Abstract
Vertebrate locomotion is executed by networks of neurons within the spinal cord. Here, we describe recent advances in our understanding of spinal locomotor control provided by work using optical and genetic approaches in mice and zebrafish. In particular, we highlight common observations that demonstrate simplification of limb and axial motor pool coordination by spinal network modularity, differences in the deployment of spinal modules at increasing speeds of locomotion, and functional hierarchies in the regulation of locomotor rhythm and pattern. We also discuss the promise of intersectional genetic strategies for better resolution of network components and connectivity, which should help us continue to close the gap between theory and function.
Introduction
“It is inessential at present whether the lumbar centres are two in number and situate on opposite sides of the spinal cord; or whether they are four in number and situated in antagonistic pairs on each side of the cord; or whether there are more than four in number.”
T. Graham Brown, 1911 [1]
The evidence that networks of neurons within the spinal cord are sufficient to generate locomotion is over a century old. Although this idea remains largely uncontested [2], work since then has led to modifications of the original model Brown put forth to explain his observations, namely the ‘half-center’ hypothesis. According to this concept, locomotion relies on pools of premotor excitatory interneurons locked in rhythmic alternation by fatigable sources of inhibition. While Brown was understandably less concerned with the number, location or scalability of neuronal half-centers, more recent models have attempted to account for the complexity of motor coordination during locomotion, where muscles are not always purely antagonistic and movements are not always at the same speed. Here, we highlight recent studies testing some of the major predictions arising from past and current models. To this end, we will focus on work using optical and genetic approaches to interrogate the spinal locomotor networks of mice and zebrafish.
Spinal network modularity
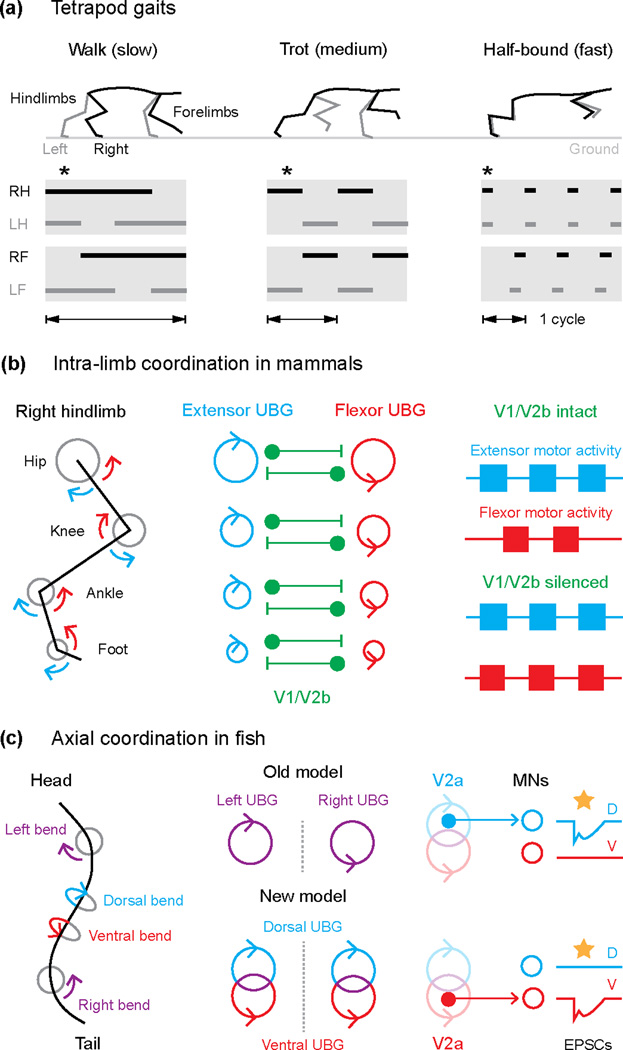
In mammals, spinal motor neurons are grouped into functionally and spatially distinct pools according to the muscles they innervate [3]. For locomotion, these pools must be appropriately coordinated within and between two sets of bilaterally paired limbs (Fig. 1A). Given the prohibitive complexity of independently controlling motor neurons, theories about premotor control have also invoked a pooled or ‘modular’ organization, as exemplified by the ‘unit burst generator’ (UBG) hypothesis of Sten Grillner [4]. According to this idea, motor pools controlling flexor or extensor movements around different joints have their own dedicated UBG made up of interconnected excitatory interneurons, whose purpose is to drive rhythmic motor activity (Fig. 1B). The UBG concept deviates from the half-center hypothesis in that reciprocal inhibition is not a prerequisite for rhythmicity, which allows UBGs to change their relative state of coupling (e.g., antagonistic versus synergistic) and provides a basis for variations in limb coordination during locomotion.
Figure 1.
Modular control of ipsilateral motor pools in mammals and fish. (a) Changes in frequency and coordination between limbs associated with tetrapod locomotion are depicted by kinematic snapshots (upper panels) showing right (R, black) and left (L, grey) hindlimbs (H) and forelimbs (F) during three different gaits. Ground contact for each limb (bottom) is indicated by solid bars and asterisks indicate time points shown in the top panels. (b) Articulations around the joints within a limb (left) are divided into extensor (blue arrows) and flexor (red arrows) movements. Intra-limb coordination based on the unit burst generator (UBG) hypothesis (adapted from [4]), likely involving the V1 and V2b ipsilateral inhibitory neurons, is illustrated in the center. Right panel summarizes results testing the involvement of V1/V2b interneurons in flexor-extensor coordination [9]. (c) Top down view of the midline of a fish swimming is presented on the left, where movements are coordinated across (left-right) and along the same side (dorsal-ventral) of the body. In the middle, an older UBG model is compared with an updated one, incorporating independent UBG control of dorsal (blue) and ventral (red) flexors along the same side of the body. Experiments demonstrating the segregation of V2a neurons into dorsal and ventral microcircuits are illustrated on the right [10]. Yellow stars indicate optogenetic activation of V2a neurons, which evokes electrical/chemical excitatory post-synaptic currents (EPSCs) in either dorsally projecting (D) or ventrally projecting (V) motor neurons (MNs).
A recent paper from the Kiehn lab has tested one of the major predictions of a UBG type organization, namely that motor pools should be able to generate rhythmic activity independently. To do so, Hagglund et al. [5] used transgenic lines of mice in which optogenetic actuators were selectively expressed in spinal glutamatergic neurons. The advantage over past work is that this approach allowed for the targeted and reversible activation and silencing of restricted regions of the spinal cord [6]. Using this method, combined with bulk recordings from ventral roots and more selective recordings from rootlets, the authors demonstrate the independent bursting capability of flexor- and extensor-related motor pools in both spatial distant and more closely apposed locations in the lumbar spinal cord.
So how might these independent UBGs be coordinated during locomotion? At least for movements within a limb, flexor-extensor alternation during locomotion has been attributed to the reciprocal actions of ipsilateral sources of inhibition [7]. A recent paper from the Goulding lab has examined the role of ipsilateral inhibitory interneurons in mediating flexor-extensor alternation in the hindlimbs. The work relied on methods to manipulate these populations based on their developmentally-derived molecular signatures [8], specifically En1-labeled V1 neurons from the p1 progenitor domain and Gata3-labeled V2b neurons from the p2 progenitor domain. Consistent with the UBG hypothesis, Zhang et al. [9] demonstrate that in the absence of ipsilateral inhibition flexor and extensor motor pools can burst rhythmically, however the pools become synchronized (Fig. 1B). The work also demonstrated a functional redundancy in that flexor-extensor alternation is only abolished after silencing both the V1 and V2b populations, suggesting that inhibitory flexor-extensor modules are found in both groups.
While the organization of separate UBGs for control of ipsilateral motor pools was considered a specialization of limb control, recent work has extended this concept to the axial networks controlling swimming in larval zebrafish. Using paired voltage-clamp recordings to compare the relative timing of excitatory and inhibitory synaptic currents during ‘fictive’ swimming, Bagnall and McLean [10] reveal that the inputs to motor neurons that innervate either dorsal or ventral trunk musculature along one side of the body are not completely shared. To link these observations to molecularly-defined spinal circuitry, the authors drove stochastic expression of a light-gated channel into a major source of ipsilateral premotor excitatory drive, namely Chx10-labeled V2a neurons [11–14]. Consistent with the assessments of network drive, optogenetic activation of sparsely labeled V2a neurons demonstrated the existence of mutually exclusive input patterns (Fig. 1C). Although the original formulation of UBGs suggested that left-right alternation represented the minimum functional module in primitive axial networks [4], there is also evidence for separate spinal drive to motor neurons innervating dorsal and ventral trunk muscles in lampreys [15,16]. Taken together, the findings suggest a finer scale modular organization of axial premotor networks than previously appreciated and also provide an early evolutionary template for independent control of musculature on the same side of the body, as proposed by the UBG concept (Fig. 1C).
Speed control
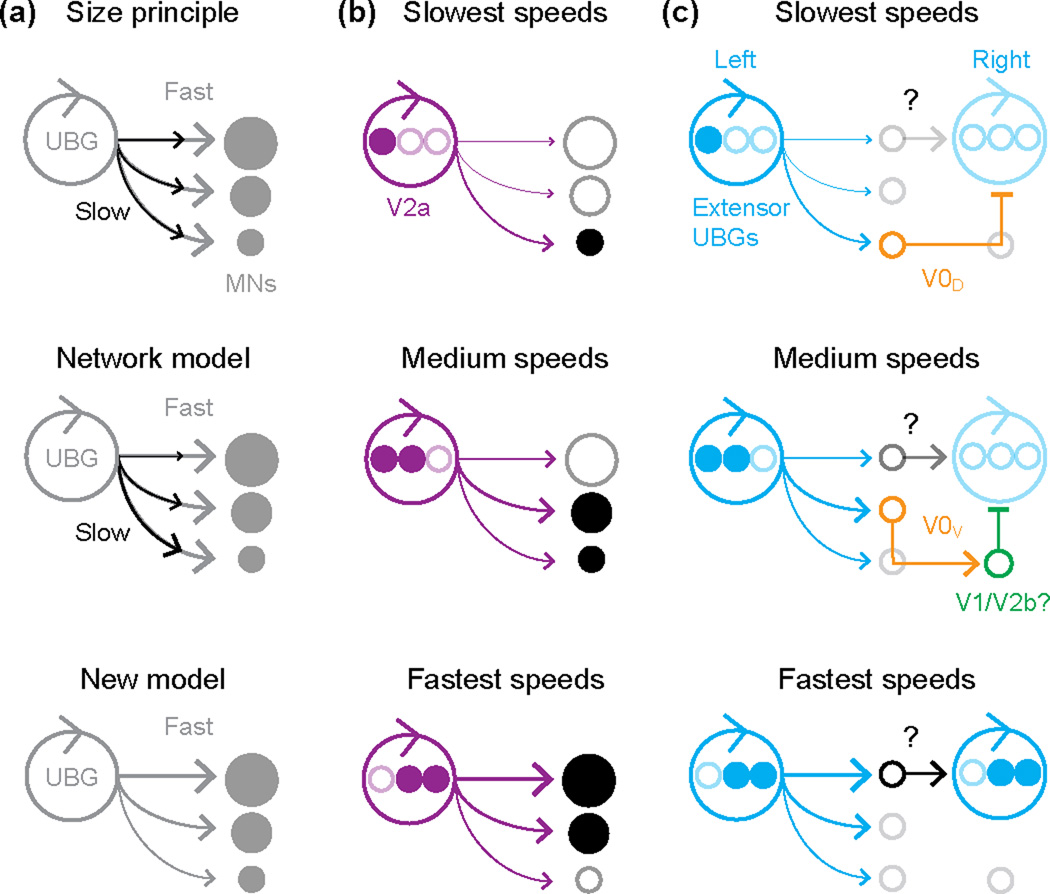
When considering the manifestation of spinal network modularity, it is important to remember that motor pools are not uniform in their composition, nor are they likely to participate equally in movements of varying speeds and strengths [17]. In this sense, the UBG hypothesis needs to be integrated with another prevailing concept, namely Elwood Henneman’s ‘size principle’ [18]. Stronger movements are associated with the recruitment of motor neurons that drive muscle fibers with more force output. These motor neurons tend to be larger and less excitable and so presumably require more drive to get them to threshold (Fig. 2A). A recent study using voltage-clamp recordings in larval zebrafish has begun to elucidate the synaptic basis for synchronizing activity among heterogeneous motor neurons during rhythmic locomotion [19]. In particular, increases in the frequency of fictive swimming are associated with a preferential increase in excitatory drive to less excitable motor neurons (Fig. 2A). This observation favors arguments for network properties determining the coordinated recruitment of motor pools [20], as opposed to the exclusive contribution of intrinsic membrane properties, as predicted by the size principle [21].
Figure 2.
Speed-dependent premotor control of motor neuron recruitment. (a) Incorporating the unit burst generator (UBG) hypothesis into models of motor neuron recruitment. According to the size principle (top), motor neurons (MNs) receive evenly distributed inputs, which are weaker at slow speeds (black arrows) and stronger at fast speeds (grey arrows), and orderly recruitment is a function of intrinsic excitability related to soma size [21]. In the network model (middle) [20], motor neurons recruited first receive biased drive (black arrows), and inputs biased to motor neurons recruited at faster speeds are added to the total excitatory drive (grey arrows). Critically, both models predict the same maximal distribution of excitatory drive across the motor pool at the fastest speeds. A new model (bottom) based on [19] has maximal drive (grey arrows) weighted to neuronal excitability across the motor pool. (b) In larval and juvenile zebrafish, V2a neurons comprise the UBG and provide appropriately biased drive to the motor pool [24,27]. V2a neurons recruited at slow speeds (top, filled purple circles) are biased in their connectivity to motor neurons active at those speeds (filled black circles). With progressively faster swimming, V2a neurons are recruited to drive the appropriate motor neurons (middle and bottom) and some V2a neurons and motor neurons fall silent (bottom, open circles) at the fastest speeds [22]. (c) Speed-related changes in left-right alternation circuitry in the mouse [31]. Left-right alternation is secured by inhibitory V0D and excitatory V0V commissural inhibitory neurons at slow (top) and medium (middle) speeds, respectively. Note the weak activation of excitatory commissural neurons at medium speeds, accounting for synchrony following V0V ablation. At the fastest locomotor speeds (bottom), synchrony between left and right sides is mediated by a currently unknown excitatory commissural connection.
To this end, recent work in zebrafish is beginning to reveal the sources of differential drive to motor neurons during increases in swimming frequency (Fig. 2B). At larval stages, premotor V2a neurons vary in their participation in swimming based on their spatial location; at the highest frequencies subsets of ventrally located V2a neurons are inhibited as more dorsal ones are engaged [12,14,22,23]. A recent anatomical study in larvae has demonstrated that more dorsal V2a neurons have the potential to make systematically more connections to less-excitable motor neurons by virtue of convergent intersegmental axonal projections [24]. This could provide the observed bias in rhythmic excitation during faster swimming [19]. In juvenile/adult zebrafish young enough to use Chx10 expression to identify V2a neurons [25], the spatial recruitment pattern is obscured by neuronal migration, but there are still differences in V2a activation patterns from low to medium frequencies of swimming evoked by bulk electrical stimulation [26]. A recent paper from the El Manira lab has used recruitment over this range of frequencies in older zebrafish to reveal functional patterns of premotor V2a connectivity. Using paired patch-clamp recordings, Ampatzis et al. [27] find that V2a neurons and motor neurons recruited around the same frequency are biased in their local connectivity to one another. Collectively, the work suggests that increases in speed are accompanied by the incorporation of V2a circuits that can drive the appropriate motor neurons (Fig. 2B).
In mammals, ground speed is not only a function of the level and frequency of motor pool activation during cyclical footfalls, but also a product of changes in inter-limb coordination (Fig. 1A). One example of a speed-dependent difference in inter-limb coupling in mice, as in many other mammals [4], is the transition from left-right alternation of the hindlimbs at slower speeds to left-right synchrony at the fastest speeds [28]. A recent paper from the Kiehn lab has revealed a functional dichotomy within the Dbx1-labeled V0 population during the maintenance of left-right hindlimb alternation at different speeds of locomotion. Most V0 neurons are commissural, and are thus well suited to regulate left-right limb coordination, but they are divided into inhibitory (V0D) and excitatory (V0V) subsets based on their spatial location and respective expression of the transcription factor, Pax7 [29–31]. Talpalar et al. [31] took advantage of the latter difference to selectively remove crossed inhibition or crossed excitation, and demonstrated that inhibitory V0D neurons are essential for maintaining left/right alternation at slow speeds, but are dispensable at faster speeds, when excitatory V0V cells appear to play a more prominent role (Fig. 2C). Specifically, in the absence of V0V cells, mice make an earlier transition to a near synchronous hopping gait than they normally would. This study adds to the growing body of evidence suggesting that in mice, as originally reported in zebrafish [22], there are different spinal circuit configurations driving increasing speeds of locomotion [32–36].
Separating rhythm from pattern
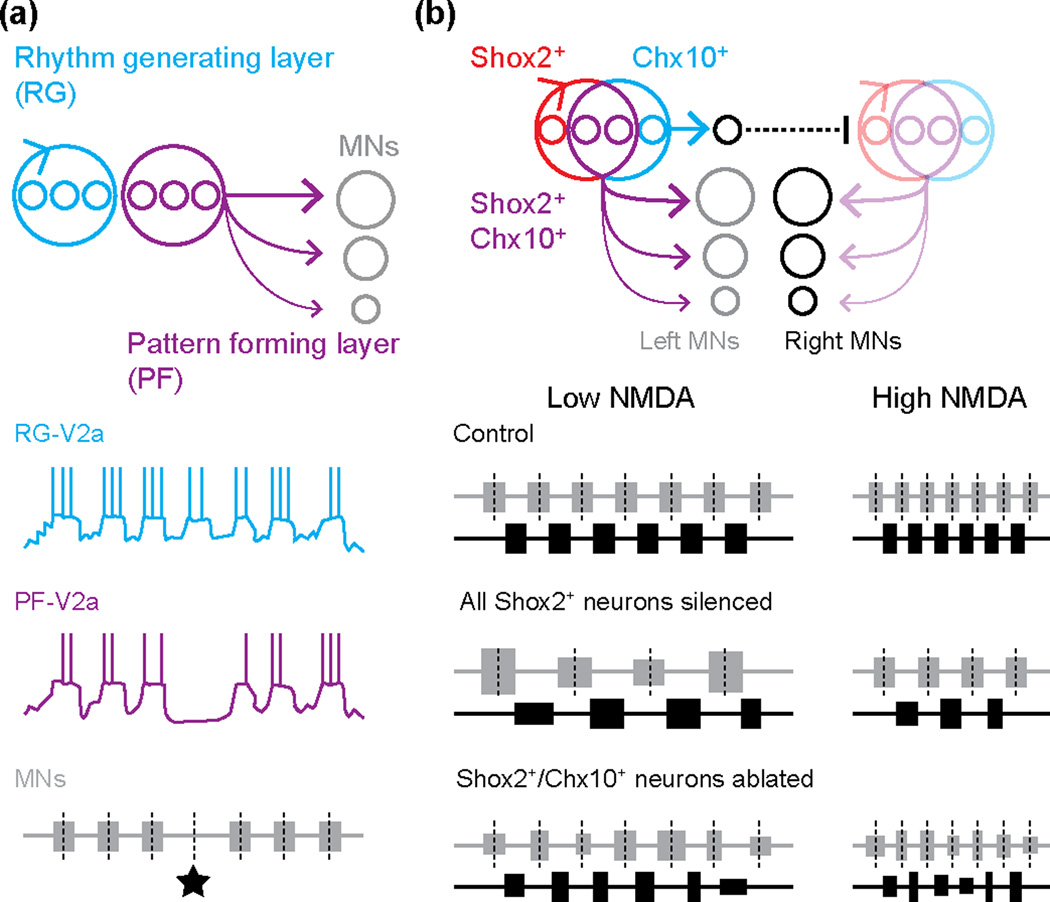
The UBG model presents the modular organization of locomotion as a single layer, where last-order interneurons (i.e., those driving motor neurons) also serve as first-order interneurons (i.e., those driving other interneurons [37]). However, in a more recent model David McCrea and Ilya Rybak argue for a multi-layer organization, in which ‘pattern’ forming interneurons with both last- and first-order function are driven by ‘rhythm’ generating interneurons that are purely first-order [38]. The multi-layered concept arose to explain the occurrence of spontaneous ‘deletions’ in motor output, when premotor synaptic drive would completely drop out [39,40]. In some cases, deletions had no impact on the timing of subsequent motor bursts (‘non-resetting deletions’), while in others the deletions were associated with a change in burst timing (‘resetting deletions’). In particular, non-resetting deletions are difficult to reconcile with a single layer UBG model, if the same neurons responsible for recruiting motor neurons also control the timing of their activity [41].
Recent work from the Harris-Warrick and Gosgnach labs has begun to look for evidence of multi-layer control in mice, by monitoring the activity patterns of spinal interneurons during non-resetting deletions. In V2a [42] and commissural dI6 [43] populations, as well as other unidentified interneurons [44], subsets fall silent during deletions, while others remain rhythmically active (Fig. 3A). These observations are consistent with the idea that neurons are embedded within either the pattern forming or rhythm generating layers, respectively. Another recent paper from the Kiehn lab has used an intersectional genetic approach to tease apart the control of rhythm versus pattern. Dougherty et al. [45] identify a novel population of ipsilaterally projecting excitatory interneurons marked by the expression of Shox2, which partially overlaps with the Chx10-labeled V2a population (Fig. 3B). Optogenetic or synaptic silencing of all Shox2+ interneurons (red and purple in Fig. 3B) prevents the generation of faster locomotor-related rhythms, while leaving the patterning of flexor-extensor and left-right alternation intact. In this case, the selective impact on frequency was used to define a rhythm generating function. Critically, ablation of Shox2+/Chx10+ V2a neurons (purple in Fig. 3B) has no dramatic effect on frequency (i.e., rhythm) or pattern. This makes it likely that the Shox2+ non-V2a neurons (red in Fig. 3B) are responsible for the observed frequency perturbation, and that they, along with other excitatory interneurons, comprise the main rhythm generators in the rhythm generating layer.
Figure 3.
Separate control of locomotor rhythm and pattern. (a) Top panel illustrates the functional separation of spinal neurons into first-order rhythm generating (RG, blue) and last-order pattern forming (PF, purple) layers. Below are representations of whole-cell patch clamp recordings from V2a neurons within each layer and motor neuron activity [42] during a ‘non-resetting’ deletion (black star). (b) Top panel illustrates the functional separation of neurons based on Shox2 and Chx10 expression. Shox2+ non-V2a neurons (red) are the rhythm generating neurons in the RG layer, while Chx10+ V2a neurons (blue) are also in the RG layer, but serve to control left-right alternation between rhythm generating neurons at certain speeds (by an as yet unidentified commissural pathway). Shox2+/Chx10+ V2a neurons are in the PF layer (purple), connecting to either extensor or flexor motor neurons (MNs). Subtraction experiments (bottom) demonstrate the contribution of Shox2+ non-V2a neurons (red) to rhythm generation [45]. When all Shox2+ neurons (red and purple) are optogenetically silenced, slower rhythms with more variable burst durations and cycle times are evoked by the same drug concentrations as compared to controls, but alternating patterns persist. When Shox2+/Chx10+ V2a neurons are ablated (purple), burst durations and cycle times are more variable, however the frequency of the rhythm is unaffected (black dashed lines).
The utility of a multi-layer organization is also bolstered by its ability to explain disruptions induced by genetic reorganization of spinal networks [46,47]. However, exploratory simulations based on UBGs also capture the major features of intra-and inter-limb coordination during locomotion [48]. Thus, the likelihood of a hierarchical UBG versus a multi-layered scheme is still debated [49–51]. More detailed maps of connectivity should help resolve this issue, by testing the last- versus first-order predictions of one concept over the other.
It is presently unclear whether axial networks would require a similar segregation of rhythm versus pattern elements. However, a recent paper from the Masino lab has demonstrated a functional dissociation in the control of the episodic structure and fine burst timing of motor activity during drug-evoked fictive swimming [52], leaving open the possibility that a multi-layer organization is also present in larval zebrafish.
Future directions
As the work described here illustrates, the investigation of spinal locomotor control has a long tradition of developing models to account for new experimental observations. Moving forward, predictions arising from increasingly detailed, biologically inspired computational models will need to be tested using methods that allow more precise control of circuit elements in behaving animals. The current state of the art uses genes identifying common early developmental origins, however there is a growing appreciation that even within progenitor domains there is considerable heterogeneity. For instance, recent work has demonstrated that time of differentiation plays a crucial role in generating anatomical and functional diversity within progenitor zones in mice [53–55] and zebrafish [56]. Consequently, identification of genes specific to neurons arising at different stages of development or better temporal control of inducible gene expression should facilitate a more targeted optogenetic assessment of circuit function.
Another important consideration relates to the distributed nature of spinal locomotor control. A recent paper has characterized the distribution of genetically identified populations along the length of spinal cord in mice [57], which reveals regional specializations resembling the columnar organization of motor pools controlling the limbs and trunk [58]. In addition, viral tracing methods have recently identified sources of premotor inputs to hindlimb motor neurons, which include long range descending V2a neurons [59] and local subsets of dl6, V0, V1, and V2 interneurons that also synapse with axial motor neurons [60]. Collectively, the work is beginning to outline molecularly-defined candidate neurons and patterns of connectivity responsible for coordinating movements of the hindlimbs with those of the forelimbs and trunk. However, it also raises important caveats for future work, namely that spinal premotor drive to lumbar motor pools is unlikely to come solely from local sources, nor are local lumbar circuits likely to be dedicated only to hindlimb movements.
A basis for the spatial and temporal integration of molecularly-defined appendage and axial circuits will also be provided by studies of metamorphosis in Xenopus frogs [61], in which optogenetics are now feasible [62], and can build on decades of work studying axial control [63]. Future optogenetic studies in zebrafish will also help in this endeavor. A recent paper from the Hale lab has demonstrated that slow swimming in larvae is associated with coordinated activation of pectoral fin and axial motor neurons, while at faster speeds the axial motor neurons take over as tonic activation of ‘flexor’ pools pins both pectoral fins against the body [64]. It will be interesting to see if such ‘gait’ transitions reflect dedicated versus shared limb/axial circuitry, whether hierarchical relationships between the two exist in speed-dependent manner, and if the patterns revealed for pectoral fin control continue on as caudal, anal, dorsal, and pelvic fins are sequentially added during zebrafish development.
Finally, if principles of spinal locomotor control are to be defined, it should be based on the breadth of their explanatory power. We have covered a few examples that appear to be conserved in mammals and fish. However, specializations related to different locomotor strategies are also informative, which will be evident not only between species, but also within them during development. In this sense, examinations of species- and age-dependent differences will need to account for differences in methodology, which often comes down to comparisons between studies performed either in vivo or in vitro. Continued advances in intersectional genetic and optical approaches that allow us to mark and monitor neurons into adulthood should help level the playing field, and as the techniques improve so too will our ability to distinguish what is inessential at present from what is essential in future.
Highlights.
Limb and axial motor pool coordination are simplified by spinal network modularity
Different spinal modules are deployed at increasing speeds of locomotion
Hierarchical networks control the rhythm and pattern of locomotor activity
Intersectional genetics promises better resolution of components and connectivity
Acknowledgements
We thank Abdel El Manira, Simon Giszter and Ilya Rybak for discussions and comments on the manuscript. DLM is supported by a National Institutes of Health R01 award (NS067299).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare no conflict of interest.
References
* of special interest
** of outstanding interest
- 1.Brown TG. The intrinsic factors in the act of progression in mammals. Proc R Soc B. 1911;84:308–319. [Google Scholar]
- 2.Akay T, Tourtellotte WG, Arber S, Jessell TM. Degradation of mouse locomotor pattern in the absence of proprioceptive sensory feedback. Proc Natl Acad Sci U S A. 2014;111:16877–16882. doi: 10.1073/pnas.1419045111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jessell TM, Surmeli G, Kelly JS. Motor neurons and the sense of place. Neuron. 2011;72:419–424. doi: 10.1016/j.neuron.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Grillner S. Control of locomotion in bipeds, tetrapods, and fish. In: Brooks VB, editor. Handbook of Physiology. American Physiological Society; 1981. pp. 1179–1236. [Google Scholar]
- 5. Hagglund M, Dougherty KJ, Borgius L, Itohara S, Iwasato T, Kiehn O. Optogenetic dissection reveals multiple rhythmogenic modules underlying locomotion. Proc Natl Acad Sci U S A. 2013;110:11589–11594. doi: 10.1073/pnas.1304365110. The authors use optogenetics to activate and inhibit restricted populations of excitatory and inhibitory neurons in the lumbar spinal cord of transgenic mice. Light activation of excitatory interneurons evokes rhythmic motor bursts independently in either flexor or extensor-related motor pools. Rhythmic activity in motor pools innervating closely related flexor muscles can also be autonomously driven, demonstrating that an organization of multiple rhythmogenic modules exists in the mammalian spinal cord, similar to the unit burst generator model proposed by Grillner [4].
- 6.Buschges A, Borgmann A. Network modularity: back to the future in motor control. Curr Biol. 2013;23:R936–R938. doi: 10.1016/j.cub.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 7.Goulding M, Bourane S, Garcia-Campmany L, Dalet A, Koch S. Inhibition downunder: an update from the spinal cord. Curr Opin Neurobiol. 2014;26:161–166. doi: 10.1016/j.conb.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arber S. Motor circuits in action: specification, connectivity, and function. Neuron. 2012;74:975–989. doi: 10.1016/j.neuron.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 9. Zhang J, Lanuza GM, Britz O, Wang Z, Siembab VC, Zhang Y, Velasquez T, Alvarez FJ, Frank E, Goulding M. V1 and V2b interneurons secure the alternating flexor-extensor motor activity mice require for limbed locomotion. Neuron. 2014;82:138–150. doi: 10.1016/j.neuron.2014.02.013. This study identifies V1 and V2b inhibitory interneurons as components of the hindlimb flexor-extensor coordinating circuitry. Synaptic silencing of either population alone is not sufficient to disrupt flexor-extensor alternation. However, when all V1 and V2b inhibitory neurons are silenced, flexor and extensor motor bursts become synchronous. Interestingly, flexor and extensor activity was also synchronous in the V2b mutants but only following hemisection, suggesting that the V1 neurons involved either receive drive from or indirectly provide drive to the contralateral side, adding further complexity to the circuit.
- 10. Bagnall MW, McLean DL. Modular organization of axial microcircuits in zebrafish. Science. 2014;343:197–200. doi: 10.1126/science.1245629. Bagnall and McLean examined synaptic inputs to pairs of motor neurons innervating the same or different muscle quadrants in zebrafish. They demonstrate that, during swimming, motor neurons innervating dorsal and ventral muscles are rarely activated or inhibited synchronously on a fine time scale, suggesting that the dorsal and ventral motor pools are controlled by distinct premotor networks. Further, they demonstrate the behavioral relevance of such modular organization during self-righting postural corrections. The study raises the intriguing possibility that the axial system served as a template for the modular control of limb motor networks.
- 11.Bhatt DH, McLean DL, Hale ME, Fetcho JR. Grading movement strength by changes in firing intensity versus recruitment of spinal interneurons. Neuron. 2007;53:91–102. doi: 10.1016/j.neuron.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Kimura Y, Okamura Y, Higashijima S. alx, a zebrafish homolog of Chx10, marks ipsilateral descending excitatory interneurons that participate in the regulation of spinal locomotor circuits. J Neurosci. 2006;26:5684–5697. doi: 10.1523/JNEUROSCI.4993-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eklof-Ljunggren E, Haupt S, Ausborn J, Ampatzis K, El Manira A. Optogenetic activation of excitatory premotor interneurons is sufficient to generate coordinated locomotor activity in larval zebrafish. J Neurosci. 2014;34:134–139. doi: 10.1523/JNEUROSCI.4087-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eklof-Ljunggren E, Haupt S, Ausborn J, Dehnisch I, Uhlen P, Higashijima S, El Manira A. Origin of excitation underlying locomotion in the spinal circuit of zebrafish. Proc Natl Acad Sci U S A. 2012;109:5511–5516. doi: 10.1073/pnas.1115377109. The authors use targeted photo-ablations to remove up to 30% of the Chx10-labeled population of V2a neurons in larval zebrafish, which prevents the generation of faster steady-state fictive swimming. Elimination of just the dorsal subset has a more selective effect on peak swimming frequencies, consistent with the dorso-ventral pattern of recruitment at this age [22]. The effect on frequency contrasts similar Chx10-V2a ablation experiments in mice [31,42] and suggests that Chx10-V2a neurons contribute to both the rhythm and the pattern at faster speeds in axial networks.
- 15.Buchanan JT. Flexibility in the patterning and control of axial locomotor networks in lamprey. Integr Comp Biol. 2011;51:869–878. doi: 10.1093/icb/icr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallen P, Grillner S, Feldman JL, Bergelt S. Dorsal and ventral myotome motoneurons and their input during fictive locomotion in lamprey. J Neurosci. 1985;5:654–661. doi: 10.1523/JNEUROSCI.05-03-00654.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heckman CJ, Enoka RM. Motor unit. Compr Physiol. 2012;2:2629–2682. doi: 10.1002/cphy.c100087. [DOI] [PubMed] [Google Scholar]
- 18. Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. This study examines inputs to axial motor neurons in larval zebrafish during swimming at different speeds. The authors found that, with increasing speed, the timing of excitatory inputs becomes more synchronous and excitation preferentially increases to motor neurons with lower input resistance (Rin). Inhibition, on the other hand, is increased to all motor neurons with increasing speeds and a preference for anti-phase inhibition switches to in-phase inhibition, especially in motor neurons with higher Rin. Together, this provides evidence for differences in the balance of excitation and inhibition appropriately tuned to the excitability of motor neurons to ensure graded recruitment within axial motor pools during faster speeds of swimming.
- 19.Kishore S, Bagnall MW, McLean DL. Systematic shifts in the balance of excitation and inhibition coordinate the activity of axial motor pools at different speeds of locomotion. J Neurosci. 2014;34:14046–14054. doi: 10.1523/JNEUROSCI.0514-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke RE. The role of synaptic organization in the control of motor unit activity during movement. Prog Brain Res. 1979;50:61–67. doi: 10.1016/S0079-6123(08)60807-9. [DOI] [PubMed] [Google Scholar]
- 21.Henneman E. The size-principle: a deterministic output emerges from a set of probabilistic connections. J Exp Biol. 1985;115:105–112. doi: 10.1242/jeb.115.1.105. [DOI] [PubMed] [Google Scholar]
- 22.McLean DL, Masino MA, Koh IY, Lindquist WB, Fetcho JR. Continuous shifts in the active set of spinal interneurons during changes in locomotor speed. Nat Neurosci. 2008;11:1419–1429. doi: 10.1038/nn.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McLean DL, Fan J, Higashijima S, Hale ME, Fetcho JR. A topographic map of recruitment in spinal cord. Nature. 2007;446:71–75. doi: 10.1038/nature05588. By stochastically labeling neurons with fluorescent markers in zebrafish, the authors describe differences in axonal projection patterns and synapse distribution within the V2a population. V2a neurons can be divided into two classes based on the presence or absence of a prominent ascending axon branch. In the purely descending V2a neurons, the length of the descending axon is related to soma position with more dorsal V2a neurons, which are recruited during faster swimming, having longer axons and a higher density of synaptic boutons. Therefore, the fast V2a neurons are capable of driving more spinal neurons than the more ventral V2a neurons active at slower swimming speeds.
- 24.Menelaou E, VanDunk C, McLean DL. Differences in the morphology of spinal V2a neurons reflect their recruitment order during swimming in larval zebrafish. J Comp Neurol. 2014;522:1232–1248. doi: 10.1002/cne.23465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuscha V, Frazer SL, Dias TB, Hibi M, Becker T, Becker CG. Lesion-induced generation of interneuron cell types in specific dorsoventral domains in the spinal cord of adult zebrafish. J Comp Neurol. 2012;520:3604–3616. doi: 10.1002/cne.23115. [DOI] [PubMed] [Google Scholar]
- 26. Ausborn J, Mahmood R, El Manira A. Decoding the rules of recruitment of excitatory interneurons in the adult zebrafish locomotor network. Proc Natl Acad Sci U S A. 2012;109:E3631–E3639. doi: 10.1073/pnas.1216256110. Ampatzis et al. use paired recordings from zebrafish V2a neurons and motor neurons to demonstrate that premotor excitatory V2a neurons activated in a modular manner at slow, intermediate, and faster speeds provide more reliable monosynaptic input only to motor neurons active at the corresponding speed. This biased connectivity allows the V2a neurons to selectively drive motor neurons relevant to the swimming speed.
- 27.Ampatzis K, Song J, Ausborn J, El Manira A. Separate microcircuit modules of distinct V2a interneurons and motoneurons control the speed of locomotion. Neuron. 2014;83:934–943. doi: 10.1016/j.neuron.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Serradj N, Jamon M. The adaptation of limb kinematics to increasing walking speeds in freely moving mice 129/Sv and C57BL/6. Behav Brain Res. 2009;201:59–65. doi: 10.1016/j.bbr.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 29. Pierani A, Moran-Rivard L, Sunshine MJ, Littman DR, Goulding M, Jessell TM. Control of interneuron fate in the developing spinal cord by the progenitor homeodomain protein Dbx1. Neuron. 2001;29:367–384. doi: 10.1016/s0896-6273(01)00212-4. The authors use genetic ablations of molecularly-specified neuronal populations to show that different commissural circuits are activated to ensure left-right alternation at different locomotor speeds. The V0D inhibitory neurons are necessary for alternation at slow speeds and the V0V excitatory neurons are required for alternation at higher speeds. This study shows that different commissural circuits are activated in a speed-dependent manner and identifies essential components of this left-right alternation circuitry.
- 30.Lanuza GM, Gosgnach S, Pierani A, Jessell TM, Goulding M. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron. 2004;42:375–386. doi: 10.1016/s0896-6273(04)00249-1. [DOI] [PubMed] [Google Scholar]
- 31.Talpalar AE, Bouvier J, Borgius L, Fortin G, Pierani A, Kiehn O. Dual-mode operation of neuronal networks involved in left-right alternation. Nature. 2013;500:85–88. doi: 10.1038/nature12286. [DOI] [PubMed] [Google Scholar]
- 32.Gosgnach S, Lanuza GM, Butt SJ, Saueressig H, Zhang Y, Velasquez T, Riethmacher D, Callaway EM, Kiehn O, Goulding M. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature. 2006;440:215–219. doi: 10.1038/nature04545. [DOI] [PubMed] [Google Scholar]
- 33.Crone SA, Zhong G, Harris-Warrick R, Sharma K. In mice lacking V2a interneurons, gait depends on speed of locomotion. J Neurosci. 2009;29:7098–7109. doi: 10.1523/JNEUROSCI.1206-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhong G, Sharma K, Harris-Warrick RM. Frequency-dependent recruitment of V2a interneurons during fictive locomotion in the mouse spinal cord. Nat Commun. 2011;2:274. doi: 10.1038/ncomms1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borowska J, Jones CT, Zhang H, Blacklaws J, Goulding M, Zhang Y. Functional subpopulations of V3 interneurons in the mature mouse spinal cord. J Neurosci. 2013;33:18553–18565. doi: 10.1523/JNEUROSCI.2005-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersson LS, Larhammar M, Memic F, Wootz H, Schwochow D, Rubin CJ, Patra K, Arnason T, Wellbring L, Hjalm G, et al. Mutations in DMRT3 affect locomotion in horses and spinal circuit function in mice. Nature. 2012;488:642–646. doi: 10.1038/nature11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miri A, Azim E, Jessell TM. Edging toward entelechy in motor control. Neuron. 2013;80:827–834. doi: 10.1016/j.neuron.2013.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCrea DA, Rybak IA. Modeling the mammalian locomotor CPG: insights from mistakes and perturbations. Prog Brain Res. 2007;165:235–253. doi: 10.1016/S0079-6123(06)65015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lafreniere-Roula M, McCrea DA. Deletions of rhythmic motoneuron activity during fictive locomotion and scratch provide clues to the organization of the mammalian central pattern generator. J Neurophysiol. 2005;94:1120–1132. doi: 10.1152/jn.00216.2005. In this combined experimental/modeling study, Zhong et al. look at spontaneous locomotor deletions during drug-evoked locomotion in the in vitro neonatal mouse spinal cord. They identify two types of V2a interneurons based on their activity (or lack of activity) during non-resetting locomotor deletions. The V2a neurons are located at different levels in the CPG organization. Type I V2a neurons maintain rhythmicity during the deletion and are located in the rhythm generating level. Type II V2a neurons, like motor neurons, do not receive synaptic input during the deletion and are in the patterning level. These results have been built into their two-level asymmetrical model of the locomotor CPG, which is validated by the electrophysiological data.
- 40.Stein PS. Motor pattern deletions and modular organization of turtle spinal cord. Brain Res Rev. 2008;57:118–124. doi: 10.1016/j.brainresrev.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev. 2008;57:134–146. doi: 10.1016/j.brainresrev.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhong G, Shevtsova NA, Rybak IA, Harris-Warrick RM. Neuronal activity in the isolated mouse spinal cord during spontaneous deletions in fictive locomotion: insights into locomotor central pattern generator organization. J Physiol. 2012;590:4735–4759. doi: 10.1113/jphysiol.2012.240895. This study identifies the transcription factor Shox2 as a marker of a population of excitatory, ipsilaterally projecting interneurons that overlaps with the V2a population. Shox2 neurons are rhythmically active during locomotion and subpopulations connect to commissural neurons, motor neurons, and other Shox2 neurons. The functional roles of Shox2 neurons are dissected using combinatorial genetics. When Shox2 neurons are synaptically silenced, the locomotor frequency decreases. However, ablation of Shox2 V2a neurons has no effect on locomotor rhythm or left-right alternation, demonstrating that the silencing of the Shox2 non-V2a neurons is responsible for the observed frequency reduction. Assuming the loss of rhythm generating neurons reduces locomotor frequency, the results place Shox2 non-V2a neurons as components of the hindlimb rhythm generator for locomotion.
- 43.Dyck J, Lanuza GM, Gosgnach S. Functional characterization of dI6 interneurons in the neonatal mouse spinal cord. J Neurophysiol. 2012;107:3256–3266. doi: 10.1152/jn.01132.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griener A, Dyck J, Gosgnach S. Regional distribution of putative rhythm-generating and pattern-forming components of the mammalian locomotor CPG. Neuroscience. 2013;250:644–650. doi: 10.1016/j.neuroscience.2013.07.070. [DOI] [PubMed] [Google Scholar]
- 45.Dougherty KJ, Zagoraiou L, Satoh D, Rozani I, Doobar S, Arber S, Jessell TM, Kiehn O. Locomotor rhythm generation linked to the output of spinal Shox2 excitatory interneurons. Neuron. 2013;80:920–933. doi: 10.1016/j.neuron.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 46.Rybak IA, Shevtsova NA, Kiehn O. Modelling genetic reorganization in the mouse spinal cord affecting left-right coordination during locomotion. J Physiol. 2013;591:5491–5508. doi: 10.1113/jphysiol.2013.261115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Borgius L, Nishimaru H, Caldeira V, Kunugise Y, Low P, Reig R, Itohara S, Iwasato T, Kiehn O. Spinal glutamatergic neurons defined by EphA4 signaling are essential components of normal locomotor circuits. J Neurosci. 2014;34:3841–3853. doi: 10.1523/JNEUROSCI.4992-13.2014. The authors suggest that, similar to mammals, the larval zebrafish locomotor network is hierarchically organized, at least at slow swimming speeds. Using preparations where different spinal segments were isolated and fictive swimming was evoked by NMDA application, Wiggin et al. reveal that the timing of episodic swimming bouts and motor bursts within those bouts can be functionally separated and, therefore, are controlled by distinct circuits.
- 48.Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron. 2006;52:751–766. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 49.Grillner S, Manira AE. The intrinsic operation of the networks that make us locomote. Curr Opin Neurobiol. 2015;31C:244–249. doi: 10.1016/j.conb.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 50.Brownstone RM, Wilson JM. Strategies for delineating spinal locomotor rhythm-generating networks and the possible role of Hb9 interneurones in rhythmogenesis. Brain Res Rev. 2008;57:64–76. doi: 10.1016/j.brainresrev.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu Rev Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. Satou et al. reveal a systematic pattern of development of different anatomical classes of V0 commissural neurons in zebrafish. Glutamatergic ascending neurons arise first, followed by both glutamatergic and GABAergic/glycinergic bifurcating neurons, and then finally glutamatergic descending neurons. This is also reflected in a systematic difference in their dorso-ventral distribution. At least for the latest born, most ventral excitatory class, a subset is known to participate exclusively in slow swimming [21,22]. When combined with work in mice [48–50], this study provides strong evidence for the contribution of neurogenesis to the ultimate functional roles of spinal interneurons during locomotor behavior.
- 52.Wiggin TD, Anderson TM, Eian J, Peck JH, Masino MA. Episodic swimming in the larval zebrafish is generated by a spatially distributed spinal network with modular functional organization. J Neurophysiol. 2012;108:925–934. doi: 10.1152/jn.00233.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tripodi M, Stepien AE, Arber S. Motor antagonism exposed by spatial segregation and timing of neurogenesis. Nature. 2011;479:61–66. doi: 10.1038/nature10538. [DOI] [PubMed] [Google Scholar]
- 54.Stam FJ, Hendricks TJ, Zhang J, Geiman EJ, Francius C, Labosky PA, Clotman F, Goulding M. Renshaw cell interneuron specialization is controlled by a temporally restricted transcription factor program. Development. 2012;139:179–190. doi: 10.1242/dev.071134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Benito-Gonzalez A, Alvarez FJ. Renshaw cells and Ia inhibitory interneurons are generated at different times from p1 progenitors and differentiate shortly after exiting the cell cycle. J Neurosci. 2012;32:1156–1170. doi: 10.1523/JNEUROSCI.3630-12.2012. Goetz et al. use monosynaptically restricted transsynaptic virus labeling to differentiate trunk and limb premotor networks. The trunk circuits are predominantly contralateral to the motor neurons. The limb circuits, on the other hand, are mostly ipsilateral and the dominance of ipsilateral over contralateral premotor neurons increases as the muscle innervated becomes more distal. Although the laterality of inputs may be distinct, neuronal classes involved in the coordination of hindlimbs during locomotion (dI6, V0, V1, and V2) are premotor to both limb and axial motor pools. The work has important implications for the relative binding of different axial and limb network modules during increases in locomotor speed.
- 56.Satou C, Kimura Y, Higashijima S. Generation of multiple classes of V0 neurons in zebrafish spinal cord: progenitor heterogeneity and temporal control of neuronal diversity. J Neurosci. 2012;32:1771–1783. doi: 10.1523/JNEUROSCI.5500-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Francius C, Harris A, Rucchin V, Hendricks TJ, Stam FJ, Barber M, Kurek D, Grosveld FG, Pierani A, Goulding M, et al. Identification of multiple subsets of ventral interneurons and differential distribution along the rostrocaudal axis of the developing spinal cord. PLoS One. 2013;8:e70325. doi: 10.1371/journal.pone.0070325. Moult et al. rapidly and reversibly silence neurons on one side of the spinal cord using optogenetics or single cell perturbations to demonstrate the necessity of commissural inhibition for the maintenance of locomotor rhythmogenesis in Xenopus tadpoles. The experiments reveal that in some circumstances the predictions arising from the half-center hypothesis hold true, since a fatigable source of reciprocal inhibition between pools of excitatory premotor interneurons is necessary to generate locomotion [1].
- 58.Philippidou P, Dasen JS. Hox genes: choreographers in neural development, architects of circuit organization. Neuron. 2013;80:12–34. doi: 10.1016/j.neuron.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ni Y, Nawabi H, Liu X, Yang L, Miyamichi K, Tedeschi A, Xu B, Wall NR, Callaway EM, He Z. Characterization of long descending premotor propriospinal neurons in the spinal cord. J Neurosci. 2014;34:9404–9417. doi: 10.1523/JNEUROSCI.1771-14.2014. The authors use patch-clamp recordings from abductor (extensor) and adductor (flexor) motor neurons of the pectoral fins combined with axial motor nerve recordings to reveal differences in their patterns of activation during fictive swimming. At slow speeds, the extensor motor neurons fire out of phase with local axial motor nerves, while flexor motor neurons fire in phase. At faster speeds, the extensor motor neurons fall silent as flexor motor neurons become tonically active. The patterns demonstrate active control of the pectoral fins during transitions from slow to fast swimming and have important implications for the functional interactions between fin and axial circuitry.
- 60.Goetz C, Pivetta C, Arber S. Distinct limb and trunk premotor circuits establish laterality in the spinal cord. Neuron. 2015;85:131–144. doi: 10.1016/j.neuron.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 61.Sillar KT, Combes D, Ramanathan S, Molinari M, Simmers J. Neuromodulation and developmental plasticity in the locomotor system of anuran amphibians during metamorphosis. Brain Res Rev. 2008;57:94–102. doi: 10.1016/j.brainresrev.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 62.Moult PR, Cottrell GA, Li WC. Fast silencing reveals a lost role for reciprocal inhibition in locomotion. Neuron. 2013;77:129–140. doi: 10.1016/j.neuron.2012.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roberts A, Li WC, Soffe SR. How neurons generate behavior in a hatchling amphibian tadpole: an outline. Front Behav Neurosci. 2010;4:16. doi: 10.3389/fnbeh.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Green MH, Hale ME. Activity of pectoral fin motoneurons during two swimming gaits in the larval zebrafish (Danio rerio) and localization of upstream circuit elements. J Neurophysiol. 2012;108:3393–3402. doi: 10.1152/jn.00623.2012. [DOI] [PubMed] [Google Scholar]