Abstract
The ON pathway mutation in nob mice is associated with altered refractive development, and an increased susceptibility to form-deprivation (FD) myopia. In this study, we used mGluR6−/− mice, another ON pathway mutant, to determine whether the nob phenotype was due to the Nyx mutation or abnormal ON pathway transmission. Refractive development under a normal visual environment for mGluR6−/− and age-matched wild-type (WT) mice was measured every 2 weeks from 4 to 16 weeks of age. The response to monocular FD from 4 weeks of age was measured weekly in a separate cohort of mice. Refraction and ocular biometry were obtained using a photorefractor and optical coherence tomography. Retinas were harvested at 16 weeks, and analyzed for dopamine (DA) and DOPAC using high-performance liquid chromatography. Under normal conditions, mGluR6−/− mice were significantly more myopic than their WT controls (refraction at 12 weeks; WT: 9.40 ± 0.16 D, mGluR6−/−: 6.91 ± 0.38 D). Similar to nob mice, two weeks of FD resulted in a significant myopic shift of −5.57 ± 0.72 D in mGluR6−/− mice compared to −1.66 ± 0.19 D in WT animals. No significant axial length changes were observed with either normal or FD visual conditions. At 16 weeks, mGluR6−/− retinas showed significantly lower DOPAC levels (111.2 ± 33.0 pg/mg) compared to their WT counterparts (197.5 ± 11.2 pg/mg). Retinal DA levels were similar between the different genotypes. Our results indicate that reduced retinal DA metabolism/turnover may be associated with increased susceptibility to myopia in mice with ON pathway defect mutations.
Keywords: refractive error, form-deprivation, ON pathway, metabotropic glutamate receptor, dopamine, myopia
Emmetropization is an active, visually-guided process whereby the axial length and the optical power of the eye precisely match each other to eliminate neonatal refractive errors, and bring the eye into perfect focus (Smith, 1998; Wallman and Winawer, 2004). Any disruption to this mechanism of ocular growth results in the development of refractive errors; eyes being either too short (hyperopia) or too long (myopia) (Wallman and Winawer, 2004).
A large body of animal research using lens-induced (Hung et al., 1995; Irving et al., 1992; Schaeffel et al., 1988; Wildsoet and Wallman, 1995) and form-deprivation (FD) (Nickla et al., 1998; Smith and Hung, 2000; Wallman et al., 1978; Wallman et al., 1995) paradigms have shown the importance of the visual environment in the regulation of ocular growth. The ocular response to FD (Troilo et al., 1987) or lens-induced defocus (Wildsoet and Wallman, 1995) after optic nerve section in chickens indicates that the visual mechanisms involved in regulating refractive development localize primarily [if not exclusively, (Troilo et al., 1987; Wildsoet, 2003)] to the retina. Furthermore, partial diffusers only cause changes in the defocused area of the visual field in chickens (Diether and Schaeffel, 1997; Hodos and Kuenzel, 1984; Wallman et al., 1987) and primates (Smith et al., 2009), resulting in focal changes in refraction. Therefore, any defect in visual transmission through the retina could potentially influence ocular growth and lead to development of refractive errors.
Several studies have suggested a role for various retinal cell types and pathways in normal eye development. In chickens, physiological and morphological changes in photoreceptors are associated with experimentally induced myopia (Crewther, 2000). Pharmacological elimination of the OFF pathway in chicken retina using the D isomer of a gliotoxin α amino adipic acid (DαAAA) resulted in an enhanced rate of axial elongation under normal visual conditions, as well as with negative lenses (Crewther and Crewther, 1990). Conversely, inhibition of the ON pathway with the L isomer (LαAAA) caused a reduction in axial eye growth for both visually normal and lens-reared conditions (Crewther and Crewther, 1990). Furthermore, alternations in ON and OFF responses using 2-amino-4 phosphonobutyric acid (APB) or cis 2,3 piperidine-dicarboxylic acid (PDA) in chickens influence the ocular growth patterns and refractive errors (Crewther et al., 1996). More recent studies using various mutant mouse models have provided stronger evidence of retinal involvement in ocular refractive development (Chakraborty et al., 2014; Pardue et al., 2008; Park et al., 2013; Park et al., 2014). Mouse models ensure complete and selective blockage of a single pathway, and allow examination of the interaction between genetic background and visual environment in refractive development (Pardue et al., 2013).
ON and OFF pathways are important for efficiently transferring information about changes in light stimuli to the higher visual centers, and processing contrast sensitivity information (Schiller, 1992; Schiller et al., 1986). These pathways have been implicated in refractive development of mutant mice with selective ON or OFF pathway defects (Chakraborty et al., 2014; Pardue et al., 2008). A non-functional ON pathway (Gregg et al., 2003; Pardue et al., 1998) in nob mice due to a mutation in Nyx (Gregg et al., 2003) causes low retinal dopamine (DA) levels and has previously been associated with a small myopic shift under normal visual conditions, and increased susceptibility to myopia in response to visual FD (Pardue et al., 2008). Conversely, non-functional OFF pathways in Vsx1−/− mice (Chow et al., 2001; Chow et al., 2004) had no significant effect on either normal or visually deprived refractive development (Chakraborty et al., 2014). These findings suggest that abnormal visual transmission through the ON pathway causes a greater refractive effect than disruption of the OFF pathway, perhaps due to changes in retinal dopaminergic activity, and may be more critical for normal ocular development in mammals.
In this study, we examined the refractive development and dopamine levels of mGluR6−/− mice (Masu et al., 1995; Sugihara et al., 1997; Tagawa et al., 1999; Takao et al., 2000), with a null mutation in the metabotropic glutamate receptor (mGluR6), which is located on the postsynaptic membrane of ON bipolar cells in both rod and cone systems (Nakajima et al., 1993; Vardi and Morigiwa, 1997). As a result of defective synaptic transmission through the ON-bipolar cells, mGluR6−/− mice have normal electroretinogram (ERG) a-waves, but non-recordable b-waves without significant change in responses from the OFF pathway (Masu et al., 1995). Additionally, mGluR6 mutants show unmeasurable ON-responses from the superior colliculus (Masu et al., 1995). Furthermore, the loss of mGluR6 produces these functional abnormalities without morphological changes in the retina (Tagawa et al., 1999; See reviews of mouse b-wave mutants McCall and Gregg, 2008; Pardue and Peachey, 2014). In humans, mGluR6 mutations are associated with complete autosomal recessive congenital stationary night blindness (CSNB) (Dryja et al., 2005; Zeitz et al., 2005), abnormal cone ERG ON responses (Dryja et al., 2005) and high myopia (Xu et al., 2009) suggesting a potential link between the genetic mutation and refractive error development. In this study, we measured refractive changes in mGluR6−/− mice to determine whether altered refractive development in this mutant was similar to nob mice, which might implicate abnormal ON pathway transmission (and related changes in retinal DA levels) in refractive development versus some other aspect of the mutations.
An in-house breeding colony with both male and female homozygous mGluR6 mutants (Jackson Laboratory, Bar Harbor, ME) on C57BL/6J background was maintained at the Atlanta Department of Veterans Affairs Medical Center. Mice were kept in 12:12 hour light cycles of ~17 lux with mouse chow and water ad libitum.
Age-matched male and female mGluR6−/− and C57BL/6J wild-type (WT) mice were subjected to one of two different experimental conditions: a normal visual environment or form- deprivation (FD). For mice raised in a normal visual environment (WT n=10; mGluR6−/− n=10), refractive measurements were obtained every 2 weeks from 4 to 16 weeks of age. For FD experiments, baseline refractive error measurements for WT (goggled n= 6; naïve controls n=6) and mGluR6−/− (goggled n= 5; naïve controls n=5) mice were obtained at 4 weeks of age and then the mice were subjected to monocular visual deprivation in the right eye using head-mounted diffuser goggles, as described previously (Faulkner et al, 2007). Weekly measurements were performed on the FD cohort for a period of 2 weeks (i.e. to 6 weeks of age). All procedures adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the local Institutional Animal Care and Use Committee.
For both a normal visual environment and FD conditions, refractive error and axial length measurements (measured from the anterior cornea to the retinal pigment epithelium) were acquired using an automated infrared photorefractor (Schaeffel et al., 2004) and a 1310 nm spectral-domain optical coherence tomography system (SD-OCT; Bioptigen Inc., Durham, NC), as described previously (Chakraborty et al., 2014; Pardue et al., 2008; Park et al., 2012; Park et al., 2013). Statistical analyses were performed using commercial software (SigmaStat 3.5, Aspire Software International, Ashburn, VA). Changes in refractive error and axial length between the mGluR6−/− and C57BL/6J WT animals across age under normal and FD visual conditions were analyzed by repeated-measures two-way analysis of variance (ANOVA), and Holm-Sidak post-hoc tests for statistical significance.
Under a normal visual environment, both mGluR6−/− and WT mice showed significantly increased hyperopic refractions (values averaged between the two eyes for each mouse) from 4 to 16 weeks of age (Figure 1A; two-way repeated-measures ANOVA main effect of age, F(6,135)=58.69, p<0.001). However, mGluR6−/− mice were significantly more myopic (mean refraction at 12 weeks ± standard error of the mean (SEM); WT: 9.40 ± 0.16 D, mGluR6−/−: 6.91 ± 0.38 D) than their age-matched WT controls throughout the developmental period (two-way repeated-measures ANOVA main effect of genotype, F(1,135)=75.04, p<0.001), suggesting that the mGluR6 mutation had a significant effect on normal refractive development of the eye.
Figure 1.
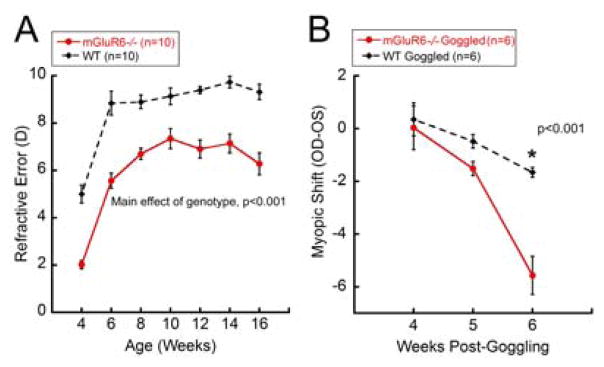
Refractive development in mGluR6−/− mice raised under normal visual environment (A) or form-deprived (B) conditions. A: Both mGluR6−/− and WT mice showed significant increases in hyperopic refraction from 4 to 16 weeks of age (two-way repeated-measures ANOVA main effect of age, F(6,135)=58.69, p<0.001). Across normal refractive development, mGluR6−/− mice were significantly more myopic than WT controls (two-way repeated-measures ANOVA main effect of genotype, F(1,135)=75.04, p<0.001). B: After two weeks of FD, mGluR6−/− mice showed a significantly greater magnitude of myopic shift compared to the WT mice (two-way repeated-measures ANOVA interaction effect, F(2,29)=16.311, p<0.001, *Holm-Sidak multiple comparisons, p<0.001).
From 4 to 16 weeks of age, both genotypes exhibited a significant increase in axial length (mean change in axial length between 4 to 16 weeks of age; WT: 0.37 ± 0.01, mGluR6−/−: 0.37 ± 0.007 mm; two-way repeated-measures ANOVA main effect of genotype, F(6,135)=1214.2, p<0.001). However, no significant differences were observed between the two genotypes at any measured time point (two-way repeated-measures ANOVA main effect of genotype, F(1,135)=0.25, p=0.623). In order to elucidate the refractive changes in mGluR6 mutants, we further examined the changes in corneal curvature using automated keratometry. However, no significant differences in corneal curvatures were observed between the mGluR6−/− and WT mice under either normal visual or FD conditions (data not shown). Given the inadequacy of the axial length and corneal curvature changes in explaining the myopic refractive error in mGluR6−/− mice, we hypothesize that it could be due to differences in other ocular optical parameters, such as changes in thickness, curvature or refractive index of the crystalline lens.
To examine the interaction between visual environment and genetic background, mice were form-deprived from 4 to 6 weeks of age, and the effect of goggling on refraction were compared between the two genotypes (Figure 1B). For the FD cohort, refractive errors are presented as “myopic shift”, the difference between right, goggled and left, opposite eyes or right minus left eyes for non-goggled naïve controls. For both genotypes, naïve untreated controls showed no significant differences in refraction between the left and right eyes (myopic shift at 6 weeks, WT: −0.32 ± 0.65 D, mGluR6−/−: −0.37 ± 0.33 D; two-way repeated-measures ANOVA main effect of genotype, F(1,30)=0.2, p=0.664). Although both goggled mGluR6−/− (−5.57 ± 0.72 D) and WT (−1.66 ± 0.19 D) mice exhibited a significant myopic shift after 2 weeks of goggling (two-way repeated-measures ANOVA interaction effect, F(2,29)=16.31, p<0.001), the magnitude of refractive shift at 6 weeks was significantly greater in mGluR6−/− compared to WT mice (Holm-Sidak multiple comparisons, p<0.001, Figure 1B).
For axial length data, “axial shift” was determined by calculating the difference in eye length between the right (FD) and the left (control) eye after normalizing the data to 4 week-old baseline values. FD did not cause significant change in axial lengths of goggled mice and no significant differences were observed in axial shift of untreated control animals for any genotype (two-way repeated measures ANOVA, p>0.05; data not shown). Please note that in the current study, the standard deviation for raw axial length measurements was ~ 35 μm, mainly due to limited resolution of the instrument to detect posterior retinal borders. This was greater than the inter-user measurement variability of our SD-OCT (21 μm) (Park et al., 2012), and predicted axial length difference based on mouse eye modeling (1 D of refractive change = ~ 5 μm change in eye length) (Schmucker and Schaeffel, 2004). Therefore, our instrument may not have been able to detect small changes in axial length, despite obvious differences in refractive errors as shown in Figure 1A and 1B.
To determine the changes in retinal dopamine activity associated with the mGluR6 mutation, levels of retinal DA and DOPAC (3,4-dihydroxyphenylacetate, the primary metabolite of DA) (Witkovsky, 2004) were quantified for both normal refractive development and FD experiments. For normal refractive development, retinas of mGluR6−/− (n=7) and WT (n=7) mice were harvested at the end of the experiment (i.e. 16 weeks of age) between 4 and 6 h after light onset. For the FD experiments, retinas of mGluR6−/− (control: n=5, goggled: n=5) were harvested after the final end point at 6 weeks of age. Given the evidence that C57BL/6 WT mice (Wu et al., 2015) or WT mice of different backgrounds (Chakraborty et al., 2014; Park et al., 2013; Park et al., 2014) do not show significant changes in retinal DA levels with imposed FD, changes in retinal dopamine activity for goggled WT animals were not measured in this study. To avoid the effects of anesthesia on measurements, all experimental mouse retinas were collected 48 hours after the final measurements. Harvested retinas were immediately frozen on dry ice and stored at −80°C. The frozen retinas were processed using high-performance liquid chromatography (HPLC), as previously described (Nir et al., 2000; Pozdeyev et al., 2008). Right and left eyes of mice with a normal visual environment were pooled together for analysis, while eyes of FD mice were analyzed individually. For normal visual environment cohorts, all HPLC measurements were compared between mGluR6−/− and WT mice using independent two-tailed t-test. For the FD experiments, DA and DOPAC values were individually analyzed for the goggled (right eye), opposite (left untreated eye) and naïve control (average of untreated right and left) eyes using one-way ANOVA with Holm-Sidak post-hoc comparisons. An estimate of retinal dopamine turnover was presented as the ratio of DOPAC to DA (DOPAC/DA ratio) for both normal visual environment and FD experiments.
For 16 week old normal visual environment cohorts, retinas from mGluR6−/− mice yielded significantly lower DOPAC levels (111.2 ± 33.0 pg/mg) compared to their WT counterparts (197.5 ± 11.2 pg/mg, Student’s t-test, t=2.761, p=0.019, Figure 2A). However, retinal DA levels were found to be similar between the two genotypes (mGluR6−/−: 1609 ± 53.5 pg/mg, WT: 1491 ± 69.1 pg/mg, Student’s t-test, t=−1.352, p=0.201, Figure 2B). The DOPAC/DA ratios in WT mice (0.14 ± 0.002) were approximately two fold greater than those in mGluR6−/− mice (0.07 ± 0.02) (Student’s t-test, t=3.001, p=0.012, Figure 2C), indicating a significantly lower dopamine turnover in mGluR6−/− retinas.
Figure 2.
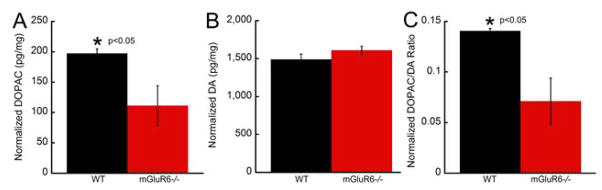
Lower retinal dopamine metabolism and turnover in mGluR6−/− mice compared to WT mice at 16 weeks of age. A: Retinas harvested from mGluR6−/− mice exhibited significantly lower DOPAC levels compared to their WT counterparts (t-test, t=2.761, p=0.019). B: Retinal DA levels were similar between the two genotypes (t-test, t=−1.352, p=0.201). C: DOPAC/DA ratios in WT mice were approximately two fold greater than those in the mGluR6−/− mice (t-test, t=3.001, p=0.012).
For the FD experiments, goggled eyes of mGluR6−/− mice did not yield significant differences in any measured dopamine variable compared to their untreated left eyes or eyes of naïve control animals (p>0.05), suggesting that goggling had no significant effect on retinal dopaminergic activity of mGluR6−/− animals.
Under normal unmanipulated visual conditions, we found mGluR6−/− mice to be significantly more myopic than their WT controls throughout the developmental period measured from 4 to 16 weeks of age (Figure 1A). Using nob mice, another genetic model for the ON pathway defect (Pardue et al., 1998), Pardue et al., reported a relative myopic shift (decrease in hyperopic refraction) in nob mice during adolescence (from 6 to 12 weeks of age) compared to WT mice (Pardue et al., 2008). Together, these findings suggest that defects in the ON visual pathway significantly disrupt normal refractive development, and may predispose the eye to myopia in rodents.
The use of FD has become a standard method to induce experimental myopia in murine eyes (see review, Pardue et al., 2013). The small magnitude of myopic shift observed in WT mice after 2 weeks of goggling was in close agreement with previous studies (see review, Pardue et al., 2013). Importantly, we found mGluR6−/− mice to be highly susceptible to FD myopia, developing about 5.5 D of myopia in response to 2 weeks of FD (Figure 1B). Previously, a similar period of FD has been shown to induce comparable magnitude of myopia (~ 5 D) in nob mice (Pardue et al., 2008). In both studies, the largest differences in refractive error were induced after imposing FD, suggesting that changes in visual environment in conjunction with the ON pathway defect (and not the ON transmission defect alone) produces the most profound visual deficits in murine eyes.
The refractive deficits observed in mGluR6−/− mice could be due to lower DA metabolism and/or turnover in mGluR6−/− retinas. A defect in the ON pathway may lead to decreased DA levels in the retina as DA release is stimulated by light exposure via the ON pathway (Boatright et al., 1994; Boelen et al., 1998; Dumitrescu et al., 2009; Newkirk et al., 2013; Voigt and Wassle, 1987). At 16 weeks, we found retinal DOPAC (Figure 2A) and DOPAC/DA (Figure 2C) ratios in mGluR6−/− mice to be significantly lower than the WT animals. Consistent with our findings, Pardue et al. (Pardue et al., 2008) also reported retinal DOPAC levels to be significantly lower in nob mice compared to WT animals. Previous studies on chickens have also reported relatively greater changes in retinal DOPAC levels compared to changes in retinal DA levels associated with visual FD (Ohngemach et al., 1997). Furthermore, lower DOPAC and DA levels have been associated with FD myopia in chickens (Stone et al., 1989) and primates (Iuvone et al., 1989). However, contrary to lower DA levels in the nob mouse (Pardue et al., 2008), the current study found no significant differences in the endogenous retinal DA levels between the two genotypes (Figure 2B). Subtle differences in DA levels between the two studies could be due to differences in the end point of the two experiments (12 weeks vs 16 weeks in our study), as age-related dynamic changes in retinal DA levels of mice have recently been reported (Park et al., 2014).
Following 2 weeks of FD in mGluR6−/− mice, no significant differences in retinal DA or DOPAC levels were observed, despite a significant myopic shift. These findings suggest that endogenous retinal DA metabolism and/or turnover are perhaps more important for determining the susceptibility of the mouse eye to experimental myopia than acute and/or induced changes in DA with FD (Park et al., 2013; Chakraborty and Pardue, 2015). Furthermore, these findings agree with the report that C57BL6J mice have unaltered dopamine with form-deprivation (Park et al., 2013; Wu et al., 2015). Reports of DA acting on different receptors to influence eye growth in rodents (Huang et al., 2014) suggest complex roles for DA in regulating visually guided ocular growth.
Our results are generally consistent with the previous study on refractive development in nob mice (Pardue et al., 2008), and further emphasize that ON pathway transmission is important for normal refractive development of the eye. However, both Nyx (Zhang et al., 2007) and mGluR6 (Xu et al., 2009) mutations have been associated with high myopia independent of night blindness, suggesting that these mutations may play a separate role in detecting visual blur than that related to the ON pathway. If the refractive changes observed in mGluR6−/− and nob mice were occurring solely as a result of the mutation (not associated with the ON pathway defect), then both mutants would be expected to show distinct patterns of refractive development under normal visual conditions. However, given the similarities in refractive phenotypes of mGluR6−/− and nob mice under both normal and visually-deprived conditions, with significant reduction in retinal DOPAC levels, it is reasonable to conjecture that refractive defects observed in these mouse mutants are caused by the ON pathway defect (and related changes in retinal DOPAC levels), and not the mutation itself. In order to confirm this hypothesis, future studies are needed to examine whether systemic treatment with L-DOPA (L-3,4-dihydroxyphenylalanine, the precursor of DA) (Witkovsky, 2004) could rescue refractive deficits caused by the retinal DA deficiency in nob and mGluR6−/− mice.
Highlights.
Functional mGluR6 receptor is important for normal refractive development in mice.
mGluR6 mutation leads to myopic eye growth in mice.
mGluR6 mutation increases the susceptibility to form-deprivation myopia in mice.
Reduced retinal dopamine may cause increased myopia susceptibility in mGluR6 mutant.
Both nob and mGluR6 ON pathway mutants exhibit similar refractive phenotypes.
Acknowledgments
This project was supported by the National Institutes of Health (NIH R01 EY016435, NIH R01 EY004864, NIH P30 EY006360), Department of Veterans Affairs (Rehabilitation R&D Service Research Career Scientist Award to MTP), and Research to Prevent Blindness (Departmental Award).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boatright JH, Gordon JR, Iuvone PM. Inhibition of endogenous dopamine release in amphibian retina by L-2-amino-4-phosphonobutyric acid (L-AP4) and trans-2-aminocyclopentane-1,3-dicarboxylate (ACPD) Brain research. 1994;649:339–342. doi: 10.1016/0006-8993(94)91084-7. [DOI] [PubMed] [Google Scholar]
- Boelen MK, Boelen MG, Marshak DW. Light-stimulated release of dopamine from the primate retina is blocked by 1-2-amino-4-phosphonobutyric acid (APB) Visual neuroscience. 1998;15:97–103. doi: 10.1017/s0952523898151040. [DOI] [PubMed] [Google Scholar]
- Chakraborty R, Park H, Aung MH, Tan CC, Sidhu CS, Iuvone PM, Pardue MT. Comparison of refractive development and retinal dopamine in OFF pathway mutant and C57BL/6J wild-type mice. Molecular vision. 2014;20:1318–1327. [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R, Pardue MT. Molecular and Biochemical aspects of the retina on refraction. In: Hejtmancik F, Nickerson JM, editors. Progress in Molecular Biology and Translational Science vol 132: Molecular Biology of Eye Disease. Elsevier; Oxford: 2015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RL, Snow B, Novak J, Looser J, Freund C, Vidgen D, Ploder L, McInnes RR. Vsx1, a rapidly evolving paired-like homeobox gene expressed in cone bipolar cells. Mechanisms of development. 2001;109:315–322. doi: 10.1016/s0925-4773(01)00585-8. [DOI] [PubMed] [Google Scholar]
- Chow RL, Volgyi B, Szilard RK, Ng D, McKerlie C, Bloomfield SA, Birch DG, McInnes RR. Control of late off-center cone bipolar cell differentiation and visual signaling by the homeobox gene Vsx1. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:1754–1759. doi: 10.1073/pnas.0306520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crewther DP. The role of photoreceptors in the control of refractive state. Progress in retinal and eye research. 2000;19:421–457. doi: 10.1016/s1350-9462(00)00004-5. [DOI] [PubMed] [Google Scholar]
- Crewther DP, Crewther SG. Pharmacological modification of eye growth in normally reared and visually deprived chicks. Current eye research. 1990;9:733–740. doi: 10.3109/02713689008999568. [DOI] [PubMed] [Google Scholar]
- Crewther DP, Crewther SG, Xie RZ. Changes in eye growth produced by drugs which affect retinal ON or OFF responses to light. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 1996;12:193–208. doi: 10.1089/jop.1996.12.193. [DOI] [PubMed] [Google Scholar]
- Diether S, Schaeffel F. Local changes in eye growth induced by imposed local refractive error despite active accommodation. Vision research. 1997;37:659–668. doi: 10.1016/s0042-6989(96)00224-6. [DOI] [PubMed] [Google Scholar]
- Dryja TP, McGee TL, Berson EL, Fishman GA, Sandberg MA, Alexander KR, Derlacki DJ, Rajagopalan AS. Night blindness and abnormal cone electroretinogram ON responses in patients with mutations in the GRM6 gene encoding mGluR6. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4884–4889. doi: 10.1073/pnas.0501233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrescu ON, Pucci FG, Wong KY, Berson DM. Ectopic retinal ON bipolar cell synapses in the OFF inner plexiform layer: contacts with dopaminergic amacrine cells and melanopsin ganglion cells. The Journal of comparative neurology. 2009;517:226–244. doi: 10.1002/cne.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner AE, Kim KK, Iuvone PM, Pardue MT. Head-mounted goggles for murine form deprivation myopia. Journal of neuroscience methods. 2007;161:96–1000. doi: 10.1016/j.jneumeth.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Gregg RG, Mukhopadhyay S, Candille SI, Ball SL, Pardue MT, McCall MA, Peachey NS. Identification of the gene and the mutation responsible for the mouse nob phenotype. Investigative ophthalmology & visual science. 2003;44:378–384. doi: 10.1167/iovs.02-0501. [DOI] [PubMed] [Google Scholar]
- Hodos W, Kuenzel WJ. Retinal-image degradation produces ocular enlargement in chicks. Investigative ophthalmology & visual science. 1984;25:652–659. [PubMed] [Google Scholar]
- Huang F, Yan T, Shi F, An J, Xie R, Zheng F, Li Y, Chen J, Qu J, Zhou X. Activation of dopamine D2 receptor is critical for the development of form-deprivation myopia in the C57BL/6 mouse. Investigative ophthalmology & visual science. 2014;55:5537–5544. doi: 10.1167/iovs.13-13211. [DOI] [PubMed] [Google Scholar]
- Hung LF, Crawford ML, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nature medicine. 1995;1:761–765. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- Irving EL, Sivak JG, Callender MG. Refractive plasticity of the developing chick eye. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians (Optometrists) 1992;12:448–456. [PubMed] [Google Scholar]
- Iuvone PM, Tigges M, Fernandes A, Tigges J. Dopamine synthesis and metabolism in rhesus monkey retina: development, aging, and the effects of monocular visual deprivation. Visual neuroscience. 1989;2:465–471. doi: 10.1017/s0952523800012360. [DOI] [PubMed] [Google Scholar]
- Masu M, Iwakabe H, Tagawa Y, Miyoshi T, Yamashita M, Fukuda Y, Sasaki H, Hiroi K, Nakamura Y, Shigemoto R, et al. Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell. 1995;80:757–765. doi: 10.1016/0092-8674(95)90354-2. [DOI] [PubMed] [Google Scholar]
- McCall MA, Gregg RG. Comparisons of structural and functional abnormalities in mouse b-wave mutants. The Journal of physiology. 2008;586:4385–4392. doi: 10.1113/jphysiol.2008.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. The Journal of biological chemistry. 1993;268:11868–11873. [PubMed] [Google Scholar]
- Newkirk GS, Hoon M, Wong RO, Detwiler PB. Inhibitory inputs tune the light response properties of dopaminergic amacrine cells in mouse retina. Journal of neurophysiology. 2013;110:536–552. doi: 10.1152/jn.00118.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickla DL, Wildsoet C, Wallman J. Visual influences on diurnal rhythms in ocular length and choroidal thickness in chick eyes. Experimental eye research. 1998;66:163–181. doi: 10.1006/exer.1997.0420. [DOI] [PubMed] [Google Scholar]
- Nir I, Haque R, Iuvone PM. Diurnal metabolism of dopamine in the mouse retina. Brain research. 2000;870:118–125. doi: 10.1016/s0006-8993(00)02409-4. [DOI] [PubMed] [Google Scholar]
- Ohngemach S, Hagel G, Schaeffel F. Concentrations of biogenic amines in fundal layers in chickens with normal visual experience, deprivation, and after reserpine application. Visual neuroscience. 1997;14:493–505. doi: 10.1017/s0952523800012153. [DOI] [PubMed] [Google Scholar]
- Pardue MT, Faulkner AE, Fernandes A, Yin H, Schaeffel F, Williams RW, Pozdeyev N, Iuvone PM. High susceptibility to experimental myopia in a mouse model with a retinal on pathway defect. Investigative ophthalmology & visual science. 2008;49:706–712. doi: 10.1167/iovs.07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue MT, McCall MA, LaVail MM, Gregg RG, Peachey NS. A naturally occurring mouse model of X-linked congenital stationary night blindness. Investigative ophthalmology & visual science. 1998;39:2443–2449. [PubMed] [Google Scholar]
- Pardue MT, Peachey NS. Mouse b-wave mutants. Documenta ophthalmologica. Advances in ophthalmology. 2014;128:77–89. doi: 10.1007/s10633-013-9424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue MT, Stone RA, Iuvone PM. Investigating mechanisms of myopia in mice. Experimental eye research. 2013;114:96–105. doi: 10.1016/j.exer.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Qazi Y, Tan C, Jabbar SB, Cao Y, Schmid G, Pardue MT. Assessment of axial length measurements in mouse eyes. Optometry and vision science : official publication of the American Academy of Optometry. 2012;89:296–303. doi: 10.1097/OPX.0b013e31824529e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Tan CC, Faulkner A, Jabbar SB, Schmid G, Abey J, Iuvone PM, Pardue MT. Retinal degeneration increases susceptibility to myopia in mice. Molecular vision. 2013;19:2068–2079. [PMC free article] [PubMed] [Google Scholar]
- Park HN, Jabbar SB, Tan CC, Sidhu CS, Abey J, Aseem F, Schmid G, Iuvone PM, Pardue MT. Visually-driven ocular growth in mice requires functional rod photoreceptors. Investigative ophthalmology & visual science. 2014;55:6272–6279. doi: 10.1167/iovs.14-14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozdeyev N, Tosini G, Li L, Ali F, Rozov S, Lee RH, Iuvone PM. Dopamine modulates diurnal and circadian rhythms of protein phosphorylation in photoreceptor cells of mouse retina. The European journal of neuroscience. 2008;27:2691–2700. doi: 10.1111/j.1460-9568.2008.06224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffel F, Burkhardt E, Howland HC, Williams RW. Measurement of refractive state and deprivation myopia in two strains of mice. Optometry and vision science : official publication of the American Academy of Optometry. 2004;81:99–110. doi: 10.1097/00006324-200402000-00008. [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision research. 1988;28:639–657. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- Schiller PH. The ON and OFF channels of the visual system. Trends in neurosciences. 1992;15:86–92. doi: 10.1016/0166-2236(92)90017-3. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Sandell JH, Maunsell JH. Functions of the ON and OFF channels of the visual system. Nature. 1986;322:824–825. doi: 10.1038/322824a0. [DOI] [PubMed] [Google Scholar]
- Schmucker C, Schaeffel F. A paraxial schematic eye model for the growing C57BL/6 mouse. Vision research. 2004;44:1857–1867. doi: 10.1016/j.visres.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Smith EL., 3rd Spectacle lenses and emmetropization: the role of optical defocus in regulating ocular development. Optometry and vision science : official publication of the American Academy of Optometry. 1998;75:388–398. doi: 10.1097/00006324-199806000-00023. [DOI] [PubMed] [Google Scholar]
- Smith EL, 3rd, Huang J, Hung LF, Blasdel TL, Humbird TL. Hemiretinal form deprivation: evidence for local control of eye growth and refractive development in infant monkeys. Investigative ophthalmology & visual science. 2009;50:5057–5069. doi: 10.1167/iovs.08-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, 3rd, Hung LF. Form-deprivation myopia in monkeys is a graded phenomenon. Vision research. 2000;40:371–381. doi: 10.1016/s0042-6989(99)00184-4. [DOI] [PubMed] [Google Scholar]
- Stone RA, Lin T, Laties AM, Iuvone PM. Retinal dopamine and form-deprivation myopia. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:704–706. doi: 10.1073/pnas.86.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara H, Inoue T, Nakanishi S, Fukuda Y. A late ON response remains in visual response of the mGluR6-deficient mouse. Neuroscience letters. 1997;233:137–140. doi: 10.1016/s0304-3940(97)00656-3. [DOI] [PubMed] [Google Scholar]
- Tagawa Y, Sawai H, Ueda Y, Tauchi M, Nakanishi S. Immunohistological studies of metabotropic glutamate receptor subtype 6-deficient mice show no abnormality of retinal cell organization and ganglion cell maturation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:2568–2579. doi: 10.1523/JNEUROSCI.19-07-02568.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao M, Morigiwa K, Sasaki H, Miyoshi T, Shima T, Nakanishi S, Nagai K, Fukuda Y. Impaired behavioral suppression by light in metabotropic glutamate receptor subtype 6-deficient mice. Neuroscience. 2000;97:779–787. doi: 10.1016/s0306-4522(00)00053-1. [DOI] [PubMed] [Google Scholar]
- Troilo D, Gottlieb MD, Wallman J. Visual deprivation causes myopia in chicks with optic nerve section. Current eye research. 1987;6:993–999. doi: 10.3109/02713688709034870. [DOI] [PubMed] [Google Scholar]
- Vardi N, Morigiwa K. ON cone bipolar cells in rat express the metabotropic receptor mGluR6. Visual neuroscience. 1997;14:789–794. doi: 10.1017/s0952523800012736. [DOI] [PubMed] [Google Scholar]
- Voigt T, Wassle H. Dopaminergic innervation of A II amacrine cells in mammalian retina. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1987;7:4115–4128. doi: 10.1523/JNEUROSCI.07-12-04115.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek LA. Local retinal regions control local eye growth and myopia. Science (New York, NY) 1987;237:73–77. doi: 10.1126/science.3603011. [DOI] [PubMed] [Google Scholar]
- Wallman J, Turkel J, Trachtman J. Extreme myopia produced by modest change in early visual experience. Science (New York, NY) 1978;201:1249–1251. doi: 10.1126/science.694514. [DOI] [PubMed] [Google Scholar]
- Wallman J, Wildsoet C, Xu A, Gottlieb MD, Nickla DL, Marran L, Krebs W, Christensen AM. Moving the retina: choroidal modulation of refractive state. Vision research. 1995;35:37–50. doi: 10.1016/0042-6989(94)e0049-q. [DOI] [PubMed] [Google Scholar]
- Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Wildsoet C. Neural pathways subserving negative lens-induced emmetropization in chicks--insights from selective lesions of the optic nerve and ciliary nerve. Current eye research. 2003;27:371–385. doi: 10.1076/ceyr.27.6.371.18188. [DOI] [PubMed] [Google Scholar]
- Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision research. 1995;35:1175–1194. doi: 10.1016/0042-6989(94)00233-c. [DOI] [PubMed] [Google Scholar]
- Witkovsky P. Dopamine and retinal function. Documenta ophthalmologica. Advances in ophthalmology. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- Wu XH, Li YY, Zhang PP, Qian KW, Ding JH, Hu G, Weng SJ, Yang XL, Zhong YM. Unaltered retinal dopamine levels in a C57BL/6 mouse model of form-deprivation myopia. Investigative ophthalmology & visual science. 2015;56:967–977. doi: 10.1167/iovs.13-13362. [DOI] [PubMed] [Google Scholar]
- Xu X, Li S, Xiao X, Wang P, Guo X, Zhang Q. Sequence variations of GRM6 in patients with high myopia. Molecular vision. 2009;15:2094–2100. [PMC free article] [PubMed] [Google Scholar]
- Zeitz C, van Genderen M, Neidhardt J, Luhmann UF, Hoeben F, Forster U, Wycisk K, Matyas G, Hoyng CB, Riemslag F, Meire F, Cremers FP, Berger W. Mutations in GRM6 cause autosomal recessive congenital stationary night blindness with a distinctive scotopic 15-Hz flicker electroretinogram. Investigative ophthalmology & visual science. 2005;46:4328–4335. doi: 10.1167/iovs.05-0526. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Xiao X, Li S, Jia X, Yang Z, Huang S, Caruso RC, Guan T, Sergeev Y, Guo X, Hejtmancik JF. Mutations in NYX of individuals with high myopia, but without night blindness. Molecular vision. 2007;13:330–336. [PMC free article] [PubMed] [Google Scholar]


