Abstract
Accumulation of unfolded or misfolded proteins in the endoplasmic reticulum (ER) leads to ER stress, which is characteristic of cells with high level of secretory activity and implicated in a variety of disease conditions. In response to ER stress, the cell elicits an adaptive process called the Unfolded Protein Response (UPR) to support cellular homeostasis and survival. However, prolonged and unsolvable ER stress also induces apoptosis. As the most conserved signaling branch of the UPR, the IRE1α-XBP1 pathway plays important roles in both physiological and pathological settings and its activity has profound effects on disease progression and prognosis. Recently, modulating this pathway with small molecule compounds has been demonstrated as a promising approach for disease therapy. In this review, we summarize a list of current investigational compounds targeting this pathway and their therapeutic features for treating human diseases.
1. Introduction
Controlling a critical step along the secretory pathway, the endoplasmic reticulum (ER) is the central organelle where newly synthesized proteins mature and are properly folded. A variety of stresses, including increased cellular demands of secretory protein production, glucose deprivation, hypoxia, and redox perturbation, causes accumulation of unfolded or misfolded proteins inside the ER. Collectively, we call these conditions as ER stress. In response to ER stress, the cell initiates a series of adaptive signaling pathways, referred to as the unfolded protein response (UPR), in order to restore protein folding homeostasis. The UPR actively reduces protein translation, increases expression of ER chaperones and enzymes facilitating protein folding, and clears misfolded proteins for degradation [1]. However, under prolonged ER stress, homeostasis cannot be restored and the UPR also induces cell death through apoptosis [2]. A number of specialized secretory cells, such as plasma cells or pancreatic β cells, rely upon the UPR for normal physiologic function because of the increased demand for protein synthesis and secretion [3].
In mammalian cells, the UPR consists of 3 primary signaling pathways. Each pathway initiates with an ER membrane-bound protein that senses the accumulation of unfolded or misfolded proteins and activates a b-ZIP (Basic Leucine Zipper domain) transcription factor. The 3 sensor protein-transcription factor pairs are (i) inositol requiring kinase 1α (IRE1α) and X-box binding protein-1 (XBP1), (ii) eukaryotic translation initiation factor 2-alpha kinase 3 (PERK) and activating transcription factor 4 (ATF4), and (iii) activating transcription factor 6 (ATF6), which serves as both a sensor and transcription factor [1]. Target genes of the IRE1α-XBP1 branch of the UPR are involved in lipid synthesis, ER-associated protein degradation (ERAD), protein folding, translocation to ER and secretion. All of these activities are characteristic of active secretory cells. The PERK-eIF2α pathway regulates a global decrease in protein translation and reduces protein flux into the ER. Paradoxically, activation of PERK and eIF2α phosphorylation also promotes translation of mRNAs with short open reading frames in the 5’-untranslated regions, including ATF4. ATF4 transactivates target genes involved in redox processes, amino acid metabolism, ER chaperones and foldases [4, 5]. ATF4 also regulates expression of pro-apoptotic genes like CHOP (C/EBP-homologous protein) [6] and GADD34 (growth arrest and DNA damage-inducible 34) [7]. The transcriptional program regulated by ATF6 is generally geared to increase the protein folding capacity of the ER, but there is considerable overlap between the target genes regulated by the other branches of the UPR [1].
2. Molecular mechanisms of the IRE1α-XBP1 pathway
Mammalian IRE1 has two isoforms - IRE1α and IRE1β, which are encoded by different genes (ERN1 and ERN2 in humans, respectively) [8]. While IRE1α is ubiquitously expressed, expression of IRE1β is limited to the epithelial cells of the gastrointestinal track [8, 9]. At the molecular level, IRE1α is a type I transmembrane protein with dual enzymatic activities, consisting of an N-terminal ER luminal domain (IRE1-LD) and a serine/threonine kinase domain plus a C-terminal ribonuclease (RNase) domain located on the cytosolic side of the protein. Upon accumulation of unfolded/misfolded proteins in the ER, IRE1α dimerizes and oligomerizes while stimulating trans-autophosphorylation, leading to activation of the RNase domain [10, 11]. The exact mechanism of IRE1 activation by unfolded/misfolded proteins is not entirely clear. In one model, activation of IRE1 is mediated through competitive binding of unfolded proteins to ER-resident chaperone binding immunoglobulin (BiP), which in the absence of unfolded proteins associates with the IRE1-LD to keep it in an inactive state. When levels of unfolded proteins increase, BIP dissociates from IRE1-LD and associates with unfolded proteins, freeing IRE1 to dimerize/oligomerize, which leads to the activation of IRE1 [12–14]. Paradoxically, however, the deletion of the KAR2/BiP binding site from yeast IRE1-LD did not result in constitutive activation of IRE1 [14]. Furthermore, crystal structure analyses of both yeast and mammalian IRE1 revealed a similarity to the peptide binding domain of MHC-I molecule [13, 15]. The subsequent study showed that IRE1-LD could bind directly to certain peptides [16, 17]. These experiments suggested the presence of an additional activation step(s) such as binding of unfolded proteins themselves to IRE1-LD. Once activated, IRE1 becomes an active kinase and autophosphorylates themselves. At this point, however, no other substrates of the IRE1 kinase have been identified. Autophosphorylation of the kinase domain and binding of ADP (or ATP in vivo) allosterically regulates dimerization/oligomerization and leads to activation of IRE1 RNase domain [18, 19].
Activated IRE1α, through the RNase domain, excises an intron from the XBP1 mRNA in metazoans (and HAC1 mRNA in yeast), which causes a translational frame shift that results in the production of the spliced/activated form of XBP1 protein in metazoans (and HAC1 in yeast), an active transcription factor responsible for the induction of a specific set of target genes [20]. Ligation of the spliced intron is mediated through tRNA ligase in yeast [21] and the RTCB/archease complex in metazoans [22].
The unconventional cleavage of an intron from the inactive form of XBP1/HAC1 mRNA happens at a stem-loop structure [23–26]. Activated IRE1 also degrades ER-bound mRNAs through cleavage at both stem-loop sites and non-stem-loop sites, a process referred to as regulated Ire1-dependent decay (RIDD). RIDD may help to reduce the folding load of nascent proteins entering the ER and thus, further alleviating ER stress [27–29]. Using in vitro evidence, a recent study revealed that while oligomerization is required for XBP1/HAC1 mRNA cleavage, RIDD activity is retained with the IRE1 monomer/dimer [30]. This differential substrate preference may be translated into cell fate determination, as activation of XBP1 splicing by IRE1 promotes cell survival while activation of RIDD leads to cell death in vivo. However, depending upon different cell types or on the kinetic relationships and magnitude of activations between XBP1 splicing and RIDD in different tissues, ultimate output of either XBP1s or RIDD might differ.
Activated IRE1α also promotes apoptosis by activating apoptosis signal-regulating kinase 1 (ASK1) and JUN N-terminal kinase (JNK) through interaction with tumor necrosis factor (TNF) receptor-associated factor 2 (TRAF2) [31–33].
3. The IRE1α-XBP1 pathway in human diseases
Studies in animal models have revealed that the IRE1α-XBP1 pathway is involved in various human pathological conditions, including neurodegenerative diseases, inflammation, metabolic disorders, liver dysfunction, brain and heart ischemia, and cancer. Targeting this pathway has emerged as a promising therapeutic strategy against these diseases [3]. As more mechanistic data regarding the regulation of this pathway emerges, modulating this pathway through inhibition or activation will likely confer different clinical benefits depending on the context of the disease state. While this is not intended to be a comprehensive review, in the disease conditions described below we will highlight relevant human diseases in which modulation of the IRE1α-XBP1 pathway may lead to the development of novel therapies.
3.1. Neurodegenerative diseases
In mouse models of amyotrophic lateral sclerosis (ALS), XBP1 deficiency leads to augmented autophagy, which enhances clearance of the mutant superoxide dismutase-1 (SOD1) protein and decreases its toxicity [34]. Similarly, in a mouse model of Huntington’s disease, XBP1 deficiency also stimulates degradation of the mutant Huntington protein through autophagy and delays disease progression [35]. The same study revealed that XBP1 deficiency promotes autophagy by induction of FOXO1 expression, which encodes a key transcription factor regulating autophagy in neurons. In contrast, XBP1 is required for locomotor recovery after spinal cord injury (SCI) [36]. However, a study modeling prion-related disorders in mice showed that XBP1 is dispensable for disease progression [37]. In addition, XBP1 splicing was detected in mice experiencing cerebral ischemia [38]. In both drosophila and mammalian cell culture models of Alzheimer’s disease, XBP1 splicing was found to have neuroprotective effects [39]. In support of this concept, both elevated XBP1 splicing and IRE1α phosphorylation were detected in disease tissues of patients with Alzheimer’s disease [40, 41]. In another study using cell culture and mouse model of Parkinson’s disease, overexpression of spliced XBP1 demonstrated cytoprotection against 1-methyl-4-phenylpyridinium (MPP+) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced cell death [42]. Moreover, elevated levels of XBP1 were also detected in multiple sclerosis demyelinated lesions [43].
3.2. Inflammatory diseases
In a mouse model of inflammatory bowel disease induced by dextran sodium sulfate, IRE1β was identified as a critical protein that mitigates perturbations of ER function during disease progression [44]. Toll-like receptor 2 (TLR2) and TLR4 signaling induce IRE1α activation and XBP1 splicing, which is required for optimal production of proinflammatory cytokines in macrophages [45]. XBP1 is also essential for dendritic cell differentiation and survival [46].
3.3. Metabolic diseases
In liver cells, XBP1 regulates the expression of genes involved in fatty acid synthesis, including stearoyl-CoA desaturase-1 (SCD1), acetyl-CoA carboxylase 2 (ACC2) and diacyl glycerol acyl transferase 2 (DGAT2). Accordingly, mice with liver-specific deficiency of XBP1 have lower levels of triglyceride and cholesterol production and are free of hepatic steatosis (fatty liver) when fed high carbohydrate diet [47]. XBP1 deficiency has also been linked to induction of insulin resistance, and mice haploinsufficient for XBP1 develop hyperinsulinemia, hyperglycemia, impaired glucose and insulin tolerance, and increase in body weight [48]. Through direct interaction with FOXO1, activated XBP1 (XBP1s) alleviates hepatic insulin resistance [49]. In addition, XBP1 interacts with the regulatory subunits of phosphoinositide 3-kinase (PI3K), which increases XBP1 nuclear translocation, ER stress resolution, and insulin sensitization [50, 51].
3.4. Cancer
Several genomic screens have identified common mutations associated with IRE1α in human cancers [52–54]. The IRE1α-XBP1 pathway plays an indispensable role in tumor growth, metastatic progression and chemo-resistance [55]. Expression and activation of XBP1 correlates with clinical outcome in breast cancer [56, 57] and angiogenesis in pancreatic cancer [58]. Furthermore, tumor growth and survival under hypoxic conditions are severely compromised when XBP1 expression is blocked [58]. In mouse models of glioblastoma, IRE1α is required for upregulation of pro-inflammatory cytokines and angiogenic factors, which contributes to tumor growth, angiogenesis and invasiveness [59, 60]. XBP1 is crucial for development of terminally-differentiated plasma cells [61, 62] and is overexpressed in multiple myeloma (MM) [63, 64], a plasma cell malignancy. Several lines of evidence demonstrate that the IRE1α-XBP1 pathway is involved in the pathogenesis of multiple myeloma [65, 66]. Moreover, XBP1 has been implicated in the development of resistance to chemotherapy [64, 66]. However, more recent evidence suggests that XBP1 inactivation may contribute to bortezomib resistance [67], underscoring the complexity of its role in regulating this function.
4. IRE1α-XBP1 inhibitors and activators
Recently, several groups have identified small molecule inhibitors that selectively block IRE1α-XBP1 activation [68–73]. Two major sites on IRE1α have been identified as targets for developing inhibitors: the catalytic core of the RNase domain and the ATP binding site of the kinase domain. Small molecules targeting the RNase domain include salicylaldehydes [70], 4μ8C [71], MKC-3946 [69], STF-083010 [68], toyocamycin [72], and hydroxyl-aryl-aldehydes (HAA) [73]. Many of these compounds were identified utilizing different chemical screening strategies. The only compound reported to inhibit IRE1α-XBP1 by directly interfering with ATP binding in the IRE1α kinase domain is “Compound 3” [74]. Although there has not been an extensive effort to identify compounds that specifically activate IRE1α-XBP1, the flavonol quercetin was reported to activate the RNAase activity of IRE1α [19].
4.1. Salicylaldehydes
Through in vitro fluorescence quenching (FQ)-based high throughput screening strategy detecting the cleavage of Cy5-labeled XBP1 stem-loop RNA substrate by purified recombinant human IRE1α-cytosolic domain (hIRE1α-cyto, amino acids 462–977), salicylaldimines and their hydrolysis products, salicylaldehydes, were identified as inhibitors of the endoribonuclease activity of IRE1α [70]. These compounds inhibited yeast IRE1α, but not RNase L or the unrelated RNase A and T1. They also blocked chemically-induced XBP1 splicing and prevented induction of known XBP1 target genes in cultured cell lines. One potent non-competitive inhibitor of XBP1 activation, 3-ethoxy-5,6-dibromosalicylaldehyde, binds to IRE1α in a specific, reversible and dose-dependent fashion revealed by surface plasmon resonance analysis. Additional analysis revealed that these compounds do not inhibit autophosphorylation of IRE1α.
4.2. 4μ8C
High throughput screening using an in vitro fluorescent-based FRET-derepression assay with purified recombinant human IRE1α-cytosolic domain (amino acids 464–977) identified compound CB5305630, an 8-formyl-7-hydroxy-4-methylcoumarin conjugated to 2-aminopyridine via an aldimine. This compound was shown to be a noncompetitive inhibitor of the endoribonuclease activity of IRE1α, with an IC50 of 60 nM in the FRET-derepression assay [71]. In an aqueous environment, CB5305630 is hydrolyzed to generate the active component 8-formyl-7-hydroxy-4-methylcoumarin, which is referred to as 4µ8c. Interestingly, 4µ8c can also be categorized as a salicylaldehyde derivative and potentially has a similar mechanism of action as those described previously. Through HPLC and MALDI-TOF mass spectrometry, 4µ8c was shown to bind to K599 (in the kinase domain) and K907 (in the RNase domain) of IRE1α protein via Schiff-base formation, thus inhibiting both the kinase and RNase activity of IRE1α. 4µ8c was shown to inhibit chemically-induced XBP1 splicing by IRE1α and induction of XBP1 target genes, but not ATF4 regulated genes. Furthermore, 4µ8c did not block RNase L activity in vitro. 4µ8c blocked RIDD activity both in vitro and in cultured cells. However, inhibition of IRE1α activity by 4µ8c did not sensitize mammalian cells to chemically-induced ER stress, but this compound did affect the expansion of the cell’s secretory capacity. Recently, the structure and mechanism of action of this class of salicylaldehyde derivatives were further characterized through protein-compound co-crystallization studies, highlighting the interaction between these compounds and a shallow pocket around K907 of the IRE1α protein [73].
4.3. MKC-3946
Through chemical optimization of the salicylaldehydes identified from [70], a more potent and soluble inhibitor, MKC-3946, was synthesized [69]. MKC-3946 inhibits chemically-induced XBP1 splicing in a dose dependent manner from a MM cell line and from patient derived samples. Furthermore, endogenous XBP1 splicing was blocked in patient derived MM cells and an MM tumor xenograft model, without affecting IRE1α phosphorylation in this context. MKC-3946 also demonstrated selective cytotoxicity against MM cell lines without cytotoxicity to normal mononuclear cells. Furthermore, in MM cell lines, MKC-3946 inhibited XBP1 splicing induced by bortezomib (a proteasome inhibitor) and 17-AAG (an HSP90 inhibitor) and enhanced cytotoxicity induced by these compounds. Mechanistically, combined treatment with MKC-3946 and bortezomib/17-AAG activates the PERK-eIF2α-ATF4 branch of the UPR and subsequently increases expression of pro-apoptotic factor CHOP, leading to apoptosis in MM cell lines. Interestingly, in this context, binding of IRE1α to TRAF2 and IRE1α/JNK phosphorylation was also enhanced by treatment of MKC-3946 alone or in combination with bortezomib. In this pre-clinical setting, MKC-3946 alone or in combination with bortezomib significantly inhibited xenograft MM cell growth in vivo.
4.4. STF-083010
As a result of a high throughput chemical screening strategy using HT1080 human fibrosarcoma cells stably expressing a luciferase-based XBP1 reporter construct [75], STF-083010 was identified as a small molecule inhibitor of XBP1 splicing [68]. Subsequently, in an in vitro cell-free IRE1α RNase reaction, this compound also inhibited XBP1 mRNA splicing by directly inhibiting IRE1α RNase activity. A later study showed that this compound forms a carbaldehyde in water and selectively binds to K907 of IRE1α [71]. STF-083010 inhibits both endogenous and chemically-induced XBP1 splicing in an MM cell line, without affecting IRE1α phosphorylation. This compound also inhibits bortezomib-induced XBP1 splicing in reporter mice expressing the same luciferase construct as a transgene, without causing detectable toxicity in the normal tissue of these mice. STF-083010 further demonstrated cytotoxicity against a panel of MM cell lines in a dose- and time-dependent manner, and selectively killed CD138+ cells from MM patients but not normal hematopoietic cells. And finally, it inhibited in vivo growth of xenograft MM tumors.
4.5. HAA (hydroxy–aryl–aldehydes) inhibitors
Stemming from the salicylaldehyde derivatives, the combination of the adjacent hydroxy–aldehyde motif and a few dual-ring structures form a class of compounds called HAA (hydroxy–aryl–aldehydes), including MKC-3946 [69], 4μ8C [71] and an experimental IRE1α inhibitor (“Compound 2”) developed by Mannkind Corporation [76]. Recently, the crystal structures of murine IRE1α in complex with three HAA inhibitors, MKC9989, OICR464 and OICR573, were solved to elucidate the mechanism of action of this group of inhibitors [73]. These inhibitors blocked XBP1 splicing in a cell-free assay while having minimum effect on the kinase activity of IRE1α. Furthermore, MKC9989 inhibits XBP1 splicing as well as the RIDD activity in RPMI8226 multiple myeloma cells. The three compound-protein co-structures reveal that these HAA inhibitors bind to a shallow pocket at the RNase-active site of IRE1α through pi-stacking interactions with His910 and Phe889 and a hydrogen bond with Tyr892. Consistent with previous studies [71], the essential interaction is a Schiff base interaction between the HAA aldehyde group and the amine group of Lys907. Surface and schematic view of the interaction between IRE1α and MKC9989 are shown in Figure 1. This study provides the only X-ray crystallographic data for the interaction between chemical compounds and the RNase active-site of IRE1α. The revealed structures not only give insight into the molecular functions of the IRE1α protein, but also suggest new strategies in designing IRE1α inhibitors.
Figure 1. Surface and schematic view of the interaction between murine IRE1α and MKC9989 adapted from Sanches et al. [73].
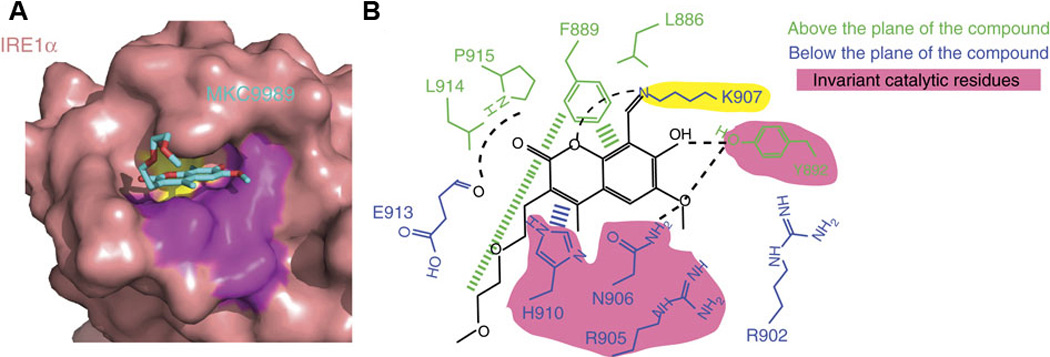
A. Surface view of the IRE1α–MKC9989 complex. Lysine 907 is colored yellow and invariant active site residues are colored purple. B. Schematic view of the contact residues of IRE1α and notable interactions between IRE1α and MKC9989.
4.6. Toyocamycin
Through the application of a similar XBP1-luciferase reporter construct overexpressed in HeLa cells, toyocamycin, a nucleoside-type antibiotic analogue of adenosine, was identified as an inhibitor of ER stress-induced XBP1 activation [72]. Toyocamycin blocked chemically-induced XBP1 splicing as well as XBP1 target gene expression in HeLa cells, without affecting PERK and ATF6 activation or IRE1α phosphorylation. Toyocamycin also prevented the splicing of an XBP1 RNA substrate by purified recombinant human IRE1α-cytosolic domain (amino acids 467–977) in vitro. Toyocamycin induced profound apoptosis in MM cell lines, including those resistant to bortezomib treatment, in a dose-dependent manner, the magnitude of which correlated with extent of XBP1 activation in the different cell lines. Toyocamycin was synergistic with bortezomib in inducing apoptosis in a MM cell line. In addition, toyocamycin inhibited XBP1 splicing in primary MM cells and demonstrated cytotoxicity to primary MM but not healthy PBMC cells. This compound showed antitumor activity in a xenograft MM tumor model, either alone or in a synergistic manner with bortezomib.
4.7. Compound 3
Compound 3 is a type II kinase inhibitor that competes for ATP-binding to IRE1α and stabilizes an inactive form of the protein [74]. This compound blocked autophosphorylation, oligomerization and XBP1 splicing capacity of IRE1α in vitroas well as chemically-induced IRE1α autophosphorylation and XBP1 splicing in cultured INS-1 insulinoma cells.
4.8. Quercetin
Through an in vitro fluorescence quenching (FQ)-based screening strategy, quercetin was identified as a compound that activates the RNase activity of IRE1α on XBP1 splicing [19]. The co-crystal structure of IRE1α, ADP and quercetin revealed that quercetin binds to the “Q site” in the RNase domain of IRE1α, defined by S984, K985, E988, K992, P1077, I1108, and F1112 of yeast IRE1α. In IRE1-null mouse embryonic fibroblasts (MEFs) expressing a chimeric human-yeast IRE1α protein (hyIRE1, with lumenal, transmembrane, and juxtamembrane domains of human IRE1α fused with the kinase and RNase domains of yeast IRE1α), quercetin induces XBP1 splicing. Additional evidence indicates that quercetin stimulates IRE1α dimerization in vitro.
In summary, multiple modulators of IRE1α-XBP1 have been described. The specific activities of these compounds and the corresponding references are summarized in Table 1. Collectively, these data indicate the tremendous enthusiasm and promise for developing therapeutic drugs targeting this pathway in human disease.
Table 1.
Summary of known IRE1α-XBP1 modulators
| Name | Structure | RNase activity | Kinase activity | RIDD | Oligomerization | IRE1α binding |
Cell culture | In vivo | Refs |
|---|---|---|---|---|---|---|---|---|---|
| Salicylaldehy des (3-methoxy-6-bromosalicyl aldehyde) | 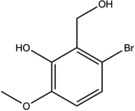 |
Inhibition (In vitro cleavage of Mini-XBP-1 stem-loop substrate) | No effect on thapsigargin-induced IRE1α phosphorylation in MM cells | Inhibition | Not tested | Binding confirmed by surface plasmon resonance assay | Inhibits XBP1 splicing in HEK293 cells | Inhibits tunicamycin-induced XBP1 splicing in CB17 SCID mice | [1] |
| 4µ8C | 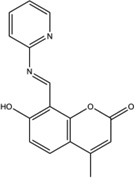 |
Inhibition (In vitro cleavage of RNA substrates) | Inhibition in vitro but not on thapsigargin-induced phosphorylation in MEFs. | Inhibition | Not tested | Binding to K599 and K907 of IRE1α detected by HPLC-MALDI-TOF | Inhibits XBP1 splicing in MEFs cells, induces cytotoxicity in MM cells | Not tested | [2] |
| MKC-3946 | 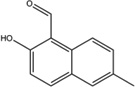 |
Not tested | No effect on IRE1α phosphorylation in MM cells | Not tested | Not tested | Not tested | Inhibits XBP1 splicing, induces cytotoxicity, enhances cytotoxicity of bortezomib and 17-AAG in MM cells | Inhibits tunicamycin-induced XBP1 splicing in SCID mice and MM xenograft growth alone or in combination with bortezomib | [3] |
| STF-083010 | 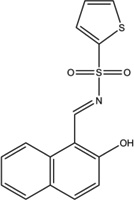 |
Inhibition (In vitro cleavage of RNA substrates) | No effect on IRE1α phosphorylation in MM cells | Inhibits RIDD activity induced by ADP but not quercetin | Not tested | Not tested | Inhibits endogenous and thapsigargin-induced XBP1 splicing in MM cells | Inhibits XBP1 splicing in luciferase reporter mice and MM xenograft growth | [4, 5] |
| Compound 2 | 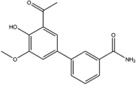 |
Not tested | Not tested | Not tested | Not tested | Not tested | Inhibits XBP1 splicing in U373 glioblastoma cells | Synergizes with oncolytic virus in reducing tumor burden in an OVCAR-4 orthotopic xenograft tumor model | [6] |
| MKC9989 | 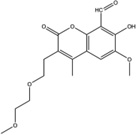 |
Inhibition (In vitro cleavage of RNA substrates) | Have minor effect on IRE1α auto-phosphorylation In vitro at highest tested concentration (100 µM) | Inhibition | Not tested | Binds to the RNase domain of murine IRE1α reveal ed by X-ray crystallograp hy | Inhibits XBP1 splicing in RPMI8226 MM cells | Not tested | [7] |
| OICR464 | 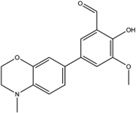 |
Inhibition (In vitro cleavage of RNA substrates) | No effect on IRE1α auto-phosphorylation In vitro at highest tested concentration (100 µM) | Not tested | Not tested | Binds to the RNase domain of murine IRE1α reveal ed by X-ray crystallograp hy | Not tested | Not tested | [7] |
| OICR573 | 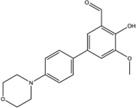 |
Inhibition (In vitro cleavage of RNA substrates) | No effect on IRE1α auto-phosphorylation In vitro at highest tested concentration (100 µM) | Not tested | Not tested | Binds to the RNase domain of murine IRE1α reveal ed by X-ray crystallograp hy | Not tested | Not tested | [7] |
| Toyocamycin | 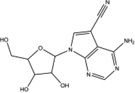 |
Inhibition (In vitro cleavage of RNA substrates) | No effect on IRE1α phosphorylation in HEK293T and MM cells | Not tested | Not tested | Not tested | Inhibits thapsigargin-, tumicamycin-and 2-DG-induced XBP1 splicing in HeLa cells and endogenous XBP1 splicing in MM cells, induces cytotoxicity and enhances cytotoxicity by bortezomib in MM cells | Inhibits MM xenograft growth alone and enhances the effects of bortezomib | [8] |
| Compound 3 | 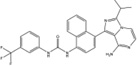 |
Inhibition (In vitro cleavage of Mini-XBP-1 stem-loop substrate) | Inhibition in vitro and on T-Rex 293 and INS-1 cells | Not tested | Inhibition in vitro | Interacts with the ATP-binding site of IRE1α detect ed by ICAT footprinting | Inhibits XBP1 splicing in T-Rex 293 and thapsigargin-induced XBP1 splicing in INS-1 cells | Not tested | [9] |
| Quercetin | 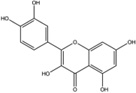 |
Activation (In vitro cleavage of Mini-XBP-1 stem-loop substrate) | Weak inhibitor | Promotes RIDD | Stimulates dimerization in vitro | Binds to the “Q site” in the RNase domain of IRE1α | Induces XBP1 splicing in IRE1-null MEFs overexpressing a chimeric hyIRE1 protein | Not tested | [5, 10] |
5. Conclusions and future perspectives
Recapitulating the complex nature of protein folding homeostasis, the UPR displays variable physiological outputs depending on the cellular context. Selective activation of or inhibition of IRE1α may be desirable depending on the disease state. Thus, therapeutic intervention based on targeting the UPR pathways should be optimized to differentially modulate the adaptive pro-survival or pro-apoptotic activity of the UPR according to the specific disease context. Within the three major branches of the UPR, the IRE1α-XBP1 pathway reveals multiple roles in neurodegenerative and metabolic diseases, while mainly serving as a pro-survival pathway in multiple human cancers. The complexity of IRE1α biology was demonstrated through a recent study suggesting that modulation of IRE1α RNase activity was possible through an allosteric mechanism using ATP-competitive kinase inhibitors APY29 and sunitinib [74]. Furthermore, a peptide derived from the IRE1α kinase domain [77] was shown to stimulate IRE1α oligomerization while inhibiting the JNK activation and RIDD activity of IRE1α. Another yet unexplored aspect of targeting IRE1α is the specificity on XBP1 splicing and RIDD activity. Finally, as RIDD activity is implicated in certain physiological and pathological settings, including lipid metabolism [78, 79], acetaminophen toxicity [80] and cell migration [81], IRE1α modulators specific for either XBP1 splicing or RIDD activity may be clinically useful depending on the therapeutic intent.
Acknowledgement
The authors would like to acknowledge the funding source P01 CA67166 (A.C.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 2.Davenport EL, Moore HE, Dunlop AS, Sharp SY, Workman P, Morgan GJ, et al. Heat shock protein inhibition is associated with activation of the unfolded protein response pathway in myeloma plasma cells. Blood. 2007;110:2641–2649. doi: 10.1182/blood-2006-11-053728. [DOI] [PubMed] [Google Scholar]
- 3.Hetz C, Chevet E, Harding HP. Targeting the unfolded protein response in disease. Nature reviews Drug discovery. 2013;12:703–719. doi: 10.1038/nrd3976. [DOI] [PubMed] [Google Scholar]
- 4.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Molecular cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 5.Lange PS, Chavez JC, Pinto JT, Coppola G, Sun CW, Townes TM, et al. ATF4 is an oxidative stress-inducible, prodeath transcription factor in neurons in vitro and in vivo. The Journal of experimental medicine. 2008;205:1227–1242. doi: 10.1084/jem.20071460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Averous J, Bruhat A, Jousse C, Carraro V, Thiel G, Fafournoux P. Induction of CHOP expression by amino acid limitation requires both ATF4 expression and ATF2 phosphorylation. The Journal of biological chemistry. 2004;279:5288–5297. doi: 10.1074/jbc.M311862200. [DOI] [PubMed] [Google Scholar]
- 7.Ma Y, Hendershot LM. Delineation of a negative feedback regulatory loop that controls protein translation during endoplasmic reticulum stress. The Journal of biological chemistry. 2003;278:34864–34873. doi: 10.1074/jbc.M301107200. [DOI] [PubMed] [Google Scholar]
- 8.Tirasophon W, Welihinda AA, Kaufman RJ. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes & development. 1998;12:1812–1824. doi: 10.1101/gad.12.12.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urano F, Bertolotti A, Ron D. IRE1 and efferent signaling from the endoplasmic reticulum. Journal of cell science. 2000;113(Pt 21):3697–3702. doi: 10.1242/jcs.113.21.3697. [DOI] [PubMed] [Google Scholar]
- 10.Lee KP, Dey M, Neculai D, Cao C, Dever TE, Sicheri F. Structure of the dual enzyme Ire1 reveals the basis for catalysis and regulation in nonconventional RNA splicing. Cell. 2008;132:89–100. doi: 10.1016/j.cell.2007.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korennykh AV, Egea PF, Korostelev AA, Finer-Moore J, Zhang C, Shokat KM, et al. The unfolded protein response signals through high-order assembly of Ire1. Nature. 2009;457:687–693. doi: 10.1038/nature07661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nature cell biology. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J, Liu CY, Back SH, Clark RL, Peisach D, Xu Z, et al. The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:14343–14348. doi: 10.1073/pnas.0606480103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimata Y, Ishiwata-Kimata Y, Ito T, Hirata A, Suzuki T, Oikawa D, et al. Two regulatory steps of ER-stress sensor Ire1 involving its cluster formation and interaction with unfolded proteins. The Journal of cell biology. 2007;179:75–86. doi: 10.1083/jcb.200704166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Credle JJ, Finer-Moore JS, Papa FR, Stroud RM, Walter P. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18773–18784. doi: 10.1073/pnas.0509487102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pincus D, Chevalier MW, Aragon T, van Anken E, Vidal SE, El-Samad H, et al. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS biology. 2010;8:e1000415. doi: 10.1371/journal.pbio.1000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardner BM, Walter P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science. 2011;333:1891–1894. doi: 10.1126/science.1209126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korennykh AV, Egea PF, Korostelev AA, Finer-Moore J, Stroud RM, Zhang C, et al. Cofactor-mediated conformational control in the bifunctional kinase/RNase Ire1. BMC biology. 2011;9:48. doi: 10.1186/1741-7007-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiseman RL, Zhang Y, Lee KP, Harding HP, Haynes CM, Price J, et al. Flavonol activation defines an unanticipated ligand-binding site in the kinase-RNase domain of IRE1. Molecular cell. 2010;38:291–304. doi: 10.1016/j.molcel.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- 21.Sidrauski C, Cox JS, Walter P. tRNA ligase is required for regulated mRNA splicing in the unfolded protein response. Cell. 1996;87:405–413. doi: 10.1016/s0092-8674(00)81361-6. [DOI] [PubMed] [Google Scholar]
- 22.Jurkin J, Henkel T, Nielsen AF, Minnich M, Popow J, Kaufmann T, et al. The mammalian tRNA ligase complex mediates splicing of XBP1 mRNA and controls antibody secretion in plasma cells. The EMBO journal. 2014;33:2922–2936. doi: 10.15252/embj.201490332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aragon T, van Anken E, Pincus D, Serafimova IM, Korennykh AV, Rubio CA, et al. Messenger RNA targeting to endoplasmic reticulum stress signalling sites. Nature. 2009;457:736–740. doi: 10.1038/nature07641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez TN, Sidrauski C, Dorfler S, Walter P. Mechanism of non-spliceosomal mRNA splicing in the unfolded protein response pathway. The EMBO journal. 1999;18:3119–3132. doi: 10.1093/emboj/18.11.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sidrauski C, Walter P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell. 1997;90:1031–1039. doi: 10.1016/s0092-8674(00)80369-4. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 27.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 28.Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. The Journal of cell biology. 2009;186:323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, et al. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138:562–575. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tam AB, Koong AC, Niwa M. Ire1 Has Distinct Catalytic Mechanisms for XBP1/HAC1 Splicing and RIDD. Cell reports. 2014;9:850–858. doi: 10.1016/j.celrep.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 32.Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, et al. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes & development. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim I, Shu CW, Xu W, Shiau CW, Grant D, Vasile S, et al. Chemical biology investigation of cell death pathways activated by endoplasmic reticulum stress reveals cytoprotective modulators of ASK1. The Journal of biological chemistry. 2009;284:1593–1603. doi: 10.1074/jbc.M807308200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hetz C, Thielen P, Matus S, Nassif M, Court F, Kiffin R, et al. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes & development. 2009;23:2294–2306. doi: 10.1101/gad.1830709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vidal RL, Figueroa A, Court FA, Thielen P, Molina C, Wirth C, et al. Targeting the UPR transcription factor XBP1 protects against Huntington's disease through the regulation of FoxO1 and autophagy. Human molecular genetics. 2012;21:2245–2262. doi: 10.1093/hmg/dds040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valenzuela V, Collyer E, Armentano D, Parsons GB, Court FA, Hetz C. Activation of the unfolded protein response enhances motor recovery after spinal cord injury. Cell death & disease. 2012;3:e272. doi: 10.1038/cddis.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hetz C, Lee AH, Gonzalez-Romero D, Thielen P, Castilla J, Soto C, et al. Unfolded protein response transcription factor XBP-1 does not influence prion replication or pathogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:757–762. doi: 10.1073/pnas.0711094105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morimoto N, Oida Y, Shimazawa M, Miura M, Kudo T, Imaizumi K, et al. Involvement of endoplasmic reticulum stress after middle cerebral artery occlusion in mice. Neuroscience. 2007;147:957–967. doi: 10.1016/j.neuroscience.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 39.Casas-Tinto S, Zhang Y, Sanchez-Garcia J, Gomez-Velazquez M, Rincon-Limas DE, Fernandez-Funez P. The ER stress factor XBP1s prevents amyloid-beta neurotoxicity. Human molecular genetics. 2011;20:2144–2160. doi: 10.1093/hmg/ddr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JH, Won SM, Suh J, Son SJ, Moon GJ, Park UJ, et al. Induction of the unfolded protein response and cell death pathway in Alzheimer's disease, but not in aged Tg2576 mice. Experimental & molecular medicine. 2010;42:386–394. doi: 10.3858/emm.2010.42.5.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoozemans JJ, van Haastert ES, Nijholt DA, Rozemuller AJ, Eikelenboom P, Scheper W. The unfolded protein response is activated in pretangle neurons in Alzheimer's disease hippocampus. The American journal of pathology. 2009;174:1241–1251. doi: 10.2353/ajpath.2009.080814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sado M, Yamasaki Y, Iwanaga T, Onaka Y, Ibuki T, Nishihara S, et al. Protective effect against Parkinson's disease-related insults through the activation of XBP1. Brain research. 2009;1257:16–24. doi: 10.1016/j.brainres.2008.11.104. [DOI] [PubMed] [Google Scholar]
- 43.Mhaille AN, McQuaid S, Windebank A, Cunnea P, McMahon J, Samali A, et al. Increased expression of endoplasmic reticulum stress-related signaling pathway molecules in multiple sclerosis lesions. Journal of neuropathology and experimental neurology. 2008;67:200–211. doi: 10.1097/NEN.0b013e318165b239. [DOI] [PubMed] [Google Scholar]
- 44.Bertolotti A, Wang X, Novoa I, Jungreis R, Schlessinger K, Cho JH, et al. Increased sensitivity to dextran sodium sulfate colitis in IRE1beta-deficient mice. The Journal of clinical investigation. 2001;107:585–593. doi: 10.1172/JCI11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nature immunology. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iwakoshi NN, Pypaert M, Glimcher LH. The transcription factor XBP-1 is essential for the development and survival of dendritic cells. The Journal of experimental medicine. 2007;204:2267–2275. doi: 10.1084/jem.20070525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 49.Zhou Y, Lee J, Reno CM, Sun C, Park SW, Chung J, et al. Regulation of glucose homeostasis through a XBP-1-FoxO1 interaction. Nature medicine. 2011;17:356–365. doi: 10.1038/nm.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park SW, Zhou Y, Lee J, Lu A, Sun C, Chung J, et al. The regulatory subunits of PI3K, p85alpha and p85beta, interact with XBP-1 and increase its nuclear translocation. Nature medicine. 2010;16:429–437. doi: 10.1038/nm.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winnay JN, Boucher J, Mori MA, Ueki K, Kahn CR. A regulatory subunit of phosphoinositide 3-kinase increases the nuclear accumulation of X-box-binding protein-1 to modulate the unfolded protein response. Nature medicine. 2010;16:438–445. doi: 10.1038/nm.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guichard C, Amaddeo G, Imbeaud S, Ladeiro Y, Pelletier L, Maad IB, et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nature genetics. 2012;44:694–698. doi: 10.1038/ng.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shajahan AN, Riggins RB, Clarke R. The role of X-box binding protein-1 in tumorigenicity. Drug news & perspectives. 2009;22:241–246. doi: 10.1358/dnp.2009.22.5.1378631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davies MP, Barraclough DL, Stewart C, Joyce KA, Eccles RM, Barraclough R, et al. Expression and splicing of the unfolded protein response gene XBP-1 are significantly associated with clinical outcome of endocrine-treated breast cancer. International journal of cancer Journal international du cancer. 2008;123:85–88. doi: 10.1002/ijc.23479. [DOI] [PubMed] [Google Scholar]
- 57.Chen X, Iliopoulos D, Zhang Q, Tang Q, Greenblatt MB, Hatziapostolou M, et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1alpha pathway. Nature. 2014;508:103–107. doi: 10.1038/nature13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romero-Ramirez L, Cao H, Regalado MP, Kambham N, Siemann D, Kim JJ, et al. X box-binding protein 1 regulates angiogenesis in human pancreatic adenocarcinomas. Translational oncology. 2009;2:31–38. doi: 10.1593/tlo.08211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drogat B, Auguste P, Nguyen DT, Bouchecareilh M, Pineau R, Nalbantoglu J, et al. IRE1 signaling is essential for ischemia-induced vascular endothelial growth factor-A expression and contributes to angiogenesis and tumor growth in vivo. Cancer research. 2007;67:6700–6707. doi: 10.1158/0008-5472.CAN-06-3235. [DOI] [PubMed] [Google Scholar]
- 60.Auf G, Jabouille A, Guerit S, Pineau R, Delugin M, Bouchecareilh M, et al. Inositol-requiring enzyme 1alpha is a key regulator of angiogenesis and invasion in malignant glioma. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15553–15558. doi: 10.1073/pnas.0914072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300–307. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 62.Iwakoshi NN, Lee AH, Vallabhajosyula P, Otipoby KL, Rajewsky K, Glimcher LH. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nature immunology. 2003;4:321–329. doi: 10.1038/ni907. [DOI] [PubMed] [Google Scholar]
- 63.Reimold AM, Ponath PD, Li YS, Hardy RR, David CS, Strominger JL, et al. Transcription factor B cell lineage-specific activator protein regulates the gene for human X-box binding protein 1. The Journal of experimental medicine. 1996;183:393–401. doi: 10.1084/jem.183.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carrasco DR, Sukhdeo K, Protopopova M, Sinha R, Enos M, Carrasco DE, et al. The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis. Cancer cell. 2007;11:349–360. doi: 10.1016/j.ccr.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clarke HJ, Chambers JE, Liniker E, Marciniak SJ. Endoplasmic reticulum stress in malignancy. Cancer cell. 2014;25:563–573. doi: 10.1016/j.ccr.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 66.Nakamura M, Gotoh T, Okuno Y, Tatetsu H, Sonoki T, Uneda S, et al. Activation of the endoplasmic reticulum stress pathway is associated with survival of myeloma cells. Leukemia & lymphoma. 2006;47:531–539. doi: 10.1080/10428190500312196. [DOI] [PubMed] [Google Scholar]
- 67.Leung-Hagesteijn C, Erdmann N, Cheung G, Keats JJ, Stewart AK, Reece DE, et al. Xbp1s-negative tumor B cells and pre-plasmablasts mediate therapeutic proteasome inhibitor resistance in multiple myeloma. Cancer cell. 2013;24:289–304. doi: 10.1016/j.ccr.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Papandreou I, Denko NC, Olson M, Van Melckebeke H, Lust S, Tam A, et al. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117:1311–1314. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mimura N, Fulciniti M, Gorgun G, Tai YT, Cirstea D, Santo L, et al. Blockade of XBP1 splicing by inhibition of IRE1alpha is a promising therapeutic option in multiple myeloma. Blood. 2012;119:5772–5781. doi: 10.1182/blood-2011-07-366633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Volkmann K, Lucas JL, Vuga D, Wang X, Brumm D, Stiles C, et al. Potent and selective inhibitors of the inositol-requiring enzyme 1 endoribonuclease. The Journal of biological chemistry. 2011;286:12743–12755. doi: 10.1074/jbc.M110.199737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cross BC, Bond PJ, Sadowski PG, Jha BK, Zak J, Goodman JM, et al. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E869–E878. doi: 10.1073/pnas.1115623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ri M, Tashiro E, Oikawa D, Shinjo S, Tokuda M, Yokouchi Y, et al. Identification of Toyocamycin, an agent cytotoxic for multiple myeloma cells, as a potent inhibitor of ER stress-induced XBP1 mRNA splicing. Blood cancer journal. 2012;2:e79. doi: 10.1038/bcj.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sanches M, Duffy NM, Talukdar M, Thevakumaran N, Chiovitti D, Canny MD, et al. Structure and mechanism of action of the hydroxy-aryl-aldehyde class of IRE1 endoribonuclease inhibitors. Nature communications. 2014;5:4202. doi: 10.1038/ncomms5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang L, Perera BG, Hari SB, Bhhatarai B, Backes BJ, Seeliger MA, et al. Divergent allosteric control of the IRE1alpha endoribonuclease using kinase inhibitors. Nature chemical biology. 2012;8:982–989. doi: 10.1038/nchembio.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spiotto MT, Banh A, Papandreou I, Cao H, Galvez MG, Gurtner GC, et al. Imaging the unfolded protein response in primary tumors reveals microenvironments with metabolic variations that predict tumor growth. Cancer research. 2010;70:78–88. doi: 10.1158/0008-5472.CAN-09-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mahoney DJ, Lefebvre C, Allan K, Brun J, Sanaei CA, Baird S, et al. Virus-tumor interactome screen reveals ER stress response can reprogram resistant cancers for oncolytic virus-triggered caspase-2 cell death. Cancer cell. 2011;20:443–456. doi: 10.1016/j.ccr.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 77.Bouchecareilh M, Higa A, Fribourg S, Moenner M, Chevet E. Peptides derived from the bifunctional kinase/RNase enzyme IRE1alpha modulate IRE1alpha activity and protect cells from endoplasmic reticulum stress. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:3115–3129. doi: 10.1096/fj.11-182931. [DOI] [PubMed] [Google Scholar]
- 78.So JS, Hur KY, Tarrio M, Ruda V, Frank-Kamenetsky M, Fitzgerald K, et al. Silencing of lipid metabolism genes through IRE1alpha-mediated mRNA decay lowers plasma lipids in mice. Cell metabolism. 2012;16:487–499. doi: 10.1016/j.cmet.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fu S, Watkins SM, Hotamisligil GS. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell metabolism. 2012;15:623–634. doi: 10.1016/j.cmet.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 80.Hur KY, So JS, Ruda V, Frank-Kamenetsky M, Fitzgerald K, Koteliansky V, et al. IRE1alpha activation protects mice against acetaminophen-induced hepatotoxicity. The Journal of experimental medicine. 2012;209:307–318. doi: 10.1084/jem.20111298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dejeans N, Pluquet O, Lhomond S, Grise F, Bouchecareilh M, Juin A, et al. Autocrine control of glioma cells adhesion and migration through IRE1alpha-mediated cleavage of SPARC mRNA. Journal of cell science. 2012;125:4278–4287. doi: 10.1242/jcs.099291. [DOI] [PubMed] [Google Scholar]


