Abstract
Autophagy is a process that maintains homeostasis during stress, although it also contributes to cell death under specific contexts. Ceramides have emerged as important effectors in the regulation of autophagy, mediating the crosstalk with apoptosis. Melatonin induces apoptosis of cancer cells; however, its role in autophagy and ceramide metabolism has yet to be clearly elucidated. This study was aimed to evaluate the effect of melatonin administration on autophagy and ceramide metabolism and its possible link with melatonin-induced apoptotic cell death in hepatocarcinoma (HCC) cells. Melatonin (2 mM) transiently induced autophagy in HepG2 cells through JNK phosphorylation, characterized by increased Beclin1 expression, p62 degradation and LC3II and LAMP2 colocalization, which translated in decreased cell viability. Moreover, ATG5-silencing sensitized HepG2 cells to melatonin induced-apoptosis, suggesting a dual role of autophagy in cell death. Melatonin enhanced ceramide levels through both de novo synthesis and acid sphingomyelinase (ASMase) stimulation. Serine palmitoyl transferase (SPT) inhibition with myriocin prevented melatonin induced autophagy and ASMase inhibition with imipramine impaired autophagy flux. However, ASMase inhibition partially protected HepG2 cells against melatonin while SPT inhibition significantly enhanced cell death. Findings suggest a cross-talk between SPT-mediated ceramide generation and autophagy in protecting against melatonin, while specific ASMase-induced ceramide production participates in melatonin-mediated cell death. Thus, dual blocking of SPT and autophagy emerge as a potential strategy to potentiate the apoptotic effects of melatonin in liver cancer cells.
Keywords: melatonin, hepatocarcinoma, autophagy, ceramides, apoptosis, serine palmitoyltransferase, acid sphingomyelinase
Introduction
Hepatocellular carcinoma is the fifth most common neoplasm and the third most frequent cause of cancer death [1]. Surgery remains the most effective treatment for patients with HCC; however, it is limited to selected cases [2], and new curative modalities are required. Hepatocarcinogenesis is a complex multistep process in which many signaling cascades are altered, leading to a heterogeneous molecular profile. The main mutations include the gene for β catenin and tumor suppressor p53. Other mutations less frequent such as chromosomal amplifications and deletions, or epigenetic alterations also affect important oncogenes and tumor suppressors [3]. As a result of these alterations, several signaling cascades related to cell survival and proliferations are modified. For instance, ceramide metabolism and signaling are usually suppressed by overexpression of ceramide-metabolizing enzymes or downregulation of ceramide-generating enzymes [4, 5]. Moreover, alterations such as deregulation of the hepatocyte growth factor (HGF) and c-MET pathway, low levels of human forkhead box class O (FoxO) transcription factors or high levels of vascular endothelial growth factor (VEGF) are associated with poor prognosis in cancer patients, and seem to confer chemotherapy resistance [6, 7]. Finally, the MTOR (mammalian target of rapamycin) pathway, related to autophagy, is disrupted in 40–50% of liver cancers owing to inactivation of the tumour suppressor PTEN, or mutations of phosphoinositide-3-kinase [8, 9].
Autophagy is a dynamic process that triggers the digestion of damaged organelles and misfolded proteins. Cell components are engulfed in double-membrane vesicles called autophagosomes, which after maturation fuse with lysosomes, leading to the formation of an autophagolysosome, whose contents are degraded by lysosomal enzymes. The role of autophagy in cancer is dual. At early stages of tumour development, autophagy acts as a suppressor mechanism [10], promoting the recycling of defective organelles and unfolded proteins, prevention of oxidative stress and maintenance of genome stability [11]. However, autophagy can also favour tumour promotion via oncogene-mediated cancer development [12] and cellular adaptation to different stress, such as hypoxia or starvation [13, 14]. Although the crosstalk between autophagy and apoptosis is not well defined, a relationship has been established due to the interaction of different autophagy and apoptosis-related proteins [15, 16]. Moreover, both processes are regulated by common transcription factors and signalling pathways, such as nuclear factor kappaB (NF-κB), tumor protein p53 (p53), phosphatidylinositide 3-kinases, protein kinase B (PI3K/Akt), or c-Jun N-terminal kinase (JNK) [17].
Ceramides are critical components of membrane bilayers and play an important role in cellular responses, including inflammation, cell proliferation, differentiation, apoptosis, cell migration, senescence and autophagy [18, 19]. Although ceramide is a well-established apoptosis inducer, more recent studies have also implicated ceramide in the induction of autophagy through the activation of JNK, upregulation of Beclin-1 or BNIP3 and downregulation of nutrient transporter proteins [20–22]. Thus, ceramides have emerged as important effectors in the regulation of the autophagic pathway, mediating the crosstalk between apoptosis and autophagy. Cells generate ceramides by the novo byosinthesis in the endoplasmic reticulum (ER) with the condensation of serine and palmitoyl-CoA catalysed by serine palmitoyl transferase (SPT) [23]. In addition, ceramides can be generated through sphingomyelin hydrolysis by sphingomyelinases (SMase) [24]. Acid SMase (ASMase) is activated in response to various proinflammatory and proapoptotic stimuli [22, 25], and it contributes to apoptotic death of tumour cells. Recent evidence demonstrates that ASMase regulates key mechanisms involved in autophagy [26]. On the other hand, although ceramide promotes early autophagy and apoptotic cell death, the cytotoxic effect of C2 ceramide is enhanced when autophagy is inhibited [27]. In addition, an increase of endogenous levels of ceramides is related to apoptosis, and these pro-apoptotic effects are mediated in part by an early autophagy induction [28].
Melatonin is the main product of the pineal gland and plays a protective role in several pathophysiological contexts, including cancer, where it acts as an effective oncostatic drug [29, 30]. Previous studies from our group showed the anti-proliferative, pro-apoptotic, anti-invasiveness and anti-angiogenic effect of melatonin in HepG2 cells [31–33]. However, it has been previously reported a dual role of melatonin in cancer cells. For instance, in glioblastoma-initiating cells, autophagy is involved in melatonin-induced cell death [34], while in hepatoma H22-bearing mice autophagy caused by melatonin is related to apoptotic cell death resistance [35]. Other antioxidants similar to melatonin such as honokiol, quercetin or resveratrol induce autophagy and apoptosis in cancer cell lines and the disruption of autophagy enhance apoptosis-dependent cell death [36, 37]. Moreover, HepG2 cells treated with low doses of a curcumin analog, exhibited increased apoptosis following autophagy inhibition through caspase-dependent and caspase-independent pathway [38].
The role of melatonin on autophagy and its effect on apoptotic cell death in liver cancer cells is not completely understood. Moreover, no information exists on the relationship between melatonin and sphingolipid metabolism. Thus, our aim was to investigate the effect of melatonin administration on autophagy and ceramide metabolism in HepG2 cells, and its possible link with melatonin-induced apoptotic cell death.
Materials and methods
Cell culture and reagents
The HepG2 human hepatocarcinoma cell line was obtained from the American Type Culture Collection (Manassas, VA). They were cultured under controlled conditions (37°C, 5% CO2) and grown in Dulbecco’s modified Eagle’s medium/low glucose, GlutaMAX™ supplement pyruvate (GIBCO, Life Technologies, Madrid, Spain) containing 10% fetal bovine serum and 100 U/mL penicillin/streptomycin. Cells were plated in 9.6 cm2 culture dishes at a density of 0.25 × 106 cells/well. Twenty four hours after plating, cells were treated with 2 mM melatonin (Sigma, St Louis, MO), 50 μM chloroquine diphosphate salt (Sigma) or 100 nM bafilomycin A1 (Tocris, Bristol, UK). In some cases, cells were pretreated two hours with 2.5 μM myriocin (Sigma), 10 μM imipramine (Sigma) or 10 μM SP600125 (Tocris).
Annexin V-propidium iodide assay
Apoptosis was assessed by Alexa Fluor 488 annexin V/Dead apoptosis kit (Invitrogen, Carlsbad, CA). HepG2 cells were seeded in a 6-well plate at density of 0.25 × 106 cells/well. Next day, the cells were treated with melatonin 2 mM for 48 hours. Cell pellets were resuspended in 100 μL buffer with 5 μl annexin V and 1 μL of propidium iodide, and incubated for 15 min at 25°C in the dark. 400 μL of buffer were added for a final volume of 500 μL. Cells were immediately analyzed by FACS SCAN flow Cytometer (Becton-Dickinson, San Jose, United States). 10,000 cells per sample were acquired and percentage of cell death was analyzed using Cell Quest software.
Cell viability assay
HepG2 cells were seeded on 96-well plates at 5,000 cells/well 24 hours before being treated with melatonin and ceramide synthesis inhibitors for 48 hours. Cells were incubated for 3 hours with 0.5 mg/ml of 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) (Sigma) dissolved in serum free medium. After this interval, cells were washed with PBS followed by the addition of DMSO. The optical densities were measured at 560 nm spectral wavelength using microtitre plate reader (SynergyTM HT Multi-Mode Microplate Reader, Bio-Tek Instruments, Inc., Winooski, VT, USA).
Western blot analysis
After treatments, cultured cells were washed twice with ice cold PBS and lysed by adding ice cold RIPA buffer containing 50 mM Tris-HCl pH 7.4, 150 mM NaCl, 2 mM EDTA, 0.1% Triton 100X, 10% sodium deoxycholate, 10% SDS, 1 mM NaF and protease cocktail inhibitor (Roche, Basel, Switzerland) and scraped off the plate. Extracts were transferred to a microfuge tube and centrifuged for 10 min at 15,000g. Equal amounts of the supernatant protein (20 μg) were separately subjected to SDS-PAGE and transferred to a PVDF membrane (Bio-Rad, Hercules, CA). Primary antibodies (Ab) were diluted in blocking solution and incubated overnight at 4°C with polyclonal Ab to p62, LC3, Atg5, phospho-JNK, (1:1000 dilution; from Cell Signalling, Bervely, MA) phospho-mTOR (Ser2448) (1:1000 dilution, Abcam, Cambridge, UK), PARP, BAX and Beclin1 (1:100, Santa Cruz Biotechnology, Dallas, TX). Equal loading of protein was demonstrated by probing the membranes with a rabbit polyclonal anti β-actin antibody (Sigma). After washing with PBS-T, the membranes were incubated for 1 hour at room temperature with secondary HRP-conjugated antibody (1:5,000; Dako, Glostrup, Denmark) and visualized using ECL detection kit (Amersham Pharmacia, Uppsala, Sweden). The density of the specific bands was quantified employing the software ImageJ (National institute of Mental Health, Bethesda, MD) with an imaging densitometer (Scion Image, Maryland, MA).
ASMase assay
ASMase activity was determined using a fluorescent sphingomyelin analog (NBD C6-sphingomyelin (Cayman Ann Harbor, MI). HepG2 cells were lysed in sodium acetate buffer (250 mM sodium acetate, 0.1% Triton X-100, pH 5.0). Samples (75 μg protein) were incubated for 1 hour 30 min at 37 °C in a buffer (250 mM sodium acetate, 0.1% Tri- ton X-100, pH 5.0) containing 10 μM NBD C6-sphingomyelin. Lipids were extracted with a methanol:chloroform (1:2), dried in a speed vacuum and separated by TLC (chloroform: methanol: H2O; 65:25:4, v/v). NBD-ceramide was visualized under UV light and the images were collected with the image capturing instrument, LAS4000 (GE Healthcare, Little Chalfont, UK) and analyzed with Image J free software.
Ceramide detection
Lipids were obtained and measured using HPLC as described previously [39]. Samples were extracted with methanol:chloroform (1:2, v/v). The chloroform phase extracts were dried, resuspended in 250 μl of 1 M KOH in methanol, and incubated at 100°C 1 hour. Once samples had cooled to room temperature, the methanol:chloroform (1:2, v/v) extraction was repeated. The free long-chain bases were recovered in the chloroform phase and dried. Lipids were dissolved in 50 μl of methanol and mixed with 100ul of naphthalene-2,3-dicarboxaldehyde (NDA) reagent 50 mM boric acid (pH 9), sodium cyanide 5 mM and NDA 5 mM (2:1:1, v/v). After 10 min of incubation at 50°C in the dark, 300 μl of methanol was added. Then samples were centrifuged and analyzed by HPLC in a reverse phase C18 column (Teknokroma, Barcelona, Spain) using a Gilson fluorimetric detector with an excitation wavelength of 252 nm and an emission wavelength of 483 nm. The mobile phase composition for the gradient system was 5 mM potassium phosphate buffer (pH=6.5):methanol (85:15, v/v) for mobile phase A and acetonitrile:methanol (75:25, v/v) for mobile phase B and the flow rate was 1 ml/min. The gradient program was 0–1 min 52.94 A, 47.06 B; 1–6 min 52.94-5.88 A, 47.06-94.11 B; 6–21 min 5.88 A, 94.11 B; 21–25 min 5.88-52.94 A, 94.11-47.06 B, 25–30 min 52.94 A, 47.06 B. Quantification of the ceramide peaks were calculated according to a calibration curve commercial standards.
Real-time reverse transcriptase polymerase chain reaction (RT-PCR)
Real-time reverse transcriptase polymerase chain reaction (RT-PCR) was performed as described [40]. Briefly, confluent HepG2 cells grown in complete media were replated in 6 wells culture plate, at a density of 0.25 × 106 cells/well in a total volume of 2 mL of complete medium. After treatment, total RNA was isolated with TRIzol reagent (Invitrogen). For reverse transcription, 1 μg of the total RNA was converted to first-strand cDNAs using a High-Capacity cDNA Archive Kit (Applied Biosystems, CA). Quantitative real-time PCR analysis was performed using SYBR Green (Invitrogen). Human primers used in the present study were: ASMase forward 5′-CTGACTCTCGGGTTCTCTGG-3′ and reverse 5′-AGGTTGATGGCGGTGAATAG-3′ and SPT forward 5′-TGGAAGAGAGCACTGGGTCT-3′ and reverse 5′-GCTACCTCCTTGATGGTGGA-3′. For ATG5 determination, quantitative real-time PCR was performed using FastStart TaqMan Probe Master (Roche Diagnostics GmbH, Mannheim, Germany). TaqMan primers and probes for ATG5 gene are derived from the commercially available TaqMan Gene Expression Assays. To normalize expression data, actin was used as an internal control gene. Relative changes in gene expression levels were determined using the 2−ΔΔCT method.
Immunofluorescence and laser confocal imaging
For immunofluorescence labeling cells were cultured on 24 wells culture plates containing glass coverslips at a seeding density of 1×104. Briefly, HepG2 cells were fixed for 15 minutes with 4% paraformaldehyde and washed twice with PBS 1X. Cells were blocked and permeabilized with PBS 1X + 0.2 % saponin and 1% fatty acid free BSA (FFA-BSA) for 15 minutes at room temperature. After washing twice with PBS 1X, cells were incubated with a rat anti-LAMP monoclonal antibody [GL2A7] (Abcam) and rabbit anti- LC3B polyclonal antibody (Cell Signaling) diluted both 1:300 in 1X PBS with 1 % ffa-BSA O/N at 4°C and washed twice with PBS 1X followed by incubation with a secondary anti-rat IgG antibody, conjugated to Alexa 488 (1:500) and anti-rabbit IgG antibody, conjugated to Alexa 647 (1:500) for 1 hour at 25°C. Coverslips were washed twice with PBS 1X and mounted on glass slides with fluorescent mounting medium Fluoroshield™ with DAPI (Sigma) and visualized in a Leica SPE confocal laser-scanning microscope.
Statistical analysis
Results are expressed as mean values ± SD of the indicated number of experiments. One-way ANOVA followed by Bonferroni post hoc test was used to determinate differences between the mean values of the different treated groups. p <0.05 was considered significant. Values were analyzed using the statistical package GraphPad Prism 5.
Results
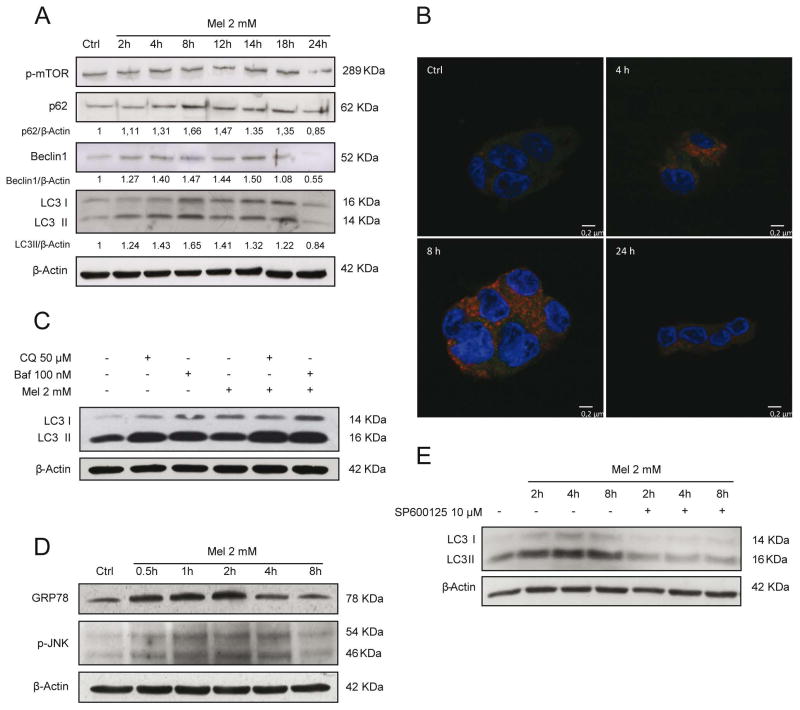
We have previously reported that melatonin induces apoptotic cell death in HepG2 cells [32]. Since autophagy-lysosome pathway is involved in the turnover of damaged organelles and misfolded proteins and due to the importance of autophagy in cell homeostasis and its relationship with apoptosis, we first assessed if melatonin was able to trigger an autophagic response in HepG2 cells. As shown in Fig. 1., when HepG2 cells were maintained in the presence of melatonin (2 mM), LC3II levels increased gradually over time, peaking at 8–12 hours, and decreasing at 24 hours after melatonin treatment. These findings were accompanied by p62 accumulation and gradual degradation over time, as well as an early increase of Beclin 1 levels (Fig. 1A). In order to confirm the functional relationship between autophagosomes and lysosomes we evaluated the colocalization of the specific markers, LC3 and LAMP-2 by confocal microscopy. Colocalization of both markers was detected at 4 hours exhibiting a maximum after 8 hours post-treatment, which decreased by 24 hours, indicating the onset of autophagy (Fig. 1B).
Fig. 1.
Melatonin (mel) administration (2 mM) induces an autophagic response in HCC HepG2 cells. Representative immunoblots of p-mTOR, p62, Beclin1 and LC3I/II after melatonin treatment for different time points showing a transient autophagic response characterized by Beclin1 and LC3II increased expression and p62 degradation. No changes were observed in p-mTOR levels (A). Representative confocal images of LAMP-2 (red) and LC3 (green) immunofluorescence labelling showing a maximal colocalization of LC3 and LAMP-2 at 8 hours after melatonin administration, which disappears at 24 hours (B). Effect of melatonin 2 mM on autophagic flux. Representative immunoblots of LC3I/II from HepG2 cells treated for 8 hours with melatonin in presence of the autophagic inhibitors chloroquine 50 μM (CQ) and bafilomycin 100 nM (Baf) for the last 4 hours of treatment. LC3 levels increased following treatment with melatonin as well as with CQ and Baf, with a higher effect following the combination of melatonin and the autophagic inhibitors (C). Expression levels of ER stress proteins. Time-course with melatonin 2 mM showed enhanced levels of BIP and p-JNK from 30 minutes of treatment until 2–4 hours (D). Prevention of JNK phosphorylation with SP600125 10 μM reduced LC3II formation (E). Images of immunoblots are representative for experiments performed in triplicate.
To define the functional impact of melatonin on autophagy, we determined autophagy flux status. We used HepG2 cells in the presence of melatonin with and without chloroquine (50 μM) or bafilomycin A1 (100 nM). After 8 hours of melatonin treatment, an increase in LC3II was observed, which further augmented by autophagy inhibitors chloroquine and bafilomycin A1. Although, chloroquine and bafilomimycin A1 increased LC3II content, this increase was lower that observed in the presence of melatonin (Fig. 1C). These data suggest that induction of autophagy by melatonin is an early and transient effect following administration of the indol.
To elucidate the mechanism for the induction of autophagy, we decided to evaluate mTOR pathway, which is a critical regulator of autophagy at the level of autophagosome formation. As seen, melatonin failed to reduce the phosphorylation of mTOR (Fig. 1A). Phosphorylation of JNK has also been reported to contribute to autophagy induction in different contexts such as reticulum stress response to unfolded proteins accumulation, so we checked if ER stress and JNK activation were altered by melatonin. Melatonin triggered an increase in GRP78 levels from 1 to 4 hours of treatment, concomitant with JNK phosphorylation (Fig. 1D). Thus, ER stress mediated JNK activation could account for initiation of autophagy in HepG2 cells in presence of melatonin. To address this question, we administered a specific JNK phosphorylation inhibitor SP600125 (10 μM) 1 hour prior to melatonin treatment. Western blot showed that the increase in LC3II levels induced by melatonin was prevented by p-JNK inhibitor, indicating that autophagy triggered by melatonin is likely caused by JNK activation (Fig 1E).
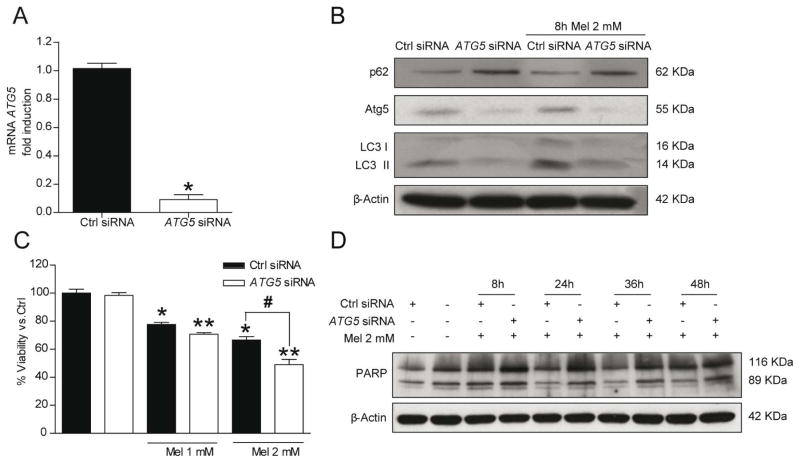
Autophagy is described as a pro-survival mechanism and it is up-regulated in response to therapeutic agents. On the other hand, autophagy is able to promote cell death in different cellular contexts. In order to address the role of autophagy in melatonin induced cell death, the initiation of autophagy was inhibited by siRNA-mediated knockdown of ATG5 in HepG2 cells. ATG5 mRNA expression and protein levels were depleted after 24 hours in ATG5-silenced HepG2 cells (Fig. 2A, B). The ATG5-silencing suppressed the increase of LC3II as well as p62 degradation induced by melatonin administration (2 mM) (Fig. 2B).
Fig. 2.
Autophagic response induced by melatonin 2 mM in HepG2 cells is essential for cell survival. Silencing of ATG5 for 24 hours reduces ATG5 mRNA levels and autophagy induced by melatonin 2 mM treatment for 8 hours (A and B). Representative immunoblots showed not increased levels of Atg5 and LC3, as well as p62 accumulation. After 48 hours, MTT assays revealed lower viability of ATG5-silenced cells in presence of melatonin in a dose-dependent manner (C). Autophagy blocks the increased apoptotic response to melatonin 2 mM treatment. Representative immunoblots of cleaved-PARP, a marker of apoptosis, showed that ATG5-silenced cells are more susceptible to apoptosis in presence of melatonin (D). Images of immunoblots are representative for experiments performed in triplicate. Data are expressed as a percentage of mean values ± SEM of three independent experiments. *P < 0.05 significant differences vs non-silenced cells **P < 0.05 significant differences between treated and untreated silenced cells #P < 0.05 significant differences between control-treated cells and silenced-treated cells cells.
Next, we assessed the effect of ATG5 siRNA in melatonin induced cell death. The down regulation of ATG5 enhanced significantly the cytotoxic effect of 2 mM melatonin in ATG5 silenced cells versus control cells after incubation with 2 mM melatonin for 48 hours (Fig. 2C). Moreover, these findings were accompanied by PARP processing, a caspase-3 target, with an increase of the cleaved 89 kDa fragment after 48 hours treatment in ATG5 silenced cells (Fig. 2D). These results suggest that autophagy induction is a survival mechanism that reduces in part apoptotic cell death induced by melatonin.
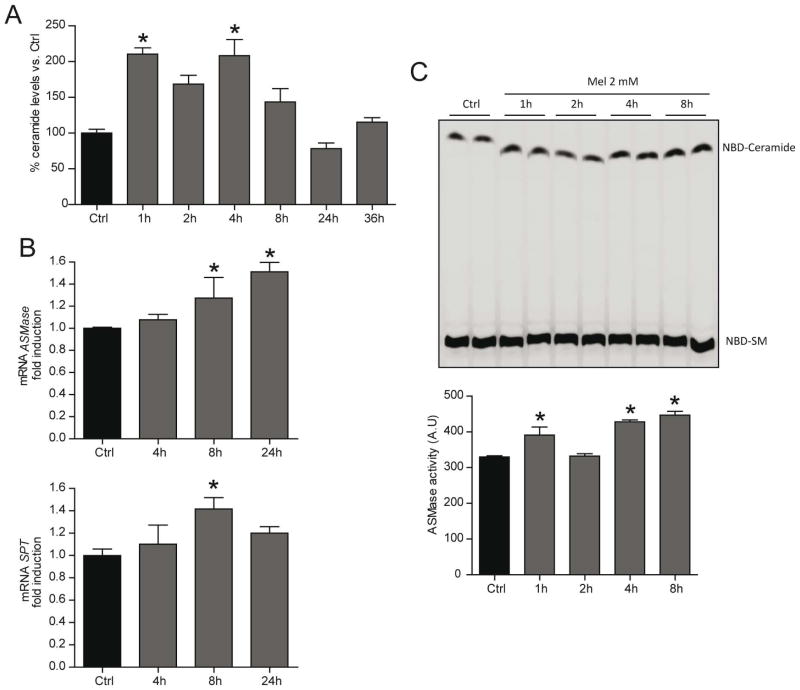
Ceramides are bioactive lipids which have been implicated in a variety of physiological functions, including cell growth arrest, differentiation, apoptosis and autophagy. In order to evaluate the role of ceramides in apoptosis and autophagy, we assessed whether melatonin increase ceramide levels. We observed an early and transient increase in total ceramide content that declined by 24–36 hours after melatonin treatment (Fig. 3A). Interestingly, ASMase activity was stimulated in presence of melatonin as early as 1 hour post-treatment (Fig. 3B), which could contribute to the initial phase of ceramide generation. This activation of ASMase, however, was accompanied by increase expression of the mRNA level detected after 8 hours of melatonin treatment (Fig. 3C). Moreover, melatonin induced the expression of SPT, which catalyzes de rate-limiting step in ceramide neo synthesis (Fig. 3C). These results suggest that melatonin is able to induce ceramide synthesis involving different pathways in HepG2 cells.
Fig. 3.
Sphingolipid metabolism is enhanced in HepG2 cells in response to melatonin 2 mM treatment. Total ceramide levels analyzed by HPLC in HepG2 cells treated with melatonin 2 mM for different time points showing enhanced ceramide synthesis from 1 hour (A). Representative TLC of ASMase activity displaying the enhanced ASMase activity from 1 hour later melatonin 2 mM treatment. Arbitrary units are densitometric values of NBD-Ceramide bands representing the specific product normalized with the values of the corresponding NBD-SM bands (B). mRNA levels of SPT and ASMase after incubation with melatonin 2 mM at the indicated time points analyzed by quantitative RT-PCR. mRNA values were normalized to β-actin RNA and reported as relative levels compared to the expression in control (Ctrl) HepG2 cells (C). Data are expressed as a percentage of mean values ± SEM of experiments performed in triplicate.*P < 0.05 versus Ctrl HepG2 cells values by unpaired one-way analysis of variance with the Bonferroni post hoc test.
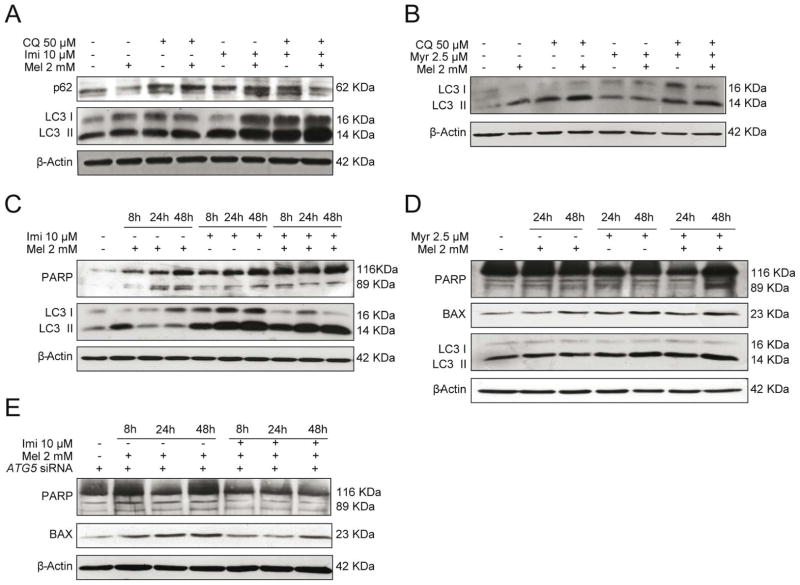
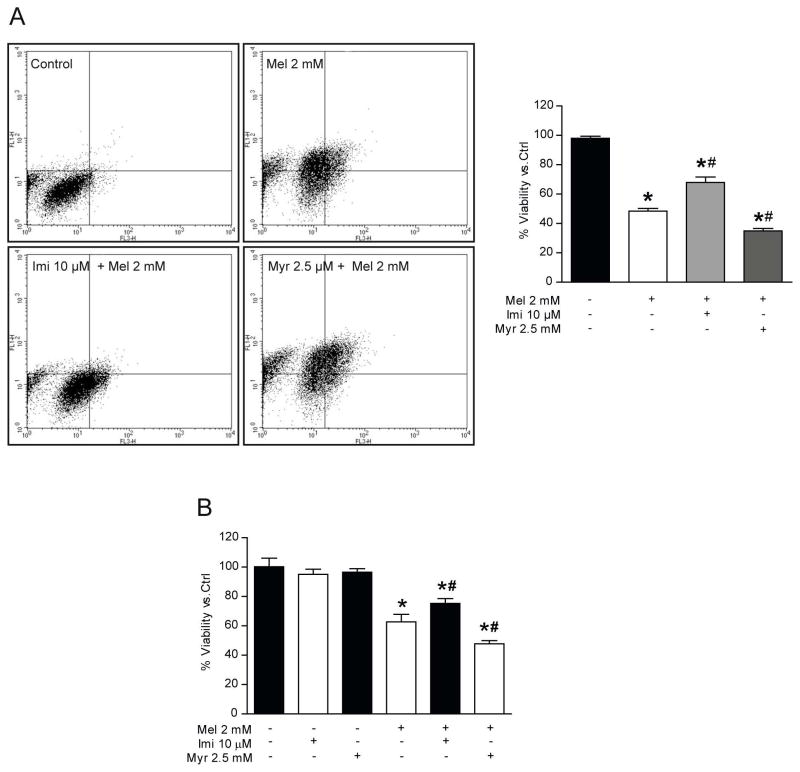
To address the role of ceramide in melatonin-induced autophagy, we tested the effect of inhibition of ASMase and SPT during melatonin treatment. Pre-incubation with imipramine, which prevents ASMase activation, led to a significant increase of LC3II levels in the presence of melatonin, chloroquine, and the combination of both (Fig. 4A). Additionally, we measured p62 levels to monitor the status of lysosomal degradation. Intriguingly, we observed that p62 levels were significantly enhanced after pre-incubation with imipramine for all conditions, indicating dysfunctional lysosomal degradation (Fig. 4A). Pre-incubation with myriocin abrogated the induction of LC3II levels caused by melatonin (Fig. 4B), suggesting that de novo ceramide shyntesis is necessary for melatonin to induce autophagy. Once established that autophagy was impaired through the inhibition of ASMase, we determined the effect of imipramine on melatonin induced apoptotic cell death. Initially, HepG2 cells were pre-incubated 2 hours with imipramine and treated with melatonin and we evaluated the effect over time. As expected, LC3II was increased over time with imipramine as well as the combination of imipramine and melatonin (Fig. 4C), which was accompanied by reduced cleaved PARP in response to melatonin administration (Fig. 4C). Moreover, in conditions of genetic autophagy antagonism by ATG5 silencing, ASMase inhibition by imipramine decreased melatonin induced PARP cleavage and BAX induction, suggesting an ASMase-induced apoptotic pathway in response to melatonin (Fig. 4E). In contrast, LC3II levels were not modified when cells were pre-treated with myriocin, although it increased PARP-cleaved and BAX levels (Fig. 4D). To further explore these effects on HepG2 viability, cells were pre-incubated with imipramine and treated with 2 mM of melatonin during 48 hours. Surprisingly, cells pre-treated with imipramine prevented the cytotoxic effect of melatonin respect to cells treated only with melatonin (Fig. 5A, B). Together, this data support the idea that ASMase inhibition impairs the autophagy-lysosomal degradation pathway, while rescuing cell viability in response to melatonin, highlighting the important role of ceramides in melatonin induced apoptotic cell death. On the other hand, myriocin pre-treatment enhanced melatonin induced cell death (Fig. 5A, B). Interestingly, ASMase expression was enhanced in cells co-treated with myriocin plus melatonin (data not shown). These results suggest that the loss of viability in HepG2 could be related not only to autophagy suppression, but also to induction of ASMase, which enhances apoptotic cell death.
Fig. 4.
Effect of ceramide synthesis inhibition in HepG2 cells on autophagy and apoptosis induced by melatonin 2 mM. Representative immunoblots of LC3 and p62 in HepG2 cells pre-incubated with imipramine 10 μM 2 hours and treated with melatonin 2 mM in the absence or presence of CQ 50 μM for the last four hours of treatment. LC3II protein levels were enhanced with imipramine pre-treatment as well as p62, suggesting an impairment in autophagic flux (A). Representative immunoblots of LC3 in HepG2 cells pre-incubated with myriocin 2.5 μM (Myr) 2 hours and treated with melatonin 2 mM in the absence or presence of CQ 50 μM for the last four hours of treatment. Pre-treatment with myriocin prevented an increase of LC3 levels in response to melatonin administration (B). Representative immunoblots of PARP and LC3 in HepG2 cells pre-incubated with imipramine (Imi) 10 μM for 2 hours and treated with melatonin 2 mM, showing that pre-incubation with imipramine enhanced LC3 levels and prevented PARP cleavage in response to melatonin administration (C). Representative immunoblots of PARP, BAX and LC3 in HepG2 cells pre-incubated with myoricin 2.5 μM for 2 hours and treated with melatonin 2 mM. Pre-incubation with myriocin enhanced LC3, PARP cleavage and Bax levels in response to melatonin treatment (D). Representative immunoblots of PARP and BAX in ATG5-silenced HepG2 cells pre-incubated with imipramine 10 μM 2 hours and treated with melatonin 2 mM, showing that pre-incubation with imipramine prevented PARP cleaved and reduced BAX levels in response to melatonin administration (E). Data are expressed as a percentage of mean values ± SEM of experiments performed in triplicate.
Fig. 5.
Effect of SPT and ASMase inhibition on cell viability and cell death in melatonin-HepG2 treated cells. Cells pre-treated with imipramine 10 μM or myriocin 2.5 μM were incubated with melatonin 2 mM until 48 hours. Annexin V-propidium iodide assay determinated low cell viability when cells were pre-treated with myriocin and a parcial rise when ASMase was inhibited (A). Viability was determined by MTT assay, showing a decrease in cell viability in myriocin plus melatonin group, but a recovery of viability in imipramine co-treated cells (B). Data are expressed as a percentage of mean values ± SEM of three independent experiments. *P < 0.05 significant differences vs control cells #P < 0.05 significant differences vs melatonin-treated cells.
Discussion
Results from the present study indicate that exposure of HepG2 to melatonin transiently induces autophagy and an increase in ceramide levels, which are related to apoptotic cell death possibly by induction of de novo synthesis and ASMase activity. Moreover, the inhibition of ceramide synthesis regulates the autophagic response and has consequences on melatonin-induced apoptosis. Thus, ceramide metabolism modulated by melatonin is proposed to play an important role in autophagy regulation as well as in apoptotic cell death.
First, we observed that melatonin increases the expression of LC3II and Beclin1, and causes the colocalization of LC3II with LAMP-2. These findings are in line with other studies in different cell types and H22 bearing mice [34, 35]. Lower doses of melatonin have been tested in other types of cells, such as HTC116 colon cancer cells or corneal dystrophy type 2 (GCD2) fibroblasts, showing a non-continuous induction of autophagy [41, 42]. On the other hand, previous studies from our laboratory indicate that melatonin administration improves survival and reduces the autophagic response in a viral model of fulminant hepatic failure [43]. In addition, treatment with melatonin also reduces autophagy in the liver of ob/ob mice, or in morphine-treated rat neurons [44, 45], and in both cases autophagy reduction is beneficial. Therefore, melatonin modulation of autophagy seems to be cell type and context-dependent, similarly to other antioxidant molecules with analog properties [38, 46].
Given the key role of mTOR in autophagy regulation [34, 35, 41, 42], we monitored the effect of melatonin in mTOR phosphorylation. Unexpectedly, no changes in p-mTOR protein levels were observed at different time points in cells treated with melatonin. Our data suggest that induction of autophagy could occur upstream of mTOR signaling. ER stress has a pivotal role in cancer and some interactions between autophagy and ER stress have been reported [47–49]. On the other hand, activation of ER stress has been related with JNK phosphorylation and autophagosome formation in SK-N-SH cells [50] or BRAFV600E melanoma cells [48]. Previous research has shown that Beclin1 interacts with anti-apoptotic proteins, such as Bcl-2 or Bcl-XL, inhibiting autophagy [20, 51, 52]. In different situations that induce autophagy, p-JNK is able to phosphorylate Bcl-2, promoting the disruption of Bcl-2/Beclin1 complex and consequently, the initiation of the autophagic process [48, 52]. Our results demonstrate that melatonin administration induces an increase in GRP78 levels paralleled by JNK phosphorylation. Moreover, Beclin1 levels are enhanced from early stages of melatonin administration. These observations are consistent with an induction of autophagy mediated by ER stress, JNK activation and high levels of Beclin1 as necessary elements for autophagosome formation. In fact, inhibition of p-JNK prevented the increment of LC3II protein levels by melatonin. Based on these findings and previous reports, we hypothesize that melatonin could induce autophagy through phosphorylation of Bcl-2 and disturbance of its interaction with Beclin1.
The role played by autophagy in cell survival and cell death in cancer is still unclear. Autophagy functions as a tumour suppressor mechanism by removing damaged organelles/proteins and limiting cell growth and genomic instability, and protects against tumorigenesis by limiting necrosis and chronic inflammation in the early stage [53]. In established tumour cells, autophagy can be used as a survival mechanism in response to chemotherapeutic or nutritional stress. Autophagy modulation as a potential therapeutic target in cancer has been reported in ovarian cell lines, colorectal cancer stem-like cells, immortalized baby mouse kidney (iBMK) cells and the triple-negative breast cancer [54–57]. In the present study, autophagic inhibition assessed by ATG5 silencing increased melatonin-induced apoptotic cell death, suggesting that autophagy favours cell survival in HepG2 cells.
Melatonin exerts its biological actions by receptor-mediated and independent-receptor mechanisms. Two plasma membrane-bounded melatonin receptors, MT1 and MT2, and a nuclear receptor of the orphan family have been described. Moreover, melatonin can also bind to calmodulin and drive antiproliferative effects [58]. MT1 and MT2 are widely expressed in different human tissues, and its implication in melatonin-related antiproliferative action has been reported in different cancer cell lines and in vivo models [59]. Our group has demonstrated that inhibition of MT1 prevents cytostatic and cytotoxic effects of pharmacological concentrations of melatonin on HepG2 cells, probably associated to alterations in cAMP intracellular concentrations [60]. Activation of MT receptors requires the formation of homo or heterodimers. It has been shown that MT1 and MT2 interaction is necessary for melatonin inhibitory action on cAMP formation when nanomolar concentrations are used [61]. However, MT2 receptor is not expressed in HepG2 cells [62], which could explain that higher concentrations of the indole are necessary for its apoptotic and autophagic effect on this HCC cell line.
Ceramides regulate many cellular processes and play an important role as a lipid mediator of apoptotic cell death. Interestingly, ceramide has been also reported to promote the formation of autophagic vacuoles by up-regulation of Beclin1, ER stress and JNK phosphorylation [63, 64]. Here, we show an early induction of ceramide synthesis in response to melatonin treatment. To our understanding, there are no previous reports that demonstrate alterations in ceramide metabolism due to melatonin, but other compounds with similar effects on cancer cells have demonstrated to induce endogenous ceramides accumulation. For instance, resveratrol induces an increase in ceramide levels via de novo biosynthetic pathway in breast cancer cells [65]. Stichoposide c also increases ceramide production through ASMase activation in mouse CT-26 subcutaneous tumours and HL-60 leukaemia xenograft models or colorectal cancer cells [66]. The increase in ceramide levels by melatonin is due in part to the ASMase activity observed early after melatonin treatment, although we cannot discard the contribution of the de novo synthesis because of the observed increase in the expression of key enzymes in the de novo synthesis. To address the implication of ceramide metabolism on autophagy induced by melatonin, we prevented activation of the enzymes SPT and ASMase with chemical inhibitors. When ASMase activity was inhibited with imipramine, we observed that melatonin was able to induce LC3 lipidation but not p62 degradation, indicating an accumulation of autophaghosomes and, consequently, an incomplete autophagic process. These results are in accordance to those observed in ASMase deficient mice, where induction of autophagy in liver due to high fat diet feeding is not complete [67]. In addition, KO ASMase mice exhibit a defect in the fusion of autophagosomes with lysosomes in arterial smooth muscle cells [67]. In line with these findings, ASMase was necessary for the upregulation of ATG5 expression and autophagy induction in HepG2 cells [64]. Moreover, other inhibitors of ASMase such as desipramine, disturbe lysosome function in HTC hepatoma cells [68]. Lysosomal membrane permeabilization (LMP) has been suggested to be related to impaired autophagic flux because of the release of lysosomal enzymes to the cytoplasm, altering lysosomes function [69, 70]. In addition, the lack of ASMase activity is also associated to LMP and blocking of autophagy [67, 71]. So, our observations in HepG2 cells treated with melatonin and imipramine suggest a LMP regulation through ASMase activity.
De novo synthesis of ceramides is necessary for autophagy induction in different cell types and contexts. For instance, inhibition of SPT with myriocin in MCF-7 suppressed Beclin1 expression and reduced resveratrol induced autophagy [63]. Moreover, myriocin pre-treatment prevents C16 ceramide accumulation, ER stress and autophagy induced by resveratrol in human nasopharyngeal cancer cells [72]. In addition, overexpression of SPT in liver has been observed to induce autophagy [73]. In line with these findings, our experiments with the SPT inhibitor myriocin showed that this enzyme is necessary for autophagy induction due to melatonin. However, the mechanism by which myriocin pre-treatment prevents melatonin-induced autophagy in HepG2 cells remains unclear.
To elucidate the interplay between autophagy and apoptosis, and the implication of both ceramide synthesis pathways in these processes, we employed myriocin 2.5 μM and imipramine 10 μM. ASMase is an enzyme that mediates stress response and apoptosis. It is activated in response to a variety of proinflammatory and proapoptotic stimuli such as cytokines, ROS and oxidative stress [25, 74, 75]. Our results show that inhibition of ASMase activity recovers partially the apoptotic cell death induced by melatonin. Moreover, ASMase inhibition in autophagy-defective cells reduces apoptotic markers such as PARP and BAX versus cells without imipramine pre-treatment. These results taken together suggest that ASMase activity plays an important role in melatonin-induced apoptotic cell death.
When SPT was blocked, cell death induced by melatonin was potentiated, which could be explained by the fact that autophagy as a survival mechanism is not induced. In human breast cancer cells, vitamin E analogs stimulate apoptosis mediated by ceramides, which in turn activate the JNK/CHOP pathway; however when de novo synthesis of ceramides is abolished, no toxic effects are observed, showing a relationship between de novo pathway and cell death [76]. In bovine endothelial cerebral cells, apoptosis induced by TNF-α is due to ceramide accumulation by de novo synthesis stimulation [77]. Resveratrol, another molecule with similar properties to melatonin, reduces the expression of cleaved PARP when SPT is inhibited with myriocin in human nasopharyngeal carcinoma cells [72]. These data contrast with our results, which demonstrate that inhibition of SPT reduces HepG2 cells viability and increases apoptosis when melatonin is administered. On the other hand, it has been observed that inhibition of de novo ceramide synthesis with fumonisin B1 in mouse liver activates ASMase at the same time that sphyngomielin levels are reduced [78]. Moreover, administration of fumonisin B1 in mice increases apoptosis although ceramide levels are not altered [79], which suggests that ASMase could enhance its activity in order to maintain ceramide amounts. In addition, SPT inhibition in insulinoma cells did not affected induced apoptosis, and it was proposed that ceramides from sphyngomieline hydrolysis was the key factor for cell death [80]. SPT inhibition in HepG2 cells causes autophagy suppression, so the protective effect induced in response to melatonin is not activated, which could also explain this enhancement in cell death. In addition, myriocin pre-treatment and melatonin administration enhanced significantly ASMase RNA levels versus only melatonin.
In summary, these results suggest that the inhibition of de novo ceramide synthesis could be activating sphingomyelin hydrolysis through ASMase activity as a compensatory mechanism. Additionally, the results presented here indicate that ASMase activity stimulated by melatonin regulates autophagy. Moreover, when both autophagy and ASMase were inhibited, apoptosis was lower compared to melatonin alone. These data suggest that ASMase changes induced by melatonin have a more powerful effect on apoptosis and cell viability that its implication in the regulation of autophagic flux. However, autophagy suppression by targeting SPT is important to enhance the cytotoxic effect of melatonin. Our findings indicate that inhibition of autophagy could increase the beneficial effects of melatonin in cancer treatment, but the blockade of this mechanism should be made with caution considering the implication of different intracellular messengers in cell death.
Acknowledgments
Raquel Ordoñez and Néstor Prieto-Domínguez are supported by the program “Formación del Profesorado Universitario” (Becas FPU, references FPU12/01433 and FPU13/04173 respectively) from the Ministry of Education (Spain). CIBERehd is funded by Instituto de la Salud Carlos III, Spain. This work was supported in part by grants BFU2013-48141-R from Plan Nacional de I+D+I Spain, LE135U13 from Consejería de Educación de la Junta de Castilla y León, SAF-2011-23031, SAF-2012-34831 from Plan Nacional de I+D Spain, Fundació Marató de TV3, La Mutua Madrileña, PI11/0325 (META) grant from the Instituto Salud Carlos III, and by the center grant P50-AA-11999 Research Center for Liver and Pancretic Diseases funded by NIAAA/NIH.
References
- 1.BLECHACZ B, MISHRA L. Hepatocellular carcinoma biology. Recent Results Cancer Res. 2013;190:1–20. doi: 10.1007/978-3-642-16037-0_1. [DOI] [PubMed] [Google Scholar]
- 2.KAPITANOV T, NEUMANN UP, SCHMEDING M. Hepatocellular Carcinoma in Liver Cirrhosis: Surgical Resection versus Transarterial Chemoembolization-A Meta-Analysis. Gastroenterol Res Pract. 2015;2015:696120. doi: 10.1155/2015/696120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.VILLANUEVA A, PORTELA A, SAYOLS S, et al. DNA Methylation-based prognosis and epidrivers in hepatocellular carcinoma. Hepatology. 2015 doi: 10.1002/hep.27732. [DOI] [PubMed] [Google Scholar]
- 4.MORALES A, PARIS R, VILLANUEVA A, et al. Pharmacological inhibition or small interfering RNA targeting acid ceramidase sensitizes hepatoma cells to chemotherapy and reduces tumor growth in vivo. Oncogene. 2007;26:905–916. doi: 10.1038/sj.onc.1209834. [DOI] [PubMed] [Google Scholar]
- 5.GARCIA-RUIZ C, MORALES A, FERNANDEZ-CHECA JC. Glycosphingolipids and cell death: one aim, many ways. Apoptosis. 2015;20:607–620. doi: 10.1007/s10495-015-1092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LU M, MA J, XUE W, et al. The expression and prognosis of FOXO3a and Skp2 in human hepatocellular carcinoma. Pathol Oncol Res. 2009;15:679–687. doi: 10.1007/s12253-009-9171-z. [DOI] [PubMed] [Google Scholar]
- 7.CHEN JA, SHI M, LI JQ, et al. Angiogenesis: multiple masks in hepatocellular carcinoma and liver regeneration. Hepatol Int. 2010;4:537–547. doi: 10.1007/s12072-010-9192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ASHWORTH RE, WU J. Mammalian target of rapamycin inhibition in hepatocellular carcinoma. World J Hepatol. 2014;6:776–782. doi: 10.4254/wjh.v6.i11.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CHA YL, LI PD, YUAN LJ, et al. EIF4EBP1 Overexpression Is Associated with Poor Survival and Disease Progression in Patients with Hepatocellular Carcinoma. PLoS One. 2015;10:e0117493. doi: 10.1371/journal.pone.0117493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.TAKAHASHI Y, COPPOLA D, MATSUSHITA N, et al. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat Cell Biol. 2007;9:1142–1151. doi: 10.1038/ncb1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LIU YQ, JI Y, LI XZ, et al. Retigeric acid B-induced mitophagy by oxidative stress attenuates cell death against prostate cancer cells in vitro. Acta Pharmacol Sin. 2013;34:1183–1191. doi: 10.1038/aps.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.GUO JY, CHEN HY, MATHEW R, et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25:460–470. doi: 10.1101/gad.2016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.BELLOT G, GARCIA-MEDINA R, GOUNON P, et al. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol. 2009;29:2570–2581. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WONG PM, PUENTE C, GANLEY IG, et al. The ULK1 complex: sensing nutrient signals for autophagy activation. Autophagy. 2013;9:124–137. doi: 10.4161/auto.23323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.JIANG H, WHITE EJ, RIOS-VICIL CI, et al. Human adenovirus type 5 induces cell lysis through autophagy and autophagy-triggered caspase activity. J Virol. 2011;85:4720–4729. doi: 10.1128/JVI.02032-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WIRAWAN E, VANDE WALLE L, KERSSE K, et al. Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis. 2010;1:e18. doi: 10.1038/cddis.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MARINO G, NISO-SANTANO M, BAEHRECKE EH, et al. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15:81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MORALES A, LEE H, GONI FM, et al. Sphingolipids and cell death. Apoptosis. 2007;12:923–939. doi: 10.1007/s10495-007-0721-0. [DOI] [PubMed] [Google Scholar]
- 19.KITATANI K, IDKOWIAK-BALDYS J, HANNUN YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008;20:1010–1018. doi: 10.1016/j.cellsig.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.PATTINGRE S, BAUVY C, CARPENTIER S, et al. Role of JNK1-dependent Bcl-2 phosphorylation in ceramide-induced macroautophagy. J Biol Chem. 2009;284:2719–2728. doi: 10.1074/jbc.M805920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.GUENTHER GG, PERALTA ER, ROSALES KR, et al. Ceramide starves cells to death by downregulating nutrient transporter proteins. Proc Natl Acad Sci U S A. 2008;105:17402–17407. doi: 10.1073/pnas.0802781105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.PERROTTA C, BIZZOZERO L, CAZZATO D, et al. Syntaxin 4 is required for acid sphingomyelinase activity and apoptotic function. J Biol Chem. 2010;285:40240–40251. doi: 10.1074/jbc.M110.139287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.GAULT CR, OBEID LM, HANNUN YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv Exp Med Biol. 2010;688:1–23. doi: 10.1007/978-1-4419-6741-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.KOLESNICK RN, KRONKE M. Regulation of ceramide production and apoptosis. Annu Rev Physiol. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- 25.COLELL A, MORALES A, FERNANDEZ-CHECA JC, et al. Ceramide generated by acidic sphingomyelinase contributes to tumor necrosis factor-alpha-mediated apoptosis in human colon HT-29 cells through glycosphingolipids formation. Possible role of ganglioside GD3. FEBS Lett. 2002;526:135–141. doi: 10.1016/s0014-5793(02)03140-x. [DOI] [PubMed] [Google Scholar]
- 26.PERROTTA C, CERVIA D, DE PALMA C, et al. The emerging role of Acid Sphingomyelinase in autophagy. Apoptosis. 2015;20:635–44. doi: 10.1007/s10495-015-1101-9. [DOI] [PubMed] [Google Scholar]
- 27.ZHU W, WANG X, ZHOU Y, et al. C2-ceramide induces cell death and protective autophagy in head and neck squamous cell carcinoma cells. Int J Mol Sci. 2014;15:3336–3355. doi: 10.3390/ijms15023336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.YANG YL, JI C, BI ZG, et al. Deguelin induces both apoptosis and autophagy in cultured head and neck squamous cell carcinoma cells. PLoS One. 2013;8:e54736. doi: 10.1371/journal.pone.0054736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.CRESPO I, MIGUEL BS, LALIENA A, et al. Melatonin prevents the decreased activity of antioxidant enzymes and activates nuclear erythroid 2-related factor 2 signaling in an animal model of fulminant hepatic failure of viral origin. J Pineal Res. 2010;49:193–200. doi: 10.1111/j.1600-079X.2010.00787.x. [DOI] [PubMed] [Google Scholar]
- 30.TUNON MJ, SAN MIGUEL B, CRESPO I, et al. Melatonin attenuates apoptotic liver damage in fulminant hepatic failure induced by the rabbit hemorrhagic disease virus. J Pineal Res. 2011;50:38–45. doi: 10.1111/j.1600-079X.2010.00807.x. [DOI] [PubMed] [Google Scholar]
- 31.CARBAJO-PESCADOR S, ORDONEZ R, BENET M, et al. Inhibition of VEGF expression through blockade of Hif1alpha and STAT3 signalling mediates the anti-angiogenic effect of melatonin in HepG2 liver cancer cells. Br J Cancer. 2013;109:83–91. doi: 10.1038/bjc.2013.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MARTIN-RENEDO J, MAURIZ JL, JORQUERA F, et al. Melatonin induces cell cycle arrest and apoptosis in hepatocarcinoma HepG2 cell line. J Pineal Res. 2008;45:532–540. doi: 10.1111/j.1600-079X.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- 33.ORDONEZ R, CARBAJO-PESCADOR S, PRIETO-DOMINGUEZ N, et al. Inhibition of matrix metalloproteinase-9 and nuclear factor kappa B contribute to melatonin prevention of motility and invasiveness in HepG2 liver cancer cells. J Pineal Res. 2014;56:20–30. doi: 10.1111/jpi.12092. [DOI] [PubMed] [Google Scholar]
- 34.MARTIN V, SANCHEZ-SANCHEZ AM, PUENTE-MONCADA N, et al. Involvement of autophagy in melatonin-induced cytotoxicity in glioma-initiating cells. J Pineal Res. 2014;57:308–316. doi: 10.1111/jpi.12170. [DOI] [PubMed] [Google Scholar]
- 35.LIU C, JIA Z, ZHANG X, et al. Involvement of melatonin in autophagy-mediated mouse hepatoma H22 cell survival. Int Immunopharmacol. 2012;12:394–401. doi: 10.1016/j.intimp.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 36.HAHM ER, SAKAO K, SINGH SV. Honokiol activates reactive oxygen species-mediated cytoprotective autophagy in human prostate cancer cells. Prostate. 2014;74:1209–1221. doi: 10.1002/pros.22837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.KIM DG, JUNG KH, LEE DG, et al. 20(S)-Ginsenoside Rg3 is a novel inhibitor of autophagy and sensitizes hepatocellular carcinoma to doxorubicin. Oncotarget. 2014;5:4438–4451. doi: 10.18632/oncotarget.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.ZHOU T, YE L, BAI Y, et al. Autophagy and apoptosis in hepatocellular carcinoma induced by EF25-(GSH)2: a novel curcumin analog. PLoS One. 2014;9:e107876. doi: 10.1371/journal.pone.0107876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.FERNANDEZ A, MATIAS N, FUCHO R, et al. ASMase is required for chronic alcohol induced hepatic endoplasmic reticulum stress and mitochondrial cholesterol loading. J Hepatol. 2013;59:805–813. doi: 10.1016/j.jhep.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.GARCIA-MEDIAVILLA MV, SANCHEZ-CAMPOS S, GONZALEZ-PEREZ P, et al. Differential contribution of hepatitis C virus NS5A and core proteins to the induction of oxidative and nitrosative stress in human hepatocyte-derived cells. J Hepatol. 2005;43:606–613. doi: 10.1016/j.jhep.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 41.HONG Y, WON J, LEE Y, et al. Melatonin treatment induces interplay of apoptosis, autophagy, and senescence in human colorectal cancer cells. J Pineal Res. 2014;56:264–674. doi: 10.1111/jpi.12119. [DOI] [PubMed] [Google Scholar]
- 42.CHOI SI, KIM KS, OH JY, et al. Melatonin induces autophagy via an mTOR-dependent pathway and enhances clearance of mutant-TGFBIp. J Pineal Res. 2013;54:361–372. doi: 10.1111/jpi.12039. [DOI] [PubMed] [Google Scholar]
- 43.SAN-MIGUEL B, CRESPO I, VALLEJO D, et al. Melatonin modulates the autophagic response in acute liver failure induced by the rabbit hemorrhagic disease virus. J Pineal Res. 2014;56:313–321. doi: 10.1111/jpi.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DE LUXAN-DELGADO B, CABALLERO B, POTES Y, et al. Melatonin administration decreases adipogenesis in the liver of ob/ob mice through autophagy modulation. J Pineal Res. 2014;56:126–33. doi: 10.1111/jpi.12104. [DOI] [PubMed] [Google Scholar]
- 45.FENG YM, JIA YF, SU LY, et al. Decreased mitochondrial DNA copy number in the hippocampus and peripheral blood during opiate addiction is mediated by autophagy and can be salvaged by melatonin. Autophagy. 2013;9:1395–1406. doi: 10.4161/auto.25468. [DOI] [PubMed] [Google Scholar]
- 46.KIM H, MOON JY, AHN KS, et al. Quercetin induces mitochondrial mediated apoptosis and protective autophagy in human glioblastoma U373MG cells. Oxid Med Cell Longev. 2013;2013:596496. doi: 10.1155/2013/596496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.SUH DH, KIM MK, KIM HS, et al. Unfolded protein response to autophagy as a promising druggable target for anticancer therapy. Ann N Y Acad Sci. 2012;1271:20–32. doi: 10.1111/j.1749-6632.2012.06739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.CORAZZARI M, RAPINO F, CICCOSANTI F, et al. Oncogenic BRAF induces chronic ER stress condition resulting in increased basal autophagy and apoptotic resistance of cutaneous melanoma. Cell Death Differ. 2014 doi: 10.1038/cdd.2014.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.HABERZETTL P, HILL BG. Oxidized lipids activate autophagy in a JNK-dependent manner by stimulating the endoplasmic reticulum stress response. Redox Biol. 2013;1:56–64. doi: 10.1016/j.redox.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.OGATA M, HINO S, SAITO A, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.MAIURI MC, LE TOUMELIN G, CRIOLLO A, et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.LI C, JOHNSON DE. Bortezomib induces autophagy in head and neck squamous cell carcinoma cells via JNK activation. Cancer Lett. 2012;314:102–107. doi: 10.1016/j.canlet.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.GUO JY, KARSLI-UZUNBAS G, MATHEW R, et al. Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis. Genes Dev. 2013;27:1447–1461. doi: 10.1101/gad.219642.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.KAO C, CHAO A, TSAI CL, et al. Bortezomib enhances cancer cell death by blocking the autophagic flux through stimulating ERK phosphorylation. Cell Death Dis. 2014;5:e1510. doi: 10.1038/cddis.2014.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MATHEW R, KARP CM, BEAUDOIN B, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.WEI MF, CHEN MW, CHEN KC, et al. Autophagy promotes resistance to photodynamic therapy-induced apoptosis selectively in colorectal cancer stem-like cells. Autophagy. 2014;10:1179–1192. doi: 10.4161/auto.28679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.LEFORT S, JOFFRE C, KIEFFER Y, et al. Inhibition of autophagy as a new means of improving chemotherapy efficiency in high-LC3B triple-negative breast cancers. Autophagy. 2014;10:2122–2142. doi: 10.4161/15548627.2014.981788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.EKMEKCIOGLU C. Melatonin receptors in humans: biological role and clinical relevance. Biomed Pharmacother. 2006;60:97–108. doi: 10.1016/j.biopha.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 59.DUBOCOVICH ML, DELAGRANGE P, KRAUSE DN, et al. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010;62:343–380. doi: 10.1124/pr.110.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.CARBAJO-PESCADOR S, GARCIA-PALOMO A, MARTIN-RENEDO J, et al. Melatonin modulation of intracellular signaling pathways in hepatocarcinoma HepG2 cell line: role of the MT1 receptor. J Pineal Res. 2011;51:463–471. doi: 10.1111/j.1600-079X.2011.00910.x. [DOI] [PubMed] [Google Scholar]
- 61.IMBESI M, UZ T, DZITOYEVA S, et al. Melatonin signaling in mouse cerebellar granule cells with variable native MT1 and MT2 melatonin receptors. Brain Res. 2008;1227:19–25. doi: 10.1016/j.brainres.2008.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.CARBAJO-PESCADOR S, MARTIN-RENEDO J, GARCIA-PALOMO A, et al. Changes in the expression of melatonin receptors induced by melatonin treatment in hepatocarcinoma HepG2 cells. J Pineal Res. 2009;47:330–338. doi: 10.1111/j.1600-079X.2009.00719.x. [DOI] [PubMed] [Google Scholar]
- 63.SCARLATTI F, BAUVY C, VENTRUTI A, et al. Ceramide-mediated macroautophagy involves inhibition of protein kinase B and up-regulation of beclin 1. J Biol Chem. 2004;279:18384–18391. doi: 10.1074/jbc.M313561200. [DOI] [PubMed] [Google Scholar]
- 64.PARK MA, ZHANG G, MARTIN AP, et al. Vorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activation. Cancer Biol Ther. 2008;7:1648–1662. doi: 10.4161/cbt.7.10.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.SCARLATTI F, SALA G, SOMENZI G, et al. Resveratrol induces growth inhibition and apoptosis in metastatic breast cancer cells via de novo ceramide signaling. FASEB J. 2003;17:2339–2341. doi: 10.1096/fj.03-0292fje. [DOI] [PubMed] [Google Scholar]
- 66.YUN SH, PARK ES, SHIN SW, et al. Stichoposide C induces apoptosis through the generation of ceramide in leukemia and colorectal cancer cells and shows in vivo antitumor activity. Clin Cancer Res. 2012;18:5934–5948. doi: 10.1158/1078-0432.CCR-12-0655. [DOI] [PubMed] [Google Scholar]
- 67.FUCHO R, MARTINEZ L, BAULIES A, et al. ASMase regulates autophagy and lysosomal membrane permeabilization and its inhibition prevents early stage non-alcoholic steatohepatitis. J Hepatol. 2014;61:1126–1134. doi: 10.1016/j.jhep.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.AUTELLI R, ULLIO C, PRIGIONE E, et al. Divergent pathways for TNF and C(2)-ceramide toxicity in HTC hepatoma cells. Biochim Biophys Acta. 2009;1793:1182–1190. doi: 10.1016/j.bbamcr.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 69.GONZALEZ P, MADER I, TCHOGHANDJIAN A, et al. Impairment of lysosomal integrity by B10, a glycosylated derivative of betulinic acid, leads to lysosomal cell death and converts autophagy into a detrimental process. Cell Death Differ. 2012;19:1337–1346. doi: 10.1038/cdd.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.YAO XF, CAO J, XU LM, et al. Perfluorooctane sulfonate blocked autophagy flux and induced lysosome membrane permeabilization in HepG2 cells. Food Chem Toxicol. 2014;67:96–104. doi: 10.1016/j.fct.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 71.GABANDE-RODRIGUEZ E, BOYA P, LABRADOR V, et al. High sphingomyelin levels induce lysosomal damage and autophagy dysfunction in Niemann Pick disease type A. Cell Death Differ. 2014;21:864–875. doi: 10.1038/cdd.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.CHOW SE, KAO CH, LIU YT, et al. Resveratrol induced ER expansion and ER caspase-mediated apoptosis in human nasopharyngeal carcinoma cells. Apoptosis. 2014;19:527–541. doi: 10.1007/s10495-013-0945-0. [DOI] [PubMed] [Google Scholar]
- 73.ALEXAKI A, GUPTA SD, MAJUMDER S, et al. Autophagy regulates sphingolipid levels in the liver. J Lipid Res. 2014;55:2521–2531. doi: 10.1194/jlr.M051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.FERNANDEZ-CHECA JC, COLELL A, MARI M, et al. Ceramide, tumor necrosis factor and alcohol-induced liver disease. Alcohol Clin Exp Res. 2005;29:151S–157S. [PubMed] [Google Scholar]
- 75.JIANG L, PAN X, CHEN Y, et al. Preferential involvement of both ROS and ceramide in fenretinide-induced apoptosis of HL60 rather than NB4 and U937 cells. Biochem Biophys Res Commun. 2011;405:314–318. doi: 10.1016/j.bbrc.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 76.GOPALAN A, YU W, JIANG Q, et al. Involvement of de novo ceramide synthesis in gamma-tocopherol and gamma-tocotrienol-induced apoptosis in human breast cancer cells. Mol Nutr Food Res. 2012;56:1803–1811. doi: 10.1002/mnfr.201200350. [DOI] [PubMed] [Google Scholar]
- 77.XU J, YEH CH, CHEN S, et al. Involvement of de novo ceramide biosynthesis in tumor necrosis factor-alpha/cycloheximide-induced cerebral endothelial cell death. J Biol Chem. 1998;273:16521–16526. doi: 10.1074/jbc.273.26.16521. [DOI] [PubMed] [Google Scholar]
- 78.HE Q, SUZUKI H, SHARMA N, et al. Ceramide synthase inhibition by fumonisin B1 treatment activates sphingolipid-metabolizing systems in mouse liver. Toxicol Sci. 2006;94:388–397. doi: 10.1093/toxsci/kfl102. [DOI] [PubMed] [Google Scholar]
- 79.TSUNODA M, SHARMA RP, RILEY RT. Early fumonisin B1 toxicity in relation to disrupted sphingolipid metabolism in male BALB/c mice. J Biochem Mol Toxicol. 1998;12:281–289. doi: 10.1002/(sici)1099-0461(1998)12:5<281::aid-jbt4>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 80.LEI X, ZHANG S, BOHRER A, et al. The group VIA calcium-independent phospholipase A2 participates in ER stress-induced INS-1 insulinoma cell apoptosis by promoting ceramide generation via hydrolysis of sphingomyelins by neutral sphingomyelinase. Biochemistry. 2007;46:10170–10185. doi: 10.1021/bi700017z. [DOI] [PMC free article] [PubMed] [Google Scholar]