Abstract
Tactile stimuli produce afferent signals that activate specific regions of the cerebral cortex. Noninvasive transcranial direct current stimulation (tDCS) effectively modulates cortical excitability. We therefore hypothesized that a single session of tDCS targeting the sensory cortices would alter the cortical response to tactile stimuli. This hypothesis was tested with a block-design fMRI protocol designed to quantify the BOLD response to controlled sinusoidal pressure stimulation applied to the right foot sole, as compared to rest, in 16 healthy young adults. Following sham tDCS, right foot sole stimulation was associated with activation bilaterally within the precentral cortex, postcentral cortex, middle and superior frontal gyri, temporal lobe (sub-gyral) and cingulate gyrus. Activation was also observed in the left insula, middle temporal lobe, superior parietal lobule, supramarginal gyrus and thalamus, as well as the right inferior parietal lobule and claustrum (FDR corrected, p < 0.05). To explore the regional effects of tDCS, brain regions related to somatosensory processing, and cortical areas underneath each tDCS electrode, were chosen as regions-of-interest (ROIs). Real tDCS, as compared to sham tDCS, increased the percent signal change associated with foot stimulation relative to rest in the left posterior paracentral lobule. These results indicate that tDCS acutely modulates the cortical responsiveness to controlled foot pressure stimuli in healthy adults. Further study is warranted, in both healthy individuals and patients with sensory impairments, to link tDCS-induced modulation of the cortical response to tactile stimuli with changes in somatosensory perception.
INTRODUCTION
Perceptible somatosensory stimuli are associated with a degree of cortical activation that is contingent upon stimulus location, size and intensity (Fregni & Pascual-Leone, 2007). For a given stimulus, the degree of cortical activation is in turn dependent upon the integrity of peripheral, spinal and subcortical circuitry, as well as the excitability of involved cortical neurons (Adolphs et al., 2000; Maldjian et al., 1999; Goldberg et al., 2006; Beauchamp, 2005). Strategies designed to facilitate or suppress the excitability of cortical neurons may thus enable modulation of somatosensation by increasing or decreasing the cortical response to a given stimulus. This might ultimately help overcome deficits of sensation in patients with peripheral neuropathies. Foot sole somatosensory impairments in particular diminish balance and heighten the risk of suffering falls, which often result in injuries and long-term disability. Therefore, strategies to enhance somatosensation from the soles of the feet offer promise as valuable therapeutic interventions. As a preliminary exploration of this potential, the present study aimed to assess the ability of noninvasive brain stimulation to augment the cortical response – as indexed by functional magnetic resonance imaging (fMRI) – to a controlled mechanical stimulus to the soles of the feet.
Transcranial direct current stimulation (tDCS) is a noninvasive, safe and painless neurophysiologic intervention that alters cortical excitability by inducing low amplitude current flow between two or more surface sponge electrodes (Schlaug & Renga, 2008). Depending upon the direction, duration and intensity of current flow, a single session of tDCS can facilitate or suppress cortical excitability in targeted brain regions for several hours following stimulation (Bindman et al., 1964; Radman et al., 2009). Recently, researchers have demonstrated that tDCS targeting the sensorimotor cortex is capable of modulating tactile acuity under certain situations (Matsunaga et al., 2004; Ragert et al., 2008; Mori et al., 2013; Rogalewski et al., 2004). However, the impact of tDCS on the underlying cortical response to a given stimulus has yet to be examined. We hypothesized that tDCS would modulate the excitability of the sensorimotor cortex and thus alter the degree of cortical activation induced by tactile stimuli. To test this hypothesis, we utilized fMRI to quantify the BOLD response to a controlled pressure stimulus applied to the right foot sole, immediately following a single session of tDCS designed to facilitate left sensorimotor cortex excitability.
METHODS AND MATERIALS
2.1 Subjects
Sixteen young adults (mean ± SD age = 22.2 ± 2.1 years; 11 males) without any known neurological or other disorders were recruited for this double-blinded, sham-controlled study. All subjects were right-handed as determined by the Edinburgh Handedness Inventory (Oldfield, 1971). Subjects provided written informed consent of the protocol as approved by the Institutional Review Board of Peking University First Hospital, Beijing.
2.2 Protocol
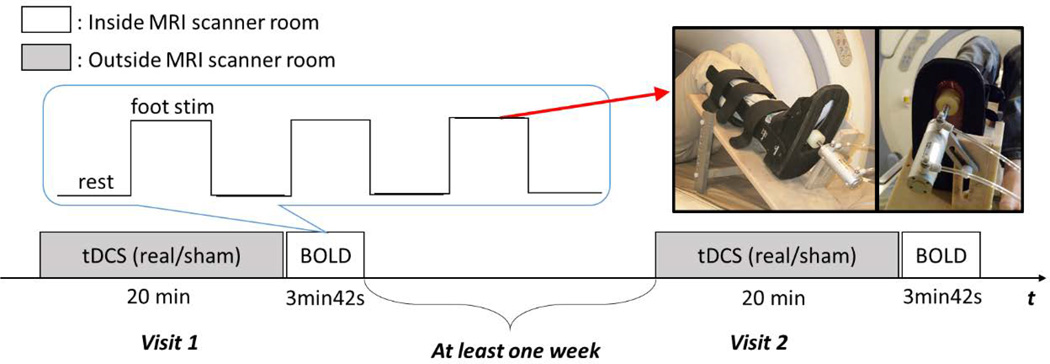
All subjects completed an fMRI protocol immediately following either real or sham (i.e., control) tDCS on two days separated by at least one week (Fig. 1). The anode was place over the left sensorimotor cortex with the aim of increasing its excitability. The order of real and sham tDCS sessions was counterbalanced. The fMRI protocol was a block-design comprising alternating blocks of foot sole pressure stimulation and rest (i.e., no stimulation). Each block was 30 seconds in duration and repeated three times (Hao et al., 2012).
Figure 1.
2.2.1 tDCS
Transcranial direct current stimulation was delivered for 20 minutes up to a maximum current intensity of 2.0 mA by personnel uninvolved in any other study procedure. A battery-driven electrical stimulator (Chattanooga Ionto, USA) was connected to a pair of saline-soaked synthetic surface sponge electrodes (surface area: 35 cm2 per electrode) placed on the scalp. The positive electrode was placed over C3 (according to the EEG international 10–20 system), which corresponds to left sensorimotor cortex. The negative electrode was placed over the contralateral supraorbital region. At the beginning of each tDCS session, stimulation intensity was increased manually from 0.1 to 2.0 mA in 0.1 mA increments. Subjects were instructed to notify study personnel if the stimulation became too uncomfortable or if they noticed continuous itching sensations. At this point, the current intensity was ramped down 0.1 mA and then fixed for 20 minutes. Across all subjects, real tDCS was applied at a mean ± SD intensity of 1.43 ± 0.39 mA (range: 0.7– 2.0 mA). Current was automatically ramped down over a 30 second period at the end of the session. During sham tDCS, current was ramped up for the first 30 seconds of the session, but then ramped back down to 0 mA over the next 30 seconds. This procedure mimics the transient skin sensation of real tDCS that is typically felt at the beginning of stimulation, yet does not produce significant modulatory effects on the brain (Brunoni et al., 2011). Impedance was continuously monitored throughout each session and never rose above 5 kΩ.
At the end of each study visit, subjects completed a short questionnaire surveying for potential adverse effects associated with tDCS (Brunoni et al., 2011). In order to determine the effectiveness of blinding procedures, subjects were asked, “Do you believe that you received real stimulation during this session?” at the end of each session.
2.2.2 Foot Sole Tactile Stimulation
A custom-built, MRI-compatible tactile stimulation system was used to apply controlled mechanical pressures to the sole of the right foot (Fig. 1). Briefly, this system consists of an air compressor and control unit located outside of the scanner room, which is connected via plastic air tubes to an MRI-compatible, aluminum pneumatic actuator attached to a support platform secured the scanner bed (Hao et al., 2012). The subject’s right leg was secured to a plastic medical boot, which was modified and attached to the support platform. This setup enabled fixation of the ankle joint at 90° of dorsiflexion as well as adjustment of both knee and hip joint angles. Our previous work has demonstrated that this setup significantly reduces translational movements of the head and related MRI motion artifact (Hao et al., 2012).
During each 30-second fMRI foot sole pressure stimulation block, continuous oscillatory pressure stimuli were applied to a circular area (4 cm in diameter) of the foot sole over the head of the first metatarsal of the right foot. Maximum force output of the actuator was set to 10% of the subject’s body mass and was applied in a 1 Hz sinusoidal waveform with a duty cycle of 80%. We have previous demonstrated that this paradigm induces a characteristic pattern of cortical activation while not influencing image quality or producing motion artifact with applied load (Hao et al., 2012).
2.2.3 MR Image Acquisition
MRIs were acquired within Peking University First Hospital using a GE 3T (Signa Excite HD; GE Medical Systems, Milwaukee, WI) whole body scanner with an 8-channel receive-only head coil. Blood oxygen level depended (BOLD) data were acquired after tDCS using a standard echo-planar imaging (EPI) sequence with the following parameters: repetition time/echo time (TR/TE): 2000/30 ms, flip angle: 90°, image matrix: 64×64, thickness/spacing: 4mm /1 mm, field of view (FOV): 230 × 230 mm2, 28 interleaved axial slices, 30 TRs. In each subject we acquired a total of 3300 BOLD images. The interval between the conclusion of tDCS and acquisition of the first BOLD image was less than 10 minutes for all subjects. A high-resolution structural image was acquired prior to tDCS by using a 3D fast spoiled gradient echo (FSPGR) sequence for anatomical localization (TR/TE: 7.8/3.0 ms; flip angles: 20°; inversion time: 450 ms; FOV: 240 × 240 mm2; slice thick: 2.0 mm with 1.0 mm overlap; in-plane resolution: 1 × 1 mm2).
2.2.4 Data and Statistical Analysis
Raw EPI data were preprocessed with Statistical Parametric Mapping software (SPM8, Wellcome Department of Imaging Neuroscience, University College, London, UK). For each subject, images were realigned to the first scan to correct for potential head movement within scans, generating six-parameter head motion curves. Each time-series was corrected to compensate for delays associated with acquisition time differences across slices. Functional images were co-registered to the corresponding structural T1 image and normalized to a 2-mm isovoxel Montreal Neurological Institute (MNI) template. Functional images in MNI space were then spatially smoothed using an 8-mm (full width/half maximum; FWHM) Gaussian kernel and subjected to a high-pass temporal filter with a cutoff of 128 seconds. For each subject and tDCS condition, foot stimulation and rest blocks were modeled as a boxcar regressor (30 seconds on, 30 seconds off) convoluted with a double-gamma hemodynamic response function (see Figure 1). These regressors were entered into a first-pass general linear model (GLM) to generate parameter estimates for each condition (rest, foot stimulation). A subject-level contrast of parameter estimates, representing the percent signal change (PSC) of foot sole pressure stimulation as compared to rest, was generated separately for each tDCS condition using paired-sample t-tests. One sample t tests were utilized to generate a group-wise statistical map of each tDCS condition, using a false discovery rate (FDR) corrected p < 0.05, with a threshold of at least 10 contiguous voxels. A paired-samples t test was then used to analyze potential whole brain differences between the real and sham conditions.
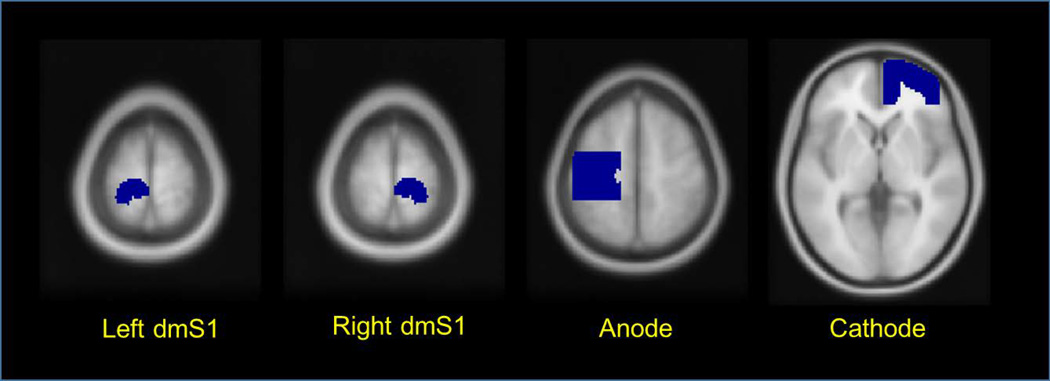
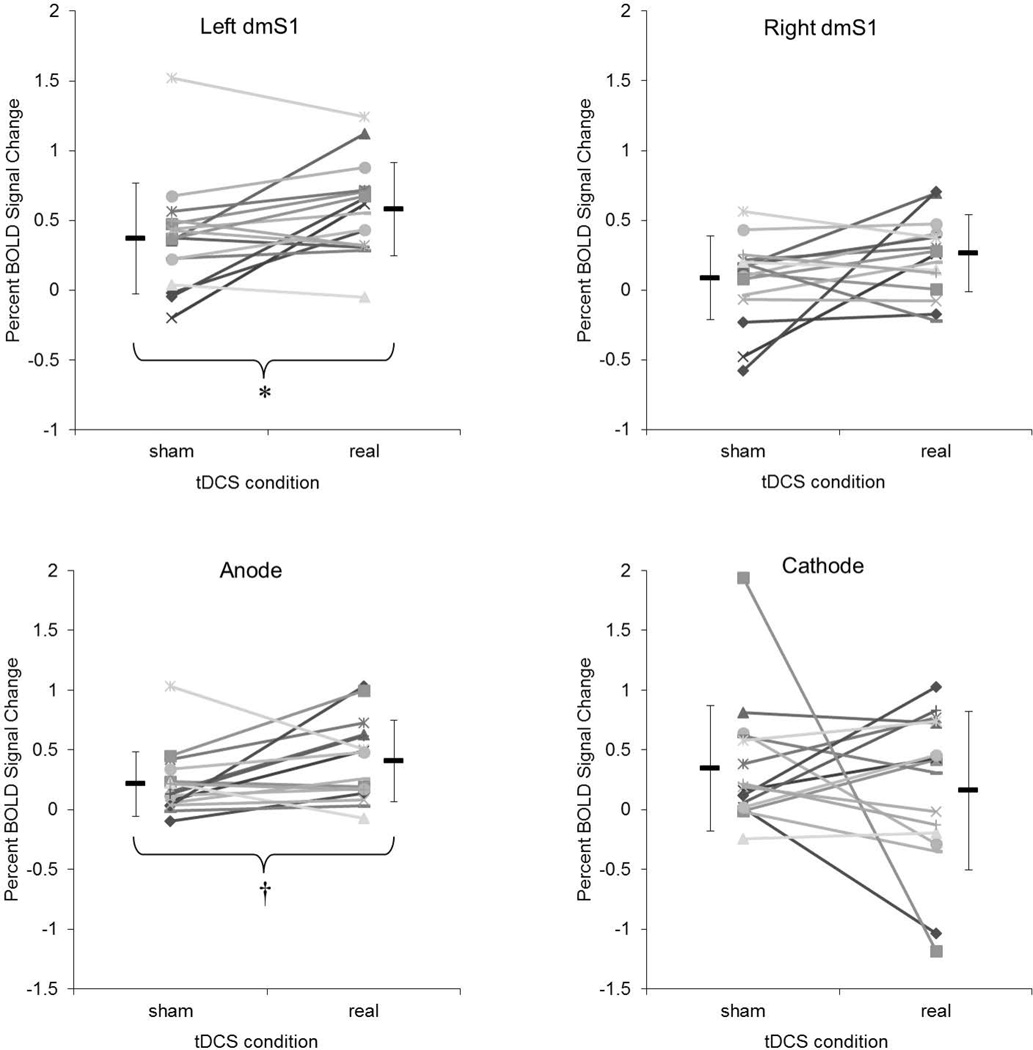
Using a standard approach (Weiskopf et al., 2003; Goble et al., 2011; Goble et al., 2012), we further examined the impact of tDCS on the cortical and subcortical response to foot sole pressure stimulation in specific regions-of-interest (ROIs). ROIs were selected a priori to include the left and right dorso-medial somatosensory cortices (dmS1) (Figure 2). Using the coordinates (−13.2, −37.8, 70) of peak activation obtained from the same fMRI protocol in a prior study (Hao et al., 2012), we created a surrounding sphere (radius = 15mm) and multiplied it by a mask of the paracentral lobule and postcentral gyri from the AAL template (Tzourio-Mazoyer et al., 2002). Anatomically, the dmS1 receives afferent signals from the contralateral foot and lower limbs. Functionally, the left dmS1 is activated by the same type of right foot sole pressure stimulation applied in the current study (Hao et al., 2012). As stimulation was applied only to the right foot, the ipsilateral dmS1 was included to provide a negative control ROI. In addition to these regions, we also included the cortical areas beneath each electrode. The positions of anodal and cathodal ROIs were defined according to EEG positions C3 (anode) and Fp2 (cathode) of the international 10 –20 electrode system. The size and depth of both anode and cathode ROIs was determined using previously-reported simulated and realistic models (Miranda et al., 2009; Sadleir et al., 2010).
Figure 2.
The average PSC from rest to foot stimulation within each ROI was calculated for each subject following each tDCS condition using established methods within the SPM Marsbar toolbox (Brett et al., 2002). The PSC value thus reflects the mean intensity of hemodynamic response within each ROI caused by foot sole pressure stimulation. The effects of tDCS condition (real, sham) on PSC values from each ROI were then analyzed using 2-tailed paired t tests. The relationship between tDCS current intensity and the PSC value within each ROI was examined using Pearson’s correlation coefficients.
RESULTS
Fifteen of 16 subjects reported minimal itching sensations beneath the tDCS electrodes during stimulation. These discomforts were independent of stimulation condition and current strength. No other discomforts or side effects were observed or reported during the study. The number of subjects who reported the correct tDCS condition (43.8%) was slightly less than that expected by chance, suggesting that subjects were adequately blinded to tDCS condition.
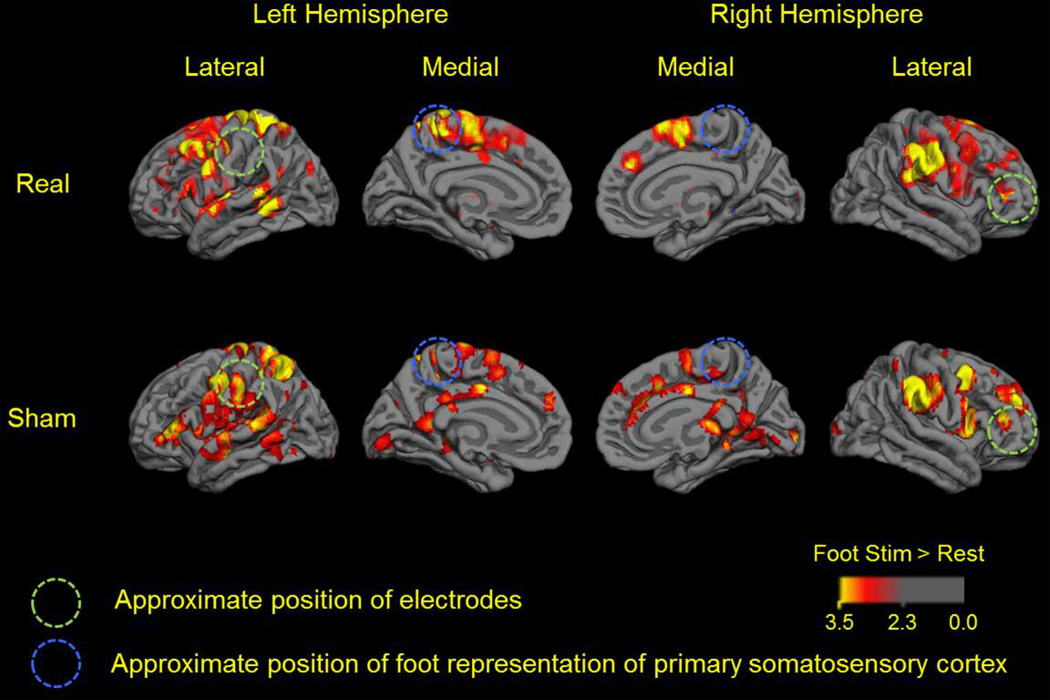
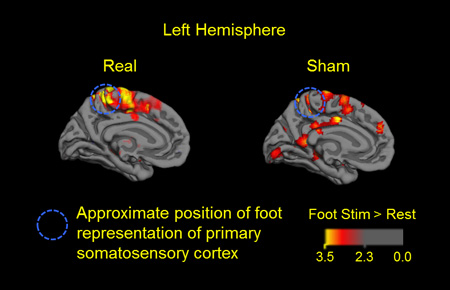
The whole brain cortical response to right foot sole stimulation following real and sham tDCS is presented in Figure 3, which portrays group-level BOLD activation maps for each condition. Following sham tDCS (i.e., the control condition), the contrast, foot stimulation > rest, yielded activation within the bilateral precentral cortex, postcentral cortex, middle and superior frontal gyri, temporal lobe (sub-gyral) and cingulate gyrus. Activation was also observed in the left insula, middle temporal lobe, superior parietal lobule, supramarginal gyrus and thalamus, as well as the right inferior parietal lobule and claustrum (FDR corrected, p < 0.05). Following real tDCS, the same contrast yielded activation within each of the aforementioned regions, with the following exceptions: no significant activation was present in the thalamus and anterior cingulate cortex, while additional areas of activation were present in the left caudate nucleus and right insula. Group-wise analysis yielded no clusters with significant deactivation following either of the tDCS conditions.
Figure 3.
Whole-brain group-level comparison revealed that as compared the sham tDCS condition, right foot stimulation following real tDCS was associated with greater activation within the left precentral gyrus, left middle frontal gyrus, left middle temporal gyrus and right postcentral gyrus (uncorrected, p < 0.005; cluster size > 10 voxels). In contrast, there were no clusters in which activation was less following real tDCS as compared to sham.
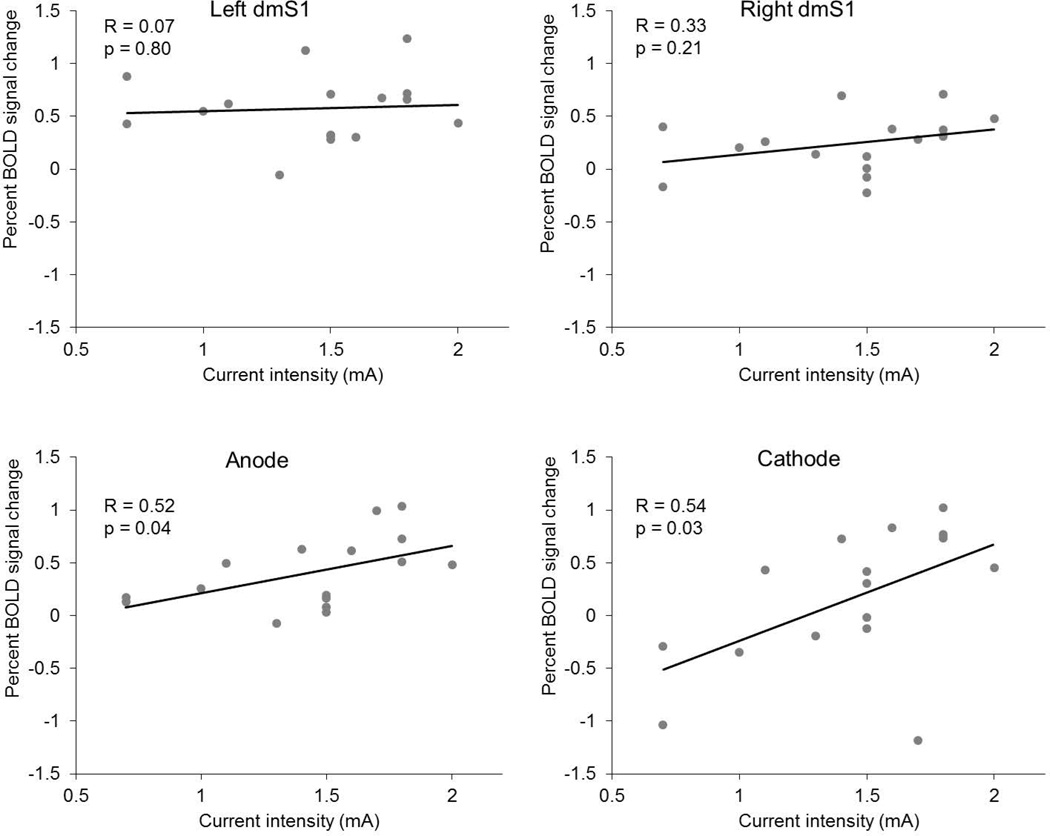
Since the intensity of applied tDCS current varied across subjects, we also examined the relationship between tDCS intensity and the PSC induced by foot stimulation within each ROI. Pearson’s correlation analysis revealed that PSC values within the anode and cathode ROIs were larger in those subjects who received tDCS current at greater intensities (Figure 5). On the other hand, the observed PSC within both the left and right dmS1 was not correlated with the intensity of tDCS.
Figure 5.
DISCUSSION
In the control condition (i.e., following sham tDCS), sinusoidal pressure stimuli applied to the right foot sole induced a distributed pattern of cortical activation within numerous brain regions linked to an array of sensorimotor and cognitive functions. Activation within these regions was expected and consistent with our previous study employing the same fMRI-compatible tactile stimulation system (Hao et al., 2012). In addition to the primary and secondary somatosensory cortex, activation was observed within multiple regions linked to somatosensory processing: the insula has been associated with the perception of light touch (Yoo et al., 2003; Nagai et al., 2007), the superior parietal lobule and the supramarginal gyrus are involved in the discrimination of multiple somatosensory stimuli and their integration with other perceptual modalities (Wolpert et al., 1998; Bohlhalter et al., 2002), the middle temporal lobe participates in tactile-motor processing (Hagen et al., 2002), the frontal gyrus has been implicated in tactile imagery (Yoo et al., 2003) and the claustrum is an important node in cross-modal matching (Hadjikhani & Roland, 1998). Moreover, foot pressure stimulation induced activation within the cingulate gyrus, which is believed to be closely involved in the regulation of attention to sensory stimuli (Baleydier & Mauguiere, 1980). While outside the scope of the present study, future research employing resting-state fMRI and/or arterial spin labeling (ASL) may shed light on the functional connectivity between these regions. Moreover, studies including block designs that alter the magnitude of foot pressure, as well as attentional focus, may help to delineate the specific role of each of the above regions in the processing of this type of somatosensory feedback.
The major novel finding of the present study was that following real tDCS, as compared to sham, right foot sole stimulation was associated with greater PSC of the BOLD signal within the left posterior paracentral lobule (i.e., the dmS1). These results suggest that a single session of tDCS effectively modulates the cortical response to controlled foot pressure stimuli in healthy adults. The dmS1 contains representations of the feet and lower limbs (Hari & Forss, 1999). Importantly, these effects were selective to the left dmS1 and not the right. tDCS is believed to modulate cortical excitability by altering the relative concentration of freely moving anions and cations in the extracellular milieu (Bikson et al., 2004), which in turn raises or lowers neuronal resting membrane potentials (Bindman et al., 1962; Purpura & McMurtry, 1965). Thus, the most likely interpretation of our findings is that increased BOLD activity following anodal tDCS results from an increase in the sensitivity of the cortex to afferent signals. BOLD fMRI is limited, however, in that nature of the activity (excitatory vs. inhibitory) cannot be ascertained. Nor is it possible to completely separate changes in cortical excitability or metabolism from changes in neurovascular coupling. Nevertheless, administering tDCS with the anode over the sensory cortex has been reported to increase both the magnitude of somatosensory evokes potentials (SEP) and cerebral blood flow (CBF) within brain regions involved in sensory processing (Matsunaga et al., 2004; Zheng et al., 2011). Since the cortical components of the SEP are due to the summation of synchronous synaptic activity, increased SEP after real tDCS may be due to an increase in efficacy of synaptic transmission or increased phase coupling in these pathways. Alternatively, if the neurons involved in processing the signal were more excitable, they would then be more easily discharged and produce a larger synaptic input in cortical processing (Matsunaga et al., 2004). The increased hemodynamic response induced by tDCS expressed an increase of oxygen and glucose availability of nervous tissue, which can also reflect stronger cortical excitability. Future studies are therefore warranted to concurrently quantify the effects of tDCS on 1) the BOLD and perfusion (e.g., using arterial spin labeling) responses to foot sole stimulation, and 2) direct measures of somatosensory cortical excitability as quantified by noninvasive neurophysiological techniques, in older adults both with and without peripheral somatosensory impairments.
Several behavioral studies have demonstrated that tDCS designed to facilitate neuronal excitability within the sensorimotor cortices alter one’s ability to perceive somatosensory stimuli (Ragert et al., 2008; Mori et al., 2013; Rogalewski et al., 2004). For example, Ragert et al (2008) reported that a single, 20-minute session of tDCS targeting the left primary sensory cortex with a current intensity of 1.0 mA enhanced tactile spatial acuity in the contralateral hand, as compared to sham tDCS. Mori et al (2013) demonstrated longer-lasting effects of tDCS on tactile sensation in patients with multiple sclerosis. Specifically, five daily sessions of tDCS over C3 or C4 (on the 10–20 EEG electrode placement system) at a target current intensity of 2.0 mA improved spatial discrimination thresholds on the hypoesthetic hand for at least two weeks after the last tDCS session. For a given individual, the BOLD response to a tactile stimulus is dependent upon the stimulus intensity (Nelson et al., 2004; Backes et al., 2000; Arthurs et al., 2000; Jousmäki & Forss, 1998). For example, Nelson et al (2004) employed an MRI-compatible magnetomechanical device to deliver controlled vibrations to the right hand of healthy young adults. They observed that the degree of activation within the primary somatosensory cortex increased in accordance with both the amplitude and frequency of vibration. A similar stimulus-response relationship has also been observed by electrically stimulating the median nerve at the wrist (Backes et al., 2000; Arthurs et al., 2000; Jousmäki & Forss, 1998). These studies therefore suggest that the magnitude of the PSC achieved by fMRI (or somatosensory-evoked magnetic fields recorded by magnetoencephalography) becomes greater as the intensity of the applied stimulus increases. The results of the present study, to our knowledge, are the first to show a tDCS-induced augmentation of the somatosensory cortical response to a controlled tactile stimuli, provide further support for this model and offer a mechanistic explanation for previous reports of tDCS-mediated enhancement of somatosensation. As somatosensation is critical to understanding and interacting with the world around us, future studies that apply sensory stimuli at intensities both above and below an individual’s threshold of perception are warranted in order to determine if tDCS increases the cortical response, and therefore perception, across all stimuli.
It is important to note that the effects of real tDCS on foot sole sensory performance are also dependent upon factors other than the cortical response as measured by BOLD fMRI in the present study. Behavioral experiments are thus still needed to examine the capacity of tDCS to alter the perception of foot sole tactile stimuli. Additionally, in the current study, fMRIs were only conducted following the administration of tDCS. As such, the magnitude of the tDCS-induced change from “baseline” cannot be compared across conditions. The acquisition of functional brain images both immediately before and after both real and sham tDCS would have strengthened our results by enabling further comparison to pre-tDCS “baseline” conditions. Nevertheless, our finding of an increase above sham supports our hypothesis that anodal tDCS is capable of modulating the cortical response to foot stimulation.
Somatosensory impairments are common in aging and disease and often lead to functional decline and falls (Allison et al., 1984; Woollacott et al., 1986; Van-Deursen & Simoneau, 1999; Horak et al., 2002; Quai et al., 2005; Shaffer & Harrison, 2007). In our study, we show that real tDCS, relative to sham, modulates the cortical response to peripheral stimulation in regions linked to somatosensory integration and interpretation. We suggest that enhanced cortical excitability might be the neural substrate for previously-report effects of tDCS on somatosensory perception (Nelson et al., 2004; Backes et al., 2000; Arthurs et al., 2000; Jousmäki & Forss, 1998). As foot-sole somatosensation is critical to gait and balance, we further contend that tDCS may be a valuable new method of improving gait and balance (Zhou et al., 2014). Future studies combining the current approach with tests of tactile perception and balance in older adults and those with somatosensory impairments are therefore encouraged.
Figure 4.
ACKNOWLEDGEMENTS
This study was supported by grants from the National Natural Science Foundation of China (grant no. 11372013), the Sidney Baer Foundation, a KL2 Medical Research Investigator Training (MeRIT) award (1KL2RR025757-04) from Harvard Catalyst/The Harvard Clinical and Translational Science Center (UL 1RR025758) and a K01 award (1K01AG044543-01A1) from the National Institute on Aging. Study funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- Adolphs R, Damasio H, Tranel D, Cooper G, Damasio AR. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. The Journal of Neuroscience. 2000;20(7):2683–2690. doi: 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison T, Hume AL, Wood CC, Goff WR. Developmental and aging changes in somatosensory, auditory and visual evoked potentials. Electroencephalography and clinical neurophysiology. 1984;58(1):14–24. doi: 10.1016/0013-4694(84)90196-2. [DOI] [PubMed] [Google Scholar]
- Arthurs OJ, Williams EJ, Carpenter TA, Pickard JD, Boniface SJ. Linear coupling between functional magnetic resonance imaging and evoked potential amplitude in human somatosensory cortex. Neuroscience. 2000;101(4):803–806. doi: 10.1016/s0306-4522(00)00511-x. [DOI] [PubMed] [Google Scholar]
- Backes WH, Mess WH, van Kranen-Mastenbroek V, Reulen JPH. Somatosensory cortex responses to median nerve stimulation: fMRI effects of current amplitude and selective attention. Clinical neurophysiology. 2000;111(10):1738–1744. doi: 10.1016/s1388-2457(00)00420-x. [DOI] [PubMed] [Google Scholar]
- Baleydier C, Mauguiere F. The duality of the cingulate gyrus in monkey. Neuroanatomical study and functional hypothesis. Brain: a journal of neurology. 1980;103(3):525–554. doi: 10.1093/brain/103.3.525. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS. Statistical criteria in FMRI studies of multisensory integration. Neuroinformatics. 2005;3(2):93–113. doi: 10.1385/NI:3:2:093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Inoue M, Akiyama H, Deans JK, Fox JE, Miyakawa H, Jefferys JG. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. The Journal of physiology. 2004;557(1):175–190. doi: 10.1113/jphysiol.2003.055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindman LJ, Lippold OCJ, Redfearn JWT. Long-lasting changes in the level of the electrical activity of the cerebral cortex produced by polarizing currents. 1962 doi: 10.1038/196584a0. [DOI] [PubMed] [Google Scholar]
- Bohlhalter S, Fretz C, Weder B. Hierarchical versus parallel processing in tactile object recognition: A behavioural–neuroanatomical study of aperceptive tactile agnosia. Brain. 2002;125(11):2537–2548. doi: 10.1093/brain/awf245. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. International Journal of Neuropsychopharmacology. 2011;14(8):1133–1145. doi: 10.1017/S1461145710001690. [DOI] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology—perspectives on the therapeutic potential of rTMS and tDCS. Nature Clinical Practice Neurology. 2007;3(7):383–393. doi: 10.1038/ncpneuro0530. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Coxon JP, Van Impe A, Geurts M, Doumas M, Wenderoth N, Swinnen SP. Brain activity during ankle proprioceptive stimulation predicts balance performance in young and older adults. The Journal of Neuroscience. 2011;31(45):16344–16352. doi: 10.1523/JNEUROSCI.4159-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goble DJ, Coxon JP, Van Impe A, Geurts M, Van Hecke W, Sunaert S, Swinnen SP. The neural basis of central proprioceptive processing in older versus younger adults: an important sensory role for right putamen. Human brain mapping. 2012;33(4):895–908. doi: 10.1002/hbm.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg RF, Perfetti CA, Schneider W. Perceptual knowledge retrieval activates sensory brain regions. The Journal of Neuroscience. 2006;26(18):4917–4921. doi: 10.1523/JNEUROSCI.5389-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Roland PE. Cross-modal transfer of information between the tactile and the visual representations in the human brain: a positron emission tomographic study. The Journal of Neuroscience. 1998;18(3):1072–1084. doi: 10.1523/JNEUROSCI.18-03-01072.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen MC, Franzén O, McGlone F, Essick G, Dancer C, Pardo JV. Tactile motion activates the human middle temporal/V5 (MT/V5) complex. European Journal of Neuroscience. 2002;16(5):957–964. doi: 10.1046/j.1460-9568.2002.02139.x. [DOI] [PubMed] [Google Scholar]
- Hari R, Forss N. Magnetoencephalography in the study of human somatosensory cortical processing. Philosophical Transactions of the Royal Society B: Biological Sciences. 1999;354(1387):1145–1154. doi: 10.1098/rstb.1999.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak FB, Dickstein R, Peterka RJ. Diabetic neuropathy and surface sway-referencing disrupt somatosensory information for postural stability in stance. Somatosensory & motor research. 2002;19(4):316–326. doi: 10.1080/0899022021000037782. [DOI] [PubMed] [Google Scholar]
- Jousmäki V, Forss N. Effects of stimulus intensity on signals from human somatosensory cortices. Neuroreport. 1998;9(15):3427–3431. doi: 10.1097/00001756-199810260-00017. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Gottschalk A, Patel RS, Pincus D, Detre JA, Alsop DC. Mapping of secondary somatosensory cortex activation induced by vibrational stimulation: an fMRI study. Brain research. 1999;824(2):291–295. doi: 10.1016/s0006-8993(99)01126-9. [DOI] [PubMed] [Google Scholar]
- Matsunaga K, Nitsche MA, Tsuji S, Rothwell JC. Effect of transcranial DC sensorimotor cortex stimulation on somatosensory evoked potentials in humans. Clinical Neurophysiology. 2004;115(2):456–460. doi: 10.1016/s1388-2457(03)00362-6. [DOI] [PubMed] [Google Scholar]
- Miranda PC, Faria P, Hallett M. What does the ratio of injected current to electrode area tell us about current density in the brain during tDCS? Clinical Neurophysiology. 2009;120(6):1183–1187. doi: 10.1016/j.clinph.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori F, Nicoletti CG, Kusayanagi H, Foti C, Restivo DA, Marciani MG, Centonze D. Transcranial direct current stimulation ameliorates tactile sensory deficit in multiple sclerosis. Brain stimulation. 2013;6(4):654–659. doi: 10.1016/j.brs.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Nagai M, Kishi K, Kato S. Insular cortex and neuropsychiatric disorders: a review of recent literature. European Psychiatry. 2007;22(6):387–394. doi: 10.1016/j.eurpsy.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Nelson AJ, Staines WR, Graham SJ, McIlroy WE. Activation in SI and SII; the influence of vibrotactile amplitude during passive and task-relevant stimulation. Cognitive brain research. 2004;19(2):174–184. doi: 10.1016/j.cogbrainres.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. Journal of Neurophysiology. 1965;28(1):166–185. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- Quai TM, Brauer SG, Nitz JC. Somatosensation, circulation and stance balance in elderly dysvascular transtibial amputees. Clinical rehabilitation. 2005;19(6):668–676. doi: 10.1191/0269215505cr857oa. [DOI] [PubMed] [Google Scholar]
- Radman T, Ramos RL, Brumberg JC, Bikson M. Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain stimulation. 2009;2(4):215–228. doi: 10.1016/j.brs.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragert P, Vandermeeren Y, Camus M, Cohen LG. Improvement of spatial tactile acuity by transcranial direct current stimulation. Clinical Neurophysiology. 2008;119(4):805–811. doi: 10.1016/j.clinph.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalewski A, Breitenstein C, Nitsche MA, Paulus W, Knecht S. Transcranial direct current stimulation disrupts tactile perception. European Journal of Neuroscience. 2004;20(1):313–316. doi: 10.1111/j.0953-816X.2004.03450.x. [DOI] [PubMed] [Google Scholar]
- Sadleir RJ, Vannorsdall TD, Schretlen DJ, Gordon B. Transcranial direct current stimulation (tDCS) in a realistic head model. Neuroimage. 2010;51(4):1310–1318. doi: 10.1016/j.neuroimage.2010.03.052. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Renga V. Transcranial direct current stimulation: a noninvasive tool to facilitate stroke recovery. 2008 doi: 10.1586/17434440.5.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer SW, Harrison AL. Aging of the somatosensory system: a translational perspective. Physical therapy. 2007;87(2):193–207. doi: 10.2522/ptj.20060083. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyerc B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Deursen RWM, Simoneau GG. Foot and ankle sensory neuropathy, proprioception, and postural stability. Journal of orthopaedic & sports physical therapy. 1999;29(12):718–726. doi: 10.2519/jospt.1999.29.12.718. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Veit R, Erb M, Mathiak K, Grodd W, Goebel R, Birbaumer N. Physiological self-regulation of regional brain activity using real-time functional magnetic resonance imaging (fMRI): methodology and exemplary data. Neuroimage. 2003;19(3):577–586. doi: 10.1016/s1053-8119(03)00145-9. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Goodbody SJ, Husain M. Maintaining internal representations: the role of the human superior parietal lobe. Nature neuroscience. 1998;1(6):529–533. doi: 10.1038/2245. [DOI] [PubMed] [Google Scholar]
- Woollacott MH, Shumway-Cook A, Nashner LM. Aging and posture control: changes in sensory organization and muscular coordination. The International Journal of Aging and Human Development. 1986;23(2):97–114. doi: 10.2190/VXN3-N3RT-54JB-X16X. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Freeman DK, McCarthy JJ, III, Jolesz FA. Neural substrates of tactile imagery: a functional MRI study. Neuroreport. 2003;14(4):581–585. doi: 10.1097/00001756-200303240-00011. [DOI] [PubMed] [Google Scholar]
- Zheng X, Alsop DC, Schlaug G. Effects of transcranial direct current stimulation (tDCS) on human regional cerebral blood flow. Neuroimage. 2011;58(1):26–33. doi: 10.1016/j.neuroimage.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Hao Y, Wang Y, Jor'dan A, Pascual-Leone A, Zhang J, Manor B. Transcranial direct current stimulation reduces the cost of performing a cognitive task on gait and postural control. European Journal of Neuroscience. 2014;39(8):1343–1348. doi: 10.1111/ejn.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]