Abstract
We examined the intriguing but controversial idea that disrupted sleep-dependent consolidation contributes to age-related memory decline. Slow-wave activity during sleep may help strengthen neural connections and provide memories with long-term stability, in which case decreased slow-wave activity in older adults could contribute to their weaker memories. One prediction from this account is that age-related memory deficits should be reduced by artificially enhancing slow-wave activity. In young adults, applying transcranial current oscillating at a slow frequency (.75 Hz) during sleep improves memory. Here, we tested whether this procedure can improve memory in older adults. In two sessions separated by 1 week, we applied either slow-oscillatory stimulation or sham stimulation during an afternoon nap in a double-blind, crossover design. Memory tests were administered before and after sleep. A larger improvement in word-pair recall and higher slow-wave activity were observed with slow-oscillatory stimulation than with sham stimulation. This is the first demonstration that this procedure can improve memory in older adults, suggesting that declarative memory performance in older adults is partly dependent on slow-wave activity during sleep.
Keywords: slow-wave sleep, aging, electrical stimulation, declarative memory
1. Introduction
As the average lifespan increases in our society, so does the incidence of age-related memory dysfunction. An integral part of effectively addressing this problem involves a more thorough understanding of how and why memory decline occurs during normal aging in individuals who otherwise appear healthy. Declarative memory refers to the ability to consciously recall or recognize facts and events, and is the focus of most age-related memory complaints. Explanations for declarative memory failures generally fault inadequate encoding and/or retrieval (Craik and Byrd, 1982; Luo and Craik, 2008). Another possibility is that memory processing during sleep operates sub-optimally in older adults, exacerbating deficits in encoding and/or retrieval.
Effective maintenance and long-term storage of declarative memories depends on a systems-level consolidation process. During consolidation, cortical representations are strengthened while dependence on the hippocampus diminishes (Diekelmann and Born, 2010; Paller, 2009). Though transformations occur gradually, notable changes in memory representations are observed after a single sleep period in young adults. For example, decreased hippocampal activity, increased cortical activity, and altered functional connectivity between these regions have been observed during post-sleep retrieval (Gais et al., 2007; Takashima et al., 2009).
Slow-wave sleep (SWS) may be particularly important in declarative memory consolidation (Marshall and Born, 2007). A reasonable speculation is that the low-frequency slow-oscillatory activity that predominates during SWS serves to coordinate dialogue between hippocampus and cortex. Synchronized firing during the up phase of each slow oscillation could lead to hippocampal-cortical transfer of information and the persistence of long-term memories in the cortex (Sirota et al., 2003). Supporting this view, retention intervals rich in SWS have been shown to preferentially benefit declarative memory (Daurat et al., 2007; Drosopoulos et al., 2005; Plihal and Born, 1997), and neural activity patterns measured with functional magnetic resonance imaging during SWS have been linked with declarative memory (Chee and Chuah, 2008; Peigneux et al., 2004). In addition to this indirect evidence, other studies have causally implicated slow-wave activity in consolidation. In young adults, electrical stimulation passed transcranially during sleep at approximately the same frequency as endogenous slow oscillations (.75 Hz) improves declarative memory (Marshall et al., 2006; Marshall et al., 2004). Similar results have been observed in patients with schizophrenia (Goder et al., 2013) and in children with attention-deficit-hyperactivity disorder (Prehn-Kristensen et al., 2014), and both conditions have been associated with abnormal SWS.
Changes in sleep are a natural consequence of aging, chiefly featuring a marked decrease in SWS (Bliwise, 1993). Given the similar trajectories of memory and SWS decline in older adults, altered sleep may impair consolidation and therefore contribute to memory decline (Altena et al., 2010; Hornung et al., 2005; Westerberg et al., 2010). Supporting this assertion, it has recently been demonstrated that sleep-memory relationships observed in young adults are similarly present in older adults (Aly and Moscovitch, 2010; Wilson et al., 2012), and that slow-wave activity during sleep predicts recall performance in older adults (Mander et al., 2013; Westerberg et al., 2012). Furthermore, experimentally disrupting SWS in older adults leads to similar changes in cognition and sleepiness levels that are present when SWS is disrupted in young adults (Dijk et al., 2010; Groeger et al., 2014).
Nonetheless, it is possible that age-related memory deficits are unrelated to sleep-dependent consolidation. Links observed in young adults between SWS and other cognitive functions such as attention are reduced in older adults (Crenshaw and Edinger, 1999; Duffy et al., 2009), and in one study, SWS-declarative memory relationships observed in young adults were not present in older adults (Scullin, 2013). These findings suggest that sleep’s contribution to maintaining memories may diminish with age (Harand et al., 2012; Pace-Schott and Spencer, 2011). Given the mixed findings in this literature, further research is necessary to determine the nature of sleep-memory relationships across the lifespan.
The goal of the present study was to examine whether reductions in slow-oscillatory activity contribute to age-related memory decline, by testing whether boosting slow-oscillatory activity during sleep in older adults improves declarative memory. We used the procedure developed by Marshall and colleagues (2006), wherein anodal current, oscillating at the typical slow-oscillation frequency (.75 Hz) was passed through frontal scalp locations during sleep. If sleep-based consolidation impairments contribute to age-related memory decline, than this stimulation should increase slow-oscillatory activity and improve recall, as in young individuals.
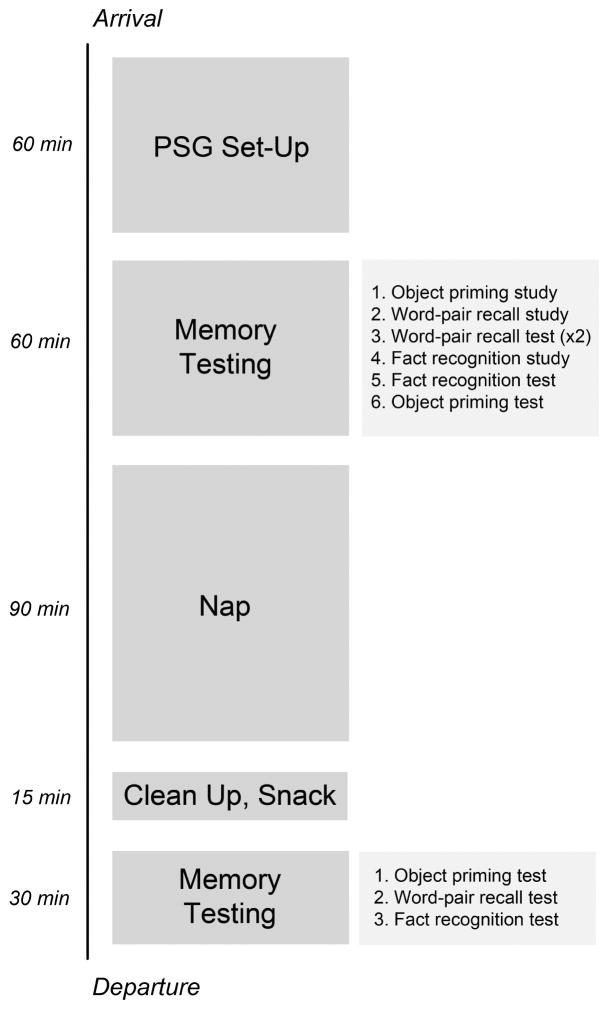
The present study was modeled after the studies of Marshall and colleagues (2004, 2006). In each of two sessions, we administered two declarative memory tests and one nondeclarative memory test before and after participants took an afternoon nap (Figure 1). Slow-oscillatory stimulation (SOS) was delivered through the scalp during one of the naps in a double-blind manner, such that neither the participant nor the experimenter conducting memory testing knew which was the SOS nap and which the sham-SOS nap.
Figure 1.
Timeline of events for each session.
2. Materials and Methods
2.1. Participants
Nineteen cognitively healthy older adults (3 male) were recruited from the Northwestern University Alzheimer’s Disease Center (age: mean 73.4, range 65–85 yrs; education: mean 15.4, range 12–20 yrs) and received monetary compensation for participation. Participants were determined to be cognitively and neurologically normal based on neuropsychological test results and research neurological examinations that were part of the Uniform Data Set of the ADC’s of the National Institute on Aging (Weintraub et al., 2009), supplemented by additional episodic memory tests. All participants scored 28 or higher on the Mini-Mental State Examination (Folstein et al., 1975), and were within 1.5 standard deviations of the mean for individuals of comparable gender, age, and education level on neuropsychological tests assessing cognitive performance across a range of domains (e.g., attention, memory, language, executive function). One additional participant was excluded for taking medication prior to testing. Eighteen others were recruited but did not complete the protocol for various reasons (i.e., failure to fall asleep, scheduling conflicts, recall scores < 20%, or technical difficulties). Standard exclusion criteria included history of central neurological disease, major psychiatric disorder, alcohol or substance abuse, serious medical illness (thyroid disorder; renal, hepatic, cardiac, or pulmonary insufficiency; unstable diabetes; uncontrolled high blood pressure; cancer), chronic psychoactive drug use, presence of a sleep disorder (sleep apnea, restless leg syndrome, narcolepsy), or poor sleep quality (Buysse et al., 1989).
2.2. General Procedure
During a phone interview, participants were screened and scheduled for two sessions, 1 week apart. Slow-oscillation stimulation (SOS) was delivered during one of the two sessions. During the other session, SOS-related equipment was in place but stimulation was never delivered (sham-SOS). SOS was first for 10 participants and sham-SOS was first for 9 participants. The experimenter conducting memory testing and all participants were blinded to which session included SOS, and no participants reported feeling stimulation.
Sessions began between 11:00 AM and 12:00 PM. At each session, participants completed a questionnaire regarding sleep quality from the previous night (Akerstedt et al., 1994) and were prepared for polysomnography. Next, participants completed the Positive and Negative Affectivity Scale or PANAS (Watson et al., 1988), answered the question “How awake do you feel right now?” on a 1–5 scale (1=very sleepy, 5=wide awake), and completed three memory tests. They were informed that memory would be tested again after a nap lasting up to 90 min. These activities took roughly 2 hrs to complete. Participants were then allowed to take a nap. Approximately 90 min after sleep onset, participants were woken up if still asleep. They were given approximately 15 min to clean up from the recording and were offered a beverage and a snack. Next, they completed the PANAS again and answered the question “How awake do you feel right now?” before taking three final memory tests (Figure 1).
2.3. Memory Tests
The three memory tests given to participants included two declarative memory tests and one nondeclarative memory test. Before the nap, an encoding phase was followed by a test phase for each of the three tests. After the nap, participants completed the three test phases again. The tests were administered in the same order before and after each nap (Figure 1). One declarative memory test was a word-pair recall test, modeled after the test used by Marshall and colleagues (2004, 2006), which showed effects of SOS in young adults. A (declarative) fact recognition test and an (non-declarative) object-priming test were also administered, to determine the specificity of SOS effects. These two tests were used in a previous investigation of sleep/memory relationships in aging (Westerberg et al., 2012), and did not show relationships with slow-wave activity during sleep. Three versions of each test were created using different stimuli. Each participant completed two versions of each test (one per session). Across participants, each version appeared in at least 5 SOS and 5 sham-SOS sessions.
2.3.1. Word-pair recall
Participants viewed 40 moderately related word pairs (e.g., weed-flower) in random order. Forward associative strength was assessed using published norms (Nelson et al., 1998). For word pairs in this database (31% of the pairs), the mean cue-to-target strength was 0.04, meaning that in the absence of prior exposure to the pair there was a 4% chance that the second word would be produced in response to the first. For word pairs not in the database, the associative strength would likely be even lower. Word pairs were presented centrally, one word above the other, for 3 s each. Two additional pairs occurred at the beginning of the list and two at the end of the list to curtail serial position effects. Next, participants completed math problems for 1 min before cued recall was initially tested. The first word appeared centrally and participants attempted to say the second word aloud. After 3.5 s, a tone sounded and the correct answer appeared below the first word. Both words remained on the screen for 4 s longer. This initial cued-recall test provided an additional learning opportunity. Immediately after, participants completed more math problems for 1 min and then took the same cued-recall test again. Scores from the second test were used in subsequent analyses as the estimate of learning prior to sleep. The same cued-recall test (without further study) was administered after sleep. Each test administration utilized a new random order.
2.3.2. Fact recognition
During encoding, participants viewed a sequence of 10 faces (5 male, 5 female), each presented for 15 s above four facts pertaining to the person depicted. After each face and associated facts, participants rated how emotional the facts made them feel on a 1–5 scale (1=very emotional, 5=not emotional) to ensure robust encoding. To curtail primacy effects, one additional face with four facts was presented at the beginning of the list but was never tested. After 1 min of math problems, participants completed the recognition test with a different random order of faces. One previously studied face appeared on the left side of the screen with a list of 10 facts on the right (the first fact presented with each studied face). Participants attempted to press a key corresponding to the one fact that was previously associated with the face on the screen. They were instructed to guess if they did not know the correct answer. After a key was pressed, a new list of 10 previously studied facts appeared on the right (all second facts), and participants were again asked to press a key corresponding to the correct fact. This procedure was repeated a total of four times, with one of the facts associated with the face in each of the four fact lists. Next, another previously studied face appeared on the left side of the screen and participants made fact recognition decisions in a similar manner. Four fact recognition decisions were made for each of the 10 previously studied faces. Immediately after the test, a final learning opportunity was provided wherein each face and its associated facts were presented on the screen for 15 s each in a different random order. The same recognition test was administered after the nap (without further study), with the exception that faces and facts appeared in new random orders.
2.3.3. Object priming
Participants viewed a sequence of 30 color pictures of common objects centrally. Participants were asked to immediately name aloud each object. After 4 s, participants were asked to rate how much they liked each object on a 1–4 scale (1=like very much, 4=dislike very much). Although this naming task served as the study phase to subsequently assess priming, participants were only instructed that this was a test of object naming, and they were not informed that memory would be assessed for these objects. The word-pair recall and fact recognition tests intervened between the study and test phases of the object-priming task. The test phase was introduced as another object-naming task that was independent of the first object-naming task, and participants were not informed that any previously viewed objects would appear in the list. Sixty color object pictures (30 old, 30 new) were presented in random order, for 102 ms each followed by a 102-ms visual mask created by randomly rearranging parts of other color objects into a square. Participants pressed the “b” key as quickly as possible if they could name the object and the “n” key if they could not. If the “b” key was pressed, participants were required to say the name of the object aloud. Trials in which the “b” key was pressed and the subsequent spoken name was accurate were counted as correct. After the nap, participants completed only the test phase. It was again introduced as an object-naming task. It followed the same format as the pre-nap test, except that 30 different new objects were presented and a new random order was used.
2.4. Memory Analyses
For each of the three different tests, we assessed memory differences across conditions using an analysis of variance (ANOVA) with test (pre-nap, post-nap) and session (SOS, sham-SOS) as within-subjects variables. For the word-pair recall test, percent of pairs correctly recalled was the dependent measure. For the fact recognition test, percent of facts correctly recognized was the dependent measure. For the object-priming test, a priming score was used as the dependent measure (data from one participant were omitted due to technical problems). The priming score was computed by subtracting the percent of new objects correctly identified from the percent of old objects correctly identified.
2.5. Polysomnography
Electroencephalography (EEG) was recorded from eight scalp sites (F3, F4, C3, C4, P3, P4, O1, O2), each referenced to the contralateral mastoid. Impedance was always below 10 kΩ. Two electrodes (one lateral to each eye) were used to record the electrooculogram, three chin electrodes for the electromyogram, and two chest electrodes for the electrocardiogram. Signals were recorded and amplified using a 200 Hz sampling rate with a .27 Hz high-pass filter and a 70 Hz low-pass filter (Neurofax EEG-1100 amplifier, Nihon Khoden).
2.6. Slow Oscillatory Stimulation
At both sessions (SOS, sham-SOS), 8-mm electrodes were placed at F7 and F8, referenced to ipsilateral mastoids placed slightly below and not overlapping with the polysomnography mastoids. Impedance was below 3 kΩ. A battery-powered constant-current stimulator was located just outside the door to the bedroom, and during both experimental sessions it was connected to electrodes via long cords running under the door. The stimulator had the same specifications as in a prior SOS study (Marshall et al., 2011). During the SOS nap, anodal sinusoidal stimulation (.75 Hz) was applied with current oscillating between 0–260 μA. Current amplitude for each hemisphere was regulated independently, and currents for each hemisphere were phase-coupled with zero phase-shift. Stimulation began 4 min after the onset of stage-2 sleep, and was delivered in five alternating 5-min “on” and 1-min “off” intervals, for a total stimulation period of 30 min (25 min on, 5 min off). The stimulation was applied manually by turning a 3-position (SOS, sham-SOS, off) switch on the stimulator at the beginning and end of each stimulation period. The only procedural difference during the sham-SOS session was that the switch was flipped to the sham-SOS position (i.e., circuits were not connected when the switch was in this position).
2.7. Sleep Analyses
Primary analyses focused on whether SOS-related effects observed in young adults (Marshall et al., 2006; Marshall et al., 2004) were also present in older adults. We thus examined three measures extracted from data during the off intervals of the 30-min stimulation period: frontal slow-oscillatory power (0.5–1 Hz), frontal slow spindles, and time spent in SWS. We also examined frontal power in the neighboring delta band (1.0–4.5 Hz), as Marshall et al. (2006) observed slight but nonsignificant SOS-related increases in a similar frequency band in young adults.
Additional analyses considered the effect of SOS on fast spindles, given that fast spindles have been implicated in declarative memory consolidation in young adults (Ngo et al., 2013; van der Helm et al., 2011). We also analyzed time spent in other sleep stages, as well as slow-oscillatory power and slow spindles at central and posterior recording sites.
Each of the above measures was also analyzed during post-stimulation sleep (i.e., from the time immediately after the stimulation period ended until the end of the nap). In this way, we sought to determine whether effects of SOS on sleep were restricted to the stimulation period, as was observed in young adults (Marshall et al., 2006).
Whereas SOS artifacts precluded examination of the EEG signal during on intervals, off intervals provided clean signals, after excluding the first 3-s due to SOS-related artifacts. For sham-SOS naps, data were marked with the times when SOS on and off intervals would have occurred. Data from these off intervals were then compared against data from off intervals for the SOS naps.
Sleep staging, spectral power computations, and spindle detection were completed with Prana software (Phitools). For all SOS-free epochs, polysomnographic data were staged according to standard criteria (Iber et al., 2007), with artifacts rejected based on visual inspection. Spectral and spindle analyses were completed for the off intervals during the stimulation (or sham-stimulation) period for all non-wake epochs, and averaged across all 5 off intervals. Spectral and spindle analyses were also completed from the time the stimulation or sham-stimulation period ended until the end of the nap for all non-wake 30-s epochs. Spectral analyses were conducted using a fast Fourier transform with a 4-s Hanning window and 50% overlap, and absolute power estimates were averaged over 30-s epochs for slow-oscillation and delta bands for all recording sites. Slow-spindle and fast-spindle densities (spindles/min) were computed at all sites using an automated algorithm to identify spindles (duration: 0.5–3.0 s, amplitude: > 2.5 SD above mean). There have been discrepancies regarding optimal boundaries for the slow spindle frequency range. Marshall et al. (2006) used a range based on spectral-power peaks observed at 10.2 Hz in young adults (Molle et al., 2011), whereas other reports suggest that slow spindles are centered around 12 Hz (Anderer et al., 2001; Schabus et al., 2007; Zygierewicz et al., 1999). Therefore, we defined two separate frequency ranges for slow spindles, 8.5–12.5 Hz and 11.5–13.5 Hz, based on these prior findings. The frequency range for fast spindles was 13.5–15.5 Hz. Paired-sample t-tests were subsequently used for all planned comparisons.
3. Results
3.1. Memory
Table 1 shows scores for word-pair recall, fact recognition, and object priming for each test (pre-nap, post-nap) and session (SOS, sham-SOS). Note, the fact that mean post-nap scores were all higher than pre-nap scores does not necessarily reflect memory change during sleep, instead these increases could have resulted from additional learning that took place during the course of pre-nap testing. Also, pre-nap memory scores were not necessarily identical on the two sessions. Nonetheless, the key question motivating this experiment is whether memory change differed between SOS and sham-SOS sessions.
Table 1.
Memory performance for word-pair recall, fact recognition, and object priming tests. Standard error in parentheses.
| SOS | Sham-SOS | |
|---|---|---|
|
| ||
| Word-Pair recall | ||
| Pre-nap (% recalled) | 62.2 (1.3) | 63.9 (1.5) |
| Post-nap (% recalled) | 68.2 (1.5) | 66.5 (1.3) |
|
| ||
| Fact recognition | ||
| Pre-nap (% recognized) | 55.8 (1.7) | 57.8 (1.7) |
| Post-nap (% recognized) | 66.4 (2.4) | 72.1 (1.9) |
|
| ||
| Object priming | ||
| Pre-nap new objects (% recognized) | 74.6 (1.4) | 78.0 (1.8) |
| Pre-nap old objects (% recognized) | 84.2 (1.8) | 86.8 (1.3) |
| Post-nap new objects (% recognized) | 76.9 (1.7) | 81.1 (.8) |
| Post-nap old objects (% recognized) | 82.8 (1.1) | 88.3 (1.2) |
3.1.1. Word-pair recall
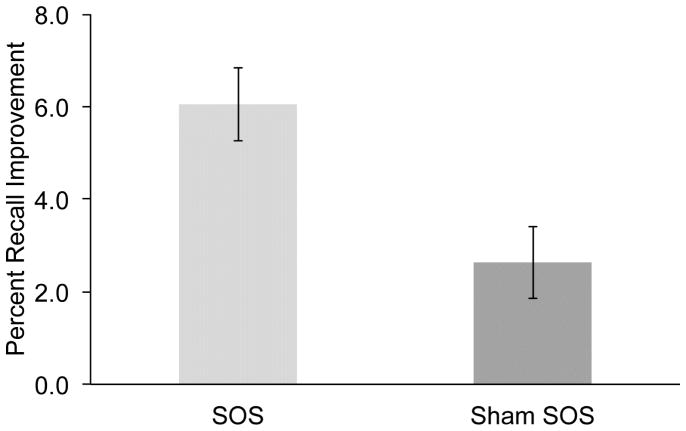
As predicted, the memory change across the nap period (Figure 2) differed as a function of stimulation [ANOVA test X session interaction; F(1,18)=4.7, p<.05]. Whereas pre-nap memory did not differ across sessions [t(18)=.7, p>.5], recall improvement from pre-nap to post-nap was larger in the SOS session compared with the sham-SOS session [6.1% vs. 2.6%, respectively; t(18)=2.5, p<.05]. This result indicates that SOS improved recall, in keeping with results from young participants (Marshall et al., 2006; Marshall et al., 2004). Across both sessions, post-nap recall was superior to pre-nap recall [67.4% vs. 63.1%, respectively; F(1,18)=8.8, p<.01]. Collapsed across pre- and post-nap testing, recall did not differ as a function of SOS [F(1,18)=0.0, p>.9].
Figure 2.
Percent recall improvement (post-nap recall – pre-nap recall) on the word-pair recall test for SOS and sham-SOS sessions. Error bars indicate standard error of the mean.
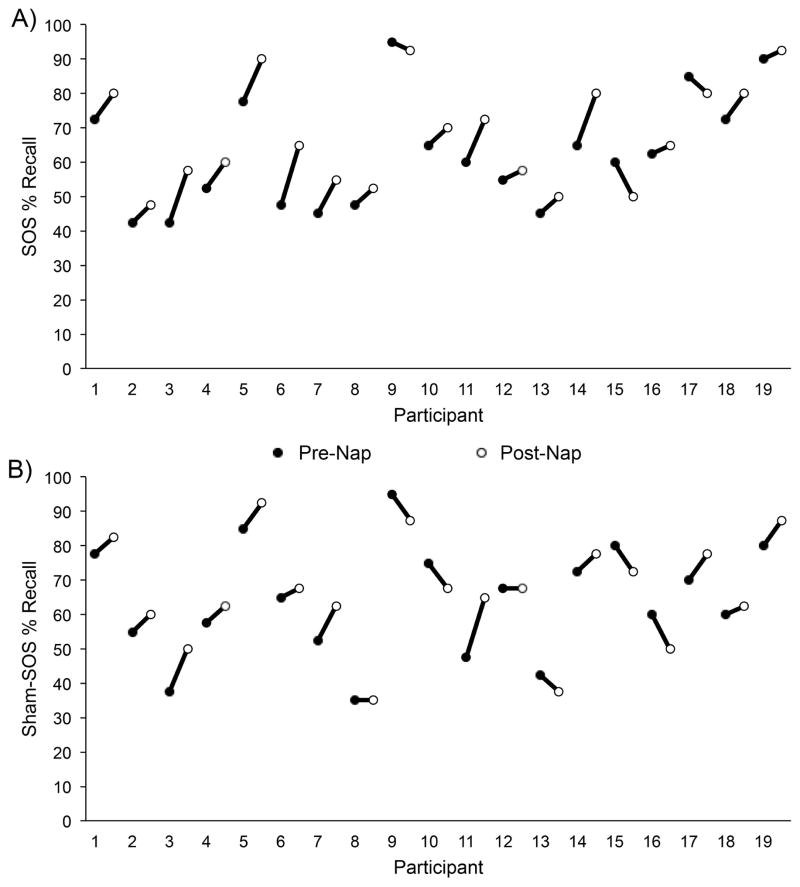
Although pre-nap word recall was not consistently different between sessions, the mean recall score was numerically lower for the SOS session than for the sham-SOS session (Table 1). Whereas this 1.7% difference was not statistically significant, further analysis was warranted because of the possibility that it still influenced memory improvement results. One step involved inspection of data from individual participants on pre- and post-nap recall (Figure 3), but there was no suggestion of ceiling effects or that outlier participants were responsible for the larger memory improvement during the SOS session. We also considered whether the trend for pre-nap differences between sessions could have reflected learning-rate differences. Specifically, if learning was slower (for nonsystematic reasons) during the SOS session, then one might expect both lower pre-nap recall and larger post-nap improvement. To investigate this possibility, we examined learning-phase results. After studying the word pairs once, participants took an initial recall test where the correct answers were revealed (immediately followed by the pre-nap recall test). There was no significant difference in initial pre-nap recall between SOS and sham-SOS sessions [t(18)=1.0, p>.3], and performance trended to be higher in the SOS session (39.2%) than the sham-SOS session (35.4%). Slower learning of word pairs in the SOS session thus did not appear to contribute to the differential memory improvement across the nap period.
Figure 3.
Percent pre- and post-nap recall for A) SOS and B) sham-SOS sessions for each participant (1–19).
An additional ANOVA was conducted to determine whether memory performance differed between the first and second experimental sessions, regardless of whether participants received SOS or sham-SOS during the first experimental session. Experimental session (first, second) and test (pre-nap, post-nap) were within-subjects variables. As expected, a main effect of test was present, indicating that post-nap recall was higher than pre-nap recall, regardless of the experimental session [F(1,18)=8.8, p<.01]. However, neither the experimental session main effect (p>.4) nor the session day X test interaction (p>.6) were significant, confirming that recall performance did not differ as a function of experimental session (first or second).
A final ANOVA was performed to determine whether gender differences in memory due to SOS were present, including gender as a between-subjects variable. It was not surprising that a main effect of gender was not present [F(1,18)=0.5, p>.4], given the small sample sizes of the groups. Further inspection of the data revealed that during both SOS and sham-SOS sessions, males showed numerically lower memory improvement across the nap compared with females (SOS: males=2.5%, females=6.7%; sham-SOS: males=-4.2%, females=3.9%), although these differences failed to reach significance (p values > .08). However, both genders showed a numerically larger memory improvement across the SOS-nap compared with the sham-SOS nap. In addition, the difference in memory improvement between the SOS and sham-SOS naps (SOS improvement - sham-SOS improvement) was numerically but not significantly (p>.3) larger in males than in females (males=6.7%, females=2.8%), tentatively suggesting that SOS may be more effective in males than in females, although future research including larger groups will be necessary to effectively test this possibility.
3.1.2. Fact recognition
Post-nap recognition (69%) was significantly better than pre-nap recognition (57%), as shown by the main effect of test [F(1,18)=38.2, p<.001]. However, the test X session interaction was not significant (p >.3), indicating that this improvement did not differ between SOS (8%) and sham-SOS (8%) sessions. The session main effect was not significant (p >.1).
3.1.3. Object priming
A test main effect revealed that pre-nap priming (9.7%) was larger than post-nap priming [6.3%; F(1,17)=6.2, p<.05]. No other effects were significant (p values >.4).
3.2. Sleep
3.2.1. General sleep characteristics
Latency to sleep onset (SOS: 15 min; sham-SOS: 11 min) did not differ between SOS and sham-SOS sessions [t(18)=1.7, p>.1], nor did time in bed [SOS: 125 min; sham-SOS: 122 min; t(18)=.7, p>.4]. SOS-related artifacts during the stimulation period precluded comparison of total sleep time and time spent in each sleep stage across the full sleep period. However, time spent in each sleep stage (wake, stage 1, stage 2) from sleep onset until the start of the stimulation period did not differ across sessions (p values > .05; analyses of post-stimulation data reported below in section 3.2.d).
3.2.2. Primary planned comparisons during stimulation off intervals
To determine whether SOS increased frontal slow-oscillation power, frontal delta power, frontal slow-spindle density, or time spent in SWS, paired sample t-tests were used. As shown in Table 2, data from the off intervals during the stimulation period were averaged and compared for SOS and sham-SOS conditions. Spectral power and spindle data were averaged across left and right sides, as laterality effects were not evident in separate analyses (p values > .1).
Table 2.
Sleep measures averaged across the five off intervals during stimulation or sham-stimulation. Standard error in parentheses.
| SOS | Sham-SOS | |
|---|---|---|
|
| ||
| Measures influenced by SOS in young adults: | ||
| Frontal slow-oscillation power (0.5–1.0 Hz; μV2) | 166 (15)* | 101 (15) |
| Frontal delta power (1.0–4.5 Hz; μV2) | 187 (12) | 164 (12) |
| Frontal slow spindle density (8.5–12.5 Hz; number/min) | 5.4 (.3) | 5.8 (.3) |
| Frontal slow spindle density (11.5–13.5 Hz; number/min) | 4.3 (.3) | 3.8 (.3) |
| Slow-wave sleep (min) | .6 (.7) | .7 (.1) |
|
| ||
| Measures not influenced by SOS in young adults: | ||
| Slow-oscillation power (0.5–1.0 Hz; μV2) | ||
| Central | 144 (13) | 103 (13) |
| Parietal | 153 (14) | 104 (14) |
| Occipital | 123 (12) | 87 (12) |
| Slow-spindle density (11.5–13.5 Hz; number/min) | ||
| Central | 3.8 (.3) | 3.3 (.3) |
| Parietal | 3.5 (.3) | 2.6 (.3) |
| Occipital | 1.5 (.2) | 1.1 (.2) |
| Slow-spindle density (8.5–12.5 Hz; number/min) | ||
| Central | 4.8 (.2) | 5.3 (.2) |
| Parietal | 4.4 (.2) | 4.2 (.2) |
| Occipital | 2.3 (.2) | 2.3 (.2) |
| Fast-spindle density (13.5–15.5 Hz; number/min) | ||
| Frontal | 1.1 (.1) | 1.1 (.1) |
| Central | 1.5 (.2) | 2.4 (.2)** |
| Parietal | 2.9 (.2) | 3.1 (.2) |
| Occipital | 1.2 (.1) | 1.1 (.1) |
SOS significantly greater than sham-SOS (p<.05).
sham-SOS significantly greater than SOS (p<.05).
SOS increased slow-oscillation activity in the 1-min off intervals. Frontal slow-oscillation power was greater for the SOS than for the sham-SOS nap [t(18)=2.2, p<.05], and the magnitude of this increase was very similar for males and females (65.9 μV2 and 64.2 μV2, respectively; p>.9). This SOS effect was restricted to very slow frequencies (0.5–1 Hz). Frontal delta power (1–4.5 Hz) did not differ reliably between naps [t(18)=1.0, p>.3]. When a slow-spindle frequency range comparable to that used by Marshall et al. (2006) was examined (8.5–12.5 Hz), frontal slow-spindle density did not differ between sessions [t(18)=.7, p>.4]. Using a frequency range (11.5–13.5 Hz) consistent with other reports (Anderer et al., 2001; Schabus et al., 2007; Zygierewicz et al., 1999), frontal slow-spindle density also did not differ between sessions [t(18)=.7, p>.4]. SOS also did not increase time spent in SWS during off intervals [t(18)=.5, p>.6], as it did in young adults (Marshall et al., 2006).
3.2.3. Other sleep analyses during stimulation off intervals
For all analyses described in this section, data were averaged across left and right sides, due to the absence of laterality differences in separate analyses (p values > .1). Given that effects of SOS on slow-oscillatory power and slow spindles were limited to frontal recording sites in young adults, we had no a priori predictions regarding the effects of SOS on these measures at more posterior recording sites. Nonetheless, given reports of topographical changes in spectral power and spindle activity in older adults (Landolt and Borbely, 2001; Martin et al., 2013), we also examined slow-oscillation power and slow-spindle density at central, parietal, and occipital recording sites. Paired t-tests confirmed that SOS did not increase slow-oscillatory power (p values >.09) or slow-spindle density in either frequency range (8.5–12.5 Hz: p values > .2; or 11.5–13.5 Hz: p values > .1) at central, parietal, or occipital sites relative sham-SOS (Table 2). Replicating results found in young adults (Marshall et al., 2006), no changes in the amount of time spent in stage 1 (SOS: .5 min; sham-SOS: .5 min; p >.9), stage 2 (SOS: 3 min; sham-SOS: 2.5 min; p >.09), REM (SOS: 0.1 min; sham-SOS: 0.2 min; p >.7) or awake (SOS: 0.8 min; sham-SOS: 1.1 min; p >.3) due to SOS were observed. To determine whether fast-spindle density was affected by SOS during the stimulation period, an ANOVA with session (SOS, sham-SOS) and site (frontal, central, parietal, occipital), as within-subjects variables was conducted (Table 2). A main effect of site was present, indicating that fast-spindle density was greater at central and parietal sites compared with frontal and occipital sites during both sessions [F(3,54)=12.7, p<.001], as is typically observed for fast spindles (De Gennaro and Ferrara, 2003). The session X site interaction was also significant [F(3,54)=3.2, p<.05]. Follow-up paired t-tests indicated that at central sites, fast-spindle density was greater for the sham-SOS nap than for the SOS nap [t(18)=2.5, p<.05], whereas significant differences were not present at frontal, parietal, or occipital sites (p values >.5).
3.2.4. Post-stimulation period and nap end
SOS did not influence post-stimulation sleep in young adults (Marshall et al., 2006), nor did it in older adults (Table 3). When sleep was examined for the period immediately following the stimulation or sham-stimulation period until the end of the nap, no difference in slow-oscillation power (p values >.1), slow-spindle density (11.5–13.5 Hz: p values >.09; 8.5–12.5 Hz: p values >.07), or fast-spindle density (p values >.1) was observed at any site across sessions. Likewise, SOS did not change the length of any sleep stages during this interval across naps (p values >.3).
Table 3.
Slow-oscillation power (μV2), slow-spindle density (number per minute), fast-spindle density (number per minute), and time spent in each sleep stage (min) for post-stimulation period and post-sham stimulation period sleep.
| SOS | Sham-SOS | |
|---|---|---|
|
| ||
| Slow-oscillation power (0.5–1.0 Hz; μV2) | ||
| Frontal | 113 (8) | 126 (8) |
| Central | 101 (9) | 122 (9) |
| Parietal | 101 (10) | 127 (10) |
| Occipital | 83 (12) | 108 (12) |
| Slow-spindle density (11.5–13.5 Hz; number/min) | ||
| Frontal | 3.7 (.4) | 3.9 (.4) |
| Central | 3.5 (.3) | 3.1 (.3) |
| Parietal | 3.6 (.3) | 2.7 (.3) |
| Occipital | 1.6 (.2) | 1.0 (.2) |
| Slow-spindle density (8.5–12.5 Hz; number/min) | ||
| Frontal | 4.8 (.3) | 4.8 (.3) |
| Central | 4.5 (.3) | 3.9 (.3) |
| Parietal | 4.4 (.3) | 3.4 (.3) |
| Occipital | 2.3 (.2) | 1.7 (.2) |
| Fast-spindle density (13.5–15.5 Hz; number/min) | ||
| Frontal | .9 (.1) | .6 (.1) |
| Central | 1.2 (.1) | 1.3 (.1) |
| Parietal | 2.1 (.1) | 2.1 (.1) |
| Occipital | .7 (.1) | .7 (.1) |
| Sleep stages (min) | ||
| Stage 1 | 5 (.5) | 10 (.5) |
| Stage 2 | 16 (1) | 14 (1) |
| SWS | 6 (.7) | 5 (.7) |
| REM | 10 (1) | 9 (1) |
| Wake | 8 (1) | 8 (1) |
After sleep onset, participants were given 90 min to sleep. After 90 min, some participants were awake whereas others were woken up if still asleep (SOS session: 8 participants; sham-SOS session: 11 participants). To ensure that differences in awakening time across sessions did not influence the results, we computed the difference between when a participant woke up and when the 90 min elapsed for each session for each participant (i.e., if the participant was still asleep after 90 min, the difference was 0 min). A paired t-test indicated that this was not a concern, as there were no significant differences in awakening times across sessions [t(18)=1.5, p>.1].
3.3. Sleep-Memory Relationships
Correlations were conducted to determine whether differences in sleep parameters between the SOS and sham-SOS naps were related to differences in memory improvement on the word-pair recall test across the SOS and sham-SOS naps. First, the difference in frontal slow-oscillation power between the SOS nap and the sham-SOS nap was computed for each subject. This measure was not related to across-subject differences in word-pair recall improvement between the SOS and sham-SOS nap (r=−.39, p>.09). The difference between the SOS and sham-SOS naps in SWS min and slow-spindle density (for both frequency ranges) was also computed. Separate correlations showed that none of these measures significantly predicted the difference in word-pair recall improvement across naps [SWS min: r=−.35, p>.1; slow-spindle density (8.5–12.5 Hz): r=−.11, p>.6; slow-spindle density (11.5–13.5 Hz): r=.01, p>.9].
3.4. Questionnaires
Self-reported measures of sleep quality from the previous night from a standard questionnaire (Akerstedt et al., 1994) were compared across sessions (data from one participant were excluded due to technical error). Responses did not differ across SOS and sham-SOS sessions for subjective reports of bed time, wake time, sleep latency, total sleep time, or awakenings (p values >.2). Seven additional sleep-quality ratings were made on a 5-point scale. No differences were present when numerical responses for these questions were totaled and compared across sessions [t(17)=0.1, p>.9].
Tabulation of PANAS data yielded a positive and negative affect score for each participant (Watson et al., 1988). An ANOVA with session (SOS, sham-SOS), time (before nap, after nap), and valence (positive, negative) as within-subject variables was conducted. Across all conditions, participants reported more positive than negative feelings [F(1,18)=118, p<.05]. No main effect of session (p>.7) or time (p>.09) was present, nor did any interaction approach significance (p values >.4).
To determine if alertness differed across conditions, responses to the question “How awake do you feel right now” were submitted to an ANOVA with session (SOS, sham-SOS) and time (before nap, after nap) as within-subject variables. A main effect of time [F(1,18)=14.7, p<.01] revealed that post-nap alertness (4.2) was higher than pre-nap alertness (3.3), indicating that post-nap sleep inertia was not a concern. There was no main effect of session (p>.9) nor was a time X session interaction present (p>.3), indicating that SOS did not influence alertness.
4. Discussion
Transcranial slow oscillation electrical stimulation during sleep improved verbal recall in young adults (Marshall et al., 2006; Marshall et al., 2004). Here, we demonstrated that SOS administered during an afternoon nap improved verbal recall in healthy older adults. After participants memorized word pairs, a larger recall improvement was observed after a nap containing SOS compared with a nap that did not. Naps with SOS also showed increased frontal slow-oscillation activity, consistent with the possibility that this enhancement underlies the memory facilitation. It appears that the dependence of declarative memories on slow-oscillatory activity during sleep persists into later adulthood. Therefore, some memory complaints may be secondary to ineffective sleep-dependent consolidation.
As word-pair learning is generally dependent on the hippocampus (Giovanello et al., 2003; Mayes et al., 2004), the present findings reinforce a causal role for slow oscillations during sleep in preserving hippocampal-dependent memories. As in young adults (Marshall et al., 2006; Marshall et al., 2004), SOS increased slow-oscillation power over frontal cortex. SOS did not affect the neighboring delta band (1.0–4.5 Hz), highlighting the specificity of the slow-oscillation effect. Slow oscillations originate in the neocortex and orchestrate widespread firing synchrony across the cortex and other brain regions (Crunelli and Hughes, 2010), which could coordinate hippocampal-cortical dialogue (Buzsaki, 1998; Sirota et al., 2003). Here, we speculate that that increased slow-oscillatory activity during sleep may have enhanced crosstalk among cortical regions involved in representing word-pair memories.
It should also be acknowledged that many factors can influence word-pair recall performance. Accordingly, the relative memory improvement could relate to other factors besides the SOS treatment. For example, pre-nap word-pair recall scores were slightly but not significantly lower during the SOS session compared with the sham-SOS session. On the other hand, additional analyses failed to reveal differences in learning rates, ceiling effects, alertness, or mood contributing to the apparent benefit of SOS. Yet, given that SOS effects were estimated using a two-session procedure, it remains possible that idiosyncratic factors on either session contributed to the differential pattern of results across the two sessions. Further studies are thus needed to confirm and extend these findings. For example, it remains important to determine whether benefits of sleep for later recall influence information maintained over longer periods of time.
These results are the first to establish a causal connection between slow oscillations and declarative memory in older adults. Previous indirect evidence on the stability of sleep/memory relationships throughout the lifespan has been mixed, with some evidence supporting the connection (Aly and Moscovitch, 2010; Wilson et al., 2012) and some against it (Scullin, 2013). In the latter study, however, SWS was examined but slow-oscillation power was not. Given that slow-oscillation amplitudes decrease with age (Bliwise, 1993), traditional sleep-stage measures may not fully capture the extent of slow-oscillatory activity. Spectral power provides a more sensitive measure of slow-oscillatory activity and so may be preferable for studies in older adults. Indeed, sleep/memory relationships in older adults have been observed using estimates of slow-wave power (Mander et al., 2013; Westerberg et al., 2012). Moreover medial prefrontal gray matter atrophy predicts slow-wave power decline (Mander et al., 2013), suggesting that aging-related structural brain changes mediate the extent of sleep-dependent consolidation impairments.
Although SOS improved word-pair recall and increased slow-oscillation power in healthy older adults here, another study found no effect of SOS on word-pair recall or slow-oscillation power in older adults (Eggert et al., 2013). Therefore, a critical aspect of the effectiveness of SOS on memory may be a concomitant increase in slow-oscillation power. Methodological differences may have contributed to differential findings for slow-oscillation power, as minor variations in stimulation paradigms can influence the efficacy of SOS (Berryhill et al., 2014). Here, SOS was administered across a 30-min period during an afternoon nap, whereas in the Eggert study SOS was administered across a 31.3-min period during the early part of an entire night of sleep (7.5 hours). The additional slow-wave sleep accrued across an entire night in the Eggert study may have mitigated any benefits the early stimulation provided. Furthermore, SOS electrodes were placed at F7 and F8 here, allowing SOS effects to be observed at F3 and F4. In the Eggert study, SOS electrodes were placed at F3 and F4, precluding detection of effects at these sites. Other differences between our study and the Eggert study included SOS electrode impedance limits (3 kΩ vs. 5 kΩ, respectively), participant populations and their homogeneity (ages 73.4±7.7 vs. 69.1±5.5 yrs, respectively, with different protocols for assessing cognitive integrity), stimulation procedures (abrupt stimulation vs. a ramping procedure at the beginning and end of stimulation), and different learning procedures. Here, participants completed two test rounds that included feedback before sleep. Participants in the Eggert study completed study-test cycles until a 60% criterion was reached and no feedback was given. This criterion may not promote robust retention of the word-pairs in older adults, especially with no feedback. Given this long list of procedural differences, future research will be necessary to determine why SOS does not always increase slow-wave power and memory.
The present results also demonstrate that SOS does not influence object priming, confirming that SOS selectively benefits hippocampally-dependent memories (Marshall and Born, 2007). In young participants, SOS during sleep did not improve procedural memory in finger-sequence tapping or mirror tracing tasks (Marshall et al., 2006; Marshall et al., 2004). Whereas motor regions can be particularly important for procedural memories (Grafton et al., 1992), there may be hippocampal contributions to these tasks in some situations (Schendan et al., 2003). Object priming primarily relies on visual cortex (Maccotta and Buckner, 2004), and occurs despite hippocampal damage (Cave and Squire, 1992). Therefore, the present results help to extend the range of hippocampal-independent memory tasks not influenced by SOS applied over frontal cortex during sleep, demonstrating for the first time that SOS has no effect on priming while concurrently improving word-pair recall.
As SOS did not improve fact recognition in the present study, the effects of SOS on declarative memory in older adults may be limited to recall tests. This would not be entirely surprising given that links between sleep and recognition in younger adults have been elusive (Daurat et al., 2007; Drosopoulos et al., 2005), although other features of our recognition test may also be relevant (e.g., memory for face-fact associations rather than word pair associations). Notably, slow-wave activity in our previous study with older adults was not correlated with recognition change scores across a sleep interval, despite a strong relationship with word-pair recall change scores in the same participants (Westerberg et al., 2012). Sleep-dependent consolidation may be less relevant for recognition than for recall for multiple reasons. Recall requires more extensive retrieval than recognition, because an answer must be produced in response to a cue. Interconnections between new and pre-existing knowledge could facilitate recall, by providing additional retrieval routes. Consolidation may have less relevance for recognition because an answer can be selected from a list based on familiarity, and familiarity has previously failed to show significant relationships with sleep (Atienza and Cantero, 2008). Also, whereas a hippocampal contribution to recall is necessary (Aggleton and Brown, 1999), recognition can be supported without it (Diana et al., 2007; Montaldi and Mayes, 2010; Norman and O’Reilly, 2003).
In addition to increased slow-oscillation power, Marshall et al. (2006) observed increased SWS and increased slow-spindle activity with SOS. In older adults, SOS did not affect SWS. Given that slow-wave amplitudes decline with age (Bliwise, 1993), amplitudes may remain too low even after SOS to reach the 75-μV amplitude criterion for SWS in older adults. Additionally, no SWS increase was observed by Marshall et al. (2004). Thus, conventional sleep staging may be less appropriate for assessing SOS-induced changes to the EEG signal than analyses of spectral power.
SOS also did not affect slow-spindle density in older adults, regardless of the frequency range used to define slow spindles (11.5–13.5 Hz or 8.5–12.5 Hz). Relationships between slow-spindle activity and declarative memory have been repeatedly documented during stage 2 sleep (Schabus et al., 2008; Schmidt et al., 2006). Increased slow-spindle density due to SOS might be expected, as slow-spindle activity is grouped with the excitatory up phase of slow oscillations and is maximal during SWS (Molle et al., 2011). In older adults, overall decreased levels of SWS and decreased spindle amplitude with age (Martin et al., 2013) may have precluded detection of any increase in slow spindles due to SOS.
Although fast spindles have also been linked with declarative memory consolidation in young adults (Ngo et al., 2013; van der Helm et al., 2011), fast-spindle density at central recording sites was actually larger for the sham-SOS nap compared with the SOS nap. Whereas slow spindles are maximal over frontal regions, fast spindles are typically greatest over central and parietal regions. Therefore, it has been suggested that slow and fast spindles may reflect independent mechanisms that contribute to consolidation processing (Molle et al., 2011; Schabus et al., 2007). Furthermore, it has been demonstrated that certain pharmacological interventions can jointly increase slow-spindle activity and slow-oscillatory activity, while simultaneously decreasing fast-spindle activity (Ayoub et al., 2013). Thus, one highly speculative possibility is that by enhancing slow-oscillatory activity with SOS in elderly adults, a concomitant decrease in fast-spindle activity might be produced, but further research is necessary to assess this possibility.
No differences in SWS or slow-oscillation activity were observed from the period after the stimulation (or sham-stimulation) until the end of the nap. This was not surprising, given that no such differences were observed in young adults (Marshall et al., 2004; Marshall et al., 2006), but it could also be attributed to the decreasing levels of SWS typically observed across a sleep period, especially in older adults (Bliwise, 1993).
5. Conclusions
The present results are the first to demonstrate that SOS during sleep improves retention of declarative memories in older adults, and the concomitant increase in slow-oscillation power implicated a mechanism through which memory storage could be facilitated. Whereas it has been previously speculated that SWS may be a “functionally meaningless remnant” in older adults (Spiegel et al., 1986), here we demonstrate a functional role for slow-wave activity in declarative memory with individuals of advancing age. However, important differences may exist between intrinsic slow oscillations and SOS-driven effects, so future research will be necessary to substantiate the presumptive link between intrinsically generated slow oscillations and age-related memory decline. Not only does the present study shed light on current theoretical controversies surrounding memory consolidation in older adults, but it also points to ways in which memory deficits may be ameliorated. Whether or not SOS or related and less-invasive techniques (Ngo et al., 2013; Oudiette et al., 2013; Santostasi et al., under review) prove to be useful tools for treating memory decline, the current results suggest that interventions targeting sleep may prove beneficial for improving memory in older adults.
Highlights.
Poor memory processing during sleep contributes to age-related memory decline
Electrical stimulation delivered transcranially to 19 healthy adults 65–85 years old
Stimulation oscillated at 0.75 Hz up to 260 μA, across 30 min, during afternoon naps
Stimulation facilitated slow waves and improved memory compared to shamstimulation
Acknowledgments
We thank Jessica Creery and Lori McGee Koch for assistance with data collection, Jan Born and Giovanni Santostasi for their guidance, and Horst Koller for designing and constructing the stimulator. This research was supported by the donors of Alzheimer’s Disease Research, a program of the Bright Focus Foundation, formerly the American Health Assistance Foundation (K.A.P, C.W.), a Senator Mark Hatfield Award from the Alzheimer’s Association (K.A.P.), the Illinois Department of Public Health Alzheimer’s Disease Research Fund (K.A.P.), the National Institute on Aging (P.C.Z., P01 AG11412), the German Ministry of Education and Research Grant 01GQ1008 (L.M.), and the Northwestern University Cognitive Neurology and Alzheimer’s Disease Center, including a Pilot Project Grant Award (M.M.M., NIH P30 AG13854).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci. 1999;22:425–44. discussion 44–89. [PubMed] [Google Scholar]
- Akerstedt T, Hume K, Minors D, Waterhouse J. The subjective meaning of good sleep, an intraindividual approach using the Karolinska Sleep Diary. Percept Mot Skills. 1994;79:287–96. doi: 10.2466/pms.1994.79.1.287. [DOI] [PubMed] [Google Scholar]
- Altena E, Ramautar JR, Van Der Werf YD, Van Someren EJW. Do sleep complaints contribute to age-related cognitive decline? In: Kerkhof GA, Van Dongen HPA, editors. Progress in Brain Research. Amsterdam: Elsevier B.V; 2010. pp. 183–205. [DOI] [PubMed] [Google Scholar]
- Aly M, Moscovitch M. The effects of sleep on episodic memory in older and younger adults. Memory. 2010;18:327–34. doi: 10.1080/09658211003601548. [DOI] [PubMed] [Google Scholar]
- Anderer P, Klosch G, Gruber G, Trenker E, Pascual-Marqui RD, Zeitlhofer J, et al. Low-resolution brain electromagnetic tomography revealed simultaneously active frontal and parietal sleep spindle sources in the human cortex. Neuroscience. 2001;103:581–92. doi: 10.1016/s0306-4522(01)00028-8. [DOI] [PubMed] [Google Scholar]
- Atienza M, Cantero JL. Modulatory effects of emotion and sleep on recollection and familiarity. J Sleep Res. 2008;17:285–94. doi: 10.1111/j.1365-2869.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- Ayoub A, Aumann D, Horschelmann A, Kouchekmanesch A, Paul P, Born J, et al. Differential effects on fast and slow spindle activity, and the sleep slow oscillation in humans with carbamazepine and flunarizine to antagonize voltage-dependent Na+ and Ca2+ channel activity. Sleep. 2013;36:905–11. doi: 10.5665/sleep.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Peterson DJ, Jones KT, Stephens JA. Hits and misses: Leveraging tDCS to advance cognitive research. Front Psychol. 2014;5:800. doi: 10.3389/fpsyg.2014.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Memory consolidation during sleep: A neurophysiological perspective. J Sleep Res. 1998;7(Suppl 1):17–23. doi: 10.1046/j.1365-2869.7.s1.3.x. [DOI] [PubMed] [Google Scholar]
- Cave CB, Squire LR. Intact and long-lasting repetition priming in amnesia. J Exp Psychol Learn Mem Cogn. 1992;18:509–20. doi: 10.1037//0278-7393.18.3.509. [DOI] [PubMed] [Google Scholar]
- Chee MW, Chuah LY. Functional neuroimaging insights into how sleep and sleep deprivation affect memory and cognition. Curr Opin Neurol. 2008;21:417–23. doi: 10.1097/WCO.0b013e3283052cf7. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Byrd M. Aging and cognitive deficits. In: Craik FIM, Trehub S, editors. Aging and Cognitive Processes. New York: Springer US; 1982. pp. 191–211. [Google Scholar]
- Crenshaw MC, Edinger JD. Slow-wave sleep and waking cognitive performance among older adults with and without insomnia complaints. Physiol Behav. 1999;66:485–92. doi: 10.1016/s0031-9384(98)00316-3. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Hughes SW. The slow (<1 Hz) rhythm of non-REM sleep: A dialogue between three cardinal oscillators. Nat Neurosci. 2010;13:9–17. doi: 10.1038/nn.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daurat A, Terrier P, Foret J, Tiberge M. Slow wave sleep and recollection in recognition memory. Conscious Cogn. 2007;16:445–55. doi: 10.1016/j.concog.2006.06.011. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7:423–40. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11:379–86. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Groeger JA, Stanley N, Deacon S. Age-related reduction in daytime sleep propensity and nocturnal slow wave sleep. Sleep. 2010;33:211–23. doi: 10.1093/sleep/33.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosopoulos S, Wagner U, Born J. Sleep enhances explicit recollection in recognition memory. Learn Mem. 2005;12:44–51. doi: 10.1101/lm.83805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Willson HJ, Wang W, Czeisler CA. Healthy older adults better tolerate sleep deprivation than young adults. J Am Geriatr Soc. 2009;57:1245–51. doi: 10.1111/j.1532-5415.2009.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert T, Dorn H, Sauter C, Nitsche MA, Bajbouj M, Danker-Hopfe H. No effects of slow oscillatory transcranial direct current stimulation (tDCS) on sleep-dependent memory consolidation in healthy elderly subjects. Brain Stimul. 2013;6:938–45. doi: 10.1016/j.brs.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gais S, Albouy G, Boly M, Dang-Vu TT, Darsaud A, Desseilles M, et al. Sleep transforms the cerebral trace of declarative memories. Proc Natl Acad Sci U S A. 2007;104:18778–83. doi: 10.1073/pnas.0705454104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanello KS, Verfaellie M, Keane MM. Disproportionate deficit in associative recognition relative to item recognition in global amnesia. Cogn Affect Behav Neurosci. 2003;3:186–94. doi: 10.3758/cabn.3.3.186. [DOI] [PubMed] [Google Scholar]
- Goder R, Baier PC, Beith B, Baecker C, Seeck-Hirschner M, Junghanns K, et al. Effects of transcranial direct current stimulation during sleep on memory performance in patients with schizophrenia. Schizophr Res. 2013;144:153–4. doi: 10.1016/j.schres.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Mazziotta JC, Presty S, Friston KJ, Frackowiak RS, Phelps ME. Functional anatomy of human procedural learning determined with regional cerebral blood flow and PET. J Neurosci. 1992;12:2542–8. doi: 10.1523/JNEUROSCI.12-07-02542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeger JA, Stanley N, Deacon S, Dijk DJ. Dissociating effects of global SWS disruption and healthy aging on waking performance and daytime sleepiness. Sleep. 2014;37:1127–42. doi: 10.5665/sleep.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harand C, Bertran F, Doidy F, Guenole F, Desgranges B, Eustache F, et al. How aging affects sleep-dependent memory consolidation? Front Neurol. 2012;3:8. doi: 10.3389/fneur.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung OP, Danker-Hopfe H, Heuser I. Age-related changes in sleep and memory: Commonalities and interrelationships. Exp Gerontol. 2005;40:279–85. doi: 10.1016/j.exger.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM manual for the scoring of sleep and associated events. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Landolt HP, Borbely AA. Age-dependent changes in sleep EEG topography. Clin Neurophysiol. 2001;112:369–77. doi: 10.1016/s1388-2457(00)00542-3. [DOI] [PubMed] [Google Scholar]
- Luo L, Craik FIM. Aging and memory: A cognitive approach. Can J Psychiat. 2008;53:346–53. doi: 10.1177/070674370805300603. [DOI] [PubMed] [Google Scholar]
- Maccotta L, Buckner RL. Evidence for neural effects of repetition that directly correlate with behavioral priming. J Cogn Neurosci. 2004;16:1625–32. doi: 10.1162/0898929042568451. [DOI] [PubMed] [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16:357–64. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Molle M, Hallschmid M, Born J. Transcranial direct current stimulation during sleep improves declarative memory. J Neurosci. 2004;24:9985–92. doi: 10.1523/JNEUROSCI.2725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–3. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- Marshall L, Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cogn Sci. 2007;11:442–50. doi: 10.1016/j.tics.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Marshall L, Kirov R, Brade J, Molle M, Born J. Transcranial electrical currents to probe EEG brain rhythms and memory consolidation during sleep in humans. PLoS One. 2011;6:e16905. doi: 10.1371/journal.pone.0016905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N, Lafortune M, Godbout J, Barakat M, Robillard R, Poirier G, et al. Topography of age-related changes in sleep spindles. Neurobiol Aging. 2013;34:468–76. doi: 10.1016/j.neurobiolaging.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Holdstock JS, Isaac CL, Montaldi D, Grigor J, Gummer A, et al. Associative recognition in a patient with selective hippocampal lesions and relatively normal item recognition. Hippocampus. 2004;14:763–84. doi: 10.1002/hipo.10211. [DOI] [PubMed] [Google Scholar]
- Molle M, Bergmann TO, Marshall L, Born J. Fast and slow spindles during the sleep slow oscillation: Disparate coalescence and engagement in memory processing. Sleep. 2011;34:1411–21. doi: 10.5665/SLEEP.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaldi D, Mayes AR. The role of recollection and familiarity in the functional differentiation of the medial temporal lobes. Hippocampus. 2010;20:1291–314. doi: 10.1002/hipo.20853. [DOI] [PubMed] [Google Scholar]
- Nelson DL, McEvoy CL, Schreiber TA. The University of South Florida word association, rhyme, and word fragment norms. 1998 doi: 10.3758/bf03195588. [DOI] [PubMed] [Google Scholar]
- Ngo HV, Martinetz T, Born J, Molle M. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron. 2013;78:545–53. doi: 10.1016/j.neuron.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Norman KA, O’Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol Rev. 2003;110:611–46. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- Oudiette D, Santostasi G, Paller KA. Reinforcing rhythms in the sleeping brain with a computerized metronome. Neuron. 2013;78:413–5. doi: 10.1016/j.neuron.2013.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Spencer RM. Age-related changes in the cognitive function of sleep. Prog Brain Res. 2011;191:75–89. doi: 10.1016/B978-0-444-53752-2.00012-6. [DOI] [PubMed] [Google Scholar]
- Paller KA. Memory consolidation: systems. In: Squire LR, editor. Encyclopedia of Neuroscience. Oxford: Academic Press; 2009. pp. 741–9. [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–45. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997;9:534–47. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- Prehn-Kristensen A, Munz M, Goder R, Wilhelm I, Korr K, Vahl W, et al. Transcranial oscillatory direct current stimulation during sleep improves declarative memory consolidation in children with attention-deficit/hyperactivity disorder to a level comparable to healthy controls. Brain Stimul. 2014;7:793–9. doi: 10.1016/j.brs.2014.07.036. [DOI] [PubMed] [Google Scholar]
- Santostasi G, Malkani R, Reidner B, Bellesi M, Tononi G, Paller KA, et al. Phase-locked loop for precisely timed acoustic stimulation during sleep. doi: 10.1016/j.jneumeth.2015.11.007. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabus M, Dang-Vu TT, Albouy G, Balteau E, Boly M, Carrier J, et al. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2007;104:13164–9. doi: 10.1073/pnas.0703084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabus M, Hoedlmoser K, Pecherstorfer T, Anderer P, Gruber G, Parapatics S, et al. Interindividual sleep spindle differences and their relation to learning-related enhancements. Brain Res. 2008;1191:127–35. doi: 10.1016/j.brainres.2007.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendan HE, Searl MM, Melrose RJ, Stern CE. An FMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron. 2003;37:1013–25. doi: 10.1016/s0896-6273(03)00123-5. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Peigneux P, Muto V, Schenkel M, Knoblauch V, Munch M, et al. Encoding difficulty promotes postlearning changes in sleep spindle activity during napping. J Neurosci. 2006;26:8976–82. doi: 10.1523/JNEUROSCI.2464-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullin MK. Sleep, memory, and aging: The link between slow-wave sleep and episodic memory changes from younger to older adults. Psychol Aging. 2013;28:105–14. doi: 10.1037/a0028830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota A, Csicsvari J, Buhl D, Buzsaki G. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci U S A. 2003;100:2065–9. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel R, Koberle S, Allen SR. Significance of slow wave sleep: Considerations from a clinical viewpoint. Sleep. 1986;9:66–79. doi: 10.1093/sleep/9.1.66. [DOI] [PubMed] [Google Scholar]
- Takashima A, Nieuwenhuis IL, Jensen O, Talamini LM, Rijpkema M, Fernandez G. Shift from hippocampal to neocortical centered retrieval network with consolidation. J Neurosci. 2009;29:10087–93. doi: 10.1523/JNEUROSCI.0799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Helm E, Gujar N, Nishida M, Walker MP. Sleep-dependent facilitation of episodic memory details. PLoS One. 2011;6:e27421. doi: 10.1371/journal.pone.0027421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg CE, Lundgren EM, Florczak SM, Mesulam MM, Weintraub S, Zee PC, et al. Sleep influences the severity of memory disruption in mild cognitive impairment: Results from sleep self-assessment and continuous activity monitoring. Alzheimer Dis Assoc Disord. 2010;24:325–33. doi: 10.1097/WAD.0b013e3181e30846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg CE, Mander BA, Florczak SM, Weintraub S, Mesulam MM, Zee PC, et al. Concurrent impairments in sleep and memory in amnestic mild cognitive impairment. J Int Neuropsychol Soc. 2012;18:490–500. doi: 10.1017/S135561771200001X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JK, Baran B, Pace-Schott EF, Ivry RB, Spencer RM. Sleep modulates word-pair learning but not motor sequence learning in healthy older adults. Neurobiol Aging. 2012;33:991–1000. doi: 10.1016/j.neurobiolaging.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygierewicz J, Blinowska KJ, Durka PJ, Szelenberger W, Niemcewicz S, Androsiuk W. High resolution study of sleep spindles. Clin Neurophysiol. 1999;110:2136–47. doi: 10.1016/s1388-2457(99)00175-3. [DOI] [PubMed] [Google Scholar]