Abstract
There is growing evidence that brooding rumination plays a key role in the intergenerational transmission of major depressive disorder (MDD) and may be an endophenotype for depression risk. However, less is known about the mechanisms underlying this role. Therefore, the goal of the current study was to examine levels of brooding in children of mothers with a history of MDD (n = 129) compared to children of never depressed mothers (n = 126) and to determine whether the variation in a gene known to influence HPA axis functioning –corticotropin-releasing hormone receptor 1 (CRHR1) –would moderate the link between maternal MDD and children’s levels of brooding. We predicted children of mothers with a history of MDD would exhibit higher levels of brooding than children of mothers with no lifetime depression history but that this link would be stronger among children carrying no copies of the protective CRHR1 TAT haplotype. Our results supported these hypotheses and suggest that the development of brooding among children of depressed mothers, particularly children without the protective CRHR1 haplotype, may serve as an important mechanism of risk for the intergenerational transmission of depression.
Keywords: Intergenerational transmission of depression, brooding rumination, CRHR1, endophenotype, Major Depressive Disorder, cognitive vulnerability
Depressive disorders are common among children. For example, among community populations, about 10% of all children will meet the diagnostic criteria for a depressive disorder by age 16 (Costello, Mustillo, Erkanli, Keeler, & Angold, 2003). Childhood depression often indicates the start of a chronic and recurrent disorder and is associated with significant impairment in interpersonal, emotional, and achievement domains (Gibb, 2014). Although there is an extensive body of literature examining the psychological correlates of major depressive disorder (MDD) in children, less is known about the mechanisms underlying risk for depression. One of the most robust predictors of the development of MDD is a family history of the disorder. For example, children of depressed mothers are 3–4 times more likely to be diagnosed with MDD by early adulthood compared to individuals in the general population (for reviews, see Goodman, 2007; Hammen, 2009), and theorists have suggested that cognitive vulnerabilities featured in cognitive models of depression risk may be a key mechanism in the intergenerational transmission of depression (Goodman, 2007; Goodman & Gotlib, 1999). Furthermore, there is evidence that one form of cognitive vulnerability, brooding rumination, may be a central factor underlying various other forms of information-processing biases highlighted in cognitive models, including attentional biases and deficits in working memory (for a review, see Gotlib & Joormann, 2010). Therefore, the aim of the current study was to examine levels of brooding rumination in children of mothers with and without a history of MDD.
According to the response styles theory of depression (Nolen-Hoeksema, 1991; Nolen-Hoeksema, Wisco, & Lyubormirsky, 2008), the tendency to ruminate in response to a sad mood will increase the severity and duration of depressive symptoms, and there is growing support for the role of rumination in depression risk among youth. For example, a number of cross-sectional studies have found a relation between rumination and depressive symptoms among children and adolescents (for reviews, see Abela & Hankin, 2008; Rood, Roelofs, Bogels, Nolen-Hoeksema, & Schouten, 2009). Prospectively, there is evidence that rumination develops before the onset of depression and is a robust predictor of the onset of new depressive episodes in children and adolescents (e.g., Abela & Hankin, 2011; Gibb, Grassia, Stone, Uhrlass, & McGeary, 2012). Research suggests there are two distinct components of rumination termed brooding and reflection (Treynor, Gonzalez & Nolen-Hoeksema, 2003). Brooding, described as passively comparing one’s perceived reality with an unachieved standard, is thought to be a more maladaptive form of rumination than is reflection, a response style that is more oriented toward reappraisal (e.g., Armey et al., 2009; Gibb et al., 2012; Grassia & Gibb, 2008, 2009; Schoofs, Hermans, & Raes, 2010; Treynor et al., 2003).
With regard to the intergenerational transmission of depression, there is evidence that children of depressed mothers exhibit higher levels of brooding rumination than children of mothers with no history of depression, and elevated levels of brooding prospectively predict the onset of new depressive episodes in children of depressed mothers (Gibb et al., 2012). Although the precise mechanisms by which maternal depression contributes to the development of brooding rumination are not known, maternal depression is associated with a number of factors that theorists have proposed increase risk for the development of rumination including the presence of rumination in the mother and the use of negative parenting styles and criticism (e.g., Nolen-Hoeksema, 1991). Children of depressed mothers are also exposed to greater levels of chronic and episodic stress in their lives than children of nondepressed mothers (e.g., Feurer, Hammen, & Gibb, in press) and there is evidence that negative life events predict future increases in rumination (e.g., Michl, McLaughlin, Shepherd, & Nolen-Hoeksema, 2013). Therefore, maternal depression can be viewed as a marker for a number of negative influences to which the child is likely exposed, all of which may combine to increase risk for the development of brooding rumination.
Although there is evidence for a link between a history of maternal depression and brooding rumination in children, there is also substantial heterogeneity of cognitive profiles among children of depressed mothers and not all children of depressed mothers develop a cognitive vulnerability to depression themselves. Recently, there has been a growing interest in identifying genetic influences on various forms of cognitive vulnerability to depression (see Gibb, Beevers, & McGeary, 2013), which may help to identify specific subgroups of children who are most at risk for developing a cognitive vulnerability to depression. When seeking to identify specific genetic influences on cognitive vulnerability, a promising approach is to focus on genes known to affect activity in biological systems thought to underlie cognitive vulnerabilities (cf. Gibb et al., 2013). Research suggests that dysfunction in neuroregulatory systems, which arise from genetic and environmental factors and their interaction, may underlie cognitive vulnerabilities in children of depressed mothers (e.g., Goodman, 2007). More specifically, there is evidence that chronic activation of the hypothalamic-pituitary-adrenal (HPA) axis can lead to dysregulation in neural circuits essential for emotion regulation (e.g., prefrontal cortex, anterior cingulate cortex, and amygdala; see Gotlib & Colich, 2014). Further, studies have shown that heightened HPA axis reactivity leads to poorer prefrontal functioning (e.g., Wingenfeld & Wolf, 2011), which impacts rumination (for a review, see Gotlib & Joormann, 2010). Therefore, in the context of maternal depression, it may be that children who are the most biologically reactive to the stress associated with maternal depression are most likely to exhibit deficits in prefrontal functioning, which contributes to the development of maladaptive emotion regulation strategies, such as brooding rumination. Therefore, when examining genetic risk factors for brooding in children of depression mothers, a promising approach would be to focus on genes that influence HPA axis reactivity to environmental stress.
One candidate is the corticotropin-releasing hormone receptor 1 gene (CRHR1). The corticotropin-releasing hormone (CRH) activates the HPA axis by binding to corticotropin-releasing hormone type 1 receptors in the anterior pituitary, which triggers the release of adrenal corticotrophic hormone (ACTH). ACTH is transmitted through the blood stream and triggers the release of glucocorticoids by binding to receptors in the adrenal gland cortex (Bale & Vale, 2004). Variation in CRHR1 genotype has been shown to affect the level of cortisol released in response to laboratory-based stressors (Sheikh, Kryski, Smith, Hayden, & Singh, 2013), an effect that is amplified by the presence of childhood maltreatment (Tyrka et al., 2009). In addition, variation in the CRHR1 genotype also moderates the effects of early life stress on working memory performance (Fuge et al., 2014), an important element of prefrontal functioning. Finally, there is evidence that three CRHR1 single nucleotide polymorphisms (SNPs) – rs7209436, rs110402, and rs242924 – form a protective TAT haplotype such that, in the context of early life stress (e.g., maltreatment), adults with no copies of the protective TAT haplotype, compared to those carrying at least one copy of the haplotype, reported significantly higher current depressive symptoms and more depression diagnoses in adulthood (Bradley et al., 2008; Kranzler et al., 2011; Polanczyk et al., 2009; but see also Laucht et al., 2013). Taken together, these studies suggest that variation in the CRHR1 genotype, particularly the TAT haplotype, may play an important role in the development of risk for depression.
Depression, however, is a heterogeneous phenotype and both theorists and researchers have become increasingly focused on identifying intermediate phenotypes or endophenotypes, which are more homogeneous constructs that should be closer to the mechanism of action for specific genes (Gottesman & Gould, 2003; Miller & Rockstroh, 2013). Gottesman and Gould (2003) outlined five criteria for any putative endophenotype, suggesting that it must be: (i) associated with the disorder in the population, (ii) heritable, (iii) primarily state-independent, (iv) co-segregate with illness within families, and (v) found in unaffected family members more than in the general population. Rumination satisfies most of these requirements. As noted earlier, it is associated with depression concurrently (for reviews, see Abela & Hankin, 2008; Rood et al., 2009) and prospectively predicts onset of the disorder (Abela & Hankin, 2011; Gibb et al., 2012). Research also suggests that rumination is moderately heritable (h2 = .20–.41) and exhibits shared genetic variability with depression (Chen & Li, 2013; Johnson et al., 2014; Moore et al., 2013), supporting its potential role as an endophenotype. A key question then is whether high levels of brooding rumination will be observed in an at-risk population such as children of depressed mothers, whether this relation is moderated by specific genetic influences such as CRHR1, and whether the effects are at least partially independent of children’s own current or past depression.
The primary goal of this study, therefore, was to examine levels of brooding rumination in children of mothers with a history of MDD compared to children of mothers with no depression history and to determine whether the presence of one or more copies of the CRHR1 TAT haplotype in children would moderate the link between maternal MDD and children’s levels of brooding. We predicted children of mothers with a history of MDD would exhibit higher levels of brooding rumination than would children of mothers with no lifetime depression history. Furthermore, we hypothesized that CRHR1 haplotype would moderate the link between maternal MDD and children’s current levels of brooding rumination, such that the link would be stronger among children with no copies of the protective CRHR1 TAT haplotype. Finally, we sought to ensure that the results were not driven by the presence of current or past depression. Therefore, we predicted that these relations would be maintained even after excluding children with a lifetime history of major or minor depression and after statistically controlling for the influence of current depressive symptom levels.
Method
Participants
Participants in the present study were 255 mother-child dyads recruited for a study examining the intergenerational transmission of depression. To qualify for the study, mothers were required to either meet criteria for an MDD episode during the child’s lifetime according to the Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition (DSM-IV; American Psychiatric Association, 1994) (n = 129) or have no lifetime diagnosis of any DSM-IV mood disorder and no current Axis I diagnosis (n = 126). Exclusion criteria for both groups included the presence of schizophrenia symptoms, organic mental disorder, a history of alcohol or substance dependence within the last six months, or lifetime history of bipolar disorder. To participate in the study, children had to be between the ages of 8–14 years old and only one child per family was allowed to participate. If more than one child was available within the age range, one child was chosen at random for participation. The average age of children in our sample was 10.89 years (SD = 1.91), 53% were female, and 81% were Caucasian. The average age of mothers in our sample was 40.36 years (SD = 6.83, Range = 24–55) and 87% were Caucasian. The median annual family income was $50,001–55,000.
Measures
The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I; First, Spitzer, Gibbon, & Williams, 1995) and the Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Version (K-SADS-PL; Kaufman, Birmaher, Brent, & Rao, 1997) were used to assess for current and past DSM-IV Axis I disorders in mothers and children, respectively. The SCID-I and K-SADS-PL were administered by separate interviewers. For the K-SADS-PL, mothers and children were interviewed separately by the same interviewer. Women were coded as having a history of MDD during the child’s lifetime (n = 129) or having no lifetime history of any mood disorder (n = 126). Of the depressed mothers, 24 met criteria for current MDD not in partial remission. Of the children, 32 met criteria for a lifetime depressive disorder (MDD: n = 20; minor depression: n = 12). Inter-rater reliability was assessed for a subset of 20 SCID and 20 K-SADS interviews and reliability for diagnoses of MDD was excellent (all κs = 1.00).
Children’s depressive symptoms were assessed using the Children’s Depression Inventory (CDI; Kovacs, 1981). The CDI is a 27-item self-report instrument that has demonstrated excellent reliability and validity in previous studies (e.g., Kovacs, 1981, 1985; Smucker, Craighead, Craighead, & Green, 1986). It exhibited good internal consistency in the current sample (α= .86).
Children’s levels of rumination were assessed with the Children’s Response Style Scale (CRSS; Ziegert & Kistner, 2002). CRSS is a 20-item self-report questionnaire with higher scores indicating greater ruminative tendencies. The CRSS asks participants to identify the frequency with which they think or do certain things when they feel sad, down, or depressed (e.g., “Go some place alone to think about your feelings”). We focused on the brooding subscale, as previously described (e.g., Lopez et al., 2009; Treynor et al., 2003). This subscale of the CRSS has demonstrated good reliability and validity in previous research (Lopez et al., 2009; Muris, Roelofs, Meesters, & Boomsma, 2004; Ziegert & Kistner, 2002) and exhibited adequate internal consistency in the current study (α = .67).
Finally, children provided DNA samples via cheek swabs. DNA was collected and isolated from buccal cells, as previously described (Freeman et al., 1997; Lench, Stanier, & Williamson, 1988). The samples were genotyped for 3 SNPs on the CRHR1 gene – rs7209436, rs110402, and rs242924 – that form a T-A-T haplotype (Bradley et al., 2008; Polanczyk et al., 2009). The three CRHR1 polymorphisms were genotyped using fluorogenic 5′ nuclease (Taqman, Applied Biosystems, Foster City, CA) method involving reagents (VIC(tm) and FAM(tm), labeled probes, and TaqMan® Universal PCR Master Mix without AMPerase® UNG) obtained from Applied Biosystems (ABI). Genotype determination was performed using primers purchased from ABI or Integrated DNA Technologies (Coralville, IA). Genotypes were obtained using an ABI Prism 7300 Sequence Detection System using both absolute quantification and allelic discrimination modes (Livak, Flood, Marmaro, Giusti, & Deetz, 1995). All markers were found to be in the Hardy-Weinberg equilibrium using default parameters in Haploview (Barrett, 2009). In order to (a) maximize the amount of information provided by the multiple markers, and (b) circumvent loss of power due to multiple testing, we utilized all of the available SNP data to identify haplotype blocks (i.e., the combinations of SNP markers that are statistically associated). Haploview was used to visualize haplotype blocks (Barrett, 2009; Barrett et al., 2005). Haplotypes for both chromosomes were then confirmed and extracted using PHASE Version 2.1 (Stephens and Donnelly, 2003; Stephens et al., 2001), requiring that the probability of a haplotype be greater than or equal to 0.80. PHASE haplotypes were used to construct diplotypes (i.e., combination of haplotypes across the pair of homologous chromosomes) that were used in the regression analyses. This scoring scheme was utilized for every haplotype that was more frequent than .20 in the study sample. Marker to marker D′ correlations are as follows: rs7209436 – rs110402 = 0.95, rs7209436 – rs242924 = 0.88, rs110402 – rs242924 = 0.93. Marker-to-marker D′ values for the CRHR1 polymorphisms varies between 0 and 1 and describe the extent of linkage disequilibrium, a measure of interdependency between genetic loci. A value of 0 for D′ suggests that the examined SNPs are independent of one another, while a value of 1 suggests that the SNPs provide redundant information. Consistent with previous research suggesting that the presence of one or more TAT haplotypes is protective (Bradley et al., 2008; Kranzler et al., 2011; Polanczyk et al., 2009) and due to the low number of children who had two copies of the haplotype (n = 11 children of depressed mothers and n = 16 children of control mothers), CRHR1 TAT haplotype was coded as presence (n = 151) or absence (n = 104) of at least one copy of the haplotype.
Procedure
Potential participants were recruited from the community through a variety of means (e.g., television, newspaper and bus ads, flyers). Mothers responding to the recruitment advertisements were initially screened over the phone to determine potential eligibility. Upon arrival at the laboratory, women were asked to provide informed consent and children were asked to provide assent to be in the study. Next, the child completed the CDI and CRSS. Following this, the mother was administered the K-SADS-PL by a trained interviewer. After completing the K-SADS-PL with the mother, the same interviewer then administered the K-SADS-PL to the child. While the child was administered the K-SADS-PL, the mother was administered the SCID-I by a separate interviewer. Families were compensated a total of $75 and children received a $10 store gift card for their participation in the study. The project was approved by the university’s internal review board.
Results
A preliminary inspection of the data revealed the presence of some missing data, with up to 2% missing for any given variable due to participant nonresponse. Given the presence of missing data, we examined whether the data were missing at random, thereby justifying the use of data imputation methods for estimating missing values (cf. Shafer & Graham, 2002). Little’s missing completely at random (MCAR) test, for which the null hypothesis is that the data are MCAR (Little & Rubin, 1987), was nonsignificant, χ2(88) = 95.94, p = .26, providing support for the imputation of missing values. Given these results, maximum likelihood estimates of missing data were created and used in all subsequent analyses (see Shafer & Graham, 2002).1 Demographic and clinical variables among children of depressed versus nondepressed mothers as a function of child CRHR1 TAT haplotype are presented in Table 1.
Table 1.
Demographic and clinical variables among children of depressed versus nondepressed mothers as a function of child CRHR1 TAT haplotype.
| 0 Copies of TAT Haplotype
|
1–2 Copies of TAT Haplotype
|
|||
|---|---|---|---|---|
| Variable | Control Mom | MDD Mom | Control Mom | MDD Mom |
|
|
|
|
||
| N | 47 | 57 | 79 | 72 |
| Age | 10.74 (1.96) | 10.96 (2.00) | 11.03 (1.72) | 10.76 (2.02) |
| % girls | 66% | 58% | 47% | 47% |
| % Caucasian | 92% | 68% | 90% | 75% |
| CRSS Brooding | 24.97 (9.46) | 30.54 (8.46) | 27.12 (9.37) | 26.36 (11.02) |
| CDI | 1.98 (1.18) | 2.62 (1.11) | 1.82 (1.19) | 2.38 (1.23) |
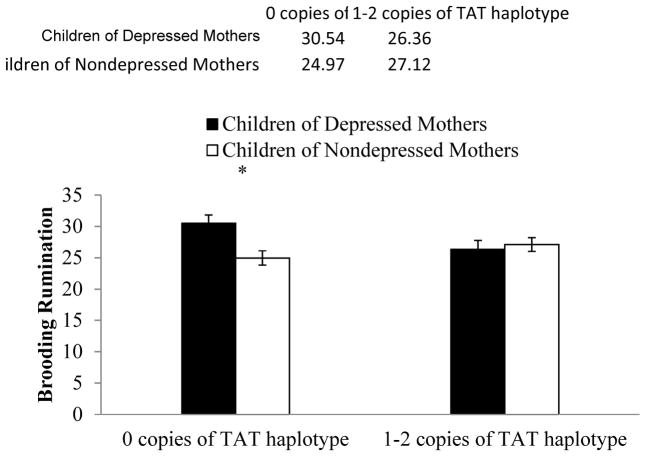
Next, we tested our hypothesis that children’s CRHR1 TAT haplotype would moderate the link between maternal MDD history and children’s levels of brooding rumination. Specifically, we conducted a 2 (maternal MDD history: yes, no) × 2 (CRHR1 TAT haplotype: 0 copies, at least 1 copy) ANOVA with children’s levels of brooding rumination serving as the dependent variable. In this analysis, the main effect of mother MDD was significant, F(1, 251) = 3.78, p = .05, ηp2 = .02, showing that children with a maternal history of MDD, compared to children with no maternal history of MDD, exhibited higher levels of brooding rumination. The CRHR1 main effect was nonsignificant, F(1, 251) = 0.67, p = .41, ηp2 = .003. Importantly, the predicted mother MDD x CRHR1 interaction was significant, F(1, 251) = 6.53, p = .01, ηp2 = .03. Examining the form of this interaction, we found that among children with no copies of the protective CRHR1 TAT haplotype, children of mothers with a history of MDD had significantly higher levels of brooding than children of mothers with no history of depression, t(102) = 3.17, p = .002, reffect size = .30. In contrast, among children with at least one copy of the TAT haplotype, the link between mothers’ MDD history and children’s levels of brooding was nonsignificant, t(149) = −0.46, p = .65, reffect size = −.04 . Indeed, levels of brooding in children of depressed mothers with at least 1 copy of the TAT haplotype did not differ significantly from those observed in children of mothers with no history of MDD (regardless of haplotype), t(196) = 0.30, p = .98, reffect size = .002. These results are depicted in Figure 1.2
Figure 1.
Levels of brooding rumination among children of depressed versus nondepressed mothers as a function of child CRHR1 TAT haplotype.
* p < .05.
Next, to evaluate the robustness of this effect, we examined whether it would be maintained even after accounting for the influence of children’s current depressive symptom levels or history of major or minor depressive disorders or mothers’ current diagnoses of MDD. Supporting the robustness of the findings, the mother MDD × CRHR1 interaction remained significant even after statistically controlling for the influence of children’s current depressive symptoms, F(1, 251) = 6.49, p = .01, ηp2 = .03, and after excluding children with a lifetime history of major or minor depression (n = 32), F(1, 219) = 8.76, p = .003, ηp2 = .04. In addition, the main effect of mother MDD among children with no copies of the protective CRHR1 TAT haplotype was maintained even after statistically controlling for the influence of children’s depressive symptoms, F (1, 101) = 6.83, p = .01, ηp2 = .06, and after excluding children with a lifetime history of major or minor depression, t(90) = −3.08, p = .003, reffect size = .31, suggesting that the relation is not due simply to current or past depression in children and is observed in children at high risk for depression prior to developing depression themselves. Finally, the mother MDD × CRHR1 interaction was also maintained after excluding dyads in which the mother met criteria for current MDD (n = 24), F (1, 227) = 6.73, p = .01, ηp2 = .03. The main effect of mother MDD among children with no copies of the protective CRHR1 TAT haplotype was also maintained when excluding dyads in which the mother met criteria for current MDD, t(91) = −2.76, p = .01, reffect size = .28, suggesting that the results were not due simply to the presence of current depression in the mothers.
Additionally, we conducted exploratory analyses to examine the effects of age or sex on the study variables. Children of depressed mothers did not differ significantly from children of nondepressed mothers in age (t(253) = .28, p = .78) or sex (t(253) = .32, p = .75), and brooding rumination was not associated with children’s age (r = −.01, p = .88) or sex (r = .04, p =.57). Although children with 0 copies of the protective TAT haplotype did not differ in age from children with at least 1 copy (t(253) = −.15, p = .89), there was a group difference in sex, χ2(1) = 5.21, p = .02, reffect size = −.14. Specifically, boys were more likely than girls to be carriers of the protective haplotype, Therefore, the univariate ANOVA described above was repeated, but with child sex added as a covariate. The mother MDD × CRHR1 interaction was maintained even when we statistically controlled for the influence of children’s sex, F(1, 250) = 6.63, p = .01, ηp2 = .03. The main effect of mother MDD among children with no copies of the protective CRHR1 TAT haplotype was also maintained even after statistically controlling for children’s sex, F(101) = 9.50, p = .003, ηp2 = .09 . Finally, we examined age and sex as moderators of our findings and found that neither age nor sex moderated any of the findings presented above (all ps > .50).
Finally, given potential concerns about population stratification, we should note that the distribution of children’s CRHR1 TAT haplotype did not differ significantly between Caucasian children and children from other racial groups, χ2(1) = 0.62, p = .43, reffect size = .05, and all of the results were maintained even after including child race as a covariate in our analyses (all ps < .05). Although the majority of our sample was Caucasian, 19% (n = 48) were from other racial/ethnic groups. Therefore, we also conducted supplemental analyses, focusing only on Caucasians and found that although the main effect of mother MDD among children with no copies of the of the haplotype was reduced to marginal, t(80) = 1.81, p = .07, reffect size = .20, the size of the effect was similar to that observed in the full sample suggesting that the reduction in significance was due to reduced power with the smaller sample size.
Discussion
The primary goal of this study was to examine individual variability in the degree to which children at high risk for depression (i.e., children of mothers with a history of MDD) exhibit a known risk factor and potential endophenotype for depression, brooding rumination. We predicted that children of depressed mothers would display higher levels of brooding rumination than children of mothers with no depression history and that this effect would be stronger among children with no copies of the protective CRHR1 TAT haplotype. Our results supported these hypotheses. Indeed, among children carrying at least one copy of the protective TAT haplotype, there was no significant difference in levels of brooding observed in children of depressed versus nondepressed mothers. Importantly, these results were maintained even when we excluded children with a past history of depression and when we statistically controlled for children’s current depressive symptoms, suggesting these results were not confounded with children’s history of depression and were observed in children at high risk for depression prior to developing depression themselves. Given the important role that brooding plays in the development of depression (see, Abela & Hankin, 2011; Gibb et al., 2012), these findings suggest that the development of brooding among children of depressed mothers, particularly children without the protective CRHR1 haplotype, may serve as an important mechanism of risk for the intergenerational transmission of depression. The current results also support recent arguments that brooding rumination may be an endophenotype for depression (e.g., Johnson et al., 2014) by showing that the elevated levels of brooding rumination observed in at-risk children are independent of children’s own current or past experiences of depression (cf. Gottesman & Gould, 2003).
These results also provide additional support for the role of specific genetic influences on cognitive vulnerabilities to depression. Although maternal depression is an important risk factor in the development of depression, and cognitive vulnerabilities are thought to be a key mediator of this risk, not all children of depressed mothers will develop a cognitive vulnerability or depression themselves. Therefore, by identifying genetic influences that moderate the link between maternal depression and risk factors such as brooding rumination, researchers and clinicians may be better able to identify specific subgroups of children who are at the highest risk for developing depression. Our results support prior findings showing that CRHR1 genotype interacts with early life stress to predict depression-relevant constructs (Bradley et al., 2008; Fuge et al., 2014; Kranzler et al., 2011; Polanczyk et al., 2009; Tyrka et al., 2009), suggesting that CRHR1 could play an important role in the identification of high risk children. Indeed, levels of brooding rumination among children of depressed mothers with at least one copy of the CRHR1 TAT did not differ from levels of brooding observed in children of never depressed mothers, suggesting that it may partially buffer the effect of maternal depression on the development of brooding rumination.
Additionally, given the role of CRHR1 in HPA axis functioning, these findings add to growing support for the link between stress reactivity and brooding rumination. Prior research has shown that high-ruminating youth experience greater psychological and physiological reactivity to stress (Borelli, Hilt, West, Weekes, & Gonzalez, 2013; Roger & Najarian, 1998; Zoccola, Dickerson, & Zaldivar, 2008; Zoccola, Quas, & Yim, 2010) and that this reactivity is associated with later depressive symptoms following negative events (Abela & Hankin, 2011; Abela, Hankin, Sheshka, Fishman, & Stolow, 2012; Cox, Funasaki, Smith, & Mezulis, 2012; Driscoll, Lopez, & Kistner, 2009). However, our study is the first to show that variation in a gene with a strong link to HPA axis reactivity may play an important role in the development of rumination in at-risk children.
Although these findings complement prior research suggesting that maternal depression contributes to brooding rumination in children (e.g., Gibb et al., 2012), one limitation of the current study is that it is unable to elucidate the precise mechanisms by which maternal depression increases risk for brooding in children. In addition to the heritable components of depression and rumination (e.g., Moore et al., 2013), theorists have suggested that depressed mothers may influence the development of brooding rumination in their children through modeling and specific parenting styles (e.g., Nolen-Hoeksema, 1991). For example, a depressed mother may be more likely to display a ruminative response style when she is sad compared to a mother without a history of depression, which children of depressed mothers may then imitate. A second hypothesis is that children of depressed mothers are more likely to display a ruminative response style because they have not been taught or shown how to utilize more adaptive forms of emotion regulation, such as distraction or cognitive reappraisal. A third possibility is children of depressed mothers react to the high levels of hostility, anger, and criticism associated with maternal depression (e.g., Lovejoy, Graczyk, O’Hare, & Neuman, 2000) by turning inward and brooding on the perceived internal traits that may elicit this reaction from their mother. Although these proposed pathways fit well with current cognitive models of the intergenerational transmission of depression, our study and others suggest that these models must begin to take into account potential genetic moderators (e.g., Gibb et al., 2013). For example, it may be that children of depressed mothers who are biologically predisposed toward greater HPA axis reactivity following the negative events associated with maternal depression are at greater risk for poor prefrontal regulation during stress, which places them at increased risk for the development of brooding rumination. Clearly, future research is needed to examine more precise mechanisms of depression risk.
Overall, the current study demonstrated several strengths, including the assessment of diagnoses among mothers and children and the focus on a well-established genetic haplotype rather than an isolated SNP. In addition, the hypothesis regarding the moderating role of the CRHR1 TAT haplotype was based on a strong foundation of previous theory and research (Bradley et al., 2008; Gotlib et al., 2010; Kranzler et al., 2011; Polanczyk et al., 2009). This said, however, there were some additional limitations that highlight areas for future research. First, our study was cross-sectional, which precludes any temporal conclusions. Future multi-wave longitudinal research is needed to examine the prospective development of brooding rumination in at-risk children. In addition, there is always the possibility in any genetic association study of an unmeasured genetic or nongenetic third variable accounting for the associations reported. For example, although we observed the predicted moderation effects of children’s CRHR1 TAT haplotype, this is only one genetic variant that influences HPA axis reactivity to stress and future studies are needed to examine other potential influences such as variation in the glucocorticoid and mineralocorticoid receptor genes (cf. DeRijk, 2009). Also, as mentioned above, maternal depression is associated with a host of negative influences on children and future research is needed to more precisely map the specific mechanisms underlying the link between maternal depression and the development of brooding rumination in children. Related to this concern, although we interpreted our findings to mean that the CRHR1 haplotype moderated the effect of environmental adversity associated with maternal depression on children’s brooding scores, which is consistent with prior research (e.g., Bradley et al., 2008; Kranzler et al., 2011; Polanczyk et al., 2009), there is always the possibility of a passive or active gene-environment correlation given that depression has a heritable component and exerts its influence through both genetic and environmental factors. A final limitation is that we focused exclusively on one genetic risk factor – CRHR1 – and it is unlikely that the observed effects are unique to CRHR1. It should be noted, however, that our decision to focus on CRHR1 was based on evidence of its impact on key biological systems thought to underlie the intergenerational transmission of depression and brooding rumination. Future research is needed to determine whether variation in other genes known to influence HPA axis reactivity also interact with maternal depression to predict rumination either alone or as part of a broader polygenic aggregate.
In summary, the current results provide important information about individual variability in the degree to which children at high risk for depression (i.e., children with a maternal history of MDD) exhibit brooding rumination. Notably, this study is the first to provide evidence that the link between maternal depression and brooding rumination is moderated by genetic influences and add to the growing body of research suggesting that rumination may be an important endophenotype for depression risk (see also Chen & Li, 2013; Johnson et al., 2014; Moore et al., 2013). Furthermore, these findings provide initial evidence that biological stress reactivity (via HPA axis disruption) is a plausible mechanism underlying the link between maternal depression and children’s brooding. Our results underscore the importance of the relation between HPA axis functioning, prefrontal functioning, and cognitive vulnerability and add to the growing body of literature suggesting HPA axis disruption as a biological mechanism in the intergenerational transmission of depression (Dougherty, Klein, Olino, Dyson, & Rose, 2009; Dougherty, Klein, Rose, & Laptook, 2011; Gotlib, Joormann, Minor, & Hallmayer, 2008). If replicated and extended with longitudinal studies examining more direct measures of stress reactivity (e.g., cortisol reactivity), these results could have important implications for understanding the development of rumination in children at high risk for depression. As future research is needed to examine the precise mechanisms underlying these relations, continued research on this topic will be essential to inform intervention and prevention programs that seek to reduce the occurrence of depression among at-risk populations.
Acknowledgments
This project was supported by National Institute of Child Health and Human Development grant HD057066 and National Institute of Mental Health grant awarded to B. E. Gibb, 1S10RR023457-01A1 and Shared equipment grants (ShEEP) from the Medical Research Service of the Department of Veteran Affairs to J. E. McGeary. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the NIH. We would like to thank Ashley Johnson, Lindsey Stone, Andrea Hanley, Katie Burkhouse, Sydney Meadows, Michael Van Wie, and Devra Alper for their help in conducting assessments for this project, and Kayla Beaucage for her help with genotyping.
Footnotes
We re-ran our analyses using listwise deletion to account for the presence of missing data, leaving us with a sample size of 244 parent-child dyads for our analyses. The mother MDD x CRHR1 interaction and the main effect of mother MDD among children with no copies of the TAT haplotype remained significant. In addition, all tests of robustness were maintained. The only difference between the original data and the estimated data was for the main effect of mother MDD across the sample, which was significant in the estimated data (p = .05) and marginally significant in the original data (p = .08).
Analyses of individual SNPs (rs7209436, rs110402, and rs242924) were identical to those of reported for the overall haplotype. Specifically, for each SNP, we found a significant mother MDD × CRHR1 SNP interaction (all ps < .04). In addition, we found significant main effects of mother MDD on children’s levels of brooding rumination among children with no copies of the rs7209436 T allele, rs110402 A allele, and rs242924 T allele (all ps < .02).
References
- Abela JRZ, Hankin BL. Cognitive vulnerability to depression in children and adolescents: A developmental psychopathology approach. In: Abela JRZ, Hankin BL, editors. Handbook of depression in children and adolescents. New York: Guilford; 2008. pp. 6–32. [Google Scholar]
- Abela JRZ, Hankin BL. Rumination as a vulnerability factor to depression during the transition from early to middle adolescence: A multiwave longitudinal study. Journal of Abnormal Psychology. 2011;120:259–271. doi: 10.1037/a0022796. [DOI] [PubMed] [Google Scholar]
- Abela JRZ, Hankin BL, Sheshko DM, Fishman MB, Stolow D. Multi-wave prospective examination of the stress-reactivity extension of Response Styles Theory of Depression in high-risk children and early adolescents. Journal of Abnormal Child Psychology. 2012;40:277–287. doi: 10.1007/s10802-011-9563-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armey MF, Fresco DM, Moore MT, Mennin DS, Turk CL, Heimberg RG, Kecmanovic J, Alloy LB. Brooding and pondering: Isolating the active ingredients of depressive rumination with exploratory factor analysis and structural equation modeling. Assessment. 2009;16:315–327. doi: 10.1177/1073191109340388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annual review of pharmacology and toxicology. 2004;44:525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Barrett JC. Haploview: Visualization and analysis of SNP genotype data. Cold Spring Harbor Protocols. 2009;10 doi: 10.1101/pdb.ip71. pdb ip71. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Borelli JL, Hilt LM, West JL, Weekes NY, Gonzalez MC. School-aged children’s depressive rumination is associated with their reactivity to sadness but not fear. Journal of Clinical Child & Adolescent Psychology. 2014;43:799–812. doi: 10.1080/15374416.2013.814542. [DOI] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Archives of General Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li X. Genetic and environmental influences on adolescent rumination and its association with depressive symptoms. Journal of Abnormal Child Psychology. 2013;41:1289–1298. doi: 10.1007/s10802-013-9757-5. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Cox S, Funasaki K, Smith L, Mezulis AM. A prospective study of brooding and reflection as moderators of the relationship between stress and depressive symptoms in adolescence. Cognitive Therapy and Research. 2012;36:290–299. [Google Scholar]
- DeRijk RH. Single nucleotide polymorphisms related to HPA axis reactivity. Neuroimmunomodulation. 2009;16:340–352. [Google Scholar]
- Driscoll KA, Lopez CM, Kistner JA. Diathesis-stress test of response styles in children. Journal of Social and Clinical Psychology. 2009;28:1050–1070. [Google Scholar]
- Dougherty LR, Klein DN, Olino TM, Dyson M, Rose S. Increased waking salivary cortisol and depression risk in preschoolers: The role of maternal history of melancholic depression and early child temperament. Journal of Child Psychology and Psychiatry. 2009;50:1495–1503. doi: 10.1111/j.1469-7610.2009.02116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty LR, Klein DN, Rose S, Laptook RS. Hypothalamic-pituitary-adrenal axis reactivity in the preschool-age offspring of depressed parents: Moderation by early parenting. Psychological Science. 2011;22:650–658. doi: 10.1177/0956797611404084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feurer C, Hammen CL, Gibb BE. Chronic and episodic stress in children of depressed mothers. Journal of Clinical Child and Adolescent Psychology. doi: 10.1080/15374416.2014.963859. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders – Patient Edition (SCID-I/P) New York: Biometrics Research Department, NY State Psychiatric Institute; 1995. [Google Scholar]
- Freeman B, Powell J, Ball D, Hill L, Craig I, Plomin R. DNA by mail: an inexpensive and noninvasive method for collecting DNA samples from widely dispersed populations. Behavior Genetics. 1997;27:251–257. doi: 10.1023/a:1025614231190. [DOI] [PubMed] [Google Scholar]
- Fuge P, Aust S, Fan Y, Weigand A, Gärtner M, Feeser M, Bajbouj M, Grimm S. Interaction of early life stress and corticotropin-releasing hormone receptor gene: Effects on working memory. Biological Psychiatry. 2014;76:888–894. doi: 10.1016/j.biopsych.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Gibb BE. Depression in children. In: Gotlib IH, Hammen CL, editors. Handbook of depression. New York: Guilford; 2014. pp. 374–390. [Google Scholar]
- Gibb BE, Beevers CG, McGeary JE. Toward an integration of cognitive and genetic models of risk for depression. Cognition and Emotion. 2013;27:193–216. doi: 10.1080/02699931.2012.712950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb B, Grassia M, Stone L, Uhrlass D, McGeary J. Brooding Rumination and Risk for Depressive Disorders in Children of Depressed Mothers. Journal of Abnormal Child Psychology. 2012;40:317–326. doi: 10.1007/s10802-011-9554-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH. Depression in mothers. Annual Review of Clinical Psychology. 2007;3:107–135. doi: 10.1146/annurev.clinpsy.3.022806.091401. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed mothers: A development model for understanding mechanisms of transmission. Psychological Review. 1999;106:458–490. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Colich NL. Children of Depressed Parents. In: Gotlib IH, Hammen CL, editors. Handbook of depression. New York: Guilford; 2014. pp. 240–258. [Google Scholar]
- Gotlib IH, Joormann J. Cognition and Depression: Current status and future directions. Annual Review of Clinical Psychology. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biological Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassia M, Gibb BE. Rumination and prospective changes in depressive symptoms. Journal of Social and Clinical Psychology. 2008;27:931–948. [Google Scholar]
- Grassia M, Gibb BE. Rumination and lifetime history of suicide attempts. International Journal of Cognitive Therapy. 2009;2:400–406. doi: 10.1521/ijct.2009.2.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Children of depressed parents. In: Gotlib IH, Hammen C, editors. Handbook of depression. 2. New York: Guilford; 2009. pp. 275–297. [Google Scholar]
- Johnson DP, Whisman MA, Corley RP, Hewitt JK, Friedman NP. Genetic and environmental influences on rumination and its covariation with depression. Cognition and Emotion. 2014;28:2170–1286. doi: 10.1080/02699931.2014.881325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Rating scales to assess depression in school-aged children. Acta Paedopsychiatrica. 1981;46:305–315. [PubMed] [Google Scholar]
- Kovacs M. The Children’s Depression Inventory. Psychopharmacology Bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- Kranzler HR, Feinn R, Ph D, Nelson EC, Covault J, Anton RF, Gelernter J. CRHR1 haplotype moderates the effect of adverse childhood experiences on lifetime risk of major depressive episode in African-American women. American Journal of Medical Genetics Part B. 2012;156B:960–968. doi: 10.1002/ajmg.b.31243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laucht M, Treutlein J, Blomeyer D, Buchmann AF, Schmidt MH, Esser G, Banaschewski T. Interactive effects of corticotropin-releasing hormone receptor 1 gene and childhood adversity on depressive symptoms in young adults: findings from a longitudinal study. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology. 2013 May; doi: 10.1016/j.euroneuro.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Lench N, Stanier P, Williamson R. Simple non-invasive method to obtain DNA for gene analysis. Lancet. 1988;331:1356–1358. doi: 10.1016/s0140-6736(88)92178-2. [DOI] [PubMed] [Google Scholar]
- Lopez CM, Driscoll KA, Kistner JA. Sex differences and response styles: Subtypes of rumination and associations with depressive symptoms. Journal of Clinical Child and Adolescent Psychology. 2009;38:27–35. doi: 10.1080/15374410802575412. [DOI] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O’Hare E, Neuman G. Maternal depression and parenting behavior: A meta-analytic review. Clinical Psychology Review. 2000;20:561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Michl LC, McLaughlin KA, Shepherd K, Nolen-Hoeksema S. Rumination as a mechanisms linking stressful life events to symptoms of depression and anxiety: Longitudinal evidence in early adolescents and adults. Journal of Abnormal Psychology. 2013;122:339–352. doi: 10.1037/a0031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, Rockstroh B. Endophenotypes in psychopathology research: Where do we stand? Annual Review of Clinical Psychology. 2013;9:177–213. doi: 10.1146/annurev-clinpsy-050212-185540. [DOI] [PubMed] [Google Scholar]
- Moore MN, Salk RH, Van Hulle CA, Abramson LY, Hyde JS, Lemery-Chalfant K, Goldsmith HH. Genetic and environmental influences on rumination, distraction, and depressed mood in adolescence. Clinical Psychological Science. 2013;1:316–322. doi: 10.1177/2167702612472884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P, Roelofs J, Meesters C, Boomsma P. Rumination and worry in nonclinical adolescents. Cognitive Therapy and Research. 2004;28:539–554. [Google Scholar]
- Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology. 1991;100:569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3:400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K, Uher R, Poulton R, Moffitt TE. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: replication and extension. Archives of General Psychiatry. 2009;66:978–985. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Roger D, Najarian B. The relationship between emotional rumination and cortisol secretion under stress. Personality and Individual Differences. 1998;24:531–538. [Google Scholar]
- Rood L, Roelofs J, Bögels SM, Nolen-Hoeksema S, Schouten E. The influence of emotion-focused rumination and distraction on depressive symptoms in non-clinical youth: A meta-analytic review. Clinical Psychology Review. 2009;29:607–616. doi: 10.1016/j.cpr.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Schoofs H, Hermans D, Raes F. Brooding and reflection as subtypes of rumination: Evidence from confirmatory factor analysis in nonclinical samples using the Dutch Ruminative Response Scale. Journal of Psychopathology and Behavioral Assessment. 2010;32:609–617. [Google Scholar]
- Sheikh HI, Kryski KR, Smith HJ, Hayden EP, Singh SM. Corticotropin-releasing hormone system polymorphisms are associated with children’s cortisol reactivity. Neuroscience. 2013;229:1–11. doi: 10.1016/j.neuroscience.2012.10.056. [DOI] [PubMed] [Google Scholar]
- Smucker MR, Craighead WE, Craighead LW, Green BJ. Normative and reliability data for the children’s depression inventory. Journal of Abnormal Child Psychology. 1986;14:25–39. doi: 10.1007/BF00917219. [DOI] [PubMed] [Google Scholar]
- Stephens M, Donnelly PA. Comparison of bayesian methods for haplotype reconstruction from population genotype data. American Journal of Human Genetics. 2003;73:1162–1169. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly PA. New statistical method for haplotype reconstruction from population data. American Journal of Human Genetics. 68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Gelernter J, Schepker C, Anderson GM, Carpenter LL. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: Effects on hypothalamic adrenal axis reactivity. Biological Psychiatry. 2009;66:681–685. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research. 2003;27:247–259. [Google Scholar]
- Wingenfeld K, Wolf OT. HPA axis alterations in mental disorders: Impact on memory and its relevance for therapeutic interventions. CNS Neuroscience & Therapeutics. 2011;17:714–722. doi: 10.1111/j.1755-5949.2010.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegert DI, Kistner JA. Response styles theory: Downward extension to children. Journal of Clinical Child Adolescent Psychology. 2002;31:325–334. doi: 10.1207/S15374424JCCP3103_04. [DOI] [PubMed] [Google Scholar]
- Zoccola PM, Dickerson SS, Zaldivar FP. Rumination and cortisol responses to laboratory stressors. Psychosomatic Medicine. 2008;70:661–667. doi: 10.1097/PSY.0b013e31817bbc77. [DOI] [PubMed] [Google Scholar]
- Zoccola PM, Quas JA, Yim IS. Salivary cortisol responses to a psychosocial laboratory stressor and later verbal recall of the stressor: The role of trait and state rumination. Stress: The International Journal on the Biology of Stress. 2010;13:435–443. doi: 10.3109/10253891003713765. [DOI] [PubMed] [Google Scholar]