Abstract
Ethnopharmacological relevance
Sutherlandia frutescens (L.) R. Br. is an indigenous plant of southern Africa that has been traditionally used for various cancers, infections, and inflammatory conditions.
Aim of the study
Our aim was to investigate the potential immuno-stimulatory activity of a polysaccharide-enriched fraction (SFPS) from a decoction of S. frutescens.
Materials and methods
RAW 264.7 cells (a murine macrophage cell line) were used to determine the activities of SFPS on macrophage function. The production of reactive oxygen species (ROS), nitric oxide (NO), and inflammatory cytokines were evaluated in the cells treated with or without SFPS. CLI-095, a toll-like receptor (TLR) 4-specific inhibitor, was used to identify whether or not SFPS exerts its effects through TLR4. An antagonist of endotoxin, polymyxin B, was used to evaluate whether endotoxin present in SFPS contributed to its immune-stimulatory activity.
Results
SFPS exhibited potent immune-stimulatory activity by macrophages. The production of ROS, NO, and tumor necrosis factor (TNF-α) were increased upon exposure to SFPS in a dose-dependent manner. All of these activities were completely blocked by co-treatment with CLI-095, but only partially diminished by polymyxin B.
Conclusion
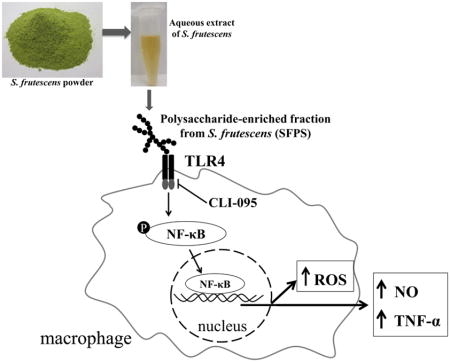
We demonstrate for the first time potent immune-stimulatory activity in a decoction prepared from S. frutescens. We believe that this immune stimulatory activity is due, in part, to the action of polysaccharides present in the decoction that acts by way of TLR4 receptors and the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway. These findings provide a plausible mechanism through which we can understand some of the medicinal properties of S. frutescens.
Keywords: Sutherlandia frutescens, polysaccharides, macrophage, toll-like receptor 4, endotoxin
Graphical Abstract
1. Introduction
Sutherlandia frutescens is a medicinal plant of southern Africa. Traditionally, infusions or decoctions of the S. frutescens leaf and bark have been used to treat patients with cancer, infections, and inflammatory conditions (van Wyk, 2008; van Wyk and Albrecht, 2008). Fernandes reported that a hot-water extract of S. frutescens had antioxidant and anti-inflammatory activities both in human neutrophils and in a cell-free system, and these findings were confirmed by other groups (Chen, 2007; Fernandes et al., 2004; Tobwala et al., 2014). A hot-water extract induces apoptosis and autophagic processes in neoplastic cells (e.g., cervical carcinoma and human breast adenocarcinoma MCF-7 cells (Chinkwo, 2005; Stander et al., 2009), which may explain S. frutescens’ claimed activity toward certain cancers.
Several plant polysaccharides have been recognized for their potent immune-stimulating activities which are often related to their ability to enhance the activation of macrophages (Schepetkin and Quinn, 2006). Polysaccharide-mediated immune cell stimulation can occur by way of binding to various cell surface receptors or following internalization and subsequent activation of intracellular signaling pathways (Hsu et al., 2004). Polysaccharides isolated from a variety of plants (e.g., Carthamus tinctoriu, Acanthopanax senticosus, Polyporus umbellatus, Astragalus membranaceus, Platycodon grandiflorum, and Ganoderma atrum) have been shown to activate the toll-like receptor 4 (TLR4) signaling pathway (Ando et al., 2002; Han et al., 2003; Li and Xu, 2011; Shao et al., 2004; Yoon et al., 2003; Zhang and Deng, 2014). Once TLR4 is activated a number of intracellular signaling pathways are triggered, including NF-κB and mitogen-activated protein kinases (MAPKs), eventually leading to the generation of reactive oxygen species (ROS), nitric oxide (NO), as well as inflammatory cytokines/chemokines (Ando et al., 2002). Though underappreciated, evidence suggesting that the immune-stimulatory activity of some botanicals is, partially or entirely, due to the presence of small amounts of contaminating bacterial lipopolysaccharide (LPS) (Pugh et al., 2008; Tabanca, 2007). Since LPS is a high-affinity agonist for the TLR4 signaling pathway (Miller et al., 2005), TLR4 signaling promotes increased production of ROS, NO, and cytokines/chemokines (Ando et al., 2002). Therefore, the presence of LPS in botanical extracts that are being screened for immune-modulatory activity has been problematic.
Hot water extracts of S. frutescens are rich in plant polysaccharides comprised of many glucose and galacturonic acid units with a pectin-like structure (Zhang et al., 2014). These polysaccharides have been found to promote complement fixation, which is a feature of the innate immune response (Zhang et al., 2014). However, little is known about other immuno-modulatory activities of polysaccharides from S. frutescens. The present study was designed to characterize the impact of a polysaccharide-enriched fraction (SFPS) from a decoction of S. frutescens on macrophage functions. We hypothesized that treatment with SFA or SFPS would stimulate innate immune cells and that this activation would be independent of the presence of LPS. To test this hypothesis a well-characterized murine macrophage cell line (i.e., RAW 264.7 cells) was chosen for our studies. We used CLI-095 (i.e., TLR4 antagonist) to explore the impact of SFPS on the TLR4 signaling pathway in these innate immune cells. Furthermore, steps were taken to distinguish between the immune-stimulating activity that arises from the presence of very small amounts of endotoxin and that which is independent of this well-studied immune cell activator. Removal of LPS in botanical extracts would be challenging and if attempted it would likely require extraction and/or fractionation steps. Fortunately, there exists an effective method for neutralizing the bioactivity of LPS without actually removing it. Polymyxin B is a cationic polypeptide antibiotic with a high affinity for the lipid A component of LPS, which results in neutralization of endotoxin-like bioactivity of most forms of LPS (Esteban et al., 2013).
2. Materials and Methods
2.1 Reagents
Ultrapure lipopolysaccharide (LPS) (from E. coli 0111:B4), CLI-095 (Cat#: tlrl-cli95), and polymyxin B (Cat#: tlrl-pmb) were purchased from Invivogen (San Diego, CA, USA). Fetal bovine serum (FBS) was purchased from Thermo Scientific (Logan, Utah, USA). 5-and-6-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) was purchased from Invitrogen, Inc. (Carlsbad, CA, USA) and used for ROS detection. For the cytokines and chemokines analysis, a Multi-Plex kit (Cat. MCYTOMAG-70K-PMX) was obtained from EMD Millipore (Billerica, MA, USA).
2.2 Preparation of Sutherlandia polysaccharides enriched fraction (SFPS)
Ground powder of vegetative parts of S. frutescens (L.) R. Br. was purchased from Big Tree Nutraceutical (Fish Hoek, South Africa). The product identity was confirmed using HPLC/ELSD and HPLC/UV (Avula, 2010), which determined that the S. frutescens used in this study contained 3.3% (w/w) of sutherlandioside B, a specific biomarker of this medicinal plant (Avula, 2010; Fu et al., 2008). An aqueous extract of S. frutescens was prepared using the method described by Fernandes (Fernandes et al., 2004). Briefly, 10 grams of finely ground S. frutescens was added to 250 mL of boiling water. This mixture was kept in a 100 °C water bath for 1 h, with stirring every 10 min. This mixture was allowed to cool overnight, in the dark. The decoction was transferred into 50 mL sterilized centrifuge tubes, and centrifuged at 2000× g for 15 min. The supernatant was recovered, filtered with a sterilized 0.2 μm nylon filter (Fisher Scientific, Pittsburgh, PA, USA), and then stored in small aliquots at −80 °C until used.
A crude polysaccharide-enriched fraction was prepared from this decoction as described by Xie and coworkers (Xie et al., 2007). In brief, 40 mL of 95% ethanol (Cat. 61509, Acros Organics, NJ, USA) was added to 10 mL aqueous extract of S. frutescens, and kept at 4°C overnight to precipitate the polysaccharides. The polysaccharides were pelleted by spinning at 2000× g for 15 min, re-suspended in endotoxin-free H2O (Cat. 7732, Alfa Aesar, Ward Hill, MA, USA), and then sonicated for 10 min to resuspend the pellet. The resuspended polysaccharides were centrifuged (i.e., 2000× g for 1 h) to remove insoluble particulates. The supernatant was collected, and filtered with a sterile (0.2 μm) nylon filter. To minimize the risk of endotoxin contamination during processing, we relied entirely on commercial sterile polypropylene tubes, bottles, and pipets for preparation of the aqueous extract and during polysaccharide enrichment. A single 40 mL fraction was freeze-dried yielding a dry weight of 7.3% for our SFPS.
2.3 Cell culture
RAW 264.7 cells were grown and maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 5% fetal bovine serum (FBS) at 37 °C with 5% CO2. For most experiments, 2 × 105 cells were seeded in each well of a flat-bottom 96-well tissue culture-treated plate (BD Falcon, Franklin Lakes, New Jersey, USA), and cultured overnight. Typically, this approach resulted in cell monolayers that were >90% confluent. As indicated, some cells were pretreated with polymyxin B (10 μg/mL) or CLI-095 (1 μg/mL) for 1 h prior to incubation with various concentrations of SFPS in DMEM/1%FBS for 18 hours (for ROS experiments) or 20 hours (for NO and cytokine/chemokines).
RAW 264.7 cells stably-transfected with a luciferase reporter construct with five copies of the NF-κB promoter and a green fluorescent protein (GFP) reporter were prepared as described previously (Mossine et al., 2013). The transfected cells were treated with SFPS for 3 h to detect the impact of SFPS on NF-κB activation.
2.4 Cell viability
The impact of treatments on cell viability was assessed using the resazurin assay (Wang, 2002). Following overnight treatments of SFPS with/without CLI-095 or polymyxin B and the collection of the medium in each well, 100 μL of a 0.1 % resazurin solution in DMEM/5% FBS was added to incubate with cells. The fluorescence (excitation 530, emission 590) was measured every 30 min for 3 h in a microplate reader (Biotek, Winooski, VT, USA).
When experiments were conducted with the stably-transfected RAW 264.7 cells, the cell viability was monitored by the expression of green fluorescent protein (GFP) as described by Elliott et al.(Elliott et al., 2000). Cells were cultured and treated as described above, and then lysed using 70 μL cell lysis buffer. Sixty microliters cell lysates were transferred into a 96-well plate with clear bottoms and black side-walls (Cat. 655096, Greiner bio-one, Monroe, NC, USA), and the fluorescence was measured (excitation 485 nm, emission 528 nm). The GFP fluorescence of lysates is a validated surrogate for cell enumeration (Elliott et al., 2000) and was used as an indication of cell loss due to treatment-induced apoptosis/death.
2.5 Reactive oxygen species (ROS) assessment
ROS production by murine macrophages was measured using CM-H2DCFDA (Choi et al., 2007). RAW 264.7 cells were seeded in 96-well plate with density of 1 × 105 cells/well and cultured overnight as described previously. Cells were treated with indicated concentrations of SFPS or 10 ng/mL of LPS as a positive control. After 18 hrs all culture medium was carefully removed, followed by the addition of 100 μL of CM-H2DCFDA at 10 μM in PBS for 30 min. After a 30 min incubation at 37°C the dye was removed and cells were washed with PBS. Fluorescence in each well was measured using a microplate reader at 485 nm (excitation) and 520 nm (emission) every 10 min for 3 h. The production of ROS was indicated by the fluorescence units, and the data were expressed as percentage of positive control.
2.6 Measurement of nitric oxide (NO) generation
The concentration of nitric oxide (NO) in the cell culture medium was measured by Griess reagents using sodium nitrite (NaNO2) as the standard (Schmidt, 1992). RAW 264.7 cells were treated with SFPS for 20 h, then aliquots of the culture medium (50 μL) were transferred into a separate 96-well ELISA plate. Griess reagents (i.e., sulfanilamide (7.5 mM); HCl (0.75 M); napthyl ethylenediamine (7.5 mM)) were added to each well, and incubated for 10 min at room temperature. The absorbance at 548 nm was measured with a microplate reader. The nitrite concentration was calculated using a NaNO2 standard curve that had a linear response in the range of 5–100 μM.
2.7 Determination of inflammatory cytokine and chemokine production
Concentrations of tumor necrosis factor (TNF)-α, interleukin (IL)-6, granulocyte-colony stimulating factor (G-CSF), macrophage inflammatory protein (MIP)-1β, MIP-1α, MIP-2, chemokine ligand 5, and keratinocyte chemoattractant were measured by multiplex magnetic bead panel kit (Milliplex murine cytokines/chemokines, Cat. MCYTOMAG-70K-PMX, EMD Millipore, Billerica, MA, USA). Aliquots (25 μL) of cell culture medium were incubated with anti-cytokine or anti-chemokine antibody-immobilized beads, detection antibodies, and streptavidin-phycoerythrin according to manufacturer’s instructions. Some samples were diluted with medium in order to bring the results into the linear portion of the standard curve. The plate was read by using a MAGPIX® reader running 4.2 xPONENT software (Luminex, Austin, TX, USA). Standards (with a range of 3.2 to 10,000 pg/mL) and high and low concentration quality controls were assayed in duplicate as provided by manufacturer. Data were analyzed using MILLIPLEX™ Analyst software version 3.5.
2.8 Analysis of NF-κB activation
The impact of SFPS on NF-κB activation was investigated using the method described by Tabanca, et al. (Tabanca, 2007). RAW 264.7 cells stably-transfected with a luciferase reporter construct with five copies of the NF-κB promoter were seeded in 96-well plate, and cultured in DMEM/5% FBS at 37°C with 5% CO2 overnight to > 95% confluence. Cells were treated with various concentrations of SFPS for 3 h, then the medium was carefully removed and 70 μL of lysis buffer was added to each well. Cell lysates (30 μL) were mixed with equal volume of luciferase substrate, and luminescence was read immediately in a microplate reader. Luminescence was normalized to the GFP fluorescence readings in the unstimulated wells.
2.9 Analysis of endotoxin in the SFPS
The presence and concentration of endotoxin in SFPS was quantified using the recombinant factor C endotoxin detection assay (Lonza Walkersville, Inc., Cat. 50-658U, Walkersville, MD, USA) following the instructions provided by the manufacturer. Aliquots of SFPS were mixed with fluorogenic substrate, assay buffer, and recombinant factor C (rFC) in an endotoxin-free plate. Using a temperature-controlled plate reader set at 37°C fluorescence at excitation and emission wavelengths of 380 nm and 440 nm, respectively, were measured immediately following mixing of the samples with the reagents and 60 min later. The endotoxin concentration was calculated using a standard curve (0.01 to 10 endotoxin units/mL). One endotoxin unit is equivalent to 0.1 ng/mL of LPS from E. coli 0111:B4 (Schwarz et al., 2014).
2.10 Statistical Analysis
All data represent the mean ± standard error of the mean from at least three independent experiments with each treatment condition conducted in duplicate or triplicate. All treatment effects were analyzed by one-way ANOVA and Tukey’s multiple comparison tests using version 9.3 software from SAS (SAS Institute Inc., Cary, NC, USA). A p < 0.05 was considered to indicate statistical significance.
3. Results
3.1 SFPS increased the production of nitric oxide (NO) and reactive oxygen species (ROS) by RAW 264.7 cells
RAW 264.7 cells were treated with/without SFPS for 20 h and cell culture medium was harvested to determine the NO concentration. Cells without any treatment were included as a negative control, while those stimulated with 10 ng/mL of LPS served as a positive control. The SFPS alone and in combination with other co-treatments (i.e., TLR4 inhibitor, CLI-095; LPS-neutralizer, polymyxin B) appeared to have no adverse effects on RAW 264.7 cell viability based on the results of the resazurin assay (Fig. 1) or GFP expression (data not shown).
Figure 1.
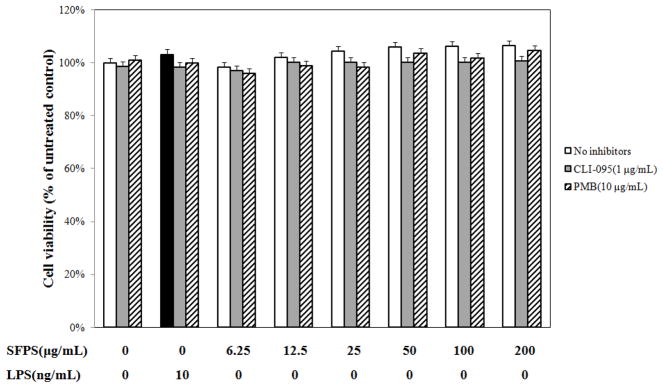
Polysaccharides-enriched fraction from S.frutescens (SFPS) was not toxic to murine macrophages, RAW 264.7. Cells were pretreated with 1 μg/mL CLI-095 or 10 μg/mL polymyxin B (PMB) for 1 h prior to incubation with various concentrations of SFPS in DMEM/1%FBS for 20 h. The cell viability was determined by resazurin assay. SFPS with/without co-treatment of CLI-095 or PMB showed no toxicity on murine macrophage (RAW 264.7 cells) after 20 h. The resazurin data was expressed as percentage of untreated control. The data were from four independent experiments conducted in triplicate.
SFPS significantly elevated the production of NO by RAW 264.7 cells, such that treatment with 200 mg/mL of SFPS (Fig. 2A) caused about 18 times greater levels than produced by cells without treatment. This NO-inducing activity was comparable to that induced by 10 ng/mL LPS (i.e., 50.9 ± 2.5 μM). RAW 267.4 cells co-treated with SFPS and CLI-095 (1 μg/mL) produced NO at levels found in unstimulated cells (i.e., < 5 μM). On the other hand, treatment with 10 μg/mL polymyxin B reduced NO production stimulated by SFPS by about 50% (Fig. 2A).
Figure 2.
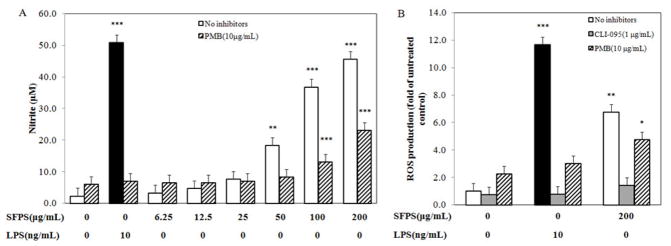
SFPS induced the production of NO and ROS via activation of TLR4 signaling pathway. NO production induced by SFPS was partially inhibited by pretreated cells with polymyxin B (PMB) (A). Meanwhile, SFPS-induced ROS production was completely blocked by treatment of CLI-095, and polymyxin B partially inhibited it (B). Data were from three independent experiments each conducted in triplicate. * p <0.05, ** p <0.01, *** p <0.0001.
SFPS also increased the production of intracellular reactive oxygen species (ROS) by RAW 267.4 cells. Fig. 2 showed that the production of ROS was elevated by ~6-fold after administration of the highest concentration (200 μg/mL) of SFPS tested, however, this induction was 50% lower than that induced by 10 ng/mL of LPS (Fig. 2B). Furthermore, the induced ROS production was inhibited by co-treatments of 1 μg/mL CLI-095 (reduced >90%) and 10 μg/mL polymyxin B (reduced ~30%).
3.2 SFPS-mediated induction of pro-inflammatory cytokines and chemokines
Exposure to SFPS increased the production of TNF-α, IL-6, G-CSF, MIP-1α, MIP-1β, and MIP-2 (Table 1). As expected, CLI-095 co-treatment completely (i.e., >99%) prevented LPS-stimulated production of cytokines and chemokines by RAW 264.7 cells. Similarly, co-treatment with CLI-095 was effective at inhibiting >95% of SFPS-mediated production of these same inflammatory mediators. Polymyxin B, an LPS neutralizing agent, was effective at inhibiting LPS stimulation of cytokine and chemokine biosynthesis. In contrast, 14%, 20%, 26%, and 15% of SFPS-induced production of TNF-α, MIP-1α, MIP-1β, and MIP-2, respectively, were resistant to polymyxin B co-treatment. Surprisingly, co-treatment with either CLI-095 or polymyxin B inhibited >95% of SFPS-induced production of IL-6 and G-CSF. Finally, SFPS failed to impact the production of chemokine ligand 5 or keratinocyte chemoattractant (data not shown).
Table 1.
SFPS induced production of various cytokines and chemokines via the TLR4 signaling pathway.
| TNF-α (ng/mL)
| ||||
|---|---|---|---|---|
| No inhibitors | + PMB | + CLI | ||
| No treatment | 0.47 ± 0.02 | |||
| LPSa | 139.35 ± 9.92 | 3.52 ± 0.85*** | 0.12 ± 0.01*** | |
| SFPS (μg/mL) | 12.5 | 2.79 ± 1.51 | 1.57 ± 0.66 | 0.15 ± 0.03 |
| 50 | 13.07 ± 3.85 | 2.50 ± 1.00 | 0.27 ± 0.01*** | |
| 200 | 43.65 ± 10.40 | 8.13 ± 1.21*** | 1.55 ± 0.06*** | |
| IL-6 (ng/mL)
| ||||
|---|---|---|---|---|
| No inhibitors | + PMB | + CLI | ||
| No treatment | <0.01b | |||
| LPSa | 54.41 ± 3.71 | <0.12b | <0.12b | |
| SFPS (μg/mL) | 12.5 | <0.12b | <0.12b | <0.12b |
| 50 | 0.18 ± 0.01 | <0.12b | <0.12b | |
| 200 | 6.31 ± 1.75 | <0.12b | <0.12b | |
| G-CSF (ng/mL)
| ||||
|---|---|---|---|---|
| No inhibitors | + PMB | + CLI | ||
| No treatment | 0.90 ± 0.11 | |||
| LPSa | 754.94 ± 28.24 | 14.35 ± 7.20*** | <0.14 | |
| SFPS (μg/mL) | 12.5 | 1.95 ± 0.59 | 0.15 ± 0.00 | 2.43 ± 3.01 |
| 50 | 44.19 ± 8.18 | 0.17 ± 0.00 | 2.70 ± 1.74 | |
| 200 | 459.60 ± 91.03 | 0.61 ± 0.12*** | 29.46 ± 7.52*** | |
| MIP-1α (ng/mL)
| ||||
|---|---|---|---|---|
| No inhibitors | + PMB | + CLI | ||
| No treatment | >21.76b,c | |||
| LPSa | 1002.19 ± 15.82 | 76.77 ± 23.64*** | 11.68 ± 1.44*** | |
| SFPS (μg/mL) | 12.5 | 55.71 ± 7.28 | 31.71 ± 7.51 | 12.56 ± 1.74 |
| 50 | 250.64 ± 24.26 | 37.67 ± 6.29 | 16.29 ± 1.24 | |
| 200 | 816.70 ± 58.49 | 160.79 ± 9.00 | 31.86 ± 2.22* | |
| MIP-1β (ng/mL)
| ||||
|---|---|---|---|---|
| No inhibitors | + PMB | + CLI | ||
| No treatment | 15.71 ± 0.80 | |||
| LPSa | 806.20 ± 125.22 | 45.01 ± 15.22*** | 7.65 ± 0.98*** | |
| SFPS (μg/mL) | 12.5 | 54.61 ± 25.14 | 26.79 ± 8.83 | 8.79 ± 1.34 |
| 50 | 245.62 ± 47.17 | 38.72 ± 14.71* | 12.5 ± 0.90* | |
| 200 | 497.67 ± 46.91 | 160.08 ± 20.74*** | 25.21 ± 1.50*** | |
| MIP-2 (ng/mL)
| ||||
|---|---|---|---|---|
| No inhibitors | + PMB | + CLI | ||
| No treatment | >19.78b,c | |||
| LPSa | >988.86 b | 66.70 ± 19.93 | 0.62 ± 0.04 | |
| SFPS (μg/mL) | 12.5 | 19.45 ± 5.94 | 14.70 ± 7.49 | 1.36 ± 0.74 |
| 50 | 107.34 ± 12.51 | 23.98 ± 8.72 | 0.68 ± 0.01 | |
| 200 | 612.22 ± 108.22 | 88.55 ± 35.64 | 1.58 ± 0.44 | |
Data are expressed as means ± standard error of mean (n = 4). Comparisons between no inhibitors values to either stimuli (i.e., LPS or SFPS).
p<0.05;
p<0.01;
p<0.001.
Ultrapure LPS from E. coli 0111:B4 used at 100 ng/mL was used as a positive control and to confirm the effectiveness of both inhibitors.
The concentrations were either lower or higher than the detection ranges of the kit.
These concentrations are higher than the detection range, however, they are lower than some of data from other treatment, this is because of different dilution factors (control: 2-fold dilution, LPS: 100-fold dilution, SFPS: 10-fold dilution).
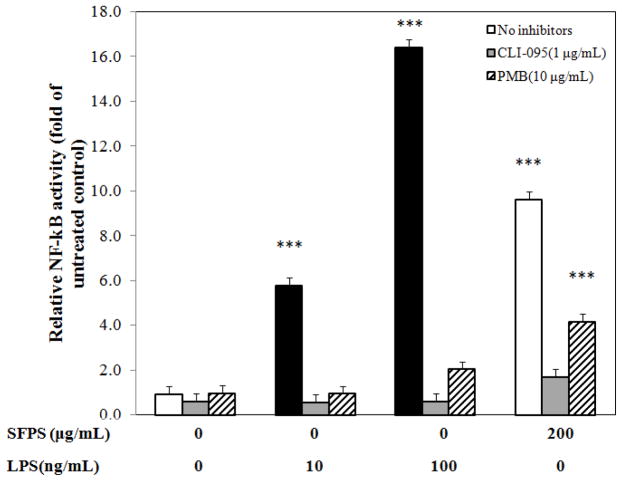
3.3 SFPS activated NF-κB signaling pathway
NF-κB activation, a key transcription factor for inflammatory responses, was assessed using the RAW 267.4 cells stably-transfected with a luciferase reporter. Our assays showed that LPS increased the activation of NF-κB by 5- and 17-fold at concentrations of 10 ng/mL and 100 ng/mL, respectively (Fig. 3), and was completely blocked by 1 μg/mL CLI-095, and reduced by >95% with treatment of 10 μg/mL polymyxin B. While SFPS stimulated the activation of NF-κB by up to 9-fold in 3 h compared to the cells without treatment with SFPS (Fig. 3), only 80% of this activity was attenuated by co-treated with CLI-095, and co-treatment with polymyxin B inhibited SFPS-induced NF-κB activation by 57%.
Figure 3.
Effect of SFPS on NF-κB activity in RAW 264.7 cells. The activation of NF-κB was induced by SFPS. This activity was completely inhibited by CLI-095 and partially diminished by polymyxin B (PMB). * p<0.05, ** p <0.01, *** p <0.0001.
3.4 Role of LPS in SFPS-mediated activation of murine macrophages
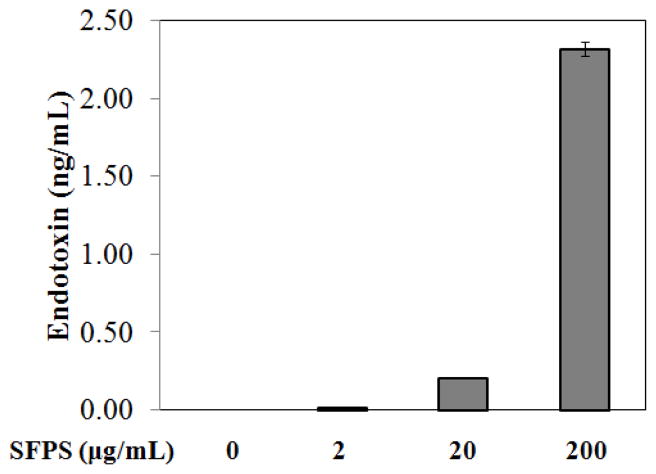
Since co-treatment with 10 μg/mL of polymyxin B partially attenuated the SFPS-mediated activation of murine macrophages we directly measured the concentration of LPS in SFPS. Using the recombinant factor C endotoxin detection assay, we found ~2.3 ng/mL endotoxin present in 200 μg/mL SFPS (Fig. 4). Therefore, a significant portion of the immune-stimulatory activity in the SFPS preparation is a consequence of endotoxin present in the extract. However, there is clear evidence of immune-stimulatory activity present in the SFPS that is independent of LPS, but still dependent upon TLR4 signaling.
Figure 4.
Endotoxin concentration in SFPS. The SFPS was analyzed in triplicate using recombinant factor C endotoxin detection assay.
4. Discussion
In this study, we demonstrate for the first time immune-stimulatory activity present in a decoction prepared from S. frutescens. Using a well-studied murine macrophage cell line we noted dose-dependent elevations in the production of nitric oxide, pro-inflammatory cytokines, and reactive oxygen species following treatment with a polysaccharide-enriched fraction from this medicinal plant. Furthermore, we provide evidence that this immune-stimulatory activity is partly a result of the action of bacterial endotoxin (LPS), however, this extract also contained immune-stimulatory activity independent of the LPS that is probably from polysaccharides.
While this is the first report of immune-stimulation by SFPS, others have documented similar activities by polysaccharides present in many plants, mushrooms, lichens, and algae (Schepetkin and Quinn, 2006). It is thought that plant polysaccharides may stimulate macrophages, and other innate immune cells, by binding to one or more cell surface receptors, including: TLR4, CD14, complement receptor 3, scavenger receptor, dectin-1, and mannose receptors. Use of peritoneal macrophages isolated from C3H/HeJ mice, which possess a null mutation in the Tlr4 gene allowed others to demonstrate TLR4-dependent immuno-stimulation by polysaccharides from Carthamus tinctoriu, Acanthopanax senticosus, Polyporus umbellatus, Astragalus membranaceus, and Platycodon grandiflorum (Ando et al., 2002; Han et al., 2003; Li and Xu, 2011; Shao et al., 2004; Yoon et al., 2003). In contrast, we used a novel and specific TLR4 inhibitor, CLI-095 (Takashima et al., 2009), to demonstrate that SFPS activated murine macrophages via the TLR4 signaling pathway.
Lipopolysaccharide (LPS), a component of gram-negative bacteria, is one of the most potent and well-studied stimulators of innate immune cells. LPS activity is entirely dependent upon activation of TLR4 signaling (Doyle and O’Neill, 2006) and can be detected in many botanical extracts, particularly in those botanicals reported to possess immune-stimulatory activity, such as Echinacea, American ginseng, alfalfa sprouts, and black walnuts (Pugh et al., 2008; Tamta et al., 2008). We detected a small amount of endotoxin in SFPS, but we demonstrated the existence of an immune-stimulatory activity within the polysaccharide-enriched fraction from S. frutescens independent of LPS.
TLR4-mediated signaling in phagocytes involves several different pathways, including the NF-κB, extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 (O’Neill et al., 2013). Botanical polysaccharide activation of macrophages via TLR4 has been reported to occur through each of these signaling pathways (Hsu et al., 2004). By using RAW 264.7 cells transfected with an NF-κB reporter we determined that much of the immune-stimulation by SFPS is mediated by NF-κB signaling. Whether other signaling pathways are involved in the immune-stimulation activity of SFPS not assessed. Macrophage activation of NF-κB and/or signal transducers and activators of transcription (STAT) 1 signaling pathways promote the production of ROS, NO, and pro-inflammatory cytokines, which in turn boosts the antimicrobial and tumoricidal activities of these cells (Wynn et al., 2013). Such activities, taken together with the complement fixing activity identified previously by others (Zhang et al., 2014), is consistent with the claimed use of S. frutescens decoctions for cancers. Unlike in the Zhang’s study, the polysaccharides-enriched fraction in this study was not characterized any further, therefore we are unable to speculate about which type of polysaccharide(s) in S. frutescens contributes to the immune-stimulatory activities we observed. Besides the polysaccharides obtained from the aqueous extract, S. frutescens is also known to contain a number of other metabolites, including a number of flavonol glycosides (Fu et al., 2010), cycloartane glycosides (Fu et al., 2008), and even compounds possessing anti-inflammatory activities (e.g., L-canavanine). This may help explain why we, and others (Jiang et al., 2014), have noted that ethanol extracts of S. frutescens inhibit LPS-induced NO and ROS production.
5. Conclusions
In conclusion, we demonstrate that a crude polysaccharide-enriched fraction isolated from a decoction of S. frutescens possesses immuno-stimulatory activity resulting in the activation of macrophages via TLR4 receptors and the NF-κB signaling pathway. This extract increased production of NO, ROS, and inflammatory cytokines/chemokines by macrophages, a cell with a central role in shaping innate immune responses in the host. These findings may help explain the use of S. frutescens for conditions where stimulating innate immune responses could be beneficial.
Acknowledgments
This publication or project was made possible by Grant Number P50AT006273 from the National Center for Complementary & Alternative Medicine (NCCAM), the Office of Dietary Supplements (ODS), and the National Cancer Institute (NCI) and the support of the University of Missouri’s College of Agriculture, Food, and Natural Resources, and the Food-for-the-21st Century Program. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, ODS, NCI, or the National Institutes of Health.
Glossary
Glossary
- CM-H2DCFDA
5, 6-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- G-CSF
granulocyte colony-stimulating factor
- GFP
green fluorescence protein
- IL-6, interleukin 6 LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MIP
macrophage inflammatory protein
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NO
nitric oxide
- PMB
polymyxin B
- ROS
reactive oxygen species
- SFA
aqueous extract of Sutherlandia frutescens
- SFPS
polysaccharide-enriched fraction from a decoction of Sutherlandia frutescens
- TLR
toll-like receptor
- TNF-α
Tumor necrosis factor-alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ando I, Tsukumo Y, Wakabayashi T, Akashi S, Miyake K, Kataoka T, Nagai K. Safflower polysaccharides activate the transcription factor NF-kappa B via Toll-like receptor 4 and induce cytokine production by macrophages. International immunopharmacology. 2002;2:1155–1162. doi: 10.1016/s1567-5769(02)00076-0. [DOI] [PubMed] [Google Scholar]
- Avula B, Wang YH, Smillie TJ, Fu X, Li XC, Mabusela W, Syce J, Johnson Q, Folk W, Khan IA. Quantitative determination of flavonoids and cycloartanol glycosides from aerial parts of Sutherlandia frutescens (L.) R. BR. by using LC-UV/ELSD methods and confirmation by using LC-MS method. Journal of pharmaceutical and biomedical analysis. 2010;52:173–180. doi: 10.1016/j.jpba.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SY, Hwang JH, Ko HC, Park JG, Kim SJ. Nobiletin from citrus fruit peel inhibits the DNA-binding activity of NF-kappaB and ROS production in LPS-activated RAW 264.7 cells. Journal of ethnopharmacology. 2007;113:149–155. doi: 10.1016/j.jep.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Doyle SL, O’Neill LA. Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochemical pharmacology. 2006;72:1102–1113. doi: 10.1016/j.bcp.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Elliott G, McGrath J, Crockett-Torabi E. Green fluorescent protein: A novel viability assay for cryobiological applications. Cryobiology. 2000;40:360–369. doi: 10.1006/cryo.2000.2258. [DOI] [PubMed] [Google Scholar]
- Esteban E, Ferrer R, Alsina L, Artigas A. Immunomodulation in sepsis: the role of endotoxin removal by polymyxin B-immobilized cartridge. Mediators of inflammation. 2013;2013:507539. doi: 10.1155/2013/507539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes AC, Cromarty AD, Albrecht C, van Rensburg CE. The antioxidant potential of Sutherlandia frutescens. Journal of ethnopharmacology. 2004;95:1–5. doi: 10.1016/j.jep.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Fu X, Li XC, Smillie TJ, Carvalho P, Mabusela W, Syce J, Johnson Q, Folk W, Avery MA, Khan IA. Cycloartane glycosides from Sutherlandia frutescens. Journal of natural products. 2008;71:1749–1753. doi: 10.1021/np800328r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Li XC, Wang YH, Avula B, Smillie TJ, Mabusela W, Syce J, Johnson Q, Folk W, Khan IA. Flavonol glycosides from the south African medicinal plant Sutherlandia frutescens. Planta medica. 2010;76:178–181. doi: 10.1055/s-0029-1186030. [DOI] [PubMed] [Google Scholar]
- Han SB, Yoon YD, Ahn HJ, Lee HS, Lee CW, Yoon WK, Park SK, Kim HM. Toll-like receptor-mediated activation of B cells and macrophages by polysaccharide isolated from cell culture of Acanthopanax senticosus. International immunopharmacology. 2003;3:1301–1312. doi: 10.1016/S1567-5769(03)00118-8. [DOI] [PubMed] [Google Scholar]
- Hsu HY, Hua KF, Lin CC, Lin CH, Hsu J, Wong CH. Extract of Reishi polysaccharides induces cytokine expression via TLR4-modulated protein kinase signaling pathways. Journal of immunology. 2004;173:5989–5999. doi: 10.4049/jimmunol.173.10.5989. [DOI] [PubMed] [Google Scholar]
- Jiang J, Chuang DY, Zong Y, Patel J, Brownstein K, Lei W, Lu CH, Simonyi A, Gu Z, Cui J, Rottinghaus GE, Fritsche KL, Lubahn DB, Folk WR, Sun GY. Sutherlandia frutescens ethanol extracts inhibit oxidative stress and inflammatory responses in neurons and microglial cells. PloS one. 2014;9:e89748. doi: 10.1371/journal.pone.0089748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Xu W. TLR4-mediated activation of macrophages by the polysaccharide fraction from Polyporus umbellatus(pers.) Fries. Journal of ethnopharmacology. 2011;135:1–6. doi: 10.1016/j.jep.2010.06.028. [DOI] [PubMed] [Google Scholar]
- Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nature reviews Microbiology. 2005;3:36–46. doi: 10.1038/nrmicro1068. [DOI] [PubMed] [Google Scholar]
- Mossine VV, Waters JK, Hannink M, Mawhinney TP. piggyBac transposon plus insulators overcome epigenetic silencing to provide for stable signaling pathway reporter cell lines. PloS one. 2013;8:e85494. doi: 10.1371/journal.pone.0085494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill LAJ, Golenbock D, Bowie AG. The history of Toll-like receptors [mdash] redefining innate immunity. Nature reviews Immunology. 2013;13:453–460. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- Pugh ND, Tamta H, Balachandran P, Wu X, Howell JL, Dayan FE, Pasco DS. The majority of in vitro macrophage activation exhibited by extracts of some immune enhancing botanicals is due to bacterial lipoproteins and lipopolysaccharides. International immunopharmacology. 2008;8:1023–1032. doi: 10.1016/j.intimp.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepetkin IA, Quinn MT. Botanical polysaccharides: macrophage immunomodulation and therapeutic potential. International immunopharmacology. 2006;6:317–333. doi: 10.1016/j.intimp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Schmidt HH, Warner TD, Nakane M, Forstermann U, Murad M. Regulation and subcellular location of nitrogen oxide synthases in RAW264.7 macrophages. Molecular Pharmocology. 1992:615–624. [PubMed] [Google Scholar]
- Schwarz H, Schmittner M, Duschl A, Horejs-Hoeck J. Residual Endotoxin Contaminations in Recombinant Proteins Are Sufficient to Activate Human CD1c(+) Dendritic Cells. PloS one. 2014;9:e113840. doi: 10.1371/journal.pone.0113840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao BM, Xu W, Dai H, Tu P, Li Z, Gao XM. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochemical and biophysical research communications. 2004;320:1103–1111. doi: 10.1016/j.bbrc.2004.06.065. [DOI] [PubMed] [Google Scholar]
- Tabanca N, Ma G, Pasco DS, Bedir E, Kirimer N, Husnu K, Baser C, Khan IA, Khan SI. Effect of essential oils and isolated compounds from Pimpinella Species on NF-κB: a target for antiinflammatory therapy. Phtotherapy Research. 2007;21:741–745. doi: 10.1002/ptr.2154. [DOI] [PubMed] [Google Scholar]
- Takashima K, Matsunaga N, Yoshimatsu M, Hazeki K, Kaisho T, Uekata M, Hazeki O, Akira S, Iizawa Y, Ii M. Analysis of binding site for the novel small-molecule TLR4 signal transduction inhibitor TAK-242 and its therapeutic effect on mouse sepsis model. Br J Pharmacol. 2009;157:1250–1262. doi: 10.1111/j.1476-5381.2009.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamta H, Pugh ND, Balachandran P, Moraes R, Sumiyanto J, Pasco DS. Variability in in vitro macrophage activation by commercially diverse bulk echinacea plant material is predominantly due to bacterial lipoproteins and lipopolysaccharides. Journal of agricultural and food chemistry. 2008;56:10552–10556. doi: 10.1021/jf8023722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyk BE. A broad review of commercially important southern African medicinal plants. Journal of ethnopharmacology. 2008;119:342–355. doi: 10.1016/j.jep.2008.05.029. [DOI] [PubMed] [Google Scholar]
- van Wyk BE, Albrecht C. A review of the taxonomy, ethnobotany, chemistry and pharmacology of Sutherlandia frutescens (Fabaceae) Journal of ethnopharmacology. 2008;119:620–629. doi: 10.1016/j.jep.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Wang J, Mazza G. Inhibitory effects of aAnthocyanins and other phenolic compounds on nitric oxide production in LPS/IFN-γ-activated RAW 264.7 macrophages. J Agric Food Chem. 2002;50:850–857. doi: 10.1021/jf010976a. [DOI] [PubMed] [Google Scholar]
- Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie G, Schepetkin IA, Quinn MT. Immunomodulatory activity of acidic polysaccharides isolated from Tanacetum vulgare L. International immunopharmacology. 2007;7:1639–1650. doi: 10.1016/j.intimp.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon YD, Han SB, Kang JS, Lee CW, Park SK, Lee HS, Kang JS, Kim HM. Toll-like receptor 4-dependent activation of macrophages by polysaccharide isolated from the radix of Platycodon grandiflorum. International immunopharmacology. 2003;3:1873–1882. doi: 10.1016/j.intimp.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Zhang B, Leung WK, Zou Y, Mabusela W, Johnson Q, Michaelsen TE, Paulsen BS. Immunomodulating polysaccharides from Lessertia frutescens leaves: Isolation, characterization and structure activity relationship. Journal of ethnopharmacology. 2014 doi: 10.1016/j.jep.2014.01.017. [DOI] [PubMed] [Google Scholar]
- Zhang L, Deng W. Structure characterization and adhesive ability of a polysaccharide from tendrils of Parthenocissus heterophylla. Natural product communications. 2014;9:541–544. [PubMed] [Google Scholar]