Abstract
Midlife may be an ideal window for intervention in Alzheimer’s disease (AD). To determine whether sleep is associated with early signs of AD neuropathology (amyloid deposition) in late midlife, we imaged brain amyloid deposits using Positron Emission Tomography (PET) with [C-11]Pittsburgh Compound B (PiB), and assessed sleep with the Epworth Sleepiness Scale (ESS) and the Medical Outcomes Study (MOS) Sleep Scale in 98 cognitively healthy adults (aged 62.4 ± 5.7 years) from the Wisconsin Registry for Alzheimer’s Prevention. We used multiple regression to test the extent to which sleep scores predicted regional amyloid burden. Participants reporting less adequate sleep, more sleep problems and greater somnolence on the MOS had greater amyloid burden in AD-sensitive brain regions (angular gyrus, frontal medial orbital cortex, cingulate gyrus and precuneus). Amyloid was not associated with reported sleep amount, symptoms of sleep disordered breathing, trouble falling asleep or ESS. Poor sleep may be a risk factor for AD and a potential early marker of AD or target for preventative interventions in mid-life.
Keywords: sleep, amyloid, Alzheimer’s disease, PET, mid-life, self-report
1. Introduction
Amyloid plaques are a hallmark of Alzheimer’s disease (AD). Accumulation and aggregation of the peptide β-amyloid (1-42) (Aβ42) into insoluble plaques is evident a decade or more before AD symptoms appear, during the preclinical phase of the disease (Jack et al., 2013; Sperling et al., 2011) and is thought to be a major cause of neural dysfunction and cognitive decline to dementia. Older adults (mean age 65.6 years) with pathological levels of Aβ42 in cerebrospinal fluid (CSF) had lower sleep efficiency as measured by actigraphy than those with normal Aβ42 levels (Ju et al., 2013). In humans, amyloid plaques can be imaged with positron emission tomography (PET) using radioligands such as [C-11]Pittsburgh Compound B (PiB). In older adults (mean age 78.2 years), greater amyloid burden was associated with self-report of poor sleep quality and shorter sleep duration (Spira et al., 2013).
The mechanism linking poor sleep with greater amyloid burden is not clear. In mice, sleep disruption increases amyloid generation (Shiota et al., 2013) and deposition (Kang et al., 2009). Amyloid levels in brain interstitial fluid follow a diurnal pattern (Kang et al., 2009; Roh et al., 2012) and clearance of exogenous amyloid is greatest during sleep (Xie et al., 2013). Aβ plaques arise from an imbalance between Aβ production and clearance (Yan et al., 2009). Thus sleep problems may reduce Aβ clearance, leading to its accumulation and aggregation into plaques.
The association between sleep and amyloid burden has not been examined in late middle age. This age range is important because amyloid accumulation begins years before AD symptoms begin, and current AD treatments targeting later-stage disease have shown disappointing results (Schneider et al., 2014). Earlier intervention may be a more effective strategy to prevent or delay clinical symptom onset due to AD pathology (Sperling et al., 2011). Sleep is an attractive therapeutic target because well-established methods already exist for improving sleep. Alternatively, if sleep is affected by amyloid deposition, sleep may harbor markers of early, preclinical AD useful for prognosis and treatment monitoring.
The objective of this study was to determine whether sleep quality and quantity are related to amyloid burden in late mid-life, and to determine which aspects of sleep are associated with increased amyloid burden. We used PiB PET imaging and validated sleep questionnaires to test the hypothesis that in cognitively healthy middle-aged adults, poorer self-reported sleep quality would be associated with greater amyloid burden in brain regions typically affected by AD.
2. Methods
2.1 Participants and Study Design
Participants were drawn from the Wisconsin Registry for Alzheimer’s Prevention (WRAP), a longitudinal cohort of >1500 cognitively healthy adults, aged 40–65 years at study entry (Sager et al., 2005). Participants were included in the present analysis if they had completed the WRAP Wave 4 visit, which included sleep assessment, and had completed a PiB PET scan; 98 individuals met inclusion criteria. Pertinent demographic and cognitive characteristics are summarized in Table 2; note that the sample was enriched with parental family history of AD and APOE4 genotype, to a similar degree as the entire WRAP cohort.
Table 2.
Participant characteristics (n=98).
| Variable | Data (n=98) |
|---|---|
| Age at PiB PET scan, y | 62.4 (5.7; 50–73) |
| Age at sleep assessment, y | 63.0 (5.6; 51–73) |
| Interval between PiB PET scan and sleep assessment, y | .69 (.98; 0–3.7) |
| Female, % | 67.3 |
| APOE4 positive, % | 34.7 |
| FH positive, % | 75.5 |
| Maternal FH positive, % | 52 |
| BMI, kg/m2 | 28.7 (5.7) |
| Education, y | 16.582 (2.832; 12–25) |
| CES-D | 5.78 (5.48; 0–27) |
| MMSE | 29.31 (1.22; 23–30) |
| AVLT total | 50.21 (8.66; 30–67) |
| AVLT delayed recall | 10.36 (2.96; 0–15) |
| Trails A Time* | 10.11 (2.18; 5–17) |
| Trails B Time* | 10.26 (2.51; 6–17) |
| Digit Symbol* | 13.35 (2.1; 9–19) |
All values are mean (SD; range) except where indicated. APOE4, the epsilon 4 allele of the apolipoprotein E gene; FH, family history of Alzheimer’s disease; BMI, Body Mass Index, CES-D, Center for Epidemiological Studies Depression Scale; MMSE, Mini-Mental State Exam; AVLT, Auditory Verbal Learning Test;
scaled for age and gender.
WRAP participants underwent comprehensive neurocognitive and medical history assessment at baseline, four years later, and every 2 years thereafter at the University of Wisconsin (Sager et al., 2005). Participants were recruited to PiB PET imaging sub-studies by telephone, letter, or in person at their WRAP visit. The scan closest to the time of the sleep questionnaires was used in this analysis. Exclusion criteria included MRI contra-indications, abnormal structural MRI, and diagnosis of significant neurological disease, medical illness or major psychiatric disorders (determined by patient self report). All study procedures were approved by the University of Wisconsin Institutional Review Board and each participant provided signed informed consent before participation.
2.2 MRI Acquisition and Processing
All participants were scanned on a GE 3.0 Tesla MR750 (Waukesha, WI) using an 8 channel head coil. A T1-weighted brain volume was acquired in the axial plane with a 3D inversion recovery prepared fast spoiled gradient-echo (3D SPGR) sequence using the following parameters: TI = 450 ms; TR = 8.1 ms; TE = 3.2 ms; flip angle = 12°; acquisition matrix = 256 × 256 ×156 mm, FOV = 256 mm; slice thickness = 1.0 mm. Voxels were 1 mm isotropic. The image acquisition protocol also included T2 weighted and FLAIR anatomical scans, which were reviewed by a neuroradiologist (H.A.R.) for exclusionary abnormalities. The T1-weighted volume was segmented into tissue classes using the updated segmentation feature in SPM12 (www.fil.ion.ucl.ac.uk/spm).
2.3 PiB PET Imaging
[C-11] PiB PET radiochemical synthesis, acquisition parameters and generation of distribution volume ratio (DVR) maps were detailed previously (Johnson et al., 2014). Briefly, after a 70 minute dynamic [C-11]PiB PET acquisition, PET data were reconstructed using a filtered back-projection algorithm (DIFT) and were corrected for random events, attenuation of annihilation radiation, deadtime, scanner normalization, and scatter radiation and were realigned and coregistered in SPM12. The data were then transformed into voxel-wise DVR maps of [C-11]PiB binding using the time activity curve in the gray matter (GM) of the cerebellum as the reference region (Logan et al., 1996).
2.4 Cortical Amyloid Burden Quantification
To reduce the number of statistical tests, amyloid binding was averaged within eight bilateral regions of interest (ROIs), selected on the basis of AD sensitivity and known amyloid binding. The eight ROIs from the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) were angular gyrus, anterior cingulate gyrus, posterior cingulate gyrus, frontal medial orbital gyrus, precuneus, supramarginal gyrus, middle temporal gyrus, and superior temporal gyrus. The inverse deformation field resulting from unified segmentation on each subject image was applied to each AAL ROI to produce ROI masks in native space. To constrain ROI analyses to grey matter only, each ROI mask was next multiplied by the binarized grey matter probability map thresholded at 0.3. A summary measure of amyloid burden was calculated by averaging all ROI means.
2.5 Sleep Assessment
Two validated questionnaires assessing sleep were completed as part of a larger standardized neuropsychological assessment, proximal to the time of the PET scan. The Epworth Sleepiness Scale (ESS) (Johns, 1991) assesses sleep propensity and daytime sleepiness. Participants rate how likely they are to doze off or fall asleep in 8 common situations that vary in their soporific qualities, such as watching TV, talking to someone or lying down. Responses are on a 4-point scale ranging from 0 = “would never doze” to 3 = “high chance of dozing”. Responses are summed to produce a total score ranging from 0 to 24, with higher scores indicating greater daytime sleepiness. The ESS has been shown to have good internal consistency (Cronbach α = 0.73–0.88) and test-retest reliability (correlation of measures across a 5-month interval = 0.82) (Johns, 1992).
The Sleep Scale from the Medical Outcomes Study (MOS) (Hays and Stewart, 1992) is summarized in Table 1. It comprises 12 questions about the past 4 weeks, from which 8 scores were computed. The first question asks how long it takes to fall asleep, with possible responses in 15 minute increments ranging from 1 = “0–15 minutes’ to 5 = “More than 60 minutes”. The second question asks the average number of hours slept each night, which is entered freely. Responses to the remaining 10 questions are on a 6-point scale ranging from 1=“All of the time” to 6=“None of the Time”. Responses were converted to a 0–100 scale, with higher values indicating more of the concept being measured, then summed to give scores for 6 sleep domains: Sleep Disturbance, Somnolence, Sleep Adequacy, Snoring, Awaking Short of Breath or with a headache and Sleep Quantity, and two indices of sleep problems summarizing 6 (Index I) or 9 (Index II) items (Spritzer and Hays, 2003). Multi-item scores show good internal consistency (Cronbach’s alpha 0.71 to 0.81) (Viala-Danten et al., 2008). Table 1 indicates which items contribute to each score, with some items contributing to more than one score.
Table 1.
Sleep Scales
| ESS | Sleep Disturbance | Snoring | Waking short of breath | Sleep Adequacy | Somnolence | Sleep Problems Index I | Sleep Problems Index II | Sleep Quantity | |
|---|---|---|---|---|---|---|---|---|---|
| EPWORTH SLEEPINESS SCALE (ESS) | |||||||||
| How likely are you to doze off or fall asleep in the following 8 situations? a | ⭘ | ||||||||
| MOS SLEEP SCALE | |||||||||
| During the past 4 weeks… | |||||||||
| 1. How long did it usually take for you to fall asleep? b | ⭘ | ⭘ | |||||||
| 2. On the average, how many hours did you sleep each night? c | ⭘ | ||||||||
| How often did you…d | |||||||||
| 3. feel that your sleep was not quiet (moving restlessly, feeling tense, speaking, etc., while sleeping)? | ⦿ | ⦿ | |||||||
| 4. get enough sleep to feel rested upon waking in the morning? | ⦿ | ⭘ | ⭘ | ||||||
| 5. awaken short of breath or with a headache? | ⦿ | ⦿ | ⦿ | ||||||
| 6. feel drowsy or sleepy during the day? | ⦿ | ||||||||
| 7. have trouble falling asleep? | ⦿ | ⦿ | ⦿ | ||||||
| 8. awaken during your sleep time and have trouble falling asleep again? | ⦿ | ⦿ | ⦿ | ||||||
| 9. have trouble staying awake during the day? | ⦿ | ⦿ | ⦿ | ||||||
| 10. snore during your sleep? | ⦿ | ||||||||
| 11. take naps (5 minutes or longer) during the day? | ⦿ | ||||||||
| 12. get the amount of sleep you needed? | ⦿ | ⭘ | ⦿ |
Responses were on a 4-point scale ranging from 0 = “would never doze” to 3 = “high chance of dozing”. Responses were summed to produce a total score ranging from 0 to 24, with higher scores indicating greater daytime sleepiness.
Possible responses were 15 minute increments from 1 = “0–15 minutes’ to 5 = “More than 60 minutes”.
Responses were free-entry.
Responses were on a 6-point scale ranging from 1=“All of the time” to 6=“None of the Time”.
Responses were converted to a 0–100 scale, then summed to give scores.
⭘ indicates item included in scale,
⦿ indicates that item was reversed before computing scale.
2.6 APOE, Family History and Cognitive Data
APOE genotype was expressed as a binary categorical variable, with participants classified as carriers (one or more ε4 alleles present) or non-carriers (no ε4 allele present). Family history of AD was determined by a multidisciplinary diagnostic consensus panel, as previously described (Sager et al., 2005). Positive family history of AD was defined as having one or both parents with autopsy-confirmed or probable AD according to the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) (McKhann et al., 2011, 1984).
Participants completed a comprehensive battery of standard psychometric tests and health and lifestyle questionnaires (Sager et al., 2005). Here we report measures known to be associated with AD and/or sleep function. Depressive symptoms were assessed with the Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff, 1977), global cognitive function was assessed with the Mini Mental State Exam (MMSE) (Folstein et al., 1975), episodic memory was assessed with the Rey Auditory Verbal Learning Task (AVLT) (Spreen and Strauss, 1998) and executive function was assessed with the Trail Making Tests A and B (Reitan and Wolfson, 1993) and the Digit Symbol Substitution subtest of the Wechsler Adult Intelligence Scale (WAIS-III) (Wechsler, 1997).
2.7 Statistical Analysis
We used multiple regression to assess the relationship between self-reported sleep and regional amyloid load, quantified as PiB DVR. Separate models were tested for each possible sleep score and ROI combination, with sleep as the predictor of interest and PiB DVR as the outcome, using SPSS version 22 (IBM Corporation, Armonk, New York). Because sleep and amyloid can be affected by age, sex, APOE4 genotype, family history of AD and body mass index (BMI) these were included as covariates. Age was taken from the PET scan. It has previously been shown that the effect of sleep disordered breathing on cognition depends on APOE genotype (Nikodemova et al., 2013), therefore for each possible combination of sleep score and ROI we tested a regression model that included a term for the interaction of sleep score with binary APOE4 status. Case analyses identified no outliers in need of removal. To explore the difference between ESS and MOS somnolence scores we used Pearson’s correlation to test the relationship between ESS and the 3 sub-items of the MOS somnolence score. Results were considered statistically significant when p<.05.
3. Results
3.1 Participant Characteristics
Participant characteristics are summarized in Table 2. The mean age was 62.4 years (SD = 5.7, range = 50–73) at the time of the PET scan. Mean interval between PET scan and questionnaire completion was 0.69 (SD.98) years, and the results did not change when interval was added as a covariate. The sample was enriched for AD risk factors: 34 (34.7%) were APOE4 carriers, 74 (75.5%) had one or both parents with AD. Four participants had MMSE scores between 23 and 26, within the range of possible mild cognitive impairment. The remaining 94 participants had MMSE scores ≥27. Six participants had a CES-D score of 15–21, indicating possible mild-moderate depressive symptoms. One participant had a CES-D score of 27, indicating possible major depressive symptoms.
3.2 Association of Self-Reported Sleep Characteristics with Regional Amyloid Load
After adjusting for covariates, poorer sleep was significantly associated (p<.05) with greater amyloid burden in most of the ROIs examined, across multiple sleep measures, with effect sizes ranging from small to medium (partial η2 = .04–.09). Sleep scores that were significantly associated with PiB DVR are in Table 3, other scores are in Supplemental Table 2. Note that beta coefficients appear small because responses to the MOS questionnaire were converted from a 6-point to 100-point scale for scoring. Data for 3 representative sleep score - ROI combinations are plotted in Figure 2.
Table 3.
Association between self-reported sleep and regional amyloid burden
| ROI | Sleep Adequacy | Sleep Problems Index I | Somnolence | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | t | p | η2p | B | SE | t | p | η2p | B | SE | t | p | η2p | ||
| Angular Gyrus | L | −.002 | .001 | −1.948 | .055 | .041 | .002 | .002 | 1.409 | .162 | .022 | .003 | .002 | 2.282 | .025 | .055 |
| R | −.003 | .001 | −2.910 | .005 | .087 | .004 | .002 | 2.299 | .024 | .056 | .004 | .001 | 2.461 | .016 | .064 | |
| Cingulum Anterior | L | −.003 | .001 | −2.565 | .012 | .069 | .004 | .002 | 2.176 | .032 | .051 | .004 | .002 | 2.258 | .026 | .054 |
| R | −.004 | .001 | −2.819 | .006 | .082 | .004 | .002 | 2.213 | .029 | .052 | .004 | .002 | 2.146 | .035 | .049 | |
| Cingulum Posterior | L | −.003 | .001 | −2.536 | .013 | .067 | .004 | .002 | 2.155 | .034 | .050 | .002 | .002 | 1.300 | .197 | .019 |
| R | −.002 | .001 | −2.373 | .020 | .059 | .003 | .002 | 2.113 | .037 | .048 | .002 | .001 | 1.291 | .200 | .018 | |
| Frontal Med Orb | L | −.003 | .001 | −2.843 | .006 | .083 | .004 | .002 | 2.234 | .028 | .053 | .004 | .002 | 2.721 | .008 | .077 |
| R | −.004 | .001 | −2.896 | .005 | .086 | .004 | .002 | 2.219 | .029 | .052 | .004 | .002 | 2.196 | .031 | .051 | |
| Precuneus | L | −.003 | .001 | −2.461 | .016 | .064 | .004 | .002 | 2.154 | .034 | .050 | .003 | .002 | 2.090 | .040 | .047 |
| R | −.003 | .001 | −2.823 | .006 | .082 | .004 | .002 | 2.331 | .022 | .058 | .003 | .002 | 2.020 | .046 | .044 | |
| Supra-marginal | L | −.001 | .001 | −1.472 | .144 | .024 | .002 | .001 | 1.180 | .241 | .015 | .003 | .001 | 2.246 | .027 | .054 |
| R | −.002 | .001 | −2.473 | .015 | .064 | .003 | .001 | 1.856 | .067 | .037 | .003 | .001 | 2.080 | .040 | .046 | |
| Temporal Mid | L | −.001 | .001 | −1.542 | .127 | .026 | .001 | .001 | 1.068 | .288 | .013 | .002 | .001 | 2.006 | .048 | .043 |
| R | −.002 | .001 | −2.067 | .042 | .046 | .002 | .002 | 1.353 | .180 | .020 | .002 | .001 | 1.711 | .090 | .032 | |
| Temporal Sup | L | −.001 | .001 | −.830 | .409 | .008 | .001 | .001 | .676 | .501 | .005 | .002 | .001 | 1.864 | .066 | .038 |
| R | −.002 | .001 | −1.998 | .049 | .043 | .002 | .001 | 1.277 | .205 | .018 | .002 | .001 | 1.663 | .100 | .030 | |
| Mean | −.002 | .001 | −2.503 | .014 | .066 | .003 | .002 | 1.973 | .052 | .042 | .003 | .001 | 2.171 | .033 | .050 | |
ROI, regions of interest; B, unstandardized regression coefficient; SE, standard error of the coefficient; p, p-value; η2p, partial eta squared. All models were adjusted for age, sex, APOE4, Family history of Alzheimer’s disease and body mass index. Statistically significant associations in bold, trends in italics.
Figure 2.
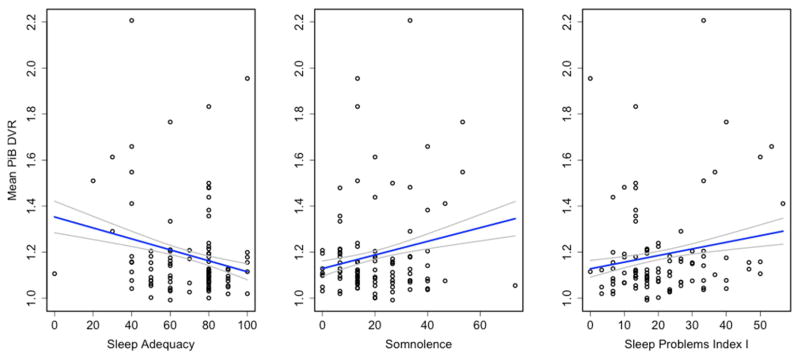
Association between sleep scores and mean PiB DVR. Raw data is plotted, regression line is adjusted for age, sex, APOE4, family history of Alzheimer’s disease and body mass index.
3.2.1 Sleep Adequacy
Sleep Adequacy is derived from two questions; one asks whether respondents are getting the amount of sleep they need and the other asks whether respondents are getting enough sleep to feel rested. Less adequate sleep was associated with greater amyloid burden in the angular gyrus, anterior and posterior cingulate, frontal medial orbital gyrus, precuneus, supramarginal gyrus, temporal middle gyrus and temporal superior gyrus, and averaged across all 8 ROIs (Table 3).
3.2.2 Sleep Problems
MOS Sleep Problems Index I is computed from six items probing a range of sleep issues including sleep disordered breathing, insomnia and restfulness. Higher scores were associated with greater amyloid burden in the angular gyrus, anterior and posterior cingulate, frontal medial orbital cortex and precuneus (Table 3). Similar results were observed for Sleep Problems Index II (Supplemental Table 1).
3.2.3 Somnolence and Sleepiness
Participants reporting greater Somnolence on the MOS had greater mean amyloid burden, and regionally in the angular gyrus, anterior cingulum, frontal medial orbital cortex, precuneus, supramarginal gyrus and middle temporal gyrus (Table 3). By contrast, there was no association between PiB DVR and sleepiness assessed by ESS (Supplemental Table 2). We explored the differences between ESS and MOS Somnolence further by testing the three MOS Somnolence sub-items individually against PiB DVR. The sub-item most similar to the ESS, “trouble staying awake during the day”, was not associated with regional amyloid load, whereas feeling drowsy and taking naps were significantly associated with greater amyloid burden in several regions (Table 4). We further examined the relationship between the ESS and MOS Somonolence with Pearson correlation. ESS was significantly correlated with the MOS Somnolence score (r=.27, p=.01) and with 2 of the 3 MOS somnolence sub-items: feeling drowsy (MOS Q6; r=.36, p=.0003) and trouble staying awake during the day (MOS Q9; r=.38, p=.0001) (Table 5). ESS was not correlated with the sub-item taking naps.
Table 4.
Association between MOS somnolence sub-items and regional amyloid burden
| ROI | Q6: feel drowsya | Q9: trouble staying awakea | Q11: take napsa | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | t | p | η2p | B | SE | t | p | η2p | B | SE | t | p | η2p | ||
| Angular Gyrus | L | .002 | .001 | 1.939 | .056 | .041 | .001 | .002 | .906 | .367 | .009 | .002 | .001 | 2.162 | .033 | .050 |
| R | .002 | .001 | 2.262 | .026 | .054 | .002 | .001 | 1.436 | .154 | .023 | .002 | .001 | 1.839 | .069 | .037 | |
| Cingulum Anterior | L | .003 | .001 | 2.026 | .046 | .044 | .003 | .002 | 1.638 | .105 | .029 | .002 | .001 | 1.520 | .132 | .025 |
| R | .003 | .001 | 2.075 | .041 | .046 | .003 | .002 | 1.595 | .114 | .028 | .002 | .001 | 1.287 | .202 | .018 | |
| Cingulum Posterior | L | .002 | .001 | 1.320 | .190 | .019 | .001 | .002 | .718 | .474 | .006 | .001 | .001 | .903 | .369 | .009 |
| R | .001 | .001 | 1.349 | .181 | .020 | .001 | .001 | .802 | .425 | .007 | .001 | .001 | .802 | .425 | .007 | |
| Frontal Med Orb | L | .003 | .001 | 2.472 | .015 | .064 | .003 | .002 | 1.629 | .107 | .029 | .002 | .001 | 2.016 | .047 | .044 |
| R | .003 | .001 | 2.221 | .029 | .053 | .003 | .002 | 1.415 | .161 | .022 | .002 | .001 | 1.374 | .173 | .021 | |
| Precuneus | L | .002 | .001 | 1.989 | .050 | .043 | .002 | .002 | 1.349 | .181 | .020 | .002 | .001 | 1.422 | .158 | .022 |
| R | .002 | .001 | 1.961 | .053 | .041 | .002 | .002 | 1.328 | .188 | .019 | .002 | .001 | 1.326 | .188 | .019 | |
| Supra-marginal | L | .001 | .001 | 1.648 | .103 | .030 | .001 | .001 | 1.065 | .290 | .013 | .002 | .001 | 2.256 | .027 | .054 |
| R | .002 | .001 | 1.944 | .055 | .041 | .002 | .001 | 1.336 | .185 | .020 | .001 | .001 | 1.453 | .150 | .023 | |
| Temporal Mid | L | .001 | .001 | 1.685 | .095 | .031 | .001 | .001 | .782 | .436 | .007 | .002 | .001 | 1.938 | .056 | .040 |
| R | .002 | .001 | 1.676 | .097 | .031 | .001 | .001 | .850 | .398 | .008 | .001 | .001 | 1.306 | .195 | .019 | |
| Temporal Sup | L | .001 | .001 | 1.424 | .158 | .022 | .001 | .001 | .610 | .543 | .004 | .002 | .001 | 2.024 | .046 | .044 |
| R | .001 | .001 | 1.562 | .122 | .027 | .001 | .001 | .921 | .360 | .009 | .001 | .001 | 1.265 | .209 | .018 | |
| Mean | .002 | .001 | 1.998 | .049 | .043 | .002 | .001 | 1.259 | .211 | .017 | .002 | .001 | 1.635 | .106 | .029 | |
ROI, regions of interest; B, unstandardized regression coefficient; SE, standard error of the coefficient; p, p-value; η2p, partial eta squared. All models were adjusted for age, sex, APOE4, Family history of Alzheimer’s disease and body mass index. Statistically significant associations in bold, trends in italics.
How often during the past 4 weeks did you… (Q6) feel drowsy or sleepy during the day? (Q9) have trouble staying awake? (Q11) take naps (5 min or longer) during the day?
Table 5.
Correlation of MOS and ESS sleepiness reports.
| MOS Qs | Correlation with ESS | |
|---|---|---|
| How often during the past 4 weeks did you… | r | p |
| Q6 …feel drowsy or sleepy during the day? | .36 | .00032 |
| Q9 …have trouble staying awake during the day? | .38 | .0001 |
| Q11 …take naps (5 min or longer) during the day? | .13 | .19 |
| Somnolence (mean of Qs 6, 9 & 11) | .27 | .01 |
r, Pearson correlation coefficient; p, p-value; statistically significant associations are in bold. ESS, Epworth Sleepiness Scale. For 8 situations, asks “How likely are you to doze off or fall asleep in the following situations?” Responses are on a 4 point scale (no chance of dozing, slight chance, moderate chance, definitely would not doze).
3.2.4 Sleep Quantity, Sleep Disturbance and Sleep Disordered Breathing
There were no significant associations between regional amyloid burden and sleep quantity, expressed as a continuous variable (number of hours spent sleeping per night). Amyloid load was not significantly associated with sleep disturbance (defined by the MOS as unrestful sleep, trouble falling and staying asleep) or symptoms of sleep disordered breathing (waking short of breath or with a headache, snoring) (Supplemental Table 2).
3.2.5 Effect of APOE, Depressive Symptoms and Cognitive Status
We found no statistically significant interaction of sleep scales with APOE4 status, age or gender (i.e., the relationship between sleep and PiB DVR did not depend on APOE4 status, age or gender). When CES-D was added as a covariate, the results did not change substantially. Three associations no longer reached significance, but remained as trends (p<.063): sleep adequacy with right middle temporal gyrus and left superior temporal gyrus PiB DVR, and sleep problems index I with left and right posterior cingulate PiB DVR.
When the 4 participants with MMSE <27 were excluded, the general pattern of results did not change substantially. Significant associations (p<.05) that became trends (p<.065) were between somnolence and PiB DVR in the right precuneus, right supramarginal gyrus and left middle temporal gyrus, between sleep adequacy and right temporal middle and superior gyri PiB DVR, and between sleep problems index I and left precuneus PiB DVR.
4. Discussion
Our objective was to determine whether sleep problems and/or specific aspects of self-reported sleep are associated with amyloid burden during late mid-life, when interventions to prevent AD may be most effective. Compared to the only previous study of sleep and direct measures of amyloid deposits (Spira et al., 2013), we studied an age group 15 years younger, and examined more brain regions and more sleep domains. We found that in cognitively healthy late middle-aged adults, greater regional amyloid burden was associated with self-report of less adequate sleep, greater sleepiness, more sleep problems and more napping. There was no apparent association between amyloid burden and sleep duration, sleep disturbance or symptoms of sleep disordered breathing.
4.1 Perceived Sleep Quality, Sleepiness and Amyloid
The sleep measure most consistently associated with increased amyloid burden across brain regions was a perception of less adequate sleep, derived from items asking whether participants were getting the amount of sleep they needed and enough sleep to feel rested. Higher amyloid levels were also associated more sleep problems. These findings are consistent with those in elderly adults, in whom worse perceived sleep quality was associated with greater PiB amyloid burden (Spira et al., 2013).
Greater amyloid burden was associated with a higher MOS somnolence score but not a higher score on the Epworth Sleepiness Scale, which may appear surprising initially. However, the ESS has been shown to underestimate daytime sleepiness in adults over 65 years (Onen et al., 2013). Furthermore, the scales assess distinct constructs: feeling sleepy (MOS) vs. propensity for falling asleep (ESS) (Kim and Young, 2005). The ESS asks about the propensity to fall asleep while performing other activities (e.g. talking to someone, reading, driving) whereas the MOS somnolence score assesses feeling sleepy without falling asleep, voluntary napping, and trouble staying awake during the day. Indeed, ESS did not correlate with the MOS sub-item ‘taking naps’. The MOS somnolence score may capture a broader definition of perceived sleepiness or sleep inadequacy that does not require falling asleep. Our findings suggest that greater amyloid burden is associated with drowsiness but not involuntary daytime sleep. It also highlights the importance of assessing a range of self-reported sleep characteristics, given that sleep is a complex, multidimensional concept.
4.2 Sleep Quantity and Amyloid
There was no association between self-reported sleep duration and amyloid burden. This is consistent with previous studies in older adults using objective measures of sleep duration (polysomnography, actigraphy) and amyloid (Aβ42) in cerebrospinal fluid (Ju et al., 2013) or plaques (Spira et al., 2014). In contrast, Spira et al. (2013) found that, in an older age group, self-reported shorter sleep was correlated with greater amyloid burden in the precuneus and averaged across the cortex. Whereas Spira et al measured sleep duration with a 4-category scale, we treated sleep duration as a continuous variable (number of hours slept) because criteria for optimal sleep quantity are widely debated in the literature. Furthermore, categorization of continuous variables in regression analyses reduces power and increases the likelihood of false positives (Royston et al., 2006). We recoded our data according to the categorical scale of Spira et al (≤5; >5 to 6; >6 to 7; >7 hours) and again found no association between sleep duration and amyloid in our sample (data not shown). However, we cannot discount the possibility that sleep duration may impact amyloid deposition differently in older vs. middle aged subjects.
4.3 Sleep Disorders and Amyloid
We found no association between reports of symptoms of sleep disordered breathing and amyloid burden, consistent with a prior finding of no relation of amyloid to reports of waking several times during the night in an elderly cohort (Spira et al., 2013). This is surprising, given that several lines of evidence link sleep disordered breathing with AD. Sleep disordered breathing is more prevalent in dementia than the general population (Frohnhofen and Roffe, 2012), increases risk of developing AD (Yaffe K et al., 2011) and is correlated with AD markers in human cerebrospinal fluid (amyloid and tau) (Osorio et al., 2014, 2013). In rodents, sleep disruption and intermittent hypoxia (features of sleep disordered breathing) increase amyloid production and deposition (Kang et al., 2009; Shiota et al., 2013). We chose not to consider diagnosis of sleep disorders in this analysis because obstructive sleep apnea is underdiagnosed (Kapur et al., 2002). We measured self-reported symptoms, however sleep disordered breathing can be present without awareness or endorsement of symptoms (Gooneratne and Vitiello, 2014; Halász et al., 2004). Therefore it is likely that some participants who did not report symptoms of sleep-disordered breathing actually did suffer from the disorder. Similarly, we found no association between brain amyloid and reports of insomnia-type symptoms (trouble falling and staying asleep). This is consistent with a study using a similar measure (self report of trouble falling asleep, trouble staying asleep and use of sleep medications), which found no association between disturbed sleep and Aβ42 in plasma, even though those reporting troubled sleep had a ~1.5 higher risk of developing AD (Benedict et al., 2014). In contrast, behavioral measurement of sleep continuity (actigraphy) found that those with pathological CSF Aβ42 levels had significantly lower sleep efficiency. Although sleep disordered breathing and insomnia were not individually associated with amyloid, both contributed to the sleep problems index, which was positively associated with greater amyloid burden. These findings highlight the need for further studies using physiological measures of sleep, breathing and hypoxia to clarify their relationship with amyloid deposition. Self-reported sleep measures remain important, given that perceptions of disturbed sleep are what drive patients to seek clinical evaluation for sleep disorders.
4.4 Possible Mechanisms Linking Sleep and Amyloid
Because this study was cross-sectional, we cannot determine whether poor sleep drives amyloid deposition or vice versa. Studies in mice suggest the relationship may be bidirectional. Sleep restriction increased amyloid plaque burden (Kang et al., 2009), and chronic intermittent hypoxia during sleep (a feature of obstructive sleep apnea) increased CSF Aβ42 (Shiota et al., 2013). Conversely, rising plaque burden was accompanied by disrupted sleep, and immunization against plaque formation preserved sleep (Roh et al., 2012).
Aβ accumulation arises from an imbalance between Aβ production and clearance, and plaque formation is dependent on regional Aβ concentrations (Thal et al., 2006). Sleep disruption may affect several steps in the process of amyloid plaque formation. Amyloid is released during synaptic activity (Cirrito et al., 2005) and brain regions with greater neuronal activity show greater Aβ concentrations in interstitial fluid, and subsequently more plaque formation (Bero et al., 2011). Synaptic activity and synaptic strength are progressively decreased during sleep (Tononi and Cirelli, 2012; Vyazovskiy et al., 2009). Therefore sleep disruption could chronically elevate neuronal activity, thereby increasing amyloid release. The resulting accumulation of extracellular amyloid would result in greater aggregation and plaque formation.
Aβ levels in CSF follow a diurnal pattern (Kang et al., 2009), with clearance greatest during sleep (Xie et al., 2013). In healthy men (40–60 years), one night of sleep deprivation abolished the overnight decline in CSF Aβ42 (Ooms et al., 2014). More wakefulness during the sleep period (i.e. lower sleep efficiency) may reduce Aβ clearance, leading to its accumulation and then aggregation into plaques. Consistent with this hypothesis, a study of older adults (mean 65.6 years) found lower sleep efficiency (measured objectively with actigraphy) and more napping (measured by sleep diary) in the group with lower CSF Aβ42, indicative of Aβ42 sequestration into plaques (Ju et al., 2013).
In addition to sleep disruption promoting amyloid deposition, amyloid may affect sleep by impairing the function of sleep-active brain regions and networks. Sleep is characterized by electrical oscillations in cortico-cortical and thalamo-cortical neural assemblies that directly contribute to neural plasticity and daytime cognitive function (Poe et al., 2010). Aβ disrupts synaptic transmission (Wang et al., 2009), network oscillations (Palop and Mucke, 2010) and functional connectivity (Bero et al., 2012; Sheline et al., 2010). Amyloid may impair the restorative functions of sleep oscillations, leading to perceptions of inadequate sleep and impaired daytime cognitive function.
4.5 Limitations
Strengths of this study include the assessment of multiple aspects of sleep, regional amyloid binding and a well-characterized cohort in midlife, an age range that may be optimal for interventions. Limitations discussed above include the use of self-report, which decreased our ability to detect sleep disorders such as sleep disordered breathing. There were multiple comparisons, however the likelihood of Type I error was minimized by restricting analyses to regions of interest chosen a priori based on published literature. The consistent relationship of mean PiB DVR (averaged across all 8 ROIs) to sleep factors adds confidence to the findings. Although the PET scan and questionnaire were completed on separate days, sleep disorders are typically chronic, and the sleep questionnaire asked for symptoms over the previous 4 weeks. Indeed, results did not change when interval was included as a covariate (data not shown). The study was cross-sectional and observational, therefore we cannot determine causality. However, this study establishes a link between sleep and amyloid in mid-life, prior to the onset of AD symptoms.
4.6 Implications
No adequate treatments exist to reverse or prevent AD (Schneider et al., 2014). Effective treatments already exist for optimizing sleep, and treating sleep disorders in AD patients improves cognition, although dementia is not resolved (Ancoli-Israel et al., 2008). Our findings suggest that earlier interventions to improve sleep quality and to treat sleep disorders could potentially impact AD progression by reducing amyloid pathology. Additionally, sleep characteristics that are modified by amyloid may provide early biomarkers of preclinical AD and may be useful for diagnosis, prognosis and for monitoring effectiveness of treatments. Prospective longitudinal studies and randomized control trials in cohorts at risk for AD are needed to determine which aspects of sleep are the most useful as treatment targets or disease markers.
Greater amyloid burden was associated with perceptions of inadequate sleep, daytime sleepiness and napping, but not with reported sleep amount or symptoms of sleep disordered breathing or insomnia. It will be important for future studies to include physiological measures of the signs and symptoms of sleep disorders. However, healthy sleep is more than the absence of sleep disorders; it can be broadly defined as patterns of sleep-wakefulness that produce physical, mental and social well-being (Buysse, 2014). Healthy sleep may take different forms across individuals and cultures. For example, sleep duration and cognitive vulnerability to sleep restriction vary between individuals in a trait-like manner (Rupp et al., 2012; Van Dongen et al., 2004), and across cultures sleep patterns vary from highly consolidated western schedules to siestas or biphasic schedules (Ekirch, 2005; Worthman and Melby, 2002). While it is essential to identify mechanisms in order to effectively target interventions, it is also important to recognize that our understanding of sleep function and regulation continues to evolve. For example it has become increasingly clear in recent years that, rather than being a global state, sleep can be considered a localized phenomenon, taking place independently in discrete neural circuits and even individual cells (Fisher and Vyazovskiy, 2014; Nir et al., 2011). Therefore perceptions of inadequate sleep may capture defective sleep processes that we have yet to identify on a physiological level. Our finding that neuropathology is associated with perceptions of inadequate sleep, but not with reported sleep duration or symptoms of sleep disorders highlight the importance of maintaining a broader view of sleep health at this time, when sleep’s role in AD pathology is only beginning to be uncovered. For example, rather than considering absolute amounts of sleep, it may be more informative to assess whether individuals are getting less sleep than they need, which is captured by questions addressing sleep adequacy.
4.7 Conclusion
We found that self-reported sleep quality, but not quantity, was associated with amyloid plaques in brain regions typically affected in AD. These relationships were present in middle-aged adults who are currently cognitively healthy, therefore sleep may be useful during the preclinical phase of AD as a biomarker or modifiable risk factor to prevent or delay AD. Future work will need to clarify which aspects of sleep are most strongly related to amyloid and other markers of AD pathology, and the mechanisms linking sleep and AD progression.
Supplementary Material
Figure 1.
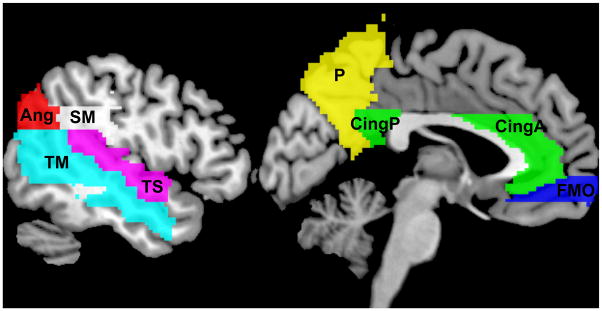
AAL Regions of Interest. Ang, angular gyrus; CingA, anterior cingulate; CingP, posterior cingulate; FMO, frontal middle orbital gyrus; P, precuneus; SM, supramarginal gyrus; TM, middle temporal gyrus; TS, superior temporal gyrus.
HIGHLIGHTS.
We examined whether sleep quality is related to amyloid burden in late midlife
Sleep was assessed with standardized questionnaires
Amyloid was imaged with PiB Positron Emission Tomography
Reports of poor, inadequate sleep were associated with higher amyloid load
Poor sleep may be an early marker or target for intervention in preclinical AD
Acknowledgments
This research was supported by National Research Service Award (NRSA) T32 GM007507 (KES), CTSA award UL1TR000427 (KES) and by the National Institute on Aging grants R01 AG027161 (SCJ), ADRC P50 AG033514 (SA), R01 AG021155 (SCJ), and R01 AG037639 (BB).
We thank Caitlin A. Cleary, BSc, Sandra Harding, MS, Jennifer Bond, BA and the WRAP psychometrists for assistance with study data collection. We gratefully acknowledge support of researchers and staff of the Waisman Center, University of Wisconsin-Madison where the brain scans took place. Finally, we thank participants in the Wisconsin Registry for Alzheimer’s Prevention for their ongoing dedication.
Footnotes
Financial Discolosures
RMB has served as a consultant to Merck and Jazz, and receives grant support from Merck. The remaining authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kate E. Sprecher, Email: ksprecher@wisc.edu.
Barbara B. Bendlin, Email: bbb@medicine.wisc.edu.
Annie M. Racine, Email: aracine@wisc.edu.
Ozioma C. Okonkwo, Email: ozioma@medicine.wisc.edu.
Bradley T. Christian, Email: bchristian@wisc.edu.
Rebecca L. Koscik, Email: rekoscik@wisc.edu.
Mark A. Sager, Email: masager@wisc.edu.
Sanjay Asthana, Email: sa@medicine.wisc.edu.
Sterling C. Johnson, Email: scj@medicine.wisc.edu.
Ruth M. Benca, Email: rmbenca@wisc.edu.
References
- Ancoli-Israel S, Palmer BW, Cooke JR, Corey-Bloom J, Fiorentino L, Natarajan L, Liu L, Ayalon L, He F, Loredo JS. Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: a randomized controlled study. J Am Geriatr Soc. 2008;56:2076–2081. doi: 10.1111/j.1532-5415.2008.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C, Byberg L, Cedernaes J, Hogenkamp PS, Giedratis V, Kilander L, Lind L, Lannfelt L, Schiöth HB. Self-reported sleep disturbance is associated with Alzheimer’s disease risk in men. Alzheimers Dement J Alzheimers Assoc. 2014 doi: 10.1016/j.jalz.2014.08.104. [DOI] [PubMed] [Google Scholar]
- Bero AW, Bauer AQ, Stewart FR, White BR, Cirrito JR, Raichle ME, Culver JP, Holtzman DM. Bidirectional Relationship between Functional Connectivity and Amyloid-β Deposition in Mouse Brain. J Neurosci. 2012;32:4334–4340. doi: 10.1523/JNEUROSCI.5845-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37:9–17. doi: 10.5665/sleep.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic Activity Regulates Interstitial Fluid Amyloid-β Levels In Vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Ekirch A. At Day’s Close. W.W. Norton & Company; New York: 2005. [Google Scholar]
- Fisher SP, Vyazovskiy VV. Local Sleep Taking Care of High-Maintenance Cortical Circuits under Sleep Restriction. Sleep. 2014;37:1727–1730. doi: 10.5665/sleep.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frohnhofen H, Roffe C. Intermittent Nocturnal Hypoxemia in Individuals with Dementia: Prevalence and Relationship with Functional Status. J Am Geriatr Soc. 2012;60:1997–1999. doi: 10.1111/j.1532-5415.2012.04183.x. [DOI] [PubMed] [Google Scholar]
- Gooneratne NS, Vitiello MV. Sleep in Older Adults: Normative Changes, Sleep Disorders, and Treatment Options. Clin Geriatr Med. 2014;30:591–627. doi: 10.1016/j.cger.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halász P, Terzano M, Parrino L, Bódizs R. The nature of arousal in sleep. J Sleep Res. 2004;13:1–23. doi: 10.1111/j.1365-2869.2004.00388.x. [DOI] [PubMed] [Google Scholar]
- Hays R, Stewart A. Sleep measures. In: Stewart A, Ware J, editors. Measuring Functioning and Well-Being: The Medical Outcomes Study Approach. Duke University Press; Durham, NC: 1992. pp. 235–259. [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–381. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Christian BT, Okonkwo OC, Oh JM, Harding S, Xu G, Hillmer AT, Wooten DW, Murali D, Barnhart TE, Hall LT, Racine AM, Klunk WE, Mathis CA, Bendlin BB, Gallagher CL, Carlsson CM, Rowley HA, Hermann BP, Dowling NM, Asthana S, Sager MA. Amyloid burden and neural function in people at risk for Alzheimer’s Disease. Neurobiol Aging. 2014;35:576–584. doi: 10.1016/j.neurobiolaging.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YES, McLeland JS, Toedebusch CD, Xiong C, Fagan AM, Duntley SP, Morris JC, Holtzman DM. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70:587–593. doi: 10.1001/jamaneurol.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Amyloid-β Dynamics Are Regulated by Orexin and the Sleep-Wake Cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath Schlaf Atm. 2002;6:49–54. doi: 10.1007/s11325-002-0049-5. [DOI] [PubMed] [Google Scholar]
- Kim H, Young T. Subjective daytime sleepiness: dimensions and correlates in the general population. Sleep. 2005;28:625–634. doi: 10.1093/sleep/28.5.625. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikodemova M, Finn L, Mignot E, Salzieder N, Peppard PE. Association of sleep disordered breathing and cognitive deficit in APOE ε4 carriers. Sleep. 2013;36:873–880. doi: 10.5665/sleep.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y, Staba RJ, Andrillon T, Vyazovskiy VV, Cirelli C, Fried I, Tononi G. Regional slow waves and spindles in human sleep. Neuron. 2011;70:153–169. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onen F, Moreau T, Gooneratne NS, Petit C, Falissard B, Onen SH. Limits of the Epworth Sleepiness Scale in older adults. Sleep Breath Schlaf Atm. 2013;17:343–350. doi: 10.1007/s11325-012-0700-8. [DOI] [PubMed] [Google Scholar]
- Ooms S, Overeem S, Besse K, Rikkert MO, Verbeek M, Claassen JAHR. Effect of 1 night of total sleep deprivation on cerebrospinal fluid β-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol. 2014;71:971–977. doi: 10.1001/jamaneurol.2014.1173. [DOI] [PubMed] [Google Scholar]
- Osorio RS, Ayappa I, Mantua J, Gumb T, Varga A, Mooney AM, Burschtin OE, Taxin Z, During E, Spector N, Biagioni M, Pirraglia E, Lau H, Zetterberg H, Blennow K, Lu SE, Mosconi L, Glodzik L, Rapoport DM, de Leon MJ. The interaction between sleep-disordered breathing and apolipoprotein E genotype on cerebrospinal fluid biomarkers for Alzheimer’s disease in cognitively normal elderly individuals. Neurobiol Aging. 2014;35:1318–1324. doi: 10.1016/j.neurobiolaging.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio RS, Pirraglia E, Gumb T, Mantua J, Ayappa I, Williams S, Mosconi L, Glodzik L, de Leon MJ. Imaging and Cerebrospinal Fluid Biomarkers in the Search for Alzheimer’s Disease Mechanisms. Neurodegener Dis. 2013 doi: 10.1159/000355063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Amyloid-β-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci. 2010;13:812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe GR, Walsh CM, Bjorness TE. Cognitive neuroscience of sleep. Prog Brain Res. 2010;185:1–19. doi: 10.1016/B978-0-444-53702-7.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. Vol. 2. Neuropsychology Press; Tucson: 1993. [Google Scholar]
- Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, Holtzman DM. Disruption of the Sleep-Wake Cycle and Diurnal Fluctuation of β-Amyloid in Mice with Alzheimer’s Disease Pathology. Sci Transl Med. 2012;4:150ra122–150ra122. doi: 10.1126/scitranslmed.3004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25:127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- Rupp TL, Wesensten NJ, Balkin TJ. Trait-Like Vulnerability to Total and Partial Sleep Loss. Sleep. 2012;35:1163–1172. doi: 10.5665/sleep.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager MA, Hermann B, La Rue A. Middle-aged children of persons with Alzheimer’s disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer’s Prevention. J Geriatr Psychiatry Neurol. 2005;18:245–249. doi: 10.1177/0891988705281882. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Mangialasche F, Andreasen N, Feldman H, Giacobini E, Jones R, Mantua V, Mecocci P, Pani L, Winblad B, Kivipelto M. Clinical trials and late-stage drug development for Alzheimer’s disease: an appraisal from 1984 to 2014. J Intern Med. 2014;275:251–283. doi: 10.1111/joim.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67:584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota S, Takekawa H, Matsumoto S, Takeda K, Nurwidya F, Yoshioka Y, Takahashi F, Hattori N, Tabira T, Mochizuki H. Chronic Intermittent Hypoxia/Reoxygenation Facilitate Amyloid-β Generation in Mice. J Alzheimers Dis. 2013 doi: 10.3233/JAD-130419. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, Zhou Y, Wong DF, Ferrucci L, Resnick SM. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70:1537–1543. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira AP, Yager C, Brandt J, Smith GS, Zhou Y, Mathur A, Kumar A, Brašić JR, Wong DF, Wu MN. Objectively Measured Sleep and β-amyloid Burden in Older Adults: A Pilot Study. SAGE Open Med. 2014:2. doi: 10.1177/2050312114546520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests. 2. Oxford University Press; New York: 1998. [Google Scholar]
- Spritzer K, Hays R. MOS Sleep Scale: A Manual for Use and Scoring, Version 10. Los Angeles, CA: 2003. [Google Scholar]
- Thal DR, Capetillo-Zarate E, Del Tredici K, Braak H. The Development of Amyloid beta Protein Deposits in the Aged Brain. Sci Aging Knowl Environ. 2006;2006:re1. doi: 10.1126/sageke.2006.6.re1. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Time to Be SHY? Some Comments on Sleep and Synaptic Homeostasis. Neural Plast. 2012;2012 doi: 10.1155/2012/415250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Dongen HPA, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–433. [PubMed] [Google Scholar]
- Viala-Danten M, Martin S, Guillemin I, Hays RD. Evaluation of the reliability and validity of the Medical Outcomes Study sleep scale in patients with painful diabetic peripheral neuropathy during an international clinical trial. Health Qual Life Outcomes. 2008;6:113. doi: 10.1186/1477-7525-6-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, Cirelli C, Tononi G. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang G, Zhou H, Barakat A, Querfurth H. Opposite Effects of Low and High Doses of Aβ42 on Electrical Network and Neuronal Excitability in the Rat Prefrontal Cortex. PLoS ONE. 2009;4:e8366. doi: 10.1371/journal.pone.0008366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale 3. The Psychological Corporation; San Antonio: 1997. [Google Scholar]
- Worthman CM, Melby M. Toward a comparative developmental ecology of human sleep. In: Carskadon MA, editor. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. Cambridge University Press; 2002. pp. 69–117. [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep Drives Metabolite Clearance from the Adult Brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Laffan AM, Harrison S, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan P, Bero AW, Cirrito JR, Xiao Q, Hu X, Wang Y, Gonzales E, Holtzman DM, Lee JM. Characterizing the appearance and growth of amyloid plaques in APP/PS1 mice. J Neurosci Off J Soc Neurosci. 2009;29:10706–10714. doi: 10.1523/JNEUROSCI.2637-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


