Abstract
Inhibition of mitochondrial complex I activity is hypothesized to be one of the major mechanisms responsible for dopaminergic neuron death in Parkinson’s disease. However, loss of complex I activity by systemic deletion of the Ndufs4 gene, one of the subunits comprising complex I, does not cause dopaminergic neuron death in culture. Here we generated mice with conditional Ndufs4 knockout in dopaminergic neurons (Ndufs4 cKO) to examine the effect of complex I inhibition on dopaminergic neuron function and survival during aging and upon MPTP treatment in vivo. Ndufs4 cKO mice did not show enhanced dopaminergic neuron loss in the SNpc or dopamine-dependent motor deficits over the 24-month lifespan. These mice were just as susceptible to MPTP as control mice. However, compared to control mice, Ndufs4 cKO mice exhibited an age-dependent reduction of dopamine in the striatum and increased α-synuclein phosphorylation in dopaminergic neurons of the SNpc. We also utilized an inducible Ndufs4 knockout mouse strain (Ndufs4 iKO) in which Ndufs4 is conditionally deleted in all cells in adult to examine the effect of adult onset, complex I inhibition on MPTP sensitivity of dopaminergic neurons. The Ndufs4 iKO mice exhibited similar sensitivity to MPTP as control littermates. These data suggest that mitochondrial complex I inhibition in dopaminergic neurons does contribute to dopamine loss and the development of α-synuclein pathology. However, it is not sufficient to cause cell- autonomous dopaminergic neuron death during the normal lifespan of mice. Furthermore, mitochondrial complex I inhibition does not underlie MPTP toxicity in vivo in either cell autonomous or non-autonomous manner. These results provide strong evidence that inhibition of mitochondrial complex I activity is not sufficient to cause dopaminergic neuron death during aging nor does it contribute to dopamine neuron toxicity in the MPTP model of Parkinson’s disease. These findings suggest the existence of alternative mechanisms of dopaminergic neuron death independent of mitochondrial complex I inhibition.
Keywords: Parkinson’s disease, mitochondrial complex I, dopamine neuron
1. Introduction
Parkinson’s disease is the second most common aging-related progressive neurodegenerative disorder (Dawson and Dawson 2003, Blesa, Phani et al. 2012, Lee, Dawson et al. 2012, Hirsch, Jenner et al. 2013). It is characterized by motor deficits resulting from loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) of the brain. Patients with Parkinson’s disease also suffer from non-motor symptoms, including impaired cognition and anxiety (Pandya, Kubu et al. 2008, Blonder and Slevin 2011, Lima, Martins et al. 2012, Aarsland, Taylor et al. 2014). Although the mechanisms underlying dopaminergic neuron death are not fully elucidated, inhibition of mitochondrial complex I activity has been one of the leading hypotheses (Abou-Sleiman, Muqit et al. 2006). This hypothesis arose from the observation that drug abusers who were accidently exposed to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) developed Parkinsonism and the discovery that MPP+, the toxic metabolite of MPTP, is a mitochondrial complex I inhibitor (Langston, Ballard et al. 1983, Lang and Blair 1984). Subsequently, reduced complex I activity was found in various tissues of Parkinson's disease patients (Mizuno, Ohta et al. 1989, Parker, Boyson et al. 1989, Schapira, Cooper et al. 1989, Haas, Nasirian et al. 1995). The complex I inhibition hypothesis was further supported by the finding that treatment of rodents with MPTP or rotenone, another well-established complex I inhibitor, induces many key features of Parkinson’s disease (Jackson-Lewis, Jakowec et al. 1995, Przedborski, Jackson-Lewis et al. 1996, Betarbet, Sherer et al. 2000, Sherer, Kim et al. 2003, Liou, Zhou et al. 2005, Inden, Kitamura et al. 2007, Pan-Montojo, Anichtchik et al. 2010, Blesa, Phani et al. 2012). A recent epidemiology study also linked rotenone exposure in humans to increased risk of Parkinson's disease (Tanner, Kamel et al. 2011). Furthermore, loss-of-function mutants of PINK1 are linked to familiar forms of Parkinson’s disease and reduced mitochondrial complex I activity (Morais, Verstreken et al. 2009, Liu, Acin-Perez et al. 2011, Vilain, Esposito et al. 2012, Morais, Haddad et al. 2014).
To test the complex I inhibition hypothesis genetically, we used a mouse strain lacking Ndufs4 in all cells starting from early embryonic development (Kruse, Watt et al. 2008). The Ndufs4 gene encodes an 18 kDa protein, one of the 46 subunits comprising mitochondrial complex I and is required for complete assembly and function of complex I (van den Heuvel, Ruitenbeek et al. 1998, Budde, van den Heuvel et al. 2000, Petruzzella and Papa 2002, Scacco, Petruzzella et al. 2003, Vogel, van den Brand et al. 2007). We reported that systemic deletion of the Ndufs4 gene abolished complex I activity but did not affect the survival of dopaminergic neurons in culture (Choi, Kruse et al. 2008, Choi, Palmiter et al. 2011). The lack of complex I activity also did not render cultured dopaminergic neurons less vulnerable to MPP+ as would be expected if MPTP acted by inhibiting complex I. Although these results does not support the mitochondrial complex I inhibition hypothesis, caution must be taken in the extrapolation of these data because in vitro results do not always reflect what occurs in the intact animal. Furthermore, Parkinson's disease is an aging-related disease. Thus, it is critical to validate the in vitro findings in vivo using aged animals.
The Ndufs4 systemic knockout mice die at postnatal week 7 (Kruse, Watt et al. 2008), precluding its use for in vivo aging studies or for MPTP treatment. In this study, we generated Ndufs4 conditional knockout mice (cKO) by deleting Ndufs4 specifically in dopaminergic neurons. These mice are viable and fertile. We utilized these Ndufs4 cKO mice to investigate the effect of mitochondrial complex I inhibition on spontaneous dopaminergic neuron death during aging as well as on dopaminergic neuron death induced by MPTP. To address the concern of potential developmental compensation that may occur in Ndufs4 cKO mice and possible non-cell autonomous effect of complex I inhibition on MPTP toxicity, we also utilized the Ndufs4 iKO mouse strain to induce Ndufs4 deletion in all adult cells (Quintana, Kruse et al. 2010).
2. Material and Methods
2.1 Generation of Ndufs4 cKO mice
Female Ndufs4loxP/loxP: Gt(ROSA)SorfsLacZ/fsLacZ mice (Kruse, Watt et al. 2008) were crossed with male Slc6a3iCre/+ mice (Turiault, Parnaudeau et al. 2007) in which the codon-improved Cre recombinase gene (iCre) is expressed under the control of the regulatory elements for the dopamine transporter (DAT) gene, encompassed in a Bacterial Artificial Chromosome(Slc6a3). The resulting male Slc6a3iCre/+∷ Ndufs4loxP/+∷Gt(ROSA)SorfsLacZ/+ mice were mated with female Ndufs4loxP/loxP ∷ Gt(ROSA)SorfsLacZ/fsLacZ mice to generate experimental animals (Ndufs4loxP/loxP∷ Gt(ROSA)SorfsLacZ/ fsLacZ ∷ Slc6a3iCre/+; Ndufs4-cKO). Ndufs4loxP/loxP∷Gt(ROSA)SorfsLacZ/fsLacZ littermates were used as controls.
2.2 Generation of Ndufs4 iKO mice
The generation of Ndufs4loxP/loxP: Ubc-Cre-ERt2 mice has been described (Quintana, Kruse et al. 2010). Tamoxifen (Sigma) was prepared fresh daily by dissolving in corn oil (Sigma) and 2% acetic acid (VWR), and orally administered to 12 week-old male mice (250 mg/kg body weight) once daily for 10 days. Tamoxifen-treated Ndufs4loxP/loxP: Ubc-Cre-ERt2 mice were designated Ndufs4 iKO, while similarly treated Ndufs4loxP/loxP littermates were used as controls.
All mice (Ndufs4 cKO, Ndufs4 iKO, and their littermate controls) were on mixed C57Bl/6 genetic background and male mice were used for this study. Mice were housed under standard conditions (12 h light/dark cycle) with food and water provided ad libitum. All experimental procedures were approved by the University of Washington Institutional Animal Care and Use Committee.
2.3 L-Dopa administration
Freshly dissolved L-Dopa (50 mg/kg, Sigma) in phosphate buffered saline (PBS) in 2.5 mg/ml ascorbic acid was administered to two-year-old male mice (i.p.). Benserazide (12.5 mg/kg) in PBS in 2.5 mg/ml ascorbic acid was administered (i.p.) 20 min before L-Dopa injection to prevent peripheral metabolism of L-Dopa.
2.4 MPTP administration
Freshly dissolved 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP, Sigma) was administered once a day for five consecutive days to 8- to10-week-old male Ndufs4 cKO and their control littermates (i.p., 30 mg/kg), or to Ndufs4 iKO and their control littermates (i.p., 25 mg/kg) at 14 days after the last dose of tamoxifen treatment. Animals were sacrificed 14 days after the last MPTP injection. A range of doses of MPTP (20–30mg/kg) have been used in mice in studies reported in the literature (Dietz, Stockhausen et al. 2008, Pinna, Tronci et al. 2010). We performed the MPTP studies in iKO mice before the cKO mice, and chose to use 25 mg/kg MPTP in iKO mice. We selected this dose of MPTP because complex I is inducibly deleted in all tissues of the iKO mice and we were concerned that this may increase the animal’s vulnerability to MPTP toxicity and increase mortality. However, this was not the case and the loss of dopamine neurons in both control and iKO mice was relatively mild using 25 mg/kg MPTP. Thus we increased the MPTP dose to 30 mg/kg MPTP when we performed the experiments later with the cKO mice in order to obtain a bigger loss of dopamine neurons after MPTP treatment.
2.5 Immunohistochemistry
Mice were perfused with heparinized saline followed by 4% paraformaldehyde as previously described (Choi, Abel et al. 2010). Harvested brains were post-fixed in 4% paraformaldehyde overnight and then incubated in PBS with 30% sucrose at 4°C for >2 d until brains sank. Brain sections (40 µm) were incubated with antibody against tyrosine hydroxylase (TH, 1:5,000, Pel-Freez Biologicals, Rogers, AR), (3-galactosidase (1:1000, Promega, Madison, WI), or phospho-a-synuclein (1:1000, Abcam, Cambridge, MA) in PBS containing 0.1% Triton X-100, 5% BSA, and 5% goat serum. Sections were washed and incubated with Alexa Fluor 488 or 568 goat anti-rabbit IgG and Alexa Fluor 488 or 568 goat anti-mouse IgG (1:200; Molecular Probes, Eugene, OR) for 1 h at room temperature.
2.6 Quantification of TH+ neurons in the SNpc
Photomicrographs were captured using a fluorescence microscope equipped with a digital camera (Axiovert 200M, Zeiss, Oberkochen, Germany). TH+ neurons were counted manually blinded to genotype and treatment. Beginning from the first slide of the SNpc section when TH+ neurons were visible, all TH+ neurons were counted on every fourth slide through the entire SNpc in one brain hemisphere. The estimated total number of TH+ neurons in each brain was calculated by multiplying the TH+ cell counts by 8 and presented as the total number of TH+ neurons in the SNpc of each brain. To quantify TH+ neurons that also contain phosphorylated α-synuclein, brain sections were co-stained for TH and phospho-α-synuclein. The numbers of TH+ and phospho-α-synuclein+ neurons were quantified.
2.7 Dopamine measurement
Dissected striatum was frozen on dry ice, stored at −80 °C , and sent to the Neurochemistry Core Laboratory at Vanderbilt University’s Center for Molecular Neuroscience Research to analyze for dopamine content, which was done without prior knowledge of the genotypes. Briefly, brain tissues were homogenized with 0.1 M trichloroacetic acid containing 10 mM sodium acetate/0.1 mM EDTA/1 mM isoproterenol (internal standard)/10.5% methanol, pH 3.8, and centrifuged at 10,000 g for 20 min. Total dopamine content in the supernatant was quantified by HPLC coupled with electrochemical detection (0.7 V). The HPLC system (Antec Leyden, Zoeterwoude, Netherlands) consisted of a 515 HPLC pump, a 717 plus autosampler, an electrochemical detector (Decade II; Antec Leyden), and an HPLC column (150 × 4.6 mm; Nucleosil C18; Phenomenex, Torrance, CA). The homogenization buffer was used as the mobile phase (0.7 ml/min), and 20 µl of the sample was injected in into an HPLC column (3.9 × 300 mm; Nova-Pak C18, Waters Milford, MA). Dopamine content was normalized to the protein concentration in each tissue.
2.8 Preparation of DAT− and DAT+ dopaminergic synaptosomes
Total synaptosomes were prepared from the striatum using a synaptic vesicle isolation kit (SV0100, Sigma, St. Louis, MO) and used to enrich DAT+ dopaminergic synaptosomes. Briefly, the striatum was dissected out and homogenized in 1 ml ice-cold homogenization buffer. The homogenate was centrifuged at 800 x g for 10 min at 4 °C. The supernatant was collected and stored on ice. The pellet was re-suspended in 1ml of homogenization buffer and centrifuged at 800 x g for 10 min at 4 °C. The supernatant was combined with the first supernatant and centrifuged at 20,000 x g for 20 min at 4 °C. The pellet, which is the total synaptosome preparation, was used to separate the DAT+ synaptosomes from the DAT− synaptosomes by magnetic activated cell sorting (MACS) per manufacturer’s instruction (Miltenyi Biotec, Bergisch Gladbach, Germany). Briefly, the total synaptosome pellet was re-suspended in buffer A, and incubated with an antibody against DAT protein (15 µg of rabbit anti-DAT antibody/each striatum lysate, Alpha Diagnostic, San Antonio, TX) for two hours and then washed twice with buffer A (Miltenyi Biotec). After centrifugation at 10,000 x g for 2 min, the pellet was resuspended in 500 µl buffer A, then incubated with magnetic beads conjugated with anti-rabbit secondary antibody (15 µl antibody/each striatum sample, Miltenyi Biotec) for one hour and washed once with buffer A. The pellet was resuspended in MACS buffer and applied onto the pre-washed magnetic column (Miltenyi Biotec). The column was washed with MACS buffer (900 µl) and the flow-through was re-applied to the column. The second flow-through was saved as DAT− fraction. The column was washed with MACS buffer (600 µl) again and then removed from the MACS stand. DAT+ synaptosomes were eluted from the column; the elutes were directly applied to the polarography assay or centrifuged at 10,000 g for 10 min and the final pellet (DAT+ fraction) was stored at - 80 °C till use.
2.9 Western analysis
For Ndufs4 protein expression in Ndufs4 cKO mice, protein lysates were prepared from DAT− (non-dopaminergic) or DAT+ (dopaminergic) synaptosomes and analyzed using anti-Ndufs4 antibody (MitoSciences, Eugene, OR). Total brain protein lysates were used for Ndufs4 protein expression in Ndufs4 iKO and control mice. Total protein lysates prepared from mouse SNpc were used for anti-phospho-α-synuclein Western blotting. Anti-β-actin was used a protein loading control.
2.10 Open field test
The open field test was performed as described to measure locomotor activity (Pan, Chan et al. 2012). A TruScan Photo Beam Tracking arena (Coulbourn, Whitehall, PA) was used for the test and TruScan 2.02 software (Coulbourn) was used for data analysis. Exploratory activity was analyzed by experimenters without the knowledge of the genotypes or treatment.
2.11 Rotarod test
Rotarod test was performed as described (Choi, Abel et al. 2010). Mice were placed on the stationary cylinder of the rotarod apparatus (San Diego Instruments, San Diego, CA) and habituated on the apparatus for at least four consecutive trials in which the rod was kept at constant speed (4 rpm). Once the animals were able to stay on the rod rotating at 4 rpm for at least 60 s, they were subjected to the rotarod test. Mice were placed individually on the rod rotating at an accelerating speed from 4 to 29 rpm in 300 s. The time before animals fell off the rod was recorded with a maximum cut-off of 300 s. Mice were tested for eight consecutive trials with at least 5-min intervals. The data from the last four trials were averaged as the latency to fall.
2.12 Catwalk assay
Catwalk assay was performed for gait analysis using Noldus system (Wageningen, Netherlands) as previously reported (Kopecky, Decook et al. 2012). Mice were placed at the end of a corridor and allowed to move freely. A walkway floor of the Noldus system contains a panel of glass enclosed with a fluorescent light. Once mice press on the surface of the glass, the light changes the dynamic property of the glass and illuminates in proportion to the intensity of pressure. A camera tracks a 40 cm × 10 cm field from below the walkway. As the mouse enters the field, a run is started and recorded on a computer. If the mouse did not stop or change direction, and exited the field of view within 25 s with less than a 60% variation in speed, the run was considered compliant. Three compliant runs were acquired for each mouse, and the mean was calculated using Noldus software per manufacturer’s recommendation. Between each trial, the glass of the walkway was cleaned. After each trial, the acquired data were classified to decide which limb induced the captured footprint.
2.13 Isolation of Purified Mitochondria
Mitochondria was isolated and purified as described (Fraker, Timmins et al. 2006). In brief, brain tissue was homogenized using a Dounce homogenizer with 10 up-and-down strokes in isolation buffer (225 mM mannitol, 75 mM sucrose, 1 mM EGTA, 5 mM Hepes (pH 7.3), and 2 mg/ml fat-free BSA). The homogenate was centrifuged at 1,000g for 10 min. The supernatant was transferred onto a layered Ficoll gradient (5 ml of 7.5% Ficoll medium on top of 5 ml of 10% Ficoll medium containing 0.3 M sucrose, 50 µM EGTA, and 10 mM Hepes) and centrifuged at 79,000 g for 30 min. The resulting mitochondrial pellet was resuspended in isolation buffer (without BSA), and the protein concentrations were assayed using the bicinchoninic assay (Pierce) with BSA standards.
2.14 Polarography
Monitoring of mitochondrial oxygen consumption was performed as described (Fraker, Timmins et al. 2006). Briefly, freshly purified mitochondria preparations (for assays of Ndufs4 iKO mice) or DAT+ synaptosomes (for assays of Ndufs4 cKO mice) were placed in respiration buffer (pH 7.3) consisting of 225 mM mannitol, 75 mM sucrose, 10 mM KCl, 5 mM Hepes, 5 mM K2HPO4, and freshly added 1 mg/ml defatted BSA at 30°C. O xygen consumption was measured in a 3-ml vessel of an oxygen monitoring apparatus (5300A system; YSI). Mitochondrial complex I activity was defined as the rotenone (1.25 µM) sensitive oxygen consumption rate in the presence of complex I-specific substrates (10 mM pyruvate /2 mM malate/1 mM ADP). State 2 or state 3 respiration was initiated by the addition of 10 mM pyruvate/2 mM malate or 1 mM ADP, respectively. The amount of oxygen consumed was calculated by assuming the initial oxygen concentration in the buffer to be 0.223 µmol O2/ml.
2.15 Statistical analysis
All data are expressed as mean ± standard error of the means (s.e.m.). Statistical analysis of data was performed using Student’s t-test within the same age group or treatment group, and one-Way ANOVA for life-span analysis. n.s. not significant; *, p < 0.05; **, p < 0.01; ***, p <0.001.
3. Results
3.1 Generation of mice with loss of Ndufs4 selectively in dopaminergic neurons
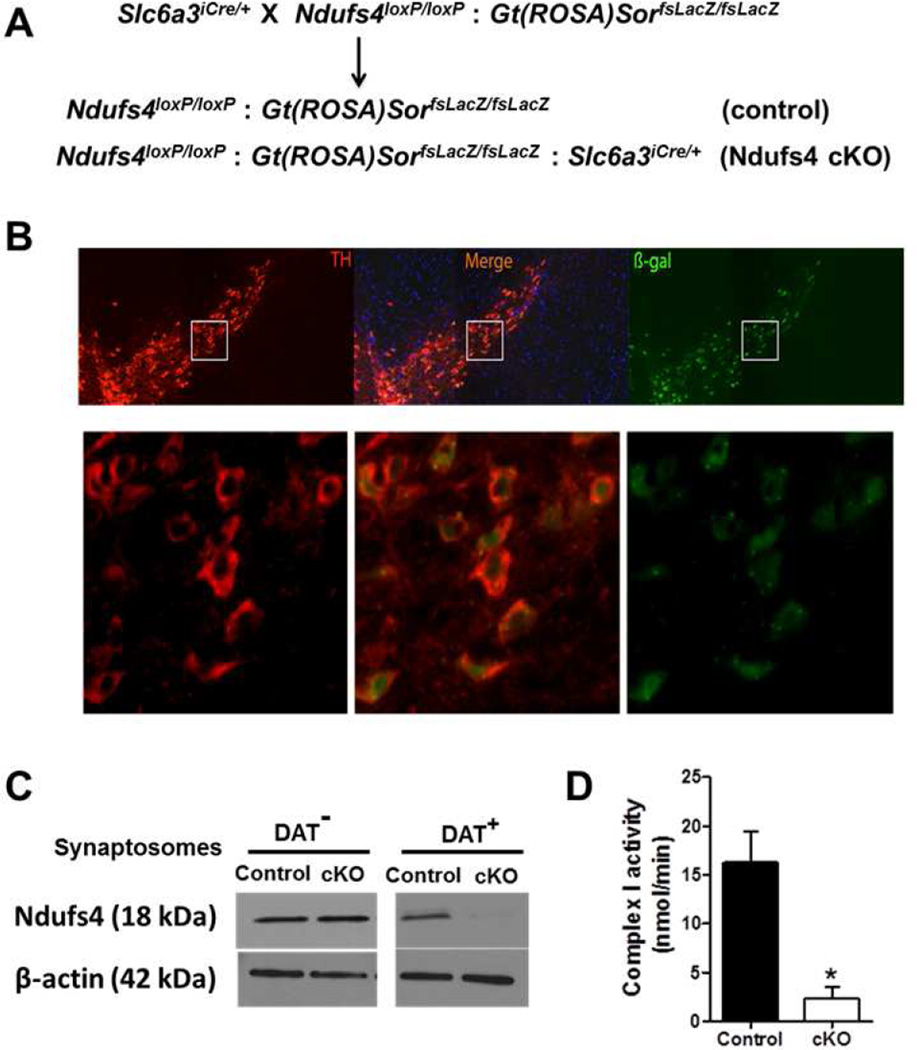
To achieve selective inhibition of mitochondrial complex I in dopaminergic neurons, we generated mice in which the Ndufs4 gene was selectivity inactivated in dopaminergic neurons. This was accomplished by crossing mice with a conditional Ndufs4 gene with mice in which Cre recombinase was expressed from the dopamine transporter (DAT) locus, Slc6a3 (Turiault, Parnaudeau et al. 2007) as described in Materials and Methods (Fig. 1A). The resulting Ndufs4 cKO mice also carried a conditional lacZ gene (Soriano 1999) that expresses β-galactosidase in a Cre-dependent manner to facilitate the detection of recombination events in vivo. Littermates with conditional Ndufs4 and LacZ genes were used as controls. The effectiveness and specificity of Cre-mediated recombination in dopaminergic neurons was confirmed by β-galactosidase expression from the Gt(ROSA)SorfsLacZ/ fsLacZ locus (Fig. 1B). We enriched dopaminergic synaptosomes from total synaptosomes prepared from the striatum by magnetic-activated cell sorting (MACS) after labeling with an antibody against DAT. While similar amounts of Ndufs4 protein were present in DAT-negative synaptosomes prepared from both control and Ndufs4 cKO mice, there was no detectable Ndufs4 protein in the dopaminergic DAT-positive synaptosomes prepared from Ndufs4 cKO mice (Fig. 1C). Purified DAT+ synaptosomes from the brains of Ndufs4 cKO mice showed 84% loss of complex I activity, measured by complex I inhibitor-sensitive oxygen consumption rate using the polarography assay (Fig. 1D). These data confirm conditional Ndufs4 deletion in dopaminergic neurons of Ndufs4 cKO mice. We previously reported that when the same Ndufs4loxP/loxP mice were crossed with Mox2-Cre driver mice to delete the Ndufs4 gene in all cells, there was no detectable complex I activity in intact cultured neurons, or in purified mitochondria prepared from either cultured neurons or from the brain, although crude mitochondria preparations from the brain or chopped brain tissues retained approximately 35% of normal complex I activity (Choi, Kruse et al. 2008). Together with the reports that mutation in the human Ndufs4 gene inhibit complex I activity (van den Heuvel, Ruitenbeek et al. 1998, Budde, van den Heuvel et al. 2000, Petruzzella, Vergari et al. 2001, Scacco, Petruzzella et al. 2003), we infer that mitochondrial complex I is inhibited in dopaminergic neurons of Ndufs4 cKO mice.
Figure 1. Generation of transgenic mice with targeted ndufs4 gene deletion in dopaminergic neurons.
(A) Breeding scheme for generating Ndufs4 cKO mice in which the Ndufs4 gene is conditionally deleted in dopaminergic neurons. (B) Representative fluorescent immunostaining images of the SNpc regions from Ndufs4 cKO mouse brain. Bottom panels are higher magnification images of the corresponding boxed areas. TH: tyrosine hydroxylase (red color); β-gal: β galactosidase (green color). (C) Western blotting analysis demonstrating that Ndufs4 protein is expressed in dopaminergic synaptosomes (DAT+) prepared from control but not Ndufs4 cKO mice. Non-dopaminergic synaptosomes (DAT) were used as controls. β-actin was used as a protein loading control. Similar results were obtained from three independent experiments. (D) Complex I activity in purified DAT+ synaptosomes from the striatums of from control or Ndufs4 cKO mouse brains was measured by the polarography method.
3.2 Ndufs4 cKO mice have reduced dopamine but dopaminergic neurons survive
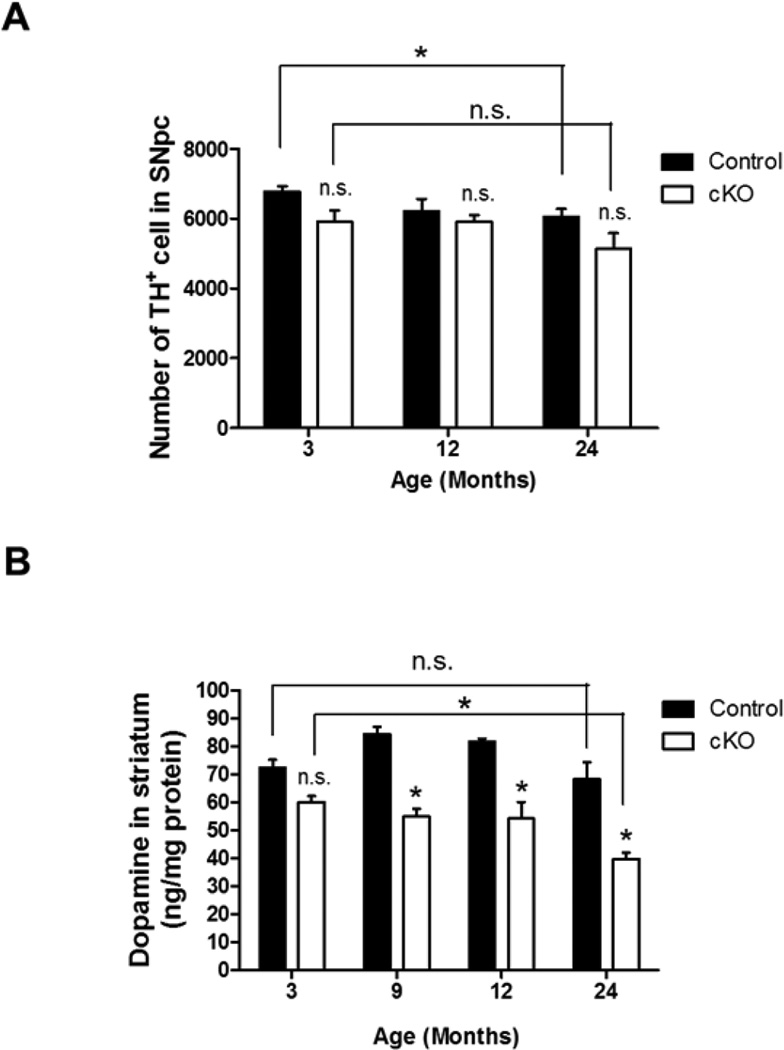
The Ndufs4 cKO mice developed normally and were fertile. Interestingly, there was no significant difference between the number of dopaminergic neurons, identified as tyrosine hydroxylase (TH)-positive cells, in the SNpc of control compared to Ndufs4 cKO mice at ages of 3, 12, or 24 months (Fig. 2A). Although there was a slight age-related decline in the number of TH+ neurons in both groups, Ndufs4 deletion did not accelerate this process. However, there was a significant age-dependent reduction in dopamine content in the striatum of Ndufs4 cKO mice compared to control littermates starting from 9 months (36% at 9 months, 42% at 24 months) (Fig. 2B). Ndufs4 cKO, but not control mice, showed a 34% reduction in striatal dopamine at 24 months of age compared to 3 months. These data suggest that complex I inhibition in dopaminergic neurons by itself is not sufficient to cause dopaminergic neuron death during the normal lifespan of mice although it does induce age-dependent dopamine depletion.
Figure 2. Effect of Ndufs4 deletion on the number of dopaminergic neurons in the SNpc and dopamine content in the striatum during aging.
(A) Number of TH+ cells in the SNpc of 3-, 12-, and 24-month-old control or cKO mice. n = 6 mice/genotype. (B) Aging-related decrease of dopamine content in the striatum of cKO mice. n = 3 mice/genotype. *, p < 0.05; n.s. not statistically significant.
3.3 Dopaminergic neuron-specific complex I inhibition does not cause dopamine-dependent motor deficits during aging
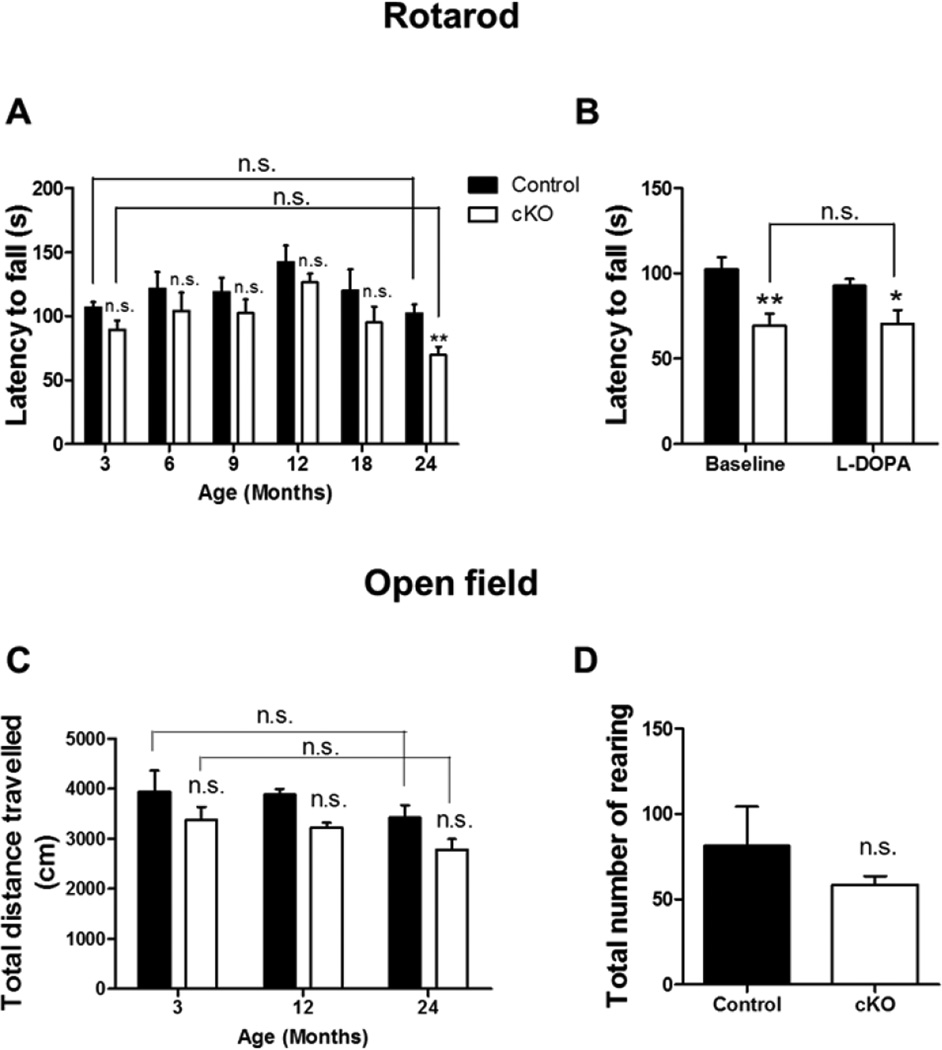
To explore the effect of complex I inhibition on motor activity, the same cohort of control and Ndufs4 cKO mice were subjected to motor behavioral tests at different ages. Both groups of mice had a similar tendency to fall in the rotarod test performed between 3 to 18 months of age (Fig. 3A). Although 24-month-old Ndufs4 cKO mice had a shorter latency to fall than control mice, this deficiency was not corrected by L-DOPA (Fig. 3B). Furthermore, the Ndufs4 cKO mice did not show a statistically significant difference in the latency to fall between 3- and 24-months of age despite a 34% reduction in striatal dopamine at 24 months. Thus, the changes in latency to fall in 24-month-old Ndufs4 cKO mice are unlikely due to reduced dopamine.
Figure 3. Age-related changes in motor activity in control and Ndufs4 cKO mice.
(A) Latency to fall in accelerated rotarod test during a 2-year lifespan. The same cohort of mice was tested at different ages as indicated. (B) Latency to fall in 2-year-old control and cKO mice treated with or without L-Dopa. (C) Locomotor activity in open field test, quantified as total distance traveled. (D) Total number of rearing in the open field test with 2-year-old mice. n = 6 mice/genotype; *, p < 0.05, **, p <0.01; n.s. not statistically significant.
Ndufs4 cKO mice behaved similarly to control mice in the open field test, including the total distance traveled (Fig. 3C) and the total number of rearing (Fig. 3D), from the ages of 3 to 24 months. Average speed and moving time also did not show any difference (data not shown). We also performed the catwalk assay with 24-month-old mice. Both control and Ndufs4 cKO mice showed similar performance in the majority of the parameters monitored, including maximum contact area, maximum contact intensity, minimum contact intensity, mean contact intensity, print length, print width, print area, and stride length, with only minor differences in the swing speed of front forepaws (Fig. 4). These results suggest that conditional complex I inhibition in dopaminergic neurons does not cause dopamine-dependent motor deficits during aging.
Figure 4. Catwalk assay to evaluate locomotion of 24-month-old control and Ndufs4 cKO mice.
(A) Maximum contact area; (B) maximum contact intensity; (C) minimum contact intensity; (D) mean contact intensity; (E) print length; (F) print width; (G) print area; (H) stride length; (I) swing speed. RF, right forepaw; RH, right hind paw; LF, left forepaw; LH, left hind paw. n = 6 mice/genotype; *, p < 0.05; n.s. not statistically significant.
3.4 Conditional Ndufs4 deletion enhances phospho-α-synuclein accumulation
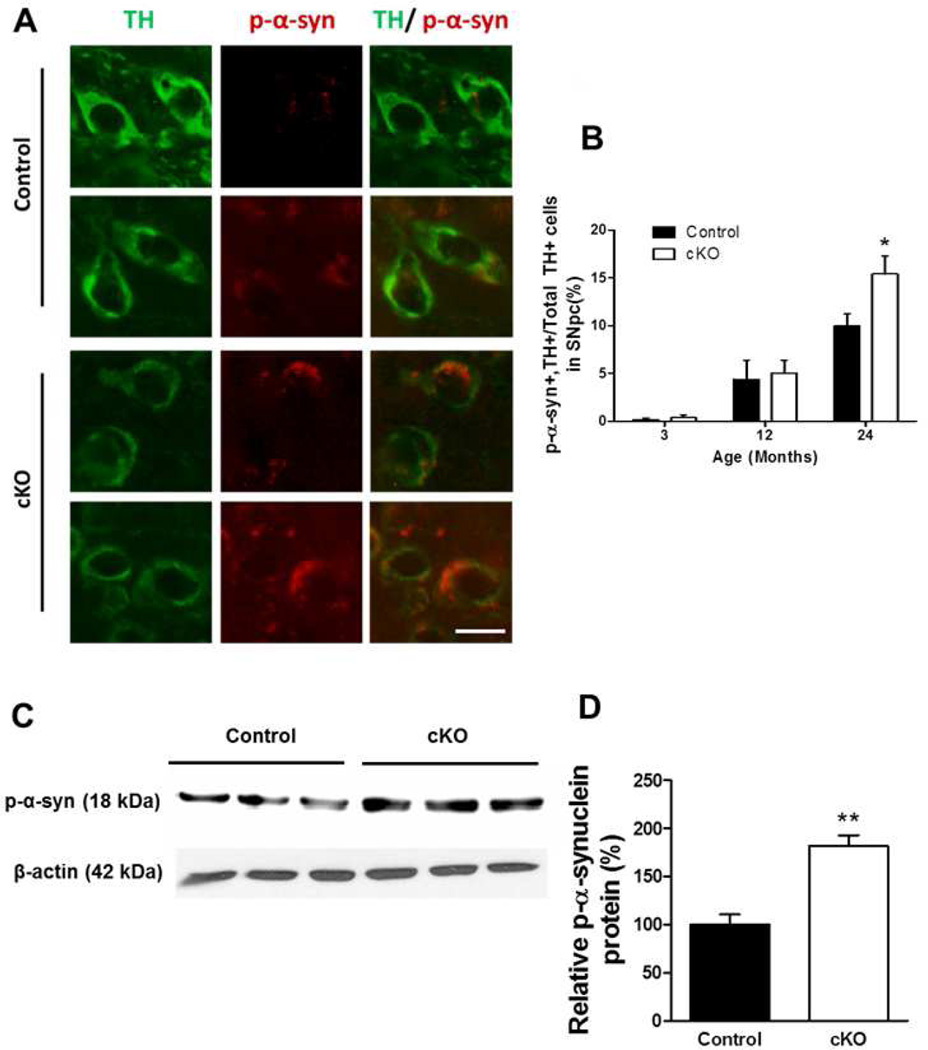
One of the cellular hallmarks of Parkinson’s disease is the presence of Lewy bodies in dopaminergic neurons of the SNpc that contain α-synuclein aggregates, most of which are phosphorylated (Iwatsubo 2003). There was a significant increase in the number of TH+ cells in the SNpc of Ndufs4 cKO mice that were also phospho- α-synuclein+ at 24 months of age (Fig. 5A and B). Western analysis also showed increased levels of phospho-α-synuclein protein in the SNpc of 24-month-old Ndufs4 cKO mice (Fig. 5C and D).
Figure 5. Age-related changes in levels of phospho-α-synuclein in control and Ndufs4 cKO mice.
(A) Representative TH (green) and phospho (p) -α-synuclein (red) immunostaining in the SNpc of 24-month-old mice. Scale bar = 20 µm. (B) Quantification of the number of phospho-α-synuclein+ cells among TH+ cell population. (C) Representative western blot analysis for phospho-α-synuclein in the SNpc of 2-year-old mice, n=3 for each genotype. (D) Quantification of phospho-α-synuclein protein, normalized to β-actin. *, p < 0.05; **, p <0.01.
3.5 Dopaminergic neuron-specific complex I inhibition does not affect the susceptibility to MPTP
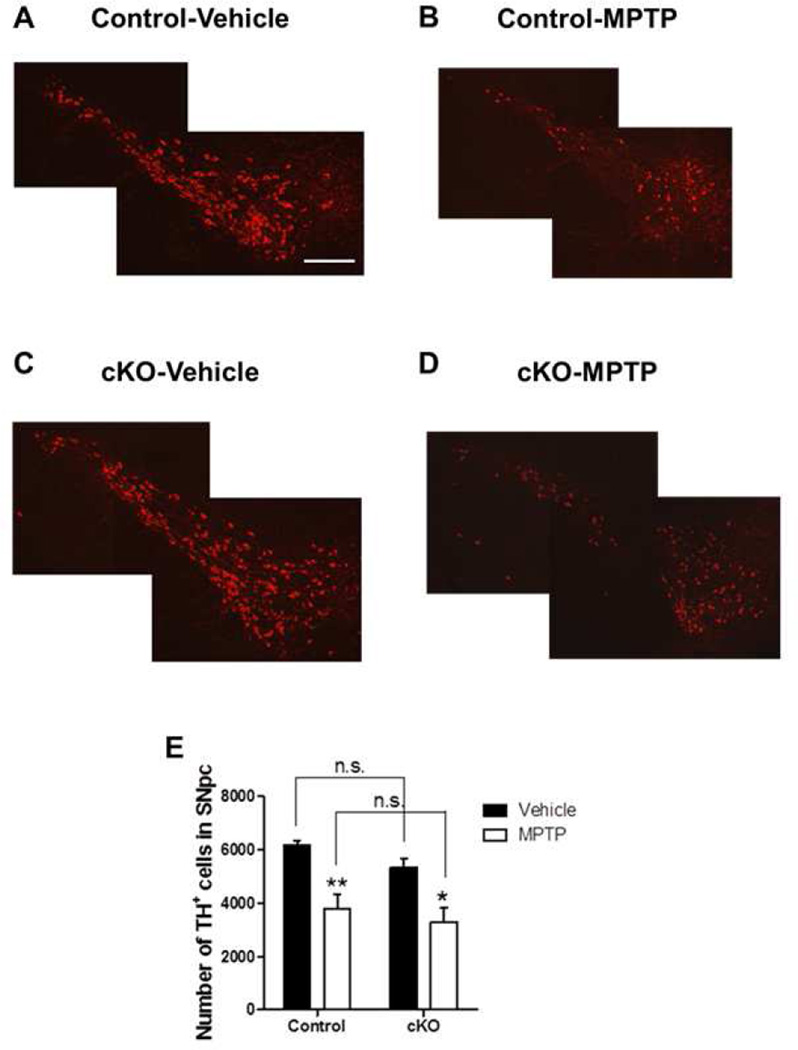
We reported that Ndufs4 inactivation in all cells does not affect the responsiveness of cultured dopaminergic neurons to MPP+ (Choi, Kruse et al. 2008). To investigate the effect of conditional loss of Ndufs4 on the susceptibility of dopaminergic neurons to MPTP in vivo, we administered MPTP to male control and Ndufs4 cKO mice. MPTP induced similar degrees of dopaminergic neuron loss in the SNpc of both groups of mice (Fig. 6). Thus, conditionally ablating Ndufs4 in dopaminergic neurons does not seem to affect the responsiveness of mice to MPTP in vivo.
Figure 6. Conditional Ndufs4 deletion in dopaminergic neurons does not affect their susceptibility to MPTP.
(A–D) Representative photomicrographs of TH immunostaining of SNpc from control mice treated with vehicle (A), MPTP (B), or from Ndufs4 cKO mice treated with vehicle (C) or MPTP (D). Scale bar = 200 µm. (E) Quantification of the total number of TH+ neurons in the SNpc of mice treated with vehicle or MPTP. n = 5–6 mice/genotype/treatment; *, p < 0.05; **, p < 0.01; n.s. not statistically significant.
3.6 Inducible complex I inhibition in all cells of adult mice does not affect their susceptibility to MPTP
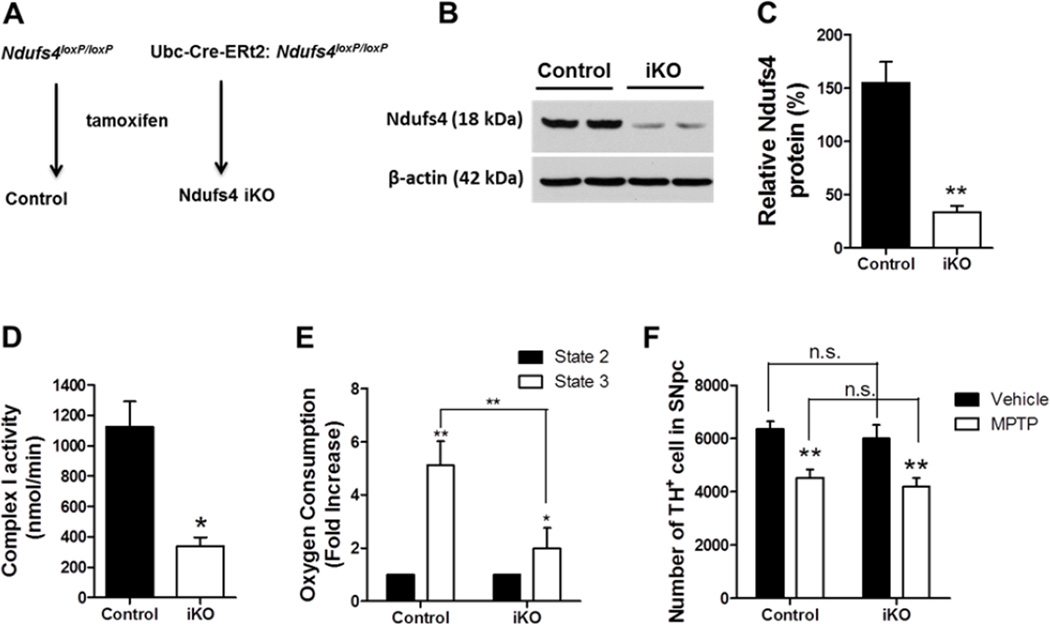
One might argue that developmental compensation may occur in Ndufs4 cKO mice. Furthermore, MPTP inhibition of complex I activity in non-dopaminergic neurons may affect MPTP toxicity to dopaminergic neurons through non-cell autonomous mechanisms. To address these concerns, we utilized the Ndufs4 iKO mice in which the Ndufs4 gene is deleted in all cells in adult mice with a tamoxifen sensitive Cre under the promoter control of the ubiquitously expressed Ubc gene (Fig. 7A). Western blot analysis showed 80% reduction of Ndufs4 protein in the brains of Ndufs4 iKO mice (Fig. 7B and C). Purified mitochondrial from the brains of Ndufs4 iKO mice showed 71% loss of complex I activity, measured by complex I inhibitor-sensitive oxygen consumption rate using the polarography assay (Fig. 7D). Complex I-driven mitochondrial respiration was also assessed by monitoring oxygen consumption in the presence of complex I substrates before (state 2 respiration) and after (state 3) addition of ADP. ADP-induced, state 3 oxygen consumption in the presence of complex I substrate is a measure of complex I-driven oxidative phosphorylation (Borutaite and Brown 1996). Unlike those from control mice, purified mitochondria preparations from Ndufs4 iKO mouse brains showed only a very small increase of oxygen consumption from state 2 to state 3 (Fig. 7E), confirming the nature of complex I inhibition in Ndufs4 iKO mouse brains. Significantly, MPTP induced similar degrees of dopaminergic neuron loss in the SNpc of both control and Ndufs4 iKO mice (Fig. 7F). Thus, inducible Ndufs4 deletion in all cells in adult does not alter the sensitivity of dopaminergic neurons to MPTP in vivo.
Figure 7. Inducible Ndufs4 deletion in all cells of adult mice does not affect their susceptibility to MPTP.
(A) Scheme for generating control and Ndufs4 iKO mice in which the Ndufs4 gene is deleted in the whole body upon tamoxifen treatment. (B) Representative Western blots of Ndufs4 proteins from the brains of two control and two Ndufs4 iKO mice. Beta-actin was used as a loading control. (C) Quantification of Ndufs4 protein, normalized to β-actin. (D) Complex I activity in mitochondria purified from control or Ndufs4 iKO mouse brains was measured by the polarography method. (E) State 2 and state 3 oxygen consumption rates in purified mitochondria. (F) Quantification of the total number of TH+ neurons in the SNpc of mice treated with vehicle or MPTP. n = 6 mice/genotype/treatment. *, p < 0.05; **, p <0.01.
4. Discussion
To examine a causal relationship between inhibition of mitochondrial complex I and dopaminergic neuronal death, we generated two lines of conditional Ndufs4 knockout mice to selectively inhibit complex I activity either only in dopaminergic neurons or in all cells but only in the adult. Our goal was to determine if complex I inhibition in dopaminergic neurons in vivo causes aging-dependent dopaminergic neuron death, dopamine-dependent motor deficits, or induces other biochemical or behavioral changes associated with Parkinson’s disease. We also aimed to determine if complex I inhibition changes the sensitivity of dopamine neurons to MPTP by either a cell autonomous or non-autonomous manner.
The Ndufs4 cKO mice did not show increased dopaminergic neuronal death in the SNpc or dopamine-dependent motor deficits up to 24 months of age. These data suggest that complex I inhibition in dopaminergic neurons per se is not sufficient to cause dopaminergic neuron degeneration or dopamine-dependent motor deficits in vivo during aging. Our data are consistent with recent reports that mesencephalic complex I deficiency does not correlate with Parkinsonism in humans (Palin, Paetau et al. 2013) or in mice (Sterky, Hoffman et al. 2012).
Nevertheless, aging Ndufs4 cKO mice showed biochemical changes commonly seen in the brains of Parkinson's disease patients, including loss of striatal dopamine at 9 months or older, and accumulation of phospho-α-synuclein in dopaminergic neurons at 24 months. The fact that ∼40% reduction in striatal dopamine did not result in motor deficits is consistent with human conditions in which up to 80% of striatal dopamine is already lost at the onset of Parkinson's disease diagnosis (Dauer and Przedborski 2003). Mice exposed to MPTP can lose 20–80% of the dopaminergic neurons without apparent motor deficits (Petroske, Meredith et al. 2001, Rommelfanger, Edwards et al. 2007, Dietz, Stockhausen et al. 2008, Gibrat, Saint-Pierre et al. 2009). Similar to our results, transgenic mice expressing a mutant LRRK2 have reduced striatal dopamine without the loss of dopaminergic neuron cell bodies or degeneration of nigrostriatal terminals (Li, Patel et al. 2010). It is not clear what caused the reduction of striatal dopamine in the absence of dopaminergic neuron death in Ndufs4 cKO mice. It is possible that complex I inhibition leads to reduced ATP synthesis (Kruse, Watt et al. 2008), which then adversely affects dopamine synthesis or the transport of dopamine vesicles to the nerve terminals in the striatum. Alternatively, since α-synuclein may regulate DAT function (Lee, Liu et al. 2001, Sidhu, Wersinger et al. 2004), increased phospho-α-synuclein in Ndufs4 cKO mice may also interfere with DAT-mediated dopamine reuptake in the nerve terminals.
If MPTP kills dopaminergic neurons by inhibiting their complex I activity, dopaminergic neurons in Ndufs4 cKO mice would be expected to be less vulnerable to MPTP. Similarly, if MPTP inhibition of complex I in non-dopaminergic cells indirectly kills dopaminergic neuron through non-cell autonomous mechanisms, fewer dopaminergic neurons in Ndufs4 iKO mice may die from MPTP treatment than those from controls. Yet, both Ndufs4 cKO and Ndufs4 iKO mice had similar sensitivity to MPTP as their respective control mice, suggesting that complex I inhibition is not the main mechanism underlying MPTP toxicity. Interestingly, mutant LRRK2-expressing mice, which have reduced striatal dopamine, also did not have altered vulnerability to MPTP (Li, Patel et al. 2010). Because the MPTP animal model is extensively used for drug testing of Parkinson's disease treatment, it will be important to identify complex I-independent, intrinsic properties of dopaminergic neurons that make them susceptible to MPTP. The fact that Ndufs4 cKO and Ndufs4 iKO mice also did not show enhanced dopaminergic neuron degeneration upon MPTP treatment suggests that complex I inhibition does not interact with MPTP in the “multiple hit” hypothesis (Carvey, Punati et al. 2006, Sulzer 2007) of Parkinson's disease etiology. Because aging is an important factor in the etiology of Parkinson’s disease, it will be interesting in the future to examine the effect of MPTP on aged Ndufs4 cKO and Ndufs4 iKO mice.
Data presented here do not exclude the role for other aspects of mitochondrial dysfunction in the etiology of dopaminergic neuron death in Parkinson's disease. Rather, our studies focus on mitochondria complex I specifically. Together with our previous in vitro reports (Choi, Kruse et al. 2008, Choi, Palmiter et al. 2011), the in vivo studies presented here provide strong evidence that inhibition of mitochondrial complex I is not sufficient to cause cell autonomous dopaminergic neuron degeneration during the normal life span of mice, nor does it underlie MPTP toxicity to dopaminergic neurons. However, it may contribute to other biochemical changes (dopamine loss and phospho-α-synuclein accumulation). Furthermore, complex I inhibition may interact with other factors, such as changes in microtubule dynamics triggered by rotenone exposure, to potentiate or accelerate dopaminergic neuron death (Ren, Liu et al. 2005, Jiang, Yan et al. 2006, Choi, Palmiter et al. 2011).
Highlights.
Mitochondrial complex I Inhibition has been one of the major hypothesis for dopaminergic neuron death in Parkinson’s disease.
We generated mice with conditional Ndufs4 knockout in dopaminergic neurons to examine the effect of complex I inhibition on dopaminergic neuron survival during aging.
Ndufs4 cKO mice did not show enhanced dopaminergic neuron loss in the SNpc or dopamine-dependent motor deficits over the 24-month lifespan.
Inhibition of mitochondrial complex I activity is not sufficient to cause dopaminergic neuron death during aging nor does it contribute to dopamine neuron toxicity in the MPTP model of Parkinson’s disease.
Acknowledgments
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2013R1A1A1059258, WSC and NRF-2014R1A1A2055836, HWK), NIH grants ES012215 and ES013696 (ZX), and facilitated by grant P30 HD02274 from the National Institute of Child Health and Human Development. We thank members of Dr. Toby Cole and the Xia laboratory for technical assistance on behavior tests, and for critical reading of the manuscript.
Abbreviations footnote
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- Ndufs4 cKO
Ndufs4 conditional Knockout
- SNpc
substantia nigra pars compacta
- TH
tyrosine hydroxylase
- DAT
dopamine transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors claim no conflict of interest.
5. References
- Aarsland D, Taylor JP, Weintraub D. Psychiatric issues in cognitive impairment. Mov Disord. 2014;29(5):651–662. doi: 10.1002/mds.25873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nat Rev Neurosci. 2006;7(3):207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3(12):1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Blesa J, Phani S, Jackson-Lewis V, Przedborski S. Classic and new animal models of Parkinson's disease. J Biomed Biotechnol. 2012;2012:845618. doi: 10.1155/2012/845618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonder LX, Slevin JT. Emotional dysfunction in Parkinson's disease. Behav Neurol. 2011;24(3):201–217. doi: 10.3233/BEN-2011-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borutaite V, Brown GC. Rapid reduction of nitric oxide by mitochondria, and reversible inhibition of mitochondrial respiration by nitric oxide. Biochem J. 1996;315(Pt 1):295–299. doi: 10.1042/bj3150295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde SM, van den Heuvel LP, Janssen AJ, Smeets RJ, Buskens CA, DeMeirleir L, Van Coster R, Baethmann M, Voit T, Trijbels JM, Smeitink JA. Combined enzymatic complex I and III deficiency associated with mutations in the nuclear encoded NDUFS4 gene. Biochem Biophys Res Commun. 2000;275(1):63–68. doi: 10.1006/bbrc.2000.3257. [DOI] [PubMed] [Google Scholar]
- Carvey PM, Punati A, Newman MB. Progressive dopamine neuron loss in Parkinson's disease: the multiple hit hypothesis. Cell Transplant. 2006;15(3):239–250. doi: 10.3727/000000006783981990. [DOI] [PubMed] [Google Scholar]
- Choi WS, Abel G, Klintworth H, Flavell RA, Xia Z. JNK3 mediates paraquat- and rotenone-induced dopaminergic neuron death. J Neuropathol Exp Neurol. 2010;69(5):511–520. doi: 10.1097/NEN.0b013e3181db8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WS, Kruse SE, Palmiter RD, Xia Z. Mitochondrial complex I inhibition is not required for dopaminergic neuron death induced by rotenone, MPP+, or paraquat. Proc Natl Acad Sci U S A. 2008;105(39):15136–15141. doi: 10.1073/pnas.0807581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WS, Palmiter RD, Xia Z. Loss of mitochondrial complex I activity potentiates dopamine neuron death induced by microtubule dysfunction in a Parkinson's disease model. J Cell Biol. 2011;192(5):873–882. doi: 10.1083/jcb.201009132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's Disease: Mechanisms and Models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302(5646):819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- Dietz GP, Stockhausen KV, Dietz B, Falkenburger BH, Valbuena P, Opazo F, Lingor P, Meuer K, Weishaupt JH, Schulz JB, Bahr M. Membrane-permeable Bcl-xL prevents MPTP-induced dopaminergic neuronal loss in the substantia nigra. J Neurochem. 2008;104(3):757–765. doi: 10.1111/j.1471-4159.2007.05028.x. [DOI] [PubMed] [Google Scholar]
- Fraker C, Timmins MR, Guarino RD, Haaland PD, Ichii H, Molano D, Pileggi A, Poggioli R, Presnell SC, Inverardi L, Zehtab M, Ricordi C. The use of the BD oxygen biosensor system to assess isolated human islets of langerhans: oxygen consumption as a potential measure of islet potency. Cell Transplant. 2006;15(8–9):745–758. doi: 10.3727/000000006783981440. [DOI] [PubMed] [Google Scholar]
- Gibrat C, Saint-Pierre M, Bousquet M, Levesque D, Rouillard C, Cicchetti F. Differences between subacute and chronic MPTP mice models: investigation of dopaminergic neuronal degeneration and alpha-synuclein inclusions. J Neurochem. 2009;109(5):1469–1482. doi: 10.1111/j.1471-4159.2009.06072.x. [DOI] [PubMed] [Google Scholar]
- Haas RH, Nasirian F, Nakano K, Ward D, Pay M, Hill R, Shults CW. Low platelet mitochondrial complex I and complex II/III activity in early untreated Parkinson's disease. Ann Neurol. 1995;37(6):714–722. doi: 10.1002/ana.410370604. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Jenner P, Przedborski S. Pathogenesis of Parkinson's disease. Mov Disord. 2013;28(1):24–30. doi: 10.1002/mds.25032. [DOI] [PubMed] [Google Scholar]
- Inden M, Kitamura Y, Takeuchi H, Yanagida T, Takata K, Kobayashi Y, Taniguchi T, Yoshimoto K, Kaneko M, Okuma Y, Taira T, Ariga H, Shimohama S. Neurodegeneration of mouse nigrostriatal dopaminergic system induced by repeated oral administration of rotenone is prevented by 4-phenylbutyrate, a chemical chaperone. J Neurochem. 2007;101(6):1491–1504. doi: 10.1111/j.1471-4159.2006.04440.x. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T. Aggregation of alpha-synuclein in the pathogenesis of Parkinson's disease. J Neurol. 2003;250(Suppl 3) doi: 10.1007/s00415-003-1303-x. III11-14. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995;4(3):257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Yan Z, Feng J. Neurotrophic factors stabilize microtubules and protect against rotenone toxicity on dopaminergic neurons. J Biol Chem. 2006;281(39):29391–29400. doi: 10.1074/jbc.M602740200. [DOI] [PubMed] [Google Scholar]
- Kopecky B, Decook R, Fritzsch B. Mutational ataxia resulting from abnormal vestibular acquisition and processing is partially compensated for. Behav Neurosci. 2012;126(2):301–313. doi: 10.1037/a0026896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse SE, Watt WC, Marcinek DJ, Kapur RP, Schenkman KA, Palmiter RD. Mice with mitochondrial complex I deficiency develop a fatal encephalomyopathy. Cell Metabolism. 2008;7(4):312–320. doi: 10.1016/j.cmet.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AE, Blair RD. Parkinson's disease in 1984: an update. Can Med Assoc J. 1984;131(9):1031–1037. [PMC free article] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219(4587):979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Liu F, Pristupa ZB, Niznik HB. Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB J. 2001;15(6):916–926. doi: 10.1096/fj.00-0334com. [DOI] [PubMed] [Google Scholar]
- Lee Y, Dawson VL, Dawson TM. Animal models of Parkinson's disease: vertebrate genetics. Cold Spring Harb Perspect Med. 2012;2(10) doi: 10.1101/cshperspect.a009324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Patel JC, Wang J, Avshalumov MV, Nicholson C, Buxbaum JD, Elder GA, Rice ME, Yue Z. Enhanced striatal dopamine transmission and motor performance with LRRK2 overexpression in mice is eliminated by familial Parkinson's disease mutation G0027S. J Neurosci. 2010;30(5):1788–1797. doi: 10.1523/JNEUROSCI.5604-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima MM, Martins EF, Delattre AM, Proenca MB, Mori MA, Carabelli B, Ferraz AC. Motor and non-motor features of Parkinson's disease - a review of clinical and experimental studies. CNS Neurol Disord Drug Targets. 2012;11(4):439–449. doi: 10.2174/187152712800792893. [DOI] [PubMed] [Google Scholar]
- Liou AK, Zhou Z, Pei W, Lim TM, Yin XM, Chen J. BimEL up-regulation potentiates AIF translocation and cell death in response to MPTP. Faseb J. 2005;19(10):1350–1352. doi: 10.1096/fj.04-3258fje. [DOI] [PubMed] [Google Scholar]
- Liu W, Acin-Perez R, Geghman KD, Manfredi G, Lu B, Li C. Pink1 regulates the oxidative phosphorylation machinery via mitochondrial fission. Proc Natl Acad Sci U S A. 2011;108(31):12920–12924. doi: 10.1073/pnas.1107332108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y, Ohta S, Tanaka M, Takamiya S, Suzuki K, Sato T, Oya H, Ozawa T, Kagawa Y. Deficiencies in complex I subunits of the respiratory chain in Parkinson's disease. Biochem Biophys Res Commun. 1989;163(3):1450–1455. doi: 10.1016/0006-291x(89)91141-8. [DOI] [PubMed] [Google Scholar]
- Morais VA, Haddad D, Craessaerts K, De Bock PJ, Swerts J, Vilain S, Aerts L, Overbergh L, Grunewald A, Seibler P, Klein C, Gevaert K, Verstreken P, De Strooper B. PINK1 loss-of-function mutations affect mitochondrial complex I activity via NdufA10 ubiquinone uncoupling. Science. 2014;344(6180):203–207. doi: 10.1126/science.1249161. [DOI] [PubMed] [Google Scholar]
- Morais VA, Verstreken P, Roethig A, Smet J, Snellinx A, Vanbrabant M, Haddad D, Frezza C, Mandemakers W, Vogt-Weisenhorn D, Van Coster R, Wurst W, Scorrano L, De Strooper B. Parkinson's disease mutations in PINK1 result in decreased Complex I activity and deficient synaptic function. EMBO Mol Med. 2009;1(2):99–111. doi: 10.1002/emmm.200900006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palin EJ, Paetau A, Suomalainen A. Mesencephalic complex I deficiency does not correlate with parkinsonism in mitochondrial DNA maintenance disorders. Brain. 2013;136(Pt 8):2379–2392. doi: 10.1093/brain/awt160. [DOI] [PubMed] [Google Scholar]
- Pan-Montojo F, Anichtchik O, Dening Y, Knels L, Pursche S, Jung R, Jackson S, Gille G, Spillantini MG, Reichmann H, Funk RH. Progression of Parkinson's disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS One. 2010;5(1):e8762. doi: 10.1371/journal.pone.0008762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YW, Chan GCK, Kuo CT, Storm DR, Xia Z. Inhibition of Adult Neurogenesis by Inducible and Targeted Deletion of ERK5 Mitogen-Activated Protein Kinase Specifically in Adult Neurogenic Regions Impairs Contextual Fear Extinction and Remote Fear Memory. J Neurosci. 2012;32(19):6444–6455. doi: 10.1523/JNEUROSCI.6076-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya M, Kubu CS, Giroux ML. Parkinson disease: not just a movement disorder. Cleve Clin J Med. 2008;75(12):856–864. doi: 10.3949/ccjm.75a.07005. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann Neurol. 1989;26(6):719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- Petroske E, Meredith GE, Callen S, Totterdell S, Lau YS. Mouse model of Parkinsonism: a comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neuroscience. 2001;106(3):589–601. doi: 10.1016/s0306-4522(01)00295-0. [DOI] [PubMed] [Google Scholar]
- Petruzzella V, Papa S. Mutations in human nuclear genes encoding for subunits of mitochondrial respiratory complex I: the NDUFS4 gene. Gene. 2002;286(1):149–154. doi: 10.1016/s0378-1119(01)00810-1. [DOI] [PubMed] [Google Scholar]
- Petruzzella V, Vergari R, Puzziferri I, Boffoli D, Lamantea E, Zeviani M, Papa S. A nonsense mutation in the NDUFS4 gene encoding the 18 kDa (AQDQ) subunit of complex I abolishes assembly and activity of the complex in a patient with Leigh-like syndrome. Hum Mol Genet. 2001;10(5):529–535. doi: 10.1093/hmg/10.5.529. [DOI] [PubMed] [Google Scholar]
- Pinna A, Tronci E, Schintu N, Simola N, Volpini R, Pontis S, Cristalli G, Morelli M. A new ethyladenine antagonist of adenosine A(2A) receptors: behavioral and biochemical characterization as an antiparkinsonian drug. Neuropharmacology. 2010;58(3):613–623. doi: 10.1016/j.neuropharm.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Yokoyama R, Shibata T, Dawson VL, Dawson TM. Role of neuronal nitric oxide in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity. Proc Natl Acad Sci U S A. 1996;93(10):4565–4571. doi: 10.1073/pnas.93.10.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana A, Kruse SE, Kapur RP, Sanz E, Palmiter RD. Complex I deficiency due to loss of Ndufs4 in the brain results in progressive encephalopathy resembling Leigh syndrome. Proc Natl Acad Sci U S A. 2010;107(24):10996–11001. doi: 10.1073/pnas.1006214107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Liu W, Jiang H, Jiang Q, Feng J. Selective vulnerability of dopaminergic neurons to microtubule depolymerization. J Biol Chem. 2005;280(40):34105–34112. doi: 10.1074/jbc.M503483200. [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Edwards GL, Freeman KG, Liles LC, Miller GW, Weinshenker D. Norepinephrine loss produces more profound motor deficits than MPTP treatment in mice. Proc Natl Acad Sci U S A. 2007;104(34):13804–13809. doi: 10.1073/pnas.0702753104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scacco S, Petruzzella V, Budde S, Vergari R, Tamborra R, Panelli D, van den Heuvel LP, Smeitink JA, Papa S. Pathological mutations of the human NDUFS4 gene of the 18-kDa (AQDQ) subunit of complex I affect the expression of the protein and the assembly and function of the complex. J Biol Chem. 2003;278(45):44161–44167. doi: 10.1074/jbc.M307615200. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial complex I deficiency in Parkinson's disease. Lancet. 1989;1(8649):1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Kim JH, Betarbet R, Greenamyre JT. Subcutaneous Rotenone Exposure Causes Highly Selective Dopaminergic Degeneration and alpha-Synuclein Aggregation. Exp. Neurol. 2003;179(1):9–16. doi: 10.1006/exnr.2002.8072. [DOI] [PubMed] [Google Scholar]
- Sidhu A, Wersinger C, Vernier P. alpha-Synuclein regulation of the dopaminergic transporter: a possible role in the pathogenesis of Parkinson's disease. FEBS Lett. 2004;565(1–3):1–5. doi: 10.1016/j.febslet.2004.03.063. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21(1):70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Sterky FH, Hoffman AF, Milenkovic D, Bao B, Paganelli A, Edgar D, Wibom R, Lupica CR, Olson L, Larsson NG. Altered dopamine metabolism and increased vulnerability to MPTP in mice with partial deficiency of mitochondrial complex I in dopamine neurons. Hum Mol Genet. 2012;21(5):1078–1089. doi: 10.1093/hmg/ddr537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson's disease. Trends Neurosci. 2007;30(5):244–250. doi: 10.1016/j.tins.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS, Kasten M, Chade AR, Comyns K, Richards MB, Meng C, Priestley B, Fernandez HH, Cambi F, Umbach DM, Blair A, Sandler DP, Langston JW. Rotenone, paraquat, and Parkinson's disease. Environ Health Perspect. 2011;119(6):866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turiault M, Parnaudeau S, Milet A, Parlato R, Rouzeau JD, Lazar M, Tronche F. Analysis of dopamine transporter gene expression pattern -- generation of DAT-iCre transgenic mice. Febs J. 2007;274(14):3568–3577. doi: 10.1111/j.1742-4658.2007.05886.x. [DOI] [PubMed] [Google Scholar]
- van den Heuvel L, Ruitenbeek W, Smeets R, Gelman-Kohan Z, Elpeleg O, Loeffen J, Trijbels F, Mariman E, de Bruijn D, Smeitink J. Demonstration of a new pathogenic mutation in human complex I deficiency: a 5-bp duplication in the nuclear gene encoding the 18-kD (AQDQ) subunit. Am J Hum Genet. 1998;62(2):262–268. doi: 10.1086/301716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilain S, Esposito G, Haddad D, Schaap O, Dobreva MP, Vos M, Van Meensel S, Morais VA, De Strooper B, Verstreken P. The yeast complex I equivalent NADH dehydrogenase rescues pink1 mutants. PLoS Genet. 2012;8(1):e1002456. doi: 10.1371/journal.pgen.1002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel RO, van den Brand MA, Rodenburg RJ, van den Heuvel LP, Tsuneoka M, Smeitink JA, Nijtmans LG. Investigation of the complex I assembly chaperones B17.2L and NDUFAF1 in a cohort of CI deficient patients. Mol Genet Metab. 2007;91(2):176–182. doi: 10.1016/j.ymgme.2007.02.007. [DOI] [PubMed] [Google Scholar]