation timing for dSPNs and iSPN during sequence initiation. Adapted from Jin et al. (2014).
The neuronal activity observed in the direct and indirect pathways during the performing of action sequences seems partly consistent with the classic models of basal ganglia function, which postulates that direct pathway is prokinetic thus activated during movement while the indirect pathway is antikinetic and would be inhibited [75,76]. Previous studies found that optogenetic stimulation of direct vs. indirect pathway can facilitate and inhibit locomotor behavior respectively [78], which seems in line with the traditional model. However, the striatal neurons in both direct and indirect pathways are activated during action initiation [79,80], and clearly exhibited a concomitant burst during both the initiation and termination of action sequences [15], which echoes the alternative models hypothesizing that balanced activity of direct and indirect pathways is critical for action selection [81,82]. All together these data suggest that direct pathway neurons could function to select the desired motor program, while indirect pathway neurons would inhibit the competing motor programs; co-activation of these pathways would allow the appropriate action selection [81,82]. Such a model is supported by recent findings showing that the iSPNs are really concomitantly activated with dSPNs before action sequence initiation [15] (Figure 3B), and that balanced activity between dSPNs and iSPNs is critical for contraversive movements [83].
Although the diverse types of sequence-initiation, termination and execution related neural activity strongly suggests that the basal ganglia might be sufficient to sequence actions, the existence of neural correlates per se does not permit us to disambiguate between different models of basal ganglia function in sequence learning and execution. Furthermore overall stimulation of direct and indirect pathways would not distinguish between the prokinetic/antikinetic or the action selection models - in either model general stimulation of direct pathway could result in more movements being supported, while general stimulation of the indirect pathway could result in more movements being suppressed. Future work employing better spatiotemporally precise manipulations of neuronal activity in specific cell types should permit the clarification of the role of basal ganglia subcircuits in sequence initiation and performance. Additionally, further work will be needed to investigate how these different types of sequence-related activity in the basal ganglia are generated at the cellular and circuit level, as well as how they are functionally connected with and distinct from the cerebral cortex [10,72,84].
Organization of action sequences
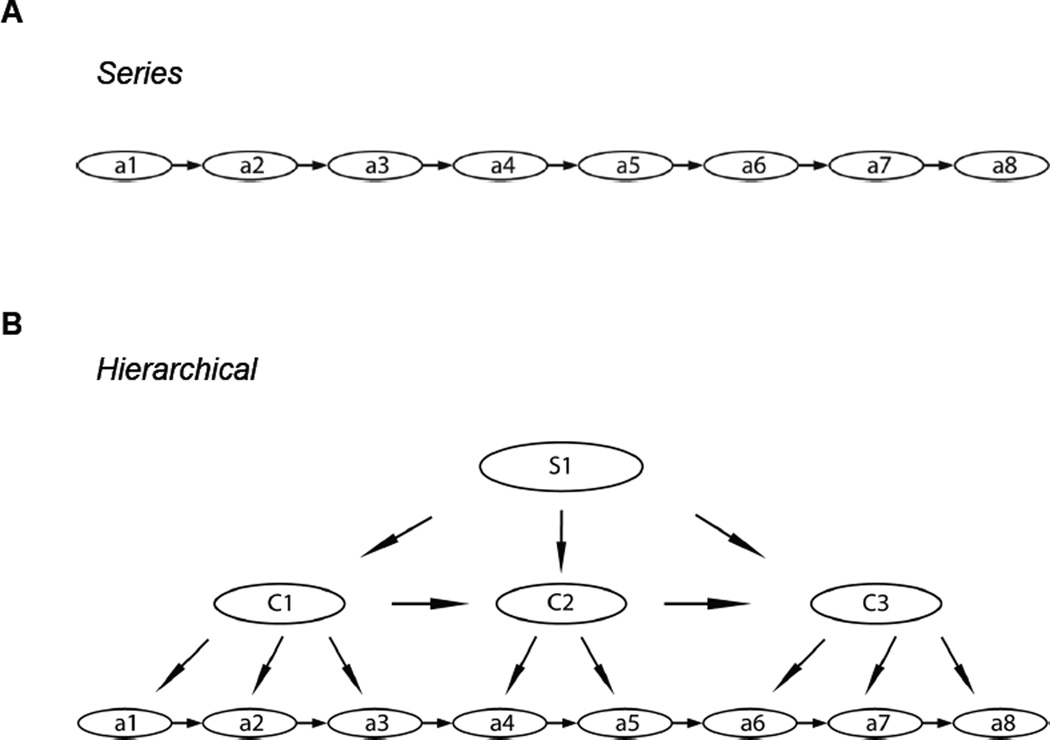
Early psychologists proposed that behavioral sequences were governed by reflex chains, where the activation of the first movement elicits the second, which triggers the third, and so on, based on a stimulation-response type principle [2]. This reflex chain theory predicts that action sequences are organized in a serial manner (Figure 4A). This theory has been called into question by the fact that the neural activity before the first movement of a sequence could sometimes correlate with the order or complexity of the whole sequence and not just the first action. However, recent studies provide evidence for sequential activity in HVC neurons responsible for temporally precise control of birdsong generated through synaptic chains [85], suggesting that the chain model might function at least in certain brain regions. A structure with hierarchical organization has also been proposed for action sequence organization (Figure 4B) [17,86]. According to this view, during skill learning, multiple movement units can be combined into larger units by “chunking”, and multiple chunks can be formed to build up the whole behavioral sequence [13,17,18,86]. Thus, one action sequence may consist of multiple sub-sequences, which in turn can contain multiple sub-sub-sequences. Hierarchical organization of action sequences can afford for example the advantage of error tolerance over serial representations during retrieval or sequence execution. In a hierarchical structure, lower level errors do not necessarily abort the sequence given the ability of the higher levels to access other representations or actions and continue sequence execution. Hierarchical representations also offer savings for re-usage of sub-modules in future action sequences. For example, behavioral studies of Sakai et al. [19] have found that the performance on a shuffled sequence was much slower and less accurate if the sub-sequence (chunk) structure was disrupted rather than preserved from the originally learned sequence. The data reviewed here further supports some type of hierarchical organization since single cortical neurons can signal both the initiation and termination (boundary) of sequences, while different subsets of neurons in the basal ganglia are related to sequence initiation, whole sequence performance, and sequence termination [13–15,17–19,22,86].
Figure 4.
Serial vs. hierarchical models for the organization of action sequences. (A) Taking the FR8 lever pressing task as an example, the serial model proposes that action sequences are governed by reflex-type chains, where the execution of a previous movement elicits the following one and the whole sequence is thus generated based on a stimulus-response type principle. (B) The hierarchical model, however, suggests the action sequences are organized hierarchically as sub-sequences or sub-chunks, which could further consist of elements of sub-sub-sequences, and the higher centers have the capability to access different components in lower levels.
Conclusions
We presented evidence that basal ganglia circuits are involved in the shaping of newly acquired action sequences. The basal ganglia circuits are important for the “chunking” of isolated motor elements into action sequences, and the shaping of behavioral variability which leads to the emergence of complex action sequences as consolidated modules or units of behavior. This behavioral “crystallization” is accompanied by a decrease in trial-to-trial variability of corticostriatal activity, most likely via plasticity in these circuits. Concomitantly, neural activity related to the initiation, execution, and termination of newly learned action sequences emerges in different cell types and pathways in the basal ganglia circuits. The data adds support to a hierarchical model of organization of newly learned action sequences, and offers insights into the mechanisms underlying the sequence learning deficits observed in Parkinson´s and Huntington´s diseases.
Highlights.
-
-
The basal ganglia are critical for chunking motor elements into action sequences
-
-
Consolidation of motor sequences is paralleled by decreases in neural variability
-
-
Neural activity related to action sequences emerges in basal ganglia circuits
-
-
Sequence-related activity is differentially expressed in basal ganglia subcircuits
-
-
Corticostriatal plasticity is critical for neural and behavioral crystallization
Acknowledgements
This work was supported by the US National Institutes of Health Grant NS083815 to X.J and European Research Council Grant 617142 to R.M.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
* Of special interest
- 1.Lorenz K. Evolution and modification of behavior. Chicago, Illinois: University of Chicago Press; 1965. [Google Scholar]
- 2.Sherrington CS. The integrative action of the nervous system. New York: C. Scribner's sons; 1906. [Google Scholar]
- 3.Von Holst E. The behavioural physiology of animals and man: the collected papers of Erich von Holst. Miami, Florida: University of Miami Press; 1973. [Google Scholar]
- 4.Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron. 2006;52:751–766. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Redgrave P, Prescott TJ, Gurney K. The basal ganglia: a vertebrate solution to the selection problem? Neuroscience. 1999;89:1009–1023. doi: 10.1016/s0306-4522(98)00319-4. [DOI] [PubMed] [Google Scholar]
- 6.Lang AE, Lozano AM. Parkinson's disease. Second of two parts. N Engl J Med. 1998;339:1130–1143. doi: 10.1056/NEJM199810153391607. [DOI] [PubMed] [Google Scholar]
- 7.Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28:343–347. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- 8.Crook ZR, Housman D. Huntington's disease: can mice lead the way to treatment? Neuron. 2011;69:423–435. doi: 10.1016/j.neuron.2010.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X, Nakamura K, Miyachi S, Doya K. Parallel neural networks for learning sequential procedures. Trends Neurosci. 1999;22:464–471. doi: 10.1016/s0166-2236(99)01439-3. [DOI] [PubMed] [Google Scholar]
- 10.Tanji J. Sequential organization of multiple movements: involvement of cortical motor areas. Annu Rev Neurosci. 2001;24:631–651. doi: 10.1146/annurev.neuro.24.1.631. [DOI] [PubMed] [Google Scholar]
- 11.Brainard MS, Doupe AJ. What songbirds teach us about learning. Nature. 2002;417:351–358. doi: 10.1038/417351a. [DOI] [PubMed] [Google Scholar]
- 12.Fee MS, Kozhevnikov AA, Hahnloser RH. Neural mechanisms of vocal sequence generation in the songbird. Ann N Y Acad Sci. 2004;1016:153–170. doi: 10.1196/annals.1298.022. [DOI] [PubMed] [Google Scholar]
- 13.Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiol Learn Mem. 1998;70:119–136. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- 14. Jin X, Costa RM. Start/stop signals emerge in nigrostriatal circuits during sequence learning. Nature. 2010;466:457–462. doi: 10.1038/nature09263. *Using a self-paced operant action sequence task in mice this study uncovers that sequence-related start/stop activity emerges in inigrostriatal circuits during sequence learning. Corticostriatal plasticity is necessary for the emergence of this sequence-related activity, and for the crystallization of action sequences.
- 15. Jin X, Tecuapetla F, Costa RM. Basal ganglia subcircuits distinctively encode the parsing and concatenation of action sequences. Nat Neurosci. 2014;17:423–430. doi: 10.1038/nn.3632. ** Using a self-paced lever pressing task this study shows that the various types of sequence-related activity are distinctevly encoded in direct and indirect basal ganglia pathways, and that activity within each basal ganglia pathway is heterogeneous.
- 16.Miller GA. The magical number seven, plus or minus two: some limits on our capacity for processing information. Psychological review. 1956;63:81. [PubMed] [Google Scholar]
- 17.Lashley KS. Cerebral mechanisms in behavior; the Hixon symposium. New York: Wiley; 1951. [Google Scholar]
- 18.Gallistel CR. The Organization of Action: A New Synthesis. Lawrence Erlbaum Associates; 1980. [Google Scholar]
- 19.Sakai K, Kitaguchi K, Hikosaka O. Chunking during human visuomotor sequence learning. Exp Brain Res. 2003;152:229–242. doi: 10.1007/s00221-003-1548-8. [DOI] [PubMed] [Google Scholar]
- 20.Hikosaka O, Rand MK, Miyachi S, Miyashita K. Learning of sequential movements in the monkey: process of learning and retention of memory. J Neurophysiol. 1995;74:1652–1661. doi: 10.1152/jn.1995.74.4.1652. [DOI] [PubMed] [Google Scholar]
- 21.Terrace H. Chunking and serially organized behavior in pigeons, monkeys and humans. Medford, MA: Comparative Cognition Press; 2001. [Google Scholar]
- 22. Wymbs NF, Bassett DS, Mucha PJ, Porter MA, Grafton ST. Differential recruitment of the sensorimotor putamen and frontoparietal cortex during motor chunking in humans. Neuron. 2012;74:936–946. doi: 10.1016/j.neuron.2012.03.038. * An elegantly designed experiment using fMRI in human subjects describes that activity in the sensorimotor putamen is related to sequence concatenation, while the frontoparietal network is related to sequence segmentation.
- 23.Stephenson-Jones M, Samuelsson E, Ericsson J, Robertson B, Grillner S. Evolutionary conservation of the basal ganglia as a common vertebrate mechanism for action selection. Current Biology. 2011;21:1081–1091. doi: 10.1016/j.cub.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Miyachi S, Hikosaka O, Miyashita K, Karadi Z, Rand MK. Differential roles of monkey striatum in learning of sequential hand movement. Exp Brain Res. 1997;115:1–5. doi: 10.1007/pl00005669. [DOI] [PubMed] [Google Scholar]
- 25.Edelman GM. Neural Darwinism: selection and reentrant signaling in higher brain function. Neuron. 1993;10:115–125. doi: 10.1016/0896-6273(93)90304-a. [DOI] [PubMed] [Google Scholar]
- 26.Skinner BF. Selection by consequences. Science. 1981;213:501–504. doi: 10.1126/science.7244649. [DOI] [PubMed] [Google Scholar]
- 27.Thelen E. Motor development. A new synthesis. Am Psychol. 1995;50:79–95. doi: 10.1037//0003-066x.50.2.79. [DOI] [PubMed] [Google Scholar]
- 28.Costa RM. A selectionist account of de novo action learning. Curr Opin Neurobiol. 2011;21:579–586. doi: 10.1016/j.conb.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Tchernichovski O, Mitra PP, Lints T, Nottebohm F. Dynamics of the vocal imitation process: how a zebra finch learns its song. Science. 2001;291:2564–2569. doi: 10.1126/science.1058522. [DOI] [PubMed] [Google Scholar]
- 30.Doya K, Sejnowski TJ. A novel reinforcement model of birdsong vocalization learning. Advances in neural information processing systems. 1995:101–108. [Google Scholar]
- 31.Willingham D, Koroshetz W. Evidence for dissociable motor skills in Huntington’s disease patients. Psychobiology. 1993;21:173–182. [Google Scholar]
- 32.Stefanova ED, Kostic VS, Ziropadja L, Markovic M, Ocic GG. Visuomotor skill learning on serial reaction time task in patients with early Parkinson's disease. Mov Disord. 2000;15:1095–1103. doi: 10.1002/1531-8257(200011)15:6<1095::aid-mds1006>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 33.Boyd LA, Edwards JD, Siengsukon CS, Vidoni ED, Wessel BD, Linsdell MA. Motor sequence chunking is impaired by basal ganglia stroke. Neurobiology of Learning and Memory. 2009;92:35–44. doi: 10.1016/j.nlm.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Costa RM, Cohen D, Nicolelis MA. Differential corticostriatal plasticity during fast and slow motor skill learning in mice. Curr Biol. 2004;14:1124–1134. doi: 10.1016/j.cub.2004.06.053. [DOI] [PubMed] [Google Scholar]
- 35.Barnes TD, Kubota Y, Hu D, Jin DZ, Graybiel AM. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437:1158–1161. doi: 10.1038/nature04053. [DOI] [PubMed] [Google Scholar]
- 36.Reynolds JN, Hyland BI, Wickens JR. A cellular mechanism of reward-related learning. Nature. 2001;413:67–70. doi: 10.1038/35092560. [DOI] [PubMed] [Google Scholar]
- 37.Yin HH, Mulcare SP, Hilario MR, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, Costa RM. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts TF, Tschida KA, Klein ME, Mooney R. Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature. 2010;463:948–952. doi: 10.1038/nature08759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peters AJ, Chen SX, Komiyama T. Emergence of reproducible spatiotemporal activity during motor learning. Nature. 2014;510:263–267. doi: 10.1038/nature13235. ** Using two-photon calcium imaging of layer 2/3 neurons in primary motor cortex of mice performing a forelimb lever-press task, this study shows dynamic activity patterns early in learning that are refined into highly reproducilble spatiotemporal patterns late in learning.
- 40.Engblom D, Bilbao A, Sanchis-Segura C, Dahan L, Perreau-Lenz S, Balland B, Parkitna JR, Lujan R, Halbout B, Mameli M, et al. Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron. 2008;59:497–508. doi: 10.1016/j.neuron.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Zweifel LS, Argilli E, Bonci A, Palmiter RD. Role of NMDA receptors in dopamine neurons for plasticity and addictive behaviors. Neuron. 2008;59:486–496. doi: 10.1016/j.neuron.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang LP, Li F, Wang D, Xie K, Wang D, Shen X, Tsien JZ. NMDA receptors in dopaminergic neurons are crucial for habit learning. Neuron. 2011;72:1055–1066. doi: 10.1016/j.neuron.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calabresi P, Pisani A, Mercuri NB, Bernardi G. Long-term Potentiation in the Striatum is Unmasked by Removing the Voltage-dependent Magnesium Block of NMDA Receptor Channels. Eur J Neurosci. 1992;4:929–935. doi: 10.1111/j.1460-9568.1992.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 44.Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dang MT, Yokoi F, Yin HH, Lovinger DM, Wang Y, Li Y. Disrupted motor learning and long-term synaptic plasticity in mice lacking NMDAR1 in the striatum. Proc Natl Acad Sci U S A. 2006;103:15254–15259. doi: 10.1073/pnas.0601758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Koralek AC, Jin X, Long JD, 2nd, Costa RM, Carmena JM. Corticostriatal plasticity is necessary for learning intentional neuroprosthetic skills. Nature. 2012;483:331–335. doi: 10.1038/nature10845. * Using a novel closed-loop brain-mechine interface paradgim, the study found that corticostriatal plasticity is required for cognitive skill learning, and for the selection of specific coritcal firing patterns.
- 47.Calabresi P, Maj R, Pisani A, Mercuri NB, Bernardi G. Long-term synaptic depression in the striatum: physiological and pharmacological characterization. J Neurosci. 1992;12:4224–4233. doi: 10.1523/JNEUROSCI.12-11-04224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lovinger DM, Tyler EC, Merritt A. Short- and long-term synaptic depression in rat neostriatum. J Neurophysiol. 1993;70:1937–1949. doi: 10.1152/jn.1993.70.5.1937. [DOI] [PubMed] [Google Scholar]
- 49.Walsh JP. Depression of excitatory synaptic input in rat striatal neurons. Brain Res. 1993;608:123–128. doi: 10.1016/0006-8993(93)90782-i. [DOI] [PubMed] [Google Scholar]
- 50.Choi S, Lovinger DM. Decreased probability of neurotransmitter release underlies striatal long-term depression and postnatal development of corticostriatal synapses. Proc Natl Acad Sci U S A. 1997;94:2665–2670. doi: 10.1073/pnas.94.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- 52.Hilário MRF, Clouse E, Yin HH, Costa RM. Endocannabinoid signaling is critical for habit formation. Frontiers in Integrative Neuroscience. 2007;1 doi: 10.3389/neuro.07.006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lai CSL, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519–523. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- 54.Groszer M, Keays DA, Deacon RM, de Bono JP, Prasad-Mulcare S, Gaub S, Baum MG, French CA, Nicod J, Coventry JA, et al. Impaired synaptic plasticity and motor learning in mice with a point mutation implicated in human speech deficits. Curr Biol. 2008;18:354–362. doi: 10.1016/j.cub.2008.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.French CA, Jin X, Campbell TG, Gerfen E, Groszer M, Fisher SE, Costa RM. An aetiological Foxp2 mutation causes aberrant striatal activity and alters plasticity during skill learning. Mol Psychiatry. 2012;17:1077–1085. doi: 10.1038/mp.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haesler S, Rochefort C, Georgi B, Licznerski P, Osten P, Scharff C. Incomplete and inaccurate vocal imitation after knockdown of FoxP2 in songbird basal ganglia nucleus Area X. PLoS Biol. 2007;5:e321. doi: 10.1371/journal.pbio.0050321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Enard W, Gehre S, Hammerschmidt K, Holter SM, Blass T, Somel M, Bruckner MK, Schreiweis C, Winter C, Sohr R, et al. A humanized version of Foxp2 affects cortico-basal ganglia circuits in mice. Cell. 2009;137:961–971. doi: 10.1016/j.cell.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 58.Schreiweis C, Bornschein U, Burguiere E, Kerimoglu C, Schreiter S, Dannemann M, Goyal S, Rea E, French CA, Puliyadi R, et al. Humanized Foxp2 accelerates learning by enhancing transitions from declarative to procedural performance. Proc Natl Acad Sci U S A. 2014;111:14253–14258. doi: 10.1073/pnas.1414542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rawlinson GE. Doctoral Thesis. University of Nottingham; 1976. The Significance of Letter Position in Word Recognition. [Google Scholar]
- 60.Aldridge JW, Berridge KC. Coding of serial order by neostriatal neurons: a "natural action" approach to movement sequence. J Neurosci. 1998;18:2777–2787. doi: 10.1523/JNEUROSCI.18-07-02777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meyer-Luehmann M, Thompson JF, Berridge KC, Aldridge JW. Substantia nigra pars reticulata neurons code initiation of a serial pattern: implications for natural action sequences and sequential disorders. Eur J Neurosci. 2002;16:1599–1608. doi: 10.1046/j.1460-9568.2002.02210.x. [DOI] [PubMed] [Google Scholar]
- 62.Cromwell HC, Berridge KC. Implementation of action sequences by a neostriatal site: a lesion mapping study of grooming syntax. J Neurosci. 1996;16:3444–3458. doi: 10.1523/JNEUROSCI.16-10-03444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, Feliciano C, Chen M, Adams JP, Luo J, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agostino R, Berardelli A, Formica A, Accornero N, Manfredi M. Sequential arm movements in patients with Parkinson's disease, Huntington's disease and dystonia. Brain. 1992;115(Pt 5):1481–1495. doi: 10.1093/brain/115.5.1481. [DOI] [PubMed] [Google Scholar]
- 65.Benecke R, Rothwell JC, Dick JP, Day BL, Marsden CD. Disturbance of sequential movements in patients with Parkinson's disease. Brain. 1987;110(Pt 2):361–379. doi: 10.1093/brain/110.2.361. [DOI] [PubMed] [Google Scholar]
- 66.Kimura M. Behaviorally contingent property of movement-related activity of the primate putamen. J Neurophysiol. 1990;63:1277–1296. doi: 10.1152/jn.1990.63.6.1277. [DOI] [PubMed] [Google Scholar]
- 67.Kermadi I, Joseph JP. Activity in the caudate nucleus of monkey during spatial sequencing. J Neurophysiol. 1995;74:911–933. doi: 10.1152/jn.1995.74.3.911. [DOI] [PubMed] [Google Scholar]
- 68.Miyachi S, Hikosaka O, Lu X. Differential activation of monkey striatal neurons in the early and late stages of procedural learning. Exp Brain Res. 2002;146:122–126. doi: 10.1007/s00221-002-1213-7. [DOI] [PubMed] [Google Scholar]
- 69.Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286:1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- 70.Fujimoto H, Hasegawa T, Watanabe D. Neural coding of syntactic structure in learned vocalizations in the songbird. J Neurosci. 2011;31:10023–10033. doi: 10.1523/JNEUROSCI.1606-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang DV, Tsien JZ. Conjunctive processing of locomotor signals by the ventral tegmental area neuronal population. PLoS One. 2011;6:e16528. doi: 10.1371/journal.pone.0016528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fujii N, Graybiel AM. Representation of action sequence boundaries by macaque prefrontal cortical neurons. Science. 2003;301:1246–1249. doi: 10.1126/science.1086872. [DOI] [PubMed] [Google Scholar]
- 73.Lu X, Ashe J. Anticipatory activity in primary motor cortex codes memorized movement sequences. Neuron. 2005;45:967–973. doi: 10.1016/j.neuron.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 74.Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 75.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 76.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 77.Lima SQ, Hromadka T, Znamenskiy P, Zador AM. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PLoS One. 2009;4:e6099. doi: 10.1371/journal.pone.0006099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. * Uisng optogenetic manipulation of striatal direct and indirect pathways in mice, this study found that stimulation of striatal direct pathway promoted locomotion, while stimulating striatal indirect pathway reduced locomotion.
- 79. Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–242. doi: 10.1038/nature11846. *This study used fiber-optic calcium imaging to show that striatal direct and indirect pathways are concurrently activated during voluntary movement initiation.
- 80. Isomura Y, Takekawa T, Harukuni R, Handa T, Aizawa H, Takada M, Fukai T. Reward-modulated motor information in identified striatum neurons. J Neurosci. 2013;33:10209–10220. doi: 10.1523/JNEUROSCI.0381-13.2013. * Using juxtacellular recordings this study shows the striatal direct and indirect pathways are co-activated during movement initiation.
- 81.Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- 82.Mink JW. The Basal Ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol. 2003;60:1365–1368. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- 83. Tecuapetla F, Matias S, Dugue GP, Mainen ZF, Costa RM. Balanced activity in basal ganglia projection pathways is critical for contraversive movements. Nat Commun. 2014;5:4315. doi: 10.1038/ncomms5315. * Using optogenetic manipulation of striatal direct and indirect pathways in mice, this study found that unilateral optogenetic inhibition of either or both pathways disrupts contraversive movements, while simultaneous activation of both pathways produces contraversive movement, suggesting the balanced activity between the two pathways is critical for contraversive movements.
- 84.Hikosaka O, Miyashita K, Miyachi S, Sakai K, Lu X. Differential roles of the frontal cortex, basal ganglia, and cerebellum in visuomotor sequence learning. Neurobiol Learn Mem. 1998;70:137–149. doi: 10.1006/nlme.1998.3844. [DOI] [PubMed] [Google Scholar]
- 85.Long MA, Jin DZ, Fee MS. Support for a synaptic chain model of neuronal sequence generation. Nature. 2010;468:394–399. doi: 10.1038/nature09514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosenbaum DA, Cohen RG, Jax SA, Weiss DJ, van der Wel R. The problem of serial order in behavior: Lashley's legacy. Hum Mov Sci. 2007;26:525–554. doi: 10.1016/j.humov.2007.04.001. [DOI] [PubMed] [Google Scholar]