Abstract
There is mounting evidence that appropriately timed neuromuscular stimulation can induce neural plasticity and generate functional recovery from motor disorders. This review addresses the idea that coordinating stimulation with a patient’s voluntary effort might further enhance neurorehabilitation. Studies in cell cultures and behaving animals have delineated the rules underlying neural plasticity when single neurons are used as triggers. However, the rules governing more complex stimuli and larger networks are less well understood. We argue that functional recovery might be optimized if stimulation were modulated by a brain machine interface, to matched the details of the patient’s voluntary intent. The potential of this novel approach highlights the need for a better understanding of the complex rules underlying this form of plasticity.
Introduction
Brain Machine Interfaces (BMIs) hold great promise for improving the lives of patients with motor disabilities caused by stroke or spinal cord injury (SCI). Over the last 15 years, BMI users, mostly non-human primates, have controlled computer cursors [1–3] or robotic devices [4] directly from their thoughts. For a small number of human patients with neurological disorders, a BMI has actually replaced lost motor function [5,6]. These neuroprostheses typically rely on ‘decoders’ that map neural activity into the desired control signals, for example, cursor or robot motion.
A much larger number of patients with SCI or stroke have benefited from functional electrical stimulation (FES), electrical stimuli applied to muscles or nerves, used to restore both arm and leg function [7]. The most common application addresses foot drop by stimulating the common peroneal nerve to generate ankle dorsiflexion at the onset of swing (Figure 1A). Current FES neuroprostheses that restore grasp rely on preprogrammed stimulation patterns that the patient can initiate by residual proximal limb movements (Figure 1C).
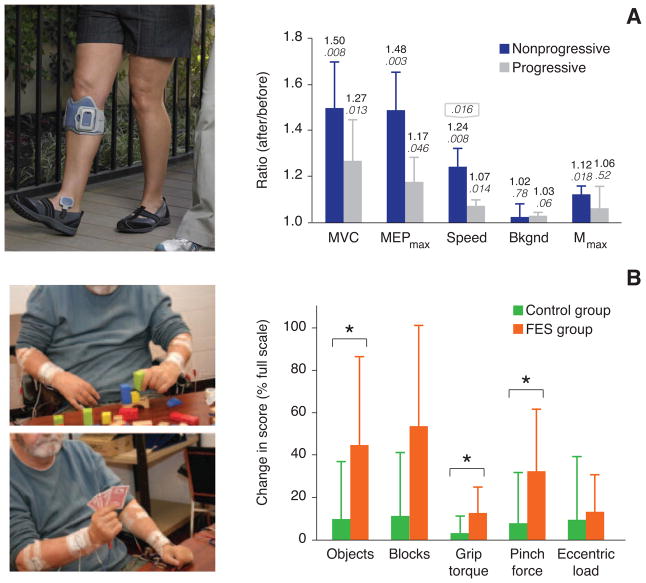
Figure 1.
Examples of the gain of function following long-term FES tested in the absence of stimulation. A: foot drop stimulator (L300, Bioness Inc., Valencia, CA, US) B: Long-term use (average: 5–6 months) improved several electrophysiological and biomechanical measures in patients with both nonprogressive (stroke and SCI) and progressive (multiple sclerosis) disorders. Improvement was greater in the former group (right panel). MVC = maximum voluntary contraction; Speed = walking speed; Bckgnd = voluntary contraction level at which the MEPs were measured; Mmax = maximum value of the M-wave. Adapted from [13]. C: Surface stimulation used to provide improved grasp function. Adapted from [14]. D: Hand function improved significantly in acute stroke patients following FES-assisted grasping (average: 13 weeks). When compared to controls, patients who underwent FES therapy exhibited greater improvement in object manipulation, palmar grip torque and pinch grip force (P < 0.05). Adapted from [16].
Recently, in experiments with monkeys, BMIs have been used to supply the control signals for FES, thereby overcoming the need to rely on residual movement [8–11]. Our group demonstrated the potential of this approach by restoring grasp in monkeys temporarily paralyzed by peripheral nerve block. We used the combined activity of nearly 100 cortical neurons to predict forearm flexor EMGs, which served as control signals driving stimulation of five electrodes [10].
There is an intriguing potential additional benefit of BMI-controlled FES: Its use in patients recovering from SCI or stroke may lead to recovered function beyond that of standard therapy. In a small number of patients with a variety of motor disorders, the use of FES to assist movement has led to recovered function that persisted after FES was discontinued in both walking (Figure 1B) [12,13] and use of the hands (Figure 1D) [14–16]. The functional recovery resulted from neural plasticity, likely including long-term potentiation (LTP) and depression (LTD) of existing synapses, axonal sprouting, and synapto- and neurogenesis, among other mechanisms [17,18]. Numerous studies involving single-neuron trigger sources have demonstrated the importance of timing of pre- and post-synaptic activity in the generation of these plastic changes [19,20] (see Figure 2A and text box). However, the importance of precise timing is less clear when numerous, continuously modulated neural pathways are involved.
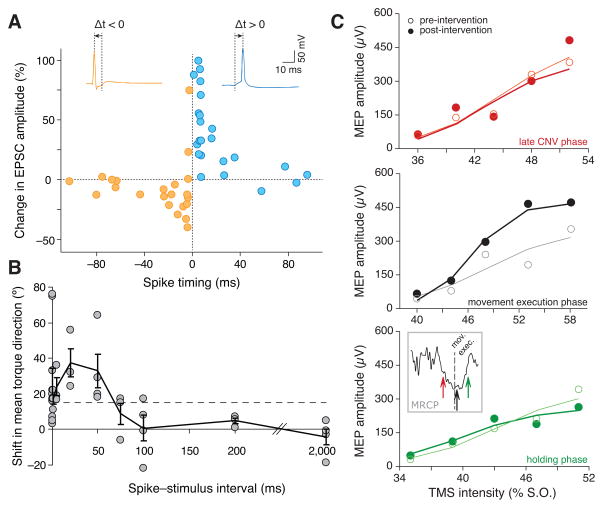
Figure 2.
Time dependence of various types of mechanisms for the induction of neural plasticity. A: Change in excitatory postsynaptic current (EPSC) as function of the inter-stimulus interval, illustrating STDP in an in vitro study involving two cells. Adapted from [20]. B: Dependence of the conditioning effect (shift in joint torque, reflecting a change in the motor output of the small population of M1 neurons activated by the stimulation) as function of the spike-stimulus interval, in an in vivo experiment in monkeys. The dashed line represents the 95th centile for controls, and shows that no significant changes are elicited if the spike-stimulus interval is > 50 ms. Adapted from [32]. C: Change in the magnitude of the MEP depending on the phase of the movement related cortical potential (MRCP) at which an afferent stimulus was delivered, in a study in healthy humans. Each panel compares the pre- and post-intervention MEP after conditioning at different phases of the MRCP (see inset). Adapted from [50]. CNV = contingent negative variation, the first deflection of the MRCP following a cue to initiate movement imagination.
Text Box. The complex timing of stimulus-driven plasticity.
In 1949, Donald Hebb suggested the postulate, now famously summarized as ‘neurons that fire together, wire together’ [64] that has formed the basis for many subsequent studies (see the reviews in [65,66]). Spike timing dependent plasticity (STDP) is an expression of a Hebbian mechanism, in which both the sign and magnitude of synaptic modification are determined by the precise timing of spikes (see Figure 2A; [19,20] and the review in [65]). Synapses tend to be strengthened as a result of a causal (i.e. greater than 0 and less than ~50ms) relation between pre- and post-synaptic activity. The opposite timing leads to decreased synaptic strength (postsynaptic activity leading presynaptic activity by <100ms; see Figure 2A). This law holds true under the relatively simple conditions that can be achieved in vitro, with constrained network properties, low stimulus rates, and a well-defined type of targeted receptor. However, it fails to describe more complex conditions, e.g., with triplets, quadruplets or trains of stimuli of higher frequencies [65,67]. These latter conditions are more similar to in vivo conditions, with more extended networks and higher firing rates. These conditions are often more accurately described by the Bienenstock-Cooper-Munro (BCM) model [68]. The BCM model is not based on individual spike events, and therefore does not consider precise timing but only the pre- and postsynaptic firing rates. Notably, the BCM and STDP models may lead to contradictory predictions even in simple scenarios. For example, when low frequency presynaptic stimulation is followed by postsynaptic stimulation, STDP predicts that the synapse will strengthen, while the BCM model predicts it will weaken because of the low frequency. The complexity of the rules underlying plasticity (see, e.g., [65–67,69,70] for more details) illustrates the challenge for inducing predictable large-scale plastic changes to promote recovery.
Neurological injury triggers widespread changes across the CNS and increases its plasticity, opening a window for therapeutic intervention soon after injury [21,22]. Unfortunately, all plasticity is not necessarily beneficial; it can also lead to maladaptive reorganization [22–26]. Potentially, the most effective way to guide adaptive plasticity would be by using a BMI to assist the patient’s attempted movements through control of a powered orthosis, or by artificially activating their own muscles through FES. The conjunction of cortical activity generated voluntarily, and movement-related afferent feedback may lead to adaptive plastic changes and improved functional recovery [11,26–31].
Induction of Synaptic Plasticity with Electrical Stimulation
In vivo, Spike-Triggered Stimulation to Induce Plastic Changes
Intracortical microstimulation (ICMS) triggered by naturally occurring action potentials has been used to induce neural plasticity in behaving animals, likely evoking mechanisms like those observed in vitro (Textbox 1). Following one or more days of spike-triggered stimulation in primary motor cortex (M1) of monkeys, test ICMS trains at the ‘trigger’ site began to activate some of the same muscles as the conditioned site, provided the trigger/target delay was less than 50 ms [32](Figure 2B). In a similar fashion, M1-triggered spinal stimulation was used to modify the strength of corticospinal projections [33]. The changes were seen in the post-spike facilitation of EMG, implicating an effect of the corticospinal terminals directly on motoneurons.
Our group evaluated the effects of spike-triggered stimulation on functional connectivity computed for small networks of neurons, and discovered that the stimulation altered not only the connectivity between trigger and target neurons, but also among neighboring cells [34]. These latter effects may have been due to preexisting network connections (e.g., horizontal fiber connections in M1 [25,35]) that distributed the stimulus effects to second-order neurons.
Spike-triggered stimulation has also been used to induce functional changes, including decreased detection thresholds for particular electrodes [36] and even recovery of reach and grasp function after motor cortical infarct in rats [37]. In that study, spike-triggered stimulation of somatosensory cortex (S1) 7.5 ms after spikes in premotor cortex significantly improved grasp function compared to rats receiving randomly triggered stimulation [37]. Results like these suggest an exciting potential application to neurorehabilitation.
Paired Stimulation to Induce Neural Plasticity
The paired associative stimulation (PAS) paradigm is a method used to induce neural plasticity non-invasively in humans. It has been applied both to healthy individuals [38] and patients suffering from stroke or other disorders (see [39] for a recent review). In a typical PAS experiment, neural plasticity is induced by the combination of transcranial magnetic stimulation (TMS) and peripheral nerve stimulation. As in STDP (Figure 2A; Textbox 1), the inter-stimulus interval can define both the sign and magnitude, and even the location of the effects.
If the inter-stimulus interval is such that the ascending afferent activity generated in motor cortex by the peripheral stimulation (blue pathway in Figure 3) coincides approximately with the post-synaptic cortical activity generated by TMS, the corresponding sensory inputs to the motor cortex will be potentiated [38,39]. In fact, a 25 ms inter-stimulus interval caused increases in the motor-evoked potentials generated in hand muscles by TMS [38], while a 10 ms interval (which reversed the timing between pre- and post-synaptic activity) caused decreases [40]. The effects are thought to be due to the induction of LTP and LTD, respectively. Similarly, TMS timed to generate presynaptic activity slightly preceding the antidromic activation of motoneurons (red pathway in Figure 3) caused the strength of corticospinal inputs to those motoneurons to be increased (black synapses on red cell in Figure 3). The changes were revealed by increased cervicomedullary motor-evoked potentials in the biceps [41]. Interstimulus intervals of either 22 or −13 ms led to the opposite effect. Finally, increases in the H-reflex following PAS have revealed changes in the spinal cord as well, potentially occurring in the synapses between Ia afferents and motoneurons, in the motoneurons themselves, or in presynaptic inhibition [42].
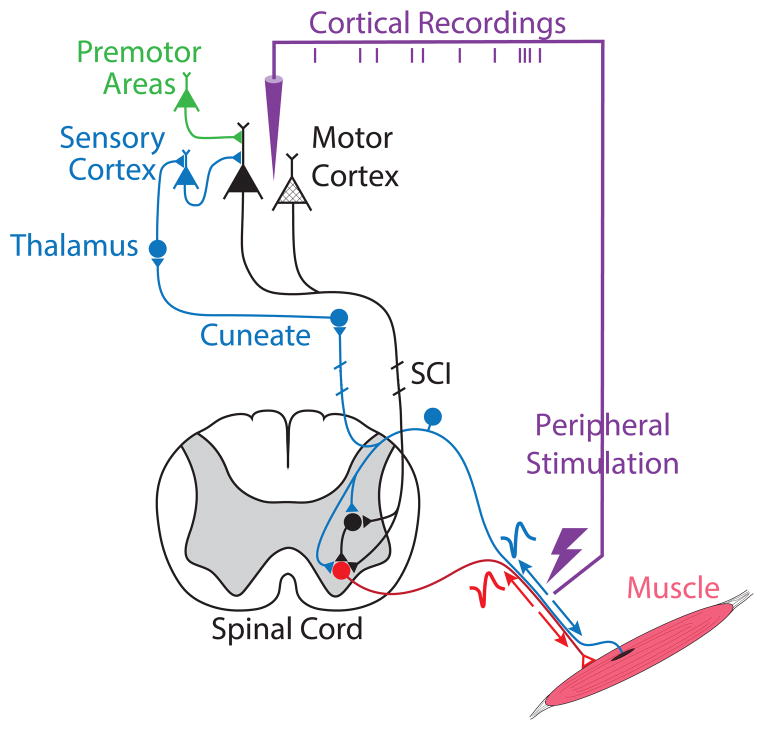
Figure 3.
After an ischemic stroke or SCI, motor deficits are caused by the death of a subpopulation of cortical neurons (hashed triangles) or the interruption of the descending pathways. Brain-controlled FES (purple line) may be used to strengthen the brain connections to the paretic muscles by inducing neural plasticity. FES-induced action potentials travelling antidromically to the motoneurons (red cell) may be made to coincide systematically with descending (black pathway) or sensory (blue) spinal cord inputs, thus altering their connectivity to motoneurons. FES-induced afferent activity (blue pathways) can also be made to coincide with the central activity related to voluntary effort (green and black cells) and induce plasticity in supraspinal networks.
Guiding Plasticity for Neurorehabilitation
Associating Stimulation and Voluntary Effort
Paired stimulation techniques such as PAS have been shown to induce cortical and spinal plasticity, with some evidence of functional recovery after SCI [43] and stroke [39] as well. There is some evidence that FES, using preprogrammed stimulus trains timed to coincide with voluntary effort and designed to effect movement, may accelerate recovery in both SCI and stroke [12–16,28,44]. Likewise, there is evidence that even continuous stimulation combined with voluntary effort can lead to improved motor function in both a rat model of SCI [31] and human SCI patients [29]. In these cases, recovery was dependent on stimulation, and progressed with continued treatment. In the rodent study, recovery was accompanied by remodeling of cortical projections to brainstem and spinal sites [31,45]. In more recent experiments, the same group monitored the gait cycle to modulate stimulation in real-time [46], in an attempt to match the resulting movements more closely to the voluntary effort.
The correspondence between voluntary effort and peripheral stimulation indeed appears to be an important factor [47]. This observation raises an important question: To what extent would functional recovery be improved by matching stimulus dynamics ever more closely to the patient’s voluntary motor commands? Answering this question is critical, as the closest match would likely be achieved only by using invasive, intracortical recordings. Unfortunately, there is little experimental evidence bearing directly on this question. Several studies have found improved finger movements in stroke patients, when EEG was used to trigger either a preprogrammed FES waveform [48,49] or orthosis movement [30] to assist function. Neither study addressed timing, and only the latter included a control group.
In healthy individuals, single peripheral stimuli paired with the onset of imagined leg movement detected by EEG induced LTP in the corticospinal pathway [50,51]. These effects were dependent on stimulus timing, but with less precision than that required by PAS (Fig. 1C). Another study in healthy individuals compared the size of MEPs following a series of grasping movements that were assisted by a fixed FES train triggered by EMG, EEG, or manually by the therapist [52]. In this study, manual triggering was least, and EMG most effective. In the following sections we review several approaches to detect motor intent, and their therapeutic potential.
EMG-Triggered Stimulation
For patients with adequate residual motor activity, EMG recordings may provide a good estimate of motor intent. Residual EMG used to trigger epidural spinal stimulation boosted the strength of muscle contraction achieved by a monkey with an incomplete SCI [11]. Stroke patients receiving preprogrammed FES patterns triggered from EMG outperformed patients who received FES only for strength training, or standard physical therapy [53–55]. Similar studies with SCI patients also report increased muscle force [56].
Despite its potential, EMG-triggered FES has at least three important limitations. First, it will be ineffective for patients who cannot voluntarily contract their muscles, or who display abnormal muscle activity patterns. Second, the use of pre-programmed stimulation patterns, necessitated in part by stimulus artifacts in EMG, may not match motor intent well. Finally, the delay between cortical and EMG activity may limit the ability to achieve optimal stimulus timing.
EEG-Triggered Stimulation
Detecting motor intent directly from the brain is an attractive alternative [26], particularly for patients with more complete loss of voluntary movement. However, detection of movement onset from EEG can be highly variable, ranging at least 100–300 ms [57,58]. Reliability can be improved with longer sampling times, but at the expense of even greater latency [59]. It is worth asking whether the apparent decreased sensitivity to timing [50,51] in these experiments compared to STDP or PAS (Figure 2) is simply due to the imprecision of movement detection by EEG. Perhaps greater detection precision would lead to even more pronounced effects, more sharply tuned in time. EEG has been used for continuous cursor control in two, and even three dimensions [3]. However, these subjects use learned arbitrary motor imagery, typically of different body parts, to control movement along different axes. As such, it would not likely represent an ideal control signal to match FES to natural motor intent.
Intracortical Control
Intracortical recordings as a means to control stimulation for restoration of function and functional recovery have been explored in monkeys by coupling LFP recordings to intraspinal stimulation, allowing limited control of voluntary movement after SCI [11]. Cortical recordings from spinal cord injured rats have also been used in real-time, to replace lever pressing [60] or control pelvic support force supplied through a robot [61]. Our group has used M1 discharge to make predictions of EMG [9,10,62]. This raises the possibility of using FES trains that are precisely modulated in time to match the motor intent for individual muscles. We speculate that this specific ‘association’ of motor intent with both peripheral afferent activity, and antidromic motoneuron activity (see neural pathways in Figure 3), might provide a much more effective stimulus for adaptive plasticity and functional recovery than the single pulses, or unmodulated FES trains used in most previous experiments. Further animal studies using invasive recordings will be required to explore the potential of this approach.
Conclusion
Interventions that promote activity-dependent plasticity by associating motor intent with artificially generated movement and afferent activity using electrical stimulation constitute a promising avenue for promoting recovery after neurological injury. However, we still have an incomplete understanding of the principles underlying stimulus-driven neural plasticity, and how to apply it optimally to promote adaptive forms of plasticity while suppressing maladaptive changes. We know that timing is critical for paradigms used to induce plasticity based on the discharge of single neurons, both in vitro, and in vivo. However, the precise timing required for these approaches may be less relevant for the adaptive plasticity that underlies functional recovery in the context of large networks of neurons and continuously modulated activity. Simply increasing overall synaptic strength is unlikely to be optimal, as it would be expected to increase reflex gains as well as the strength of descending inputs, potentially increasing symptoms of spasticity. Rather, we speculate that by more closely reproducing the normal patterns of pre- and post-synaptic activity in corticospinal and reflex circuits, an optimal combination of synaptic potentiation and depression might be achieved.
However, there is as of yet no experimental evidence bearing directly on this possibility, nor on the time course after injury for which such an intervention might be most effective. It is not obvious that a stroke patient or an SCI patient with residual function would elect to receive an intracortical implant for the purposes of rehabilitation, particularly in the early stages of recovery. Compelling experimental evidence of superior therapeutic benefit compared to the current best clinical practice would be essential to tip the balance in favor of this substantially more invasive approach. The choice is made more difficult by the recognition that the mechanisms leading to recovery are complex and dependent on many factors, including the type and severity of the neurological injury and the time after the lesion. However, it should be recognized that deep brain stimulation for Parkinson’s disease, a considerably more invasive procedure, is now a well accepted procedure, despite remaining controversy surrounding its mechanisms of action [63]. We assert that the results from studies that aim at driving plastic changes to improve recovery after SCI or stroke are sufficiently encouraging to warrant further research.
Brain-controlled Functional Electrical Stimulation (FES) can restore motor function
Appropriately timed neuromuscular electrical stimulation drives plastic changes
Site, sign, and magnitude of changes depend on coordination with central activity
Hence, brain-controlled FES may cause long-lasting recovery following stroke or SCI
Acknowledgments
This work was supported in part by grant #NS053603 from the National Institute of Neurological Disorder and Stroke (L. Miller), and a Marie Curie post-doctoral fellowship FP7-PEOPLE-2013-IOF-627384 (J. Gallego)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Serruya MD, Hatsopoulos NG, Paninski L, Fellows MR, Donoghue JP. Instant neural control of a movement signal. Nature. 2002;416:141–2. doi: 10.1038/416141a. [DOI] [PubMed] [Google Scholar]
- 2.Taylor DM, Tillery SI, Schwartz AB. Direct cortical control of 3D neuroprosthetic devices. Science (80-) 2002;296:1829–1832. doi: 10.1126/science.1070291. [DOI] [PubMed] [Google Scholar]
- 3.McFarland DJ, Sarnacki WA, Wolpaw JR. Electroencephalographic (EEG) control of three-dimensional movement. J Neural Eng. 2010;7:36007. doi: 10.1088/1741-2560/7/3/036007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Velliste M, Perel S, Spalding MC, Whitford AS, Schwartz AB. Cortical control of a prosthetic arm for self-feeding. Nature. 2008;453:1098–1101. doi: 10.1038/nature06996. [DOI] [PubMed] [Google Scholar]
- 5.Hochberg LR, Serruya MD, Friehs GM, Mukand Ja, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–71. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 6••.Collinger JL, Wodlinger B, Downey JE, Wang W, Tyler-Kabara EC, Weber DJ, McMorland AJC, Velliste M, Boninger ML, Schwartz AB. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet. 2013;381:557–64. doi: 10.1016/S0140-6736(12)61816-9. This is the current state of the art in control of a robotic arm for reaching and grasping by a paralyzed individual using an intracortical BMI. By controlling seven dimensions (three for positioning the hand, three for orienting it, and one for opening/closing it), the patient was able to grasp and move a variety of objects. Performance improved steadily from the time of implant to publication, a span of approximately 100 days. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peckham PH, Kilgore KLK. Challenges and Opportunities in Restoring Function After Paralysis. Biomed Eng IEEE. 2013;60:602–609. doi: 10.1109/TBME.2013.2245128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moritz CT, Perlmutter SI, Fetz EE. Direct control of paralysed muscles by cortical neurons. Nature. 2008;456:639–42. doi: 10.1038/nature07418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pohlmeyer Ea, Oby ER, Perreault EJ, Solla Sa, Kilgore KL, Kirsch RF, Miller LE. Toward the restoration of hand use to a paralyzed monkey: brain-controlled functional electrical stimulation of forearm muscles. PLoS One. 2009;4:e5924. doi: 10.1371/journal.pone.0005924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••.Ethier C, Oby ER, Bauman MJ, Miller LE. Restoration of grasp following paralysis through brain-controlled stimulation of muscles. Nature. 2012;485:368–71. doi: 10.1038/nature10987. Intracortically-controlled electrical stimulation of transiently paralysed forearm flexor muscles was used to restore grasp in a non-human primate model of paralysis. The stimulation of each muscle was independently controlled based on a detailed prediction of the monkey’s intended muscle activity, derived from the recordings of as many as one hundred motor cortical neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishimura Y, Perlmutter SI, Fetz EE. Restoration of upper limb movement via artificial corticospinal and musculospinal connections in a monkey with spinal cord injury. Front Neural Circuits. 2013;7:57. doi: 10.3389/fncir.2013.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daly JJ, Roenigk K, Holcomb J, Rogers JM, Butler K, Gansen J, McCabe J, Fredrickson E, Marsolais EB, Ruff RL. A randomized controlled trial of functional neuromuscular stimulation in chronic stroke subjects. Stroke. 2006;37:172–8. doi: 10.1161/01.STR.0000195129.95220.77. [DOI] [PubMed] [Google Scholar]
- 13.Everaert DG, Thompson AK, Chong SL, Stein RB. Does functional electrical stimulation for foot drop strengthen corticospinal connections? Neurorehabil Neural Repair. 2010;24:168–77. doi: 10.1177/1545968309349939. [DOI] [PubMed] [Google Scholar]
- 14.Popovic MR, Kapadia N, Zivanovic V, Furlan JC, Craven BC, McGillivray C. Functional electrical stimulation therapy of voluntary grasping versus only conventional rehabilitation for patients with subacute incomplete tetraplegia: a randomized clinical trial. Neurorehabil Neural Repair. 2011;25:433–42. doi: 10.1177/1545968310392924. [DOI] [PubMed] [Google Scholar]
- 15.Popovic MB, Popovic DB, Sinkjaer T, Stefanovic A, Schwirtlich L. Clinical evaluation of Functional Electrical Therapy in acute hemiplegic subjects. J Rehabil ResDev. 2003;40:443–53. doi: 10.1682/jrrd.2003.09.0443. [DOI] [PubMed] [Google Scholar]
- 16.Thrasher TA, Zivanovic V, McIlroy W, Popovic MR. Rehabilitation of reaching and grasping function in severe hemiplegic patients using functional electrical stimulation therapy. Neurorehabil Neural Repair. 2008;22:706–14. doi: 10.1177/1545968308317436. [DOI] [PubMed] [Google Scholar]
- 17.Wolpaw JR. What can the spinal cord teach us about learning and memory? Neuroscientist. 2010;16:532–49. doi: 10.1177/1073858410368314. [DOI] [PubMed] [Google Scholar]
- 18.Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol. 2011;7:76–85. doi: 10.1038/nrneurol.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markram H, Lübke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–5. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- 20.Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–72. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10:861–72. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 22•.Dietz V, Fouad K. Restoration of sensorimotor functions after spinal cord injury. Brain. 2014;137:654–67. doi: 10.1093/brain/awt262. This recent review discusses the promise of new rehabilitation techniques to promote sensorimotor recovery after SCI. The authors review clinical approaches aimed at driving neuroplasticity through functional training. They also present pre-clinical studies exploring the use of electrical and pharmacological stimulation of the spinal cord networks to restore function by compensating for the loss of descending inputs. [DOI] [PubMed] [Google Scholar]
- 23•.Moxon KA, Oliviero A, Aguilar J, Foffani G. Cortical reorganization after spinal cord injury: always for good? Neuroscience. 2014;283:78–94. doi: 10.1016/j.neuroscience.2014.06.056. This review describes plastic changes in the cortex following spinal cord injury, including studies in both animal models and human patients The authors discuss the effect of age and time after the injury, as well as how pharmacological treatment and physical therapy promote cortical reorganization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dancause N, Nudo RJ. Shaping plasticity to enhance recovery after injury. Prog Brain Res. 2011;192:273–95. doi: 10.1016/B978-0-444-53355-5.00015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Nudo RJ. Recovery after brain injury: mechanisms and principles. Front Hum Neurosci. 2013;7:887. doi: 10.3389/fnhum.2013.00887. This review paper discusses studies of the neurophysiological and neuroanatomical (structural) changes triggered by behavioral experience, brain injury, and the interaction of these processes. It emphasizes how plastic changes following the injury resemble those during normal brain development, and how experience can make them adaptive or maladaptive. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daly JJ, Wolpaw JR. Brain-computer interfaces in neurological rehabilitation. Lancet Neurol. 2008;7:1032–43. doi: 10.1016/S1474-4422(08)70223-0. [DOI] [PubMed] [Google Scholar]
- 27•.Jackson A, Zimmermann JB. Neural interfaces for the brain and spinal cord - restoring motor function. Nat Rev Neurol. 2012;8:690–9. doi: 10.1038/nrneurol.2012.219. The authors describe how, based on our current understanding of activity-dependent neural plasticity, a variety of neural interfaces could be used to drive plastic changes and promote recovery afer SCI. They discuss recent evidence of the therapeutic effect of neuroelectronic systems, and they propose mechanisms by which closed-loop devices, such as brain-machine interfaces that combine neural recording and electrical stimulation, may extend our ability to control neural plasticity for therapeutic applications. [DOI] [PubMed] [Google Scholar]
- 28.Jung R, Belanger A, Kanchiku T, Fairchild M, Abbas JJ. Neuromuscular stimulation therapy after incomplete spinal cord injury promotes recovery of interlimb coordination during locomotion. J Neural Eng. 2009;6:55010. doi: 10.1088/1741-2560/6/5/055010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain. 2014 doi: 10.1093/brain/awu038. Four patients with SCI (two with motor and sensory complete, two with motor complete but sensory incomplete injury) regained voluntary control of their paralyzed leg muscles through subthreshold neurostimulation of the spinal cord below the injury. As training progressed, the stimulation intensity necessary to enable volitional control decreased, the maximum voluntary force increased, and the patient’s volitional motor control improved. These promising results raise important questions about the neurophysiological substrate of recovery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Ramos-Murguialday A, Broetz D, Rea M, Läer L, Yilmaz O, Brasil FL, Liberati G, Curado MR, Garcia-Cossio E, Vyziotis A, et al. Brain-machine interface in chronic stroke rehabilitation: a controlled study. Ann Neurol. 2013;74:100–8. doi: 10.1002/ana.23879. Sixteen chronic stroke patients with severe hand weakness received training with a robotic orthosis that assisted movement execution when the patient’s intention to move was detected from changes in sensorimotor rhythms in the ipsilesional EEG. The therapy led to improved hand function, as assessed with a standard clinical scale, compared to a control group in which movement of the orthoses occurred randomly. The recovery was paralled by an increase in paretic muscle activity and a shift of motor and premotor cortical activity towards a more normal pattern, according to fMRI observations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31••.Van den Brand R, Heutschi J, Barraud Q, DiGiovanna J, Bartholdi K, Huerlimann M, Friedli L, Vollenweider I, Moraud EM, Duis S, et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012;336:1182–5. doi: 10.1126/science.1217416. In this study, rats with spinal cord injury that received a combination of pharmacological and electrical stimulation of the spinal cord recovered supraspinal control of locomotion. However, the recovery was contigent upon the rat’s active attempts to initiate stepping movements. The spinalized rats were supported by a robotic postural interface, but needed to generate active leg movements to move toward a reward. This recovery relied on extensive remodeling of cortical projections, including the formation of new brainstem and intraspinal connections, as observed by microscopy. Rats with similar spinal stimulation that were placed on a treadmill did not achieve similar recovery. In that case, automated hindlimb stepping may have been produced simply by the sensory inputs elicited by the treadmill motion. These results highlight that the subject’s voluntary effort is very important when trying to induce plasticity and recovery. [DOI] [PubMed] [Google Scholar]
- 32.Jackson A, Mavoori J, Fetz EE. Long-term motor cortex plasticity induced by an electronic neural implant. Nature. 2006;444:56–60. doi: 10.1038/nature05226. [DOI] [PubMed] [Google Scholar]
- 33.Nishimura Y, Perlmutter SI, Eaton RW, Fetz EE. Spike-timing-dependent plasticity in primate corticospinal connections induced during free behavior. Neuron. 2013;80:1301–9. doi: 10.1016/j.neuron.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Rebesco JM, Stevenson IH, Körding KP, Solla Sa, Miller LE. Rewiring neural interactions by micro-stimulation. Front Syst Neurosci. 2010;4:1–15. doi: 10.3389/fnsys.2010.00039. The authors investigated how the functional connectivity within a network of motor cortical neurons changed following two-three days of continuous spike-triggered stimulation. The precisely timed stimulation not only increased the connectivity between the trigger and target sites, but also induced more general reorganization of the network. As in previous studies, these observations were contingent on using a short (5 ms) spike-stimulus interval. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capaday C, Ethier C, Brizzi L, Sik A, van Vreeswijk C, Gingras D. On the nature of the intrinsic connectivity of the cat motor cortex: evidence for a recurrent neural network topology. J Neurophysiol. 2009;102:2131–41. doi: 10.1152/jn.91319.2008. [DOI] [PubMed] [Google Scholar]
- 36.Rebesco JM, Miller LE. Enhanced detection threshold for in vivo cortical stimulation produced by Hebbian conditioning. J Neural Eng. 2011;8:016011. doi: 10.1088/1741-2560/8/1/016011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.Guggenmos DJ, Azin M, Barbay S, Mahnken JD, Dunham C, Mohseni P, Nudo RJ. Restoration of function after brain damage using a neural prosthesis. Proc Natl Acad Sci U SA. 2013;110:21177–82. doi: 10.1073/pnas.1316885110. This study in a rodent model of focal brain injury provides the first demonstration of functional recovery induced by spike-triggered stimulation of the cortex. Single stimuli delivered to a site within somatosensory cortex, 7 ms after a spike was detected in the premotor cortex, led to improved grasping compared to controls receiving no stimulation or stimulation uncorrelated to premotor activity. The recovered grasping abilities compared to pre-lesion performance. Post-hoc analysis revealed that the improvement was paralleled by an increase in the functional connectivity between the two cortical sites. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123(Pt 3):572–84. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- 39•.Carson RG, Kennedy NC. Modulation of human corticospinal excitability by paired associative stimulation. Front HumNeurosci. 2013;7:823. doi: 10.3389/fnhum.2013.00823. This paper provides a thorough review of peri-associative stimulation (PAS), one of the most common paradigms used to induce plasticity in the human nervous system. Together with an extensive description of the literature, the authors discuss the neural pathways that may be conditioned through the application of PAS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, Benecke R, Classen J. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–45. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- 41.Taylor JL, Martin PG. Voluntary motor output is altered by spike-timing-dependent changes in the human corticospinal pathway. J Neurosci. 2009;29:11708–16. doi: 10.1523/JNEUROSCI.2217-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meunier S, Russmann H, Simonetta-Moreau M, Hallett M. Changes in spinal excitability after PAS. J Neurophysiol. 2007;97:3131–5. doi: 10.1152/jn.01086.2006. [DOI] [PubMed] [Google Scholar]
- 43.Bunday KL, Perez MA. Motor recovery after spinal cord injury enhanced by strengthening corticospinal synaptic transmission. Curr Biol. 2012;22:2355–61. doi: 10.1016/j.cub.2012.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kafri M, Laufer Y. Therapeutic Effects of Functional Electrical Stimulation on Gait in Individuals Post-Stroke. Ann Biomed Eng. 2014 doi: 10.1007/s10439-014-1148-8. [DOI] [PubMed] [Google Scholar]
- 45.Borton D, Bonizzato M, Beauparlant J, DiGiovanna J, Moraud EM, Wenger N, Musienko P, Minev IR, Lacour SP, Millán JDR, et al. Corticospinal neuroprostheses to restore locomotion after spinal cord injury. Neurosci Res. 2014;78:21–9. doi: 10.1016/j.neures.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Wenger N, Moraud EM, Raspopovic S, Bonizzato M, DiGiovanna J, Musienko P, Morari M, Micera S, Courtine G. Closed-loop neuromodulation of spinal sensorimotor circuits controls refined locomotion after complete spinal cord injury. Sci TranslMed. 2014;6:255ra133–255ra133. doi: 10.1126/scitranslmed.3008325. [DOI] [PubMed] [Google Scholar]
- 47.Stein RB, Everaert DG, Roy FD, Chong S, Soleimani M. Facilitation of corticospinal connections in able-bodied people and people with central nervous system disorders using eight interventions. J Clin Neurophysiol. 2013;30:66–78. doi: 10.1097/WNP.0b013e31827ed6bd. [DOI] [PubMed] [Google Scholar]
- 48.Daly JJ, Cheng R, Rogers J, Litinas K, Hrovat K, Dohring M. Feasibility of a new application of noninvasive Brain Computer Interface (BCI): a case study of training for recovery of volitional motor control after stroke. J Neurol PhysTher. 2009;33:203–11. doi: 10.1097/NPT.0b013e3181c1fc0b. [DOI] [PubMed] [Google Scholar]
- 49.Biasiucci A, Leeb R, Al-Khodairy A. Motor recovery after stroke by means of BCI-guided functional electrical stimulation. 5th Int. BCI; 2013. [Google Scholar]
- 50•.Mrachacz-Kersting N, Kristensen SR, Niazi IK, Farina D. Precise temporal association between cortical potentials evoked by motor imagination and afference induces cortical plasticity. J Physiol. 2012;590:1669–82. doi: 10.1113/jphysiol.2011.222851. The authors induced long-term potentiation of corticospinal projections in intact humans by delivering an afferent stimulus during a precise phase of the movement related cortical potential (MRCP) that occurs during movement imagination. They demonstrated that the association of presynaptic afferent input and postsynaptic volitional motor commands can be used to induce neural plasticity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niazi IK, Mrachacz-Kersting N, Jiang N, Dremstrup K, Farina D. Peripheral electrical stimulation triggered by self-paced detection of motor intention enhances motor evoked potentials. IEEE Trans Neural Syst Rehabil Eng. 2012;20:595–604. doi: 10.1109/TNSRE.2012.2194309. [DOI] [PubMed] [Google Scholar]
- 52.McGie SC, Zariffa J, Popovic MR, Nagai MK. Short-Term Neuroplastic Effects of Brain-Controlled and Muscle-Controlled Electrical Stimulation. Neuromodulation. 2014 doi: 10.1111/ner.12185. [DOI] [PubMed] [Google Scholar]
- 53.Francisco G, Chae J, Chawla H, Kirshblum S, Zorowitz R, Lewis G, Pang S. Electromyogram-triggered neuromuscular stimulation for improving the arm function of acute stroke survivors: a randomized pilot study. Arch Phys Med Rehabil. 1998;79:570–5. doi: 10.1016/s0003-9993(98)90074-0. [DOI] [PubMed] [Google Scholar]
- 54.De Kroon JR, Ijzerman MJ, Chae J, Lankhorst GJ, Zilvold G. Relation between stimulation characteristics and clinical outcome in studies using electrical stimulation to improve motor control of the upper extremity in stroke. J Rehabil Med. 2005;37:65–74. doi: 10.1080/16501970410024190. [DOI] [PubMed] [Google Scholar]
- 55•.Hara Y, Obayashi S, Tsujiuchi K, Muraoka Y. The effects of electromyography-controlled functional electrical stimulation on upper extremity function and cortical perfusion in stroke patients. Clin Neurophysiol. 2013;124:2008–15. doi: 10.1016/j.clinph.2013.03.030. This study provides the first direct evidence that EMG-triggered stimulation of paretic muscles to restore grasp after cerebrovascular accident improves function by increasing the activity in the ipsilesional brain hemisphere. Sixteen patients with moderate hemiparesis underwent five months of weekly or bi-weekly EMG-controlled FES therapy, resulting in higher grip strength and improved Fugl-Meyer score. EMG-controlled FES was shown by near-infrared spectroscopy to cause a greater activation of the ispsilesional sensorimotor cortex than voluntary effort or electrical stimulation alone. [DOI] [PubMed] [Google Scholar]
- 56.Van Overeem Hansen G. EMG-controlled functional electrical stimulation of the paretic hand. Scand J Rehabil Med. 1979;11:189–93. [PubMed] [Google Scholar]
- 57.Ibáñez J, Serrano JI, Del Castillo MD, Monge-Pereira E, Molina-Rueda F, Alguacil-Diego I, Pons JL. Detection of the onset of upper-limb movements based on the combined analysis of changes in the sensorimotor rhythms and slow cortical potentials. J Neural Eng. 2014;11:056009. doi: 10.1088/1741-2560/11/5/056009. [DOI] [PubMed] [Google Scholar]
- 58.Xu R, Jiang N, Lin C, Mrachacz-Kersting N, Dremstrup K, Farina D. Enhanced low-latency detection of motor intention from EEG for closed-loop brain-computer interface applications. IEEE Trans Biomed Eng. 2014;61:288–96. doi: 10.1109/TBME.2013.2294203. [DOI] [PubMed] [Google Scholar]
- 59.Do AH, Wang PT, King CE, Abiri A, Nenadic Z. Brain-computer interface controlled functional electrical stimulation system for ankle movement. J Neuroeng Rehabil. 2011;8:49. doi: 10.1186/1743-0003-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60•.Manohar A, Flint RD, Knudsen E, Moxon KA. Decoding hindlimb movement for a brain machine interface after a complete spinal transection. PLoS One. 2012;7:e52173. doi: 10.1371/journal.pone.0052173. This study investigated brain machine interface (BMI) control of hindlimb movement after complete lumbar spinal cord transection. Rats were trained to modulate their hindlimb motor cortical activity to receive a reward. After the injury, and despite the reduced modulation of cortical activity, the animals regained the ability to modulate their cortical activity to get a reward. Their performance improved with training. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song W, Giszter SF. Adaptation to a cortex-controlled robot attached at the pelvis and engaged during locomotion in rats. J Neurosci. 2011;31:3110–28. doi: 10.1523/JNEUROSCI.2335-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pohlmeyer EA, Solla Sa, Perreault EJ, Miller LE. Prediction of upper limb muscle activity from motor cortical discharge during reaching. J Neural Eng. 2007;4:369–79. doi: 10.1088/1741-2560/4/4/003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, Horak FB, Okun MS, Foote KD, Krack P, et al. Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol. 2011;68:165. doi: 10.1001/archneurol.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hebb DO. The Organization of Behavior. Wiley; 1949. [DOI] [PubMed] [Google Scholar]
- 65•.Froemke RC, Debanne D, Bi G-Q. Temporal modulation of spike-timing-dependent plasticity. Front Synaptic Neurosci. 2010;2:19. doi: 10.3389/fnsyn.2010.00019. After describing classic studies of STDP, this review discusses experimental evidence for the more complex interactions underlying plastic changes when using higher stimulation frequencies (> 5 Hz) or patterns of pre- and postsynaptic activity reflecting more closely those generated during execution of typical activities of daily living. They discuss potential underlying processes explaining synaptic plascitiy in these conditions, as well as mathematical models predicting the effects of complex trains on synaptic strength. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66••.Feldman DE. The spike-timing dependence of plasticity. Neuron. 2012;75:556–71. doi: 10.1016/j.neuron.2012.08.001. This article provides a broad review of synaptic plasticity, spanning cellular mechanisms underlying plastic changes seen in both in vitro and in vivo experiments. It underscores the fact that spike timing is one of several factors governing the induction of neural plasticity, along with firing rate and synaptic cooperativity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pfister J-P, Gerstner W. Triplets of spikes in a model of spike timing-dependent plasticity. J Neurosci. 2006;26:9673–82. doi: 10.1523/JNEUROSCI.1425-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2:32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dan Y, Poo M-M. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44:23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 70.Kleberg FI, Fukai T, Gilson M. Excitatory and inhibitory STDP jointly tune feedforward neural circuits to selectively propagate correlated spiking activity. Front Comput Neurosci. 2014;8:53. doi: 10.3389/fncom.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]