Abstract
Objectives
Substance use disorder is characterized by impaired decision-making, impulsivity, and risk-taking. Pathological gambling shares many of these characteristics and having both diagnoses may be associated with greater problems than either diagnosis alone. We investigated whether among substance dependent individuals, co-morbid pathological gambling would be associated with worse decision-making, greater impulsivity, risk-taking, and drug severity.
Methods
Ninety-six substance dependent individuals were recruited from a residential treatment program and divided into one of two groups depending on whether they met DSM-IV criteria for pathological gambling (SDPG, n=26) or not (SD, n=70). Ninety-two controls were recruited from the community. Participants completed a decision-making task (modified Iowa Gambling Task), measures of impulsivity (Barratt Impulsivity Scale and Delay Discounting), and risk-taking (Balloon Analog Risk Task). Decision-making was analyzed using a computational model. We tested for group differences using ANCOVA or Kruskal-Wallis and appropriate post-hoc tests.
Results
The groups differed in decision-making parameters (p<0.001) and self-report impulsivity (p<0.001). All post-hoc comparisons were significant on these measures, and indicated stepwise changes in controls, followed by SD, followed by SDPG, with SDPG performing worse on decision-making and being more impulsive. Compared to SD, SDPG had greater drug severity (p<0.001). No group differences were observed in delay discounting or risk-taking.
Conclusions
Compared to individuals with substance dependence without pathological gambling, those with both disorders demonstrated worse decision-making and significantly more drug-related symptoms. When evaluating patients with substance dependence, clinicians should consider diagnostic assessments for gambling, as the co-occurrence of both disorders may impact clinical characteristics.
Keywords: Substance Dependence, Pathological Gambling, Decision-Making, Impulsivity, Computational Modeling
Individuals with substance use disorders make poor decisions that involve seeking and taking drugs despite negative long-term consequences. Such patterns of poor decision-making can result from elevated impulsivity (Leeman & Potenza, 2012; Tomassini et al., 2012), high levels of risk-taking (Lejuez et al., 2003; Crowley et al., 2006), and/or deficits in goal-directed learning (Stout et al., 2004; Stout et al., 2005; Thompson et al., 2012). The Iowa Gambling Task (IGT; Bechara, 2003), a task of decision-making under conditions of uncertainty and risk, has been shown to be sensitive to many clinical populations. Compared to controls, people who us substances perform poorly because they persist in making choices that, while yielding large immediate rewards, over time ultimately result in net losses (Bechara, 2003). Computational modeling of behavior on the IGT may identify psychological processes that could account for impaired decisions. For example, Stout et al. (2004) found that, in cocaine users, impaired decision-making on the IGT was due to hyposensitivity to loss and response inconsistency (Stout et al., 2004). In contrast, patients with ventral medial prefrontal lesions also perform poorly but their decision-making deficits may be related to impairments in updating expectations (Yechiam et al., 2005).
Poor decisions are also related to impulsivity and risk taking. A vast literature has shown that drug and alcohol addictions are associated with impulsivity (Lejuez et al., 2010; Leeman & Potenza, 2012). One measure of impulsivity, temporal discounting, indicates that individuals with substance use disorders devalue long-term rewards in favor of short term rewards to a greater extent than controls (Petry, 2001; Ledgerwood et al., 2009; Andrade & Petry, 2012; Leeman & Potenza, 2012). Individuals with substance use disorders also take greater risks than controls (Rogers et al., 1999; Lejuez et al., 2003). Impulsivity and risk-taking, together, have been shown to increase the probability of initial drug experimentation over either construct alone (Poulos, Le, & Parker, 1995; Dayan et al., 2010; Lejuez et al., 2010). However, poor decision-making, impulsivity and risk-taking are not unique to drug use disorders. Individuals who have gambling problems show similar behaviors (Leeman & Potenza, 2012). As with drugs and alcohol, most people can gamble without becoming preoccupied with or jeopardizing their family or professional relationships due to gambling. Prevalence estimates for pathological gambling are more difficult to ascertain than for substance dependence but have been reported in the range of .42% - 3% (Petry) (National Research Council 1999) compared to 10% for substance dependence (Miller, T.R. and Hendrie, D. 2009). However, both disorders share clinical and biological features that have led to the reclassification of “pathological gambling” from a disorder of impulse-control in DSM-IV (4th ed.; DSM-IV; The Diagnostic and Statistical Manual of Mental Disorders, American Psychiatric Association (APA) 1999) to a behavioral addiction in the DSM-V (5th ed.; DSM–V; APA, 2013). Both disorders are characterized by tolerance, unsuccessful efforts to stop, and continued engagement despite long-term negative consequences (Leeman & Potenza, 2012). In addition, substance use and pathological gambling are highly comorbid.
Compared to controls, individuals with pathological gambling are five to seven times more likely to have alcohol, nicotine, and substance dependence (Petry, Stinson, & Grant, 2005). In spite of this high co-occurrence, the clinical impact of having both diagnoses is not clear. Gamblers with substance use disorders show more rapid temporal discounting than gamblers without substance use disorders, suggesting an additive risk on impulsivity (Andrade & Petry, 2012; Petry & Casarella, 1999). Greater impulsivity may have adverse effects on finances, employment, and social relationships, and has been associated with increased legal problems (Ledgerwood et al., 2009). Individuals with both diagnoses may require more intensive management. Suicide attempts were reported to be more common among people who gamble who also had unhealthy alcohol use than among those who did not. (Potenza, Steinberg, & Wu, 2005). Individuals with gambling and alcohol problems were more likely to have non-gambling related arrests compared to individuals with only gambling problems (Potenza, Steinberg, & Wu, 2005).
Previous studies investigating co-morbidity of these disorders have recruited participants primarily from centers specializing in gambling problems (Ledgerwood et al., 2009; Andrade & Petry, 2012). As mentioned previously pathological gambling is significantly less common than substance dependence. Thus, examining the consequences of co-morbidity from the perspective of substance use populations would be informative. Moreover, a recent meta-analysis suggested that in drug treatment settings, the prevalence of problem gambling is significantly higher than the general population (Cowlishaw et al., 2014).
The goal of this study was to investigate decision-making, impulsivity, and drug severity in substance dependent individuals with and without pathological gambling compared to controls. We used a novel computational model to identify mechanisms underlying decision-making performance and then evaluated potential differences in model parameters among the three groups.
Methods
Participants
Patients
Ninety-six drug-abstinent substance-dependent individuals resided for at least two months in the University of Colorado Denver’s Addiction Research and Treatment Services long-term residential treatment programs. Referrals are mainly through the criminal justice system and all patients have long-term substance and antisocial behavior problems that prompted criminal justice involvement. The program requires abstinence from drugs, alcohol, and nicotine. On average, these patients have been abstinent from substances for more than one year. Once patients are admitted to the treatment program, abstinence is monitored and enforced through close observations and random urinalysis tests. The inclusion criterion was dependence on psycho-stimulants based on DSM-IV criteria (4th ed.; American Psychiatric Association, 1994). Patients were then subdivided into one of two groups: 26 patients met lifetime DSM-IV criteria for pathological gambling (SDPG group 18M/8F, Mage=35.6, SD=6.8) and 70 did not (SD group, 38M/32F, Mage=34.3, SD=8.0). Self-reported abstinence was, on average, 1.4 years. All patients also met criteria for Antisocial Personality Disorder.
Controls
Ninety-two controls (55M/37F, Mage=33.4, SD=9.3) were recruited from the community through newspaper ads, flyers, a marketing company, and a database of community members interested in participating in research. Controls were excluded if they met criteria for pathological gambling or dependence on any drug or alcohol. Smoking was not exclusionary.
Exclusions for all participants were history of head trauma with loss of consciousness exceeding 15 minutes, neurological illness, schizophrenia, bipolar disorder, or major depression, and IQ < 80 (Wechsler Abbreviated Scale of Intelligence, 2-subtest version; Psychological Corporation).
All subjects were reimbursed $65 USD and were paid the same amount. All subjects provided written informed consent approved by the Colorado Multiple Institutional Review Board.
Structured Interviews
All interviews and assessments were administered by trained lay personnel on two separate days. On the first day subjects were given CIDI-SAM, DIS-IV, BIS-11, delay discount task, and BART. On the second day, separated by about two weeks, subjects performed the modified IGT.
Composite International Diagnostic Interview-Substance Abuse Module (CIDI-SAM): This computerized structured interview was administered to patients and controls to provide dependence diagnoses on eleven substances (amphetamines, cocaine, marijuana, alcohol, tobacco, hallucinogens, opioids, inhalants, sedatives, club drugs, and PCP) and to ensure that controls did not meet dependence criteria on substances.
Diagnostic Interview Schedule – Version IV (DIS-IV): This computerized structured interview provides diagnostic and symptom information about psychiatric diagnoses according to the DSM-IV. The module for pathological gambling was used for inclusion/exclusion into the SDPG group. Controls were excluded if they met criteria for pathological gambling. Modules were administered to exclude participants with schizophrenia, bipolar disorder, or current major depression, as well as to test for the presence of antisocial personality.
Behavioral Tests
Decision-making
Modified Iowa Gambling Task (mIGT; Thompson et al., 2012): To measure decision-making we administered the modified IGT. Data, not including computational parameters, on 31% (58 of 188) of the participants have been published (Thompson et al., 2012). Participants were shown four decks of cards and instructed to win as much money as possible. For each trial, a deck was selected by the computer and the subject chose to “Play” or “Pass” by pushing one of two response buttons. If the subject chose “Play” the outcome was a single positive or negative monetary value, along with the running total. If he/she chose “Pass” the running total remained the same. The decks were balanced on the frequency and magnitude of wins and losses. The dependent measure of overall performance was the total passes on bad decks (Thompson et al., 2012). To further investigate cognitive processes underlying overall performance, data were analyzed with a computational model of expected valence (Stout et al., 2004). This model estimates three parameters: sensitivity to loss relative to win (ω), updating (α), and response consistency (θ). The expectancy valence model has been previously adapted to the modified IGT Details of the model can be found in Tanabe et al. (2013).
Impulsivity
Barratt Impulsiveness Scale (BIS-11; Patton, Stanford, & Barratt, 1995): The BIS-11 is a 30-item self-report questionnaire that measures multiple facets of impulsivity. Participant state how often phrases describing aspects of impulsivity pertain to themselves along a 4 point Likert-like scale. The BIS-11 has been shown to be a reliable measure of impulsiveness (Patton et al., 1995; Stanford et al., 2009).
Delay Discounting: As a measure of temporal impulsivity, participants completed a computerized discounting task in which they chose between a hypothetical $1000 reward at some time in the future or a lesser amount now. There were seven delays ranging from 1 day to 10 years and 30 possible immediate amounts ranging from $1 to $999 (Green et al., 1996). We considered removing non-systematic data as recommended by Johnson and Bickel (2008) and determined that 57 participants would be excluded by their criteria (Johnson & Bickel, 2008). Therefore to avoid potential bias by omitting or poorly fitting approximately one-third of our data, we computed area under the discounting curve (AUC) for each participant’s response trajectory. The AUC approach avoids assuming that data are fit by a hyperbolic or other function (Myerson, Green, & Warusawitharana, 2001) and has been used in populations similar to ours (Ledgerwood et al., 2009). Secondarily, we estimated and plotted discounting rates from averaging hyperbolic curves fit to each participant.
Risk-taking
Balloon Analogue Risk Task (Lejuez et al., 2002): BART is a computerized task in which participants earn hypothetical money by incrementally increasing the size of a balloon. If the balloon “pops” earnings for that balloon are lost. Each trial requires a decision between increasing earnings versus “collecting” money already earned. The dependent variable was average number of pumps, excluding balloons that popped (Lejuez et al., 2002).
Drug use severity
CIDI-SAM assesses four abuse and seven dependence symptoms for each of the eleven substances tested. Drug severity was determined using a dimensional approach by adding abuse and dependence symptom counts across all drugs (Gelhorn et al., 2008; Hartman et al., 2008).
Data analysis
Dependent variables were inspected for normality. For normally-distributed variables, one-way ANCOVAs, adjusted for education (which differed between controls and patients but not between SD and SDPG), were performed with post-hoc comparisons between each two group combination (e.g. SD vs. SDPG) when indicated by a significant group effect. Categorical variables were analyzed with chi-square and Fisher Exact tests, as recommended (Campbell, 2007). For variables that were not approximately normally distributed, Kruskal-Wallis tests were performed and when the group effect was significant, post-hoc comparisons between each two-group combination were conducted with Mann-Whitney U tests. When normally-distributed variables demonstrated non-homogenous variability, a reciprocal transformation was performed.
We compared drug severity between SD and SDPG using an independent t-test. Drug severity was correlated with other variables using Pearson’s R for parametric and Spearman’s rho for non-parametric variables. Analyses were conducted using SPSS 20 (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp).
Results
Demographics
Demographic data are shown in Table 1. Sex and age did not differ by group, but education [F(2,186)=30.3, p<0.001] and IQ [F(2,186)=8.5, p<0.001] did. Controls had more education than SD (controls 14.1±1.9; SD 12.2±2.1, p<0.001) and SDPG (SDPG 11.3±1.9, p<0.001). There was no difference in education between SD and SDPG (p=0.11). Controls had higher IQ than SD (controls 108.0±12.5; SD 101.2±10.1, p<0.001) and SDPG (SDPG 101.1±10.0, p=0.02). There was no difference in IQ between SD and SDPG (p=1.00). Because of these group differences education was entered as a covariate in the analyses that included the control group (i.e. ANCOVAs), while IQ was not because it strongly correlated with education.
Table 1.
Means and Standard Deviations for demographic data, drug dependence, and abstinence.
| Controls N=92 |
SD N=70 |
SDPG N=26 |
p-value | |
|---|---|---|---|---|
| Sex | 55M/37F | 38M/32F | 18M/8F | ns |
| Age | 33.4 ± 9.3 | 34.3 ± 8.0 | 35.6 ± 6.8 | ns |
| Educationa | 14.1 ± 1.9 | 12.2 ± 2.1 | 11.3 ± 1.9 | <0.001 |
| IQa | 108.0 ± 12.5 | 101.2± 10.1 | 101.1 ± 10.0 | <0.001 |
| Stimulants | 0 (0%) | 70 (100%) | 26 (100%) | ns |
| Amphetamines | 0 (0%) | 53 (76%) | 24 (93%) | ns |
| Cocaine | 0 (0%) | 41 (59%) | 20 (77%) | ns |
| Tobacco | 15 (16%) | 53 (76%) | 22 (85%) | ns |
| Alcohol | 0 (0%) | 40 (57%) | 16 (62%) | ns |
| Cannabis | 0 (0%) | 19 (27%) | 15 (58%) | <0.05 |
| Opioids | 0 (0%) | 13 (19%) | 9 (35%) | ns |
| Hallucinogens | 0 (0%) | 3 (4%) | 4 (15%) | ns |
| Club Drugs | 0 (0%) | 3 (4%) | 3 (12%) | ns |
| Sedatives | 0 (0%) | 2 (3%) | 0 (0%) | ns |
| PCP | 0 (0%) | 1 (1%) | 0 (0%) | ns |
| Inhalants | 0 (0%) | 0 (0%) | ns | |
| Abstinence (years) |
1.2 ± 0.9 | 1.7 ± 1.7 | ns |
Controls differ from SD and SDPG. No difference between SD and SDPG.
Decision-making performance
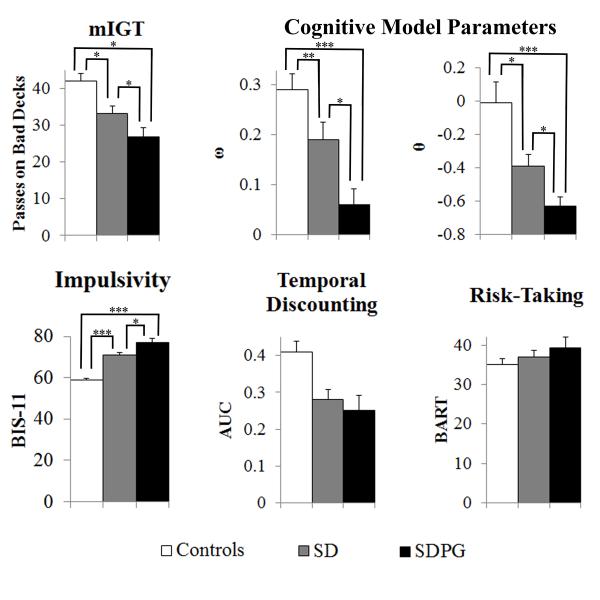
One-way ANCOVA on Passing on Bad Decks revealed a significant difference across all groups [F(2,156)=6.78, p=0.002]. Post-hoc analysis revealed that controls passed on bad decks more than SD (controls 42.01±18.3; SD 33.30±15.1, p=0.01) and SDPG (SDPG 26.85±11.2, p<0.001). SD passed more on the bad decks than SDPG (p=0.04).
Decision-making computational parameters (ω, θ, α)
The Kruskal-Wallis test revealed a significant group differences [H(2,157)=17.63, p<0.001] in sensitivity to loss (ω). Post-hoc analysis revealed significant differences between controls and SD (controls 0.29±0.28; SD 0.19±0.27, p=0.01), between controls and SDPG (SDPG 0.06±0.14, p<0.001), and between SD and SDPG (p=0.03). Controls were most sensitive to loss, followed by SD, and then followed by SDPG. The Kruskal-Wallis test revealed a significant group difference [H(2,157)=16.01, p<0.001] in response consistency (θ). Post-hoc analysis revealed significant differences between controls and SD (controls -0.01±1.1; SD -0.39±0.53, p=0.02), between controls and SDPG (SDPG -0.63±0.23, p<0.001), and between SD and SDPG (p=0.03). Controls had the highest response consistency, followed by SD, then followed by SDPG. No significant group differences were found in the update parameter (α).
Barratt Impulsiveness Scale
ANCOVA revealed a significant group difference [F(2,185)=56.59, p<0.001] on the Barratt Impulsiveness Scale. Post-hoc analysis revealed controls reported less impulsivity than both SD (controls 58.97±7.3; SD 70.89±10.8, p<0.001) and SDPG (SDPG 77.23±10.3, p<0.001). SD reported significantly less impulsivity than SDPG (p=0.01) (Table 2; Figure 1).
Table 2.
Behavioral and cognitive measures
| Controls | SD | SDPG | P-Value | |
|---|---|---|---|---|
| Impulsivity | ||||
| BIS-11a | 57.8 ± 7.8 | 70.5 ± 11.4 | 77.1 ± 11.0 | <0.001 |
| Delay Discount | 0.41 ± 0.27 | 0.28 ± 0.24 | 0.25 ± 0.21 | 0.09 |
| Risk taking | ||||
| BART | 35.1 ± 14.3 | 37.0 ± 13.9 | 39.4 ± 13.7 | 0.17 |
| Drug severityb | - | 25.49 ± 10.9 | 35.27 ± 12.4 | <0.001 |
| Decision-making | ||||
| Pass bada | 42.01 ± 18.3 | 33.30 ± 15.1 | 26.85 ± 11.2 | 0.02 |
| ω a | 0.29 ± 0.28 | 0.19 ± 0.27 | 0.06 ± 0.14 | <0.001 |
| α | 0.12 ± 0.15 | 0.12 ± 0.14 | 0.13 ± 0.13 | 0.40 |
| θ a | −0.01 ± 1.1 | −0.39 ± 0.53 | −0.63 ± 0.23 | <0.001 |
all pair-wise groups differ significantly
SD and SDPG differ significantly
All p-values are adjusted for education
Figure 1.
Behavioral and cognitive measures across all groups, *p<0.05, **p<0.01, ***p<0.001
Delay Discounting
AUC did not differ by group [F(2,185)=7.57, p=0.09], but the trend suggested that controls (0.41±0.27) discounted less than either SD (0.28±0.24) or SDPG (0.25±0.21). Comparing all substance users (SD+SDPG) with controls showed that substance users discounted at a more rapid rate (p=0.03, adjusting for education). Figure 2 shows discounting curves for each group, estimated from averaging hyperbolic curves fit to each subject.
Figure 2.
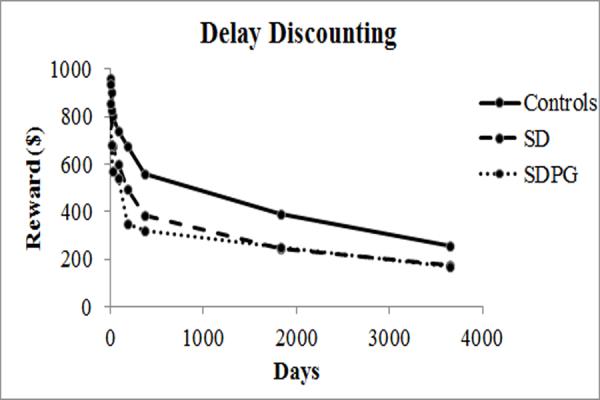
Delay discounting curves for controls, SD, and SDPG.
Balloon Analogue Risk Task
There were no significant group differences in average number of pumps, excluding balloons that popped (Controls: 35.1±14.3; SD: 37.0±13.9; SDPG: 39.4±13.7, p=0.17) (Table 2; Figure 1).
Drug severity and correlation with dependent variables
Compared to SD, SDPG had significantly greater drug severity (SDPG 35.27±12.4, SD 25.49±10.9, p<0.001; Table 2). Across all substance users, drug severity correlated significantly with BIS-11 scores (r=0.27, p=0.01; Figure 3). Drug severity did not correlate with other variables (i.e., BART, Delay Discount, ω, α, and θ).
Figure 3.
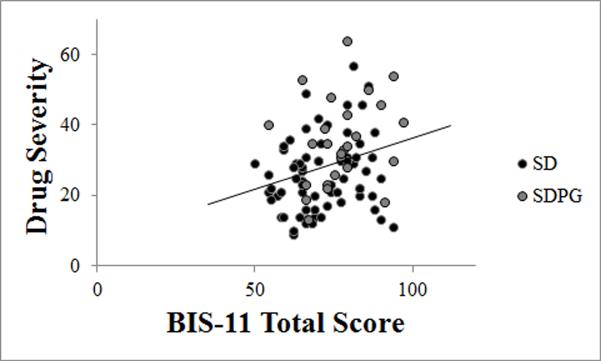
Correlation between drug severity and BIS-11 in all substance users (r=0.27, p=0.01).
Discussion
Compared to SD, SDPG had poorer decision-making driven by low sensitivity to loss and response inconsistency. Second, compared to SD, SDPG reported greater impulsivity and greater drug severity. These results suggest that among substance users, a co-occurring diagnosis of pathological gambling may be a marker for greater deficits in decision-making and more drug-related symptoms.
We found significant differences between all three groups on decision-making performance. There was a stepwise increase in passing on bad cards on the modified Iowa Gambling Task (mIGT) in SDPG, SD, and controls, respectively. Prior work has shown impaired passing on bad decks in substance dependent individual compared to control (Thompson et al., 2012). We extend that work by showing for the first time that co-occurring PG further impairs performance. A caveat is that these samples partially overlapped. The current sample size of 188 participants has greater power to examine the effect of PG compared to the prior study of 58 participants.
To evaluate the cognitive processes that underlie decision-making, we implemented a computational model of the Iowa Gambling Task (Stout et al., 2004) and found that the groups differed significantly on sensitivity to loss relative to gain. Controls were the most influenced by losses followed by SD, followed by SDPG who were the least influenced by losses. This same pattern was observed for response consistency which indicates the extent to which an individual's responses reflect his/her expectations over time. Controls showed the greatest response consistency, followed by SD, followed by SDPG. In contrast, no group difference was found on the third parameter, α, which reflects updating expectancies based on prior trials. Our results are consistent with Stout et al. (2004) who reported lower sensitivity to loss and less consistent responses, while updating did not differ, in cocaine users compared to controls (Stout et al., 2004; Stout et al., 2005). We extend those results and suggest that compared to substance dependence alone, those with co-occurring pathologic gambling are even less sensitive to loss and less consistent in their decision-making choices.
We found significant differences between all three groups on self-reported impulsivity, increasing in score from controls to SD to SDPG, consistent with some (Petry & Casarella, 1999; Andrade & Petry, 2012) but not all prior studies (Tanabe et al., 2007; Ledgerwood et al., 2009; Stea, Hodgins, & Lambert, 2011). The most notable difference between our study and those that found no difference is in the criteria for gambling and substance use problems. Previous work utilized the South Oaks Gambling Screen (SOGS), which is a 16-item self-report questionnaire. Although widely used and easily administered, SOGS is a screening tool and not a diagnostic instrument, and therefore may result in false positives (Lesieur & Blume, 1987; Gambino, 1997). Our findings of increased impulsivity in SDPG may be related to greater specificity of DSM-IV.
Drug severity was measured using a dimensional approach (Gelhorn et al., 2008; Hartman et al., 2008) by calculating total symptom count across drugs. Compared to SD, SDPG had significantly greater drug severity, in spite of the fact that, except for cannabis, dependence diagnoses were not significantly different between the groups. In addition, impulsivity correlated with drug severity and is consistent with the notion that greater impulsivity is associated with a worse clinical course. While our study cannot determine causality (e.g. impulsivity may lead to greater drug severity and pathological gambling; alternatively greater drug exposure may induce more impulsivity and lead to pathological gambling), our SD and SDPG patients had been abstinent from drug use for a prolonged period, approximately 1.4 years. This indicates that regardless of the causal relationships, high levels of impulsivity are likely to persist even with sustained full remission in a controlled environment. Our data support a recommendation to assess for co-morbid pathological gambling in substance abuse treatment populations, given the very high rates of co-morbidity and because SDPG individuals differ from SD patients in clinical meaningful ways. A co-occurring diagnosis of pathological gambling should raise the clinician's concern for very high levels of impulsiveness that may require more intensive intervention. As new treatments to improve forms of impulsiveness are developed, this patient population may particularly benefit.
We demonstrate two negative findings that require comment. While other studies have shown higher discounting of delayed rewards in substance users and/or pathological gamblers compared to controls (Petry & Casarella, 1999; Leeman & Potenza, 2012), our results demonstrated only a non-significant trend for group differences in AUC. The lack of a statistical difference across the 3 groups may reflect differences in demographics. Education and IQ were well matched between our SD and SDPG groups. Petry and Casarella (1999) did not adjust for a 15-point difference in IQ between non-problem gamblers with and without substance abuse. If we do not adjust for education, our group difference in AUC is significant (p=0.001), suggesting that education is an important factor in discounting. Moreover, a comparison of controls to all substance users (e.g., SD + SDPG) revealed significantly lower discounting by controls (p=0.03). Inconsistent results may reflect differences in delay discounting tasks and analyses (Reynolds, 2006). Previous studies compared groups by fitting hyperbolic curves to estimate discounting rates. This analysis assumes that the data fit a hyperbolic curve, though this assumption is not necessarily true. Johnson and Bickel (2008) devised a method for removing data that violated this assumption (Johnson & Bickel, 2008). This method would have excluded nearly one-third of our data, suggesting that discounting in our participants did not meet this assumption. Thus, our primary analysis measured AUC which does not assume the data are hyperbolic. Ledgerwood et al. (2006) also found no difference between individuals with substance use and gambling compared to individuals with gambling problems using AUC (Ledgerwood et al., 2009).
We found no group differences on BART, a measure of risk-taking. Other studies have also shown mixed results using this task in patients with substance use disorders (Lejuez et al., 2003; Crowley et al., 2006) and pathological gambling (Ledgerwood et al., 2009). Not providing real monetary rewards for behavior on the BART may have reduced the sensitivity of this task.
Patients with substance dependence and pathological gambling share symptoms of tolerance, withdrawal, repeated attempts to stop, and continued engagement despite long-term negative consequences (Leeman & Potenza, 2012). Here we show that patients with both diagnoses demonstrate even greater decision-making impairment, impulsiveness, and drug use severity. The decision-making impairment may be driven by greater insensitivity to loss and choice inconsistency. It is possible that both disorders are manifestations of an underlying behavioral disinhibitory trait. Traits such as antisocial behavior, impulsivity, risk-taking, and issues in personality and temperament may signify an overall problem in behavioral control. For example, early problems with behavioral control have predicted adult SD risk (Moffitt et al., 2011) and under-controlled temperament in children has been shown to predict gambling problems later in life (Slutske et al., 2012).
Our study was limited by the lack of a group with pathological gambling without substance dependence. The SDPG group was relatively small compared to the other groups; however, it is representative of gambling disorders in patients with substance dependence. Pathological gambling is relatively common among substance dependent patients in treatment, 27% in this study, slightly lower than 38% as reported by some (Petry et al., 2005; Agrawal et al., 2007; el-Guebaly et al., 2012) and much more common than 3% in the general population (National Research Council 1999; Petry et al., 2005; Topf et al., 2009). Unequal group size can affect the homogeneity of the sample. To prevent violating assumptions for parametric analyses, nonparametric analyses were utilized when necessary and variables were transformed to homogenize variability among groups. Welch’s Fs were also calculated, though this did not change the results at all.
In conclusion, individuals with substance dependence and pathological gambling have poorer decision-making, driven by low sensitivity to loss and response inconsistency, than substance dependent individuals without pathological gambling. These characteristics are measurable in the laboratory, appear to persist even after years of abstinence, and suggest targets at which to direct treatment. Furthermore, pathological gambling is prevalent among substance dependent individuals in treatment and associated with more drug-related symptoms. Our results suggest that when evaluating patients with substance use, clinicians should also consider diagnostic assessments for gambling problems.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the clients and staff at the Addiction Research Treatment Services (ARTS), University of Colorado School of Medicine and Jeremy Reynolds. PhD for helping with the model. This project was funded by grants DA024104 and DA02774
Funding: National Institute of Drug Abuse DA024104 and DA02774.
Footnotes
Conflict of Interest
Drs. Sakai and Tanabe each received reimbursement in 2012 for completing policy review for the WellPoint Office of Medical Policy & Technology Assessment (OMPTA), WellPoint, Inc., Thousand Oaks, CA. Dr. Sakai also serves as a board member of the ARTS Foundation. All other authors declare they have no conflicts of interests.
References
- Agrawal A, Lynskey MT, Madden PAF, Bucholz KK, Heath AC. A latent class analysis of illicit drug abuse/dependence: results from the National Epidemiological Survey on Alcohol and Related Conditions. Addiction. 2007;102(1):94–104. doi: 10.1111/j.1360-0443.2006.01630.x. doi:10.1111/j.1360-0443.2006.01630.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- Andrade LF, Petry NM. Delay and probability discounting in pathological gamblers with and without a history of substance use problems. Psychopharmacol. 2012;219(2):491–499. doi: 10.1007/s00213-011-2508-9. doi:10.1007/s00213-011-2508-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol. 1994;2(3):244–268. doi:10.1037/1064-1297.2.3.244. [Google Scholar]
- Bechara A. Risky business: emotion, decision-making, and addiction. J Gambl Stud. 2003;19(1):23–51. doi: 10.1023/a:1021223113233. doi:10.1023/A:1021223113233. [DOI] [PubMed] [Google Scholar]
- Campbell I. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat Med. 2003;26(19):3661–3675. doi: 10.1002/sim.2832. doi:10.1002/sim.2832. [DOI] [PubMed] [Google Scholar]
- Cowlishaw S, Merkouris S, Chapman A, Radermacher H. Pathological and problem gambling in substance use treatment: a systematic review and meta-analysis. J Subst Abuse Treat. 2104;46(2):98–105. doi: 10.1016/j.jsat.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Crowley TJ, Raymond KM, Mikulich-Gilbertson SK, Thompson LL, Lejuez CW. A risk-taking “set” in a novel task among adolescents with serious conduct and substance problems. J Am Acad Child Adolesc Psychiatry. 2006;45(2):175–183. doi: 10.1097/01.chi.0000188893.60551.31. doi:10.1097/01.chi.0000188893.60551.31. [DOI] [PubMed] [Google Scholar]
- Dayan J, Bernard A, Olliac B, Mailhes A-S, Kermarrec S. Adolescent brain development, risk-taking and vulnerability to addiction. J Physiol. 2010;104(5):279–286. doi: 10.1016/j.jphysparis.2010.08.007. doi:10.1016/j.jphysparis.2010.08.007. [DOI] [PubMed] [Google Scholar]
- el-Guebaly N, Mudry T, Zohar J, Tavares H, Potenza MN. Compulsive features in behavioural addictions: the case of pathological gambling. Addiction. 2012;107(10):1726–1734. doi: 10.1111/j.1360-0443.2011.03546.x. doi:10.1111/j.1360-0443.2011.03546.x (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambino B. The correction for bias in prevalence estimation with screening tests. J Gambl Stud. 1997;13(4):343–351. doi: 10.1023/a:1024971521887. [DOI] [PubMed] [Google Scholar]
- Gelhorn H, Hartman C, Sakai J, et al. Toward DSM-V: an item response theory analysis of the diagnostic process for DSM-IV alcohol abuse and dependence in adolescents. J Am Acad Child Adolesc Psychiatry. 2008;47(11):1329–1339. doi: 10.1097/CHI.0b013e318184ff2e. doi:10.1097/CHI.0b013e318184ff2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J, Lichtman D, Rosen S, Fry A. Temporal discounting in choice between delayed rewards: the role of age and income. Psychol Aging. 1996;11(1):79–84. doi: 10.1037//0882-7974.11.1.79. [DOI] [PubMed] [Google Scholar]
- Hartman CA, Gelhorn H, Crowley TJ, et al. Item response theory analysis of DSM-IV cannabis abuse and dependence criteria in adolescents. J Am Acad Child Adolesc Psychiatry. 2008;47(2):165–173. doi: 10.1097/chi.0b013e31815cd9f2. doi:10.1097/chi.0b013e31815cd9f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp . Released 2011. IBM SPSS Statistics for Windows. IBM Corp; Armonk, NY: Version 20.0. [Google Scholar]
- Johnson MW, Bickel WK. An algorithm for identifying nonsystematic delay discounting data. Exp Clin Psychopharmacol. 2008;16(3):264–274. doi: 10.1037/1064-1297.16.3.264. doi:10.1037/1064-1297.16.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood DM, Alessi SM, Phoenix N, Petry NM. Behavioral assessment of impulsivity in pathological gamblers with and without substance use disorder histories versus healthy controls. Drug Alcohol Depend. 2009;105(1-2):89–96. doi: 10.1016/j.drugalcdep.2009.06.011. doi:10.1016/j.drugalcdep.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Potenza MN. Similarities and differences between pathological gambling and substance use disorders: a focus on impulsivity and compulsivity. Psychopharmacol. 2012;219(2):469–490. doi: 10.1007/s00213-011-2550-7. doi:10.1007/s00213-011-2550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Zvolensky MJ, Pedulla CM. Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. J Adolesc. 2003;26(4):475–479. doi: 10.1016/s0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Magidson JF, Mitchell SH, Sinha R, Stevens MC, de Wit H. Behavioral and biological indicators of impulsivity in the development of alcohol use, problems, and disorders. Alcohol Clin Exp Res. 2010;34(8):1334–1345. doi: 10.1111/j.1530-0277.2010.01217.x. doi:10.1111/j.1530-0277.2010.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, et al. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J Exp Psychol. Applied. 2002;8(2):75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Lesieur HR, Blume SB. The South Oaks Gambling Screen (SOGS): A new instrument for the identification of pathological gamblers. Am J Psychiatry. 1987;144(9):1184–1188. doi: 10.1176/ajp.144.9.1184. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Belsky D, et al. A gradient of childhood self-control predicts health, wealth, and public safety. Proc Natl Acad Sci U S A. 2011;108(7):2693–2698. doi: 10.1073/pnas.1010076108. doi:10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76(2):235–243. doi: 10.1901/jeab.2001.76-235. doi:10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psycho. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. doi:10.1002/1097 4679(199511)51:6<768::AIDJCLP2270510607>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Petry NM. Pathological gamblers, with and without substance use disorders, discount delayed rewards at high rates. J Abnorm Psychol. 2001;110(3):482–487. doi: 10.1037//0021-843x.110.3.482. [DOI] [PubMed] [Google Scholar]
- Petry NM, Casarella T. Excessive discounting of delayed rewards in substance abusers with gambling problems. Drug Alcohol Depend. 1999;56(1):25–32. doi: 10.1016/s0376-8716(99)00010-1. doi:10.1016/S0376-8716(99)00010-1. [DOI] [PubMed] [Google Scholar]
- Petry NM, Stinson FS, Grant BF. Comorbidity of DSM-IV Pathological Gambling and Other Psychiatric Disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2005;66(5):564–574. doi: 10.4088/jcp.v66n0504. doi:10.4088/JCP.v66n0504. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Steinberg MA, Wu R. Characteristics of gambling helpline callers with self-reported gambling and alcohol use problems. J Gambl Stud. 2005;21(3):233–254. doi: 10.1007/s10899-005-3098-4. doi:10.1007/s10899-005-3098-4. [DOI] [PubMed] [Google Scholar]
- Poulos CX, Le AD, Parker JL. Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol. 1995;6(8):810–814. [PubMed] [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behav Pharmacol. 2006;17(8):651–667. doi: 10.1097/FBP.0b013e3280115f99. doi:10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, et al. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacol. 20(4):322–339. doi: 10.1016/S0893-133X(98)00091-8. 1119. doi:10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Moffitt TE, Poulton R, Caspi A. Undercontrolled temperament at age 3 predicts disordered gambling at age 32: a longitudinal study of a complete birth cohort. Psychol Sci. 2012;23(5):510–516. doi: 10.1177/0956797611429708. doi:10.1177/0956797611429708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. Fifty years of the Barratt Impulsiveness Scale: An update and review. Personality and Individual Differences. 2009;47(5):385–395. [Google Scholar]
- Stea JN, Hodgins DC, Lambert MJ. Relations between delay discounting and low to moderate gambling, cannabis, and alcohol problems among university students. Behav Process. 2011;88(3):202–205. doi: 10.1016/j.beproc.2011.09.002. doi:10.1016/j.beproc.2011.09.002 (2011) [DOI] [PubMed] [Google Scholar]
- Stout JC, Busemeyer JR, Lin A, Grant SJ, Bonson KR. Cognitive modeling analysis of decision-making processes in cocaine abusers. Psychon Bull Rev. 2004;11(4):742–747. doi: 10.3758/bf03196629. [DOI] [PubMed] [Google Scholar]
- Stout JC, Rock SL, Campbell MC, Busemeyer JR, Finn PR. Psychological processes underlying risky decisions in drug abusers. Psychol Addict Behav. 2005;19(2):148–157. doi: 10.1037/0893-164X.19.2.148. doi:10.1037/0893-164X.19.2.148. [DOI] [PubMed] [Google Scholar]
- Tanabe J, Reynolds J, Krmpotich T, et al. Reduced neural tracking of prediction error in substance-dependent individuals. Am J Psychiatry. 2013;170(11):1356–63. doi: 10.1176/appi.ajp.2013.12091257. doi:10.1176/appi.ajp.2013.12091257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J, Thompson L, Claus E, Dalwani M, Hutchison K, Banich MT. Prefrontal cortex activity is reduced in gambling and nongambling substance users during decision-making. Hum Brain Mapp. 2007;28(12):1276–1286. doi: 10.1002/hbm.20344. doi:10.1002/hbm.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LL, Claus ED, Mikulich-Gilbertson SK, et al. Negative reinforcement learning is affected in substance dependence. Drug Alcohol Depend. 2012;123(1-3):84–90. doi: 10.1016/j.drugalcdep.2011.10.017. doi:10.1016/j.drugalcdep.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassini A, Struglia F, Spaziani D, Pacifico R, Stratta P, Rossi A. Decision making, impulsivity, and personality traits in alcohol-dependent participants. Am J Addic. 2012;21(3):263–267. doi: 10.1111/j.1521-0391.2012.00225.x. doi:10.1111/j.1521-0391.2012.00225.x. [DOI] [PubMed] [Google Scholar]
- Topf JL, Yip SW, Potenza MN. Pathological gambling: biological and clinical considerations. J Addict Dis. 2009;3(3):111–119. doi: 10.1097/ADM.0b013e31819b7bff. doi:10.1097/ADM.0b013e31819b7bff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yechiam E, Busemeyer JR, Stout JC, Bechara A. Using cognitive models to map relations between neuropsychological disorders and human decision-making deficits. Psychol Sci. 2005;16(12):973–978. doi: 10.1111/j.1467-9280.2005.01646.x. doi:10.1111/j.1467-9280.2005.01646.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.