Abstract
Quality control systems in the endoplasmic reticulum (ER) mediated by unfolded protein response (UPR) and endoplasmic reticulum associated degradation (ERAD) ensure cellular function and organismal survival. Recent studies have suggested that ER quality-control systems in cancer cells may serve as a double-edged sword that aids progression as well as prevention of tumor growth in a context-dependent manner. Here we review recent advances in our understanding of the complex relationship between ER proteostasis and cancer pathology, with a focus on the two most conserved ER quality-control mechanisms – the IRE1α-XBP1 pathway of the UPR and SEL1L-HRD1 complex of the ERAD.
Keywords: Protein folding, ER homeostasis, UPR, IRE1α, XBP1, ERAD, SEL1L-HRD1, tumorigenesis
1. Introduction
In eukaryotic cells, approximately one third of the total proteome is folded to maturity in the endoplasmic reticulum (ER) prior to transportation to various subcellular or extracellular compartments. A myriad of chaperones, folding enzymes and nascent proteins crowd the molecular environment of the ER lumen all the while maintaining a delicate homeostasis in its protein folding machinery. Various perturbations to this equilibrium, including both physiological and pathological stimuli, can lead to an accumulation of misfolded proteins inside the ER, subjecting the cell to a condition called “ER stress” and activating a series of adaptive mechanisms to alleviate the stress and restore ER homeostasis. These mechanisms consist of two major ER quality control machineries, including unfolded protein response (UPR) and ER-associated degradation (ERAD) [1–3].
Originally discovered as a response to nutrient depletion, autophagy is a cellular process involved in the lysosomal degradation of cellular components and in the maintenance of energy homeostasis through recycling of amino acids and nutrients [4]. Several studies suggest that autophagy is activated as an adaptive mechanism in cells experiencing ER stress and may play a role in the maintenance of ER homeostasis in cancer [5, 6]. However, as the role of autophagy goes beyond the ER [7], whether the effect of autophagy in cancer is related to its function in the ER remains to be established. Hence, as the role of autophagy in cancer has recently been extensively reviewed [8–10], it will not be the focus here.
Owing to a high proliferation rate, cancer cells often experience impaired ATP generation, hypoxia, hypoglycemia and specific mutations which may perturb ER homeostasis and trigger the activation of UPR [2]. Persistent ER stress often activates pathways that lead to cell death, effectively eliminating cells with a potential to go rogue. On the other hand, tumor cells may hijack the ER quality control machineries to provide survival signals required for neoplasm growth and eventually avoid cell death [11]. Researchers have considered targeting various components of UPR and ERAD as potent therapeutic means to specifically modulate the survival of cancer cells [12]. In this review, we will discuss the involvement of two most highly conserved branches of the ER quality control systems – the IRE1α signaling pathway of the UPR and the SEL1L-HRD1 complex of the ERAD – in cancer pathogenesis.
2. The IRE1α signaling pathway
IRE1 is a type-1 ER-resident membrane protein with bifunctional cytosolic kinase and endoribonuclease (RNase) domains [13, 14]. In mammals, IRE1 exists in two isoforms, IRE1α [15] and IRE1β [16]. IRE1α is ubiquitously expressed and global knockout of the gene results in early embryonic lethality [17, 18]. In contrast, IRE1β expression is limited to the gastrointestinal epithelial cells [19] and has no RNase activity towards the classical IRE1α substrate X-box binding protein 1 (Xbp1) mRNA [20]. While IRE1β knockout mice are viable, they are hypersensitive to experimental colitis [19], which may be in part due to reduced mucin biosynthesis [20].
Upon ER stress, IRE1α undergoes dimerization and/or oligomerization and trans-autophosphorylation, which triggers conformational change and activation of its RNase domain. Activated IRE1α splices 26 nucleotides from Xbp1 mRNA, leading to translational frameshift and the generation of an active transcription factor XBP1s. Subsequently, XBP1s enters the nucleus, where it transactivates various target genes, including those involved in protein folding, ERAD, protein trafficking, and lipid biosynthesis (Figure 1) [21]. Additionally, IRE1α has been shown to degrade a subset of mRNAs via a process called Regulated IRE1-Dependent Decay (RIDD) (Figure 1) [22–25]. Moreover, IRE1α cleaves some premature microRNAs as a means of regulating apoptosis [26] as well as its own mRNA level [27, 28]. The physiological significance of these extra-Xbp1 activities of IRE1α in vivo remains poorly characterized.
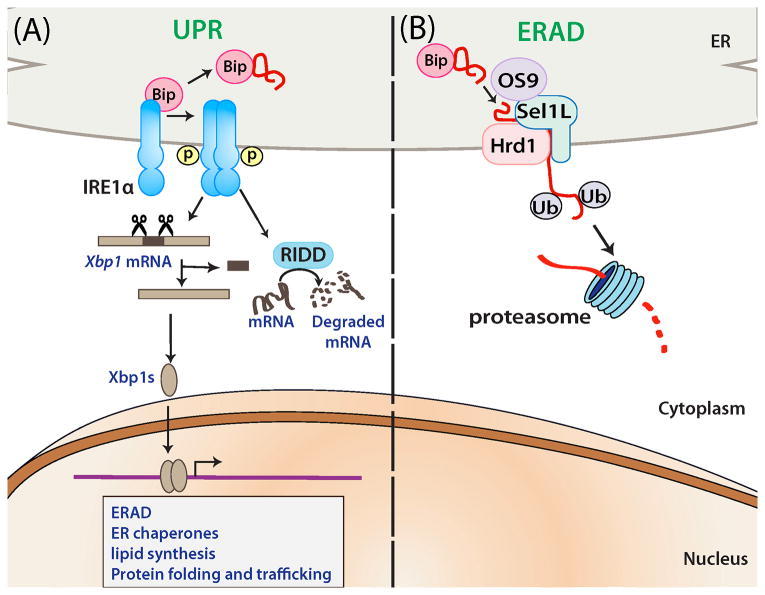
Fig. 1. Schematic diagrams depicting the roles of IRE1α in UPR and SEL1L-HRD1 in ERAD.
Upon sensing ER stress, IRE1α undergoes dimerization or oligomerization, and trans-autophosphorylation, activating its cytosolic endonuclease activity. Subsequently, IRE1αalternatively splices Xbp1 mRNA to generate Xbp1s which translocates into the nucleus and regulates different genes. Furthermore, activated IRE1α can selectively degrade particular mRNAs by a process called regulated IRE1-dependent decay (RIDD). Unlike IRE1α-XBP1 pathway, physiological significance of other IRE1α pathways are not well established. (B) Misfolded proteins in the ER lumen are recognized, ubiquitinated and retrotranslocated by the HRD1-SEL1L ERAD complex to the cytosol for proteasomal degradation. Bip and OS9 may be involved in the recognition of misfolded substrates.
Similar to IRE1α-deficient mice, global deletion of XBP1 leads to embryonically lethal in mice [17, 18, 29]. Using cell type-specific knockout mouse models, studies have demonstrated a critical role of IRE1α-XBP1 pathway in secretory cells, most notably B cell-derived plasma cells and pancreatic β cells. Mice with B cell-specific Xbp1 deficiency show a profound defect in plasma cell production, along with decreased levels of antigen-specific immunoglobulin [30–32]. Intriguingly, IRE1α deficiency in B cells affects not only plasma cell differentiation, but also early stage of B cell development [17]. While VDJ rearrangement occurs normally in XBP1−/− B cells [30], this event is severely defective in the pro-B cell stage of IRE1α−/− B cells [17]. The authors propose that the cytoplasmic domain of IRE1α may directly regulate transcriptional activation of genes involved in VDJ recombination such as Rag1 (recombination-activating gene 1), Rag2 (recombination-activating gene 2), and TdT (terminal deoxynucleotidyl transferase).
In vitro, IRE1α can be activated by glucose in a concentration-dependent manner [33] and hyperactivation of IRE1α by high glucose may lead to insulin mRNA degradation in pancreatic β cells [34]. Intriguingly, β cell-specific deletion of Xbp1 in mice results in islet atrophy and hyperglycemia associated with impaired β cell proliferation, insulin maturation and secretion at basal level [35]. Moreover, deficiency of XBP1 caused constitutive hyperactivation of IRE1α, leading to attenuation of insulin mRNA via RIDD. On the other hand, while IRE1α deficiency in β cells causes disruption in glucose homeostasis and impairs β cell proliferation under metabolic stress, it did not affect pancreatic structure or islet area [36]. These differential phenotypes observed in β cell specific IRE1α- and XBP1- null mice suggest that each component of this pathway may have its own unique function in cellular physiology. Alternatively, it points to a possible role of the unspliced form of XBP1u, whose physiological role awaits further investigation. Taken together these studies highlight the indispensible role of the IRE1α-XBP1 pathway in ER expansion and survival of highly secretory cell types.
3. The role of IRE1α-XBP1s signaling pathway in cancer
Figure 2 depicts various possible molecular mechanisms underlying the role of IRE1α in cancer. The role of IRE1α in cancer is best illustrated and characterized in multiple myeloma (MM). MM is a malignant proliferation of plasma cells in the bone marrow and share phenotypical characteristics with long-lived plasma cells. Due to abundant synthesis of secretory proteins in the ER, MM cells are hypersensitive to the activation of UPR that aggravates as the disease advances [37]. Thus, these cells require a large capacity of folding and disposal in the ER and are particularly sensitive to compounds targeting proteostasis. IRE1α activation can contribute to cancer progression in several pathways mediated by its substrate XBP1s, which is highly expressed in MM [38]. Blocking of IRE1αRNase activity by IRE1 inhibitors such as STF-083010 or 4μ8C or similarly reducing XBP1 expression by proteasome inhibitor or toyocamycin, an XBP1 inhibitor, attenuates the growth of MM cells, via apoptosis [39–42]. Conversely, forced expression of XBP1s in B cells promotes multiple characteristics of myeloma pathogenesis with lytic bone lesions, plasmacytosis and increased monoclonal antibodies [43]. More than 1,000 genes are upregulated in XBP1s-transgenic myeloma cells compared to non-transgenic B cells, including Cyclin D1, Cyclin D2, MAF and MAFB, many of which are known to be involved in human MM pathogenesis. In clinical studies, human MM patients with high ratio of Xbp1s mRNA to Xbp1u mRNA have a significantly lower survival rate [38]. Collectively, these studies suggest a potential causative role of XBP1s in diseases pathogenesis in some MM patients and implicate IRE1α-XBP1 axis as a potential therapeutic target in MM.
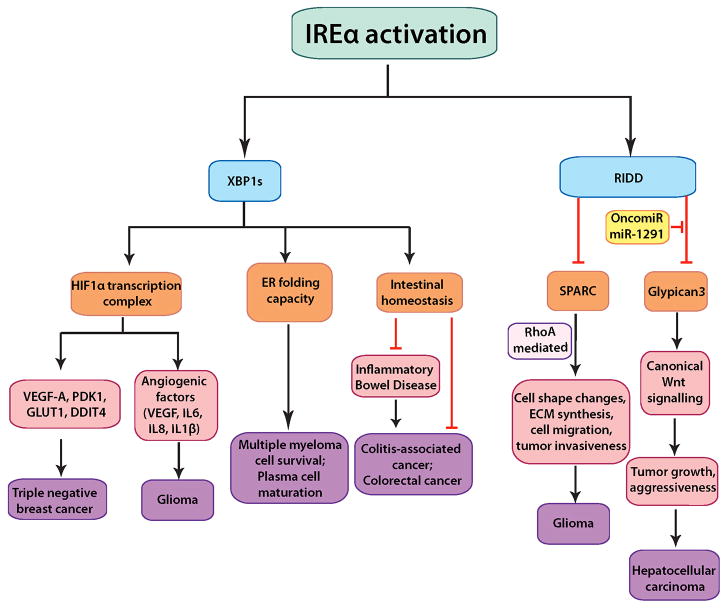
Fig. 2. The role of IRE1α-mediated signaling pathways in cancer pathogenesis.
IRE1α can exert XBP1s–dependent and –independent functions in cancer cells. In XBP1s-dependent pathways, IRE1α activation can trigger plasma cell maturation and multiple myeloma cell survival; protection from colon cancer via maintenance of intestinal homeostasis; induction of transcription of angiogenic factors and other tumor promoting components in complex formation with HIF1α, as discovered in gliomas and breast cancer. Via RIDD, IRE1α downregulates SPARC mRNA thereby preventing RhoA-mediated increase in glioma invasiveness. In liver cancer, the oncogenic miR-1291 downregulates IRE1α, thereby allowing its otherwise RIDD substrate Glypican-3-to promote tumor growth and aggressiveness via canonical Wnt signaling.
In addition to MM, the IRE1α-XBP1 signaling pathway has been implicated in colon and breast cancers. As substantial evidence is lacking in most cases, we will discuss these studies in brief. XBP1 has been implicated in colon carcinogenesis in a 2007 clinical study, where higher levels of total Xbp1 mRNA and protein assessed via RT-PCR and immunohistochemistry, respectively, were found in colorectal polyps, colon carcinomas and colon cancer cell lines as compared to normal and stromal tissue [44]. It should be pointed out that this study was limited by a small dataset of only 11 patients. In mice, loss of Xbp1 in the intestinal epithelium leads to an increase in intestinal stem cell numbers in an IRE1α-dependent manner and Stat3-dependent hyper-proliferation of intestinal epithelial cells [45]. Consequently, these Xbp1 null mice are more susceptible to colitis-associated cancer as well as genetic-induced colorectal cancer associated with the mutation of adenomatous polyposis coli (Apc-min).
In addition to its role in ER maintenance, the IRE1α-XBP1 signaling pathway may aid in the regulation of hypoxia in highly aggressive triple-negative breast cancer (TNBC) [46]. In breast cancer cell lines and xenograft models, loss of Xbp1 reduces tumor growth and metastasis due to impaired angiogenesis, independently of cell proliferation or apoptosis. ChIP-seq analysis coupled with co-IP experiments reveals that XBP1s and HIF1α may function within the same transcriptional complex to regulate the expression of genes involved in survival and angiogenesis such as VEGFA (Vascular endothelial growth factor A), PDK1 (Phosphoinositide-dependent kinase 1), GLUT1 (Glucose transporter 1), DDIT4 (DNA-damage-inducible transcript 4) via the recruitment of RNA polymerase II [46]. In line with this notion, inhibition of IRE1α in gliomas reduces the expression of pro-angiogenic genes such as VEGF-A, IL-6, IL-8 and IL-1β, while having an opposite effect on anti-angiogenic factors and matrix proteins such as thrombospondin-1, decorin and osteonectin (also known as secreted protein acidic and rich in cysteine or SPARC) [47, 48]. Indeed, in tumors expressing a dominant negative IRE1α, where IRE1α transmembrane and luminal domains (aa 1–555) is fused upstream to full length Nck-1 (non-catalytic region of tyrosine kinase adaptor protein 1), there is a marked decrease in angiogenesis, tumor vascular density and growth [47, 49]. Taken together, these studies point to a critical role of IRE1α in mediating hypoxia and angiogenesis in tumor growth and identify the IRE1α-XBP1s signaling pathway as a candidate for therapeutic intervention in targeting the angiogenic switch in tumor development.
IRE1α may be involved in cancer pathogenesis via Xbp1-independent pathways as well (Figure 2). Various RIDD targets have recently been implicated in pathways promoting tumor growth and metastasis. Among them lies SPARC, a matrix-associated protein that retards cell-cycle progression, triggers changes in cell shape and induces synthesis of extracellular matrix, thereby promoting tumor cell invasiveness. [22, 50]. IRE1α, via its RNase activity, leads to a downregulation of Sparc mRNA levels as shown in a rat glioma model. Expression of the same aforementioned dominant negative IRE1α transgene leads to an increase in tumor cell attachment and migration along with upregulation of SPARC and activation of its mediator RhoA, a cytoskeleton regulator protein [49, 51].
Glypican-3 (GPC3) is an RIDD substrate as its mRNA is cleaved at the 3’ UTR by IRE1α in an ER stress-independent manner [53]. GPC3 is a glycosylphosphatidylinositol (GPI)-anchored membrane protein that has been associated in diseases such as Wilms tumors and Simpson-Golabi-Behmel syndrome, and is highly overexpressed in hepatoblastoma and hepatocellular carcinoma (HCC) [54]. GPC3 stimulates canonical Wnt signaling by forming complexes with Wnts, thereby promoting aggressiveness, tumor growth and poor prognosis [55]. In the GPC3high HCC subgroups, the oncogenic microRNA, miR-1291, is particularly up-regulated [53]. Intriguingly, miR-1291 downregulates IRE1α via destabilization of its mRNA by targeting a site in its 5’ UTR region [53]. This regulatory circuit whereby the oncomiR-1291 downregulates IRE1α leading to high levels of GPC3 may promote tumor growth and invasiveness via stimulation of canonical Wnt signaling in liver cancer cells.
Finally, IRE1α has also been implicated in the pathogenesis of several cancers via mechanisms yet unknown. Studies conducted using glioblastoma, serous ovarian cancer and lung adenocarcinoma models have demonstrated that mutations often accrue in IRE1α genomic loci [56]. We recently analyzed the activities of some cancer-associated IRE1α mutants (P830L, S769F, L474R, and R635W) and found that a highly conserved proline residue at position 830 (Pro830) is crucial for maintaining IRE1α structural integrity [57]. This residue seems to act as a structural linker with adjacent tyrosine (Tyr945) and tryptophan (Trp833) residues to link the kinase and RNase domains of IRE1α. The P830L mutation destabilizes IRE1α and renders both kinase and RNase domains of IRE1α inactive. Similarly, the Ser769Phe mutation abolishes IRE1α activation and signaling, although the mechanism remains unclear [57]. It is possible that cancer cells may accrue these loss-of-function IRE1α mutations to attenuate the pro-apoptotic function of IRE1α as recently proposed [58]. However, as whether or not IRE1αsignaling exerts pro- and/or anti-apoptotic effects remains controversial [58, 59], further investigations are required to delineate physiological significance of IRE1α mutations in tumorigenesis.
4. ERAD
ERAD is responsible for the recognition, retrotranslocation and ubiquitination of misfolded proteins in the ER for proteasomal degradation in the cytosol [60]. The ERAD system revolves around transmembrane ubiquitin E3 ligase proteins that connect together the substrate recognition machinery in the ER lumen and the ubiquitin-proteasome system in the cytosol. Failure to remove misfolded ER proteins may result in their accumulation and aggregation, which may account for the pathogenesis of various diseases such as cystic fibrosis, α1-antitrypsin deficiency and type-1 diabetes [61, 62]
There are two principle E3 ERAD complexes in yeast (Hrd1p and Doa10p), and at least half a dozen in metazoans, each of which recognizes a subset of misfolded proteins in the ER [60]. Hrd1p forms a complex with Hrd3p in yeast [63, 64] and with Suppressor/Enhancer of Lin-12-like (Sel1L) in mammals [65, 66]. As shown in Figure 1, Sel1L nucleates the Hrd1 ERAD complex by interacting with multiple ERAD components such as Hrd1, Derlin1/2, p97, OS9, and E2 enzyme UBC6e [65–67]. A recent proteomic analysis has implicated both Sel1L-dependent and -independent Hrd1-mediated degradation, which may be dictated by substrate topology or accessibility of specific E3 ligases [67].
Physiological importance of SEL1L and HRD1 in vivo is recently emerging. Global deletion of Sel1L causes embryonic lethality in mice [68, 69]. In the absence of Sel1L, the development of embryonic pancreatic epithelial cell was blocked [70]. Using inducible and adipocyte-specific Sel1L -deficient mouse and cell models, we recently demonstrated that Sel1L plays a critical role in the stabilization of HRD1 protein in mammals [69, 71] and that the Sel1L –Hrd1 complex plays a critical role in mammalian ERAD, ER homeostasis and survival in vivo [69]. Acute loss of Sel1L in adult mice causes premature lethality and severe pathologies of secretory tissues with striking abnormalities of the ER structure integrity, suggesting a crucial role of Sel1L in secretory cell types in particular. On the other hand, loss of Sel1L in adipocytes leads to resistance to diet-induced obesity and postprandial hypertriglyceridemia due to the ER retention of lipoprotein lipase [71]. In addition, variants in the SEL1L gene have also been identified in humans with Alzheimer’s diseases [72] and SEL1L mutations have been linked to early-onset cerebellar ataxia in canines [73], pointing to a possible role of SEL1L in maintaining homeostasis in neuronal/glial cells.
Global deletion of Hrd1, also known as Synoviolin (encoded by the gene Syvn1), also causes embryonic lethality in mice [74]. Loss of Hrd1 in the liver upregulates the expression of Nrf2 (Nuclear factor (erythroid-derived 2)-like 2) protein and its target genes Nqo1 (NAD(P)H quinone oxidoreductase 1) and Gclm (glutamate-cysteine ligase, modifier subunit). This study demonstrated that Nrf2 is a substrate for Hrd1-mediated proteasomal degradation in the pathogenesis of liver cirrhosis. In this context, ER and oxidative stress response signaling pathways converge as ubiquitination of Nrf2 by HRD1 results in downregulation of the Nrf2-mediated antioxidant response pathway [75]. In dendritic cells (DC), Hrd1 seems to regulate the expression of MHC (major histocompatibility complex) class II via regulating the protein turnover of a key transcriptional repressor BLIMP1 (B lymphocyte induced maturation protein 1) [76]. Loss of Hrd1 causes the accumulation of BLIMP1, which represses gene transcription of MHC class II. Dendritic cell (DC)-specific Hrd1 knockout mice exhibit splenomegaly with increased B cell numbers and defects in CD4+ T cell priming in the autoimmune inflammatory response [76]. How Hrd1 mediates the degradation of nuclear transcription factor and whether this function of Hrd1 is Sel1L dependent remain to be demonstrated.
5. The role of SEL1L-HRD1 ERAD in cancer
While the role of ERAD in cancer remains largely unknown, SEL1L has been implicated in cancer pathogenesis. Ectopic SEL1L induction in pancreatic cancer cells leads to G1 phase cell cycle arrest via the induction of PTEN – a phosphoinositide-3-phosphatase and well-known tumor suppressor that normally inhibits cell proliferation, growth and motility. High levels of SEL1L in these cells also leads to reduction in invasiveness possibly via negative modulation of genes encoding matrix metalloproteinase inhibitors [77]. Furthermore, a 2012 study discovered that the SEL1L SNP (rs12435998) shares a close association with the age at diagnosis of pancreatic ductal adenocarcinoma and the patient survival time with or without pancreaticoduodenectomy by analysis of DNA obtained from Caucasian (non-smoker) patients [78].
In the context of colorectal cancer, while basal SEL1L expression level in normal mucosa of the epithelial lining is low, it is elevated in adenoma and adenocarcinoma cells [79]. Nonetheless, SEL1L expression pattern has so far been found to lack correlation with patient survival and the grade of colon cancer. In an investigation involving glioma stem cell lines and valproic acid (VPA), a histone deacetylase inhibitor, the same group reported that SEL1L downregulation in glioma stem cell lines leads to an impairment of neurosphere size and proliferative rate, while inducing differentiation towards a neuronal fate via Notch1 signaling [80]. VPA, a promising therapeutic agent owing to its anti-cancer and minimally toxic properties, was found to upregulate the expression of SEL1L and other UPR genes in glioma stem cells. siRNA-mediated SEL1L knockdown in these cells negatively affects their self-renewal potential and exacerbates the cytotoxic effects of VPA. These data suggest that SEL1L may protect against VPA-mediated cytotoxicity via the maintenance of cancer stem cell properties [81]. In addition, correlations between low SEL1L protein levels (detected by monoclonal antibody staining) and poor prognosis have been reported in breast carcinoma patients [82]. In the context of esophageal cancers, while absent in normal cells, SEL1L is expressed in early neoplastic events and persists in later stages of esophageal cancer [83].
It should be noted that, as most of these studies implicating SEL1L in cancer pathogenesis are based on association studies, interpretation of their findings should proceed with caution. Many outstanding questions remain, for example, what is the mechanism by which SEL1L is involved in tumorigenesis? How do changes in SEL1L level in tumor cells affect ER homeostasis or specific ERAD substrates? How ER homeostasis affects tumorigenesis? Nonetheless, these studies are important because they have opened avenues for more definitive future investigations using animal models.
Another well-studied ERAD component is the lectin protein osteosarcoma amplified-9 (OS9) involved in the recognition and recruitment of misfolded glycoprotein or nonglycoproteins to the ERAD complex [84, 85]. Under hypoxic conditions, OS-9-mediated ubiquitination and subsequent degradation of HIF-1α, aided by an E3 ligase and tumor suppressor von Hippel–Lindau (VHL), is instrumental in downregulating genes that promote cell survival, proliferation, invasion, angiogenesis and metastasis [86, 87]. A recently identified gene CIM (Cancer Invasion or Metastasis-related) also known as ERLEC1 (Endoplasmic Reticulum Lectin-1) has been found to sequester OS-9 away from the HIF-1α complex in lung cancer cells, causing HIF-1α stabilization and accumulation thereby aiding tumor growth and metastasis [88]. This study proposes OS9 to be an important link between hypoxia regulation and cancer progression.
Intriguingly, we have recently shown that in the absence of Sel1L, OS9 accumulates and is stabilized [69, 71], suggesting that OS9 is a substrate of the Sel1L-Hrd1 ERAD complex. Thus, the Sel1L-Hrd1 ERAD complex, along with other ERAD components (such as VHL) may exert its role in tumorigenesis via the regulation of either ER homeostasis in general or specific substrates such as OS9.
6. Therapeutics
Maintaining ER proteostasis assumes high importance in cancer cells due to the increased pressure on protein folding owing to their enhanced metabolic needs, as is evident from the high basal level of expression of UPR markers in these cells. Consequently, developing interventions that aim to sensitize tumor cells to various anti-cancer agents by selectively inhibiting UPR has become a popular therapeutic strategy of late.
A recent endeavor that tested the efficacy of several IRE1α inhibitors (STF-083010, 3-Ethoxy-5,6-dibromosalicylaldehyde, 2-Hydroxy-1-naphthaldehyde, toyocamycin etc.) in a dosage and time-dependent manner on 14 pancreatic cancer cell lines observed growth retardation due to either cell cycle arrest or induced apoptosis as well as reduction in invasiveness demonstrated by soft agar assays and xenograft experiments [89]. Irestatin is another IRE1α endonuclease activity inhibitor that has been seen to impair proliferation survival under starvation conditions of malignant myeloma cells [90]. Interestingly, while there is considerable variability in cellular responses to these IRE1α inhibitors, synergistic effects have been observed when using various drug treatments in combination [90].
The use of oncolytic virus therapy (OVT) – viral induction of tumor cell lysis and recruitment of the immune system to the infected tissue – is quickly rising in popularity owing to its selectivity in targeting malignancies based on the inherent abnormalities of cancer cells. A major drawback of this approach lies in the great variation in the response rates of these viruses on patients. In this context, a genome-wide RNAi screen recently identified various ER stress pathway components including IRE1α whose inhibition results in preconditioning of cancer cells to undergo apoptosis when challenged with rhabdoviral oncolysis. This sensitization to caspase-2-dependent cell death occurs via pro-apoptotic factors such as MCL1 (myeloid cell leukemia 1), RAIDD (RIP-Associated ICH1/CED3-Homologous Protein With Death Domain) and PIDD (p53-induced death domain) [91]. This “one-two punch” tactic was validated using primary patient samples and can be useful in combating cancers that are otherwise individually resistant to UPR inhibition and oncolytic viruses.
Other means of sensitizing tumor cells towards proteotoxicity involve drugs that block proteasomal activity. Popular among these are the following – Bortezomib (BTZ) which leads to abrogation of NF-κB function and increases sensitivity to TNFα-related and caspase-mediated apoptosis; Nelfinavir which blocks cellular proteasomal activity by virtue of its own protease property and elicits pro-apoptotic effects marked by amassing of polyubiquitinated proteins [92]; Eeyarestatin I (EerI) which is an agent that targets p97 (ATPase functioning in the transportation of ubiquitinated proteins) as a means to blocking ERAD [93]. Most of these therapeutic agents and their modes of action in combating cancer have been summarized in Table 2.
Table 2.
Therapeutic interventions in cancer targeting IRE1α and ERAD.
| Agent | Mechanism | Effect | Functional partners | Reference |
|---|---|---|---|---|
| STF-083010, 3-Ethoxy-5,6-dibromosalicylaldeh yde, 2-Hydroxy-1-naphthaldehyde, toyocamycin | IRE1α inhibition | Growth retardation owing to cell cycle arrest or induced apoptosis; reduction in invasiveness | Synergistic effects seen when used in combination or with gemcitabine/borte zomib | [89] |
| Rhabdovirus | Oncolytic virus therapy plus IRE1α inhibition | IRE1α inhibition preconditions cancer cells specifically and sensitizes them to apoptosis following rhabdovirus infection – called “one-two punch” | shRNA or small molecule inhibitors targeting IRE1a | [91] |
| Bortezomib | Blocks proteasome activity | Leads to abrogation of NF-κB function, increased sensitivity to apoptosis, thereby pushing tumor cells towards proteotoxicity | TNFα and caspase (mediating apoptosis) | [90] |
| Nelfinavir | Blocks proteasome activity | Exercises protease property in blocking cellular proteasome activity and inducing pro-apoptotic effects via amassing polyubiquitinated proteins | BiP/GRP78, CHOP and caspase activation induced | |
| Eeyarestatin I | Targeting p97 ATPase | Blocks ERAD by preventing de-ubiquitination of ERAD substrates and preferentially pushing cancer cells towards cytotoxicity | ||
| Irestatin | IRE1α RNase inhibitor | Blocks UPR by abrogating XBP1s transcription, thereby impairing proliferation or inhibiting tumor cell survival in oxygen-starvation conditions |
Abbreviations – BiP: Binding immunoglobulin protein; CHOP: C/EBP homologous protein; ERAD: Endoplasmic reticulum associated degradation; IRE1α: Inositol requiring enzyme 1 alpha; GRP78: 78 kDa glucose-regulated protein; NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells; shRNA: short hairpin ribonucleic acid; TNFα: Tumor necrosis factor alpha; UPR: Unfolded protein response; XBP1s: Xbox binding protein 1 spliced.
A 2003 study by Lee et al. [41] using MM cell line demonstrated XBP1 to be an important therapeutic target in cancer as proteasome inhibition in MM cells suppresses IRE1α RNase activity and XBP1s generation, resulting in an increased apoptotic cell death [41]. However, despite recent advances in therapy with proteasome inhibitors (e.g. bortezomib, carfilzomib), MM remains incurable due to the resistance to most drugs [94]. A recent study by Leung-Hagesteijn and colleagues demonstrated that silencing of IRE1α or XBP1 in MM cell lines confers resistance to proteasome inhibitors [95]. The loss of XBP1 in MM results in attenuation of Ig production and a decline in ER stress and ERAD function, which reduces ER stress hypersensitivity in MM cells and accounts for the resistance to proteasomal inhibitors. Moreover, a subset of Xbp1s-negative MM cells lacks plasma cell features [95]. These findings may explain the inability of proteasomeal inhibitors in treating MM patients, while underscoring the importance of targeting both the committed plasma cells and the progenitors in therapeutic treatment to overcome drug resistance.
7. Conclusions
When normal cells experience stress due to the accumulation of misfolded proteins inside the ER, a series of adaptive mechanisms are initiated, namely, UPR and ERAD. These pathways lead to global translational attenuation, the induction of specific signaling cascades and clearance of misfolded proteins in the ER aimed at restoring ER homeostasis in the cell. However, in the context of cancer cells, insults from hypoxia, nutrient deprivation, genetic mutations, and enhanced metabolic needs, can often lead to a gross accumulation of faulty proteins in the ER. Therefore, the survival of tumor cells may become especially dependent, relative to normal cells, on the adaptation to stressful conditions via UPR and ERAD. This can potentially serve as the Achilles heel of cancer cells and allow us to view UPR and ERAD components as lucrative therapeutic targets for cancer treatment. On the other hand, it is the same UPR activation that is wielded by the tumor cells in order to mount resistance to various anti-cancer drugs, thus becoming a double edged sword in tumor cell survival. Linking ER homeostasis to cancer progression may shed further light on the cell-intrinsic processes in tumor cells and allow us to potentially target them specifically over surrounding normal cells. However, most of the studies implicating various UPR and ERAD components in cancer pathogenesis are limited by their model system of study (cell culture based experiments) and small sample sizes (sampling of patient tissue). Hence our understanding of the mechanisms by which the ER quality control systems contribute to the function and survival of cancer cells still warrants further thoughtful investigations.
8. Perspectives
Although ER stress is thought to occur in many physiological and pathological conditions, what is lacking in most studies to date is the direct and accurate measurement of stress levels in the ER. Since cancer cells probably have a elevated protein turnover rate and are likely to accost this enhanced metabolic need by adaption via UPR and ERAD, it is quite possible that the basal levels of various components of ER chaperones are higher in tumor cells. Therefore, the induction or inhibition of these so-called “UPR markers” such as GRP78 and CHOP may not serve as a reliable indicator of ER stress. Thus, although many studies have suggested various possible roles of ER stress and IRE1α signaling pathways in tumor progression, we still lack a deep and accurate understanding of exactly what the status of ER stress and the contribution of UPR pathways in cancer pathogenesis are. Hence we would like to emphasize the urgent need to directly quantitate ER stress at the level of UPR sensors such as IRE1α and PERK activation. Using the phos-tag-based approach, one can directly measure and quantitate the extent of IRE1α phosphorylation (Fig. 3), which we have shown to correlate with the stress level in the ER. As this method is very sensitive and can detect mild ER stress under physiological and pathological conditions [57, 69, 71, 96–98], it promises to provide insights into several outstanding questions, including when and to what extent UPR and IRE1α are activated during tumorigenesis, how small molecules affect IRE1α signaling and ER homeostasis in tumors, and how perturbation of ER homeostasis affects the survival and death of cancer cells in humans.
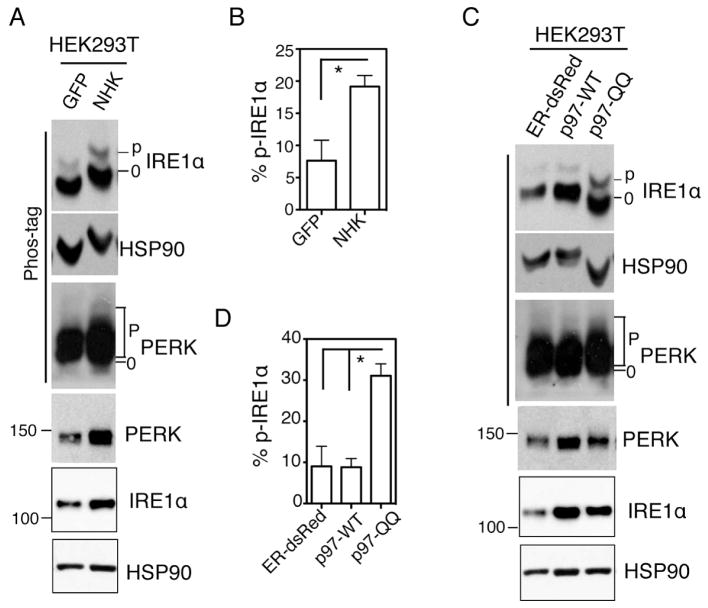
Figure 3. Methods for quantitation of IRE1α activation and stress levels in the ER.
(A and C) Immunoblots of IRE1α and PERK in HEK293T cells transfected with the indicated plasmids for 24 h. NHK, the unfolded form of a1-antitrypsin; p97-QQ, dominant negative form of p97-WT. ER-dsRed and GFP, negative control plasmids. HSP90, a position and loading control. (B and D) Quantitation of percent of phosphorylated IRE1α in total IRE1α protein in Phos-tag gels shown in A, C. Values are mean ± SEM *, P < 0.05 using unpaired two-tailed Student’s t-test. This data is taken from [98].
Table 1.
The role of IRE1α in various types of cancer.
| Type of cancer | System | Mechanism | Reference |
|---|---|---|---|
| Multiple myeloma | B cell specific Xbp1s transgenic mice and patient samples | Aiding in secretory maturation of plasma cells and resistance to anti-cancer drugs | [38, 43, 94, 95] |
| Triple negative breast cancer | Breast cancer lines and mice with mammary glands injected with tumor cells | High Xbp1s levels aid in sustaining hypoxia (co-localizing with hypoxia markers), promoting angiogenesis and invasion in collaboration with HIF1α via transcriptional regulation of various genes including VEGFA, PDK1, GLUT1, DDIT4 | [46] |
| Colon cancer | Eleven patient primary tumor samples | High levels of Xbp1 expression in colorectal carcinoma and adenoma | [44] |
| Glioma | Human U87 glioma cell line; mouse orthotopic brain model; chick chorioallantoic membrane | Supports tumor vascularity, blood vessel cooption and invasiveness by promoting pro-angiogenic VEGFA, IL-6, IL-8, IL-1β, and by inhibiting anti-angiogenic thrombospondin1, decorin. | [48] |
| Several cancers | Cell lines | IRE1α mutations identified in tumor cells are defective in signaling | [57, 58] |
| Liver cancer | Hepatoma HuH7 cell line | Downregulates GPC3 (mediator of tumor growth via canonical Wnt signaling) by RIDD; itself silenced in cancer cells by oncomiR miR-1291 | [53] |
| Colon cancer | In vivo Xbp1 intestine-specific-null mouse model | Protects against colitis-associated-cancer and Apc-mediated colorectal cancer; depletion of Xbp1 predisposes intestinal epithelium to inflammatory diseases and tumorigenesis | [45] |
| Glioma | Human U87 glioma cell line | Suppresses attachment and migration properties by downregulating RhoA and SPARC (a matrix protein that retards cell cycle, induces cell shape change and promotes invasiveness) | [51] |
Abbreviations – Apc: Adenomatous polyposis coli; DDIT4: DNA-damage-inducible transcript 4; GLUT1: Glucose transporter 1; GPC3: Glypican 3; HIF1α: Hypoxia inducible factor 1 alpha; IL-6, -8, -1β: Interleukin 6, 8, 1beta; IRE1α: Inositol requiring enzyme 1 alpha; miR: microRNA; PDK1: Phosphoinositide-dependent kinase 1; RIDD: Regulated IRE1-dependent decay; RhoA: Ras homolog gene family member A; SPARC: Secreted protein acidic and rich in cysteine; VEGFA: Vascular endothelial growth factor A; XBP1s: Xbox binding protein 1 spliced.
Acknowledgments
The work in the Qi laboratory has been supported by NIH NIDDK R01DK082582, NIGMS R01GM113188, NIAAA R21AA020351, American Diabetes Association (ADA) 1-12-CD-04 and 7-08-JF-47, Juvenile Diabetes Research Foundation 47-2012-767 and 1-SRA-2014-251-Q-R, American Federation of Aging Research (RAG08061), Cornell University, HHMI International Student Research Fellowship and AHA Pre-doctoral Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 2.Tsai YC, Weissman AM. The Unfolded Protein Response, Degradation from Endoplasmic Reticulum and Cancer. Genes Cancer. 2010;1(7):764–778. doi: 10.1177/1947601910383011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hetz C, Chevet E, Harding HP. Targeting the unfolded protein response in disease. Nat Rev Drug Discov. 2013;12(9):703–19. doi: 10.1038/nrd3976. [DOI] [PubMed] [Google Scholar]
- 4.Ryter SW, Mizumura K, Choi AM. The impact of autophagy on cell death modalities. Int J Cell Biol. 2014;2014:502676. doi: 10.1155/2014/502676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogata M, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26(24):9220–31. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40(2):280–93. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez-Mancera PA, Young AR, Narita M. Inside and out: the activities of senescence in cancer. Nat Rev Cancer. 2014;14(8):547–58. doi: 10.1038/nrc3773. [DOI] [PubMed] [Google Scholar]
- 9.Nagelkerke A, et al. Therapeutic targeting of autophagy in cancer. Part I: Molecular pathways controlling autophagy. Semin Cancer Biol. 2014 doi: 10.1016/j.semcancer.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Gewirtz DA. The four faces of autophagy: implications for cancer therapy. Cancer Res. 2014;74(3):647–51. doi: 10.1158/0008-5472.CAN-13-2966. [DOI] [PubMed] [Google Scholar]
- 11.Croft A, et al. Oncogenic activation of MEK/ERK primes melanoma cells for adaptation to endoplasmic reticulum stress. J Invest Dermatol. 2014;134(2):488–97. doi: 10.1038/jid.2013.325. [DOI] [PubMed] [Google Scholar]
- 12.Clarke HJ, et al. Endoplasmic reticulum stress in malignancy. Cancer Cell. 2014;25(5):563–73. doi: 10.1016/j.ccr.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73(6):1197–206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- 14.Mori K, et al. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell. 1993;74(4):743–56. doi: 10.1016/0092-8674(93)90521-q. [DOI] [PubMed] [Google Scholar]
- 15.Tirasophon W, Welihinda AA, Kaufman RJ. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 1998;12(12):1812–24. doi: 10.1101/gad.12.12.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang XZ, et al. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 1998;17(19):5708–17. doi: 10.1093/emboj/17.19.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang K, et al. The unfolded protein response sensor IRE1alpha is required at 2 distinct steps in B cell lymphopoiesis. J Clin Invest. 2005;115(2):268–81. doi: 10.1172/JCI21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwawaki T, et al. Function of IRE1 alpha in the placenta is essential for placental development and embryonic viability. Proc Natl Acad Sci U S A. 2009;106(39):16657–62. doi: 10.1073/pnas.0903775106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertolotti A, et al. Increased sensitivity to dextran sodium sulfate colitis in IRE1beta-deficient mice. J Clin Invest. 2001;107(5):585–93. doi: 10.1172/JCI11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuru A, et al. Negative feedback by IRE1beta optimizes mucin production in goblet cells. Proc Natl Acad Sci U S A. 2013;110(8):2864–9. doi: 10.1073/pnas.1212484110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sha H, et al. Stressed out about obesity: IRE1alpha-XBP1 in metabolic disorders. Trends Endocrinol Metab. 2011;22(9):374–81. doi: 10.1016/j.tem.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollien J, et al. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186(3):323–31. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.So JS, et al. Silencing of lipid metabolism genes through IRE1alpha-mediated mRNA decay lowers plasma lipids in mice. Cell Metab. 2012;16(4):487–99. doi: 10.1016/j.cmet.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakaki K, et al. RNA surveillance is required for endoplasmic reticulum homeostasis. Proc Natl Acad Sci U S A. 2012;109(21):8079–84. doi: 10.1073/pnas.1110589109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313(5783):104–7. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 26.Upton JP, et al. IRE1alpha cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase-2. Science. 2012;338(6108):818–22. doi: 10.1126/science.1226191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai BH, et al. microRNA-199a-5p protects hepatocytes from bile acid-induced sustained endoplasmic reticulum stress. Cell Death Dis. 2013;4:e604. doi: 10.1038/cddis.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maurel M, Chevet E. Endoplasmic reticulum stress signaling: the microRNA connection. Am J Physiol Cell Physiol. 2013;304(12):C1117–26. doi: 10.1152/ajpcell.00061.2013. [DOI] [PubMed] [Google Scholar]
- 29.Reimold AM, et al. An essential role in liver development for transcription factor XBP-1. Genes Dev. 2000;14(2):152–7. [PMC free article] [PubMed] [Google Scholar]
- 30.Reimold AM, et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412(6844):300–7. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 31.Shaffer AL, et al. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity. 2004;21(1):81–93. doi: 10.1016/j.immuni.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Todd DJ, et al. XBP1 governs late events in plasma cell differentiation and is not required for antigen-specific memory B cell development. J Exp Med. 2009;206(10):2151–9. doi: 10.1084/jem.20090738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lipson KL, et al. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 2006;4(3):245–54. doi: 10.1016/j.cmet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Lipson KL, Ghosh R, Urano F. The role of IRE1alpha in the degradation of insulin mRNA in pancreatic beta-cells. PLoS One. 2008;3(2):e1648. doi: 10.1371/journal.pone.0001648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee AH, et al. Dual and opposing roles of the unfolded protein response regulated by IRE1alpha and XBP1 in proinsulin processing and insulin secretion. Proc Natl Acad Sci U S A. 2011;108(21):8885–90. doi: 10.1073/pnas.1105564108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu T, et al. The IRE1alpha-XBP1 pathway regulates metabolic stress-induced compensatory proliferation of pancreatic beta-cells. Cell Res. 2014;24(9):1137–40. doi: 10.1038/cr.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura M, et al. Activation of the endoplasmic reticulum stress pathway is associated with survival of myeloma cells. Leuk Lymphoma. 2006;47(3):531–9. doi: 10.1080/10428190500312196. [DOI] [PubMed] [Google Scholar]
- 38.Bagratuni T, et al. XBP1s levels are implicated in the biology and outcome of myeloma mediating different clinical outcomes to thalidomide-based treatments. Blood. 2010;116(2):250–3. doi: 10.1182/blood-2010-01-263236. [DOI] [PubMed] [Google Scholar]
- 39.Papandreou I, et al. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117(4):1311–4. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cross BC, et al. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc Natl Acad Sci U S A. 2012;109(15):E869–78. doi: 10.1073/pnas.1115623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee AH, et al. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc Natl Acad Sci U S A. 2003;100(17):9946–51. doi: 10.1073/pnas.1334037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ri M, et al. Identification of Toyocamycin, an agent cytotoxic for multiple myeloma cells, as a potent inhibitor of ER stress-induced XBP1 mRNA splicing. Blood Cancer J. 2012;2(7):e79. doi: 10.1038/bcj.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carrasco DR, et al. The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis. Cancer Cell. 2007;11(4):349–60. doi: 10.1016/j.ccr.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujimoto T, et al. Overexpression of human X-box binding protein 1 (XBP-1) in colorectal adenomas and adenocarcinomas. Anticancer Res. 2007;27(1A):127–31. [PubMed] [Google Scholar]
- 45.Niederreiter L, et al. ER stress transcription factor Xbp1 suppresses intestinal tumorigenesis and directs intestinal stem cells. J Exp Med. 2013;210(10):2041–56. doi: 10.1084/jem.20122341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X, et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1alpha pathway. Nature. 2014;508(7494):103–7. doi: 10.1038/nature13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drogat B, et al. IRE1 signaling is essential for ischemia-induced vascular endothelial growth factor-A expression and contributes to angiogenesis and tumor growth in vivo. Cancer Res. 2007;67(14):6700–7. doi: 10.1158/0008-5472.CAN-06-3235. [DOI] [PubMed] [Google Scholar]
- 48.Auf G, et al. Inositol-requiring enzyme 1alpha is a key regulator of angiogenesis and invasion in malignant glioma. Proc Natl Acad Sci U S A. 2010;107(35):15553–8. doi: 10.1073/pnas.0914072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen DT, et al. Nck-dependent activation of extracellular signal-regulated kinase-1 and regulation of cell survival during endoplasmic reticulum stress. Mol Biol Cell. 2004;15(9):4248–60. doi: 10.1091/mbc.E03-11-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaddam D, Stevens N, Hollien J. Comparison of mRNA localization and regulation during endoplasmic reticulum stress in Drosophila cells. Mol Biol Cell. 2013;24(1):14–20. doi: 10.1091/mbc.E12-06-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dejeans N, et al. Autocrine control of glioma cells adhesion and migration through IRE1alpha-mediated cleavage of SPARC mRNA. J Cell Sci. 2012;125(Pt 18):4278–87. doi: 10.1242/jcs.099291. [DOI] [PubMed] [Google Scholar]
- 52.Kunigal S, et al. SPARC-induced migration of glioblastoma cell lines via uPA-uPAR signaling and activation of small GTPase RhoA. Int J Oncol. 2006;29(6):1349–57. [PMC free article] [PubMed] [Google Scholar]
- 53.Maurel M, et al. MicroRNA-1291-mediated silencing of IRE1alpha enhances Glypican-3 expression. RNA. 2013;19(6):778–88. doi: 10.1261/rna.036483.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jakubovic BD, Jothy S. Glypican-3: from the mutations of Simpson-Golabi-Behmel genetic syndrome to a tumor marker for hepatocellular carcinoma. Exp Mol Pathol. 2007;82(2):184–9. doi: 10.1016/j.yexmp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 55.Capurro MI, et al. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 2005;65(14):6245–54. doi: 10.1158/0008-5472.CAN-04-4244. [DOI] [PubMed] [Google Scholar]
- 56.Korennykh AV, et al. The unfolded protein response signals through high-order assembly of Ire1. Nature. 2009;457(7230):687–93. doi: 10.1038/nature07661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xue Z, et al. A conserved structural determinant located at the interdomain region of mammalian inositol-requiring enzyme 1alpha. J Biol Chem. 2011;286(35):30859–66. doi: 10.1074/jbc.M111.273714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghosh R, et al. Allosteric inhibition of the IRE1alpha RNase preserves cell viability and function during endoplasmic reticulum stress. Cell. 2014;158(3):534–48. doi: 10.1016/j.cell.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu M, et al. Cell death. Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science. 2014;345(6192):98–101. doi: 10.1126/science.1254312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olzmann JA, Kopito RR, Christianson JC. The mammalian endoplasmic reticulum-associated degradation system. Cold Spring Harb Perspect Biol. 2013;5(9) doi: 10.1101/cshperspect.a013185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–66. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 62.Guerriero CJ, Brodsky JL. The delicate balance between secreted protein folding and endoplasmic reticulum-associated degradation in human physiology. Physiol Rev. 2012;92(2):537–76. doi: 10.1152/physrev.00027.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hampton RY, Gardner RG, Rine J. Role of 26S proteasome and HRD genes in the degradation of 3-hydroxy-3-methylglutaryl-CoA reductase, an integral endoplasmic reticulum membrane protein. Mol Biol Cell. 1996;7(12):2029–44. doi: 10.1091/mbc.7.12.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gardner RG, et al. Endoplasmic reticulum degradation requires lumen to cytosol signaling. Transmembrane control of Hrd1p by Hrd3p. J Cell Biol. 2000;151(1):69–82. doi: 10.1083/jcb.151.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mueller B, Lilley BN, Ploegh HL. SEL1L, the homologue of yeast Hrd3p, is involved in protein dislocation from the mammalian ER. J Cell Biol. 2006;175(2):261–70. doi: 10.1083/jcb.200605196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mueller B, et al. SEL1L nucleates a protein complex required for dislocation of misfolded glycoproteins. Proc Natl Acad Sci U S A. 2008;105(34):12325–30. doi: 10.1073/pnas.0805371105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Christianson JC, et al. Defining human ERAD networks through an integrative mapping strategy. Nat Cell Biol. 2012;14(1):93–105. doi: 10.1038/ncb2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Francisco AB, et al. Deficiency of suppressor enhancer lin12 1 like (SEL1L) in mice leads to systemic endoplasmic reticulum stress and embryonic lethality. J Biol Chem. 2010;285(18):13694–703. doi: 10.1074/jbc.M109.085340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun S, et al. Sel1L is indispensable for mammalian endoplasmic reticulum-associated degradation, endoplasmic reticulum homeostasis, and survival. Proc Natl Acad Sci U S A. 2014;111(5):E582–91. doi: 10.1073/pnas.1318114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li S, et al. SEL1L deficiency impairs growth and differentiation of pancreatic epithelial cells. BMC Dev Biol. 2010;10:19. doi: 10.1186/1471-213X-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sha H, et al. The ER-associated degradation adaptor protein Sel1L regulates LPL secretion and lipid metabolism. Cell Metab. 2014;20(3):458–70. doi: 10.1016/j.cmet.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saltini G, et al. A novel polymorphism in SEL1L confers susceptibility to Alzheimer's disease. Neurosci Lett. 2006;398(1–2):53–8. doi: 10.1016/j.neulet.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 73.Kyostila K, et al. A SEL1L mutation links a canine progressive early-onset cerebellar ataxia to the endoplasmic reticulum-associated protein degradation (ERAD) machinery. PLoS Genet. 2012;8(6):e1002759. doi: 10.1371/journal.pgen.1002759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yagishita N, et al. Essential role of synoviolin in embryogenesis. J Biol Chem. 2005;280(9):7909–16. doi: 10.1074/jbc.M410863200. [DOI] [PubMed] [Google Scholar]
- 75.Wu T, et al. Hrd1 suppresses Nrf2-mediated cellular protection during liver cirrhosis. Genes Dev. 2014;28(7):708–22. doi: 10.1101/gad.238246.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang H, et al. Hrd1-mediated BLIMP-1 ubiquitination promotes dendritic cell MHCII expression for CD4 T cell priming during inflammation. J Exp Med. 2014;211(12):2467–79. doi: 10.1084/jem.20140283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cattaneo M, et al. SEL1L affects human pancreatic cancer cell cycle and invasiveness through modulation of PTEN and genes related to cell-matrix interactions. Neoplasia. 2005;7(11):1030–8. doi: 10.1593/neo.05451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Q, et al. A single-nucleotide polymorphism in tumor suppressor gene SEL1L as a predictive and prognostic marker for pancreatic ductal adenocarcinoma in Caucasians. Mol Carcinog. 2012;51(5):433–8. doi: 10.1002/mc.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ashktorab H, et al. SEL1L, an UPR response protein, a potential marker of colonic cell transformation. Dig Dis Sci. 2012;57(4):905–12. doi: 10.1007/s10620-011-2026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cardano M, et al. mSEL-1L (Suppressor/enhancer Lin12-like) protein levels influence murine neural stem cell self-renewal and lineage commitment. J Biol Chem. 2011;286(21):18708–19. doi: 10.1074/jbc.M110.210740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cattaneo M, et al. Down-modulation of SEL1L, an unfolded protein response and endoplasmic reticulum-associated degradation protein, sensitizes glioma stem cells to the cytotoxic effect of valproic acid. J Biol Chem. 2014;289(5):2826–38. doi: 10.1074/jbc.M113.527754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Orlandi R, et al. SEL1L expression decreases breast tumor cell aggressiveness in vivo and in vitro. Cancer Res. 2002;62(2):567–74. [PubMed] [Google Scholar]
- 83.Granelli P, et al. SEL1L and squamous cell carcinoma of the esophagus. Clin Cancer Res. 2004;10(17):5857–61. doi: 10.1158/1078-0432.CCR-04-0075. [DOI] [PubMed] [Google Scholar]
- 84.Christianson JC, et al. OS-9 and GRP94 deliver mutant alpha1-antitrypsin to the Hrd1-SEL1L ubiquitin ligase complex for ERAD. Nat Cell Biol. 2008;10(3):272–82. doi: 10.1038/ncb1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hosokawa N, et al. Human OS-9, a lectin required for glycoprotein endoplasmic reticulum-associated degradation, recognizes mannose-trimmed N-glycans. J Biol Chem. 2009;284(25):17061–8. doi: 10.1074/jbc.M809725200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baek JH, et al. OS-9 interacts with hypoxia-inducible factor 1alpha and prolyl hydroxylases to promote oxygen-dependent degradation of HIF-1alpha. Mol Cell. 2005;17(4):503–12. doi: 10.1016/j.molcel.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 87.Ivan M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292(5516):464–8. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 88.Yanagisawa K, et al. Novel metastasis-related gene CIM functions in the regulation of multiple cellular stress-response pathways. Cancer Res. 2010;70(23):9949–58. doi: 10.1158/0008-5472.CAN-10-1055. [DOI] [PubMed] [Google Scholar]
- 89.Chien W, et al. Selective inhibition of unfolded protein response induces apoptosis in pancreatic cancer cells. Oncotarget. 2014;5(13):4881–94. doi: 10.18632/oncotarget.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li X, Zhang K, Li Z. Unfolded protein response in cancer: the physician's perspective. J Hematol Oncol. 2011;4:8. doi: 10.1186/1756-8722-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mahoney DJ, et al. Virus-tumor interactome screen reveals ER stress response can reprogram resistant cancers for oncolytic virus-triggered caspase-2 cell death. Cancer Cell. 2011;20(4):443–56. doi: 10.1016/j.ccr.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 92.Schonthal AH. Endoplasmic reticulum stress: its role in disease and novel prospects for therapy. Scientifica (Cairo) 2012;2012:857516. doi: 10.6064/2012/857516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Q, et al. The ERAD inhibitor Eeyarestatin I is a bifunctional compound with a membrane-binding domain and a p97/VCP inhibitory group. PLoS One. 2010;5(11):e15479. doi: 10.1371/journal.pone.0015479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alexanian R, et al. Curability of multiple myeloma. Bone Marrow Res. 2012;2012:916479. doi: 10.1155/2012/916479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leung-Hagesteijn C, et al. Xbp1s-negative tumor B cells and pre-plasmablasts mediate therapeutic proteasome inhibitor resistance in multiple myeloma. Cancer Cell. 2013;24(3):289–304. doi: 10.1016/j.ccr.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.He Y, et al. Nonmuscle myosin IIB links cytoskeleton to IRE1alpha signaling during ER stress. Dev Cell. 2012;23(6):1141–52. doi: 10.1016/j.devcel.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sha H, et al. The IRE1alpha-XBP1 pathway of the unfolded protein response is required for adipogenesis. Cell Metab. 2009;9(6):556–64. doi: 10.1016/j.cmet.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang L, et al. A Phos-tag-based approach reveals the extent of physiological endoplasmic reticulum stress. PLoS One. 2010;5(7):e11621. doi: 10.1371/journal.pone.0011621. [DOI] [PMC free article] [PubMed] [Google Scholar]