Abstract
BACKGROUND/OBJECTIVES
Inflammation is associated with various types of acute and chronic alcohol liver diseases. In this study, we examined whether umbelliferone (7-hydroxycoumarin, UF) ameliorates chronic alcohol-induced liver damage by modulating inflammatory response and the antioxidant system.
METHODS
Rats were fed a Liber-Decarli liquid diet containing 5% alcohol with or without UF (0.05 g/L) for 8 weeks, while normal rats received an isocaloric carbohydrate liquid diet.
RESULTS
Chronic alcohol intake significantly increased serum tumor necrosis factor-α (TNF-α) and interleukin 6 levels and decreased interleukin 10 level; however, UF supplementation reversed the cytokines related to liver damage. UF significantly suppressed hepatic lipopolysaccharide binding protein, toll-like receptor 4 (TLR4), nuclear factor kappa B, and TNF-α gene expression increases in response to chronic alcohol intake. Masson's trichrome staining revealed that UF improved mild hepatic fibrosis caused by alcohol, and UF also significantly increased the mRNA expressions and activities of superoxide dismutase and catalase in liver, and thus, decreased lipid peroxide and mitochondrial hydrogen peroxide levels.
CONCLUSIONS
The findings of this study indicate that UF protects against alcohol-induced liver damage by inhibiting the TLR4 signaling pathway and activating the antioxidant system.
Keywords: Alcohol, antioxidant, inflammation, TLR4 signaling, umbelliferone
INTRODUCTION
Alcoholic liver disease (ALD) is a major cause of mortality and morbidity worldwide [1]. The liver is the main organ affected because it is the major site of alcohol metabolism and produces toxic metabolites from alcohol, such as, acetaldehyde, acetate, and reactive oxygen species [2]. The oxidative metabolism of alcohol produced acetaldehyde, which contributes to cell and tissue damage and increased oxidative stress [3], and increased oxidative stress decreases antioxidant enzymes and glutathione (GSH), resulting in DNA damage and the necrosis and apoptosis of hepatic cells [4].
Alcohol-induced oxidative stress promotes inflammation, which is aggravated by an increase in pro-inflammatory cytokines levels and by the up-regulation of the inflammatory cascade [5]. Circulating levels of the endotoxin, lipopolysaccharide (LPS), increase in rodents on a chronic alcohol diet [6]. Furthermore, LPS recognition by toll-like receptor 4 (TLR4) results in the recruitments of adaptor molecules that activate nuclear factor kappa B (NF-κB), which increases the productions of pro-inflammatory cytokines, such as, 'tumor necrosis factor α (TNF-α)' and 'interleukin 6 (IL-6)' [6]. In particular, these two cytokines are the principle mediators of early alcohol-induced liver injury. Treatment of ALD is accomplished using anti-inflammatory agents, antioxidants, and agents directed against the progression to fibrosis [7]. However, owing to the adverse side effects associated with many agents, alternative natural therapeutics are needed.
Umbelliferone (UF; 7-hydroxycoumarin; Fig. 1) is present in fruits and roots plants, such as, the golden apple, the bitter orange, and carrot [8,9,10], and several studies have shown that UF can exert potent antioxidant, antidiabetic and antitumor effects [11,12]. Kassim et al. [13] demonstrated the antioxidant property of UF by the 1,1-diphenyl-2-picrylhydrazyl free radical scavenging, and Ramesh and Pugalendi [11,14] have reported that UF has significant glucose reducing and antioxidant properties, as demonstrated by reductions in gluconeogenic enzyme levels and lipid peroxidation. In a previous study, we found that UF supplementation decreased hepatic lipid peroxide and activated antioxidant enzymes levels in high-fat fed mice [15], and it has also been reported the UF demonstrated potent anti-inflammatory activity in an ovalbumin-induced mouse model of allergic airway inflammation [16]. However, the antiinflammatory and antioxidant properties of UF have not been investigated in the context of alcohol-induced liver damage. Therefore, we investigated the effect of UF on chronic alcoholinduced inflammation and on the antioxidant system in rats.
Fig. 1. Chemical structures of UF (Umbelliferone).
MATERIALS AND METHODS
Animals and diets
Twenty-four male Sprague-Dawley rats (4-week-old) were purchased from Orient Bio Inc. (Seoul, Korea). After a one week adaptation period, animals were randomly divided into a normal control group (NC), an alcohol control group (Al), or a UF (0.05 g/L in diet, Sigma, St. Louis, MO, USA) supplemented with alcohol diet group (Al-UF). Animals were placed on these diets for 8 weeks, and were individually housed in stainless-steel cages in an air-conditioned environment at 20 ± 2℃, and relative humidity 50 ± 5% under a 12-h light. The compositions of diets are shown in Table 1. The rats in the two alcohol groups were given a liquid alcohol diet (36% of energy) in which ethanol was introduced progressively. Specifically, animals were provided with 3% ethanol for the first 2 days (21% of energy), 4% for the next 2 days (28% of energy) and 5% (36% of energy) thereafter [17]. Normal control rats received an isocaloric liquid diet containing dextrin-maltose instead of ethanol. The rats in the Al and Al-UF groups received food ad libitum, whereas NC group received the same amount of diet that the alcohol control rats consumed the previous day. The study was approved by the Sunchon National University Institutional Animal Care and Use Committee (SCNU-IACUC-2012-7).
Table 1. Composition of liquid diet (g/L/1,000 calories)1).
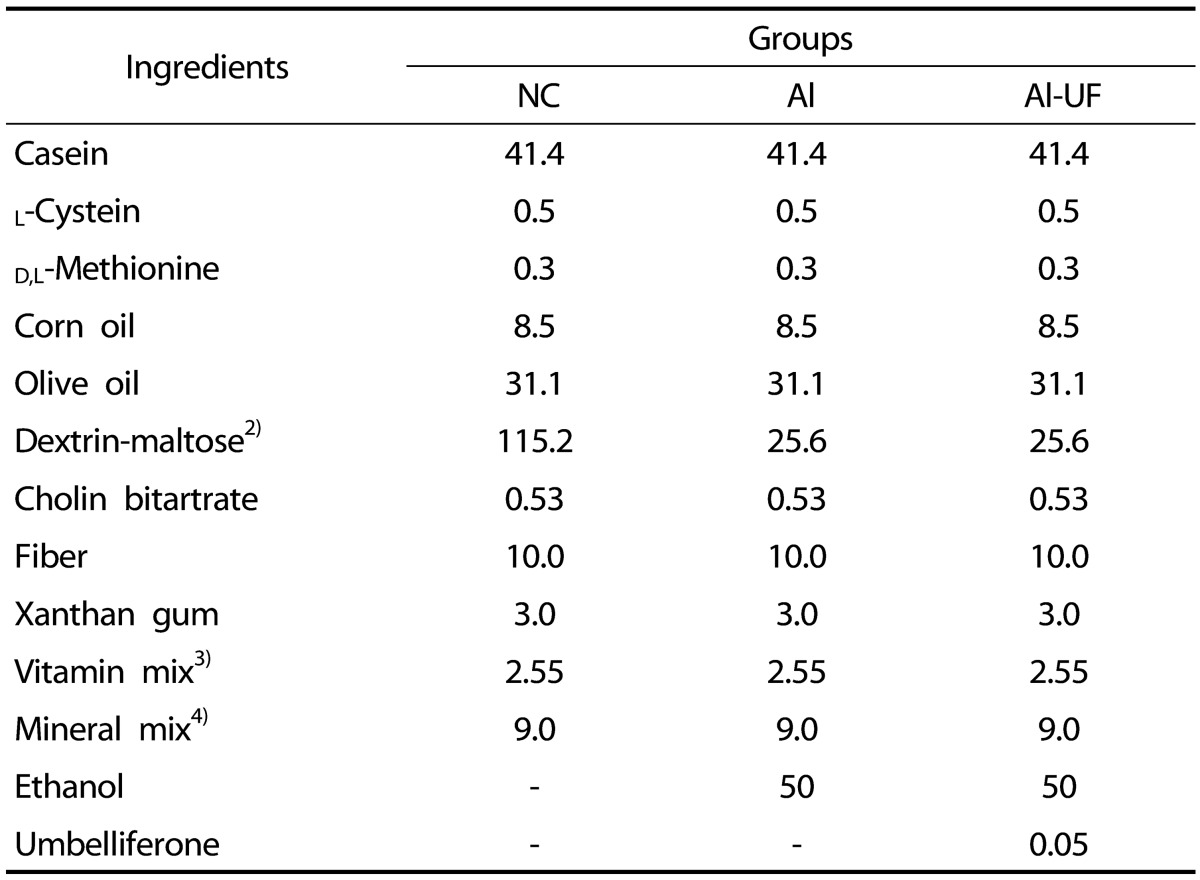
1)The liquid diet was mixed in 1 L of distilled water.
2)The ethanol in the alcohol diet was replaced with additional dextrin maltose in the NC group.
3)AIN-76 vitamin mixture.
4)AIN-76 mineral mixture.
At the end of the 8-week experimental period, animals were anesthetized with CO2 gas after a 12-h fast. Blood was then drawn from inferior vena cava into tubes, and serum was obtained by centrifuging the blood at 900 ×g for 15 min at 4℃. The organs were then removed, rinsed with physiological saline, and immediately weighed. Serum and organ samples were stored at -70℃ until required for analysis.
Serum cytokines levels
Serum cytokines levels were measured using a multiplex detection kit (M60-009RDPD, Bio-Rad, Hercules, CA, USA). Capture antibodies directed against the cytokines, TNF-α, IL-6, interleukin 10 (IL-10), interleukin 1β (IL-1β) and interferon γ (IFNγ), were covalently coupled to beads, and then reacted with serum. After washing several times to remove unbound protein, a biotinylated detection antibody was added to create a sandwich complex. The final detection complex was formed by adding streptavidin-phycoerythrin conjugate. Phycoerythrin served as a fluorescent indicator or reporter. All samples were assayed in duplicate and analyzed using a Luminex 200 Labmap system (Luminex, Austin, TX, USA). Data analysis was performed using Bio-Plex Manager software version 4.1.1 (Bio-Rad, Hercules, CA, USA).
Genes expression of inflammation and antioxidant enzymes
Liver was homogenized in Trizol reagent (Invitrogen Life Technologies, Grand Island, NY, USA), and total RNA was then isolated according to the manufacturer's instructions. DNase was used to remove DNA contamination, and the RNA was then re-precipitated in ethanol to ensure the absence of phenol contamination. For quality control, RNA purity and integrity were evaluated using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, USA). Total RNA (1 µg) was reverse-transcribed into cDNA using a QuantiTect® reverse transcription kit (Qiagen, Hilden, Germany), and then mRNA expressions were quantified by real-time quantitative PCR using a SYBR green PCR kit (Qiagen, Hilden, Germany) and the CFX96TM real-time system (Bio-Rad, Hercules, CA, UAS). The sequences of the primers used are shown in Table 2. Cycle thresholds were determined using SYBR green emission intensities during the exponential phase. Fold changes were determined using the 2-ΔΔCt method [18]. GAPDH was used as the internal control.
Table 2. Primer sequences for Real time RT-PCR.
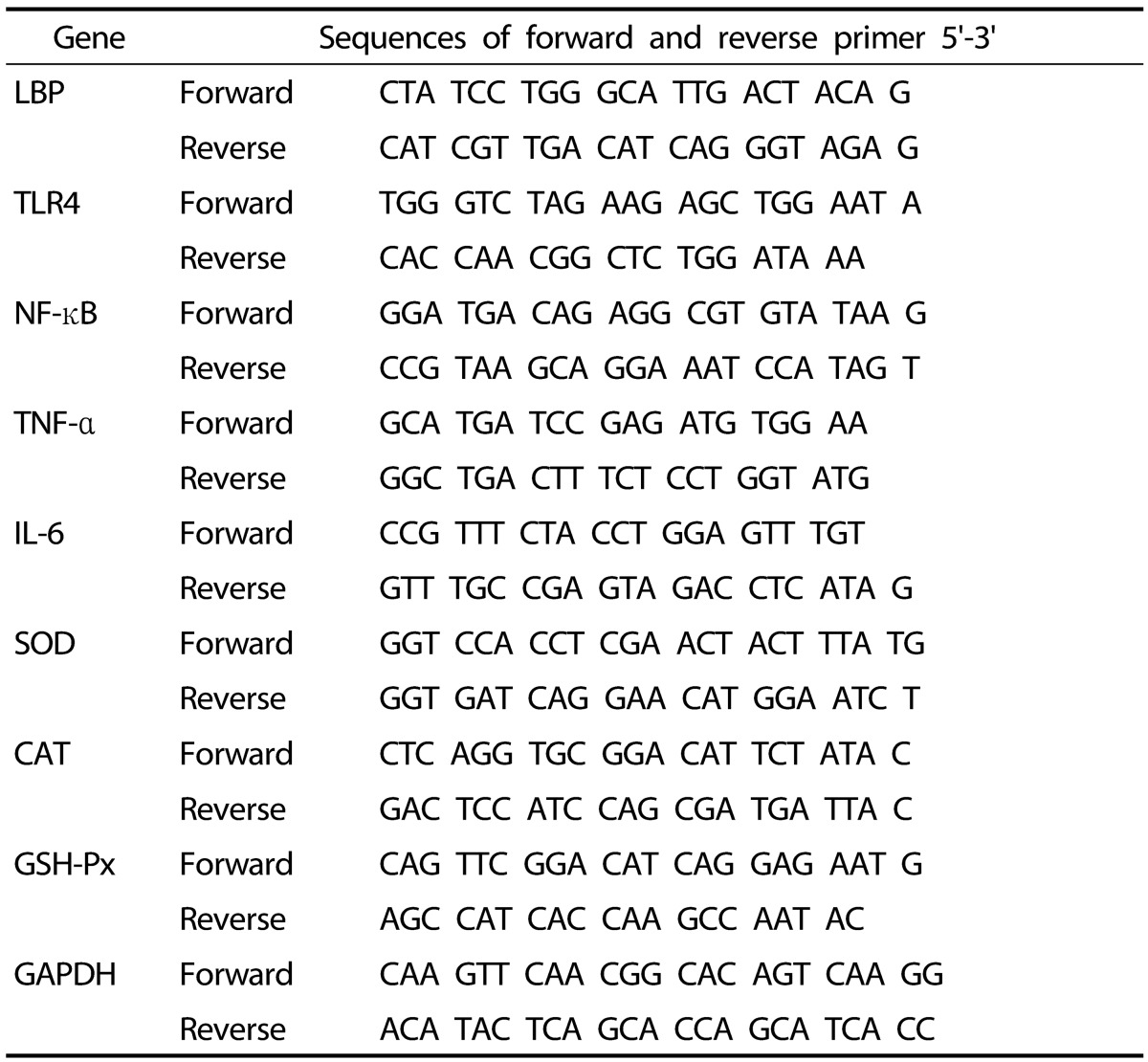
LBP: lipopolysaccharide binding protein; TLR4: toll-like receptor 4; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; TNF-α: tumor necrosis factor alpha; IL-6: interleukin 6; SOD: superoxide dismutase; CAT: catalase; GSH-Px: glutathione peroxidase; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
Antioxidant enzyme activities
Superoxide dismutase (SOD) activity was measured spectro-photometrically using a method based on the inhibition of superoxide-mediated reduction [19]. One unit was defined to be the amount of enzyme that inhibited the oxidation of pyrogallol by 50%. Catalase (CAT) activity was measured by monitoring the disappearance of hydrogen peroxide spectro-photometrically at 240 nm for 5 min, as previously described [19]. Glutathione peroxidase (GSH-Px) activity was also measured spectrophotometrically using a reaction mixture containing 1 mM glutathione, 0.2 mM NADPH and 0.24 units of glutathione reductase in 0.1 M Tris-HCl (pH 7.2) buffer. The reaction was initiated by adding 0.25 mM H2O2 and absorbance was measured at 340 nm for 5 min [19]. Glutathione-S-transferase (GST) activity was evaluated, as described by Habig et al. [20] using 1-chloro-2,4-dinitrobenzen (CDNB) as substrate. For analysis, the absorbances were measured at 340 nm in 0.1 M potassium phosphate buffer (pH 6.5) containing 60 mM CDNB, and 10 mM GSH.
Levels of GSH, H2O2, and lipid peroxide
GSH level was measured as previously described by Ellman [21]. Mitochondrial hydrogen peroxide (H2O2) levels in liver were measured using Wolff's method [22], and results are expressed as micromoles of H2O2 per milligram of mitochondrial protein. Malondialdehyde (MDA) concentration in the liver (a marker of lipid peroxidation) was measured as described previously [19].
Hepatic histology
Hepatic tissue was removed from each rat and connective tissues were removed. Samples were then fixed in 10% (v/v) paraformaldehyde/PBS, embedded in paraffin, and sectioned at 3-5 µm, and stained with Masson's trichrome to visualize collagen fibers in connective tissues. Sections were viewed under a microscope at 200×.
Statistical analysis
All results are presented as means ± SE. The analysis was performed using the student's t-test in SPSS (Chicago, IL). Statistical significance was accepted for P values of < 0.05.
RESULTS
Effects on serum cytokines levels
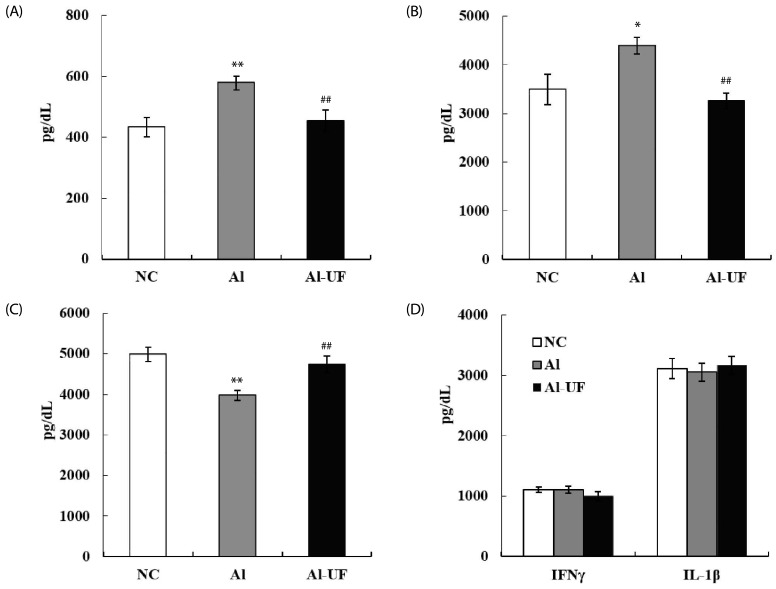
Chronic alcohol intake induced significant increases in serum TNF-α and IL-6 levels and decreased IL-10 level; however, these changes were prevented by UF supplementation. IL-1β and IFN γ levels did not differ between groups (Fig. 2).
Fig. 2. Effects of umbelliferone on serum TNF-α (A), IL-6 (B), IL-10 (C), IFNγ and IL-1β (D) levels in chronic alcohol-fed rats.
Values are expressed as the means ± SE. Values are significantly different between groups according to Student's t-test. *P < 0.05, **P < 0.01 NC : Al, ##P < 0.01 Al : Al-UF.
Effects on the expressions of inflammatory genes
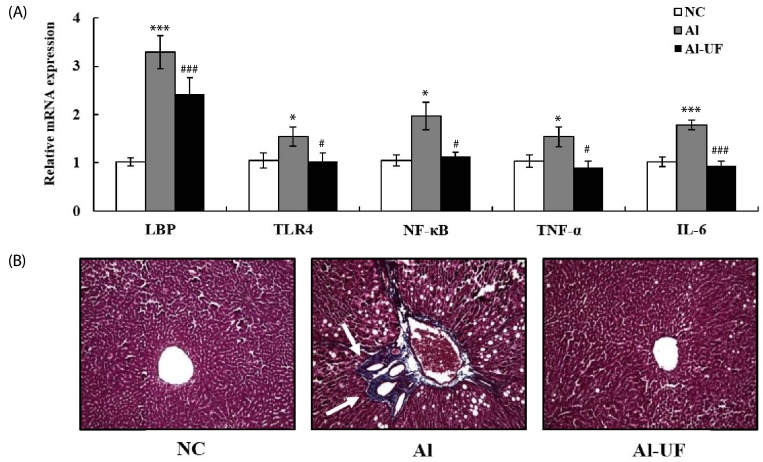
The mRNA expressions of LBP, TLR4, and NF-κB in the Al group were higher than in the NC group, but these changes were attenuated by UF supplementation. TNF-α and IL-6 mRNA expressions were up-regulated in the Al group as compared with the NC group; however, UF supplementation inhibited these up-regulations (Fig. 3A).
Fig. 3. Effects of umbelliferone on hepatic inflammatory genes expression (A) and Masson's trichrome stain (B) in chronic alcohol-fed rats.
Values are expressed as the means ± SE. Values are significantly different between groups according to Student's t-test. * P < 0.05, *** P < 0.001 NC : Al, # P < 0.05, ### P < 0.001 Al : Al-UF. Relative mRNA expression of each gene was normalized to the GAPDH and compared with NC level. White arrows indicate collagen deposition (200× magnification).
Effects on hepatic histology
Liver sections in the Al group revealed increased deposition of collagen fibers around congested central vein, indicating alcohol-induced fibrosis; however, collagen fiber deposition was less severe in the Al-UF group (Fig. 3B).
Effects on the genes expression and activities of antioxidant enzymes
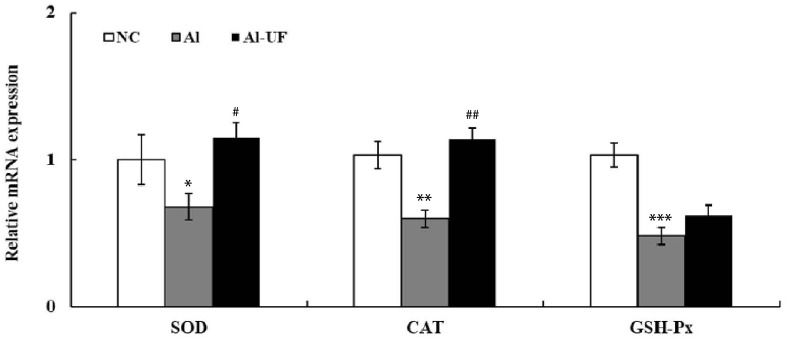
The mRNA expressions of SOD, CAT, and GSH-Px were significantly down-regulated in the Al group as compared with the NC group, but UF up-regulated SOD and CAT mRNA levels compared to the Al group (Fig. 4). The activities of SOD, CAT, GSH-Px, and GST were also significantly lower in the Al group than in the NC group. However, SOD and CAT activities were significantly elevated by UF supplementation (Table 3).
Fig. 4. Effects of umbelliferone on hepatic antioxidant enzyme genes expression in chronic alcohol-fed rats.
Values are expressed as the means ± SE. Values are significantly different between groups according to Student's t-test. *P < 0.05, **P < 0.01, ***P < 0.001 NC : Al, #P < 0.05, ##P < 0.01 Al : Al-UF. Relative mRNA expression of each gene was normalized to the GAPDH and compared with NC level.
Table 3. Effects of umbelliferone on hepatic antioxidant defense system in chronic alcohol-fed rats.
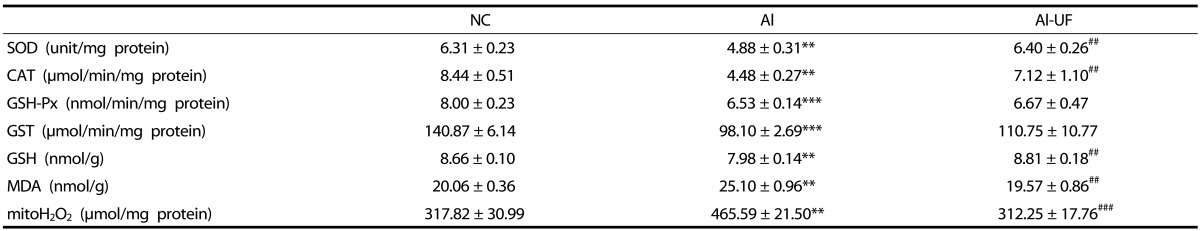
Values are mean ± SE.
Values are significantly different between groups according to Student's t-test. **P < 0.01, ***P < 0.001 NC : Al. ##P < 0.01, ###P < 0.001 Al : Al-UF.
SOD: superoxide dismutase, CAT: catalase, GSH-Px: glutathione peroxidase, GST: glutathione-S-transferase GSH: glutathione, MDA: malondialdehyde.
Effects on levels of GSH, H2O2, and lipid peroxide
H2O2 levels were significantly lower in the Al-UF group than in the Al group, in which levels were similar to those in the NC group. Hepatic MDA levels in the Al group were 25% higher than in the NC group, whereas GSH levels were by 8% lower in the Al group. However, UF supplementation attenuated these changes (Table 3).
DISCUSSION
UF is a coumarin derivative with antioxidant and anti-inflammatory effects [23,24]. Mohamed et al. [25] found that UF ameliorated CCl4-induced oxidative stress by activating nuclear factor erythroid 2-related factor 2-mediated heme oxygenase-1 in rats. However, anti-inflammatory and antioxidant properties of UF have not been previously investigated in the context of alcohol-induced liver damage. The current study shows that LBP and TLR4 mRNA levels were significantly elevated in the liver of chronic alcohol-fed rats, but that these changes were attenuated by UF supplementation. LPS is a major factor in the pathogenesis of ALD and an inducer inflammation [6]. The recognition of this endotoxin is mainly initiated by LBP, a soluble serum lipid transfer protein synthesized by the liver, whose function is to extract LPS monomers from aggregated endotoxin structures for subsequent delivery to membrane-bound or soluble CD14 [26]. Thus, the hepatic expression of the LBP gene reflects LPS influx into liver. Furthermore, the activation of TLR4 by LBP leads to the rapid activation of NF-κB, and thus, to the production of several inflammation mediators, such as, TNF-α and IL-6 [27]. In the present study, NF-κB mRNA levels were reduced by UF, which indicates a reduction in inflammatory response, as was confirmed by significant decreases in hepatic expression of TNF-α mRNA.
Pro-inflammatory cytokines, such as, TNF-α, have recently been shown to play major roles in pathogenesis of liver disease, and their serum levels have been shown to be enhanced in ALD patients and in animal models of alcohol-induced liver damage [5]. TNF-α has been suggested to mediate the early stage of fatty liver disease and transition to more advanced disease [28]. Pro-inflammatory cytokines (IL-1β, TNF-α, IL-6 or IFNγ) and anti-inflammatory cytokines (IL-10) are produced in response to LPS [29,30]. In the present study, we also found that UF supplementation decreased serum TNF-α and IL-6 levels, but increased IL-10 levels in chronic alcohol-fed rats. IL-10 is one of the most important anti-inflammatory cytokines and has been associated with the amelioration of liver inflammation in different models [31]. In addition, IL-10 controls the productions of other cytokines, such as, IL-6 and TNF-α, decreases T-cell activation [32,33], and exerts hepatic protective effects against proliferation and fibrosis [34]. On the other hand, elevated hepatic and serum levels of IL-6 have been associated with the pathogenesis of alcoholic liver injury in animal models and ALD patients, in whom serum IL-6 levels were found to be positively correlated with disease severity [27,35]. In the present study, these augmenting effects of UF on the anti-inflammatory cytokine production and pro-inflammatory cytokine suppression strongly enhanced its anti-inflammatory effect in chronic alcohol-fed rats. Thus, our findings suggest UF could protect against alcohol-induced liver fibrosis by suppressing alcohol-induced TLR4-mediated hepatic fibrosis.
Excessive chronic exposure to alcohol usually reduces antioxidant defenses in hepatic tissue and blood [36], and thus, maintenance of the antioxidant system is essential in relation to the prevention of ALD [37]. Our results show that chronic alcohol feeding decreased the hepatic genes expression and activities of antioxidant enzymes (SOD, CAT and GSH-Px) and GSH levels. Previous studies have shown diminished SOD levels are associated with the risk of cell injury [38]. SOD may play an important role in protecting cells and tissues against the toxic effects of superoxide radicals [5]. CAT, a heme protein enzyme, has been reported to remove H2O2 [39], and thus, reductions in CAT activity might reflect reduced production and/or its inhibition as a result of the increased production of free radicals [40]. We found that, UF significantly increased the activities and mRNA levels of SOD and CAT, but not of GST and GSH-Px, as compared with the Al group, and that this was associated with reduced mitochondrial H2O2 and MDA levels. MDA is a secondary metabolite produced by lipid peroxidation in cell membranes, and the amount of MDA is used as an indicator of lipid peroxidation [41]. Interestingly, in the present study, we found that hepatic MDA levels were lower in the UF-supplemented alcoholic rats, which suggests the protection afforded by UF against alcohol-induced liver injury via enhancement of antioxidant defense system. In our previous study, UF supplementation was found to decrease serum ALT and γGTP activities effectively and to suppress body weight loss by alcohol intake without changing food intake or liver weight in chronic alcohol-fed rats [42].
In conclusion, the results of the present study indicate that UF protected against alcohol-induced liver damage by inhibiting the TLR4 signaling pathway and improving the antioxidant defense system. Therefore, we suggest UF be viewed as a promising therapeutic strategy for the treatment of alcoholic liver damage.
Footnotes
This work was supported by Suncheon Research Center for Natural Medicines.
References
- 1.Frazier TH, Stocker AM, Kershner NA, Marsano LS, McClain CJ. Treatment of alcoholic liver disease. Therap Adv Gastroenterol. 2011;4:63–81. doi: 10.1177/1756283X10378925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, Wang S, Ni HM, Huang H, Ding WX. Autophagy in alcohol-induced multiorgan injury: mechanisms and potential therapeutic targets. Biomed Res Int. 2014;2014:498491. doi: 10.1155/2014/498491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44:723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albano E. Alcohol, oxidative stress and free radical damage. Proc Nutr Soc. 2006;65:278–290. doi: 10.1079/pns2006496. [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi S, Takahashi S, Sasaki T, Kumagai T, Nagata K. Progression of alcoholic and non-alcoholic steatohepatitis: common metabolic aspects of innate immune system and oxidative stress. Drug Metab Pharmacokinet. 2011;26:30–46. doi: 10.2133/dmpk.dmpk-10-rv-087. [DOI] [PubMed] [Google Scholar]
- 6.Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16:1321–1329. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tome S, Lucey MR. Review article: current management of alcoholic liver disease. Aliment Pharmacol Ther. 2004;19:707–714. doi: 10.1111/j.1365-2036.2004.01881.x. [DOI] [PubMed] [Google Scholar]
- 8.Kanimozhi G, Prasad NR, Ramachandran S, Pugalendi KV. Umbelliferone modulates gamma-radiation induced reactive oxygen species generation and subsequent oxidative damage in human blood lymphocytes. Eur J Pharmacol. 2011;672:20–29. doi: 10.1016/j.ejphar.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Jung HA, Park JJ, Islam MN, Jin SE, Min BS, Lee JH, Sohn HS, Choi JS. Inhibitory activity of coumarins from Artemisia capillaris against advanced glycation endproduct formation. Arch Pharm Res. 2012;35:1021–1035. doi: 10.1007/s12272-012-0610-0. [DOI] [PubMed] [Google Scholar]
- 10.Zhao D, Islam MN, Ahn BR, Jung HA, Kim BW, Choi JS. In vitro antioxidant and anti-inflammatory activities of Angelica decursiva. Arch Pharm Res. 2012;35:179–192. doi: 10.1007/s12272-012-0120-0. [DOI] [PubMed] [Google Scholar]
- 11.Ramesh B, Pugalendi KV. Antihyperglycemic effect of umbelliferone in streptozotocin-diabetic rats. J Med Food. 2006;9:562–566. doi: 10.1089/jmf.2006.9.562. [DOI] [PubMed] [Google Scholar]
- 12.de Lima FO, Nonato FR, Couto RD, Barbosa Filho JM, Nunes XP, Ribeiro dos Santos R, Soares MB, Villarreal CF. Mechanisms involved in the antinociceptive effects of 7-hydroxycoumarin. J Nat Prod. 2011;74:596–602. doi: 10.1021/np100621c. [DOI] [PubMed] [Google Scholar]
- 13.Kassim NK, Rahmani M, Ismail A, Sukari MA, Ee GC, Nasir NM, Awang K. Antioxidant activity-guided separation of coumarins and lignan from Melicope glabra (Rutaceae) Food Chem. 2013;139:87–92. doi: 10.1016/j.foodchem.2013.01.108. [DOI] [PubMed] [Google Scholar]
- 14.Ramesh B, Pugalendi KV. Antioxidant role of Umbelliferone in STZ-diabetic rats. Life Sci. 2006;79:306–310. doi: 10.1016/j.lfs.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Sim MO, Ham JR, Lee HI, Seo KI, Lee MK. Long-term supplementation of umbelliferone and 4-methylumbelliferone alleviates high-fat diet induced hypertriglyceridemia and hyperglycemia in mice. Chem Biol Interact. 2014;216:9–16. doi: 10.1016/j.cbi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Vasconcelos JF, Teixeira MM, Barbosa-Filho JM, Agra MF, Nunes XP, Giulietti AM, Ribeiro-Dos-Santos R, Soares MB. Effects of umbelliferone in a murine model of allergic airway inflammation. Eur J Pharmacol. 2009;609:126–131. doi: 10.1016/j.ejphar.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 17.Lieber CS, DeCarli LM. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol. 1989;24:197–211. [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Seo KI, Choi MS, Jung UJ, Kim HJ, Yeo J, Jeon SM, Lee MK. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol Nutr Food Res. 2008;52:995–1004. doi: 10.1002/mnfr.200700184. [DOI] [PubMed] [Google Scholar]
- 20.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 21.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 22.Wolff SP. [18] Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol. 1994;233:182–189. [Google Scholar]
- 23.Park WH, Park SY, Kim HM, Kim CH. Effect of a Korean traditional formulation, Hwaotang, on superoxide generation in human neutrophils, platelet aggregation in human blood, and nitric oxide, prostaglandin E2 production and paw oedema induced by carrageenan in mice. Immunopharmacol Immunotoxicol. 2004;26:53–73. doi: 10.1081/iph-120029945. [DOI] [PubMed] [Google Scholar]
- 24.Qin F, Sun HX. Immunosuppressive activity of Pollen Typhae ethanol extract on the immune responses in mice. J Ethnopharmacol. 2005;102:424–429. doi: 10.1016/j.jep.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 25.Mohamed MR, Emam MA, Hassan NS, Mogadem AI. Umbelliferone and daphnetin ameliorate carbon tetrachloride-induced hepatotoxicity in rats via nuclear factor erythroid 2-related factor 2-mediated heme oxygenase-1 expression. Environ Toxicol Pharmacol. 2014;38:531–541. doi: 10.1016/j.etap.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Tobias PS, Soldau K, Gegner JA, Mintz D, Ulevitch RJ. Lipopolysaccharide binding protein-mediated complexation of lipopolysaccharide with soluble CD14. J Biol Chem. 1995;270:10482–10488. doi: 10.1074/jbc.270.18.10482. [DOI] [PubMed] [Google Scholar]
- 27.Yoon SJ, Koh EJ, Kim CS, Zee OP, Kwak JH, Jeong WJ, Kim JH, Lee SM. Agrimonia eupatoria protects against chronic ethanol-induced liver injury in rats. Food Chem Toxicol. 2012;50:2335–2341. doi: 10.1016/j.fct.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- 29.Aono K, Isobe K, Kiuchi K, Fan ZH, Ito M, Takeuchi A, Miyachi M, Nakashima I, Nimura Y. In vitro and in vivo expression of inducible nitric oxide synthase during experimental endotoxemia: involvement of other cytokines. J Cell Biochem. 1997;65:349–358. [PubMed] [Google Scholar]
- 30.Luster MI, Germolec DR, Yoshida T, Kayama F, Thompson M. Endotoxin-induced cytokine gene expression and excretion in the liver. Hepatology. 1994;19:480–488. [PubMed] [Google Scholar]
- 31.An L, Wang X, Cederbaum AI. Cytokines in alcoholic liver disease. Arch Toxicol. 2012;86:1337–1348. doi: 10.1007/s00204-012-0814-6. [DOI] [PubMed] [Google Scholar]
- 32.Lee SH, Lee E, Ko YT. Anti-inflammatory effects of a methanol extract from Pulsatilla koreana in lipopolysaccharide-exposed rats. BMB Rep. 2012;45:371–376. doi: 10.5483/bmbrep.2012.45.6.018. [DOI] [PubMed] [Google Scholar]
- 33.Thompson KC, Trowern A, Fowell A, Marathe M, Haycock C, Arthur MJ, Sheron N. Primary rat and mouse hepatic stellate cells express the macrophage inhibitor cytokine interleukin-10 during the course of activation In vitro. Hepatology. 1998;28:1518–1524. doi: 10.1002/hep.510280611. [DOI] [PubMed] [Google Scholar]
- 34.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 35.Hill DB, Marsano L, Cohen D, Allen J, Shedlofsky S, McClain CJ. Increased plasma interleukin-6 concentrations in alcoholic hepatitis. J Lab Clin Med. 1992;119:547–552. [PubMed] [Google Scholar]
- 36.Zima T, Kalousová M. Oxidative stress and signal transduction pathways in alcoholic liver disease. Alcohol Clin Exp Res. 2005;29:110S–115S. doi: 10.1097/01.alc.0000189288.30358.4b. [DOI] [PubMed] [Google Scholar]
- 37.Suhail M, Suhail S, Gupta BK, Bharat V. Malondialdehyde and antioxidant enzymes in maternal and cord blood, and their correlation in normotensive and preeclamptic women. J Clin Med Res. 2009;1:150–157. doi: 10.4021/jocmr2009.07.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu H, Jia Z, Misra H, Li YR. Oxidative stress and redox signaling mechanisms of alcoholic liver disease: updated experimental and clinical evidence. J Dig Dis. 2012;13:133–142. doi: 10.1111/j.1751-2980.2011.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohd Yusof H, Ali NM, Yeap SK, Ho WY, Beh BK, Koh SP, Long K, Abdul Aziz S, Alitheen NB. Hepatoprotective effect of fermented soybean (Nutrient Enriched Soybean Tempeh) against alcohol-induced liver damage in mice. Evid Based Complement Alternat Med. 2013;2013:274274. doi: 10.1155/2013/274274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park HY, Ha SK, Eom H, Choi I. Narirutin fraction from citrus peels attenuates alcoholic liver disease in mice. Food Chem Toxicol. 2013;55:637–644. doi: 10.1016/j.fct.2013.01.060. [DOI] [PubMed] [Google Scholar]
- 41.Niemelä O, Alatalo P. Biomarkers of alcohol consumption and related liver disease. Scand J Clin Lab Invest. 2010;70:305–312. doi: 10.3109/00365513.2010.486442. [DOI] [PubMed] [Google Scholar]
- 42.Kim MJ, Sim MO, Lee HI, Ham JR, Seo KI, Lee MK. Dietary umbelliferone attenuates alcohol-induced fatty liver via regulation of PPARα and SREBP-1c in rats. Alcohol. 2014;48:707–715. doi: 10.1016/j.alcohol.2014.08.008. [DOI] [PubMed] [Google Scholar]