Abstract
BACKGROUND/OBJECTIVES
Fermentation of dietary fiber results in production of various short chain fatty acids in the colon. In particular, butyrate is reported to regulate the physical and functional integrity of the normal colonic mucosa by altering mucin gene expression or the number of goblet cells. The objective of this study was to investigate whether butyrate modulates mucin secretion in LS174T human colorectal cells, thereby influencing the adhesion of probiotics such as Lactobacillus and Bifidobacterium strains and subsequently inhibiting pathogenic bacteria such as E. coli. In addition, possible signaling pathways involved in mucin gene regulation induced by butyrate treatment were also investigated.
MATERIALS/METHODS
Mucin protein content assay and periodic acid-Schiff (PAS) staining were performed in LS174T cells treated with butyrate at various concentrations. Effects of butyrate on the ability of probiotics to adhere to LS174T cells and their competition with E. coli strains were examined. Real time polymerase chain reaction for mucin gene expression and Taqman array 96-well fast plate-based pathway analysis were performed on butyrate-treated LS174T cells.
RESULTS
Treatment with butyrate resulted in a dose-dependent increase in mucin protein contents in LS174T cells with peak effects at 6 or 9 mM, which was further confirmed by PAS staining. Increase in mucin protein contents resulted in elevated adherence of probiotics, which subsequently reduced the adherent ability of E. coli. Treatment with butyrate also increased transcriptional levels of MUC3, MUC4, and MUC12, which was accompanied by higher gene expressions of signaling kinases and transcription factors involved in mitogen-activated protein kinase (MAPK) signaling pathways.
CONCLUSIONS
Based on our results, butyrate is an effective regulator of modulation of mucin protein production at the transcriptional and translational levels, resulting in changes in the adherence of gut microflora. Butyrate potentially stimulates the MAPK signaling pathway in intestinal cells, which is positively correlated with gut defense.
Keywords: Butyrate, LS174T cell, mucin protein, MUC gene, MAPK
INTRODUCTION
Fermentation of dietary fiber by the colonic microflora results in production of various short chain fatty acids (SCFA), mainly acetate, propionate, and butyrate, which contribute to normal large bowel function and prevent pathology [1]. In particular, butyrate is reported to be a major regulator of homeostasis of the normal colonic mucosa by regulation of cell proliferation and differentiation for maintenance of physical and functional integrity [2,3,4,5].
Butyrate is a well-known inhibitor of histone deacetylase with anti-carcinogenic activity. Butyrate was also shown to activate three major mitogen-activated protein kinase (MAPK) signaling pathways [6,7,8,9]. Therefore, butyrate treatment could induce both pro- and anti-growth effects depending on the cell lines and experimental conditions.
The mucus layer is composed of a complex mixture containing large amounts of mucin, a high molecular weight glycoprotein, which is considered the first line of defense against noxious substances and pathogens, and participates indirectly in the immune response [10,11]. Among different mucins, the oligosaccharide chains of mucin vary in both length and antigenic structure [12].
In the colonic mucosa, the main mucin gene is MUC2, a secretory mucin, and to a lesser extent MUC1, MUC3, and MUC4, which are both transmembrane mucins and secretory mucins from the splicing variants [13,14]. Apart from their distinct protective functions, some membrane-located mucins such as MUC1 and MUC4 possess adhesion and cell signaling properties.
The microbial community in the colon is considered important for the maintenance of gut health; dominant anaerobes such as lactobacilli and bifidobacteria confer numerous benefits to the host. However, beneficial bacteria must survive in the gastrointestinal tract (GIT) in order to establish functional microbe-host interactions [15]. Components of the gastrointestinal mucus could be substrates or adhesion factors for gut microbiota. Therefore, ability of bacteria to adhere to the host mucus layer is the most crucial attribute to perpetuation of colonization [16,17].
The importance of the mucin-expressing epithelium layer in the GIT has been well established, particularly in the area of innate immunity and protective antibacterial barrier formation [18,19,20]. However, the molecular mechanism of how SCFA affects the intestinal cellular response is not well understood.
Therefore, the objective of this study was to investigate whether butyrate modulates mucin secretion in LS174T human colorectal cells, thereby influencing adhesion of probiotics such as lactobacilli and bifidobacteria, and subsequently inhibiting pathogenic bacteria such as E. coli. In addition, possible signaling pathways involved in butyrate-dependent mucin gene expression were also investigated using real time polymerase chain reaction (PCR) assays.
MATERIALS AND METHODS
Cell culture and butyrate treatment
The LS174T human colorectal cancer cell line was purchased from the Korean Cell Line Bank (Seoul, Korea). All media and reagents were purchased from Life Technologies (Thermo Fisher Scientific Inc., MA, USA). Cells were grown in Minimum Essential Medium Eagle (MEM) supplemented with 10% fetal bovine serum, 1% non-essential amino acids, penicillin (100 U/ml), and streptomycin (100 U/ml) at 37℃ with 5% CO2 in a humidified atmosphere. Confluent cells were subcultured every 3-4 days by treatment with trypsin-EDTA solution. For mucin protein assay, periodic acid-Schiff (PAS) staining and bacterial adherence assay, LS174T cells (4 × 106 cells in 4 mL) were cultured in a 6-well plate (Nunc, USA) overnight, followed by addition of 100 uL of sodium butyrate in MEM media or only MEM media to cultures as a control at final concentrations of 0, 3, 6, and 9 mM in triplicate. Cells were cultured for an additional 2 days. For RNA extraction, LS174T cells (1 × 105 cells in 1 mL) were cultured in a 24-well plate (Nunc, USA) overnight, followed by addition of 50 uL of sodium butyrate solution to the cultures at a final concentration of 6 mM in triplicate. As a control, 50 uL of MEM media was added to the cultures instead of sodium butyrate solution. Cells were then cultured for an additional 2 days.
Bacterial strains and culture condition
The bacterial strains used in this study were obtained from the Korean Culture Center of Microorganisms (Seoul, Korea). Lactobacillus acidophilus ATCC 4356 were grown in MRS broth (BD Difco) at 37℃ for 18 hours, Bifidobacterium longum ATCC 15707 were grown in reinforced clostridial medium (RCM, Oxoid) and Escherichia coli ATCC 43896 were grown in tryptic soy broth (BD Difco). Prior to the experiments, all bacteria were subcultured at least three times. For long-term storage, the stock culture was stored at -80℃ in fresh broth containing 20% glycerol.
Mucin protein assay
Butyrate-treated and control cells were collected in 1.5 mL of phosphate-buffered saline (PBS) and then sonicated using a sonicator (Branson 8150, USA). After centrifugation at 12,000 rpm for 10 min, mucin protein in the supernatant was measured using an enzyme-linked immunosorbent assay kit (USCN Life Science Inc., USA) according to the manufacturer's instructions. Briefly, various dilutions of standards and samples were added to a pre-coated, ready-to-use 96-well strip plate and incubated for 2 hours at 37℃. After removal of the liquid from each well, 100 uL of detection reagent A was added, followed by incubation for 1 hour at 37℃. The plate was washed four times with wash solution, followed by addition of 100 uL of detection reagent B to each well, and incubation for 30 min at 37℃, and then washed four times. Then, 90 uL of TMB substrate solution was added to each well, followed by incubation for 10-15 min at 37℃, and addition of 50 uL of stop solution; the absorbance was read at 450 nm using an EMax microplate reader (Molecular devices, USA).
Periodic acid-Schiff staining
Cell staining was performed using a PAS stain kit (ScyTek Laboratories Inc., USA) according to the manufacturer's guidelines. Briefly, cells were immersed in periodic acid solution for 5 min and rinsed four times with distilled water. Cells were immersed in Schiff's solution for 5 min, in Hematoxylin & Mayer's for 1 min, and then in Bluing reagent for 10 sec. Cells were washed four times after each step. Cells were then visualized using a microscope (Olympus BX51, Japan).
Bacterial adherence assay
Lactobacillus acidophilus ATCC 4356 or B. longum ATCC 15707 (5 × 107 cfu per well) were added to butyrate-treated LS174T cells at final concentrations of 0, 3, 6, and 9 mM, respectively, followed by incubation for 5 hours at 37℃. For removal of non-bound bacteria, the plate was washed three times with PBS at 200 rpm for 3 min using a shaker (Heidoph Instruments, Germany). Serial dilutions of adherent bacteria were plated on MRS agar or RCM agar for subsequent CFU quantification. For analysis of the inhibitory effect of L. acidophilus or B. longum on adherence of E. coli ATCC 43896 to LS174T cells, butyrate-treated cells were incubated with L. acidophilus or B. longum for 5 hours and washed three times with PBS, followed by addition of E. coli ATCC 43896 at 5 × 107 cfu per well and then incubation for an additional 5 hours. The plate was washed three times with PBS at 200 rpm for 3 min for removal of non-bound E. coli. Adherent E. coli were counted on MacConkey agar (BD difco) using the standard plate count method. All experiments were run in triplicate and results are based on three separate experiments.
Total RNA extraction and real time polymerase chain reaction
The experiments were performed with modification of methods described by Han et al. [11]. Total RNA was extracted from cells using Trizol reagent and a PureLink RNA mini kit (Ambion, Life Technologies, USA) according to the manufacturer's guidelines. The first strand of cDNA was synthesized using a High capacity RNA-to-cDNA kit (Applied Biosystems, Thermo Fisher Scientific Inc., MA, USA). One microgram of total RNA, 1 uL of 20X RT enzyme mix, 10 uL of 2 × RT buffer, and nuclease-free H2O were added to yield a final volume of 20 uL. The reaction mixture was then incubated at 37℃ for 60 min and at 95℃ for 5 min.
For real time PCR, all kits were purchased from Life Technology (Thermo Fisher Scientific Inc., MA, USA). The synthesized cDNA was diluted (1:3) with RNA-free water. Real time PCR was performed using the StepOnePlus™ Real-Time PCR system with Taqman Real-Time PCR master mix. Taqman gene expression assays were used as primers and probes for mucin genes (MUC2, MUC3, MUC4, and MUC12) along with an internal reference, GAPDH. Their catalog numbers were HS03005092_m1, HS03649367_mH, HS00366414_m1, HS00419779_m1, and HS99999905_m1, respectively. The reaction mixture contained 4 uL of diluted cDNA, 1 uL of Taqman gene expression assays, 5 uL of DNase-free water and 10 uL of Taqman Real-Time PCR master mix in a final volume of 20 uL. The cycling conditions of PCR were as follows: preincubation at 50℃ for 2 min and then at 95℃ for 10 min, followed by 45 amplification cycles (95℃, 15 s and 60℃, 1 min) and cooling (40℃, 10 s). For real time monitoring, the fluorescence was measured at the end of the elongation phase.
The pathway of gene expression induced by butyrate treatment was examined by application of synthesized cDNA to a TaqMan® array 96-well fast plate (Hs MAP Kinase). The reaction mixture contained 4 uL of diluted cDNA, 1 uL of DNase-free water, and 5 uL of Taqman master mix. The cycling conditions of PCR were the same as described above. The comparative Ct method was used for relative quantification. The delta Ct value was determined by subtracting the average GAPDH Ct value from the individual Ct value of the target gene. The delta delta Ct value was determined by subtracting the delta Ct of the control sample from the individual delta Ct of the test sample. The fold change of the test sample relative to the control sample was determined by 2-delta delta Ct. Significant changes in butyrate-induced gene expression were analyzed based on more than 5-fold changes compared to the control.
Statistical analysis
Data are given as mean ± SEM. Analysis of mucin protein and bacterial adhesion across treatments was performed using ANOVA (GLM procedures of SAS, SAS Institute, Cary, NC). Duncan's multiple range test was used for comparison of means. The SAS/PROC t-test was used for analysis of mucin gene expression. Statistical significance was accepted at P < 0.05.
RESULTS
Mucin production and PAS staining
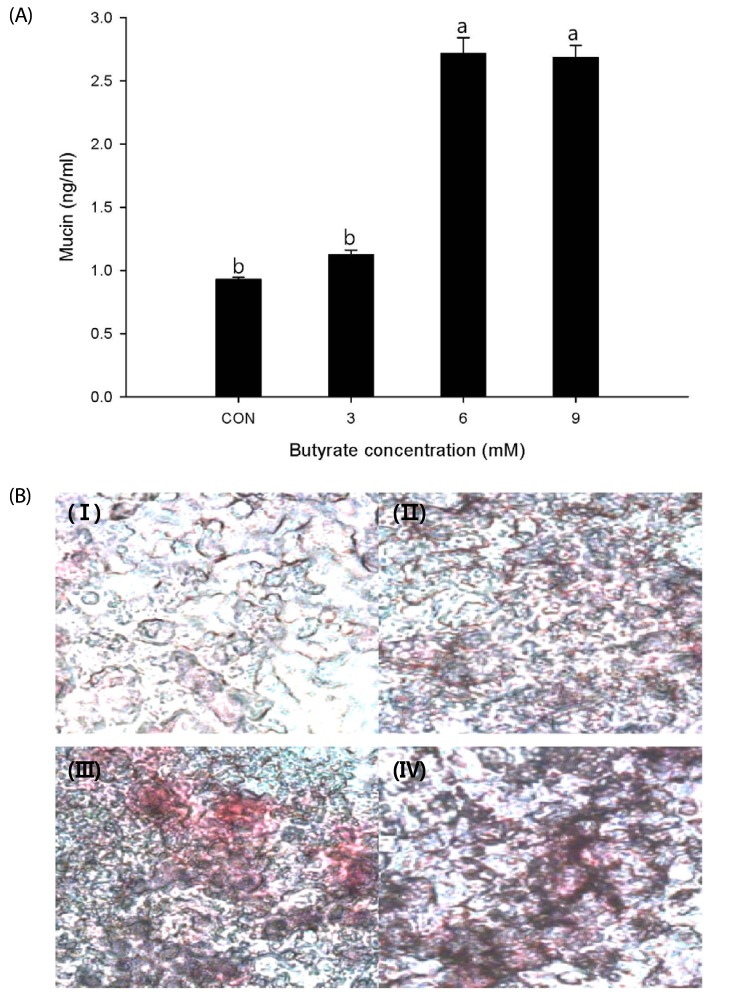
The effect of butyrate treatment on mucin protein production is shown in Fig. 1A. Cells treated with butyrate at 6 or 9 mM produced significantly (P < 0.05) higher mucin protein content of more than 2.5-fold compared to the control (0 mM) or cells treated with 3 mM butyrate.
Fig. 1. Effect of sodium butyrate treatment on mucin protein production in LS174T cells.
(A) Cells were treated with sodium butyrate at final concentrations of 0, 3, 6 and 9 mM. Mucin protein contents were measured by enzyme-linked immunosorbent assay. Values are expressed as mean ± SEM. Means without a common letter differ, P < 0.05. Con, control. (B) Periodic acid-Schiff staining of cells treated with sodium butyrate at final concentrations of 0 (I), 3 (II), 6 (III), and 9 mM (IV).
Polysaccharides of mucin glycoproteins were identified based on their red coloration by conventional PAS staining (Fig. 1B). Similar to mucin protein levels, treatment with butyrate resulted in a dose-dependent increase in the number of PAS-positive cells. A clearly visible increase in the cell population with higher mucin production was observed after treatment with butyrate at 6 or 9 mM, to a lesser extent at 3 mM.
Bacterial adherence
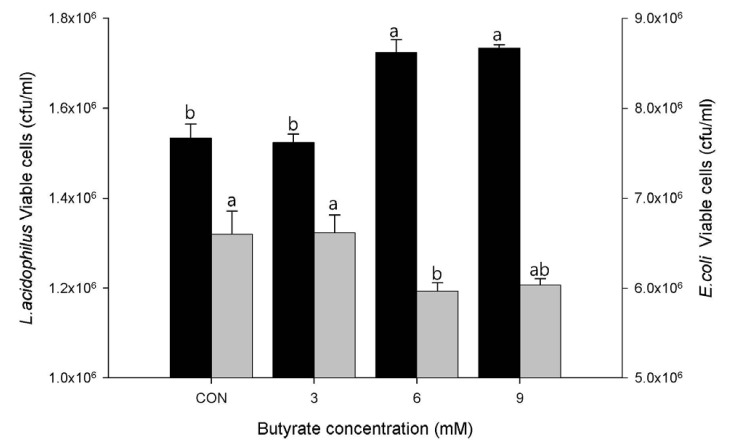
Adherence of L. acidophilus ATCC 4356 to LS174T cells treated with butyrate was measured at various concentrations (Fig. 2). Treatment with butyrate at 6 or 9 mM resulted in significantly (P < 0.01) increased adherence of L. acidophilus compared to the control (0 mM) or 3 mM butyrate treatment. On the other hand, increased adherence of L. acidophilus by treatment with 6 mM butyrate resulted in significantly (P < 0.05) reduced adherence of E. coli ATCC 43896, suggesting competition at the binding sites.
Fig. 2. Adherence of Lactobacillus acidophilus ATCC 4356 to LS174T cells and its inhibitory effect on Escherichia coli ATCC 43896.
LS174T cells were treated with sodium butyrate at final concentrations of 0, 3, 6, and 9 mM. Values are expressed as mean ± SEM. Means without a common letter differ, P < 0.01 for L. acidophilus (black bar) and P < 0.05 for E. coli (grey bar).
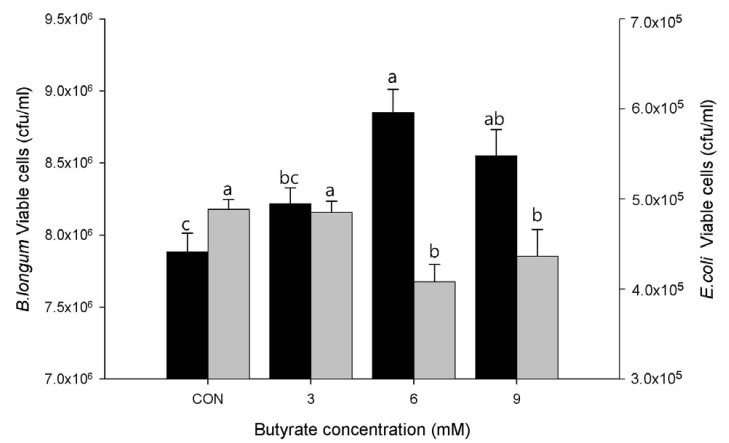
Similarly, treatment with butyrate at 6 or 9 mM resulted in significantly (P < 0.01) increased adherence of B. longum ATCC 15707 compared to the control (Fig. 3). Increased adherence of B. longum by treatment with 6 or 9 mM butyrate resulted in significantly (P < 0.05) decreased adherence of E. coli ATCC 43896 compared to the control or 3 mM butyrate treatment.
Fig. 3. Adherence of Bifidobacterum longum ATCC 15707 to LS174T cells and its inhibitory effect on Escherichia coli ATCC 43896.
LS174T cells were treated with sodium butyrate at final concentrations of 0, 3, 6, and 9 mM. Values are expressed as mean ± SEM. Means without a common letter differ, P < 0.01 for B. longum (black bar) and P < 0.05 for E. coli (grey bar).
Mucin gene expression and pathway
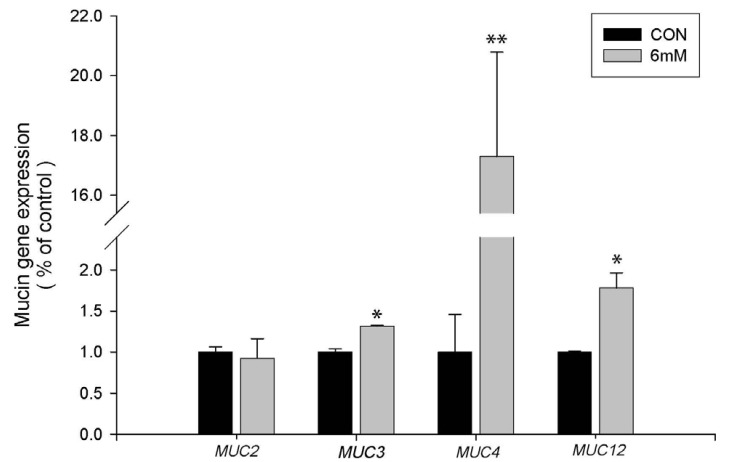
The purified RNA samples from 6 mM butyrate-treated LS174T cells were converted into cDNAs, which were subjected to real time PCR for mucin gene expression and Taqman array 96-well fast plate-based pathway analysis. Butyrate-treated LS174T cells showed significantly (P < 0.05) increased expression of MUC3 and MUC 12 mRNA compared to the control (approximately 130 and 170% increase, respectively). In particular, MUC4 mRNA expression showed a significant (P < 0.01) increase of more than 17-fold compared to the control. However, butyrate treatment did not affect MUC2 gene expression, suggesting a gene-specific regulatory effect (Fig. 4).
Fig. 4. Effect of 6 mM sodium butyrate treatment on mRNA expression levels of four mucin genes (MUC2, MUC3, MUC4, and MUC12) in LS174T cells.
The mRNA expression of mucin genes was normalized to the GAPDH mRNA level in each sample. Real time PCR was performed in triplicate at each concentration. The results are expressed as percentage increases as compared to the control (0 mM). Graphs represent mean ± SEM of three separate experiments; * P < 0.05 and ** P < 0.01 relative to control (CON) cells.
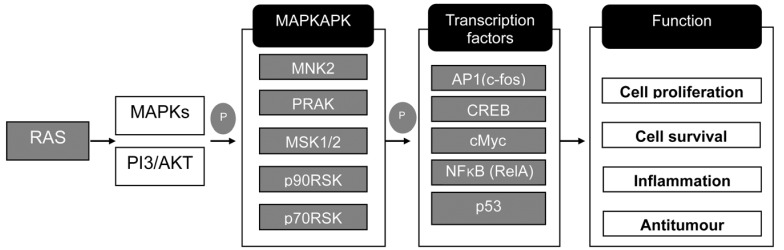
As shown in Fig. 5, butyrate treatment induced significant changes in gene expression levels related to MAPK signaling intermediates and targets. Treatment with butyrate resulted in a significant increase of mRNA levels in key downstream kinases of MAPK and PI3/AKT signaling pathways, including MNK2, PRAK, MSK1/2, p90RSK and p70RSK. Consistent with higher expression levels of these kinases, key transcription factors in regulatory gene expression appeared to be upregulated. However, it remains to be determined whether higher mRNA levels of transcription factors could be directly translated to the higher protein levels of AP1, CREB, cMyc, NFκB and p53. Alterations due to activation of these transcription factors in colonic cells would contribute to cell proliferation survival, inflammation, and antitumor activity in the epithelial layer.
Fig. 5. Analysis of signaling pathways in 6 mM butyrate-treated LS174T cells.
The synthesized cDNA was applied to a TaqMan array 96-well fast plate, and comparative Ct method for relative quantification was performed. The delta delta Ct value was determined by subtracting the delta Ct of the control sample from the individual delta Ct of the test sample. Significant changes in butyrate-induced gene expression were analyzed based on more than 5-fold changes compared to the control group. MAPKAPK, mitogen-activated protein kinase activated protein kinase; P, protein
DISCUSSION
The total concentration of SCFA in the human large intestine is mainly derived from microbial fermentation of dietary carbohydrates and the molar proportion of three main SCFA (acetate, propionate, butyrate) appears to be dependent on different factors including composition of gut microbiota and diet [21]. Acetate comprises approximately 60-75% of the total SCFA detected in human feces; however, due to their potentially beneficial effects on health, propionate and butyrate are of greater interest. In particular, butyrate, which is the major energy source for colonocytes, is implicated in prevention of colitis and colorectal cancer [22]. Butyrate, an inhibitor of histone-deacetylase, can modulate gene expression, signal transduction, and protein degradation pathways. Butyrate could also modulate mucin glycosylation by regulating the expression of certain enzymes involved in cellular glycosylation [5].
To date, the molecular mechanism of effects evoked by butyrate on colonic microbiota had not been well characterized; however, in this study, interaction between bacterial adherence and mucin secretion by treatment with butyrate was demonstrated. Cells rarely tolerate butyrate concentrations over 10 mM In vitro; therefore, concentration of butyrate below 10 mM were used. The preliminary cell viability tests confirmed that butyrate treatment below 10 mM did not significantly affect the cell growth (data not shown). The effect of butyrate on mucin protein content was clearly visible, whereas acetate and propionate had no effect.
So far, based on various in vitro and in vivo studies, butyrate appears to be the most effective SCFA in modulation of mucin production [13,23]. Butyrate has also been shown to increase mRNA expression levels of mucin in several cell lines derived from human colorectal cancers [24]. Dietary compounds including fiber and SCFA modulate the dynamics of mucus by altering the mucin gene expression or the numbers of goblet cells in the isolated vascularly perfused rat colon [25].
In this study, butyrate increased gene expression levels of MUC3, MUC4, and MUC12 but not MUC2 (Fig. 4). These results are in agreement with the findings of Gaudier et al. [13]. MUC1, MUC3, MUC4, and MUC12 are members of the membrane-bound family of mucins. In particular, certain membrane-associated mucins such as MUC1 and MUC4 exhibit specific functions in bacterial adhesion [26].
Pathogenic E. coli strains cause food-borne illness, which is generally characterized by abdominal pain and diarrhea [27]. Endogenous secretions, such as mucin, may selectively provide diet-independent substrates for gut microbiota. Mucin might be a vital defense factor for removal of pathogens and support of commensal bacteria of the GIT [28]. Mack et al. [29] reported that probiotic Lactobacillus species can adhere to intestinal epithelial cells, and induce MUC3 mucin expression. Subsequently, upregulated expression of the MUC3 gene can inhibit adherence of enteric pathogen to epithelial cells. One possible mechanism involves competition of mucin binding sites between E. coli and Lactobascillus strains.
Probiotics are known to enhance human health and well-being, and their beneficial effects include competition for pathogen-binding and receptor sites in the gut [30]. Addition of mucin and probiotics, such as L. casei GG adhering to surface receptors of enterocytes, was reported to inhibit bacterial translocation of various pathogens in different in vitro models [31]. In this study, modulation of mucin secretion by butyrate treatment resulted in alteration of adherence of Lactobacillus or Bifidobacterium strains by providing favorable binding sites.
Ras, a small GTP-binding protein, plays a critical role in propagation of extracellular signals from cell membrane receptors to downstream kinase cascades. Because it plays a pivotal role in cellular proliferation and survival, gain of activating mutations or amplification of the ras gene is commonly observed in one third of all human cancers [32,33]. Consistent with the critical role of Ras, significant upregulation of k-ras by butyrate treatment appears to stimulate all three MAPK pathways. As shown in Fig. 5, treatment with butyrate resulted in upregulated expression of most downstream kinase genes of MAPK signaling pathways, including MNK1/2, RSK2, PRAK, and MSK1/2. In addition, ras upregulation resulted in the activation of p70S6K expression through the PI3K/AKT cell survival pathway (data not shown). These downstream kinases are known to promote protein synthesis and cellular growth. Upregulated MNK1/2, p70S6K, and RSK2 stimulate protein synthesis by modifying ribosomal proteins or translation initiation machinery [34,35]. These kinases also indirectly stimulate cellular proliferation and survival by post-translational modification of key transcription factors such as Myc, CREB, c-Fos, and NF-κB. In addition to these modifications, results of our Taqman array 96-well fast plate analysis showed that butyrate treatment resulted in direct upregulation of the gene expression levels of these transcription factors, resulting in pro-cellular growth and cell survival [36,37,38]. p53 tumor suppressor is a genome guardian that protects normal cells from abnormal cellular growth and cancer development [39]. p53 protein is activated by oncogenic stress involving upregulation of ras or myc [40]. Given the hyperactive cellular growth signals induced by ras and myc, LS174 cells appear to undergo a protective cellular response through upregulation of the p53 tumor suppressor pathway. Consistent with upregulated p53, anti-cancer activity of butyrate was demonstrated in various studies [41,42]. Taken together, butyrate treatment would increase cell mass and promote cellular growth along with anti-tumor activity by altering gene expression levels of MAPK signaling intermediates and key transcription factors. This is in good agreement with reports of Hatayama et al. [24] and Perris et al. [43].
In conclusion, butyrate treatment increased mucin secretion through upregulation of the transcriptional and translational levels of mucin gene expression, which stimulates bacterial adherence to the mucus layer. The underlying molecular mechanism of enhanced mucin secretion by butyrate remains to be determined, however, the activation of MAPK signaling pathways is likely to play a role in the gene activation process. Our results will provide further molecular understanding of the contribution of butyrate to homeostasis of the normal colonic mucosa and epithelial cell health by modulation of bacterial adherence and MAPK signaling pathway.
Footnotes
This paper was supported by Sahmyook University Research Fund in 2013.
References
- 1.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 2.Shimotoyodome A, Meguro S, Hase T, Tokimitsu I, Sakata T. Short chain fatty acids but not lactate or succinate stimulate mucus release in the rat colon. Comp Biochem Physiol A Mol Integr Physiol. 2000;125:525–531. doi: 10.1016/s1095-6433(00)00183-5. [DOI] [PubMed] [Google Scholar]
- 3.Blottière HM, Buecher B, Galmiche JP, Cherbut C. Molecular analysis of the effect of short-chain fatty acids on intestinal cell proliferation. Proc Nutr Soc. 2003;62:101–106. doi: 10.1079/PNS2002215. [DOI] [PubMed] [Google Scholar]
- 4.Young GP, Hu Y, Le Leu RK, Nyskohus L. Dietary fibre and colorectal cancer: a model for environment--gene interactions. Mol Nutr Food Res. 2005;49:571–584. doi: 10.1002/mnfr.200500026. [DOI] [PubMed] [Google Scholar]
- 5.Olmo N, Turnay J, Pérez-Ramos P, Lecona E, Barrasa JI, López de Silanes I, Lizarbe MA. In vitro models for the study of the effect of butyrate on human colon adenocarcinoma cells. Toxicol In Vitro. 2007;21:262–270. doi: 10.1016/j.tiv.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Kawai Y, Hanson RW, Arinze IJ. Sodium butyrate induces transcription from the G alpha(i2) gene promoter through multiple Sp1 sites in the promoter and by activating the MEK-ERK signal transduction pathway. J Biol Chem. 2001;276:25742–25752. doi: 10.1074/jbc.M102821200. [DOI] [PubMed] [Google Scholar]
- 7.Shah P, Nankova BB, Parab S, La Gamma EF. Short chain fatty acids induce TH gene expression via ERK-dependent phosphorylation of CREB protein. Brain Res. 2006;1107:13–23. doi: 10.1016/j.brainres.2006.05.097. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Zhou L, Bao YL, Wu Y, Yu CL, Huang YX, Sun Y, Zheng LH, Li YX. Butyrate induces cell apoptosis through activation of JNK MAP kinase pathway in human colon cancer RKO cells. Chem Biol Interact. 2010;185:174–181. doi: 10.1016/j.cbi.2010.03.035. [DOI] [PubMed] [Google Scholar]
- 9.Zuo L, Lu M, Zhou Q, Wei W, Wang Y. Butyrate suppresses proliferation and migration of RKO colon cancer cells though regulating endocan expression by MAPK signaling pathway. Food Chem Toxicol. 2013;62:892–900. [PubMed] [Google Scholar]
- 10.Corfield AP, Myerscough N, Longman R, Sylvester P, Arul S, Pignatelli M. Mucins and mucosal protection in the gastrointestinal tract: new prospects for mucins in the pathology of gastrointestinal disease. Gut. 2000;47:589–594. doi: 10.1136/gut.47.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han KS, Deglaire A, Sengupta R, Moughan PJ. Hydrolyzed casein influences intestinal mucin gene expression in the rat. J Agric Food Chem. 2008;56:5572–5576. doi: 10.1021/jf800080e. [DOI] [PubMed] [Google Scholar]
- 12.Allen A, Hutton DA, Pearson JP. The MUC2 gene product: a human intestinal mucin. Int J Biochem Cell Biol. 1998;30:797–801. doi: 10.1016/s1357-2725(98)00028-4. [DOI] [PubMed] [Google Scholar]
- 13.Gaudier E, Jarry A, Blottière HM, de Coppet P, Buisine MP, Aubert JP, Laboisse C, Cherbut C, Hoebler C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1168–G1174. doi: 10.1152/ajpgi.00219.2004. [DOI] [PubMed] [Google Scholar]
- 14.Trompette A, Blanchard C, Zoghbi S, Bara J, Claustre J, Jourdan G, Chayvialle JA, Plaisancé P. The DHE cell line as a model for studying rat gastro-intestinal mucin expression: effects of dexamethasone. Eur J Cell Biol. 2004;83:347–358. doi: 10.1078/0171-9335-00391. [DOI] [PubMed] [Google Scholar]
- 15.Delcenserie V, Martel D, Lamoureux M, Amiot J, Boutin Y, Roy D. Immunomodulatory effects of probiotics in the intestinal tract. Curr Issues Mol Biol. 2008;10:37–54. [PubMed] [Google Scholar]
- 16.Ouwehand AC, Salminen S. In vitro adhesion assays for probiotics and their in vivo relevance: a review. Microb Ecol Health Dis. 2003;15:175–184. [Google Scholar]
- 17.Valeriano VD, Parungao-Balolong MM, Kang DK. In vitro evaluation of the mucin-adhesion ability and probiotic potential of Lactobacillus mucosae LM1. J Appl Microbiol. 2014;117:485–497. doi: 10.1111/jam.12539. [DOI] [PubMed] [Google Scholar]
- 18.Bucki R, Namiot DB, Namiot Z, Savage PB, Janmey PA. Salivary mucins inhibit antibacterial activity of the cathelicidin-derived LL-37 peptide but not the cationic steroid CSA-13. J Antimicrob Chemother. 2008;62:329–335. doi: 10.1093/jac/dkn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kober OI, Ahl D, Pin C, Holm L, Carding SR, Juge N. gammadelta T-cell-deficient mice show alterations in mucin expression, glycosylation, and goblet cells but maintain an intact mucus layer. Am J Physiol Gastrointest Liver Physiol. 2014;306:G582–G593. doi: 10.1152/ajpgi.00218.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louis P, Scott KP, Duncan SH, Flint HJ. Understanding the effects of diet on bacterial metabolism in the large intestine. J Appl Microbiol. 2007;102:1197–1208. doi: 10.1111/j.1365-2672.2007.03322.x. [DOI] [PubMed] [Google Scholar]
- 22.Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217:133–139. doi: 10.1111/j.1574-6968.2002.tb11467.x. [DOI] [PubMed] [Google Scholar]
- 23.Gaudier E, Rival M, Buisine MP, Robineau I, Hoebler C. Butyrate enemas upregulate Muc genes expression but decrease adherent mucus thickness in mice colon. Physiol Res. 2009;58:111–119. doi: 10.33549/physiolres.931271. [DOI] [PubMed] [Google Scholar]
- 24.Hatayama H, Iwashita J, Kuwajima A, Abe T. The short chain fatty acid, butyrate, stimulates MUC2 mucin production in the human colon cancer cell line, LS174T. Biochem Biophys Res Commun. 2007;356:599–603. doi: 10.1016/j.bbrc.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 25.Barcelo A, Claustre J, Moro F, Chayvialle JA, Cuber JC, Plaisancié P. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut. 2000;46:218–224. doi: 10.1136/gut.46.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carraway KL, Ramsauer VP, Haq B, Carothers Carraway CA. Cell signaling through membrane mucins. Bioessays. 2003;25:66–71. doi: 10.1002/bies.10201. [DOI] [PubMed] [Google Scholar]
- 27.Rangel JM, Sparling PH, Crowe C, Griffin PM, Swerdlow DL. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982-2002. Emerg Infect Dis. 2005;11:603–609. doi: 10.3201/eid1104.040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deplancke B, Gaskins HR. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr. 2001;73:1131S–1141S. doi: 10.1093/ajcn/73.6.1131S. [DOI] [PubMed] [Google Scholar]
- 29.Mack DR, Ahrne S, Hyde L, Wei S, Hollingsworth MA. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut. 2003;52:827–833. doi: 10.1136/gut.52.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujiwara S, Hashiba H, Hirota T, Forstner JF. Proteinaceous factor(s) in culture supernatant fluids of bifidobacteria which prevents the binding of enterotoxigenic Escherichia coli to gangliotetraosylceramide. Appl Environ Microbiol. 1997;63:506–512. doi: 10.1128/aem.63.2.506-512.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattar AF, Teitelbaum DH, Drongowski RA, Yongyi F, Harmon CM, Coran AG. Probiotics up-regulate MUC-2 mucin gene expression in a Caco-2 cell-culture model. Pediatr Surg Int. 2002;18:586–590. doi: 10.1007/s00383-002-0855-7. [DOI] [PubMed] [Google Scholar]
- 32.Adjei AA. Blocking oncogenic Ras signaling for cancer therapy. J Natl Cancer Inst. 2001;93:1062–1074. doi: 10.1093/jnci/93.14.1062. [DOI] [PubMed] [Google Scholar]
- 33.Wu KL, Huang EY, Jhu EW, Huang YH, Su WH, Chuang PC, Yang KD. Overexpression of galectin-3 enhances migration of colon cancer cells related to activation of the K-Ras-Raf-Erk1/2 pathway. J Gastroenterol. 2013;48:350–359. doi: 10.1007/s00535-012-0663-3. [DOI] [PubMed] [Google Scholar]
- 34.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123:569–580. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 36.Reber L, Vermeulen L, Haegeman G, Frossard N. Ser276 phosphorylation of NF-κB p65 by MSK1 controls SCF expression in inflammation. PLoS One. 2009;4:e4393. doi: 10.1371/journal.pone.0004393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gancz D, Lusthaus M, Fishelson Z. A role for the NF-kappaB pathway in cell protection from complement-dependent cytotoxicity. J Immunol. 2012;189:860–866. doi: 10.4049/jimmunol.1103451. [DOI] [PubMed] [Google Scholar]
- 38.Daniel P, Filiz G, Brown DV, Hollande F, Gonzales M, D'Abaco G, Papalexis N, Phillips WA, Malaterre J, Ramsay RG, Mantamadiotis T. Selective CREB-dependent cyclin expression mediated by the PI3K and MAPK pathways supports glioma cell proliferation. Oncogenesis. 2014;3:e108. doi: 10.1038/oncsis.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 40.Horn HF, Vousden KH. Coping with stress: multiple ways to activate p53. Oncogene. 2007;26:1306–1316. doi: 10.1038/sj.onc.1210263. [DOI] [PubMed] [Google Scholar]
- 41.Williams EA, Coxhead JM, Mathers JC. Anti-cancer effects of butyrate: use of micro-array technology to investigate mechanisms. Proc Nutr Soc. 2003;62:107–115. doi: 10.1079/PNS2002230. [DOI] [PubMed] [Google Scholar]
- 42.Wang HG, Huang XD, Shen P, Li LR, Xue HT, Ji GZ. Anticancer effects of sodium butyrate on hepatocellular carcinoma cells in vitro. Int J Mol Med. 2013;31:967–974. doi: 10.3892/ijmm.2013.1285. [DOI] [PubMed] [Google Scholar]
- 43.Perrais M, Pigny P, Copin MC, Aubert JP, Van Seuningen I. Induction of MUC2 and MUC5AC mucins by factors of the epidermal growth factor (EGF) family is mediated by EGF receptor/Ras/Raf/extracellular signal-regulated kinase cascade and Sp1. J Biol Chem. 2002;277:32258–32267. doi: 10.1074/jbc.M204862200. [DOI] [PubMed] [Google Scholar]