Abstract
Hypertension is a common chronic disease and a key risk factor in the development of cardiovascular disease. The Western Alaska Tribal Collaborative for Health study consolidates baseline data from four major cohorts residing in the Norton Sound and Yukon‐Kuskokwim regions of western Alaska. This consolidated cohort affords an opportunity for a systematic analysis of high blood pressure and its correlates in a unique population with high stroke rates over a wide age range. While the prevalence of hypertension among western Alaska Native people (30%, age‐standardized) is slightly less than that of the US general population (33%), cardiovascular disease is a leading cause of mortality in this rural population. The authors found that improvement is needed in hypertension awareness as about two thirds (64%) of patients reported awareness and only 39% with hypertension were controlled on medication. Future analyses assessing risk and protective factors for incident hypertension in this population are indicated.
Alaska Native people, once thought to have very low prevalence of cardiovascular disease (CVD), have experienced considerable increases in CVD mortality, prevalence, and risk factors.1, 2, 3, 4, 5 However, few population‐based data are available to compare prevalence of hypertension and its associated risk factors among Alaska Native people living in multiple remote rural communities. Although remote, each community has a clinic with trained and certified community health aids (CHAs) who provide primary and emergency care. CHAs manage patient monitoring, prescriptions, and medication refills through physicians and pharmacists located at the regional hospital. Hypertension is a major independent risk factor for CVD, such as stroke, myocardial infarction, and heart failure.6 CVD risk increases as blood pressure (BP) increases,6, 7 and high systolic BP (SBP) is a strong independent risk factor for CVD.6
Longitudinal cohort studies provide an opportunity for systematic analyses of population‐specific health outcomes and risk factors. However, the large numbers of participants who must be followed over an extended period are problematic in smaller, remote populations. The Western Alaska Tribal Collaborative for Health (WATCH) study has consolidated baseline data from four western Alaska Native cohorts. These data provide important baseline information about high BP and its associated risk factors in this unique population living in more than 40 communities. Consistent with recent published mortality data,5 previous WATCH analyses have shown that stroke mortality is high in these communities.8
We sought to determine the prevalence of hypertension and prehypertension overall, by sex, age, and region. We examined known risk factors, including age, body mass index (BMI)/obesity, smoking status, lipids, and diabetes. In addition, we assessed the proportion of participants who were aware of having hypertension, and among those for whom we had records of being treated for their BP, we assessed the proportion who had met their BP targets.
Methods
The WATCH study was approved by the Alaska Area institutional review board (IRB) as well as by the IRBs of all participating institutions. Tribal approval was granted by the Alaska Native Tribal Health Consortium, the Norton Sound Health Corporation, and the Yukon‐Kuskokwim Health Corporation.
Study Population
The WATCH study combines four major cohorts residing in rural, remote Alaska Native communities of the Norton Sound and Yukon‐Kuskokwim regions of western Alaska. The methods have been previously described.9 The four cohorts used to create the combined dataset were the Alaska Siberia Project (ASP),10 Genetics of Coronary Artery Disease in Alaska Natives (GOCADAN),11 Center for Alaska Native Health Research (CANHR),12, 13 and Alaska Education and Research Toward Health (EARTH).14 Briefly, all studies were initiated between 1994 and 2004. Objectively measured and self‐reported data from each of the studies were consolidated, through either direct combination or harmonization, into a large dataset to increase power and provide reliable population‐based assessments of health and risk.8, 9 For WATCH, the last two of three resting BPs obtained by all studies were averaged for a baseline BP.
Hypertension and Prehypertension
Hypertension was defined as a resting systolic BP (SBP) ≥140 mm Hg or a diastolic BP (DBP) ≥90 mm Hg6 as measured at baseline by each study, an International Classification of Diseases, Ninth Edition code 401 in the medical record, or use of antihypertensive medication confirmed by chart review or interviewer observation of medication labels. Prehypertension was defined as not meeting the above definition of hypertension but having a resting SBP measurement of 120 mm Hg to 139 mm Hg or a DBP measurement of 80 mm Hg to 89 mm Hg.6
Covariates
Covariates included self‐reported age in years (age categories: 18–34, 35–44, 45–54, 55–64, and ≥65 years), sex, smoking status, obesity determined by BMI (in kg/m2) using baseline weight and height measurements, and impaired fasting glucose, prevalent diabetes, high‐density lipoprotein cholesterol (HDL‐C), and triglycerides at baseline. All covariates were measured using standardized methods.9 Impaired fasting glucose (100–125 mg/dL) was defined according to 2010 American Diabetes Association criteria.15 Prevalent diabetes was defined as a fasting glucose ≥126 mg/dL, a laboratory‐confirmed diagnosis of diabetes in the medical record, or a documented prescription for diabetes medication. Definitions for low HDL‐C (<40 mg/dL for men, <50 mg/dL for women) and high triglycerides (≥150 mg/dL) were based on the 2002 National Cholesterol Education Program criteria for metabolic syndrome.16
Medications
Use of hypertension medication was assessed at the time of entry into the original study by study personnel and/or by chart review in all studies except for the EARTH study. For a subset excluding EARTH participants, we were able to determine whether medication use was effective in controlling hypertension.
Hypertension Awareness, Treatment, and Control
The proportion of patients with hypertension awareness was calculated among those who met our definition of having prevalent hypertension. Hypertension awareness was defined as answering “yes” to the question “Do you have or has a health care provider told you that you have high blood pressure or hypertension?” Among the subset of persons who met our definition of prevalent hypertension and for whom we had data on antihypertensive medication use, the proportion with hypertension treatment and control was determined. Hypertension treatment was defined as taking antihypertensive medication. Hypertension control was defined as taking antihypertensive medication for high BP/hypertension and having a measured BP <140/90 mm Hg at baseline examination.
Statistical Methods
SAS version 9.3 (SAS Institute Inc, Cary, NC) was used for all analyses. Descriptive statistics for participant characteristics were calculated by using means and standard deviations for continuous variables and by percentages for categorical variables. Significance was determined by 95% confidence intervals (CIs) not containing 1.0 and by nonoverlapping 95% CIs. Overall prevalence was sex‐ and age‐standardized to the standard 2000 US population by the direct method. We then calculated the prevalence of hypertension separately by age group, sex, and region.
Logistic regression analyses were used to determine the unadjusted odds ratios (ORs) for hypertension and prehypertension with each of the covariates. We then fit multivariate models with age, sex, smoking status, BMI, impaired fasting glucose, prevalent diabetes, high triglycerides, and low HDL‐C to determine their independent associations with hypertension prevalence.
In an additional analysis among patients with prevalent hypertension, we determined the proportion with hypertension awareness, hypertension treatment, and hypertension control by sex, age group, and region.
Results
Demographic information overall and by region is provided in Table 1. Of the 4569 participants, 54% (n=2453) were women. The mean age of the total cohort was 41 years (standard deviation ±16). Prevalent diabetes was low at 5% (n=219). Impaired fasting glucose was 23% (n=1047; Table 1).
Table 1.
WATCH Study Demographics
| Characteristic | Yukon Kuskokwim (n=2650) | Norton Sound (n=1919) | P Value | WATCH Total (N=4569) |
|---|---|---|---|---|
| Sociodemographics | ||||
| Age, mean (SD), y | 39.1 (16.1) | 43.1 (16.2) | <.001 | 40.8 (16.3) |
| Sex | ||||
| Female, No. (%) | 1409 (53) | 1044 (54) | .409 | 2453 (54) |
| Male, No. (%) | 1241 (47) | 875 (46) | 2116 (46) | |
| Last grade completed, mean (SD) | 9.8 (3.1) | 11.7 (2.3) | <.001 | 10.6 (2.9) |
| Clinical factors | ||||
| Glycemic status | ||||
| Normal fasting glucose, (<100 mg/dL), No. (%) | 1898 (72) | 1405 (73) | .471 | 3303 (72) |
| Impaired fasting glucose (100–125 mg/dL), No. (%) | 624 (24) | 423 (22) | 1047 (23) | |
| Diabetes (≥126 mg/dL), No. (%) | 128 (5) | 91 (5) | 219 (5) | |
| Hypertensiona | ||||
| Normal, No. (%) | 1234 (47) | 897 (47) | <.001 | 2131 (47) |
| Prehypertension, No. (%) | 684 (26) | 616 (32) | 1300 (28) | |
| Hypertension, No. (%) | 732 (28) | 406 (21) | 1138 (25) | |
| Body mass index, mean (SD) | 28.1 (6.1) | 27.3 (5.8) | <.001 | 27.8 (6.0) |
| Total cholesterol, mean (SD), mg/dL | 207.5 (44.1) | 206.9 (42.9) | .634 | 207.2 (43.6) |
| High‐density lipoprotein cholesterol, mean (SD), mg/dL | 60.3 (17.8) | 58.2 (17.9) | <.001 | 59.4 (17.9) |
| Low‐density lipoprotein cholesterol, mean (SD), mg/dL | 128.2 (38.5) | 126.8 (41.6) | .247 | 127.6 (39.9) |
| Triglycerides, mean (SD), mg/dL | 95.1 (61.0) | 111.7 (83.5) | <.001 | 101.9 (71.7) |
| Smoking statusb | ||||
| Never, No. (%) | 968 (37.0) | 325 (17.1) | <.001 | 1293 (29) |
| Current/former smoker, No. (%) | 1646 (63.0) | 1576 (82.9) | 3222 (71) | |
Abbreviations: SD, standard deviation; WATCH, Western Alaska Tribal Collaborative for Health. aHypertension was defined as measured systolic blood pressure ≥140 mm Hg, International Classification of Diseases, Ninth Edition code 401 in the medical record, or use of antihypertensive medication. bMissing=54.
Among the WATCH cohort, 25% (n=1138) of people had prevalent hypertension and 28% (n=1300) had prevalent prehypertension (Table 1). The age‐standardized hypertension prevalence rate was 29.9%, and the age‐standardized prehypertension prevalence rate was 27%.
Hypertension increased with age, with 50% of participants aged 55 to 64 years and nearly 70% of those aged 65 years and older having hypertension (1A). The prevalence of hypertension in the Yukon‐Kuskokwim region was 28%, significantly higher than the 21% prevalence found in the Norton Sound region (1 B).
Figure 1.
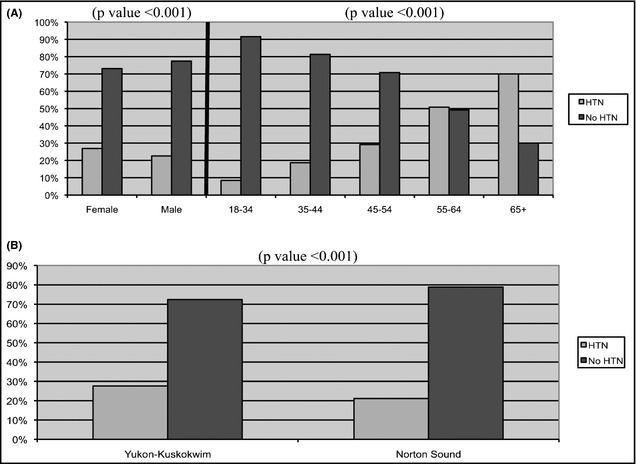
Prevalence of hypertension by sex and age group (A). Prevalence of hypertension by region (B).
In the univariate analysis, female sex, aging, and living in the Yukon‐Kuskokwim region were associated with having hypertension as well as obesity, impaired fasting glucose, prevalent diabetes, and elevated triglycerides. However, in the multivariate analysis, being female and having prevalent diabetes were no longer significant correlates. Increasing age, obesity, elevated triglycerides, impaired fasting glucose, and living in the Yukon‐Kuskokwim region were independently associated with hypertension prevalence (Table 2).
Table 2.
Factors Associated With Having Prevalent Hypertension
| Odds Ratios With 95% CIa for Prevalent Hypertension | ||
|---|---|---|
| Univariate (n=4569) | Multivariate (n=4400)b | |
| Sex (female vs male) | 1.26 (1.10–1.44) | 0.95 (0.80–1.13) |
| Age (referent is 18–34 y), y | ||
| 35–44 | 2.50 (1.99–3.14)c | 2.41 (1.89–3.08) |
| 45–54 | 4.45 (3.58–5.62) | 4.23 (3.30–5.42) |
| 55–64 | 11.24 (8.83–14.30) | 11.41 (8.72–14.94) |
| 65+ | 25.37 (19.52–32.97) | 27.12 (20.12–36.51) |
| Region (referent is Norton‐Sound) | ||
| Yukon Kuskokwim | 1.43 (1.24–1.64) | 1.96 (1.64–2.38) |
| Covariates | ||
| Obesityd (body mass index >30 kg/m2) | 2.82 (2.45–3.24) | 2.31 (1.93–2.78) |
| Impaired fasting glucosee | 2.07 (1.77–2.42) | 3.29 (2.30–4.71) |
| Prevalent diabetes | 9.04 (6.71–12.18) | 1.11 (0.92–1.34) |
| Smoking history | 0.55 (0.48–0.64) | 0.74 (0.61–0.88) |
| High triglyceridesf | 2.32 (1.95–2.75) | 2.19 (1.76–2.72) |
| Low high‐density lipoprotein cholesterolg | 1.05 (0.88–1.24) | 0.89 (0.71–1.12) |
a95% Confidence intervals (CIs) that do not include 1.0 are statistically significant. bMultivariate = adjusted for sex, age, obesity, impaired fasting glucose, prevalent diabetes, smoking history, triglycerides, and high‐density lipoprotein cholesterol. cNonoverlapping CIs indicate statistically significant differences between age groups. dObesity = body mass index >30 kg/m2. eImpaired fasting glucose=glucose 100–125 mg/dL. fHigh triglycerides ≥150 mg/dL. gLow high‐density lipoprotein cholesterol <40 mg/dL in men and <50 mg/dL in women.
Of the participants with hypertension, 64% (n=731) were aware of their condition (Table 3). Of the 700 participants with hypertension and for whom medication data were available, 61% (n=428) were taking BP medication and 39% (n=273) were at their targets for BP.
Table 3.
Prevalent Hypertension Awareness, Treatment, and Control
| Characteristic | Aware (n=1138), No. (%) | Treated (n=700), No. (%) | Controlled (n=700), No. (%) |
|---|---|---|---|
| Overall | 731 (64) | 428 (61) | 273 (39) |
| Sex | |||
| Female | 443 (67) | 283 (70) | 176 (44) |
| Male | 288 (60) | 145 (49) | 97 (33) |
| Age group, y | |||
| 18–34 | 74 (48) | 18 (24) | 11 (14) |
| 35–44 | 109 (58) | 42 (42) | 21 (21) |
| 45–54 | 145 (61) | 80 (55) | 51 (35) |
| 55–64 | 178 (70) | 124 (73) | 88 (52) |
| 65+ | 225 (73) | 164 (78) | 102 (49) |
| Region | |||
| Yukon‐Kuskokwim | 441 (60) | 185 (63) | 129 (44) |
| Norton sound | 290 (71) | 243 (60) | 144 (35) |
Hypertension (HTN) awareness = answered “yes” to self‐report question of “Do you have high blood pressure or HTN?” HTN treatment = participant is taking blood pressure medication. HTN control = participant is taking blood pressure medication and has blood pressure “at goal,” defined as <140/90 mm Hg.
Discussion
The WATCH dataset provides a unique opportunity to estimate the prevalence of hypertension and examine its risk factors in western Alaska Native people at the population level. This resource is important, given that CVD is a leading cause of mortality among all Alaska Native people and because this western Alaskan population has particularly high stroke rates. Associated risk factors for prevalent hypertension were age, obesity, impaired fasting glucose, and elevated triglycerides. Hypertension awareness was modest and among patients taking antihypertensive medication, hypertension control was low.
Compared with the US general population, in which nearly one in three adults or about 33% has hypertension,17 we found a marginally lower age‐standardized prevalence of about 30%. Likewise, nearly one in three adults has prehypertension in the US general population.18 We found a lower (27%) age‐standardized prevalence rate of prehypertension in our study population overall. Despite the known higher stroke rates among Alaska Native people, we did not find concomitant high rates of prevalent hypertension. We also found a significantly lower hypertension rate in the Norton Sound region. Tobacco use may play a role in these differences, as tobacco‐type preferences differ between the two regions. However, tobacco was not found to be independently associated with prevalent hypertension in the current study.
Our findings are consistent with other smaller studies among Alaska Native people.2, 19, 20 Using Indian Health Service ambulatory care data from the 1990s, researchers have also found a relatively low prevalence rate of hypertension in Native Americans compared with the US general population.21, 22 Other researchers who examined older Native American primary care patients receiving treatment at an urban Indian Health Service clinic found a somewhat higher hypertension prevalence rate of 38%.23 In the Strong Heart Study, an ongoing examination of CVD risk among American Indians, the prevalence rate of hypertension at baseline was approximately 27% in North and South Dakota and 44% in Oklahoma and Arizona, indicating regional differences in hypertension prevalence among Native American populations.24 Regional differences in hypertension have also been described for the indigenous Arctic populations.25 In addition, trends of increasing CVD risk factors, including hypertension, have been described among American Indian and Alaska Native populations.26
Traditional Alaska Native people have relied for centuries on physically demanding subsistence practices to obtain a diet rich in game and marine life over a vast geography.2 There is a positive relationship between traditional food consumption and protection from cardiovascular disease.27 Early nutrition studies conducted 50 years ago found that traditional foods accounted for half of the calories consumed28; however, more recent studies conducted 5 to 15 years ago found that only about 25% or less of calories consumed come from traditional food sources.29, 30 Previous work found nontraditional lifestyle factors, such as consumption of Western foods and use of motorized transport, to be independently associated with increased hypertension prevelance.2 The increase in Western food consumption is highest among younger age groups, which may have an impact on hypertension prevalence as the population ages.31, 32
Few studies exist on hypertension awareness, treatment, and control among American Indian or Alaska Native peoples. One study of American Indians seen at an urban Indian Health Service clinic between 1994 and 1995 found via chart review that BP control was suboptimal, with almost two thirds (65%) having two or more elevated SBP, DBP, or combined readings.23 Among the US general population, one study of hypertension treatment and control at the county level found that awareness, treatment, and control were highest in the southeastern United States.33 Furthermore, among the US general population from 1999 to 2010, the overall proportions remained low, with hypertension awareness at 74%, treatment at 71.6%, and control at 46.5%.34 Our study found that the proportions of those with hypertension who were aware of their diagnosis (64%), treated (61%), and had achieved their BP targets (39%) were substantially lower than those of the US general population. The remoteness of the communities may be an impediment to prompt diagnosis and management of hypertension. Hypertension, which is generally asymptomatic, is a condition identified and managed by health care providers. In addition to diet and physical activity, access to routine BP screening, health literacy levels pertaining to hypertension, and the availability of social support systems that foster treatment compliance are among multiple environmental and sociocultural factors that may play a substantial role in hypertension prevalence in rural remote Alaskan communities.35 Further research into these and other contributing factors is needed to guide interventions and practices that will improve hypertension awareness, treatment, and control in this population.
Strengths/Limitations
The WATCH study has a number of strengths. The data are from a large representative sample of Alaska Native people living in the Norton Sound and Yukon‐Kuskokwim communities of western Alaska. Based on these data, we were able to reliably estimate hypertension and prehypertension prevalence at the population level. We were also able to examine important population‐specific risk factors for hypertension. To our knowledge, this is the first study in this population to report on hypertension awareness, medication use, and BP control.
The data used in these analyses are cross‐sectional and have inherent limitations. While it was not possible to examine predictors in this cross‐sectional dataset, we did examine correlates of control. Power was limited for these analyses, but we did observe that women had better control than men, and that control increased with age. Longitudinal analyses will be needed to explore the determinants of control. We lacked harmonized data for dietary and physical activity factors, which may explain some of our findings. Measurements for diabetes status included only one value, fasting glucose at baseline, which may underestimate diabetes prevalence. Because a 2‐hour glucose tolerance test was not available, we could not explore impaired glucose tolerance. Additionally, we did not have data on organic pollutants, which have been shown to be associated with hypertension among some Arctic populations.36, 37 Population estimates of hypertension awareness were limited to a subset of 50% of participants for whom data were available. Medication data were available for about 30% of the sample. Details on the medications used or repeated measures used to examine trends were unavailable.
Conclusions
We found hypertension and prehypertension prevalence levels of the western Alaska native population just under those for the US general population. The risk factors for development of hypertension are likely to increase as this population ages and so attention to prevention is needed as well as cooperation with the communities to improve hypertension awareness, treatment, and control. Cultural identification and social support are important concepts in these communities, which will need to be considered during future studies.38 Prospective analyses conducted in conjunction with community prevention programs are needed to determine factors associated with incident hypertension.
Disclosures
The authors have nothing to declare.
Acknowledgments
Portions of this manuscript were presented in abstract form at the Society for General Internal Medicine Annual Meeting, April 23–26, San Diego, CA. The WATCH study was funded in part by an American Recovery and Reinvestment Act Administrative Supplement to a grant funded by the National Center for Research Resources (NCRR), National Institutes of Health (NIH; P20 RR16430); with federal funds (grant number UL1RR031975) from the NCRR and the National Center for Advancing Translational Sciences, NIH, through the Clinical and Translational Science Awards Program, a trademark of the US Department of Health and Human Services, part of the Roadmap Initiative, “Re‐Engineering the Clinical Research Enterprise”; with funds from the National Institute of Diabetes and Digestive and Kidney Diseases (DK097307); and with funds made available by the President of the University of Alaska from unrestricted donations by British Petroleum and ConocoPhillips. Dr Jolly is supported by a career development award (1K23DK091363) from the National Institute of Diabetes and Digestive and Kidney Diseases. We thank Rachel Schaperow, MedStar Health Research Institute, for editing the manuscript. The WATCH team would like to express our sincere appreciation to all of our study participants and their communities for welcoming us and teaching us about the Alaska Native way of life.
J Clin Hypertens (Greenwich). 2015:812–818. DOI: 10.1111/jch.12483. © 2015 Wiley Periodicals, Inc.
References
- 1. Howard BV, Comuzzie A, Devereux RB, et al. Cardiovascular disease prevalence and its relation to risk factors in Alaska Eskimos. Nutr Metab Cardiovasc Dis. 2010;20:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murphy NJ, Schraer CD, Theile MC, et al. Hypertension in Alaska Natives: association with overweight, glucose intolerance, diet and mechanized activity. Ethn Health. 1997;2:267–275. [DOI] [PubMed] [Google Scholar]
- 3. Rhoades DA. Racial misclassification and disparities in cardiovascular disease among American Indians and Alaska Natives. Circulation. 2005;111:1250–1256. [DOI] [PubMed] [Google Scholar]
- 4. Schumacher C, Davidson M, Ehrsam G. Cardiovascular disease among Alaska Natives: a review of the literature. Int J Circumpolar Health. 2003;62:343–362. [DOI] [PubMed] [Google Scholar]
- 5. Day GM, Holck P, Provost EM. Alaska Native Mortality Update: 2004–2008. Anchorage, AK: Alaska Native Epidemiology Center; 2011. http://www.anthctoday.org/epicenter/publications/mortality/index.html. Accessed January 14, 2014. [Google Scholar]
- 6. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 7. Lewington S, Clarke R, Qizilbash N, et al. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 8. Howard BV, Metzger JS, Koller KR, et al. All‐cause, cardiovascular, and cancer mortality in Western Alaska native people: Western Alaska Tribal Collaborative for Health (WATCH). Am J Public Health. 2014;104:1334–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koller KR, Wolfe AW, Metzger JS, et al. Utilizing harmonization and common surveillance methods to consolidate 4 cohorts: the Western Alaska Tribal Collaborative for Health (WATCH) study. Int J Circumpolar Health. 2013. May 2;72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ebbesson SO, Ebbesson LO, Swenson M, et al. A successful diabetes prevention study in Eskimos: the Alaska Siberia project. Int J Circumpolar Health. 2005;64:409–424. [DOI] [PubMed] [Google Scholar]
- 11. Howard BV, Devereux RB, Cole SA, et al. A genetic and epidemiologic study of cardiovascular disease in Alaska natives (GOCADAN): design and methods. Int J Circumpolar Health. 2005;64:206–221. [DOI] [PubMed] [Google Scholar]
- 12. Boyer BB, Mohatt GV, Lardon C, et al. Building a community‐based participatory research center to investigate obesity and diabetes in Alaska Natives. Int J Circumpolar Health. 2005;64:281–290. [DOI] [PubMed] [Google Scholar]
- 13. Mohatt GV, Plaetke R, Klejka J, et al. The Center for Alaska Native Health Research Study: a community‐based participatory research study of obesity and chronic disease‐related protective and risk factors. Int J Circumpolar Health. 2007;66:8–18. [DOI] [PubMed] [Google Scholar]
- 14. Slattery ML, Schumacher MC, Lanier AP, et al. A prospective cohort of American Indian and Alaska Native people: study design, methods, and implementation. Am J Epidemiol. 2007;166:606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American Diabetes Association . Standards of medical care in diabetes–2010. Diabetes Care. 2010;33(Suppl 1):S11–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) . Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention (CDC) . Vital signs: awareness and treatment of uncontrolled hypertension among adults—United States, 2003–2010. MMWR Morb Mortal Wkly Rep. 2012;61:703–709. [PubMed] [Google Scholar]
- 18. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Redwood DG, Lanier AP, Johnston JM, et al. Chronic disease risk factors among Alaska Native and American Indian people, Alaska, 2004–2006. Prev Chronic Dis. 2010;7:A85. [PMC free article] [PubMed] [Google Scholar]
- 20. Schraer CD, Ebbesson SO, Boyko E, et al. Hypertension and diabetes among Siberian Yupik Eskimos of St. Lawrence Island, Alaska. Public Health Rep. 1996;111(Suppl 2):51–52. [PMC free article] [PubMed] [Google Scholar]
- 21. Acton KJ, Preston S, Rith‐Najarian S. Clinical hypertension in Native Americans: a comparison of 1987 and 1992 rates from ambulatory care data. Public Health Rep. 1996;111(Suppl 2):33–36. [PMC free article] [PubMed] [Google Scholar]
- 22. Broussard BA, Valway SE, Kaufman S, et al. Clinical hypertension and its interaction with diabetes among American Indians and Alaska Natives. Estimated rates from ambulatory care data. Diabetes Care. 1993;16:292–296. [DOI] [PubMed] [Google Scholar]
- 23. Rhoades DA, Buchwald D. Hypertension in older urban Native‐American primary care patients. J Am Geriatr Soc. 2003;51:774–781. [DOI] [PubMed] [Google Scholar]
- 24. Howard BV, Lee ET, Yeh JL, et al. Hypertension in adult American Indians. The Strong Heart Study. Hypertension. 1996;28:256–264. [DOI] [PubMed] [Google Scholar]
- 25. Bjerregaard P, Dewailly E, Young TK, et al. Blood pressure among the Inuit (Eskimo) populations in the Arctic. Scand J Public Health. 2003;31:92–99. [DOI] [PubMed] [Google Scholar]
- 26. Jernigan VB, Duran B, Ahn D, Winkleby M. Changing patterns in health behaviors and risk factors related to cardiovascular disease among American Indians and Alaska Natives. Am J Public Health. 2010;100:677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eilat‐Adar S, Mete M, Nobmann ED, et al. Dietary patterns are linked to cardiovascular risk factors but not to inflammatory markers in Alaska Eskimos. J Nutr. 2009;139:2322–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heller CA, Scott EM. The Alaska Dietary Survey, 1956–61. PHS Publication No. 999‐AH‐2. Washington, DC: US Department of Health, Education and Welfare; 1967. [Google Scholar]
- 29. Nobmann ED, Ebbesson SO, White RG, et al. Associations between dietary factors and plasma lipids related to cardiovascular disease among Siberian Yupiks of Alaska. Int J Circumpolar Health. 1999;58:254–271. [PubMed] [Google Scholar]
- 30. Kolahdooz F, Simeon D, Ferguson G, Sharma S. Development of a Quantitative Food Frequency Questionnaire for Use among the Yup'ik People of Western Alaska. PLoS One. 2014;9:e100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bersamin A, Luick BR, King IB, et al. Westernizing diets influence fat intake, red blood cell fatty acid composition, and health in remote Alaskan Native communities in the center for Alaska Native health study. J Am Diet Assoc. 2008;108:266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nobmann ED, Ponce R, Mattil C, et al. Dietary intakes vary with age among Eskimo adults of Northwest Alaska in the GOCADAN study, 2000–2003. J Nutr. 2005;135:856–862. [DOI] [PubMed] [Google Scholar]
- 33. Olives C, Myerson R, Mokdad AH, et al. Prevalence, awareness, treatment, and control of hypertension in United States counties, 2001–2009. PLoS One. 2013;8:e60308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guo F, He D, Zhang W, Walton RG. Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. J Am Coll Cardiol. 2012;60:599–606. [DOI] [PubMed] [Google Scholar]
- 35. Brega AG, Ang A, Vega W, et al. Mechanisms underlying the relationship between health literacy and glycemic control in American Indians and Alaska Natives. Patient Educ Couns. 2012;88:61–68. [DOI] [PubMed] [Google Scholar]
- 36. Valera B, Ayotte P, Poirier P, Dewailly E. Associations between plasma persistent organic pollutant levels and blood pressure in Inuit adults from Nunavik. Environ Int. 2013;59:282–289. [DOI] [PubMed] [Google Scholar]
- 37. Valera B, Jorgensen ME, Jeppesen C, Bjerregaard P. Exposure to persistent organic pollutants and risk of hypertension among Inuit from Greenland. Environ Res. 2013;122:65–73. [DOI] [PubMed] [Google Scholar]
- 38. Boden‐Albala B, Roberts ET, Hopkins S, et al. Predictors of risk and protection for hypertension in Yup'ik people from Southwest Alaska. Ethn Dis. 2013;23:484–491. [PubMed] [Google Scholar]


